Abstract
Which neural mechanisms provide the functional basis of top-down guided cognitive control? Here we review recent evidence that suggest that the neural basis of attention is inherently rhythmic. In particular, we discuss two physical properties of self-sustained networks, namely entrainment and resonance, and how these shape the timescale of attentional control. Several recent findings revealed theta-band (3-8 Hz) dynamics in top-down guided behavior. These reports were paralleled by intracranial recordings, which implicated theta oscillations in the organization of functional attention networks. We discuss how the intrinsic network architecture shapes covert attentional sampling as well as overt behavior. Taken together, we posit that theta rhythmicity is an inherent feature of the attention network in support of top-down guided goal-directed behavior.
Keywords: Attention, Predictions, Expectations, Top-down control, Cognitive control, Entrainment, Resonance, Network Neuroscience, intracranial EEG, Electrophysiology, Connectivity, Cross-Frequency Coupling, Phase-dependent perception, Perceptual cycles, Rhythmic Attention, Neuronal oscillations
Introduction
Neurophysiological recordings reveal the ever-changing nature of brain activity. But how does time-varying (i.e. non-constant), non-stationary (i.e. changing statistical properties, such as mean, variance or autocorrelation) neuronal activity support our seemingly continuous and stable perception of the world [1,2]? At the population level, rhythmic activity patterns dominate neuronal recordings [3]. Decades of research linked these periodic activity fluctuations to specific canonical computations [4] and indicated their pivotal role for network organization and inter-areal information transfer [2,5]. However, the direct link between endogenous, spontaneously generated (in contrast to task evoked) brain activity and moment-to-moment behavioral fluctuations remains to be determined.
For perception and attention, a basic concept is that ‘spontaneous’ rhythmic activity might index moments in which a given neural circuit is more or less efficient in performing its computation [1,2,5]. In particular, certain phases of the oscillatory cycle are associated with better performance than others [6–8]. While other oscillatory features, such as amplitude and frequency, also impact neuronal excitability [9], we mainly focus on phase-dependent effects, which, unlike power modulations, capture behavioral fluctuations on a millisecond time scale. Recently, several studies promoted the relevance of neuronal oscillations for behavior by demonstrating periodicities in behavioral time courses relative to an external reference event. Notably, the observed periodicities in behavior closely matched the timescales of rhythmic brain activity [1]. For instance, several reports showed that visual perception cycles as a function of parietooccipital alpha phase (8-12 Hz; [7,10–13]), while higher cognitive functions, such as attention and predictions exhibit slower delta/theta signatures (3-8 Hz; [14–16]). Crucially, observing oscillations in behavioral time courses, which are often constructed across several hundred trials, implies that there is an underlying neuronal process that exhibits a constant phase relationship relative to a reference event, e.g. sensory cue, across all trials. Previous studies indicated that this across-trial phase organization could be induced when ongoing cortical rhythms become ‘phase-aligned’ relative to the reference external event.
Here we review recent findings that clarify the neural basis of perceptual and attentional cycles in behavior. We discuss two concepts that have often been used to explain such cycles, namely entrainment and resonance, both grounded in non-linear systems theory, but lacking a clear definition in cognitive neuroscience. We further make specific predictions about the properties of the observed spectral signatures and explore the question how the brain might utilize its inherent physical structure to support top-down guided allocation of attention.
What constitutes neuronal entrainment?
A common scenario in which periodicities in behavior can be observed at a consistent frequency occurs after exposure to a (quasi-) periodic stimulus stream, where performance is typically better at on-beat times relative to off-beat times [8,17–19]. The prevalent mechanistic explanation for observed cyclic behavioral patterns is the concept of neural entrainment, most commonly defined in cognitive neuroscience as phase alignment of ongoing oscillations to a (quasi-) periodic stimulus stream [20]. Consistent with this model, exposure to a rhythmic stream leads to increased phase locking of neural activity at the stream frequency [18,21,22]. In the field of attention research, oscillatory entrainment is considered a powerful neural mechanism to maximize the predictability of future events and precisely time the allocation of resources [18].
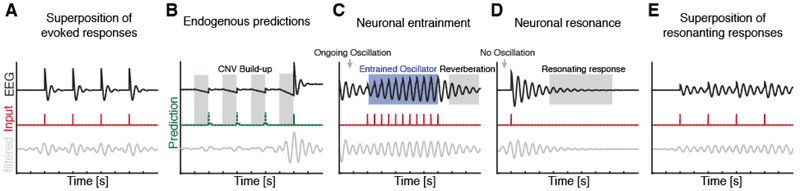
However, entrainment as defined by dynamical systems theory [23] can only be considered if several pre-requisites are met. In particular, neuronal entrainment always requires two oscillators, one in the input stream and one neural oscillator, which interact through directed synchronization. The challenge to entrainment models is that phase locking during presentations of a periodic stream can stem from other, non-oscillatory sources making it questionable whether an existing intrinsic oscillator is being entrained. One such pattern is the evoked response that is triggered by the entraining stream (Figure 1A). Given that temporal and spectral changes are inherently correlated, any evoked response will appear as an alignment of phases in the frequency domain [24–26].
