Abstract
Objective:
Reduced activation to print in the left ventral, dorsal and anterior pathways has been implicated in readers with dyslexia (DR) but is also characteristic for typical beginning readers. As the majority of studies compared DR to their age-matched peers, the observed results could either represent a dyslexia phenotype or a developmental delay. We aimed to disentangle reading and dyslexia effects by employing two control groups: age and skill matched, and a longitudinal design.
Method:
We compared brain response for print in DR with typical readers (TR) who at the beginning of schooling (TP1, 6-7 years) read on average 3 words per minute, such as DR at TP1, but improved reading to an average level; and advanced readers (AR) who at TP1 read as well as DR two years later (TP3, 8-9 years). The TR and DR groups were tracked longitudinally to observe neurodevelopmental changes.
Results:
At TP1, DR did not differ from TR. Along with time, only TR developed neural circuit for reading in the left inferior frontal and fusiform gyri. At TP3, DR hypoactivated these areas compared to both age- (TR TP3) and reading-matched (AR TP1) controls. At TP3, TR hypoactivated left frontal and bilateral ventral occipital regions when compared to AR, but these effects were non-overlapping with DR hypoactivations and are partly explained by IQ.
Conclusion:
Decreased activation of the left fusiform and inferior frontal gyri to print in DR results from an atypical developmental trajectory of reading and cannot be explained solely by lower reading skills.
Keywords: dyslexia, reading acquisition, reading development, dyslexia debate, longitudinal fMRI
INTRODUCTION
The neural processes underlying reading have long been at the center of dyslexia research and debate. However, most neuroimaging studies examining children with dyslexia compare them to age-matched peers who have significantly better reading skills. In such comparisons, differences found in patterns of brain activity could reflect dyslexia-specific effects but may also be a consequence of reduced reading expertise, reading acquisition failure, reduced exposure to print, compensatory mechanisms or the deviant learning path of readers with dyslexia (DR)1. To overcome the natural issues related to experiential and performance related differences in some studies, an additional, younger reading-matched control group was examined. The idea behind this approach was to study dyslexia-specificity, not just reading-performance dependent differences, including those that are neuroanatomical2-3 and functional4-5. To our knowledge, only two studies address the question of dyslexia specificity in the context of print processing: one with an emphasis on phonological awareness6 and the other on sentence comprehension7.
A coherent picture has emerged from a number of publications reviewing the neural pathways employed for reading in dyslexia8-11: DR fail to reorganize posterior and anterior cortical regions of the left hemisphere (LH) in a highly organized way that allows typically developing controls to effortlessly and rapidly process printed words and sentences. It is agreed that three LH neural systems tuned for reading are hypoactivated when processing print in DR: the anterior (inferior frontal gyrus, IFG), ventral (ventral occipitotemporal area, vOT) and dorsal systems (angular/supramarginal gyri). However, the importance and involvement of each of these systems may also depend on developmental changes, reading experience, stimuli type and the experimental instruction.
For example, initial work suggested that the left IFG activity can be both reduced (in children12) and increased (in adults10,13-14 and children15) in response to print in DR. Increased activation of the left IFG was linked to covert articulatory processes16, phonological recoding9, and more effortful reading13. However more recent work based on two metaanalyses8,17 provided evidence for a distinction between left IFG underactivation and a close-by left precentral region overactivation in DR. The former is implicated in access to lexical and sublexical phonological representations, and the latter in silent articulatory reading processes18. Activation in left IFG during reading is also positively correlated with reading skills in young children and adolescents19. Many studies have shown that DR or poor readers hypoactivate the left vOT during reading20-21. However, reduced activation in the left vOT is reported more often in adults with dyslexia than children with dyslexia, when compared to their age matched controls17, implicating developmental and/or reading proficiency factors. Generally, the ventral stream is regarded as the most mature and efficient system for reading, allowing for rapid recognition of visual word forms20 and linking visual or other sensory information to higher-order representations22. Thus, it seems natural that the specialization for print in this system emerges only with higher reading expertise and is positively related with performance23-24. Nonetheless, there are also findings of early recruitment of the left vOT for non-impaired reading development25-28 , also in response to phonological task 29 Finally, the implicated dysfunction of the phonological left dorsal system in dyslexia is also quite complex, as consistent hypoactivation in the posterior superior temporal cortex was found only in adults, whereas inferior parietal hypoactivation has only been shown in studies on children17. Additionally, the latter result was mostly driven by an increase in task-negative activation characterizing DR7. Dorsal pathway involvement can also be related to the orthographic transparency of the given language: it was shown that transparent orthography (Italian) readers employed dorsal pathways, while opaque orthography (English) readers employed ventral and anterior areas30.
In sum, all three LH subsystems for reading have somewhat different functions, and their activation seems (1) orthography- or task-dependent, (2) to be related to the current reading expertise and/or developmental stage of the participant, and (3) to reflect a functional disruption characteristic for dyslexia. In many cases, it is difficult or even impossible to disentangle the effects of poor (but improving) reading skills of a young reader from dyslexia specificity itself, especially at the early stages of literacy acquisition. In other words, as impaired reading is present in all DR (since dyslexia is a reading impairment per se), it is often impossible to assess whether the observed effects stem from the disorder, or possibly from other factors, such as smaller exposure to print and limited reading experience.
In the current study, we attempted to separate the effects of reading skill and dyslexia on the brain network that supports reading by comparing DR to age-matched and skill-matched controls (typical readers: TR). We retrospectively matched the groups so that at the beginning of reading acquisition TR read on the same level as DR, but outperformed DR two years later. We longitudinally traced the non-impaired and disrupted developmental trajectories of the neural processes underlying reading. A direct retrospective comparison between TR and DR allowed an assessment of the developmental phase in which differences can be observed. However, the effects observed in this comparison (like in many other studies on reading in dyslexia) could be considered both as some trait typical for dyslexia (dyslexia-specific effect) but also as an effect evoked by the unequal reading level of the groups (reading-specific effect). In this study, we attempted to separate these effects by including an additional age-matched control group which outperformed both TR and DR in reading in the first stage of the study, and was able to read as many words as DR two years later (advanced readers; AR). To observe reading-specific effects, we compared two control groups of the same age but with reading at a significantly different level (TR and AR). For the dyslexia-specific effects, we aimed to compare DR and the control groups who were reading at the same level at the selected time point (TP1 AR and TP3 DR).
We hypothesized that dyslexia specific effects (differences between DR and controls) are present only after acquisition of basic literacy, as we cannot expect differences in response to print between TR and DR at the pre-reading stage. Reading related effects emerge at the very beginning of literacy and are also present later on, reflecting either reading acquisition or proficiency effects. Dyslexia related effects are reflected in underactivation of left IFG (anterior), vOT (ventral) and inferior parietal (dorsal) regions in line with the model of impaired LH reading network in DR based on quantitative meta-analyses18. We further explore if dyslexia and reading related effects are distinct. Different neurodevelopmental trajectories are expected in DR and TR matched for age and reading skills at the start of formal reading acquisition, with higher activity in the LH neural systems tuned for reading develop only in the non-impaired group25.
