Abstract
Background
Investigating adolescents and young adults may provide a unique opportunity to understand developmental aspects of the neurobiology of depression. During adolescence, a considerable physiologic reorganization of both grey and white matter of the brain takes place, and it has been suggested that differences in grey-matter volumes during adolescence may reflect different maturational processes.
Methods
We investigated grey-matter volumes in a comparatively large sample (n = 103) of adolescents and young adults (aged 12 to 27 years), 60 of them with a diagnosis of current depression.
Results
Replicating previous studies, we found a clear whole-brain effect of age: the older the participants, the lower their global grey-matter volumes, particularly in the paracingulate and prefrontal cortices. Contrasting depressed and healthy youth in a whole-brain approach, we found greater grey-matter volumes in the dorsolateral prefrontal cortex of those with depression. Furthermore, a region-of-interest analysis indicated lower grey-matter volumes in the hippocampus in participants with depression compared with healthy controls.
Limitations
The present study was limited because of a skewed sex distribution, its cross-sectional design and the fact that some participants were taking an antidepressant.
Conclusion
During adolescence, restructuring of the brain is characterized by marked decreases in prefrontal grey-matter volumes, interpreted as a correlate of brain maturation. Findings of greater volumes in the prefrontal cortex, particularly in younger adolescents with depression, may suggest that these participants were more prone to delayed brain maturation or increased neuroplasticity. This finding may represent a risk factor for depression or constitute an effect of developing depression.
Introduction
Current research has provided evidence for neurobiological correlates of depression in terms of brain function and structure. Typical findings involve reduced hippocampal volumes and lower grey-matter volumes in cortical regions, particularly the subgenual anterior cingulate cortex (ACC). These findings have been reported in a review,1 and findings of lower hippocampal and cingulate cortex volumes have been replicated in a large meta-analysis that sampled more than 1900 adults with depression.2 Because neurobiological traces of depression are still detectable after remission, it has been suggested that these are trait markers indicating increased vulnerability to depressive illness.3,4 However, it remains to be determined whether these findings in adults reflect neurodevelopmental influences that may have increased people’s basic predisposition for depression years before.
The first signs of depression in many patients are observed during adolescence, and the cumulative prevalence of depression by the end of adolescence is approximately 20%.5,6 Particularly striking is the rapid rise of the incidence of depression in early adolescence,7 leading to suggestions of potential causative mechanisms underlying this increased risk. Investigating adolescents and young adults may provide a unique opportunity to understand the developmental aspects of the neurobiology of depression.8 So far, however, results have been conflicting. The typical findings in adults related to the subgenual ACC and hippocampus have also been reported in a larger study in depressed adolescents and young adults (n = 168),9 but a meta-analysis that investigated cortical thickness as another aspect of grey-matter structure in adolescents did not replicate the result pattern.10 Instead, in a subsample of approximately 200 adolescents and young adults with a mean age of approximately 19 years, the authors showed that the cortical thinning seen in older patients (> 21 years of age) did not appear in younger participants.10
During adolescence, a considerable reorganization of both grey and white matter occurs. Childhood and adolescence are characterized by overall increases in white-matter volumes and a regionally specific development of grey-matter volumes in an inverted U-shape.11 While subcortical, phylogenetically “older” structures such as the basal ganglia seem to reach peak volumes earlier in life (around age 7 to 8 in girls and around age 10 in boys), grey-matter volume in frontal and parietal cortices peaks around age 10 to 12 and in the temporal cortex only around age 16, with girls showing earlier peaks than boys. Consequently, areas related to motor or sensory functioning, such as somatosensory or visual cortices, seem to mature earlier than higher-order association areas. 11 Similarly, in terms of cortical thickness (as a measure of another aspect of grey-matter structure) the dorsolateral prefrontal cortex (dlPFC) reaches adult levels particularly late.12 Differences in grey-matter volumes during adolescence in depressed versus healthy young people may therefore reflect different maturational processes.13 Influences of age and sex on grey-matter volumes could explain different results for grey-matter volumes in prefrontal areas. There is meta-analysis evidence for volume reductions in adults,14 and evidence of increased and decreased volumes in different groups of adolescents.15,16 However, for most brain regions, it has been suggested that lower grey-matter volumes may reflect greater maturity of the brain during adolescence.11 Findings of greater prefrontal cortex volumes, particularly in younger depressed adolescents, may suggest that these individuals are more prone to delayed brain maturation as a risk factor for developing depression. Alternatively, increased prefrontal volumes may also be linked to neuroplasticity processes. In this vein, the rumination and compulsive symptoms frequently seen in depression have been suggested to relate to increased prefrontal volumes.17,18
For subcortical regions such as the hippocampus, the situation is different: in healthy adolescents and young adults, hippocampal volumes grow continuously.19 However, in depressed adolescents (as in adults), decreased volumes20–23 have been reported fairly consistently. A possible reason for this may be that maturation in this structure follows other principles, and a decrease in volume is not a sign of maturational processes but of already affected structural integrity as a consequence of depression. A further explanation for lower hippocampal volumes might be that the compulsive brooding commonly seen in depression could interfere with the functioning of episodic memory, which in turn could adversely affect the volume of the hippocampus.17
In the current study, we investigated grey-matter volumes in a reasonably large sample of 103 adolescents, all after the onset of puberty, and young adults (age range 12 to 27 years). We hypothesized that including both adolescents and young adults would allow us to replicate earlier findings related to maturational processes in the brain, irrespective of diagnosis. We expected to find significant effects of age in prefrontal and temporal brain regions, where restructuring is assumed to take place during this period of life (with greater grey-matter volumes in younger participants). We expected the somatosensory and visual cortices, as well as phylogenetically older brain areas such as the hippocampus and amygdala, to show no such age effects irrespective of diagnosis, because maturational processes in these areas should be less evident after the onset of puberty.11 Furthermore, we expected to replicate the findings of 2 previous studies on grey-matter volumes in larger samples of depressed adolescents and youth:9,24 in addition to the decreased hippocampal volumes found by Jaworska and colleagues,9 Hagan and colleagues24 reported effects of depression on grey-matter volume, particularly in the thalamus and ACC.
