Abstract
Background
We investigated the association between white matter hyperintensity location and depressive symptoms in a memory-clinic population using lesion–symptom mapping.
Methods
We included 680 patients with vascular brain injury from the TRACE-VCI cohort (mean age ± standard deviation: 67 ± 8 years; 52% female): 168 patients with subjective cognitive decline, 164 with mild cognitive impairment and 348 with dementia. We assessed depressive symptoms using the Geriatric Depression Scale. We applied assumption-free voxel-based lesion–symptom mapping, adjusted for age, sex, total white matter hyperintensity volume and multiple testing. Next, we applied exploratory region-of-interest linear regression analyses of major white matter tracts, with additional adjustment for diagnosis.
Results
Voxel-based lesion–symptom mapping identified voxel clusters related to the Geriatric Depression Scale in the left corticospinal tract. Region-of-interest analyses showed no relation between white matter hyperintensity volume and the Geriatric Depression Scale, but revealed an interaction with diagnosis in the forceps minor, where larger regional white matter hyperintensity volume was associated with more depressive symptoms in subjective cognitive decline (β = 0.26, p < 0.05), but not in mild cognitive impairment or dementia.
Limitations
We observed a lack of convergence of findings between voxel-based lesion–symptom mapping and region-of-interest analyses, which may have been due to small effect sizes and limited lesion coverage despite the large sample size. This warrants replication of our findings and further investigation in other cohorts.
Conclusion
This lesion–symptom mapping study in depressive symptoms indicates the corticospinal tract and forceps minor as strategic tracts in which white matter hyperintensity is associated with depressive symptoms in memory-clinic patients with vascular brain injury. The impact of white matter hyperintensity on depressive symptoms is modest, but it appears to depend on the location of white matter hyperintensity and disease severity.
Introduction
Late-life depression is highly prevalent in older people and in patients with cognitive impairment or dementia.1 It has been associated with vascular dementia, stroke and white matter hyperintensity.2–4 This link between vascular disease and late-life depression has led to the “vascular depression hypothesis.” 5–7 The clinical profile of vascular depression includes loss of interest and motivation, executive dysfunction and psychomotor retardation.8 The vascular depression hypothesis has been investigated intensively in population-based studies. Late-life depression is consistently associated with severity of white matter hyperintensity (i.e., visual rating scores; Fazekas or Scheltens scale) and larger total white matter hyperintensity volumes in healthy elderly people.2,9 Meanwhile, studies in memory-clinic populations are scarce, even though this could be a clinically important population, considering their generally higher vascular lesion burden and frequent occurrence of depressive symptoms. We recently showed that in memory-clinic patients with Alzheimer disease, the severity of white matter hyperintensity (measured using the Fazekas scale) was not related to depressive symptoms. 10 However, we found a borderline significantly increased propensity for depressive symptoms in patients with subjective cognitive decline and white matter hyperintensity. Apart from the severity of white matter hyperintensity, recent studies suggest that specific locations of white matter hyperintensity could predispose people for depressive symptoms. The LADIS study found that deep white matter hyperintensities specifically located in the frontal and temporal locations were associated with depressive symptoms in nondisabled older people.11 Frontal white matter hyperintensities have been associated with higher depression scores on a questionnaire in patients with dementia.12 Furthermore, white matter hyperintensities in the prefrontal and temporal regions, and in specific white matter tracts such as the cingulum bundle, uncinate fasciculus and superior longitudinal fasciculus, have been associated with severity of depression in patients with major depression.13,14 These results suggest disruption of prefrontal–subcortical pathways in particular as an underlying mechanism of late-life depressive symptoms in elderly people.5 Identifying specific white matter tracts in which white matter hyperintensities have the most impact on depressive symptoms would improve our understanding of the consequences of cerebral vascular injury.