METHOD
Participants
The children selected for the current analysis were part of a cohort examined in a longitudinal study on early diagnosis of dyslexia, approved by the Warsaw University Ethical Committee. All participants were Polish-speaking monolinguals, born at term, right-handed and characterized by normal IQ (controlled with Raven’s Colored Progressive Matrices31). None of the participants reported any history of neurological illness, brain damage or symptoms of ADHD. Children were tested at three time points (TP1, TP2, TP3), each one year apart. Since TP1 was conducted during the introduction of educational reforms in Poland, some of the six-year-old children were in the first grade and some were in kindergarten (based on parental decision). Formal literacy training was supposed to start in elementary school, but children were already taught letters in kindergarten. TP1 and TP3 involved both behavioral and MRI sessions and TP2 was limited to behavioral testing.
A total of 120 children were recruited for the study at TP1, and 109 completed all three TPs. At TP3, a formal diagnosis of dyslexia was conducted to enable the selection of DR (n = 25). Next, the Hungarian optimization algorithm was implemented and a custom MATLAB script was used to select subjects suitable for the control groups32 (The Math-Works Inc. Natick, MA, USA). The algorithm was set to find a pair for each DR such that a total distance between variables of interest in paired subjects (words per minute (WPM), age, IQ, SES) would be minimal. For the typical readers group (TR; n = 25), the algorithm sought children who would match DR for WPM score at TP1, as well as demographic measures (age, IQ, SES). After TR group assignment, the script was run again to look for advanced readers (AR; n = 25) among the remaining children. Children for the AR group were supposed to match DR across time points, with the AR TP1 reading score being similar to the TP3 reading score of the DR. However, we failed to match AR and DR for IQ and SES, with AR having significantly higher scores than DR (SES) or both DR and TR (IQ). As a result, a total of 75 children were included in the behavioral analysis. Demography of the groups is given in Table 1.
TABLE 1:
Participant Demographic Information
| Characteristic | DR | TR | AR | F/_χ2 | direction |
|---|---|---|---|---|---|
| n | 25 | 25 | 25 | ||
| Age in years at TP1 | M = 6.75 SD = 0.54 |
M = 6.75 SD = 0.45 |
M = 6.98 SD = 0.44 |
F(2,72) = 2.032 ns |
|
| Age in years at TP3 | M = 8.79 SD = 0.53 |
M = 8.78 SD = 0.45 |
M = 9.03 SD = 0.46 |
F(2,72) = 2.192 ns |
|
| Sex | B = 14 G = 11 |
B = 9 G = 16 |
B = 9 G = 16 |
χ2= 2.725 ns |
|
| School grade at TP1 | K = 12 E = 13 |
K = 8 E = 17 |
K = 5 E = 20 |
χ2= 4.440 ns |
|
| FHD status | FHD+ = 19 FHD− = 6 |
FHD+ = 13 FHD− = 12 |
FHD+ = 14 FHD− = 11 |
χ2= 3.486 ns |
|
| ARHQ mother | M = 35.84 SD = 17.74 |
M = 29.60 SD = 15.51 |
M = 33.40 SD = 14.30 |
F(2,72) = 0.969 ns |
|
| ARHQ father | M = 42.05 SD = 14.71 |
M= 36.23 SD = 17.42 |
M = 32.83 SD = 17.01 |
F(2,63) = 2.079 ns |
|
| SES | M = 40.37 SD = 13.31 |
M = 45.71 SD = 11.63 |
M = 51.79 SD = 8.78 |
F(2,72) = 6.286 § |
AR>DR§ |
| Raven IQ (sten score) | M = 6.84 SD = 1.52 |
M = 7.08 SD =1.26 |
M = 8.44 SD = 0.96 |
F = 11.617 *** |
AR> DR *** AR>TR§ |
Note: Demographic information of DR, TR and AR are reported, with M and SD. Statistical tests used to compare the groups are ANOVA and Chi2. ANOVA = analysis of variance; AR = advanced readers; ARHQ = adult reading history questionnaire; B = boys; Chi2 = chi-squared test; χ2 = chi-squared test score; DR = readers with dyslexia; E = first grade of elementary school; F = f-test statistics; FHD = familial history of dyslexia; G = girls; IQ = intelligence quotient; K = kindergarten; M = group mean; n = number of individuals in the subsample; ns = non significant; SD = standard deviation; SES = socioeconomic status; sten = sten scores; TP1 = time point 1; TP2 = time point 2; TR = typical readers.
p < .001;
p < .005.
Behavioral tasks and questionnaires
This report is a part of a larger study and only selected the most relevant tests and questionnaires. However, in Supplements 1-2 and Tables S1-S4, available online, a brief overview of all the applied tools is given.
At TP1, all parents were asked to complete the Adult Reading History Questionnaire33 (ARHQ). When possible, we collected ARHQ from both biological parents. Children defined as FHD+ (with familial history of dyslexia) had at least one parent who reported reading difficulties and scored greater than 40 points in the ARHQ questionnaire34. Socioeconomic status score was based on Hollingshead’s index of social position, where education level and occupation reported by both parents are considered35. At TP1, IQ was assessed with Raven’s Colored Progressive Matrices31. A low score in Raven could be used as an exclusion criterion at this time, but all screened children were in the normal or above-normal range.
Every year, children participated in 1-2 individual experimental sessions in which cognitive abilities were tested, and the most relevant tests were repeated throughout all TPs: a Decoding Test with subscales of sight word and pseudoword reading per minute, phonological awareness with phoneme analysis and phoneme deletion36; The Orthographic Awareness Test in which children were asked to select a letter string that looks most familiar to Polish37; and the Rapid Automatized Naming Test38 (subscales of objects and colors). For the cross-TPs group comparisons, raw scores were used (norms were not available or not applicable due to the educational reforms). The results of the behavioral tests applied at each TP or at only one of the TPs are provided, respectively, in Table S2 and Table S4, available online.
At TP3, all participants were tested with the standardized battery for the dyslexia diagnosis39. Detailed information about the diagnostic tool and methods used for dyslexia identification are presented in Supplement 1, available online.
fMRI task and procedure
After completing the TP1 and TP3 behavioral sessions, children were familiarized with the task in a mock scanner and successively took part in the fMRI session. The same experimental procedure in the same laboratory environment was used at TP1 and TP3. The whole experimental procedure, with both visual and auditory stimuli, is described in our previous publication,23 Supplement 3 and Table S5, available online. Only visual conditions of the fMRI task were analyzed in the current study, and thus only those will be described in this section.
Children were asked to pay attention to the stimuli appearing on the screen: high-frequency printed Polish words (e.g., “banan”) were matched with symbols (e.g., “ ”). No explicit task was given to the participants. On each trial, four different stimuli from the same condition were presented in rapid succession in a ‘tetrad’ designed to evoke strong activation with a relatively short imaging time. Each visual stimulus was presented for 250 ms, followed by a 200 ms blank screen. ‘Jittered’, intertrial intervals were employed with occasional ‘null’ trials resulting in ITIs ranging from 4 to 13 s (6.25 s on average). The whole audio-visual task was performed in two runs, each lasting 5 minutes and 2 seconds. All conditions were presented in each run, with 48 trials per run presented pseudorandomly, with no condition allowed to repeat more than three times in a row. This results in 24 total trials per condition, and 96 total stimuli per condition. Stimuli were presented using Presentation software (Neurobehavioral Systems, Albany, CA).