We formulated 2 main hypotheses. First, using whole-brain analysis, we expected to replicate an effect of age in the entire sample: that cortical volumes (particularly in phylogenetically newer areas of the brain such as the frontal, parietal and temporal cortices) decrease with age, in line with previous studies (hypothesis 1).24 In a second whole-brain analysis, we investigated the hypothesis that depressed adolescents would have relatively increased prefrontal cortex volumes compared to healthy controls, as has been found inconsistently in smaller samples (hypothesis 2).15 We expected that our sample — which had a skewed distribution toward younger participants, where maturational effects should be relatively large — would be well suited to detecting such differences.
As a secondary goal, we hypothesized that we would replicate the finding of decreased hippocampal volumes in depressed adolescents relative to controls using a region-of-interest (ROI) analysis, as consistently reported by Jaworska and colleagues9 and in several studies with smaller sample sizes (hypothesis 3).20–23 We also investigated the effects of illness on grey-matter volume in the ACC (ROI analysis) and thalamus (whole-brain analysis) as previously reported by Hagan and colleagues,24 predicting decreased grey-matter volumes in depressed participants (hypothesis 4).
Methods
Participants
We obtained participants’ morphological and clinical data as part of 2 functional imaging projects on the effects of cognitive behavioural therapy in depressed adolescents25 and on depression and nonsuicidal self-injury in adolescents and young adults (part of the project published in Groschwitz and colleagues26). From these studies, we included data from depressed patients (before cognitive behavioural therapy) and healthy controls aged 12 to 27 years. After excluding 1 patient and 1 control because of poor data quality, we had morphological data for 103 participants. All 60 participants in the depression group met DSM-IV criteria for current major depressive disorder at the time of the MRI. All were inpatients or outpatients of the Department of Child and Adolescent Psychiatry and Psychotherapy or of the Department of Psychiatry and Psychotherapy of Ulm University, or they were outpatients from a private child and adolescent psychiatry practice in Ulm, Germany. Exclusion criteria were a current or previous diagnosis of bipolar disorder, schizophrenia or substance abuse; an intellectual disability; or a major somatic or neurologic disorder. Of the patients with depression, 20 were taking psychotropic medication at the time of the scan, usually antidepressants for their current episode. Of these, 9 had a history of current or previous long-term medication of more than 2 months. Four additional patients were currently unmedicated but had a history of previous long-term medication. In the control group, we included only participants who had never been diagnosed with any psychiatric disorder and who were matched for age, education and sex. None of the controls had a history of current or previous psychotropic medication. All participants had reached puberty as indicated by Tanner stage 3 in males and menstruation in females. All participants and caregivers (in the case of minors under age 18 years) provided written informed consent. Procedures were carried out in accordance with the Declaration of Helsinki (2013), and the studies were approved by the Institutional Review Board of Ulm University.
Diagnoses were assessed using the German version of the clinical interview Schedule for Affective Disorders and Schizophrenia for School-Age-Children-Present and Lifetime (K-SADS-PL) for DSM diagnoses27 in adolescents up to age 18 years, or the Structured Clinical Interview for DSM diagnoses (SCID) in young adults.28 To assess current depressive symptoms, we used the Beck Depression Inventory second edition (BDI-II),29 German version.30
Structural MRI data acquisition
We used a 3.0 T Siemens MAGNETOM Allegra Scanner (Siemens) equipped with a head coil to obtain MRI data. We acquired anatomic high-resolution T1-weighted images using a magnetically prepared rapid acquisition gradient echo sequence (MPRAGE: 1 × 1 × 1 mm voxels, band width 130 Hz/pixel, repetition time 2500 ms, inversion time 1.1 s, echo time 4.57 ms, flip angle 12°, field of view 256 × 256, 192 sagittal slices) as part of a larger imaging protocol.
