Abstract
Background
Approximately one-third of people with schizophrenia have elevated levels of antigliadin antibodies of the immunoglobulin G type (AGA IgG) — a higher rate than seen in healthy controls. We performed the first double-blind clinical trial of gluten-free versus gluten-containing diets in a subset of patients with schizophrenia who were positive for AGA IgG.
Methods
In this pilot feasibility study, 16 participants with schizophrenia or schizoaffective disorder who had elevated AGA IgG (≥ 20 U) but were negative for celiac disease were admitted to an inpatient unit for a 5-week trial. All participants received standardized gluten-free meals and were randomized in a double-blind fashion to receive a shake containing 10 g of gluten flour or 10 g of rice flour each day. Participants were rated for psychiatric, cognitive and gastrointestinal symptoms at baseline and end point.
Results
Of the 16 participants, 14 completed the 5-week trial (2 discontinued early for administrative reasons). Compared with participants on the gluten-containing diet, participants on the gluten-free diet showed improvement on the Clinical Global Impressions scale (Cohen d = −0.75) and in negative symptoms (Cohen d = −0.53). We noted no improvement in positive or global cognitive symptoms, but did observe an improvement in attention favouring the gluten-free diet (Cohen d = 0.60). Robust improvements in gastrointestinal adverse effects occurred in the gluten-free group relative to the gluten-containing group. Adverse effects were similar between groups.
Limitations
This study was limited by its small sample size; larger studies are needed.
Conclusion
This feasibility study suggests that removal of gluten from the diet is associated with improvement in psychiatric and gastrointestinal symptoms in people with schizophrenia or schizoaffective disorder.
Introduction
A connection between schizophrenia and inflammation is emerging in the literature, supported by many research approaches. 1,2 Research also supports the idea that individual risk for schizophrenia is increased with prenatal infection exposure, 3 and that a genetic contribution may also increase vulnerability to prenatal infection.4 Imaging studies have found increased microglia activation binding in people with schizophrenia,5–7 and genome-wide association studies have linked schizophrenia to the major histocompatibility locus.8–10 Epidemiological studies have shown higher rates of auto-immune disease in people with schizophrenia. For example, data from a Danish registry reveal a 50% greater likelihood of autoimmune disease in people with schizophrenia, and a 45% greater risk of schizophrenia in people with a history of autoimmune disease.11 Moreover, people with schizophrenia — even drug-naïve and first-episode patients — have been found to exhibit elevated levels of both central and peripheral cytokines.12
However, there are components of schizophrenia that remain unexplained by this inflammation hypothesis. For example, not all those with schizophrenia present with the same symptoms, not all experience inflammation, and research into inflammatory markers such as cytokines and chemokines yields mixed results. As with all major psychiatric disorders, many genetic, environmental and biological factors contribute to a diagnosis. In this paper, we suggest that inflammation plays a considerable role for a specific subgroup of people with schizophrenia.13 This subgroup, once identified, may benefit from particular treatments that influence the immune system.
Evidence for a connection between the gut and the brain is emerging.14 Gut–brain connections may involve microbiota or gut-related immune reactions, including links to the gastro-intestinal effects of wheat. The notion that schizophrenia may be connected to wheat consumption is not new,15 but it is still not yet well understood. The first epidemiological studies looking at the association between wheat and schizophrenia use data from World War II, when a positive correlation between wheat consumption and admissions for schizophrenia was first documented: in Scandinavia, as wheat consumption decreased, so did schizophrenia-related admissions; on the other hand, as wheat consumption rose in the United States, so did admissions for schizophrenia.16 Further, in populations that traditionally consume little to no grain, schizophrenia has been found to be almost nonexistent, but to exist after westernization.13,17,18
Several trials have explored the removal of wheat from the diets of people with schizophrenia, with mixed results: several showed improvement in psychiatric symptoms, but others did not.19–27 These findings were not well understood, because there were no biological markers to help define a subpopulation that might have benefited from the removal of wheat. Further, in the 1970s and 1980s the components of wheat were not well understood, and only a limited number of hypotheses existed as to why this connection may have been important.
Foods containing wheat, barley, rye and triticale (a wheat and rye cross) possess a protein composite called gluten. Gliadin, the protein component from gluten that gives bread the ability to rise, has a low ratio of surface area to volume, making it difficult to digest. The autoimmune reaction to wheat is called celiac disease and has been recognized for about half a century, but more recently a new gluten-associated disorder has been described, distinct from celiac disease, that results from an innate immune reaction to gliadin. This disorder has been called “gluten sensitivity,” but the understanding of what it constitutes remains somewhat controversial.28–34
About one-third of people with schizophrenia have antigliadin antibodies of the immunoglobulin G (IgG) type,35 a rate about 3 times higher than seen in healthy controls.34,36–38 Also, the presence of AGA IgG antibodies in schizophrenia is related to a chronic inflammatory state associated with elevated peripheral cytokines (e.g., interleukin-1β and tumour necrosis factor α)39 and levels of neurochemicals in the anterior cingulate cortex thought to be associated with inflammation (as measured by magnetic resonance spectroscopy).40 Inflammation and immune activation with gluten sensitivity have been reported beyond schizophrenia as well.41
These findings in people with schizophrenia may be due to leakage in the blood–brain barrier. Severance and colleagues42 found a strong correlation between AGA IgG levels in the blood and cerebral spinal fluid in people with schizophrenia but not in healthy controls. Antibodies to tissue transglutaminase (tTG), indicative of celiac disease, are seen in only about 3%–5% of people with schizophrenia, representing a slightly higher risk than in the general population.34,35,43 Those with high AGA IgG levels represent a subgroup who may have gluten sensitivity.