Lesion–symptom mapping is frequently used to investigate the relationship between lesion location and specific clinical symptoms in patients with vascular brain injury such as white matter hyperintensity, infarcts and lacunes. Most lesion–symptom mapping studies on white matter hyperintensity have focused on the association between white matter hyperintensity location and cognitive function,15–17 while psychological symptoms of subcortical vascular lesions, such as depression and anxiety, have not been addressed. In this first-ever lesion–symptom mapping study on depressive symptoms, we aimed to determine the extent to which specific white matter hyperintensity locations contribute to depressive symptoms in memory-clinic patients with vascular brain injury on MRI, and to identify strategic white matter tracts in which white matter hyperintensities affect depressive symptoms.
Methods
The TRACE-VCI (Utrecht–Amsterdam clinical features and prognosis in vascular cognitive impairment) study is a prospective observational follow-up study of 860 consecutive memory-clinic patients from Dutch outpatient clinics at 2 university hospitals: VU University Medical Centre and University Medical Centre Utrecht.18 All patients visited the memory clinic between September 2009 and December 2013 and underwent a 1-day standardized dementia screening process that included a medical history, physical and neurologic examinations, screening laboratory tests, an MRI scan of the brain and a neuropsychological assessment. Patients with cognitive complaints and any burden of vascular brain injury on MRI were prospectively included. Further inclusion and exclusion criteria are described in detail elsewhere.18 Patients were divided into 3 categories related to the extent of their cognitive impairment: dementia, mild cognitive impairment and subjective cognitive decline. Patients with evidence of co-occurring neurodegenerative disease or depression were accepted because these are common comorbid etiologies in patients with vascular cognitive impairment. We excluded patients with a nonvascular or nondegenerative primary cause of cognitive impairment, such as a brain tumour, extensive traumatic head injury, substance or alcohol abuse, or multiple sclerosis. We also excluded patients with a primary psychiatric disease other than depression. The study was approved by the medical ethics committee of VU University Medical Centre and University Medical Centre Utrecht. We obtained written informed consent from participants (or their responsible guardians if they were incapable of consent) before conducting research-related procedures.
Participants
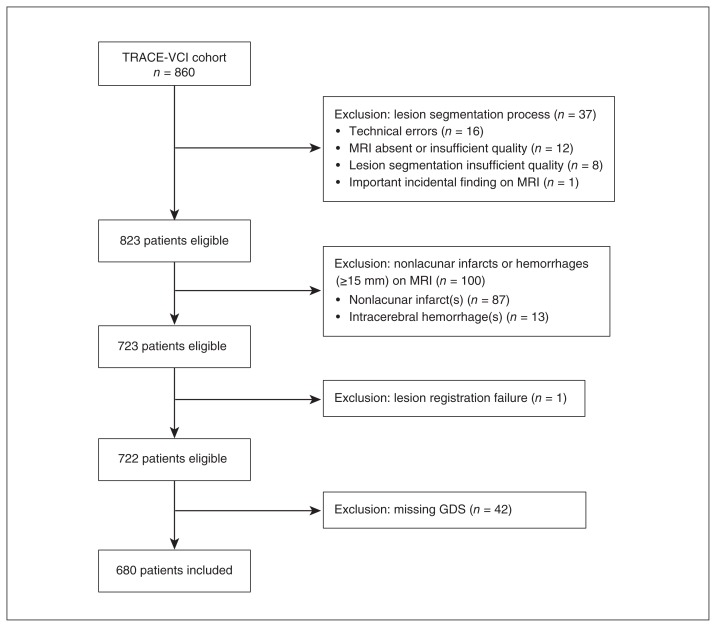
A flow chart of patient selection for the present study is presented in Figure 1. Of the 860 patients in TRACE-VCI, 37 were excluded during the vascular lesion segmentation process, mostly because the available MRI data were of insufficient quality, or because of technical errors during data processing. Next, 100 of the remaining patients were excluded based on the presence of nonlacunar infarcts or hemorrhages other than microbleeds on MRI, because such large lesions can result in the complete obliteration of white matter tracts and could have interfered with our analysis in which white matter hyperintensity volume with specific tracts is related to depressive symptoms at a group level. One additional patient was excluded because of failed lesion registration. Finally, 42 patients were excluded because a Geriatric Depression Scale (GDS) score was not available. This resulted in a study sample of 680 patients (168 subjective cognitive decline, 164 mild cognitive impairment and 348 dementia).