Statistical analysis of behavioural data
We performed statistical analyses using the Statistical Package for the Social Sciences 21 (SPSS Inc.). We computed between-group differences by means of 2-sample t tests and χ2 tests. For correlational analyses, we applied the Pearson coefficient. All tests were performed with levels of significance established at p < 0.05 (2-tailed).
Statistical analysis of structural MRI data
Preprocessing
We conducted image preprocessing using the Computational Anatomy Toolbox for SPM 12 (CAT12, http://dbm.neuro.uni-jena.de/cat12/) with the following steps: normalization, segmentation and quality check for sample homogeneity. Using standard settings of the toolbox, we normalized data into Montreal Neurological Institute (MNI) space and segmented them into grey matter, white matter and cerebrospinal fluid using the SPM12 tissue probability maps for spatial registration and segmentation. We conducted spatial smoothing as the final step of preprocessing with a Gaussian kernel of 6 mm full width at half maximum using SPM 12 standard routines.
Whole-brain analyses
We assessed the effect of age (hypothesis 1: whole-brain analysis) on grey-matter volumes in the whole brain using a simple regression analysis across the entire group of participants, irrespective of diagnosis. We tested whole-brain group differences between healthy controls and patients (hypothesis 2: whole-brain analysis) for significance using the 2-sample t test module for unpaired samples in SPM 12. For both analyses, we included age and sex as covariates, because previous investigations showed a clear influence of these variables on grey-matter volumes.11,24,31 Sex was a nuisance covariate in both analyses; age was the variable of interest for hypothesis 1 and a nuisance covariate for hypothesis 2. We added long-term psychotropic medication (> 2 months currently or in the past; n = 13) as another covariate of no interest to the model. Thresholds for both analyses were set at p < 0.001 at the voxel level, together with a family-wise error (FWE) correction for multiple comparisons at pFWE < 0.05 at the cluster level. We extracted estimates of grey-matter volumes from regions with a significant statistical effect to visualize effects.
Replication of results from previous MRI studies
To further investigate the validity of our data, we directed additional analyses toward replication of the main results of previous studies from other research groups in similar samples, particularly those by Jaworska and colleagues9 and Hagan and colleagues.24 As in Jaworska and colleagues,9 we tested an a priori hypothesis (hypothesis 3, ROI analysis) for differential effects in the hippocampus using an ROI approach and expecting smaller volumes in depressed youth. Because the tracing procedures used by Jaworska and colleagues9 to delineate the left and right hippocampus were not available at our site, we used the hippocampus templates from the atlas for automated anatomic labelling accessible via the WFU Pick Atlas for SPM (http://fmri.wfubmc.edu/software/PickAtlas). We thresholded the map at p < 0.01 at the voxel level. To control for multiple comparisons, we used a cluster-extent threshold of pFWE < 0.05 in combination with the small-volume correction (SVC) in SPM, applying the hippocampus masks bilaterally.
To study the differential effects in the thalamus as one main finding of Hagan and colleagues24 (hypothesis 4, whole-brain analysis) we first performed an analysis at the whole-brain level, expecting greater thalamic volumes, particularly in younger depressed participants than in controls. The second main finding in that study was a differential effect in the ACC (hypothesis 4, ROI analysis). Like Hagan and colleagues, we used the atlas for automated anatomic labelling accessible via the WFU pickatlas for SPM and combined templates for “Cingulum_Ant_R” and “Cingulum_Ant_L” to delineate a bilateral ACC ROI. At the voxel level, we used the same threshold for the ROI analysis as Hagan and colleagues (p < 0.004), combined with an FWE correction for multiple comparisons in small volumes (pFWE < 0.05). Because the sample studied by Hagan and colleagues included only participants younger than 18 years, we investigated group differences between patients with depression and healthy controls in the whole group, but calculated a separate analysis in participants up to age 18 years (n = 79). For hypotheses 3 and 4, we again included age, sex and medication as covariates of no interest in the analyses.
Subgroup analyses: patients with psychiatric codiagnoses excluded
To investigate whether psychiatric codiagnoses had an impact on our data, we recalculated each analysis. To exclude effects of a history of anorexia nervosa (as found in 2 patients), we designed a model with 58 patients versus 43 controls. To exclude effects of any psychiatric codiagnosis, we excluded the 18 patients with a history of psychiatric codiagnosis (including anorexia nervosa) from calculations, setting up a model with 42 patients versus 43 controls.