We believe that schizophrenia is a heterogeneous multifactorial disease; the final symptoms may manifest similarly, but the underlying mechanisms producing psychiatric symptoms may differ by subgroup. Therefore, it may be key to use specific biomarkers to increase the efficacy signal of any intervention (in our case, a dietary one). Based on this premise, we decided to implement a gluten-free diet only in participants who showed elevated levels of AGA IgG. We believe that the reason for the mixed results in clinical trials during the 1970s and 1980s was the lack of ability to identify people at risk for this immune reaction and the resulting high inflammatory state.
Prior to this study, we conducted a 2-week gluten-free in-patient study for 2 people who had elevated AGA IgG and schizophrenia, and we noted robust symptom improvements, particularly in the domain of negative symptoms.44 The aims of this 5-week feasibility study were to create a gluten-free study design and successfully enrol more participants to examine effect sizes and plan a future trial of sufficient size with appropriate instrumentation. To our knowledge, this is the first double-blind, randomized, strictly controlled study exploring the removal of gluten from the diet in an inpatient setting that focused on a subgroup showing antibodies related to wheat. We hypothesized that we would see improvements in this group with effect sizes (Cohen d) of > 0.5 in psychiatric symptoms.
Methods
Study procedures
Those who were eligible for screening had a diagnosis of schizophrenia or schizoaffective disorder, were not currently on a gluten-free diet and were between the ages of 18 and 64 years. Screening laboratory tests included AGA IgG, AGA IgA and tTG. If participants tested positive for tTG, they were excluded, and they and their clinical team were notified of their potential for celiac disease. Participants who tested positive for AGA IgG (> 20 U) were eligible for study enrolment, which involved 5 weeks of randomized, double-blind treatment with a gluten-free or gluten-containing diet in an inpatient setting with strict dietary procedures. Participants who opted to enrol in the clinical trial were admitted to the research hospital unit and continued previous antipsychotic treatment. At the end of the 5-week double-blind trial, half of the discharged participants were randomly selected to continue a gluten-free diet in the community; they were called at week 4 for follow-up and returned at 8 weeks postdischarge for assessments and blood work.
Ethical approval
This clinical trial was conducted at the Maryland Psychiatric Research Center, University of Maryland School of Medicine. Screening took place at the center, its affiliate sites and Johns Hopkins University. All participants signed informed consent after passing the Evaluation to Sign Consent45 to ensure they were able to provide consent.
The study was conducted between 2014 and 2017 and was approved by the University of Maryland Baltimore institutional review board as the primary institutional review board (HP-00056339); the Johns Hopkins University and State of Maryland Department of Health institutional review boards officially relied on the University of Maryland Baltimore institutional review board for the conduct of this study. The study was reviewed annually by a data safety and monitoring board and was registered in ClinicalTrials.gov (NCT01927276).
Participants
Inclusion criteria
Women and men (ages 18–64 years) who met DSM-IV-TR46 criteria for schizophrenia or schizoaffective disorder were eligible for the study. All participants must have had positive results (> 20 U) on their AGA IgG screening. Participants must have been taking the same antipsychotic for at least 4 weeks prior to the study. As noted earlier, all participants were required to score at least a 10 out of 12 on the Evaluation to Sign Consent,45 which documented their capacity to provide informed consent.
Exclusion criteria
All those who participated in screening and tested positive for tTG were excluded so that their presumed celiac disease could be treated appropriately. Those who tested positive for AGA IgA but negative for AGA IgG were excluded. Also excluded were participants who were already on a gluten-free diet, were pregnant or lactating, had an organic brain disorder or intellectual disability, had a medical condition whose pathology or treatment could alter the presentation or treatment of schizophrenia or significantly increase the risk associated with the proposed treatment protocol, and who met DSM-IV criteria for alcohol or substance abuse (other than nicotine) within the last month. Participants were excluded if they had gluten ataxia, determined by a physician with the aid of the Brief Ataxia Rating Scale.47 To increase the possibility of detecting positive change, participants who had a Brief Psychiatric Rating Scale (BPRS)48 total score of 29 or lower (lower quartile) were excluded.
Screening assessments
Screening involved 1–2 visits and a variety of assessments to determine study eligibility. Participants were educated about the study and possible adverse effects or consequences of participation. Psychiatric diagnosis was confirmed by the Structured Clinical Interview for Diagnosis of DSM-IV (SCID).49 A medically accountable physician reviewed participants’ medical history and conducted a physical examination to confirm study eligibility. A standard blood chemistry panel, complete blood count, urinalysis and electrocardiography were also conducted. We also evaluated existing gastrointestinal disorders and dermatologic disorders, because gluten sensitivity may be related to both.41 For outpatients admitted to the unit and subsequently discharged following study participation, we worked closely with their community providers and supports to ensure full continuity of care.