Figure 1. Oscillatory entrainment and resonance.
EEG oscillator model response (black; [29]) relative to external sensory input (red) and concomitant band-pass filtered version (grey). Note that all 5 subpanels were derived from the same underlying oscillator model. (A) Evoked responses to rhythmic stimuli when no neural oscillator is present. Note that band-pass filtering renders the signal sinusoidal despite the absence of an ongoing oscillation. For an in-depth discussion of the relationship of evoked responses and phase-alignment see [25,26]. (B) No external sensory stream is present, but events are predicted based on top-down priors. Predicted events can either occur (green solid line) or not (green dashed line). However, the build-up of the ramping neural activity (such as contingent negative variation EEG potential, CNV) and subsequent return to baseline might mimic phase entrainment after band-pass filtering a linearly trending signal [29] due to filter-ringing. (C) True entrainment: An ongoing oscillator is entrained by a rhythmic input at a slightly different frequency. The entrained oscillation becomes phase-locked and the amplitude increases. After the entraining stream stops the oscillator exhibits a reverberation at the driving frequency for several cycles. (D) Resonating response to a single stimulus might mimic reverberations of a true oscillator. Note that band-pass filtering even renders the pre-stimulus period sinusoidal due to the single evoked response. Hence, phase estimates at stimulus onset might appear to be biased. However, phase estimates, after the initial evoked response, accurately track the phase of the decaying response. (E) Superposition of resonating responses to multiple stimuli mimics entrainment signatures as well as for phase-reset phenomena. Phase estimates after the initial response reflects a good approximation of the underlying signal. Note that neither this scenario nor panel A capture true, cognitively driven, phase alignment but mimic oscillatory patterns in response to a sensory stimulus.
Even when the impact of evoked responses is reduced by using close-to-threshold stimuli [27] or comparing to aperiodic streams [28], a recent study showed that phase locking can be fully explained by repeated ramping activity, driven by the temporal predictability that is inherent to the periodic stream ([29]; Figure 1B). This was based on observing similar phase-locking metrics in a stream that was substantially less periodic, but was as predictable as a rhythmic stream. Therefore, phase locking during stream presentation cannot be taken as evidence or an indicator or a pure entrainment process [30,31].
An additional defining property of true neural entrainment entails that phase consistency outlasts the stimulation offset by several cycles, reverberating at the entrained frequency at predictable phases before dispersion ([23,29,32]; Figure 1C). Several recent studies have observed reverberation, accompanied by corresponding periodicities in behavioral time courses, in a range of frequencies from delta to alpha bands [10,33]. Such findings corroborate that exposure to a periodic stream creates attentional cycles in a bottom-up (i.e. sensory driven) manner, possibly mediated by entrainment of endogenous rhythms.
However, recent observations have questioned that periodic stimulation generates attentional cycles in a purely bottom-up manner. One study demonstrated that observers can voluntarily orient their attention to the ‘gaps’ in the entraining stream, leading to facilitated performance to off-beat relative to on-beat targets [34]. This implies a voluntary process and not an automatic bottom-up driven response, despite similar neural signatures [29]. In line with this finding, another recent study modulated both the rhythmicity and the cognitive content of a preceding visual stream [10]. The authors demonstrated that bottom-up entrainment was only effective when the stream was presented rhythmically and no additional top-down information was available (Figure 2). However, top-down information (reflecting cognitive priors, i.e. expectations and predictions) altered bottom-up, purely sensory driven, entrainment effects in behavior. Importantly, in addition to visual perception varying over time at the frequency of the sensory rhythm, this study revealed that the ability to utilize top-down information also fluctuates as a function of a low-theta rhythm. Hence, this observation strongly suggests that theta is an intrinsically generated rhythm that only emerges when top-down attention is deployed. Collectively, these studies indicate a complex interaction between bottom-up entrainment and top-down attention, which operates at its own intrinsic timescale and might be oscillatory in nature.
Figure 2. Theta mediates rhythmic top-down control.