Results
Behavioural data
Both groups (patients and healthy controls) included more females than males, and the majority were right-handed. One patient did not complete the BDI-II questionnaire. In the remaining group of 59 patients, the mean BDI-II score indicated a moderate degree of depressive symptoms (mean ± standard deviation = 24.31 ± 10.37). Age, handedness and sex did not differ between groups. Samples were roughly matched for education level. For more details, see Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Characteristic | Depressed patients (n = 60) | Healthy controls (n = 43) | Group differences |
|---|---|---|---|
| Sex, no. (%) | 48 F (80) | 38 F (88.4) | NS* |
| BDI-II score, mean ± SD | 24.31 ± 10.37 | 3.07 ± 3.44 | t74 = 14.66, p < 0.001 |
| Age, yr, mean ± SD (range) | 17.30 ± 3.44 (13–27) | 17.62 ± 3.85 (12–27) | t101 = −0.44, p = 0.66 |
| Secondary diagnosis, no.† | Anorexia nervosa: 2 Social phobia: 6 Attention-deficit/hyperactivity disorder: 4 Socialized conduct disorder: 3 Specific phobia: 2 Borderline personality disorder: 1 |
— | — |
| Handedness, no. (%)‡ | 58 right-handed (96.7) | 40 right-handed (93) | NS* |
| Intake of psychotropic medication currently or in the past > 2 months, no.§ | Antidepressant (mirtazepine: 3; fluoxetine: 2; escitalopram: 2; sertraline: 3) Antipsychotic (quetiapine: 1) Psychostimulant (methylphenidate: 4) |
— | — |
| Education level, no.¶ | Hauptschule: 10 Realschule: 23 Gymnasium: 20 Berufsschule: 5 Missing: 2 |
Hauptschule: 5 Realschule: 13 Gymnasium: 22 Berufsschule: 3 |
NS* |
NS = not significant; SD = standard deviation.
χ2 test.
Diagnosis according to DSM-IV; n = 18.
Edinburgh Handedness Inventory; a independent sample t test.
n = 13.
A Gymnasium is a type of school providing secondary education, comparable to English grammar schools and United States college preparatory high schools. Hauptschule and Realschule are secondary schools. Berufsschule is a professional school that students attend along with an apprenticeship.
Whole-brain analyses
Effect of age: hypothesis 1
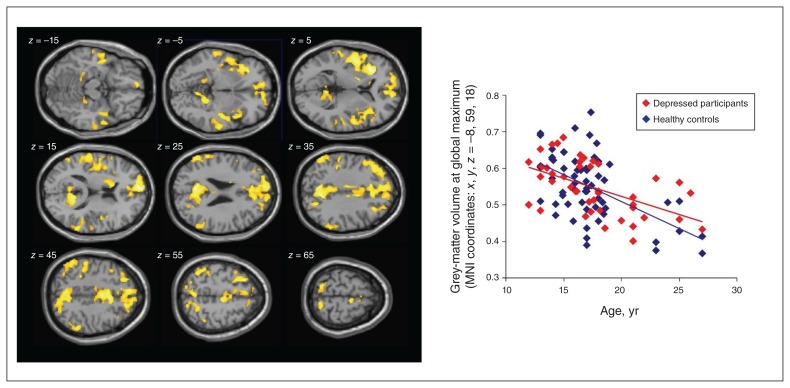
Regression analysis revealed a significant effect of age over a large array of cortical regions (Fig. 1): the older the participant, the lower the grey-matter volumes. The global maximum in this analysis was in the paracingulate and medial prefrontal cortex (MNI coordinates: x, y, z = −8, 59, 18; Z = 5.57; number of voxels = 28 181), very close to the region with the maximum effect of age on grey-matter volume found by Hagan and colleagues (MNI coordinates: x, y, z = −2, 52, 2).24 We observed further age effects in the ACC and medial prefrontal cortex, insula, lateral prefrontal cortex, inferior and superior parietal regions, and precuneus. As expected, we found no significant effects of age in the visual or somatosensory cortices, the amygdala or the hippocampus. We found no significant positive relationship between larger grey-matter volume and increasing age.
Fig. 1.
Left: Results of the whole-brain regression analysis over the entire group of participants (n = 103); p < 0.001 at the voxel level, pFWE < 0.05 at the cluster level. Right: Correlation of individual grey-matter volume (estimated relative concentration) and age at the global maximum of the age effect, with depressed participants and healthy controls in different colour coding. FWE = family-wise error; MNI, Montreal Neurological Institute.
Group differences: hypothesis 2
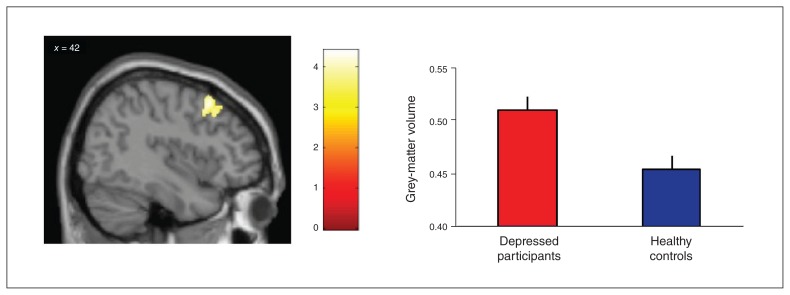
The comparison of patients with depression and healthy controls revealed a significant group difference in the dlPFC, with greater grey-matter volumes in patients than in healthy controls (MNI coordinates: x, y, z = 44, 18, 50; Z = 4.19; number of voxels = 548; Fig. 2). We found no other significant clusters for this comparison, and testing for the opposite direction (smaller grey-matter volume in patients) provided no significant results. An interaction analysis of group × age revealed no significant results (data not shown).