Study assessments
Psychiatric symptoms
We measured psychiatric symptoms using the BPRS,48 the Scale for the Assessment of Negative Symptoms (SANS),49 the Calgary Depression Scale (CDS)50 and the Clinical Global Impression scale (CGI).51 We measured positive symptoms using the sum of the following BPRS items: conceptual disorganization, suspiciousness, hallucinatory behaviour and unusual thought content. We assessed negative symptoms using the total score of the SANS52 but subtracted the global items inappropriate affect, poverty of content of speech and attention to be consistent with other studies that measured negative symptoms in schizophrenia.53,54 All of these assessments are widely used in schizophrenia research and have good validity and reliability for use in this population. We administered the assessments each week during the 5 weeks of the study. All raters were trained and reliable, with an intraclass correlation coefficient of > 0.7.
We used the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) to assess cognitive function.55 This battery was developed specifically to be the gold standard for assessing cognition in schizophrenia.55 We also administered the Brief Assessment of Cognition in Schizophrenia Tower of London Test to assure adequate coverage of executive function. 56 In recent multicentre clinical trials that our research team has participated in, individual tests of the MCCB have had intraclass correlation coefficients between 0.6 and 0.8, and the overall MCCB composite score has had an intraclass correlation coefficient of 0.9.
Adverse effect measures
We measured gastrointestinal effects using the Gastrointestinal Symptom Rating Scale (GSRS) at baseline and end point.57 This 15-item scale has 5 domains (reflux, constipation, diarrhea, pain and indigestion), and has been used in people with psychiatric symptoms.58
To assess medication adverse effects, we administered the Simpson–Angus Extrapyramidal Symptom Rating Scale,59 the Barnes Akathisia Rating Scale60 and a 25-item adverse effect checklist each week.59,60
Laboratory assessments
At screening and each week throughout the trial, we assessed weight and vital signs (heart rate, pulse, blood pressure). We drew a chemistry panel, including liver enzymes, lipids and fasting blood glucose, along with complete blood counts at baseline and end point, all analyzed by LabCorp. We also measured AGA IgG at baseline and end point in the Čiháková Laboratory at Johns Hopkins University using the Inova Diagnostics kit 708655. Units were determined by the manufacturer based on standard curve calculations. For this study, AGA IgG negative status was defined as < 20 U, and positive status as ≥ 20 U.
Randomization procedures and medications
Participants, researchers and clinical team members were blinded to intervention assignment. Participants received 10 g of either rice flour (treatment) or gluten flour (control; Bob’s Red Mill) mixed in a protein shake each afternoon. A hospital nurse mixed the blinded powder in a high-power blender with water, ice, protein powder and optional syrup of the participants’ choice to make a protein shake (Sunwarrior Plant-Based Protein). The research staff ensured that the entire shake was ingested. Each participant had a separate colour-coded container that was never shared to avoid cross-contamination. Treatments were assigned at random, using computer-generated permuted block randomization sequences with randomly varied block sizes to limit imbalance in the number of patients assigned to each group, while making it difficult for staff to predict what treatment patients were receiving. All participants received a gluten-free meal plan during the 5 weeks of the study. The hospital kitchen had fully functioning operating procedures for the creation of gluten-free meals, including 21 days of meals in a rotating schedule. During the study, participants attended weekly group sessions about gluten-free nutrition and diet counselling, and they had the opportunity to taste, shop for and prepare new gluten-free foods in this setting. We maintained a strict regimen and oversight for maintaining a gluten-free diet. All staff in the inpatient setting were required to ensure that all participants remained gluten-free. During the day, the staff:participant ratio was 1:1, and all nursing staff and aides were required to report if any deviation occurred. Each participant had an individual snack bucket with gluten-free snacks if needed, and no other free food was given out on the inpatient unit. All packaged food consumed was certified gluten-free. All participants received a gluten-free cookbook. After the end of their trial participation, study participants were discharged if they were considered stable according to the treating psychiatrist, and all were encouraged to follow a gluten-free diet.
The study intervention was added to participants’ ongoing antipsychotic regimen. Study physicians were instructed to avoid changing doses of other somatic and psychotropic medications during the study. Anticholinergic medications for extrapyramidal side effects (e.g., benztropine and diphenhydramine), propranolol for akathisia and benzodiazepines for anxiety or agitation (e.g., lorazepam) could be prescribed as needed.
Statistical analysis
This was a pilot feasibility study funded by the National Institutes of Mental Health, aiming to ensure that we could develop and maintain blinding, recruit participants into the inpatient setting and assess a diet intervention in this population in a valid way. This study was not powered to find an effect, but SANS and BPRS scores were primary outcomes for symptom improvement, and the total GSRS score was the primary outcome for gastrointestinal changes. We evaluated change in primary outcomes by calculating effect sizes using Cohen d that can be used to guide future confirmatory trials. 61 An effect size is a quantitative measure of the magnitude of a difference — in this case change in findings from baseline to end point with a gluten-free diet relative to a gluten-containing diet. This was defined as treatment group mean – placebo group mean)/SDpooled.