”). No explicit task was given to the participants. On each trial, four different stimuli from the same condition were presented in rapid succession in a ‘tetrad’ designed to evoke strong activation with a relatively short imaging time. Each visual stimulus was presented for 250 ms, followed by a 200 ms blank screen. ‘Jittered’, intertrial intervals were employed with occasional ‘null’ trials resulting in ITIs ranging from 4 to 13 s (6.25 s on average). The whole audio-visual task was performed in two runs, each lasting 5 minutes and 2 seconds. All conditions were presented in each run, with 48 trials per run presented pseudorandomly, with no condition allowed to repeat more than three times in a row. This results in 24 total trials per condition, and 96 total stimuli per condition. Stimuli were presented using Presentation software (Neurobehavioral Systems, Albany, CA).
fMRI Data Acquisition and Analyses
fMRI data were acquired on a 3T Siemens Trio scanner using a whole-brain echo planar imaging sequence with a 12-channel head coil (32 slices, slice-thickness 4 mm, TR = 2000 ms, TE = 30 ms, flip angle = 80°, FOV= 220 mm2, matrix size: 64 × 64, voxel size 3 x 3 x 4 mm). Anatomical data were acquired using a T1 weighted sequence (176 slices, slice-thickness 1 mm, TR = 2530 ms, TE = 3.32 ms, flip angle= 7°, matrix size: 256 × 256, voxel size 1 x 1 x 1 mm).
The neuroimaging data pre-processing and analyses were performed using Statistical Parametric Mapping (SPM12, Welcome Trust Center for Neuroimaging, London, UK) run on MATLAB R2016b (The Math-Works Inc. Natick, MA, USA). Images in all four runs (2 x TP1, 2 x TP3) were realigned to the mean. Next, a pairwise longitudinal registration was performed on T1-weighted images from two TPs and a midpoint average image was created. The outcome of the pairwise longitudinal registration was co-registered to the mean functional image. Co-registered images were segmented using pediatric tissue probability maps, while the Template-O-Matic toolbox40 was used for this purpose with the matched pairs option. The functional images were normalized using compositions of flow fields and a group-specific template. Finally, the normalized functional images were smoothed with an 8 mm isotropic Gaussian kernel. The data were modeled for each fMRI run using the canonical hemodynamic response function convolved with the experimental conditions. Artifactual volumes were identified in the ART toolbox using a scan-to-scan movement threshold of 3 mm and a rotation threshold of 0.05 radians, similarly to previous publication41, and modeled in the design matrix (with each artifactual volume represented as a separate regressor). Participant data were excluded from the fMRI data analysis if greater than 20% of volumes in one run exceeded these motion tolerances, and in effect one TR and one DR child was excluded from the fMRI analysis leaving 24 DR, 24 TR and 25 AR children in the whole-brain analyses. In other subjects, motion-affected volumes were modeled in the single-subject GLM and excluded from the analysis. Detailed description of this step is given in Supplement 4, available online, and statistical tests used to compare motion between the groups are reported in the Table S5 available online.
The general linear approach was used to analyze the data, contrasting experimental and rest trials in each subject. For each subject, contrasts were computed to examine word (print>rest) and word-specific effects (print>symbols). Additionally, the response for the control condition (symbols>rest) was estimated. At the group level, two sample t-tests were applied to analyze the effect of reading (not associated with reading problems) and the effects of dyslexia (not associated with reading performance). The developmental trajectories of the DR and TR groups were analyzed by means of paired t-tests. Additionally, interactions between group and time were tested with a 2×2 flexible factorial model. The results are reported at a significance level of p < .005 uncorrected, and an extent threshold of 50 voxels41 corresponding to the threshold of p < .05, corrected for multiple comparisons using a cluster size algorithm resulting from Monte Carlo simulations (3dClustSim, AFNI, http://afni.nimh.nih.gov). For all models, we report only task positive activations42 – all the results were masked with the map of positive activations for all subjects in the respective condition (print, symbols, print > symbols). Significant clusters were labeled using the Automated Anatomical Labeling (AAL) Atlas implemented in xjView toolbox (http://www.alivelearn.net/xjview).
RESULTS
Behavioral Results
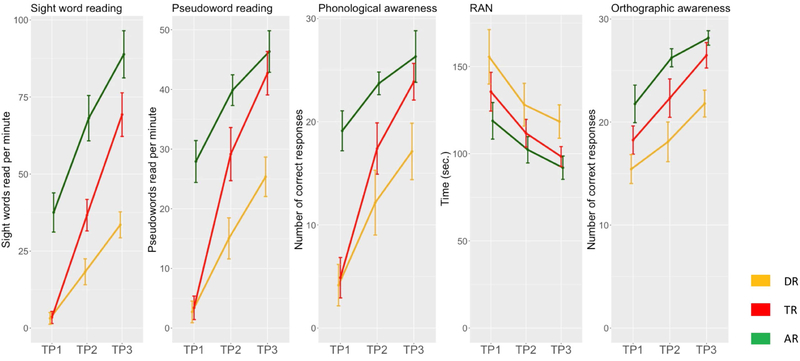
Depending on the variable either ANOVA or Ch2 was applied to test for behavioral and demographic differences between the three groups (see Table 1 for demographics and Table S2 [available online] for behavioral scores). Age, SES, Raven’s IQ and TP1 sight word reading were similar between DR and TR, since those variables were used as the pairwise matching criteria. Other reading-related skills at TP1 were similar between the DR and TR groups. No differences were found in letter knowledge, phonological awareness tasks (phoneme deletion, phoneme analysis), vocabulary and verbal working memory (digit and syllable span). However, the DR group was slower in the rapid automatized naming task and less accurate in the orthographic awareness task. At TP2, 12 months after the first testing, the groups differed in several other tests, i.e., sight word reading, pseudoword reading and phonological awareness, with the DR group lagging behind TR. Differences in orthographic awareness persisted, along with longer naming times in RAN in the DR. At TP3, the DR group scored lower in sight word and pseudoword reading, phonology, RAN, selective visual attention task, and orthographic awareness, as presented in Figure 1.
Figure 1: Changes in Reading and Reading-Related Skills Across Time.
Note: Reading (sight word reading and pseudoword reading) and reading-related skills (phonological awareness, rapid automatized naming, and phonological awareness) of DR, TR and AR across three time points. 95% confidence intervals are represented for each data point. Raw scores are reported for all measures. AR = advanced readers; DR = readers with dyslexia; TP1 = time point 1; TP2 = time point 2; TP3 = time point 3; TR = typical readers.
AR children were of similar age to DR and TR children at the TP1. However, throughout the experiment, they were consistently better than the two other groups in reading, phonological awareness, rapid automatized naming, and orthographic awareness. However, their performance in reading and reading-related tests at TP1 was similar to that of DR at TP3 (see Table S3, available online).
