Abstract
Alpha-emitters can be pharmacologically delivered for irradiation of single cancer cells, but cellular lethality could be further enhanced by targeting alpha-emitters directly to the nucleus. Poly(ADP-ribose) polymerase (PARP) 1 is a druggable protein in the nucleus that is overexpressed in neuroblastoma compared with normal pediatric tissues and is associated with decreased survival in high-risk patients. To exploit this, we have functionalized a PARP inhibitor with an alpha-emitter astatine-211. This approach offers enhanced cytotoxicity from conventional PARP inhibitors by not requiring enzymatic inhibition of PARP-1 to elicit DNA damage; instead, the alpha-particle directly induces multiple double strand DNA breaks across the particle track. Here, we explored the efficacy of [211At]MM4 in multiple cancers and found neuroblastoma to be highly sensitive in vitro and in vivo. Furthermore, alpha-particles delivered to neuroblastoma show antitumor effects and durable responses in a neuroblastoma xenograft model, especially when administered in a fractionated regimen. This work provides the pre-clinical proof-of-concept for an alpha-emitting drug conjugate that directly targets cancer chromatin as a therapeutic approach for neuroblastoma and perhaps other cancers.
Neuroblastoma is the most common malignancy diagnosed in the first year of life.[1] While approximately half of neuroblastomas are cured with little or no cytotoxic therapy, the remaining “high-risk” cases are characterized by an aggressive clinical phenotype with widespread hematogenous metastases and de novo or acquired therapy resistance.[1] Neuroblastomas are typically highly radiosensitive, requiring relatively low doses of external beam radiation to prevent local recurrence.[2] Iodine-131-meta-iodobenzylguanidine ([131I]MIBG) is a beta-emitting radiotherapeutic that shows single agent response rates of 30–40% in the relapse setting[3–5], and is presently being tested for efficacy when integrated into frontline therapy. The primary drawback of [131I]MIBG is the inability of beta radiation to induce sufficient cytotoxic DNA damage in single cells or micrometastatic clusters, which is likely the primary reason for its failure to induce durable remission.[1, 6] Newer approaches are being developed to specifically target single cancer cells using highly cytotoxic radionuclides that emit high linear energy transfer (high-LET) radiation such as alpha particles.[7, 8]
Alpha radiation is emission of charged particles that each consists of 2 protons and 2 neutrons. When emitted from the nucleus of an atom, alpha particles travel 50–70 μm depositing all of their energy over a short path-length, resulting in high-LET.[7, 8] Astatine-211 is an alpha-emitting radiohalogen with a 7.2 hour half-life that can be produced using cyclotron particle accelerators.[9] As a radiohalogen, astatine-211 can be chemically incorporated to different drug constructs to deliver highly cytotoxic alpha particles with single cell or sub-cellular specificity. Interestingly, the sub-cellular specificity of the alpha-emitter delivery may hold significant biological relevance as it has been previously shown that fewer than 20 alpha particles traversing a nucleus is sufficient to induce irreparable DNA damage and cell death.[8] A single alpha particle crossing the nucleus of a cell is estimated to interact with DNA and induce double strand DNA breaks at approximately 50 different sites.[10] Furthermore, targeting the nucleus with an alpha-emitter has a secondary radiation effect known as recoil, where after the emission of the alpha particle the ionized atomic parent nucleus is accelerated in the opposite direction with energies of 1600–1800 keV/μm.[10] This recoil is considered high-LET as well and has a path-length of approximately 100 nm which is less than the diameter of a nucleus.[10] So when an alpha-emitter is delivered to the nucleus of a cell, the emitted alpha-particle and recoiled atomic parent nuclei will both deposit high-LET to the DNA, effectively increasing the radiation dose compared to alpha-emitters having sub-cellular localization outside of the nucleus. Therefore, if DNA damage is the primary target for alpha particle therapy, then the sub-cellular delivery of alpha-emitters to cell nuclei will increase the cytotoxicity due to the high probability of both the alpha particle and its atomic parent nuclei recoil radiation traversing the cell nucleus.
Poly(ADP-ribose) polymerase 1 (PARP-1) is a suitable molecular target for the specific delivery of alpha-particles to DNA due to its nuclear sub-cellular location and typical overexpression in cancer cells compared to normal tissue, thus offering a potential therapeutic index for radiopharmaceutical therapy.[11] In addition, since PARP-1 expression is increased in response to DNA damage[12], targeting PARP-1 with cytotoxic radiation may up-regulate the target and increase binding of the therapeutic radiopharmaceutical in subsequent doses. Furthermore, PARP-1 is the second most abundantly expressed protein in the nucleus after histones, and is a well-established druggable target with anticancer agents known as PARP inhibitors (PARPi) that biochemically inhibit PARP-1 and other PARP enzymes.[13, 14]
The biochemical function of PARP-1 is to metabolize nicotinamide adenine dinucleotide (NAD+) into poly-adenosine diphosphate ribose (PAR) for post-translational modification of itself and its partner proteins. In the DNA damage response pathway, PARP-1 strongly binds to a variety of DNA lesions, subsequently undergoing allosteric activation of PAR synthesis and self-modification until it forcefully dissociates from DNA due to negative repulsive forces between PAR and the sugar phosphate backbone of DNA.[15] The mechanism that PARPis induce cellular lethality is based on catalytic inhibition of PARP-1 in DNA repair deficient cancers, which leads to PARP trapping, DNA damage, and cell death. Previously, it has been shown that even the most potent PARPi, talazoparib, is ineffective in pediatric mouse tumor models including neuroblastoma despite showing efficacy in vitro.[16] However, strong synergy was observed when talazoparib was combined with temozolamide or topotecan.[16] Other drawbacks of PARPi, aside for multiple resistance mechanisms [13], are their dependence on catalytic inhibition to induce PARP-1 trapping which blocks pro-inflammatory or cell death pathways such as ribosylation, parthanatos, and necrosis which may be important for therapeutic response. For these reasons, we hypothesize that mechanisms to induce DNA damage without disrupting physiological cell signaling pathways of PARP-1 will have the potential not only to kill cancer cells through inducing irreparable DNA damage, but also to promote cell death and inflammatory pathways dependent on PARP-1.[17]
In the present study, we utilize the druggable properties of PARP-1 that allow for cancer targeting and delivery of alpha-emitting radiopharmaceutical conjugates to the nucleus of cancer cells for therapy. This unique approach is distinct from conventional PARP-1 inhibition due to radiopharmaceuticals being below the pharmacologically active concentrations of PARPis. Previously, we reported [125I]KX1 (1-(4-(tributylstannyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one) as a PARP inhibitor radioligand that can be used for evaluating pharmacological binding properties of PARP inhibitors in vitro and in vivo.[18, 19] Herein we describe a small molecule radiopharmaceutical PARPi derived from [125I]KX1 that instead of iodine-125 has an alpha-emitting radionuclide, astatine-211 (211At), chemically incorporated to afford [211At]MM4 (1-(4-astatophenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one).[18, 19] We report the pre-clinical evaluation of [211At]MM4 for efficacy as a radiopharmaceutical therapeutic in high-risk neuroblastoma models.