Fig. 2.
Differential effect with greater grey-matter volumes (relative concentration) in the dorsolateral prefrontal cortex of depressed participants (n = 60) compared to matched healthy controls (n = 43). Whole-brain analysis, thresholded p < 0.001 at the voxel level, pFWE < 0.05 at the cluster level. FWE = family-wise error.
Analyses according to a priori hypotheses from other studies
Differential effect in the hippocampus (ROI analysis): hypothesis 3)
Comparing grey-matter volumes in the left and right hippocampus ROIs, we confirmed the finding of Jaworska and colleagues.9 We found significantly lower grey-matter volumes in the posterior hippocampus in depressed youth versus healthy controls (MNI coordinates: x, y, z = −18, −35, −2; Z = 2.54; number of voxels = 10).
Group differences in the thalamus (whole-brain analysis) and the ACC (ROI analysis): hypothesis 4
We found a differential effect in the thalamus, with greater volumes in depressed participants than in healthy controls (MNI coordinates: x, y, z = −12, −17, 20; Z = 3.37; number of voxels = 6) at thresholds of p < 0.001 at the voxel level and pFWE,SVC < 0.05, controlling for multiple comparisons. This effect was not significant when data from participants up to age 18 years were analyzed. However, as described by Hagan and colleagues24 in participants up to age 18 years, and using the ROI approach we found decreased grey-matter volumes in depressed patients compared with healthy controls in the ACC, midcingular portion (MNI coordinates: x, y, z = −6, 24, 20; Z = 3.21; number of voxels = 72). This effect was not significant for the whole group.
Subgroup analyses: patients with psychiatric codiagnoses excluded
Exclusion of the 2 patients with a history of anorexia nervosa revealed results that were not different from those of the whole sample. Exclusion of all 18 patients with any psychiatric codiagnosis revealed unchanged results for hypotheses 1 and 3, as well as for hypothesis 4 in the thalamus. For hypothesis 2, findings in the dlPFC survived correction for multiple comparisons, but only when applying a simple cluster-level correction at p < 0.05 (no FWE correction). The results of these analyses are reported in greater detail in Appendix 1, available at jpn.ca/170233.
Discussion
Investigating grey-matter volumes in a comparatively large sample of peri- and postpubertal adolescents and young adults with depression, we found further support for the observation of relatively increased prefrontal volumes in this population; we also replicated findings from previous studies. Across all participants in the present study, results revealed the expected age effect (hypothesis 1): the older the participants, the lower their global grey-matter volumes, particularly in the paracingulate and the prefrontal cortex. A comparison of both groups (hypothesis 2) revealed that depressed patients had greater grey-matter volumes in the dlPFC than healthy controls. The interaction of group × age (which could support assumptions of different maturation velocities) was not significant. Replicating the findings of Jaworska and colleagues9 and Hagan and colleagues,24 our results indicated lower grey-matter volumes in the hippocampus in depressed patients (hypothesis 3) and in the ACC in depressed patients under the age of 18 (hypothesis 4) compared with healthy controls.
Our observation of increased dlPFC grey-matter volumes in depressed youth compared with healthy controls aligned well with the results of previous studies and could indicate that developmental processes in depressed adolescents follow different trajectories in brain regions known to be involved in the regulation of emotions and stress. Because increased dlPFC brain volumes are not typically seen in adults,10 we think that a delayed onset of developmental processes is a plausible explanation for our finding in depressed adolescents. However, our sample did not include preadolescent participants in each group, necessary to span the entire range of brain maturation and monitor full-scale growth curves of grey-matter volumes. Therefore, this interpretation must remain tentative. Still, previous studies on cortical thickness — a different aspect of grey-matter structure in depression — have delivered results in a similar direction to our findings. Studies investigating cortical thickness in older adults (age > 60 years)32 and in younger adults (age 24 to 35 years)33,34 have tended to support cortical (in particular, prefrontal) thinning in patients with depression, but investigations in younger groups (males average age 17 to 21.5 years) have shown thicker cortices in depressed participants compared to healthy controls in the lateral frontal cortex32 and in frontal regions/prefrontal cortex.35 Our results point toward a similar situation in a larger sample with respect to grey-matter volume, which is a related, but not overlapping measure of grey-matter structure.