Figure 1 presents five tests repeated with the same items and testing procedures at each measurement point (sight word reading, pseudoword reading, phonological awareness task, rapid automatized naming task, orthographic awareness task). Other testing procedures, as well as the results of the tests used throughout the experiment, are reported in Supplement 2 and Tables S2-S4, available online
fMRI Results
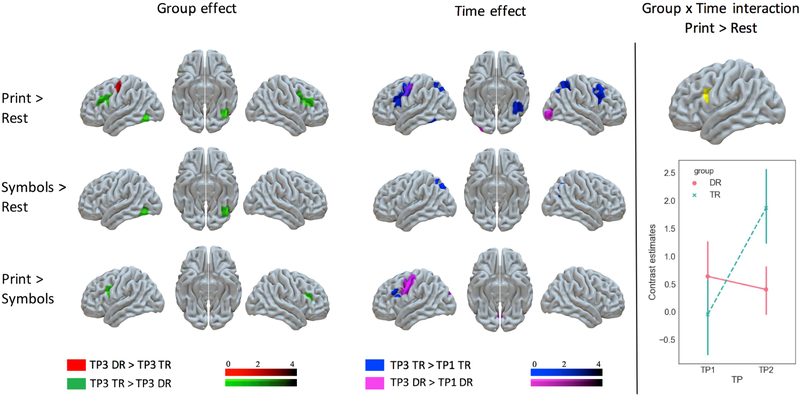
Two-sample t tests performed for the DR and TR group at TP1 revealed no differences between the groups in any of the conditions. Significant differences were found at TP3 (Table 2 and Figure 2). For print, TR showed a stronger response in the bilateral IFG and left vOT, while for the word specific contrast (print > symbols), their response was stronger only in the bilateral IFG. An opposite pattern with DR being more active than TR was found only for print in the left Precentral gyrus (PrCG). Since a difference between DR and TR in the left vOT was expected and present in response to words (print > rest contrast) but no longer significant in the word specific contrast (print > symbols), we computed an additional two-sample t-test to examine group differences for symbols. We found that TR activated the left vOT for symbols more than the DR group. This cluster was in the same anatomical location as the cluster showing a group difference for words (print > rest contrast).
Table 2:
Brain Activation for Effects of Group, Time Point, and Their Interaction
| Brain region | H | x | y | z | t | V | |
|---|---|---|---|---|---|---|---|
| PRINT > REST | |||||||
| Group effects | |||||||
| TR TP3 > DR TP3 | Inferior Frontal (tri, oper), Precentral | L | −34 | 10 | 32 | 4.13 | 472 |
| Fusiform | L | −42 | −62 | −12 | 3.85 | 52 | |
| Inferior Frontal (oper), Precentral | R | 40 | 2 | 26 | 3.29 | 97 | |
| Inferior and Middle Frontal | R | 48 | 28 | 22 | 3.21 | 152 | |
| DR TP3 > TR TP3 | Precentral | L | −46 | −12 | 50 | 3.97 | 63 |
| AR TP1 > TR TP1 | Supplementary Motor Area | L | −6 | 6 | 56 | 3.80 | 109 |
| Inferior Frontal (oper, tri), Precentral | L | −36 | 8 | 22 | 3.49 | 121 | |
| Inferior frontal (oper), Middle frontal | R | 44 | 20 | 36 | 3.33 | 64 | |
| Precentral | L | −48 | 0 | 56 | 3.24 | 133 | |
| AR TP3 > TR TP3 | Inferior Frontal (tri, orb) | L | −46 | 30 | −4 | 4.17 | 459 |
| Inferior Occipital, Lingual, Cerebellum (crus 1), Fusiform | L | −38 | −80 | −18 | 3.74 | 407 | |
| Middle Temporal | L | −50 | −38 | 4 | 3.59 | 51 | |
| Middle Frontal | L | −30 | 58 | 22 | 3.58 | 68 | |
| Lingual, Inferior Occipital | R | 26 | −88 | −8 | 3.56 | 108 | |
| AR TP1 > DR TP3 | Inferior Occipital, Fusiform | L | −40 | −64 | −12 | 4.88 | 147 |
| Inferior Temporal | L | −36 | −38 | −12 | 3.96 | 50 | |
| Inferior Occipital | L | −30 | −86 | −8 | 3.48 | 70 | |
| Inferior Frontal (oper, tri), Middle Frontal | L | −30 | 10 | 30 | 3.37 | 144 | |
| Time effects | |||||||
| DR TP3 > TP1 | Inferior and Middle Occipital | R | 38 | −96 | −4 | 4.62 | 96 |
| Precentral | L | −56 | 2 | 40 | 3.80 | 116 | |
| TR TP3 >TP1 | Precentral | L | −56 | 4 | 46 | 6.99 | 254 |
| Inferior Frontal (oper), Precentral, Middle Frontal | R | 42 | 2 | 26 | 4.52 | 322 | |
| Inferior Temporal, Fusiform | L | −44 | −50 | −20 | 4.39 | 137 | |
| Superior Parietal Lobule | L | −30 | −74 | 56 | 4.17 | 100 | |
| Supplementary Motor Area | L | −4 | 6 | 58 | 4.12 | 166 | |
| Superior Parietal Lobule, Angular, Inferior Parietal | R | 40 | −60 | 62 | 4.08 | 268 | |
| Inferior Frontal (oper, tri), Precentral | L | −34 | 6 | 26 | 3.97 | 362 | |
| TP x group interaction | |||||||
| TR and | Inferior Frontal (oper) | L | −44 | 8 | 22 | 12.11 | 126 |
| DR | |||||||
| TP1 and | |||||||
| TP3 | |||||||
| SYMBOLS > REST | |||||||
| Group effects | |||||||
| TR TP3 > DR TP3 | Inferior Occipital Gyrus, Fusiform Gyrus, Inferior Temporal Gyrus | L | −42 | −64 | −12 | 3.93 | 82 |
| AR TP1 > DR TP3 | Inferior Occipital Gyrus, Fusiform Gyrus, Middle Occipital Gyrus | L | −42 | −64 | −14 | 4.41 | 311 |
| DR TP3 > AR TP1 | Middle Occipital Gyrus | L | −48 | −84 | 12 | 3.83 | 72 |
| Insula, Inferior Frontal Gyrus (orb, tri) | L | −36 | 6 | 0 | 3.63 | 156 | |
| AR TP3 > TR TP3 | Parahippocampal Gyrus, Fusiform Gyrus, Hippocampus | L | −28 | −10 | −28 | 4.39 | 108 |
| Lingual Gyrus, Fusiform Gyrus, Cerebellum (crus I) | L | −36 | −82 | −18 | 3.55 | 146 | |
| Middle Orbital Frontal | L | −38 | 54 | −8 | 3.33 | 53 | |
| Middle Occipital, Calcarine | R | 32 | −98 | 2 | 3.55 | 73 | |
| Time effects | |||||||
| TR TP3 > TP1 | Superior and Inferior Parietal Lobule | L | −32 | −74 | 54 | 5.78 | 91 |
| Angular | R | 34 | −60 | 28 | 4.35 | 95 | |
| PRINT > SYMBOLS | |||||||
| Group effects | |||||||
| TR TP3 > DR TP3 | Inferior Frontal (oper), Precentral, Middle Frontal | L | −28 | 8 | 30 | 4.36 | 230 |
| Inferior Frontal (tri) | R | 36 | 26 | 22 | 3.74 | 106 | |
| AR TP1 > TR TP1 | Precentral, Inferior Frontal (oper, tri) | L | −50 | 6 | 42 | 5.57 | 117 2 |
| Middle and Superior Temporal, Supramarginal | L | −58 | −44 | 10 | 4.22 | 382 | |
| Supplementary Motor Area | L&R | −8 | 8 | 54 | 4.21 | 306 | |
| Superior Temporal | R | 60 | −38 | 14 | 4.19 | 209 | |
| Middle and Superior Occipital, Superior Parietal | L | −26 | −64 | 36 | 3.66 | 202 | |
| Inferior Frontal (tri, oper) | R | 40 | 16 | 26 | 3.05 | 87 | |