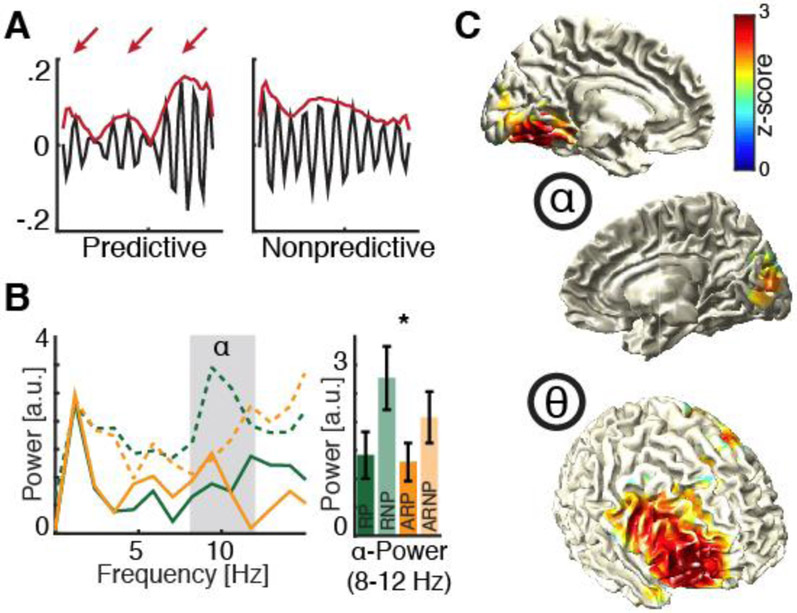
(A) Behavioral data from a target detection experiment where a 2×2 design (predictive vs. non-predictive and 10 Hz flicker vs. arrhythmic stimulation) was employed. Single subject behavioral time courses for predictive and non-predictive conditions filtered in the alpha-band. Note the rhythmic theta modulation (arrows) of the alpha envelope (red) in the predictive (left) but not the non-predictive (right) condition. (B) Left: Spectral analysis of behavioral hit rate time courses reveal elevated alpha power for non-predictive contexts (dashed lines). Note that alpha power is higher when the preceding stream was flickered at 10 Hz (green dashed lines). This increase was interpreted as evidence for alpha entrainment through the 10 Hz flicker. However, top-down guided processing (solid lines) exhibited markedly reduced alpha power, which was not modulated by the entraining stream (right panel), thus, indicating that top-down control alleviates effects of bottom-up sensory entrainment. (C) Simultaneous EEG recordings revealed distinct sources reflecting perception (posterior alpha) and cognitive content (frontal low theta). The graphs in A-C are reproduced from [10].
Network resonance of the neural attention circuitry
A different property of dynamical systems that can lead to phase-consistent behavioral and neural periodicity is the concept of resonance, which is closely related to entrainment, but differs in several important ways [23]. In contrast to entrainment, resonance does not require an oscillator in the input stream (Figure 1D). Even a singular event might trigger a frequency-specific response, which dampens over several cycles as the network returns to its baseline state. Several studies demonstrated this principle and described periodic patterns in behavior following a single transient sensory event independent of a periodic stimulus stream [15,16,35,36].
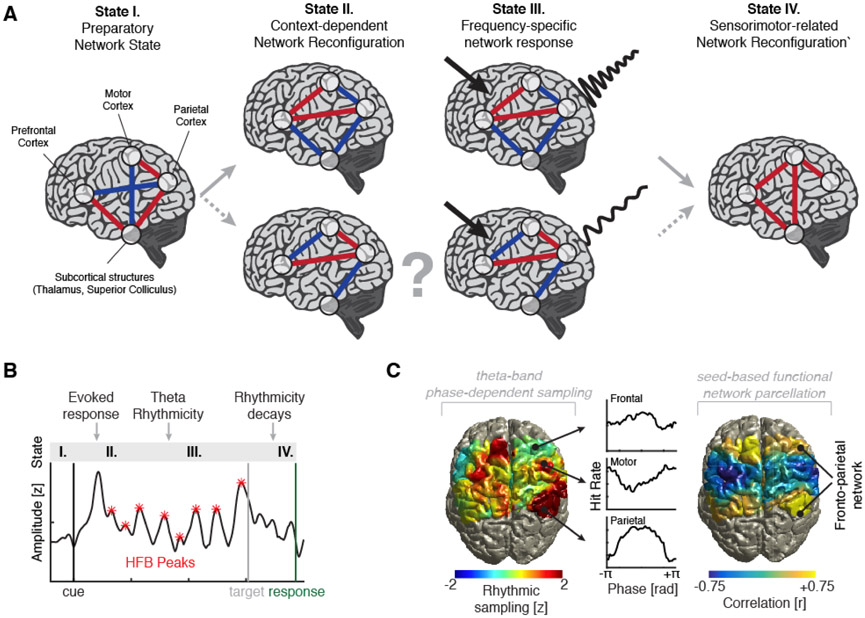
Recently, two comparative intracranial studies in macaques [35] and in humans [36] revealed that rhythmic behavioral sampling is directly related to activity patterns in the fronto-parietal attention network (Figure 3). Both studies used the same variant of a spatial attention task where subjects had to monitor three different locations and detect the presence of a close-to-threshold target, which appeared at varying cue-target-intervals [15]. These two studies, as well as a recent study in ferrets [37], demonstrated prominent theta oscillations coupled to high frequency or spiking activity during the cue-target-interval, which directly predicted behavioral outcome on a trial-by-trial basis. Notably, these theta signatures were transient and most pronounced during the covert sampling of the visual display. Distant areas in the fronto-parietal attention network exhibited similar phase-behavior dependencies supporting a common functional network organization. Given that attentional theta signatures can be observed in behavior and electrophysiological recordings across species in several sustained attention tasks, theta oscillations may be an inherent feature of the self-organization of the attention network and may reflect its eigenfrequency, defined as the preferred intrinsic resonance frequency, arising from physical constraints [38].