Materials and Methods
Clinical samples
The neuroblastoma tumor mini-array was prepared with single punches from formalin-fixed paraffin-embedded neuroblastoma tumors of 23 patients with high-risk neuroblastoma from the Children’s Hospital of Philadelphia (CHOP) tissue bank. This study was approved by the CHOP institutional review board (IRB).
PARP-1 expression in neuroblastoma and pediatric normal tissue
To determine the relative expression of PARP-1 in neuroblastoma vs. pediatric normal tissue, we used RNA sequencing data from the Therapeutically Applicable Research to Generate Effective Treatments project (TARGET data matrix; https://ocg.cancer.gov/programs/target/data-matrix) and the Genotype Tissue Expression (GTEx) project. Two publicly available databases (GSE49711 and GSE49711) were used to evaluate PARP-1 expression in low-risk and high-risk patients and were used to evaluate the overall survival.
Overall survival analysis
Kaplan-Meier curves were used to analyze overall survival in low-risk and high-risk neurobastoma of two large data sets with survival endpoints (GSE49711; high risk n= 176, low-risk n=322, GSE49711; high-risk= 176, low-risk n=322). Low-risk and high-risk groups were separately analyzed for survival.
Cell culture
All cell lines were cultured using standard conditions at 37 °C with 5% CO2 and 15% O2. For a complete list of cell lines evaluated in these studies please refer to SI table 1. Neuroblastoma cell lines were obtained from the CHOP cell line bank and were routinely tested for mycoplasma and genomic identity using the AmpFLSTR IDentifiler Kit (Thermo Fischer Scientific). All other cell lines were obtained from ATCC and the Basser Center for BRCA at the University of Pennsylvania. Breast and ovarian cancer cell lines were not authenticated in this study. Cell lines were thawed and evaluated after 6 passages and up to 20 passages for the experiments described.
Radiochemistry
[211At]MM4 was synthesized identically as previously reported.[18] This method was identical to the radiosynthesis of [125I]KX1 except for the final step of radiohalogenation where astatine-211 was used instead of iodine-125.[19]
Evaluating PARP-1 affinity
The affinity of [211At]MM4 was measured using a reverse competitive inhibition experiment. The experimental conditions are reported in the Supplemental Information Materials and Methods.
Cytotoxicity
Using [211At]MM4 at a single dose of 185 kBq/mL, we screened a panel of cancer cell lines. Cells were plated at 5,000 cells/well in black wall coated 96-well plates in quadruplicates 24 h prior to the addition of [211At]MM4 or 0.1% ethanol vehicle control, and cells were exposed to treatment for 72 h. Next, cell numbers were quantified using CellTiter-Glo (Promega, Waltham MA) and luminescence was quantified on an Enspire (Perkin Elmer, Waltham MA) multi-mode plate reader. The surviving fraction of cells was quantified by dividing the luminescence intensity in treated wells vs. the average control wells for each respective cell line. We then characterized the dose response to [211At]MM4 in nineteen neuroblastoma cell lines. Briefly, cells were plated as described above and treated with concentrations of [211At]MM4 ranging from 0.000037 – 370 kBq/mL. Surviving fractions were quantified as stated above and effective concentrations for 50% reduction in cell viability (EC50) were calculated using non-linear fit sigmoidal dose response curves (Graph Pad Version 7.0, Prism). Cytotoxicity experiments with non-radioactive PARP inhibitors were carried out in an identical manner. Experiments were completed in triplicates, independently three times.
Immunofluorescence and Western blot analysis
We performed immunofluorescence for PARP-1 and γH2AX at baseline and after treatment with [211At]MM4 in neuroblastoma cell lines (SI table 1). Experiments were completed in duplicates, independently three times and fluorescent images were taken under identical exposure settings. Western blot analysis was performed in neuroblastoma cell lines under identical conditions at the same time points to measure PARP-1 and γH2AX using methods previously described.[18] See Supplemental Information Materials and Methods for detailed methods.
DNA damage and cell cycle analysis by flow cytometry
Double stranded DNA breaks were quantified by flow cytometry through measuring phosphorylation of ATM and γH2AX using a commercially available kit (Muse Multi-Color DNA Damage Kit, Millipore) on a Muse Cell Analyzer (Millipore, Billerica MA). Neuroblastoma cells were treated with 37 kBq/mL of [211At]MM4 or 0.1% ethanol vehicle control for 1, 4, or 24 h, and then handled according to the commercial protocol for the Multi-Color DNA Damage Kit. Cell cycle analysis was performed using the same treatment followed by propidium iodide staining using a Muse Cell Cycle Assay Kit on the Muse Cell Analyzer. Experiments were completed three independent times.
In vivo studies
All animal studies were conducted under approved IACUC protocols at the University of Pennsylvania. IMR-05 tumor cells were subcutaneously injected into the left flank of 10-week-old female SHC mice and were allowed to grow for two weeks. Approximately 370 kBq of [211At]MM4 was injected intravenously in IMR-05 tumor bearing mice and the biodistribution was evaluated at time points of 2 min, 1 and 2 h (n=4). At each time point, the animals were sacrificed and their organs were removed for radioactivity measurement on a Perkin Elmer Wizard gamma counter. Ex vivo autoradiography methods are reported in the Supplemental Information Materials and Methods. We performed two in vivo efficacy studies using IMR-05 xenografts implanted on 10-week-old female SHC mice. See Supplemental Information Materials and Methods for more information.