Despite these consistencies, it must be noted that our findings were in contrast to those of a smaller study (n = 22 per group) by Shad and colleagues,16 which found lower grey-matter volumes in the dlPFC in depressed adolescents compared with healthy controls. One explanation for this inconsistency, besides the smaller sample size, might be that depressed participants in that study were younger (mean age 15 years; range 12 to 20 years) than those in our study (mean age 17.3 years; range 13 to 27 years). Furthermore, Hagan and colleagues,24 investigating a similarly large sample, did not report group differences in grey-matter volume between depressed and healthy adolescents. A reason for their finding might be that participants were again younger (mean age 15.65 years; range 11.83 to 17.96) than those in our study but older than those in the study of Shad and colleagues.16 These observations may guide conclusions that differences in brain maturation reflected in different prefrontal grey-matter volumes may be more prominent in slightly older adolescents. Different findings might also have been due to sex differences — Hagan and colleagues24 included more males (27.8%) than we did (20%) — and the fact that participants in that study were more often medicated (approximately 33%) than participants in our study (13.3%).
The dlPFC is an important structure when it comes to the processing of risk and fear, emotion regulation, cognitive control, monitoring of performance, response inhibition and behavioural adjustment.32,35,36 It has been suggested that maturational processes in the frontal lobe provide the neurophysiological basis for the acquisition of skills and knowledge related to higher cognitive functioning and social behaviour.37 Significant increases in the development of attentional control are seen around age 15 years,38 and development of executive functions and problem-solving abilities are found from this age into early adulthood.39 Neurobiological models of depression, hand in hand with impaired frontal lobe functioning, conceptualize some of the key phenomena observed in depression. These include biased attention to and increased processing of negative stimuli, as well as rumination that is related to impaired or dysfunctional attentional and executive control.40 In particular, cognitive behavioural therapy explicitly targets these aspects. Delayed maturational processes in the frontal lobe may be a risk factor for adolescent depression. Immature self-regulatory competence in combination with increased novelty and sensation-seeking (which have been suggested to drive commonly observed risky behaviours and emotional imbalance in adolescents) may also facilitate depression.41 Furthermore, because symptoms of depression such as rumination and compulsive behaviours have been associated with increased prefrontal volumes,17,18 neuroplasticity effects may stave off the normal age-related decline and keep dlPFC volumes larger. However, it remains unclear whether delayed cortical development predisposes a person to depression or whether depression delays the brain maturation trajectories.
As well as demonstrating greater grey-matter volumes in the prefrontal regions of depressed participants, where maturational processes peak during adolescence, we also replicated previous findings of reduced grey-matter volumes in regions such as the hippocampus.9,24 In these regions, brain maturation peaks earlier in development and is already much more advanced in the age group we investigated.11 More advanced maturational processes may be the reason why results from the hippocampus in adolescents are highly similar to those in adults.2 As in adults, prolonged exposure to stress (which induces high glucocorticoid levels and appears to harm the hippocampus in terms of neurogenesis and loss of dendritic spines42–44) has been suggested as a mechanism underlying these findings. In line with this idea, 77% of depressed minors report that a stressful life preceded or triggered the onset of their first depressive episode.45 Furthermore Rao and colleagues46 found that early-life stress was associated with both the onset of depression and smaller hippocampal volumes. Besides that, younger age at onset of major depressive disorder has been associated with smaller hippocampi,9 and a longitudinal study noted that attenuated hippocampal growth was associated with the onset of depression.47 These findings suggest that smaller hippocampal volumes may predispose for depression, possibly because of impaired mnestic processes,42,48 impaired executive functioning and affect regulation.49 Indeed, the meta-analysis from the ENIGMA group including data from 1728 adult patients with major depression identified smaller hippocampal volume as the most robust marker of depression, driven primarily by either earlier age of onset or recurrent depression, while recurrent depression did not moderate hippocampal volumes in early-onset patients.2 The authors concluded that early onset of depression, as in our adolescent sample, may be independently associated with lower hippocampal volumes. An alternative explanation for lower hippocampal volumes could be a lack of effective epistemic foraging and information being consigned to nonconscious episodic memory, with depressed patients getting stuck on compulsive, negative perspectives on the self, world and others. Such excessive top–down rumination might limit the normal volumetric development of the hippocampus. Indeed, it has been shown that hippocampus volume accounted for impaired memory function in depressed versus healthy participants.50 Supporting this, Wang and colleagues17 found that depressed patients revealed a significant decrease of regional grey-matter volume in the parahippocampal gyrus versus healthy controls and that rumination had a mediating effect on the relationship between depression and regional grey-matter volume in the parahippocampal gyrus.
In the present study, we also found reduced ACC grey-matter volume in depressed patients versus healthy controls, again in line with the findings of Jaworska and colleagues9 and Hagan and colleagues24 in adolescents. Although the maturation peak for the ACC is less clear, it seems to occur earlier than in the dlPFC.12 Smaller ACC volumes (particularly in the subgenual portion) have been seen consistently in adults,1 but not in a longitudinal study on adolescent depression.47 The ACC is associated with conflict monitoring, social decision-making and determination of the source of social information.51,52 It also plays a key role in the processing, regulation and appraisal of emotions.51 Therefore, reduced grey-matter volume in the ACC might relate to interpersonal and emotional impairments in depressed patients.