Figure 3. Theta rhythmicity in the fronto-parietal attention network.
(A) Schematic of context-dependent network reconfigurations: Different network configurations might exhibit characteristic frequency-specific response when input into the system is provided (solid lines indicate observations; dashed lines indicate suspected network motifs). Responses often decay over time or are terminated by another network reconfiguration. (B) Single trial example of a intracranial electrode placed over parietal cortex: After a cue-evoked response, high frequency band activity, a surrogate for multi-unit spiking activity, fluctuated at a theta frequency. This rhythmicity was terminated after target presentation and subsequent overt response. (C) Topographical depiction of theta rhythmic sampling. Note parietal, frontal and motor areas contribute significantly to the behavioral rhythmic sampling. Lower right: Behavior-phase relationships are coherent in the fronto-parietal attention network and can be delineated from the sensorimotor network. The graphs in B-C are reproduced with permission from [36].
The observations of periodic neural and behavioral patterns following a single cue raise another potential challenge for rhythmic entrainment models. Repeated input into a resonating network can give rise to a pattern that closely resembles a truly entrained response (Figure 1E) at both the neural and the behavioral level. If no additional input is provided, the initial response will be dampened, leading to markedly reduced amplitudes after several cycles (Figure 1D), hence, diminishing the impact of the instantaneous phase on subsequent behavior [6]. Therefore, it is possible that behavioral and neural periodicities, which were observed after exposure to periodic streams and were attributed to neural entrainment, in fact reflect resonance phenomena.
A related issue that is currently unclear is whether the network always resonates at its preferred eigenfrequency, or whether the precise resonance frequency is under top-down control. For instance, could the same network resonate at different frequencies depending on the cognitive content or previous sensory input? We speculate that the resonance frequency could change depending on the instantaneous network configuration. Hence, the same network could resonate at different frequencies depending on its current configuration and task requirements ([39]; Figure 3A), i.e. exhibit state-specific spectral signatures that modulate behavior [4]. Methods recently employed to illuminate hidden-states in working memory might prove beneficial to dissect the functional basis of attention. In line with this, spontaneous oscillatory bursts might precisely index the current network state [40], and activity at the eigenfrequency might be amplified when ‘pinged’ by a non-informative supra-threshold stimulus [41] providing an instantaneous readout of the current network configuration.
The emergence of theta rhythms in attention
Notably, behavioral and neural periodicities during top-down guided attention exhibited prominent theta signatures, irrespective of whether they followed a single attentional cue [18,35,36] or a preceding entraining stream [10]. Previously, theta oscillations (3-8 Hz) have been mainly associated with hippocampus-dependent processing [2,3]. Hence, it is surprising that the same spectral signatures are also prevalent in attention tasks. Several scenarios could give rise to theta fluctuations. One idea is that a supra-threshold stimulus phase-aligns ongoing activity [42]. However, theta oscillations are not commonly observed during rest [2,4], making it less likely that this is the case. Another possibility is that the theta signatures index the engagement of the underlying attention network, which might resonate at its preferred eigenfrequency in the theta range. Currently, it remains unclear whether theta signatures reflect a ‘active’ sampling mechanism by which the brain discretely explores the environment [1] or whether theta resonance constitutes a ‘passive’ network property, which constrains the environmental exploration [38].
One direct question arising from the first consideration is which structure could implement such a distributed scanning process? Recently, it has been speculated that the pulvinar, a nucleus of the visual thalamus, does not constitute a passive relay station, but might actively orchestrate attention networks [43]. Direct thalamic recordings in humans, monkeys and ferrets provide further support for this consideration: Thalamo-cortical theta synchrony [44] is increased during attentional engagement [45,46]. Importantly, theta synchrony precisely indexes attentional states and mediates feed-forward influences from the thalamus to the cortex [46,47]. This cascade where theta indexes feed-forward signaling was also apparent for cortico-cortical connections from V1 to V4 [48]. While theta modulations have been observed as early as in V1 [42], it is less clear where these modulatory signals emerge. Both, frontal [10,35,37] as well as thalamic [43,46,47,49] regions have been implicated in the long-range control of parieto-occipital activity in states of top-down attention deployment. However, it is uncertain how these structures interact.
Evidence for the latter consideration stems from a recent study that introduced a framework to explain the emergence of behaviorally relevant theta oscillations from balanced interactions in local circuits [38]. This study reported that behaviorally relevant theta modulations can also arise from competing receptive field interactions in cortical area V4 [38]. Here, intrinsic time constants of the dynamic interplay of excitation and inhibition are suggested to give rise to theta rhythmicity. It is currently unclear if similar constraints give rise to theta activity in thalamo-fronto-parietal networks, or how afferent inputs [48] into V4 shape the local [38] and long-range [35,36] theta interactions and their relationship to behavior.