We also analyzed SANS results by week using least squares mean, the group mean calculated from an analysis of covariance model after controlling for covariates. We used the following model for the current study: change from baseline = baseline + treatment + week + treatment × week. This allowed us to control for baseline differences in the presentation of the data and was defined a priori. Statistical tests for analysis of covariance findings were not performed because of the small sample size and underpowering to show effect.
Results
Screening and participant information
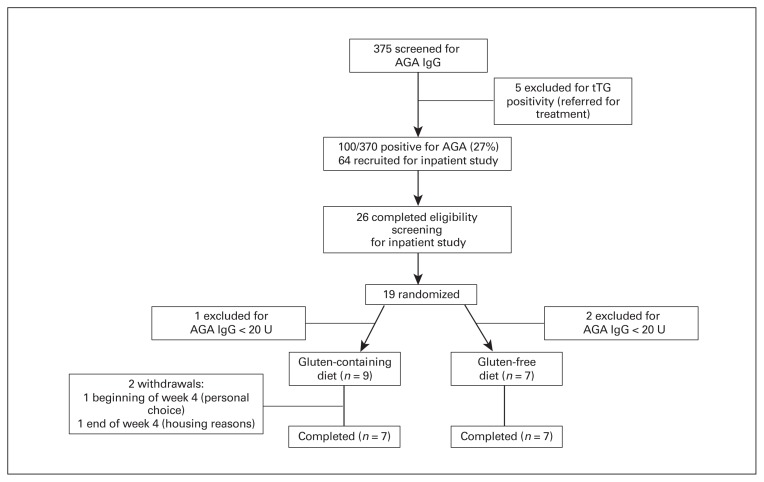
During study recruitment, we screened 375 people with schizophrenia or schizoaffective disorder, of whom 100 (27%) screened positive for AGA IgG (> 20 U). Five of those were also positive for tTG (1.3%), indicating celiac disease; they were referred for treatment with their clinicians. In total, 64 people were interested in enrolling in the clinical trial, 26 of those met the eligibility criteria and 19 were randomized. Three were excluded after randomization because of low AGA IgG levels (< 20 U), leading to an enrolment total of 16 (9 in the gluten-containing group and 7 in the gluten-free group). Of those, 14 completed the study; 2 from the gluten-containing group discontinued the study early, 1 because of personal choice and 1 for housing reasons (Fig. 1). The mean age of the 16 participants was 37.9 ± 13.2 years. Table 1 lists the demographic and clinical characteristics of the groups. All participants maintained their doses of antipsychotics and other treatments during the 5-week study, and all were discharged on the regimen on whibh they had been initiated.
Fig. 1.
Participant flow diagram. AGA = antigliadin antibodies; IgG = immunoglobulin G; tTG = tissue transglutaminase.
Table 1.
Demographic and clinical information*
| Characteristic | Gluten-containing diet (n = 9) | Gluten-free diet (n = 7) |
|---|---|---|
| Mean age, yr | 42.0 ± 14.6 | 32.5 ± 9.7 |
| M/F, no. (%) | 5/4 (56/44) | 4/3 (57/43) |
| Age of onset, yr | 16.9 ± 3.4 | 18.2 ± 2.4 |
| Level of education, yr | 11.8 ± 1.3 | 12.4 ± 2.1 |
| Smoker Y/N, no. (%) | 5/4 (56/44) | 6/1 (86/14) |
| Body mass index, kg/m2 | 28.5 ± 4.7 | 31.4 ± 8.9 |
| Baseline AGA IgG, U | 55.8 ± 28.6 | 43.8 ± 12.2 |
| Baseline AGA IgA, U | 23.6 ± 21.1 | 32.9 ± 28.3 |
| Medications, no. (%)† | ||
| FGA | < 5 | < 5 |
| SGA | < 5 | < 5 |
| Clozapine | < 5 | < 5 |
| FGA + SGA | < 5 | < 5 |
| Antidepressant | 6 (67) | < 5 |
| Anticholinergic | 7 (78) | 6 (86) |
| Comorbid disorders, no. (%)† | ||
| Gastrointestinal disorder | < 5 | < 5 |
| Dermatologic disorder‡ | < 5 | < 5 |
FGA = first-generation antipsychotic; IgA = immunoglobulin A; IgG = immunoglobulin G; SGA = second-generation antipsychotic.
Data with counts fewer than 5 have been suppressed owing to data privacy guidelines.
Distribution of medications and comorbid disorders did not differ between groups.
Dermatologic disorders included eczema, urticaria and rash.
Psychiatric and neuropsychologic symptoms
We calculated the results of the primary analyses as effect sizes (Cohen d). Positive effect sizes indicated a greater average reduction in psychiatric scores with the gluten-containing diet; negative effect sizes indicated greater average reduction in psychiatric scores with the gluten-free diet.