Finally, we found tentative evidence for aberrant thalamic volume characteristics in depressed patients versus healthy controls, similar to findings reported by Hagan and colleagues.24 The thalamus undergoes a significant amount of reorganization from early childhood through adolescence to early adulthood.53 However, structural changes in the thalamus are not consistently found in adult depression,1 despite findings of functional changes.54,55
Limitations
The present study was limited because of a skewed sex distribution biased toward female participants, which limits its generalizability to male patients. Furthermore, it was limited owing to its cross-sectional design. Although even short-term psychotropic medication might have affected brain development and volume, we were able to control only for the long term (> 2 months currently or in the past), because the only available data for short-term medication in the past were inconsistent. We did not systematically explore experiences of triggering stressors such as childhood maltreatment/trauma in our study despite their well-known influence on the limbic system. Normalization of images to a standard template in voxel-based morphometry may result in some deformation of the original brain structure and possible errors in detecting small-volume differences. One strength of the present study was the inclusion of relatively young participants, which reduced the likelihood that long-term medication or chronicity of the disorder had already induced marked changes in gross brain anatomy. Another strength was the comparably large sample size obtained from a single MRI scanner.
Conclusion
We found further evidence to support different developmental trajectories in brain regions relevant for top–down processing — particularly the dlPFC — in adolescents and young adults with depression. Other brain regions, such as the hippocampus, did not show signs in support of such a rationale. Insufficient top–down control has been suggested as an explanation for the increased incidence of substance abuse, risk behaviour and affective disorders during adolescence. 56 However, it remains unclear whether these changes are the cause of these disorders or an effect of already ongoing disorders. In our study, we focused on depressed participants, although some might develop other affective disorders later on (for example, initial presentation with depression is common in bipolar disorder). Longitudinal studies in a large sample of younger adolescents are needed to shed further light on these processes and better understand natural brain maturation in healthy controls and predictors of depression and other disorders later in life. Another approach could be to calculate normative ranges of grey-matter volume in adolescents and young adults, taking into account data from healthy populations in huge data samples (e.g., ENIGMA, Human Connectome Project). In a next-step deviation, scores of depressed participants versus normative data could be calculated by age range to learn whether the brain age of depressed participants is lower than their biological age. Besides analyses of grey-matter volume, it would be interesting to focus on other aspects of grey-matter structure in cortical thickness and surface analyses.
Footnotes
Competing interests: P. Plener declares grants from Servier and Lundbeck for clinical studies outside the submitted work. No other competing interests declared.
Contributors: J. Straub, P. Plener and B. Abler designed the study. J. Straub, R. Brown, K. Malejko and M. Bonenberger acquired the data, which J. Straub, G. Grön and B. Abler analyzed. J. Straub, G. Grön and B. Abler wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2016;21:806–12. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–51. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 4.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 5.Garrison CZ, Addy CL, Jackson KL, et al. Major depressive disorder and dysthymia in young adolescents. Am J Epidemiol. 1992;135:792–802. doi: 10.1093/oxfordjournals.aje.a116366. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker A, Johnson J, Shaffer D, et al. Uncommon troubles in young people: prevalence estimates of selected psychiatric disorders in a nonreferred adolescent population. Arch Gen Psychiatry. 1990;47:487–96. doi: 10.1001/archpsyc.1990.01810170087013. [DOI] [PubMed] [Google Scholar]
- 7.Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin Psychol Rev. 1998;18:765–94. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 8.Allen NB, Sheeber LB. Adolescent emotional development and the emergence of depressive disorders. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 9.Jaworska N, Yucel K, Courtright A, et al. Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: the role of comorbidity and age. J Affect Disord. 2016;190:726–32. doi: 10.1016/j.jad.2015.10.064. [DOI] [PubMed] [Google Scholar]
- 10.Schmaal L, Hibar DP, Samann PG, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2016;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan CL, Moore GJ, Madden R, et al. Prefrontal cortical volume in childhood-onset major depression: preliminary findings. Arch Gen Psychiatry. 2002;59:173–9. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- 16.Shad MU, Muddasani S, Rao U. Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J Child Adolesc Psychopharmacol. 2012;22:190–7. doi: 10.1089/cap.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Wei D, Yang J, et al. Individual differences in rumination in healthy and depressive samples: association with brain structure, functional connectivity and depression. Psychol Med. 2015;45:2999–3008. doi: 10.1017/S0033291715000938. [DOI] [PubMed] [Google Scholar]