Statistical analysis
Standard error of the mean (SEM) was used to determine differences between groups. The number (n) is listed next to each experiment to note the size of replicates. All in vitro experiments were completed three independent times. Data was deemed statistically significant if p-values were below 0.05. When comparing two groups, a two-sided t-test was used and if there were multiple groups, a nonparametric ANOVA analysis was performed comparing all groups to a single control group. In vivo efficacy studies were evaluated using Kaplan-Meier curves and tumor growth was modeled using a mixed linear equation to determine statistical significance between curves. All statistical analyses were performed using GraphPad Prism Version 6.0.
Results
PARP-1 expression in neuroblastoma and impact on survival
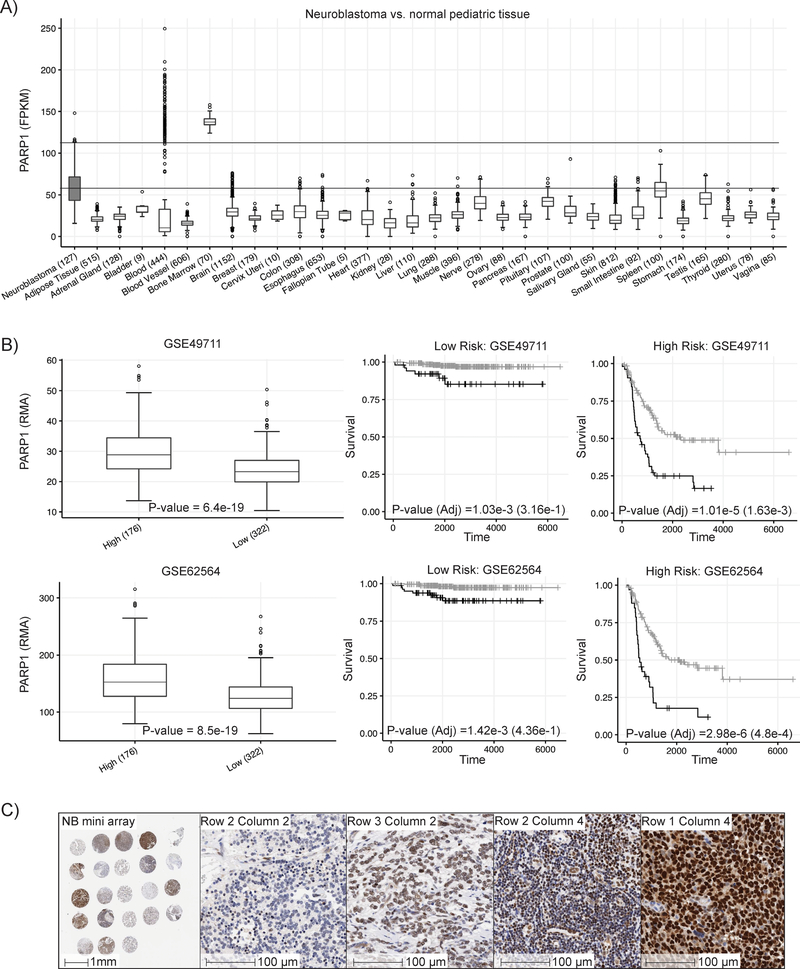
To evaluate PARP-1 as a candidate for targeted radiotherapy in neuroblastoma, we evaluated 126 high-risk primary neuroblastomas profiled via the Therapeutically Applicable Research to Generate Effective Treatments project (TARGET; https://ocg.cancer.gov/programs/target) with normal tissue RNA-sequencing data (n = 7,859 samples across 31 unique normal tissues, range = 5 – 1,152 samples per tissue; GTEx https://www.gtexportal.org ) (figure 1a). We found PARP-1 to be elevated in neuroblastoma compared to normal tissues. Further analyzing two public databases of neuroblastoma we found high PARP-1 expression is associated with decreased survival in high-risk patients (figure 1b). As the same effect was not observed in low-risk patients, this highlights the need and opportunity of utilizing PARP-1 as a therapeutic target in high-risk neuroblastoma. To confirm differential expression within the high-risk subtype, we performed immunohistochemical staining for PARP-1 on a single punch mini array of 23 cases of high-risk neuroblastoma taken from the CHOP tissue bank (figure 1c). We observed a sliding grading scale in the mini array that showed some patients highly overexpressed PARP-1 and could be candidates for a PARP-1 targeted radiotherapeutic as described in this work.
Figure 1:
Expression patterns of PARP-1 at the mRNA and protein level in normal pediatric tissues and neuroblastoma. A) PARP-1 mRNA is elevated in neuroblastoma vs. normal pediatric tissues allowing for the selective delivery of PARP-1 targeted radiopharmaceutical therapeutic. B) Two publicly available databases showed elevated PARP-1 mRNA expression was positively correlated with high-risk and worse prognosis in patients with neuroblastoma, highlighting the need and opportunity for developing a PARP-1 targeted radiopharmaceutical therapeutic. C) A single punch mini array from 23 clinical cases of neuroblastoma confirmed that PARP-1 expression is variable and that its overexpression can be targeted for delivery of alpha-emitting radiopharmaceutical therapeutic.
Radiochemistry
Astatine-211-MM4 ([211At]MM4) was synthesized using a stannylated precursor and electrophilic aromatic substitution (SI figure 1). The final product was purified by semi-preparative high-performance liquid chromatography (HPLC) with reaction recovery yields of 70 ± 10% with total radioactivity amounts of 37–72 MBq. The radiochemical purity of [211At]MM4 was greater than 95% with the specific activity ranging from 950,801–16,021,000 GBq/mmol, with lower limit defined by the limit of detection for the HPLC and the upper limit defined by the theoretical maximum. Quality control confirmed using the theoretical specific activity of astatine-211 was appropriate when no mass was detectable in the final product by HPLC equipped with a ultra-violet (UV) detector with a limit of detection of 10 ng for iodinated analogue KX1. Saline or cell culture media was used to dilute the final product to a final ethanol concentration below 10% for intravenous administration to animals or below 0.1% for cell culture experiments. Nuclear Magnetic Resonance spectroscopy and mass spectrometry were not performed due to low molar mass of [211At]MM4 and the fact that no stable isotopes of astatine-211 exist in nature which prevents the synthesis of analytical quantities of non-radioactive standards.