| AR TP1 > DR TP3 | Inferior Frontal (tri, oper), Precentral, Middle Frontal | L | −28 | 6 | 30 | 5.55 | 505 |
| Fusiform, Inferior Temporal | L | −34 | −34 | −16 | 5.36 | 111 | |
| Middle and Superior Temporal | R | 60 | −8 | −12 | 4.92 | 67 | |
| Middle and Superior Temporal | R | 50 | −26 | 2 | 4.53 | 365 | |
| Inferior Frontal (tri, oper), Middle Frontal | R | 40 | 24 | 30 | 4.36 | 489 | |
| Medial Superior Frontal | R | 10 | 70 | 12 | 4.14 | 79 | |
| Precuneus | R | 6 | −50 | 60 | 3.30 | 53 | |
| Time effects | |||||||
| DR TP3 > TP1 | Precentral, Postcentral | L | −42 | −8 | 50 | 5.39 | 595 |
| Calcarine (L&R), Cuneus (L) | L&R | −8 | −88 | 36 | 3.81 | 377 | |
| TR TP3 > TP1 | Inferior Frontal (tri) | L | −52 | 10 | 28 | 4.60 | 57 |
| Supplemenary Motor Area | L | −8 | 12 | 54 | 3.42 | 57 | |
Note: Results of word (print > rest), symbol (symbol > rest) and word-specific (print > symbols) contrasts are reported, including hemisphere, MNI coordinates, t-statistic and the number of voxels. Direct comparisons are shown for DR, TR and AR at TP1, TP3 and across time points (group effects). Time-related changes are shown in DR and TR (time effects). Results are reported at a significance level of p < .005 uncorrected, and an extent threshold of 50 voxels. AR = advanced readers; DR = readers with dyslexia; H = hemisphere; L = left hemisphere; MNI coordinates = Montreal Neurological Institute coordinates, x, y, z; orb = pars orbitalis; oper = pars opercularis; R = right hemisphere; t = t-test statistic; TP1 = time point 1; TP3 = time point 3; TR = typical readers; tri = pars triangularis; V = number of voxels.
FIGURE 2. Group Effects, Time Effects, and Their Interaction.
Note: Group effect: Word (Print > Rest), Symbol (Symbol > Rest) and word-specific (Print > Symbols) activations contrasted for DR and TR at TP3. Time effect: BOLD signal increases over time (TP3 > TP1) in the word activation (Print > Rest) and word specific activation (Print > Symbols) in DR and TR. Interaction: interaction between group (DR, TR) and time (TP1, TP3) for Print > Rest. 95% confidence intervals are represented for each data point. AR = advanced readers; BOLD = blood-oxygen level dependent; DR = readers with dyslexia; TP1 = time point 1; TP3 = time point 3; TR = typical readers.
To examine the developmental trajectories of DR and TR children who were behaviorally and neurally similar at TP1 and diverged later on, we performed a series of paired t-tests that compared the patterns of activation at T1 and T3 (Table 2 and Figure 2). For words, TR children in the TP3 > TP1 comparison showed more activation in the left vOT, bilateral IFG and precentral gyri (PrCG), left supplementary motor area (SMA), bilateral superior parietal lobule and right angular gyrus, while DR children showed more activation in the left PrCG and inferior and middle occipital gyri on the right. Word specific activation was enhanced with time in the left PrCG in the DR group and in the left IFG in the TR group. Additionally, DR showed more activity in the bilateral calcarine gyrus in the word specific contrast, and TR - in the left superior parietal lobule and right angular gyrus for symbols.
Group x TP interactions were examined with a 2×2 flexible factorial model with the DR and TR groups and both TPs included. A significant interaction between group and time was found only for words. In TR, the activity of the left IFG grew with time, while it remained on a similar level in the DR group (Figure 2, Table 2).
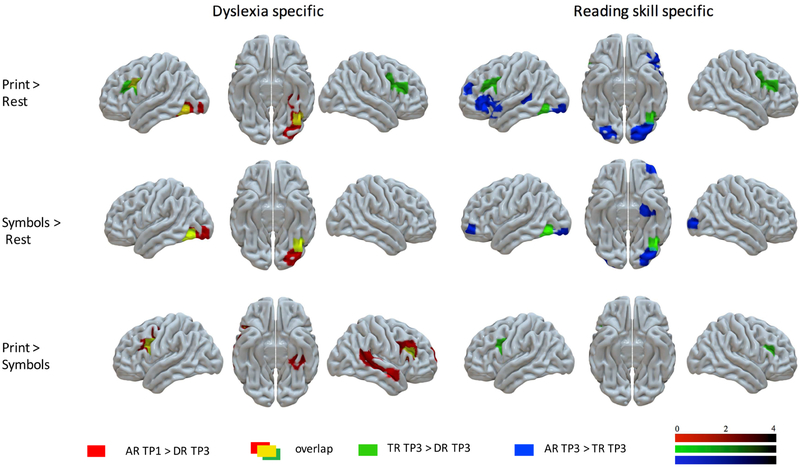
To tease apart the differences that reflect reading skills from those that reflect dyslexia, we performed another series of two-sample t-tests comparing all three groups. To extract the neural regions related to dyslexia, we compared the neural response of the DR group at TP3 to the AR group at TP1 when their reading performance was similar (TP3 DR – Mwpm= 33.52, SD = 10.78, range = 3-49; TP1 AR – Mwpm= 37.52, SD = 16.16, range = 16-69), and the TR group, who read better than DR at TP3 (Mwpm = 69.28, SD = 18.04, range = 37-107) but were more similar in all demographic measures (i.e., age, IQ and SES). For words, DR compared to the control groups (TR and AR) showed overlapping hypoactivation in the left IFG (108 voxels) and left vOT (44 voxels), while for the word specific contrast an overlapping hypoactivation was present in the bilateral IFG (left IFG - 201 voxels, right IFG – 102 voxels). Interestingly, for symbols, an overlapping hypoactivation (62 voxels) was present in the same left vOT cluster as that observed for words activation (see Figure 3 and Table 2). To examine whether the differences observed for the DR and TR groups are related to different reading skills, we compared AR with TR at TP3 (when both groups acquired reading). The AR group was consistently more proficient in reading and reading-related skills than TR at each TP. At the brain level, for words, even though AR showed stronger activity in the bilateral vOT and left IFG when compared to TR, the clusters were in somewhat different locations than the hypoactivation clusters in DR compared to control groups. We did not find any areas of overlap in any of the contrasts. For symbols, a similar pattern of was found as for words. No significant differences between AR and TR groups were found for the word specific contrast (see Figure 3 and Table 2).