In summary, theta signatures constitute an important physiological feature of attention networks. Future research must address if the exact network features, such as e.g. peak frequencies varying as a function of cognitive states or rather if they reflect a trait-like hardwiring of the underlying anatomical structures determining the resolution of the attention system. This distinction is also relevant for rhythms and entrainment theories, as resonance at the network eigenfrequency cannot explain observations of behavioral and neural periodicities across several canonical frequency bands, such as delta/theta (3-8 Hz), alpha (8-12 Hz) and beta (13-30 Hz). However, resonance with varying peak frequencies (Figure 3A) could provide a parsimonious explanation to behavioral and neural reverberation after stream termination, without relying on a preexisting oscillator (Figure 1E).
How does covert rhythmic sampling support overt behaviors?
The reviewed evidence here established that covert rhythmic sampling behaviors can arise from endogenous oscillatory processes, which do not depend on the existence of periodicities in the sensory input stream. It is currently unclear if similar dependencies exist between ongoing oscillations and overt rhythmic sampling behaviors, such as (micro-) saccadic eye movements or whisking movements in rodents [50,51]. Furthermore, it poses the question if and how oscillations support the transformation of covertly sampled information into overt behaviors.
Recent findings indicated covert rhythmic sampling is actually diminished during overt behaviors such as (micro-) saccadic eye movements [35,48]. One possible mechanistic explanation is that oscillations can also be phase-reset relative to other reference events besides external sensory stimuli, e.g. by motor output [15,16]. Support for this consideration stems from two recent observations: First, it has been demonstrated that microsaccades phase-reset cortical rhythmic sampling [52]. This was reflected in prominent behavioral fluctuations only emerging when behavioral performance was assessed relative to the microsaccade, but not to the external reference event [52,53]. This is in line with the notion that the most significant changes within the oculo-motor system occur ±100ms around a (micro-saccade), i.e. within a single theta cycle (~ 5Hz; [54]). Similar effects were observed relative to a button press in human subjects, again, being associated with prominent theta rhythmicity in behavioral time courses [55]. In addition, neuronal response gain was found to be enhanced prior to an eye movement [54,56], that is, at a time when the targeted location has already been covertly sampled.
Jointly, these findings raise the intriguing possibility that covert and overt processes are not independent but exhibit a reciprocal relationship that is mediated by neural rhythms. An external sensory event might trigger the covert rhythmic sampling, which subsequently informs overt behavior. The movement execution then restarts this cycle, i.e. covert sampling of the environment selects the most salient stimulus for the next (micro-) saccade. This consideration implicates the (oculo-) motor system, including the visual thalamus [43,45,47] as well as the superior colliculus [57,58], in organizing the network to coordinate covert and overt behaviors.
Conclusions
The evidence we reviewed here collectively demonstrates that spontaneous network activity shapes and constrains allocation of attention. We argue that periodicities in human behavior directly reflect the underlying frequency-specific network organization, which is surprisingly well preserved across species (ferrets, macaques and humans) and preferentially operates at a theta timescale (3-8 Hz). Future research will have to determine if theta rhythmicity constitutes a voluntary active-sampling process or if theta reflects the intrinsic biophysical structure of the brain directly determining covert and overt behaviors.
The concept of entrainment as a mechanism to extract important temporal regularities from the environment has gained popularity in recent years. Here, we define the limitations of this concept and highlight the similarities of and differences from pure network resonance. With this, we overcome discrepancies in the entrainment literature and directly link periodicities as observed in behavior to large-scale network organization. We reviewed recent evidence that suggests that distinct network configurations exhibit distinct resonance phenomena at their eigenfrequency, which decays over time or when the network configuration is changed. Taken together, the endogenous network architecture concept could constitute the functional unit of cognition [59,60], and might be readily visible in the behavioral outcome when probed on a fine-grained temporal scale.
Highlights.
Behavioral and neural periodicities reflect phase-aligned oscillations
Entrainment and resonance as distinct possible mechanisms
Physical network properties shape attentional control
Theta rhythmicity in behavior and in the attention network
Theta oscillations reciprocally link covert and overt behavior
Acknowledgements
This work was supported by the National Institute of Health (NINDS Javits Award R37NS21135; R.T.K.) and CONTE Center (5P50MH109429; R.T.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None.