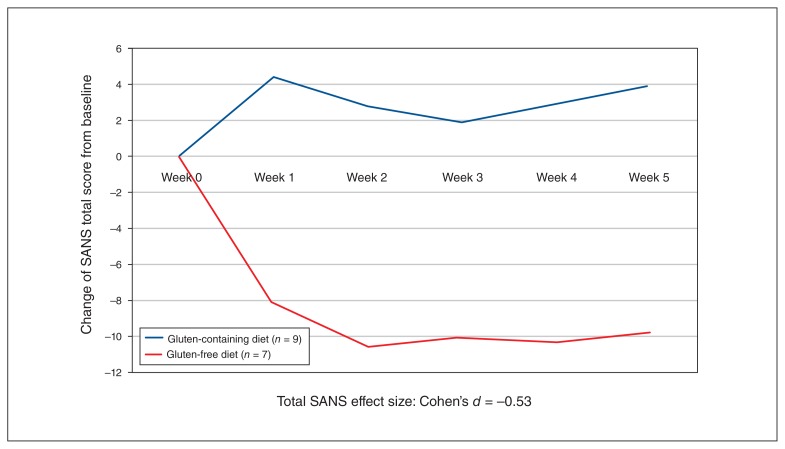
Changes on the CGI showed a robust improvement in global symptoms (Cohen d = −0.75). We observed no improvement in the BPRS or CDS, which showed low effect sizes. However, notably for negative symptoms, we found an effect size of −0.53 for total SANS scores, suggesting a moderate improvement in negative symptoms (Fig. 2). We found medium to large effect sizes in 2 of the SANS negative symptom domains: avolition (Cohen d = −0.43) and affective blunting (Cohen d = −0.71). We found small effect sizes for anhedonia (Cohen d =−0.24) and alogia (Cohen d = −0.12).
Fig. 2.
Change in SANS score by group (least squares mean). SANS = Scale for the Assessment of Negative Symptoms.
For the MCCB, a positive effect size score indicates greater average reduction in psychiatric scores with the gluten-free diet, because improvement in scores is in a positive direction (opposite of SANS, BPRS, CGI and CDS). The MCCB composite score effect size was not notable (Cohen d = −0.18). However, 2 of the domain scores had medium to large effect sizes favouring the gluten-free group: attention (Cohen d = −0.66) and verbal learning (Cohen d = −0.37).
Gastrointestinal and other adverse effects
All participants tolerated the diet well. The gluten-free group showed a robust improvement in total gastrointestinal symptoms, as measured by total GSRS score (Cohen d = −0.81), and medium to large improvements in domains of abdominal pain (Cohen d = −0.86), diarrhea (Cohen d = −0.59), constipation (Cohen d = −0.40) and indigestion (Cohen d = −0.46). The domain of reflux did not show any improvement (Cohen d = 0.18).
For adverse effects other than those on the adverse effect checklist, we found no differences between the 2 groups on any reported event, including dermatologic complaints. Three people in the gluten-free group had headaches, as did 2 in the gluten-containing group. Two people in the gluten-free group reported sedation, as did 1 person in the gluten-containing group. One person in each group reported dizziness and salivation. For extrapyramidal symptoms, we observed no notable changes in scores on the Simpson–Angus Extrapyramidal Symptom Rating Scale or the Barnes Akathisia Rating Scale.
Laboratory measures
We observed no notable changes in findings for vital signs, electrocardiogram, complete blood count or chemistry panel, except for a small improvement in fasting blood glucose (Cohen d = −0.36), which favoured the gluten-free diet over the gluten-containing diet. Over the 5 weeks, AGA IgG levels decreased by 34% in the gluten-free group relative to 16% in the gluten-containing group (Cohen d = −0.34). As part of the protocol, the investigators had serum frozen for future analysis of inflammatory markers, kynurenine pathway metabolites62 and potential measures of gut permeability (i.e., zonulin).63
Eight-week follow-up
Eight randomly selected participants agreed to continue eating gluten-free for 8 weeks postdischarge. Five were from the gluten-containing group, and 3 from the gluten-free group. During follow-up, AGA IgG levels dropped 22% (mean ± standard deviation: 62.8 ± 36.9 at discharge to 49.0 ± 29.6 at 8 weeks) in participants from the gluten-containing group and 19% (27.8 ± 17.9 at discharge to 22.6 ± 13.5 at 8 weeks) in participants from the gluten-free group. The total SANS score remained relatively stable in each group over the 8 weeks: the gluten-containing group went from 25.0 ± 5.2 to 24.5 ± 4.1, and the gluten-free group maintained their benefits, going from 16.0 ± 8.2 to 14.0 ± 5.3.