Fig. 1.
Flow chart for patient selection. GDS = Geriatric Depression Scale.
For all patients, we determined history of depression and use of antidepressant medication (e.g., selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors) based on self-reported medical history and medication use. We identified the presence of hypertension based on self-reported medical history, medication use or newly diagnosed hypertension, defined as a blood pressure of 140/90 mm Hg or higher and measured with a sphygmomanometer. We identified hypercholesterolemia based on self-reported medical history or medication use. We identified diabetes mellitus based on self-reported medical history, medication use or newly diagnosed diabetes mellitus, defined as a nonfasting blood glucose of 11.1 mmol/L or greater, or a glycosylated hemoglobin higher than 48 mmol/mol (or ≥ 6.5%). We defined obesity as a body mass index higher than 30 kg/m2.
Evaluation of depressive symptoms
We assessed depressive symptoms using the 15-item self-reported GDS,19 which has a maximum score of 15; higher scores indicate the presence of depressive symptoms. The GDS is used frequently in clinical practice and research, and is a valid and reliable screening instrument for depressive symptoms in older adults.19 In our study, the GDS was verbally administered to patients by a neuropsychologist. We classified patients as having depressive symptoms if their GDS score was 5 or higher. In our analyses we used the continuous GDS score, because it offers the highest power to detect associations.
Alzheimer disease biomarkers
Cerebrospinal fluid markers β-amyloid1–42 (Aβ1–42) and total tau were available for 446 patients. We assessed cerebrospinal fluid biomarkers using Sandwich enzyme-linked immunosorbent assays (Fujirebio).20 Assays were considered positive for Alzheimer disease when the tau/Aβ1–42 ratio was > 0.52.21 In the patients selected for this study, cerebrospinal fluid biomarkers were measured only in those included at the VU University Medical Centre, as a standard procedure of the memory clinic.
MRI protocol
Brain MRI scans were performed on 1.5 T (n = 39) or 3.0 T (n = 641) MRI scanners. Scans were acquired on GE (n = 527, 77.5%) or Philips (n = 153, 22.5%) MRI scanners using a standardized protocol that included 3D T1-weighted, T2-weighted, T2*-weighted/susceptibility-weighted imaging (SWI) and T2 fluid-attenuated inversion recovery (FLAIR) sequences. For some patients, 3D T1 and/or FLAIR sequences were not available, so 2D T1 or FLAIR sequences were used instead. Slice thickness, voxel size and other details for each scanner type are described in detail in Appendix 1, Table S1, available at jpn.ca/180136-a1.
Lesion segmentation
We rated vascular brain injury in accordance with the internationally established STRIVE criteria, which provide neuroimaging standards for classification of cerebral small vessel disease. 22 Ratings were performed by or under the supervision of a neuroradiologist. Lesion segmentation was performed on T2 FLAIR images, using the T1 modality as a reference for proper lesion classification. Automated white matter hyperintensity segmentation was performed using the k nearest neighbour classification with tissue type priors (kNN-TTPs) method.23 This method showed no systematic errors across the different MRI scanners. The resulting white matter hyperintensity lesion maps underwent a visual check for accuracy by 2 independent raters. Subsequent manual corrections were required in 6 participants (0.9%) because of segmentation inaccuracies (i.e., missed white matter hyperintensity or incorrect or incomplete white matter hyperintensity segmentation). These corrections were performed by a single rater. In addition, we determined the presence of other lesion types: lacunes were defined as sharply demarcated deep lesions with cerebrospinal fluid–like signal on all sequences; microbleeds were defined as small dot-like hypointense lesions on T2*-weighted or SWI images. We performed manual segmentation of these lesions using software developed in house based in MeVisLab (MeVis Medical Solutions AG).24,25
Generation of lesion maps
All lesion maps were transformed to the T1 1 mm Montreal Neurological Institute (MNI-152) brain template,26 using an image registration pipeline developed in house that applies the elastix toolbox.27 This standardized pipeline has been recently developed and will soon be made publicly available at www.metavcimap.org. The registration procedure consisted of linear registration followed by nonlinear registration. As an intermediate step, we performed registration to an age-specific MRI template,28 which has been shown to result in more successful registration of brains from patients with severe atrophy. These registration steps were combined into a single step, through which the original lesion maps were registered directly to the MNI-152 space to prevent intermediate interpolations and improve registration accuracy. Quality checks of all registration results were performed by 1 rater (N.A.W.), who compared the lesion location in the MNI-152 space with the original scans. One patient (0.14%) was excluded because of unsuccessful lesion registration.