In vitro cytotoxicity
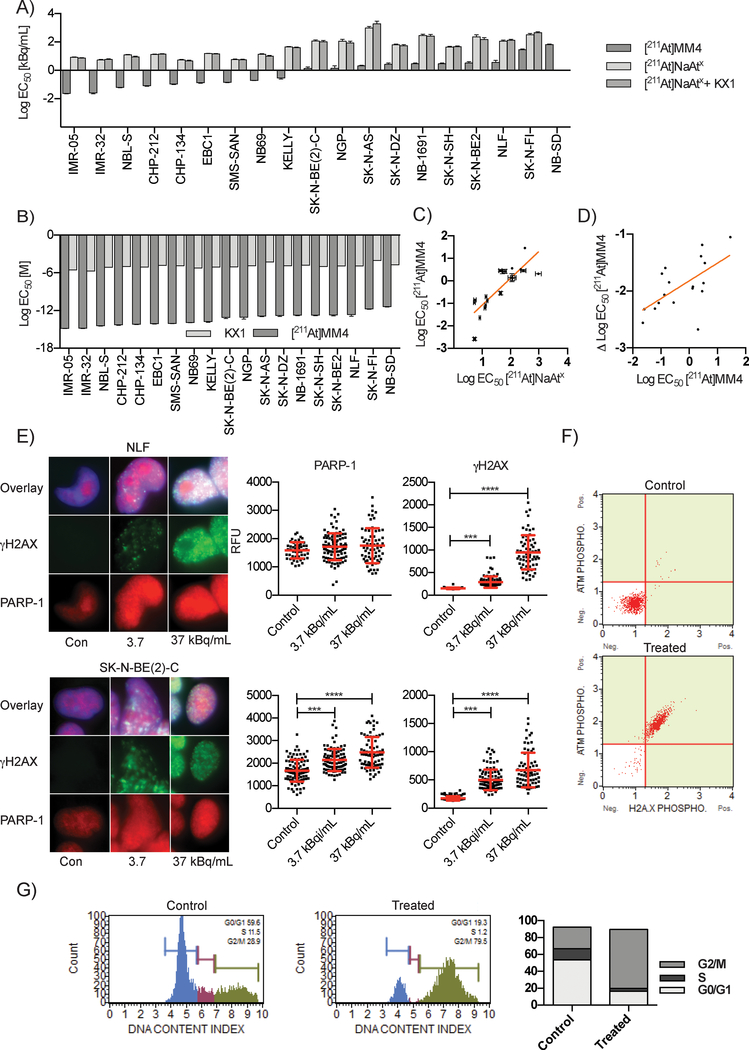
Before evaluating the cytotoxicity of [211At]MM4, we measured the affinity of [211At]MM4 to PARP-1 and calculated a dissociation constant (Kd) of 0.1 ± 0.08 nM (SI figure 2). Next, we chose to evaluate the in vitro cytotoxicity of [211At]MM4 in ovarian, breast, and neuroblastoma cancer cell lines due to strong pre-clinical and clinical evidence for sensitivity to DNA damaging therapies, including PARP inhibitors.[20–22] We found that [211At]MM4 showed broad activity in ovarian, breast, and neuroblastoma cancer cell lines (SI figure 3a and SI table 1). Neuroblastoma and ovarian cancer cell lines were the most sensitive.
Further characterizing the in vitro sensitivity using escalating doses, we evaluated nineteen neuroblastoma and four ovarian cancer cell lines. We identified IMR-05 as the most sensitive cell line and NB-SD as the most resistant neuroblastoma cell line (figure 2a, SI figure 3b, SI figure 3c, SI table 2, and SI table 3). We were able to show that the sensitivity to [211At]MM4 was PARP-1 specific by treating neuroblastoma cells with equal doses of un-conjugated free astatine-211 ([211At]NaAtx) and [211At]NaAtx with non-radioactive PARP inhibitor analogue KX1, both of which had significantly reduced effects on cell kill (figure 2a, ANOVA p-value < 0.0001, SI figure 3d, and SI figure 3e). We found that [211At]MM4 caused cell death at concentrations over 107 times lower than analogue PARPi KX1 (figure 2b, SI figure 3f, and SI table 4) and below concentrations required for catalytic inhibition of PARP-1. The cytotoxic sensitivity of the neuroblastoma cell lines to [211At]MM4 and [211At]NaAtx showed a strong positive correlation (R2 = 0.708, p<0.0001 for non-zero slope) (Figure 2c). However, more sensitive cell lines demonstrated greater sensitivity to [211At]MM4 even when normalized against their [211At]NaAtx sensitivity (R2 = 0.400, p<0.01 for non-zero slope) (figure 2d). This suggested that while the cytotoxic potency of [211At]MM4 depends on sensitivity to alpha radiation in general, additional mechanisms are at play in the sensitive cell lines to confer greater [211At]MM4 toxicity than what can be explained by radiosensitivity alone.
Figure 2:
In vitro studies evaluating [211At]MM4 cytotoxicity and DNA damage in neuroblastoma cell lines. A) Waterfall plot of EC50 values for a panel of 19 neuroblastoma cell lines that were used to test the in vitro cytotoxicity of [211At]MM4 vs. controls: free astatine-211 ([211At]NaAtx), and [211At]NaAtx with non-radioactive analogue PARP inhibitor KX1 (ANOVA p-value < 0.0001 for [211At]MM4 vs. controls in all cell lines). B) The comparison of EC50 values in molar units for [211At]MM4 vs. KX1 (ANOVA p- value < 0.0001 for all cell lines). C) Neuroblastoma cell line radiosensitivity correlation between [211At]MM4 and [211At]NaAtx (linear regression R2 = 0.708, p-value <0.0001 for non-zero slope). D) Neuroblastoma cell line radiosensitivity correlation between [211At]MM4 and [211At]MM4 normalized with [211At]NaAtx (linear regression R2 = 0.400, p-value <0.0033 for non-zero slope). E) Immunofluorescence of γH2AX and PARP-1 after 24 h treatment with [211At]MM4. PARP-1 was increased (t-test, p-value < 0.001) in SK-N-BE(2)-C cells and both cell lines showed increased γH2AX (t-test, p-value < 0.001). F) NLF cells treated with [211At]MM4 were analyzed by flow cytometry for ATM and H2AX phosphorylation. There was a 98% increase in ATM and H2AX phosphorylation in treated cells from control indicating double strand DNA breaks were the major form of DNA damage. G) Cell cycle analysis of NLF cells treated cells showed accumulation at the G2/M boundary.