Discussion
This is, to our knowledge, the first randomized, double-blind clinical trial of gluten withdrawal in people with schizophrenia that enrolled only participants likely to respond to a gluten-free diet (i.e., those who tested positive for AGA IgG). The design was rigorous, including strict rules and a consistent environment for gluten-free food preparation, as well as detailed blinding procedures for participants and staff. This study used a small sample to test feasibility, but we had robust findings in a few interesting domains. We noted an overall improvement on the CGI, suggesting that overall clinical impressions during the study were better in the gluten-free group than in the gluten-containing group. We also saw moderate beneficial effects for negative symptoms. In our previous open-label study,44 1 person had a decrease of more than 19 points in SANS total score, leading us to anticipate the possibility of improvements in negative symptoms with a gluten-free diet in the present study. Because there are currently no consistently effective treatments for negative symptoms, the possible benefit of a gluten-free diet is of considerable potential clinical importance.
Other points worthy of discussion include the 27% rate of AGA IgG positivity in our study sample. Accumulating evidence suggests these antibodies are present in higher rates among people with schizophrenia than in healthy controls,37,64 with about 30% of people with schizophrenia having AGA IgG seropositivity.35,36 Additionally, it is unclear whether people with schizophrenia can maintain a gluten-free diet. Of the participants randomly selected to be followed postdischarge, improvements in AGA IgG levels and SANS total scores were maintained in the 3 participants who had been randomized to the gluten-free group during the 5-week inpatient study.
Limitations
A 5-week study may be insufficient to see amelioration of AGA IgG, psychiatric symptoms and cognitive function; longer-term studies may be needed to observe a full effect. Medications developed to modify these processes may exist as alternatives to strict gluten-free diets. We did not evaluate antibodies to casein, which have also been studied in schizophrenia,65 and we also did not evaluate foods containing fermentable oligo/di/monosaccharides and polyols, which also may contribute to gastrointestinal symptoms or immune reactivity. 66 We were limited by the small sample and lack of power to show effect. Our study purpose was to obtain the first go/no-go signal for a full-size, double-blind, randomized clinical trial of this nature.
Conclusion
Our findings suggest that a subgroup of people with schizophrenia may benefit from a gluten-free diet for global improvement, negative symptoms and gastrointestinal benefits. They also provide a better understanding of why some groups of people with schizophrenia have high levels of inflammation. If one-third of people with schizophrenia who have AGA IgG were to benefit substantially from a gluten-free diet, it could provide a new transformative treatment option for an identifiable subpopulation of people with schizophrenia and be of enormous benefit to patients, families and society. These results could also be used to encourage screening for AGA IgG in people at high risk of schizophrenia or with first-episode schizophrenia.
As a follow-up to this pilot study, we are conducting a larger randomized double-blind confirmative clinical trial targeting negative symptoms (NCT03183609), which will help confirm the utility of gluten removal in schizophrenia patients who are positive for AGA IgG if these findings are replicated. We are also studying mechanisms related to zonulin measurement for gut integrity,63 neuroimaging and inflammatory markers.
Acknowledgements
The authors thank the clinical and research team of the Treatment Research Program (TRP) at the Maryland Psychiatric Research Center (MPRC). The authors thank the nursing staff, who were instrumental in helping to maintain a gluten-free environment and helping with blood draws for screening, and the Outpatient Research Program (ORP) at the MPRC for their help in recruitment and screening. They also thank the many students, residents and fellows who spent countless hours with participants that helped with cooking classes and study integrity. They thank numerous students and trainees for their help over the years with the study, manuscript preparation and data analysis. Lastly, the authors thank the Research Pharmacist for his work in the preparation of the intervention materials. Preliminary data were presented at the Schizophrenia International Research Society Meeting in Florence, Italy, in April 2018.
Footnotes
Funding: This study was funded by NIMH R34 (NIMH R34 MH100776; PIs: Eaton and Kelly). Preparation of this paper was supported by NIMH grant R01 MH113617; PIs: Kelly and Eaton. Clinical Trials.gov NCT01927276.
Competing interests: D. Kelly served as an advisor to Lundbeck and HLS Therapeutics. A. Fasano is the founder and a stock holder of Alba Therapeutics. R. Buchanan served on the advisory boards for Astellas Pharma, Avanir, Boehringer Ingelheim-RCV, ITI, Inc., Lundbeck and Roche. He was a consultant for Takeda and Upsher-Smith Laboratories and on the DSMB for Pfizer. W. Carpenter has served as an advisor to Boehringer Ingelheim, Allergan, Health Analytics and Teva. All other authors have nothing to disclose.