References
- [1].VanRullen R, Perceptual Cycles, Trends Cogn. Sci. 20 (2016) 723–735. doi: 10.1016/j.tics.2016.07.006. [DOI] [PubMed] [Google Scholar]
- [2].Helfrich RF, Knight RT, Oscillatory Dynamics of Prefrontal Cognitive Control, Trends Cogn. Sci 20 (2016) 916–930. doi: 10.1016/j.tics.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buzsáki G, Logothetis N, Singer W, Scaling brain size, keeping timing: evolutionary preservation of brain rhythms, Neuron. 80 (2013) 751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siegel M, Donner TH, Engel AK, Spectral fingerprints of large-scale neuronal interactions, Nat. Rev. Neurosci. 13 (2012) 121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- [5].Fries P, Rhythms for Cognition: Communication through Coherence, Neuron. 88 (2015) 220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T, To see or not to see: prestimulus alpha phase predicts visual awareness, J. Neurosci. Off. J. Soc. Neurosci. 29 (2009) 2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Busch NA, Dubois J, VanRullen R, The phase of ongoing EEG oscillations predicts visual perception, J. Neurosci. Off. J. Soc. Neurosci. 29 (2009) 7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Flenry MJ, Obleser J, Frequency modulation entrains slow neural oscillations and optimizes human listening behavior, Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 20095–20100. doi: 10.1073/pnas.1213390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schalk G, Marple J, Knight RT, Coon WG, Instantaneous Voltage as an Alternative to Power- and Phase-Based Interpretation of Oscillatory Brain Activity, Neuroimage. (2017). doi: 10.1016/j.neuroimage.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Helfrich RF, Fluang M, Wilson G, Knight RT, Prefrontal cortex modulates posterior alpha oscillations during top-down guided visual perception, PNAS. 1705965114v1-201705965 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This study demonstrates that top-down control operates rhythmically and alleviates bottom-up entrained effects.
- [11].Spaak E, de Lange FP, Jensen O, Local entrainment of α oscillations by visual stimuli causes cyclic modulation of perception, J. Neurosci. Off. J. Soc. Neurosci. 34 (2014) 3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de Graaf TA, Gross J, Paterson G, Rusch T, Sack AT, Thut G, Alpha-band rhythms in visual task performance: phase-locking by rhythmic sensory stimulation, PloS One. 8 (2013) e60035. doi: 10.1371/journal.pone.0060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mathewson KE, Prudhomme C, Fabiani M, Beck DM, Lleras A, Gratton G, Making waves in the stream of consciousness: entraining oscillations in EEG alpha and fluctuations in visual awareness with rhythmic visual stimulation, J. Cogn. Neurosci. 24 (2012) 2321–2333. doi: 10.1162/jocn_a_00288. [DOI] [PubMed] [Google Scholar]
- [14].Dugué L, Marque P, VanRullen R, Theta oscillations modulate attentional search performance periodically, J. Cogn. Neurosci. 27 (2015) 945–958. doi: 10.1162/jocn_a_00755. [DOI] [PubMed] [Google Scholar]
- [15].Fiebelkorn IC, Saalmann YB, Kastner S, Rhythmic sampling within and between objects despite sustained attention at a cued location, Curr. Biol. CB. 23 (2013) 2553–2558. doi: 10.1016/j.cub.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Landau AN, Fries P, Attention samples stimuli rhythmically, Curr. Biol. CB. 22 (2012) 1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- [17].Jones MR, Moynihan H, MacKenzie N, Puente J, Temporal aspects of stimulus-driven attending in dynamic arrays, Psychol. Sci. 13 (2002) 313–319. doi: 10.1111/1467-9280.00458. [DOI] [PubMed] [Google Scholar]
- [18].Henry MJ, Herrmann B, Obleser J, Entrained neural oscillations in multiple frequency bands comodulate behavior, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 14935–14940. doi: 10.1073/pnas.1408741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mathewson KE, Fabiani M, Gratton G, Beck DM, Lleras A, Rescuing stimuli from invisibility: Inducing a momentary release from visual masking with pre-target entrainment, Cognition. 115 (2010) 186–191. doi: 10.1016/j.cognition.2009.11.010. [DOI] [PubMed] [Google Scholar]
- [20].Calderone DJ, Lakatos P, Butler PD, Castellanos FX, Entrainment of neural oscillations as a modifiable substrate of attention, Trends Cogn. Sci. 18 (2014) 300–309. doi: 10.1016/j.tics.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zion Golumbic EM, Ding N, Bickel S, Lakatos P, Schevon CA, McKhann GM, Goodman RR, Emerson R, Mehta AD, Simon JZ, Poeppel D, Schroeder CE, Mechanisms underlying selective neuronal tracking of attended speech at a “cocktail party,” Neuron. 77 (2013) 980–991. doi: 10.1016/j.neuron.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stefanics G, Hangya B, Hernádi I, Winkler I, Lakatos P, Ulbert I, Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed, J. Neurosci. Off. J. Soc. Neurosci. 30 (2010) 13578–13585. doi: 10.1523/JNEUROSCI.0703-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pikovsky A, Rosenblum M, Kurths J, Kurths J, Synchronization: a universal concept in nonlinear sciences, Cambridge university press, 2003. [Google Scholar]
- [24].Capilla A, Pazo-Alvarez P, Darriba A, Campo P, Gross J, Steady-state visual evoked potentials can be explained by temporal superposition of transient event-related responses, PloS One. 6 (2011) e14543. doi: 10.1371/journal.pone.0014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mazaheri A, Picton TW, EEG spectral dynamics during discrimination of auditory and visual targets, Brain Res. Cogn. Brain Res. 24 (2005) 81–96. doi: 10.1016/j.cogbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- [26].van Diepen RM, Mazaheri A, The Caveats of observing Inter-Trial Phase-Coherence in Cognitive Neuroscience, Sci. Rep. 8 (2018) 2990. doi: 10.1038/s41598-018-20423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ten Oever S, Schroeder CE, Poeppel D, van Atteveldt N, Mehta AD, Mégevand P, Groppe DM, Zion-Golumbic E, Low-Frequency Cortical Oscillations Entrain to Subthreshold Rhythmic Auditory Stimuli, J. Neurosci. Off. J. Soc. Neurosci. 37 (2017) 4903–4912. doi: 10.1523/JNEUROSCI.3658-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This study suggested that the brain can entrain to stimuli that are not consciously perceived.