We then tested whether restoration of the homologous recombination DNA repair pathway caused resistance to [211At]MM4 using BRCA1 mutant UWB1.289 homologous recombination deficient (HRD) cells and their isogenic pair with a functional copy of BRCA1 restored. We found no difference in sensitivity between isogenic ovarian cancer cell lines UWB1.289 and UWB1.289-BRCA1 restored. In addition, a similar sensitivity was observed in BRCA1 mutant cell line SNU-251 although greater than 10-fold reduced sensitivity was observed in BRCA1 wild-type SKOV3 cells (SI Figure 3b and SI table 3). This data suggest that restoration of BRCA1 does not cause resistance to [211At]MM4 in this model of HRD, but DNA repair proficient cells, like SKOV3, are comparably de novo resistant. Lastly, SKOV3 cells may have other pro-survival genes activated that would increase their fitness against [211At]MM4.
Finally, The neuroblastoma cell lines were not sensitive to clinically used non-radioactive PARPi (SI figure 4, SI table 5, and SI table 6). These combined results showed that [211At]MM4 not only binds to PARP-1 with high affinity, but also effectively targets neuroblastoma, ovarian, and breast cancer cells in vitro to induce cell death at concentrations well below those required for pharmacological inhibition of PARP-1.
DNA damage response
After we evaluated the cytotoxicity of [211At]MM4, we then evaluated its ability to induce DNA damage in a dose-dependent manner in neuroblastoma cell lines. We found that [211At]MM4 caused dose-dependent increases in DNA damage as measured by γH2AX (figure 2e, SI figure 5, and SI figure 6). In addition, we observed significant increases in PARP-1 in the SK-N-BE(2)-C cell line after 24 h of treatment (ANOVA, p-value < 0.0001). Other cell lines also showed significant increases in PARP-1 after treatment with [211At]MM4 at 1, 4 and 24 h (SI figure 5 and SI figure 6). Western blot analysis showed PARP-1 increased from control at 1 and 4 h in NLF treated cells but also revealed that PARP-1 was cleaved when cells were treated for 24 h indicating apoptosis (SI figure 7). Together this shows that [211At]MM4 causes dose-dependent DNA damage and PARP-1 becomes up-regulated in response to DNA damage.
Next, in order to further characterize the level of double strand DNA breaks induced by [211At]MM4, we evaluated the phosphorylation of H2AX and ATM simultaneously. NLF cells treated with [211At]MM4 showed that 98% of cells were positive for double strand DNA breaks, as measured by phosphorylation of ATM and H2A.X (figure 2f). Similar results were seen in other neuroblastoma cell lines at the 1, 4, and 24 h time points (SI figure 8). Cell cycle analysis showed that after treatment with [211At]MM4, cells accumulated at the G2M checkpoint which is consistent with DNA damage-induced cell cycle arrest (figure 2g and SI figure 9). These experiments confirmed [211At]MM4 causes high levels of double strand DNA breaks resulting in cell cycle arrest at the G2/M checkpoint.
Through analyzing DNA damage induced by [211At]MM4 we were able to show high levels of double strand DNA damage in a dose dependent manner leading to up-regulation of the drug target PARP-1 and cell cycle arrest at the G2/M phase.
In vivo biodistribution and ex vivo autoradiography
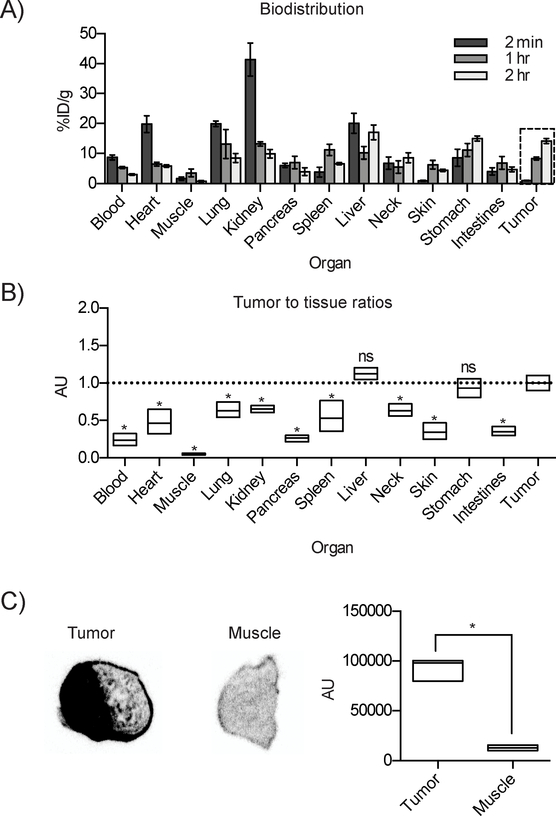
Biodistribution of [211At]MM4 in an IMR-05 tumor bearing mouse model showed tumor uptake of 14.1 ± 6.2% injected dose/gram (ID/g) at 2 h (figure 3a). Low muscle uptake was observed at all time points (<3% ID/g). High levels of radioactivity were not observed in organs known to accumulate free astatine-211 including the neck (thyroid) and stomach. These results indicate low levels of free astatine-211 and in vivo stability of [211At]MM4. Renal uptake at 2 minutes was > 40% ID/g although it rapidly decreased by 1 h. Tumor-to-tissue ratios showed higher amounts of radioactivity present in the tumor compared to most normal tissues (ANOVA, p-value < 0.05), the two exceptions being the liver and stomach that showed similar level of uptake (figure 3b). The liver uptake is most likely caused by hepatobiliary excretion of [211At]MM4 into the gut, which is a transient process. Ex vivo autoradiography of IMR-05 tumor bearing mice showed high uptake in the tumor compared to muscle, consistent with tumor-to-muscle ratio of 10 (t-test, p-value < 0.0001) as observed in biodistribution studies (figure 3c). Overall, [211At]MM4 exhibited biodistribution patterns similar to its analogues radiolabeled with fluorine-18 or iodine-125.[23] These results suggest that the kinetics of a small molecule PARP inhibitor are uniquely suited to match the half-life of astatine-211, providing quick tumor targeting and fast clearance from normal tissues.