Contributors: D. Kelly, H. Demyanovich, D. Čiháková, M. Talor, R. McMahon, A. Fasano, N. Cascella and W. Eaton conceived and designed the study. D. Kelly, H. Demyanovich, K. Rodriguez, M. Talor, C. Richardson, G. Vyas, H. Adams, S. August, S. Feldman, M. Sayer and M. Powell acquired the data, which D. Kelly, H. Demyanovich, R. McMahon, F. Liu, H. Wehring, R. Buchanan, J. Gold, W. Carpenter and W. Eaton analyzed. D. Kelly, H. Demyanovich, D. Čiháková, M. Talor, R. McMahon, H. Adams, M. Sayer, H. Wehring, R. Buchanan, J. Gold, W. Carpenter and W. Eaton wrote the article, which D. Kelly, H. Demyanovich, K. Rodriguez, D. Čiháková, M. Talor, C. Richardson, G. Vyas, H. Adams, S. August, A. Fasano, N. Cascella, S. Feldman, F. Liu, M. Powell, H. Wehring, R. Buchanan, J. Gold, W. Carpenter and W. Eaton reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–66. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Flinkkila E, Keski-Rahkonen A, Marttunen M, et al. Prenatal inflammation, infections and mental disorders. Psychopathology. 2016;49:317–33. doi: 10.1159/000448054. [DOI] [PubMed] [Google Scholar]
- 4.Clarke MC, Tanskanen A, Huttunen M, et al. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–30. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 5.Doorduin J, de Vries EF, Willemsen AT, et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–7. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 6.Van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C] PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–2. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield PS, Selvaraj S, Veronese M, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergen SE, O’Dushlaine CT, Ripke S, et al. Genome-wide association study in a Swedish population yields support for greater CNV and MHC involvement in schizophrenia compared with bipolar disorder. Mol Psychiatry. 2012;17:880–6. doi: 10.1038/mp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia Working Group of the Psychiatric Genomics C. Daly MJ, Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefansson H, Ophoff RA, Steinberg S, et al. Outcome in P. Melle I, Djurovic S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton WW, Byrne M, Ewald H, et al. Association of schizophrenia and autoimmune diseases: linkage of danish national registers. Am J Psychiatry. 2006;163:521–8. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- 12.Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Os J. “Schizophrenia” does not exist. BMJ. 2016;352:i375. doi: 10.1136/bmj.i375. [DOI] [PubMed] [Google Scholar]
- 14.Severance EG, Prandovszky E, Castiglione J, et al. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17:27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohan F. Cereals and schizophrenia data and hypothesis. Acta Psychiatr Scand. 1966;42:125–52. doi: 10.1111/j.1600-0447.1966.tb01920.x. [DOI] [PubMed] [Google Scholar]
- 16.Dohan FC. Wartime changes in hospital admissions for schizophrenia. A comparison of admission for schizophrenia and other psychoses in six countries during World War II. Acta Psychiatr Scand. 1966;42:1–23. doi: 10.1111/j.1600-0447.1966.tb01912.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan KY, Zhao FF, Meng S, et al. Prevalence of schizophrenia in China between 1990 and 2010. J Glob Health. 2015;5:010410. doi: 10.7189/jogh.05.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohan FC, Harper EH, Clark MH, et al. Is schizophrenia rare if grain is rare? Biol Psychiatry. 1984;19:385–99. [PubMed] [Google Scholar]
- 19.Dohan FC, Grasberger JC, Lowell FM, et al. Relapsed schizophrenics: more rapid improvement on a milk- and cereal-free diet. Br J Psychiatry. 1969;115:595–6. doi: 10.1192/bjp.115.522.595. [DOI] [PubMed] [Google Scholar]
- 20.Singh MM, Kay SR. Wheat gluten as a pathogenic factor in schizophrenia. Science. 1976;191:401–2. doi: 10.1126/science.1246624. [DOI] [PubMed] [Google Scholar]
- 21.Rice JR, Ham CH, Gore WE. Another look at gluten in schizophrenia. Am J Psychiatry. 1978;135:1417–8. doi: 10.1176/ajp.135.11.1417. [DOI] [PubMed] [Google Scholar]
- 22.Vlissides DN, Venulet A, Jenner FA. A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry. 1986;148:447–52. doi: 10.1192/bjp.148.4.447. [DOI] [PubMed] [Google Scholar]
- 23.Sheldon W. Celiac disease. Pediatrics. 1959;23:132–45. [PubMed] [Google Scholar]
- 24.Walsh BM, Walsh D. Mental illness in the Republic of Ireland: first admissions. J Ir Med Assoc. 1970;63:365–70. [PubMed] [Google Scholar]
- 25.Potkin SG, Weinberger D, Kleinman J, et al. Wheat gluten challenge in schizophrenic patients. Am J Psychiatry. 1981;138:1208–11. doi: 10.1176/ajp.138.9.1208. [DOI] [PubMed] [Google Scholar]
- 26.Storms LH, Clopton JM, Wright C. Effects of gluten on schizophrenics. Arch Gen Psychiatry. 1982;39:323–7. doi: 10.1001/archpsyc.1982.04290030055010. [DOI] [PubMed] [Google Scholar]
- 27.Osborne M, Crayton JW, Javaid J, et al. Lack of effect of a gluten-free diet on neuroleptic blood levels in schizophrenic patients. Biol Psychiatry. 1982;17:627–9. [PubMed] [Google Scholar]
- 28.Elli L, Villalta D, Roncoroni L, et al. Nomenclature and diagnosis of gluten-related disorders: a position statement by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) Dig Liver Dis. 2017;49:138–46. doi: 10.1016/j.dld.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai JC, Ciacci C. World gastroenterology organisation global guidelines: celiac disease February 2017. J Clin Gastroenterol. 2017;51:755–68. doi: 10.1097/MCG.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 32.Testing for celiac disease. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [accessed 2019 Mar 13]. Available: https://www.niddk.nih.gov/-/media/Files/Diagnostic-Tests/Celiac_Testing_CDAC_PP_508.pdf. [Google Scholar]