- [28].Kayser SJ, Ince RAA, Gross J, Kayser C, Irregular Speech Rate Dissociates Auditory Cortical Entrainment, Evoked Responses, and Frontal Alpha, J. Neurosci. Off. J. Soc. Neurosci. 35 (2015) 14691–14701. doi: 10.1523/JNEUROSCI.2243-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Breska A, Deouell LY, Neural mechanisms of rhythm-based temporal prediction: Delta phase-locking reflects temporal predictability but not rhythmic entrainment, PLoS Biol. 15 (2017) e2001665. doi: 10.1371/journal.pbio.2001665. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Important study that demonstrates entrainment signatures do not only reflect entrainment but also top-down predictions.
- [30].Obleser J, Henry MJ, Lakatos P, What do we talk about when we talk about rhythm?, PLoS Biol. 15 (2017) e2002794. doi: 10.1371/journal.pbio.2002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Breska A, Deouell LY, Dance to the rhythm, cautiously: Isolating unique indicators of oscillatory entrainment, PLoS Biol. 15 (2017) e2003534. doi: 10.1371/journal.pbio.2003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Notbohm A, Kurths J, Herrmann CS, Modification of Brain Oscillations via Rhythmic Light Stimulation Provides Evidence for Entrainment but Not for Superposition of Event-Related Responses, Front. Hum. Neurosci. 10 (2016) 10. doi: 10.3389/fnhum.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lakatos P, Musacchia G, O’Connel MN, Falchier AY, Javitt DC, Schroeder CE, The spectrotemporal filter mechanism of auditory selective attention, Neuron. 77 (2013) 750–761. doi: 10.1016/j.neuron.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Breska A, Deouell LY, When Synchronizing to Rhythms Is Not a Good Thing: Modulations of Preparatory and Post-Target Neural Activity When Shifting Attention Away from On-Beat Times of a Distracting Rhythm, J. Neurosci. Off. J. Soc. Neurosci. 36 (2016) 7154–7166. doi: 10.1523/JNEUROSCI.4619-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fiebelkorn IC, Pinsk MA, Kastner S, A Dynamic Interplay within the Frontoparietal Network Underlies Rhythmic Spatial Attention, Neuron. 99 (2018) 842–853.e8. doi: 10.1016/j.neuron.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Primate study using the same paradigm as [36] revealing how theta oscillations structure attentional rhythmic sampling.
- [36].Helfrich RF, Fiebelkorn IC, Szczepanski SM, Lin JJ, Parvizi J, Knight RT, Kastner S, Neural Mechanisms of Sustained Attention Are Rhythmic, Neuron. 99 (2018) 854–865.e5. doi: 10.1016/j.neuron.2018.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Intracranial study in humans using the same paradigm as [35] revealing that attentional rhythmic sampling occurs independent of task structure.
- [37].Sellers KK, Yu C, Zhou ZC, Stitt I, Li Y, Radtke-Schuller S, Alagapan S, Fröhlich F, Oscillatory Dynamics in the Frontoparietal Attention Network during Sustained Attention in the Ferret, Cell Rep. 16 (2016) 2864–2874. doi: 10.1016/j.celrep.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kienitz R, Schmiedt JT, Shapcott KA, Kouroupaki K, Saunders RC, Schmid MC, Theta Rhythmic Neuronal Activity and Reaction Times Arising from Cortical Receptive Field Interactions during Distributed Attention, Curr. Biol. CB. 28 (2018) 2377–2387.e5. doi: 10.1016/j.cub.2018.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Primate study revealing that behaviorally relevant rhythmic sampling can emerge from local interactions.
- [39].Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD, Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval, Nat. Neurosci. 16 (2013) 349–356. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK, Gamma and Beta Bursts Underlie Working Memory, Neuron. 90 (2016) 152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Important primate study that revealed that the apparent delay activity during working memory reflects a trial-averaging artifact.