FIGURE 3: Dyslexia Specific Effects and Reading Skill Specific Effects.
Note: Differences between groups of interest showing regions discriminating groups for dyslexia (Dyslexia specific: AR TP1 > DR TP3) or poorer/better reading (Reading skill specific: AR TP3 > TR TP3). AR = advanced readers; DR = readers with dyslexia; TP1 = time point 1; TP3 = time point 3; TR = typical readers.
To test if neural differences related to the reading level can be observed at any stage of reading development, we compared our two control groups at the first stage of the study (TP1, when TR were still mostly pre-readers). We found that AR, when reading words, activated bilateral IFG and left SMA more strongly than TR children. The word specific contrast showed stronger activity of the bilateral IFG and STG in the AR group (for TP3, see Figure 3; for TP1, and see Supplement 5 and Figure S1, available online).
Finally, since IQ and reading are tightly connected during typical reading development43, children selected to the AR group had higher IQ scores than the TR and DR groups. These children also had significantly higher parental SES than DR children. To control for these confounds, we introduced covariates to the group analyses (see Supplement 6, Figure S2, and Table S6, available online). At TP1, after controlling for IQ, the two control groups no longer differed in the bilateral IFG for word or right IFG and left STG for word specific activation. At TP3, IQ also partially accounted for the difference between AR and TR, but at the same time additional clusters appeared in the left IFG for words and symbols.
Interestingly, controlling for IQ and SES did not affect differences in the left vOT activation between AR and DR. However, the difference in response to symbols between AR and DR shifted from the left vOT to the left inferior temporal cortex when IQ and SES were covaried. Similarly, differences between AR and DR in the left IFG for print were no longer significant after controlling for IQ and SES.
DISCUSSION
We explored the neurodevelopmental trajectories for reading in children matched for age and reading skill at the beginning of formal literacy acquisition, half of whom developed dyslexia two years later, and half of whom developed typical reading skills. Having an additional group of advanced readers who at the beginning of the study had reading skills similar to DR with two years of literacy education enabled us to separate dyslexia specific effects from reading skill effects in the neural processes underlying reading.
Typical readers matched with DR for reading skills, age and other demographics at the beginning of the study read better and had superior phonological skills than DR one and two years later. At the beginning of literacy acquisition, DR lagged behind TR in rapid naming and orthographic awareness tasks, and this pattern persisted over the next years (for details see Figure 1, Supplements 1, 2, and Table S4, available online). No differences in brain response to print were found at the first stage of the study, when the groups read on average 3 words per minute and 14 children in each group could not yet read. A possible explanation of this finding is that at this stage the neural circuit for reading is not yet “tuned up” in both TR and DR children. Even though two previous studies reported reduced activation for print processing in pre-readers at risk for dyslexia44-45, smaller number of children, more liberal thresholds and the lack of the follow-up with the dyslexia diagnosis limit their implications. Here, all DR children indeed developed dyslexia two years later (since this was the fundamental principle of the groups assignment), and at this time clear differences emerged. TR, when exposed to print, employed the bilateral IFG and left vOT more strongly than DR, while PrCG showed more activity in DR than TR. Time effect analysis show that two years of reading instruction increased activation to print in the left vOT, SMA, PrCG, and bilateral IFG in typical readers. In DR, increased activation was observed only in the left PrCG and occipital cortex. Additionally, an interaction between group and time was found in the left IFG: while the engagement of this area in the reading process increased in the TR group, it remained at a similar level in DR across two measurement points.
As expected, we observed the emergence of higher activity in the LH anterior (IFG) and ventral (vOT) neural systems tuned for reading only in children who developed typical reading skills. DR instead employed the left PrCG to a larger extent. We have previously shown that young readers widely employ the left PrCG for print processing, and its activity is positively correlated with the level of literacy at the beginning of literacy acquisition23. Processes underlying left PrCG activity may be related to serial letter-by-letter decoding and effortful covert articulatory processes, but in normal reading development, this strategy is replaced early on by phonological recoding and whole word recognition processes19,24,46-48. Our results show that DR still use this strategy when non-impaired readers already build up more mature pathways for reading in the ventral (vOT) and anterior (IFG) areas of the left hemisphere. The inefficiency of the strategy employed by DR is reflected by slower reading speed and lower accuracy, as observed in the behavioral data.
For word-specific (print > symbols) contrast, the group difference in the left vOT together with an increase of left vOT activity with time in TR were no longer significant, in line with evidence of equivalent sensitivity, but reduced specificity in the left vOT at the beginning compared to adult readers49. Further examination of the control condition (symbols) showed that the left vOT in typical readers was similarly active for symbols as for print, thus in the print > symbols contrast this effect was cancelled out. This result suggests that the left vOT in non-impaired readers is responsive not only to stimuli other than visual50, but also to those other than words, such as symbol strings51. However, in DR, the left vOT was not activated by symbols, and was the only region that differentiated the groups for symbols when they were directly compared. Nonetheless, the control condition of symbols was not purely non-linguistic, since some of the symbols could be named easily (e.g., ) We may hypothesize that children could engage themselves in some sort of implicit, automatized naming, a process that is often disrupted in DR52-53.
) We may hypothesize that children could engage themselves in some sort of implicit, automatized naming, a process that is often disrupted in DR52-53.
In line with previous studies, we found clear differences between typical readers and DR in the neural processes underlying reading8,13,17. However, these effects could be potentially attributed to the fact that DR at this point were already reading significantly poorer than TR, using strategies suitable for the earlier stages of reading development. To identify the neural differences that are characteristic of the DR sample and not just of the lower literacy level, we compared brain responses of the DR group with advanced readers (AR) who already two years earlier were able to read at a similar level as DR at TP3. This comparison revealed hypoactivation in several brain areas in DR, which partially overlapped with the effects found in comparison with TR. Overlap was present in the left IFG in a similar anatomical location for word and word-specific contrasts. Additionally, for print and symbols, an overlap was observed in the left vOT. Controlling for IQ and SES removed differences between AR and DR in the left IFG for words but not for word specific contrast, while the difference in the left vOT for symbols was shifted more anteriorly to the inferior temporal gyrus. The observed differences between younger AR and older DR cannot be accounted for by a varying level of literacy, because both groups were reading with a similar speed and accuracy of approximately 30 words per minute. The differences could be ascribed to neural functioning characteristic of the DR group, who hypoactivate the anterior language system specifically for written words. This result is not surprising given the broad literature implicating the left IFG in early reading stages46. It is thought to reflect the problem that DR have in accessing phonological output representations54-55. In addition, the left IFG has strong reciprocal connections and interacts with the left vOT cortex during non-impaired reading56-58. The left vOT cortex, associated with both visual-orthographic whole-word processing and serial grapheme-phoneme conversion18, also showed hypoactivation in DR compared to both control groups. Surprisingly, the hypoactivation was not specific to words, but to some extent was also present for symbols, suggesting a more general visual object processing deficit in dyslexia. The word-specificity of the left vOT has been disputed before59-61, and higher activation for unfamiliar compared with familiar letter strings was interpreted as a reflection of sustained task-related top-down processing20 or of greater prediction error (i.e., the difference between bottom-up visual information and top-down predictions51. The top-down predictions are assumed to be generated automatically from prior experience in higher cortical levels that contribute to representing phonology, semantics, and actions. In this context, hypoactivation in the left vOT in DR could reflect a failure to establish hierarchical connections and access top-down predictions.