Statistical analysis
We used PASW Statistics 25.0 for Mac (SPSS Inc.) to conduct statistical analyses. We performed analyses of variance and Pearson χ2 tests to compare groups when appropriate. We applied 2 independent hypothesis-free analysis methods to identify white matter hyperintensity locations associated with depressive symptoms: voxel-based lesion–symptom mapping (VLSM), which analyzed the relation between presence of white matter hyperintensities and depressive symptoms for each voxel in the brain;29 and exploratory region-of-interest (ROI) analyses, which analyzed the impact of lesion volume in predefined white matter tracts on depressive symptoms.
Voxel-based lesion–symptom mapping
We performed VLSM using nonparametric mapping software (NPM, version May 2016; settings: univariate analysis, Brunner-Munzel test),29 which is suitable for non-normally distributed data. To ensure that our analyses were not biased by voxels that are only rarely affected, we set a minimum number of patients with a lesion in a particular voxel and included only voxels that were affected by white matter hyperintensity in at least 14 participants (2%).30 We performed VLSM analyses using a z-score of the GDS as a measure for depressive symptoms after individualized correction for age and sex using linear regression. We repeated the analyses after additional correction for normalized total white matter hyperintensity volume (i.e., calculated from lesion maps after transformation to MNI-152 space). We applied false discovery rate control (q < 0.05) to correct for multiple testing. We performed VLSM in the whole group and then stratified it for syndrome diagnosis.
Region-of-interest analysis
We created ROIs using the John Hopkins University diffusion tensor imaging–based white matter atlas31 with a probability threshold of 10%. We calculated regional white matter hyperintensity volumes in millilitres for each patient for 20 white matter tracts. Next, we merged bilateral white matter tracts to create a single ROI by combining the volumes. The GDS was standardized into a z-score. White matter hyperintensity volumes in the resulting 11 ROIs were added as independent variables to linear regression models, which included age, sex and memory-clinic centre of inclusion as covariates (Model 1). When we found a significant association, we repeated the analysis with additional adjustment for normalized total white matter hyperintensity volume (Model 2). We also performed extra analyses with adjustments for antidepressant medication and MRI field strength and vendor (Model 3). To investigate whether associations with the ROIs differed according to diagnostic group (subjective cognitive decline, mild cognitive impairment or dementia), we included interaction terms (dummy diagnosis × ROI) in the model. When we found an interaction between diagnosis and the ROI (p < 0.10), we stratified the results for syndrome diagnosis and displayed the standardized βs (β) for each diagnostic group separately. When no significant interaction was found, the interaction term was removed from the model and the overall β was reported.
Finally, we performed an additional linear regression analysis in a subgroup of patients with cerebrospinal fluid biomarkers (n = 446) to determine whether the impact of white matter hyperintensity location was influenced by co-occurring Alzheimer disease pathology. To investigate whether associations differed among patients with positive versus negative cerebrospinal fluid biomarkers, we used interaction terms (amyloid status × ROI).
Results
Demographic data and MRI measures are summarized in Table 1. We noted no differences between the original TRACE-VCI cohort and the present study sample (data not shown). Patients with subjective cognitive decline were younger than patients with mild cognitive impairment or dementia. Patients with dementia had lower scores on the GDS than patients with subjective cognitive decline (dementia 3.2 ± 2.7 v. subjective cognitive decline 4.6 ± 3.5; p < 0.001). Patients with mild cognitive impairment or dementia used antidepressant medications less often that patients with subjective cognitive decline (dementia 12% and mild cognitive impairment 11% v. subjective cognitive decline 20%; p < 0.05). The total white matter hyperintensity volume was highest in patients with dementia and mild cognitive impairment, versus patients with subjective cognitive decline: dementia (median [IQR]) 11.5 [22.8] and mild cognitive impairment 11.6 [18.7] v. subjective cognitive decline 5.0 [10.7], p < 0.001).