- [41].Wolff MJ, Jochim J, Akyürek EG, Stokes MG, Dynamic hidden states underlying working-memory-guided behavior, Nat. Neurosci. (2017). doi: 10.1038/nn.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Landau AN, Schreyer HM, van Pelt S, Fries P, Distributed Attention Is Implemented through Theta-Rhythmic Gamma Modulation, Curr. Biol. CB. 25 (2015) 2332–2337. doi: 10.1016/j.cub.2015.07.048. [DOI] [PubMed] [Google Scholar]
- [43].Halassa MM, Kastner S, Thalamic functions in distributed cognitive control, Nat. Neurosci. 20 (2017) 1669–1679. doi: 10.1038/s41593-017-0020-1. [DOI] [PubMed] [Google Scholar]
- [44].Sweeney-Reed CM, Zaehle T, Voges J, Schmitt FC, Buentjen L, Borchardt V, Walter M, Hinrichs H, Heinze H-J, Rugg MD, Knight RT, Anterior Thalamic High Frequency Band Activity Is Coupled with Theta Oscillations at Rest, Front. Hum. Neurosci. 11 (2017) 358. doi: 10.3389/fnhum.2017.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yu C, Li Y, Stitt IM, Zhou ZC, Sellers KK, Fröhlich F, Theta Oscillations Organize Spiking Activity in Higher-Order Visual Thalamus during Sustained Attention, ENeuro. 5(2018). doi: 10.1523/ENEUR0.0384-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stitt I, Zhou ZC, Radtke-Schuller S, Frohlich F, Arousal dependent modulation of thalamo-cortical functional interaction, Nat. Commun. 9 (2018) 2455. doi: 10.1038/s41467-018-04785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Simultaneous thalamic and cortical recordings in ferrets reveal that theta connectivity precisely indexes arousal levels and attentional engagement.
- [47].Fiebelkorn IC, Pinsk MA, Kastner S, Thalamo-cortical interactions define functional dissociations across the macaque attention network, BioRxiv. (2018) 398917. doi: 10.1101/398917. [DOI] [Google Scholar]
- [48].Spyropoulos G, Bosman CA, Fries P, A theta rhythm in macaque visual cortex and its attentional modulation, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) E5614–E5623. doi: 10.1073/pnas.1719433115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S, The pulvinar regulates information transmission between cortical areas based on attention demands, Science. 337 (2012) 753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bosman CA, Womelsdorf T, Desimone R, Fries P, A microsaccadic rhythm modulates gamma-band synchronization and behavior, J. Neurosci. Off. J. Soc. Neurosci. 29 (2009) 9471–9480. doi: 10.1523/JNEUROSCI.1193-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lowet E, Gips B, Roberts MJ, De Weerd P, Jensen O, van der Eerden J, Microsaccade-rhythmic modulation of neural synchronization and coding within and across cortical areas V1 and V2, PLoS Biol. 16 (2018) e2004132. doi: 10.1371/journal.pbio.2004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bellet J, Chen C-Y, Hafed ZM, Sequential hemifield gating of alpha and beta behavioral performance oscillations after microsaccades, J. Neurophysiol. (2017) jn.00253.2017. doi: 10.1152/jn.00253.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Important study that demonstrated that microsaccades could phase reset behaviorally relevant cortical oscillations.
- [53].Helfrich RF, The Rhythmic Nature of Visual Perception, J. Neurophysiol. (2017). doi: 10.1152/jn.00810.2017. [DOI] [PubMed] [Google Scholar]
- [54].Hafed ZM, Chen C-Y, Tian X, Vision, Perception, and Attention through the Lens of Microsaccades: Mechanisms and Implications, Front. Syst. Neurosci. 9 (2015) 167. doi: 10.3389/fnsys.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Benedetto A, Burr DC, Morrone MC, Perceptual Oscillation of Audiovisual Time Simultaneity, ENeuro. 5 (2018). doi: 10.1523/ENEURO.0047-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lowet E, Gomes B, Srinivasan K, Zhou H, Schafer RJ, Desimone R, Enhanced Neural Processing by Covert Attention only during Microsaccades Directed toward the Attended Stimulus, Neuron. 99 (2018) 207–214.e3. doi: 10.1016/j.neuron.2018.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stitt I, Galindo-Leon E, Pieper F, Engler G, Fiedler E, Stieglitz T, Engel AK, Intrinsic coupling modes reveal the functional architecture of cortico-tectal networks, Sci. Adv. 1 (2015) e1500229. doi: 10.1126/sciadv.1500229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hafed ZM, Goffart L, Krauzlis RJ, A neural mechanism for microsaccade generation in the primate superior colliculus, Science. 323 (2009) 940–943. doi: 10.1126/science.1166112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Eichenbaum H, Barlow versus Hebb: When is it time to abandon the notion of feature detectors and adopt the cell assembly as the unit of cognition?, Neurosci. Lett. (2017). doi: 10.1016/j.neulet.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yuste R, From the neuron doctrine to neural networks, Nat. Rev. Neurosci. 16 (2015) 487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]