No differences between DR and controls were found in the dorsal stream, in contrast to previous studies, where dyslexia-specific effects were found in the bilateral parietal areas in the basic sentence reading7 and visual word rhyming task6. In contrast to these studies, where reading comprehension or phonological decisions were required, we employed passive single word reading, which is less likely to involve the dorsal stream62. Furthermore, we focused on task positive activations42, as dyslexia specific hypoactivation in the inferior parietal cortex could be partly explained by task negative activations6-7. Hypoactivation due to deactivation in dyslexia may have a different functional role compared to reduced activation17. However, the significance of this difference has yet to be understood and some studies excluded foci which resulted from differences in deactivation22.
Finally, we examined reading skill related effects by comparing two control groups at two stages of reading development. At TP1, when TR were still mostly pre-readers, AR presented increased activity in in bilateral IFG for words and also in the bilateral STG for word specific contrasts. Differences in IFG were in a similar location to changes related to reading acquisition in TR, supporting the notion that the emergence of a neural circuit for reading parallels typical reading acquisition63. At TP3, no differences were observed for word specific contrast, while for words, increased activity was found in left IFG and bilateral visual cortex. Interestingly, when differences in IQ between the two control groups were controlled for, IFG overactivation in was no longer significant for words in the TP1. Thus, it seems that among typically reading children, brain response to print is related to their cognitive capacities, but given the general nature of this measure the mechanism is not clear. At the same time, differences in IQ or SES cannot account for reduced left vOT activation to print in children with dyslexia.
Current study has several limitations. Most importantly, the results of the comparisons, including AR, should be interpreted with caution, since this group has a significantly higher IQ than the other two groups and comes from high SES families. However, it seems natural that children who were able to master reading very quickly are characterized by high cognitive functioning and probably have considerable support from their highly educated parents. There is also evidence that IQ and reading are tightly connected in typical reading development43. After IQ or IQ and SES were controlled for, some of the effects changed, especially in the comparison between the two control groups. Another potentially limiting factor is the high number of FHD+ children present in the control groups. We cannot exclude the possibility that by using ARHQ as a measure of parental risk instead of a dyslexia diagnosis (which was not common in Poland when participants’ parents were attending school), we overestimated “dyslexia risk”. Even though the number of FHD+ children was similar across the groups, the observed results could be more robust if only FHD- children were included as controls. An additional disadvantage of the current study is that no normalized tests measuring reading and reading-related skills were available for children at TP1 or TP2. Therefore, group assignment, including dyslexia diagnosis, was based only on TP3 scores from a normalized battery for the dyslexia diagnosis. Finally, the break between fMRI scan and the behavioral sessions when reading and other skills were measured was on average 44 days (SD 25.32, range 5-127) at the TP1 and 23 days (SD = 23.73, range = −45-85) at the TP3. Future studies on reading acquisition in children should aim at reducing this gap, since at this developmental stage one could expect a steep increase in reading performance within the period of 2-3 months.
To conclude, this study’s results indicate that hypoactivation of the left ventral occipito-temporal and inferior frontal cortex characteristic of the brain response to print in dyslexia cannot be explained solely by lower reading skills. We thus confirm that these two brain modules, which emerge in parallel to reading development in typical readers, are impaired in dyslexia. Therefore, the classical models of reading-related brain activation that involve the dorsal tempo-parietal cortex need to be updated.
Supplementary Material
Acknowledgments
The authors would like to thank all of the families that participated in this study.
Funding
This work was funded by grants from the Polish Ministry of Science and Higher Education (IP2011 020271), the National Science Center (2014/N/HS6/03515, 2011/03/D/HS6/05584, 2015/17/N/HS6/03013 and 2014/14/A/HS6/00294), grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01 HD 001994 to Haskins Laboratories and P01 HD 070837 to Georgia State University). The project was realized with the aid of CePT research infrastructure purchased with funds from the European Regional Development Fund as part of the Innovative Economy Operational Programme, 2007–2013. Funding sources were not involved in the experiment realization, data collection, data analysis, or writing of the report.
Footnotes
Disclosures: Drs. Dębska, Łuniewska, Marchewka, Pugh, Jednoróg, Ms. Chyl, and Mr. Kossowski report no biomedical financial interests or potential conflicts of interest.
All authors served as statistical experts for this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ms. Katarzyna Chyl, Polish Academy of Sciences, Nencki Institute of Experimental Biology, Warsaw, Poland..
Dr. Agnieszka Dębska, Polish Academy of Sciences, Nencki Institute of Experimental Biology, Warsaw, Poland..
Dr. Magdalena Łuniewska, Polish Academy of Sciences, Nencki Institute of Experimental Biology, Warsaw, Poland., Warsaw University, Poland.
Dr. Artur Marchewka, Polish Academy of Sciences, Nencki Institute of Experimental Biology, Warsaw, Poland..
Mr. Bartosz Kossowski, Polish Academy of Sciences, Nencki Institute of Experimental Biology, Warsaw, Poland..
Kenneth R. Pugh, Haskins Laboratories, New Haven, CT; Yale University School of Medicine, New Haven, CT; and the University of Connecticut, Storrs, CT..
Katarzyna Jednoróg, Polish Academy of Sciences, Nencki Institute of Experimental Biology, Warsaw, Poland..