Table 1.
Demographics of the study population
| Characteristic | Total sample (n = 680) | SCD (n = 168) | MCI (n = 164) | Dementia (n = 348) | Statistical test* | p value | Post hoc differences |
|---|---|---|---|---|---|---|---|
| Age, yr, mean ± SD | 67.1 ± 8.2 | 62.9 ± 7.5 | 68.2 ± 8.5 | 68.6 ± 7.7 | F = 31.27 | < 0.001 | SCD < MCI = D |
| Education level, mean ± SD† | 4.9 ± 1.3 | 5.1 ± 1.4 | 5.2 ± 1.2 | 4.7 ± 1.3 | F = 9.47 | < 0.001 | SCD = MCI > D |
| MMSE score, mean ± SD | 24.3 ± 4.8 | 27.7 ± 2.2 | 26.5 ± 2.3 | 21.7 ± 5 | F = 169.15 | < 0.001 | SCD > MCI > D |
| GDS score, mean ± SD | 3.7 ± 3 | 4.6 ± 3.5 | 3.7 ± 2.8 | 3.2 ± 2.7 | F = 12.66 | < 0.001 | SCD > MCI = D |
| Female, n (%) | 320 (47) | 83 (49) | 72 (44) | 165 (47) | χ2 = 1.045 | 0.593 | NS |
| Depressive symptoms, n (%)‡ | 200 (29) | 72 (42) | 51 (31) | 77 (22) | χ2 = 23.750 | < 0.001 | SCD > MCI > D |
| Alzheimer disease biomarkers, n (%)§ | |||||||
| Available | 446 (66) | 112 (67) | 100 (61) | 234 (67) | χ2 = 2.054 | 0.358 | NS |
| Positive | 242 (54) | 26 (23) | 52 (32) | 164 (70) | χ2 = 67.315 | < 0.001 | SCD < MCI < D |
| History of depression, n (%)¶ | 86 (12) | 34 (20) | 16 (9) | 36 (10) | χ2 = 11.673 | < 0.01 | SCD > MCI = D |
| Use of antidepressant medication, n (%)¶ | 97 (14) | 35 (20) | 19 (11) | 43 (12) | χ2 = 7.926 | < 0.05 | SCD > MCI = D |
| Vascular risk factors, n (%)¶ | |||||||
| Hypertension | 577 (84) | 133 (79) | 141 (86) | 303 (87) | χ2 = 5.717 | 0.057 | NS |
| Hypercholesterolemia | 287 (42) | 66 (39) | 81 (49) | 140 (40) | χ2 = 4.615 | 0.100 | SCD < MCI > D |
| Diabetes mellitus | 123 (18) | 23 (13) | 39 (23) | 61 (17) | χ2 = 5.853 | 0.054 | SCD < MCI > D |
| Obesity (BMI = 30 kg/m2) | 144 (21) | 43 (25) | 34 (20) | 67 (19) | χ2 = 3.687 | 0.450 | NS |
| Currently smoking | 132 (19) | 36 (21) | 32 (19) | 64 (18) | χ2 = 1.205 | 0.877 | NS |
| Imaging characteristics, n (%) | |||||||
| Patients with at least 1 lacune | 124 (18) | 22 (13) | 44 (26) | 58 (16) | χ2 = 11.675 | < 0.01 | SCD < MCI > D |
| Patients with at least 1 microbleed** | 296 (44) | 62 (37) | 76 (46) | 158 (45) | χ2 = 4.388 | 0.111 | NS |
| Total WMH volume in mL, median (IQR)†† | 9.1 (18.0) | 5.0 (10.7) | 11.6 (18.7) | 11.5 (22.8) | F = 15.418 | < 0.001 | SCD < MCI = D |
BMI = body mass index; D = dementia; GDS = Geriatric Depression Scale; IQR = interquartile range; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; NS = not significant; SCD = subjective cognitive decline; SD = standard deviation; WMH = white matter hyperintensity.