Figure 3:
In vivo biodistribution and ex vivo autoradiography of [211At]MM4 in IMR-05 tumor bearing mice. A) Radioactivity measured in organs and tumor (dashed-line box) at time points of 2 min, 1 and 2 h post injection. B) Average relative uptake in organs compared to tumor at 2 h showed higher activity in tumor compared to all organs except liver and stomach (p-value < 0.05). C) Ex vivo autoradiographs of tumor vs. muscle showed a tumor:muscle ratio of 10 (p-value < 0.05).
Anti-tumor efficacy and tumor histology
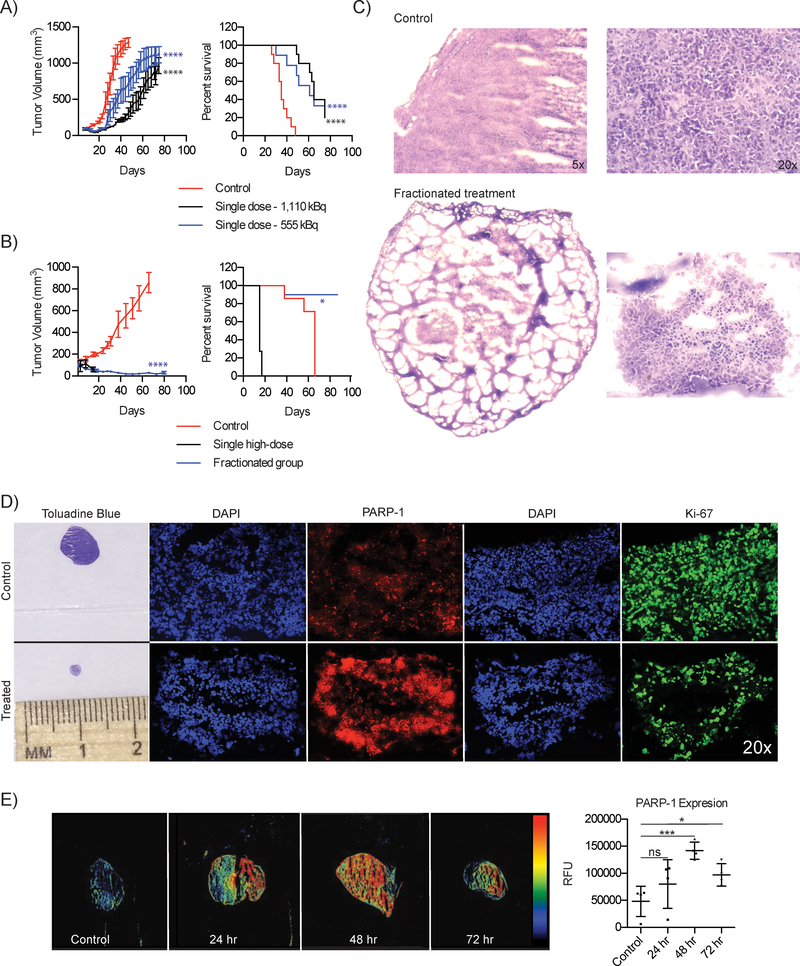
Next, we evaluated the in vivo anti-tumor efficacy of [211At]MM4 using a single dose administration of 555 and 1,110 kBq of [211At]MM4, which showed initial reductions in tumor volume and delayed tumor growth compared to controls (figure 4a). A significant survival benefit was observed for both groups vs. control (median survival 35 vs. 61 and 65 days, p-value: 0.0007). No toxicities defined by weight loss were observed at either dose (SI figure 9a). We then explored single high dose vs. fractionated administration of 1,480 kBq vs. 370 kBq doses given twice weekly. The fractionated regimen showed tumor regression with no evidence of bulk tumor regrowth at the end of the study on day 80 (figure 4b) without appreciable toxicity. However, the single high dose of 1,480 kBq proved to be toxic, despite rapid tumor regression, and resulted in all animals being removed from the study due to weight loss within 10 days of receiving the treatment (SI figure 10b). Furthermore, a significant survival benefit was seen in the fractionated group with median survival in the control group of 66 days vs. 87 days in the fractionated group (p-value: 0.0332).
Figure 4:
In vivo efficacy studies evaluating single and fractionated dosing regimens of [211At]MM4 in an IMR-05 xenograft mouse model. Tumor growth and Kaplan-Meier curves for IMR-05 tumor bearing mice treated with A) single dose of 555 or 1,110 kBq of [211At]MM4 (control vs. 555 kBq and 1110 kBq mixed linear model p-value < 0.0001; control vs. 555 kBq and 1110 kBq survival mantel cox test p-value < 0.0001, 555 KBq vs. 1110 kBq not significant), and B) single high dose of 1480 kBq vs. a fractionated dose of 370 kBq twice weekly for a cumulative dose of 1480 kBq (control vs. fractionated mixed linear model p-value < 0.0001, fractionated vs. high dose not significant; survival mantel cox test high dose vs. control p-value < 0.0001, fractionated vs. control p-value = 0.03). C) Toluadine blue stains on control vs. fractionated therapy treated tumor sections. D) Control vs. treated tumor from the in vivo efficacy study evaluating fractionated vs. single high dose therapy. Immunofluorescence on tumor sections were performed for PARP-1 and Ki-67. E) Tumor sections fluorescently stained for PARP-1 taken from IMR-05 tumor bearing mice treated with 370 kBq of [211At]MM4 at 24, 48, and 72 h after treatment (t-test, p-value < 0.001 (24 h), 0.05 (72 h).
* denote statistical significance described in the figure legend.
To assess whether residual disease was present in the tumors of animals treated with fractionated therapy, we analyzed both control and treated tumors using histopathology. Control tumors showed prolific tumor growth (removed at day 37) while treated tumors removed at day 80 showed minimal residual disease with small numbers of Ki-67 positive tumor cells (figure 4c and figure 4d). We also observed that the tumors treated in fractions showed elevated PARP-1 compared to control. To further explore this effect, we used IMR-05 tumor bearing mice and administered 370 kBq of [211At]MM4 then assessed PARP-1 expression at 24, 48, and 72 h (figure 4e). We found that after treatment with [211At]MM4, there was an increase in tumor PARP-1 compared to controls, peaking around 48 h and remaining elevated at 72 h.
These results provide the first proof of evidence that [211At]MM4 has a therapeutic window offering strong anti-tumor effects with avoidable toxicities. Most strikingly, the fractionated regimen was able to greatly reduce tumor burden and prevent regrowth with little to no toxicities. Finally, histopathological analysis of tumors treated with [211At]MM4 showed an increase in PARP-1 providing in vivo data to support what was observed in vitro.