REFERENCES
- 1.Ramus F, Altarelli I, Jednoróg K, Zhao J, Scotto di Covella L. Neuroanatomy of developmental dyslexia: Pitfalls and promise. Neurosci Biobehav Rev. 2018;84:434–452. [DOI] [PubMed] [Google Scholar]
- 2.Altarelli I, Monzalvo K, Iannuzzi S, et al. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: evidence for differential effects in boys and girls. J Neurosci. 2013;33(27): 11296–11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. An investigation into the origin of anatomical differences in dyslexia. J Neurosci. 2014;34(3):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeft F, Meyler A, Hernandez A, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci U S A. 2007;104(10):4234–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olulade OA, Napoliello EM, Eden GF. Abnormal visual motion processing is not a cause of dyslexia. Neuron. 2013;79(1):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoeft F, Hernandez A, McMillon G, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26(42): 10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz E, Maurer U, van der Mark S, et al. Reading for meaning in dyslexic and young children: distinct neural pathways but common endpoints. Neuropsychologia. 2009;47(12):2544–2557. [DOI] [PubMed] [Google Scholar]
- 8.Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30(10):3299–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34(6):479–492. [DOI] [PubMed] [Google Scholar]
- 10.Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum Brain Mapp 2015;36(5):1963–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao F, Bitan T, Chou T-L, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 2006;47(10):1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95(5):2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122 ( Pt 10):1901–1917. [DOI] [PubMed] [Google Scholar]
- 15.Georgiewa P, Rzanny R, Gaser C, et al. Phonological processing in dyslexic children: a study combining functional imaging and event related potentials. Neurosci Lett. 2002;318(1):5–8. [DOI] [PubMed] [Google Scholar]
- 16.Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. [DOI] [PubMed] [Google Scholar]
- 17.Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56(3):1735–1742. [DOI] [PubMed] [Google Scholar]
- 18.Richlan F Functional neuroanatomy of developmental dyslexia: the role of orthographic depth. Front Hum Neurosci. 2014;8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6(7):767–773. [DOI] [PubMed] [Google Scholar]
- 20.Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15(6):254–262. [DOI] [PubMed] [Google Scholar]
- 21.van der Mark S, Bucher K, Maurer U, et al. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 2009;47(4): 1940–1949. [DOI] [PubMed] [Google Scholar]
- 22.Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-byletter reading? PLoS One. 2010;5(8):e12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chyl K, Kossowski B, Dębska A, et al. Pre-reader to beginning reader: changes induced by reading acquisition in print and speech brain networks. J Child Psychol Psychiatry. 2018;59(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaywitz BA, Shaywitz SE, Pugh KR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52(2):101–110. [DOI] [PubMed] [Google Scholar]
- 25.Brem S, Bach S, Kucian K, et al. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci U S A. 2010;107(17):7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parviainen T, Helenius P, Poskiparta E, Niemi P, Salmelin R. Cortical sequence of word perception in beginning readers. J Neurosci. 2006;26(22):6052–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A Developmental fMRI Study of Reading and Repetition Reveals Changes in Phonological and Visual Mechanisms Over Age. Cereb Cortex. 2008;18(9):2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003;60(1):94–100. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Joanisse MF, Booth JR. Reading skill related to left ventral occipitotemporal cortex during a phonological awareness task in 5-6-year old children. Dev Cogn Neurosci. 2018;30:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulesu E, McCrory E, Fazio F, et al. A cultural effect on brain function. Nat Neurosci. 2000;3(1):91–96. [DOI] [PubMed] [Google Scholar]
- 31.Szustrowa T, Jaworowska A. TMK-Test Matryc Ravena w Wersji Kolorowej [CPM-Raven’s Coloured Progressive Matrices]. Warszawa, Poland: PTP; 2003. [Google Scholar]
- 32.Kuhn HW. The Hungarian method for the assignment problem. Nav Res Logist. 1955;2(1–2):83–97. [Google Scholar]
- 33.Lefly DL, Pennington BF. Reliability and validity of the adult reading history questionnaire. J Learn Disabil. 2000;33(3):286–296. [DOI] [PubMed] [Google Scholar]
- 34.Black JM, Tanaka H, Stanley L, et al. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. Neuroimage. 2012;59(3):3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollingshead AB. Four factor index of social status. New Haven, CT, USA: Yale University; 1975. [Google Scholar]
- 36.Szczerbiński M, Pelc-Pękał O Zestaw metod do diagnozy trudności w czytaniu - Test Dekodowania [The Decoding Test - A Set of Tools for Diagnosing Reading Difficulties]. Gdańsk, Poland; PTPiP; 2013. [Google Scholar]
- 37.Awramiuk E, Krasowicz-Kupis G. Reading and spelling acquisition in Polish: Educational and linguistic determinants. L1 Educational Studies in Language and Literature. 2013;14:1–24 [Google Scholar]
- 38.Fecenec D, Jaworowska A, Matczak A, Stań J, Zalewska E. Test szybkiego nazywania (TSN) [Rapid Automatized Naming Task]. Warszawa, Poland: Podręcznik Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego; 2013. [Google Scholar]
- 39.Bogdanowicz M, Jaworowska A, Krasowicz-Kupis G, et al. Diagnoza dysleksji u uczniów klasy III szkoły podstawowej. Warszawa, Poland: Przewodnik diagnostyczny Pracownia Testów Psychologicznych; 2008. [Google Scholar]
- 40.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41(3):903–913. [DOI] [PubMed] [Google Scholar]
- 41.Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci U S A. 2012;109(6):2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan C, Cao F, Pedroarena-Leal N, McNorgan C, Booth JR. Reading acquisition reorganizes the phonological awareness network only in alphabetic writing systems. Hum Brain Mapp. 2013;34(12):3354–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrer E, Shaywitz BA, Holahan JM, Marchione K, Shaywitz SE. Uncoupling of reading and IQ over time: empirical evidence for a definition of dyslexia. Psychol Sci 2010;21(1):93–101. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y, Stevens C, Dow M, Harn BA, Chard DJ, Neville HJ. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. Neuroimage. 2011;57(3):704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Specht K, Hugdahl K, Ofte S, et al. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children. Scand J Psychol 2009;50(1):79–91. [DOI] [PubMed] [Google Scholar]
- 46.Pugh KR, Frost SJ, Sandak R, et al. Mapping the word reading circuitry in skilled and disabled readers. The neural basis of reading. 2010:281–305. [Google Scholar]
- 47.Blomert L The neural signature of orthographic–phonological binding in successful and failing reading development. Neuroimage. 2011;57(3):695–703. [DOI] [PubMed] [Google Scholar]
- 48.Sandak R, Mencl WE, Frost SJ, Pugh KR. The Neurobiological Basis of Skilled and Impaired Reading: Recent Findings and New Directions. Sci Stud Read. 2004;8(3):273–292. [Google Scholar]
- 49.Centanni TM, King LW, Eddy MD, Whitfield-Gabrieli S, Gabrieli JDE. Development of sensitivity versus specificity for print in the visual word form area. Brain Lang. 2017;170:62–70. [DOI] [PubMed] [Google Scholar]
- 50.Siuda-Krzywicka K, Bola Ł, Paplńska M, et al. Massive cortical reorganization in sighted Braille readers. Elife. 2016;5:e10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn Sci 2011;15(6):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denckla MB, Cutting LE. History and significance of rapid automatized naming. Ann Dyslexia. 1999;49(1):29. [Google Scholar]
- 53.Misra M, Katzir T, Wolf M, Poldrack RA. Neural Systems for Rapid Automatized Naming in Skilled Readers: Unraveling the RAN-Reading Relationship. Sci Stud Read. 2004;8(3):241–256. [Google Scholar]
- 54.Boets B, Op de Beeck HP, Vandermosten M, et al. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342(6163):1251–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramus F, Szenkovits G. What phonological deficit? Q J Exp Psychol . 2008;61(1):129–141. [DOI] [PubMed] [Google Scholar]
- 56.Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, Kronbichler M. Resting-State and Task-Based Functional Brain Connectivity in Developmental Dyslexia. Cereb Cortex. 2015;25(10):3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia:neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(Pt 3):935–948. [DOI] [PubMed] [Google Scholar]
- 58.Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013; 125(2):146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brem S, Bucher K, Halder P, et al. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage. 2006;29(3):822–837. [DOI] [PubMed] [Google Scholar]
- 60.Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996;6(1):62–70. [DOI] [PubMed] [Google Scholar]
- 61.Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999; 10(2):181–192. [DOI] [PubMed] [Google Scholar]
- 62.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. [DOI] [PubMed] [Google Scholar]
- 63.Ozernov-Palchik O, Gaab N. Tackling the “dyslexia paradox”: reading brain and behavior for early markers of developmental dyslexia. Wiley Interdiscip Rev Cogn Sci. 2016;7(2):156–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.