- 18.Montigny C, Castellanos-Ryan N, Whelan R, et al. A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS One. 2013;8:e80151. doi: 10.1371/journal.pone.0080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krogsrud SK, Tamnes CK, Fjell AM, et al. Development of hippocampal subfield volumes from 4 to 22 years. Hum Brain Mapp. 2014;35:5646–57. doi: 10.1002/hbm.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caetano SC, Fonseca M, Hatch JP, et al. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci Lett. 2007;427:142–7. doi: 10.1016/j.neulet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 21.MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Med. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMaster FP, Mirza Y, Szeszko PR, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63:385–90. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacMillan S, Szeszko PR, Moore GJ, et al. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 24.Hagan CC, Graham JM, Tait R, et al. Adolescents with current major depressive disorder show dissimilar patterns of age-related differences in ACC and thalamus. Neuroimage Clin. 2015;7:391–9. doi: 10.1016/j.nicl.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straub J, Plener PL, Sproeber N, et al. Neural correlates of successful psychotherapy of depression in adolescents. J Affect Disord. 2015;183:239–46. doi: 10.1016/j.jad.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Groschwitz RC, Plener PL, Groen G, et al. Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: an fMRI study. Psychiatry Res. 2016;255:43–9. doi: 10.1016/j.pscychresns.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Delmo C, Weiffenbach O, Gabriel M, et al. Affective disorders and schizophrenia for school-age children – present and lifetime version (K-SADS-PL) Frankfurt: Klinik für Psychiatrie und Psychotherapie des Kindes-und Jugendalters; 2000. [Google Scholar]
- 28.Ventura J, Liberman RP, Green MF, et al. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–73. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Steer RA, Brown GK. Beck depression inventory, manual. 2nd ed. San Antonio (TX): The Psychological Corporation; 1996. [Google Scholar]
- 30.Hautzinger M, Keller F, Kühner C. BDI-II Beck Depressions-Inventar Revision. Frankfurt am Main: Harcourt Test Services; 2006. [Google Scholar]
- 31.Bray S. Age-associated patterns in gray matter volume, cerebral perfusion and BOLD oscillations in children and adolescents. Hum Brain Mapp. 2017;38:2398–407. doi: 10.1002/hbm.23526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonseka BA, Jaworska N, Courtright A, et al. Cortical thickness and emotion processing in young adults with mild to moderate depression: a preliminary study. BMC Psychiatry. 2016;16:1–9. doi: 10.1186/s12888-016-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvadore G, Nugent AC, Lemaitre H, et al. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2010;54:2643–51. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truong W, Minuzzi L, Soares CN, et al. Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Res Neuroimaging. 2013;214:204–11. doi: 10.1016/j.pscychresns.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds S, Carrey N, Jaworska N, et al. Cortical thickness in youth with major depressive disorder. BMC Psychiatry. 2014;14:1–8. doi: 10.1186/1471-244X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel R, Spreng RN, Shin LM, et al. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–42. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Grattan LM, Eslinger PJ. Frontal lobe damage in children and adults: a comparative review. Dev Neuropsychol. 1991;7:283–326. [Google Scholar]
- 38.Anderson VA, Anderson P, Northam E, et al. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- 39.De Luca CR, Wood SJ, Anderson V, et al. Normative data from the CANTAB: I. Development of executive function over the lifespan. J Clin Exp Neuropsychol. 2003;25:242–54. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- 40.Disner SG, Beevers CG, Haigh EAP, et al. Neural mechanisms of the cognitive model of depression. Natl Rev. 2011;12:467–77. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs E, Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur J Neurosci. 2000;12:2211–4. doi: 10.1046/j.1460-9568.2000.00130.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22:411–6. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essau CA, Conradt J, Petermann F. Frequency, comorbidity, and psychosocial impairment of depressive disorders in adolescents. J Adolesc Res. 2000;15:470–81. [Google Scholar]
- 46.Rao H, Betancourt L, Giannetta JM, et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–50. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittle S, Lichter R, Dennison M, et al. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am J Psychiatry. 2014;171:564–71. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- 48.Peres JFP, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37:1481–91. doi: 10.1017/S003329170700997X. [DOI] [PubMed] [Google Scholar]
- 49.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 50.Jayaweera HK, Hickie IB, Duffy SL, et al. Episodic memory in depression: the unique contribution of the anterior caudate and hippocampus. Psychol Med. 2016;46:2189–99. doi: 10.1017/S0033291716000787. [DOI] [PubMed] [Google Scholar]
- 51.Aupperle RL, Allard CB, Simmons AN, et al. Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Res Neuroimaging. 2013;214:48–55. doi: 10.1016/j.pscychresns.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Behrens TE, Hunt LT, Woolrich MW, et al. Associative learning of social value. Nature. 2008;456:245–9. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from chidhood to adulthood. Neuroimage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 54.Graham J, Salimi-Khorshidi G, Hagan C, et al. Meta-analytic evidence for neuroimaging models of depression: state or trait? J Affect Disord. 2013;151:423–31. doi: 10.1016/j.jad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton JP, Etkin A, Furman DJ, et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]