Discussion
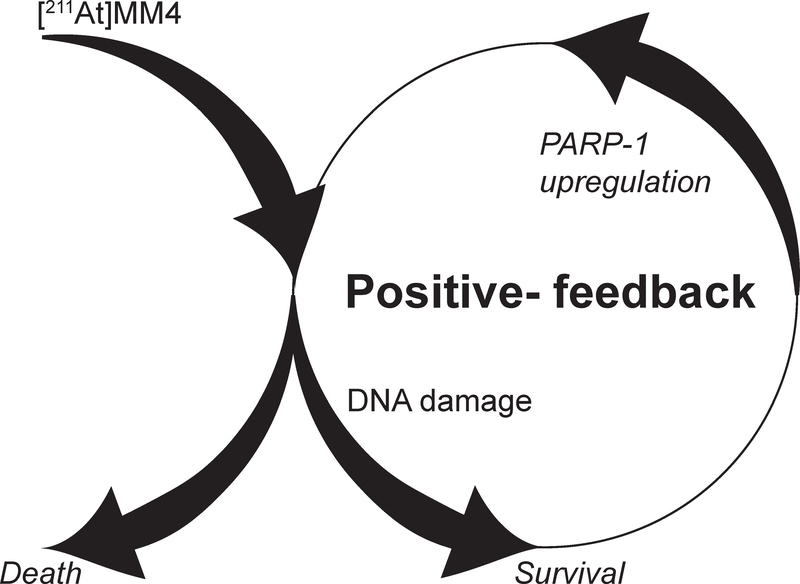
Here, we have evaluated a novel construct capable of delivering alpha particles directly to cancer chromatin by pharmacologically targeting PARP-1 with the astatinated PARP inhibitor [211At]MM4. We found that different cancer cell lines show differential sensitivity to [211At]MM4 in vitro, suggesting that cellular lethality is dependent on inherent biological properties. We propose that there are likely four factors that greatly influence the response of cancer cells to [211At]MM4: drug target density, nucleus geometry, DNA repair status, and primary cell death pathway. Identifying how each of these properties contribute to de novo resistance will likely aid in discovering new drug combinations that can sensitize cells to [211At]MM4. We observed a unique effect of drug target up-regulation after treatment with [211At]MM4 that provides a strong rationale for the added benefit observed in vivo with fractionated dosing regimens (Figure 5). Like other DNA damaging therapies, alpha particles show relative differences in biological models, the investigation of which will be of critical importance in understanding the impact on clinical translation.
Figure 5:
The positive feedback of DNA damage induced by [211At]MM4 increases PARP-1 expression and enhances drug targeting with fractionated dosing regimens.
We observed the IMR-05 cell line that was the most sensitive to [211At]MM4 was also sensitive to non-radioactive PARP inhibitors in vitro, especially to talazoparib which is a potent PARP trapper.[24, 25] Previous studies have shown that talazoparib has potent cytotoxic effects in vitro with EC50 values in the nanomolar range, but is ineffective in vivo as monotherapy in pediatric cancers including Ewing’s sarcoma, medulloblastoma, leukemia and neuroblastoma.[26] Smith et al. further described these results as unexpected due to talazoparib’s strong potency in vitro. Later it was shown that combining talazoparib with either temozolomide or topotecan induced synergistic effects in vitro that translated to in vivo anti-tumor response.[16] Collectively, these data demonstrate that even though PARP inhibitors show in vitro cytotoxicity as single agents, they lack in vivo efficacy in these tumor types, supporting our rationale for the development of [211At]MM4 as a novel therapeutic distinct from conventional PARPis. On a molar scale, [211At]MM4 is greater than 10,000 times more potent than talazoparib, which provides further evidence that the likely mechanism of cell kill does not rely on pharmacological PARP inhibition but instead on DNA damage induced by alpha-particles emitted from astatine-211 that is chemically incorporated into the structure of [211At]MM4.
In vivo studies showed [211At]MM4 has a durable anti-tumor response and potential for clinical translation as a treatment for neuroblastoma. While PARP-1 expression is heterogeneous within neuroblastoma, overall this cancer subtype has elevated median PARP-1 expression compared to other cancers. Furthermore, neuroblastoma is a highly radiosensitive cancer and current radiopharmaceutical therapy with [131I]MIBG has shown remarkable response rates in clinical trials. We propose that [211At]MM4 could be used in combination with conventional therapies, but primarily utilized to target residual disease as an adjuvant therapy. There is recent evidence that neuroblastoma micrometastases in the bone marrow may lead to relapse. The targeting properties of [211At]MM4 to the bone marrow may offer a mechanism to deliver curative doses to single or small groups of cancer cells thereby preventing relapse.
The result of normal tissue toxicity induced by [211At]MM4 is currently unknown and future studies are underway to characterize the safety profile of this agent. Based on the expression of PARP-1 in normal tissues, we expect on-target normal tissue toxicities in the spleen and bone marrow. It should be noted that bone marrow suppression and hematological toxicities are common with clinically used radiopharmaceutical therapeutics, and therefore are not expected limit the application of [211At]MM4. Other potential sites for toxicity include the liver and gastrointestinal track which are involved in the biological clearance of [211At]MM4. However, both the liver and gastrointestinal track can regenerate after sublethal radiation-induced injury and therefore are not likely to preclude the development of [211At]MM4. Low dose fractionated therapy improved the toxicity profile in mouse models and provided more durable anti-tumor responses in vivo providing evidence that [211At]MM4 can be administered safely with efficacy.
In summary, we discovered a novel alpha-emitting radiopharmaceutical by incorporating astatine-211 into the chemical structure of a small molecule PARPi. In doing so, we demonstrated the capability of targeting cancer chromatin through PARP-1 with promising efficacy both in vitro and in vivo. Here we evaluated [211At]MM4 in neuroblastoma models, but there is high potential for a broader application in cancer therapy.
Supplementary Material
Acknowledgements
This work was supported by the national institute of health and national cancer institute (1R01CA219006–01A1, D.A. Pryma; dpryma@pennmedicine.upenn.edu). The production of astatine-211 used in this proposal was supported by the Bromine and Astatine isotope production and research development grant funded by the department of energy nuclear physics isotope program (DE-SC0017646, R.H. Mach; rmach@pennmedicine.edu). Mehran Makvandi was supported by the Paul Calabresi K12 award for clinical oncology (K12CA076931, L.M. Schuchter; lynn.schuchter@uphs.upenn.edu).