- 33.Volta U, Tovoli F, Cicola R, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–5. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 34.Jackson J, Eaton W, Cascella N, et al. Gluten sensitivity and relationship to psychiatric symptoms in people with schizophrenia. Schizophr Res. 2014;159:539–42. doi: 10.1016/j.schres.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Čiháková D, Eaton WW, Talor MV, et al. Gliadin-related antibodies in schizophrenia. Schizophr Res. 2018;195:585–6. doi: 10.1016/j.schres.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 36.Sidhom O, Laadhar L, Zitouni M, et al. Spectrum of autoantibodies in Tunisian psychiatric inpatients. Immunol Invest. 2012;41:538–49. doi: 10.3109/08820139.2012.685537. [DOI] [PubMed] [Google Scholar]
- 37.Okusaga O, Yolken RH, Langenberg P, et al. Elevated gliadin antibody levels in individuals with schizophrenia. World J Biol Psychiatry. 2013;14:509–15. doi: 10.3109/15622975.2012.747699. [DOI] [PubMed] [Google Scholar]
- 38.Dickerson F, Stallings C, Origoni A, et al. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophr Bull. 2016;42:134–41. doi: 10.1093/schbul/sbv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly DL, Demyanovich HK, Eaton W, et al. Anti gliadin antibodies (AGA IgG) related to peripheral inflammation in schizophrenia. Brain Behav Immun. 2018;69:57–9. doi: 10.1016/j.bbi.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowland LM, Demyanovich HK, Wijtenburg SA, et al. Antigliadin antibodies (AGA IgG) are related to neurochemistry in schizophrenia. Front Psychiatry. 2017;8:104. doi: 10.3389/fpsyt.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Losurdo G, Principi M, Iannone A, et al. Extra-intestinal manifestations of non-celiac gluten sensitivity: an expanding paradigm. World J Gastroenterol. 2018;24:1521–30. doi: 10.3748/wjg.v24.i14.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Severance EG, Gressitt KL, Alaedini A, et al. IgG dynamics of dietary antigens point to cerebrospinal fluid barrier or flow dysfunction in first-episode schizophrenia. Brain Behav Immun. 2015;44:148–58. doi: 10.1016/j.bbi.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cascella NG, Kryszak D, Bhatti B, et al. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37:94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson J, Eaton W, Cascella N, et al. A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or antigliadin antibodies. Schizophr Res. 2012;140:262–3. doi: 10.1016/j.schres.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeRenzo EG, Conley RR, Love R. Assessment of capacity to give consent to research participation: state-of-the-art and beyond. J Health Care Law Policy. 1998;1:66–87. [PubMed] [Google Scholar]
- 46.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition: SCID-I/P. 2002. [Google Scholar]
- 47.Schmahmann JD, Gardner R, MacMore J, et al. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24:1820–8. doi: 10.1002/mds.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:812. [Google Scholar]
- 49.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–8. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 50.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- 51.Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville (MD): US Department of Health and Human Services; 1976. DHEW Publication No. ADM 76-338. [Google Scholar]
- 52.Buchanan RW, Carpenter WT. Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J Nerv Ment Dis. 1994;182:193–204. [PubMed] [Google Scholar]
- 53.Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35:2274–83. doi: 10.1038/npp.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchanan RW, Kelly DL, Weiner E, et al. A randomized clinical trial of oxytocin or galantamine for the treatment of negative symptoms and cognitive impairments in people with schizophrenia. J Clin Psychopharmacol. 2017;37:394–400. doi: 10.1097/JCP.0000000000000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 56.Keefe RS, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Revicki DA, Wood M, Wiklund I, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75–83. doi: 10.1023/a:1008841022998. [DOI] [PubMed] [Google Scholar]
- 58.Soderquist F, Sundberg I, Ramklint M, et al. The relationship between daytime salivary melatonin and gastrointestinal symptoms in young adults seeking psychiatric care. Psychosom Med. 2019;81:51–6. doi: 10.1097/PSY.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 60.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan GM, Feinn R. Using effect size — or why the p value is not enough. J Grad Med Educ. 2012;4:279–82. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okusaga O, Fuchs D, Reeves G, et al. Kynurenine and tryptophan levels in patients with schizophrenia and elevated antigliadin immunoglobulin G antibodies. Psychosom Med. 2016;78:931–9. doi: 10.1097/PSY.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollon J, Puppa EL, Greenwald B, et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7:1565–76. doi: 10.3390/nu7031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickerson F, Stallings C, Origoni A, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68:100–4. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Niebuhr DW, Li Y, Cowan DN, et al. Association between bovine casein antibody and new onset schizophrenia among US military personnel. Schizophr Res. 2011;128:51–5. doi: 10.1016/j.schres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Roncoroni L, Elli L, Doneda L, et al. A retrospective study on dietary FODMAP intake in celiac patients following a gluten-free diet. Nutrients. 2018;10 doi: 10.3390/nu10111769. pii: E1769. [DOI] [PMC free article] [PubMed] [Google Scholar]