Footnotes
Financial Disclosure statement
The authors of this manuscript have no conflicts of interest.
References
- [1].Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, Weiss WA, Neuroblastoma, Nat Rev Dis Primers, 2 (2016) 16078. [DOI] [PubMed] [Google Scholar]
- [2].Haas-Kogan DA, Swift PS, Selch M, Haase GM, Seeger RC, Gerbing RB, Stram DO, Matthay KK, Impact of radiotherapy for high-risk neuroblastoma: a Children’s Cancer Group study, Int J Radiat Oncol Biol Phys, 56 (2003) 28–39. [DOI] [PubMed] [Google Scholar]
- [3].Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, Veatch J, Goldsby R, Brophy P, Kersun LS, Hawkins RA, Maris JM, Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma, J Clin Oncol, 25 (2007) 1054–1060. [DOI] [PubMed] [Google Scholar]
- [4].Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, Villablanca JG, Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study, J Clin Oncol, 27 (2009) 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matthay KK, Quach A, Huberty J, Franc BL, Hawkins RA, Jackson H, Groshen S, Shusterman S, Yanik G, Veatch J, Brophy P, Villablanca JG, Maris JM, Iodine-131--metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: a new approaches to neuroblastoma therapy phase I study, J Clin Oncol, 27 (2009) 1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maris JM, Recent advances in neuroblastoma, N Engl J Med, 362 (2010) 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lloyd EL, Gemmell MA, Henning CB, Gemmell DS, Zabransky BJ, Cell survival following multiple-track alpha particle irradiation, Int J Radiat Biol Relat Stud Phys Chem Med, 35 (1979) 23–31. [DOI] [PubMed] [Google Scholar]
- [8].Munro TR, The relative radiosensitivity of the nucleus and cytoplasm of Chinese hamster fibroblasts, Radiat Res, 42 (1970) 451–470. [PubMed] [Google Scholar]
- [9].Zalutsky MR, Pruszynski M, Astatine-211: production and availability, Curr Radiopharm, 4 (2011) 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Azure MT, Archer RD, Sastry KS, Rao DV, Howell RW, Biological effect of lead-212 localized in the nucleus of mammalian cells: role of recoil energy in the radiotoxicity of internal alpha-particle emitters, Radiat Res, 140 (1994) 276–283. [PMC free article] [PubMed] [Google Scholar]
- [11].Knight JC, Koustoulidou S, Cornelissen B, Imaging the DNA damage response with PET and SPECT, Eur J Nucl Med Mol Imaging, 44 (2017) 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu X, Dong Z, Wang CJ, Barlow LJ, Fako V, Serrano MA, Zou Y, Liu JY, Zhang JT, FASN regulates cellular response to genotoxic treatments by increasing PARP-1 expression and DNA repair activity via NF-kappaB and SP1, Proc Natl Acad Sci U S A, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lord CJ, Ashworth A, PARP inhibitors: Synthetic lethality in the clinic, Science, 355 (2017) 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krishnakumar R, Kraus WL, The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets, Mol Cell, 39 (2010) 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Langelier MF, Planck JL, Roy S, Pascal JM, Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1, Science, 336 (2012) 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Smith MA, Reynolds CP, Kang MH, Kolb EA, Gorlick R, Carol H, Lock RB, Keir ST, Maris JM, Billups CA, Lyalin D, Kurmasheva RT, Houghton PJ, Synergistic activity of PARP inhibition by talazoparib (BMN 673) with temozolomide in pediatric cancer models in the pediatric preclinical testing program, Clin Cancer Res, 21 (2015) 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fatokun AA, Dawson VL, Dawson TM, Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities, Br J Pharmacol, 171 (2014) 2000–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Makvandi M, Xu K, Lieberman BP, Anderson RC, Effron SS, Winters HD, Zeng C, McDonald ES, Pryma DA, Greenberg RA, Mach RH, A Radiotracer Strategy to Quantify PARP-1 Expression In Vivo Provides a Biomarker That Can Enable Patient Selection for PARP Inhibitor Therapy, Cancer Res, 76 (2016) 4516–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reilly SW, Makvandi M, Xu K, Mach RH, Rapid Cu-Catalyzed [(211)At]Astatination and [(125)I]Iodination of Boronic Esters at Room Temperature, Org Lett, 20 (2018) 1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A, Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy, Nature, 434 (2005) 917–921. [DOI] [PubMed] [Google Scholar]
- [21].Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T, Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase, Nature, 434 (2005) 913–917. [DOI] [PubMed] [Google Scholar]
- [22].Colicchia V, Petroni M, Guarguaglini G, Sardina F, Sahun-Roncero M, Carbonari M, Ricci B, Heil C, Capalbo C, Belardinilli F, Coppa A, Peruzzi G, Screpanti I, Lavia P, Gulino A, Giannini G, PARP inhibitors enhance replication stress and cause mitotic catastrophe in MYCN-dependent neuroblastoma, Oncogene, 36 (2017) 4682–4691. [DOI] [PubMed] [Google Scholar]
- [23].Makvandi M, Lieberman BP, LeGeyt B, Hou C, Mankoff DA, Mach RH, Pryma DA, The pre-clinical characterization of an alpha-emitting sigma-2 receptor targeted radiotherapeutic, Nucl Med Biol, 43 (2016) 35–41. [DOI] [PubMed] [Google Scholar]
- [24].Pommier Y, O’Connor MJ, de Bono J, Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action, Sci Transl Med, 8 (2016) 362ps317. [DOI] [PubMed] [Google Scholar]
- [25].Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, Morris J, Teicher B, Doroshow JH, Pommier Y, Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib, Mol Cancer Ther, 13 (2014) 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smith MA, Hampton OA, Reynolds CP, Kang MH, Maris JM, Gorlick R, Kolb EA, Lock R, Carol H, Keir ST, Wu J, Kurmasheva RT, Wheeler DA, Houghton PJ, Initial testing (stage 1) of the PARP inhibitor BMN 673 by the pediatric preclinical testing program: PALB2 mutation predicts exceptional in vivo response to BMN 673, Pediatr Blood Cancer, 62 (2015) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.