One-way analysis of variance or χ2 were performed.
Level of education was classified according to the Verhage system, ranging from 1 (low education) to 7 (highly educated).
Presence of depressive symptoms indicates a score of ≥ 5 on the Geriatric Depression Scale.
Alzheimer disease biomarkers are available as cerebrospinal fluid total tau/βA1–42 (abnormal when > 0.5221).
Determined based on self-reported medical history and medication use or new diagnosis (for hypertension and diabetes mellitus).
Data missing in 5 patients.
Standardized WMH volumes were calculated from lesion maps after transformation to the MNI-152 standard space.
Voxel-based lesion–symptom mapping
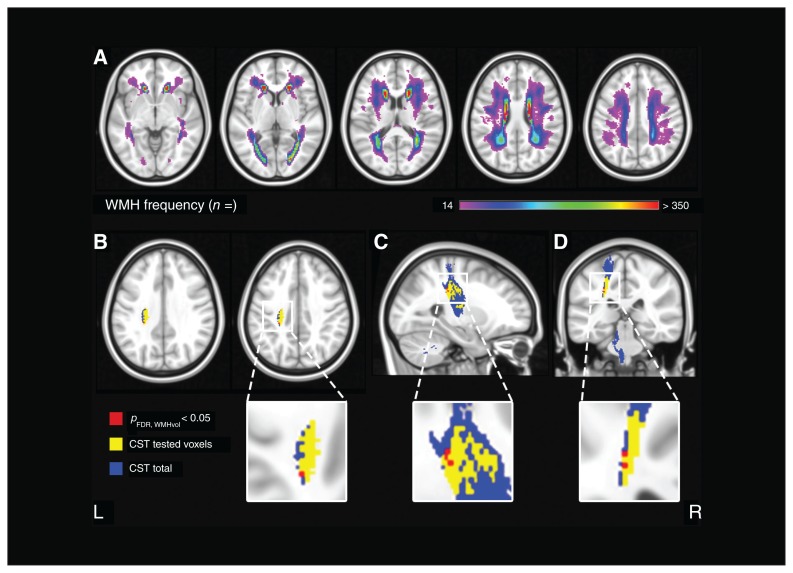
We used VLSM as an assumption-free method of investigating whether the presence of white matter hyperintensity in specific voxels of the brain was significantly associated with depressive symptoms on the GDS, independent of total white matter hyperintensity volume. The distribution of white matter hyperintensity is illustrated by the lesion prevalence map in Figure 2A. White matter hyperintensities showed a symmetric distribution, and were most prevalent in the periventricular and frontoparietal regions.
Fig. 2.
Voxel-based lesion–symptom mapping: lesion prevalence map and results. (A) Voxel-wise lesion prevalence of white matter hyperintensities in the study population, projected on the Montreal Neurological Institute 152 T1 template. A minimum threshold of 14 participants with damage in a given voxel was applied; z-coordinates: −5, 5, 15, 25, 35. Voxel-based lesion–symptom mapping results for the Geriatric Depression Scale score, shown in (B) axial, (C) sagittal and (D) coronal planes. Significant voxels after correction for multiple comparisons, age, sex and normalized total white matter hyperintensity volume are shown in red (settings: Brunner–Munzel test; FDR q < 0.05). Significant voxels were located in the corticospinal tract. Regions of interest were derived from the Johns Hopkins University diffusion tensor imaging atlas with a probability threshold of 10%. The corticospinal tract derived from the Johns Hopkins University atlas is shown in blue; the voxels included in the voxel-based lesion–symptom mapping analysis (i.e., damaged in ≥ 14 participants) are shown in yellow. Coordinates: sagittal: x = −25; coronal: y = −32; axial: z = 33, 38. CST = corticospinal tract; FDR = false detection rate; WMH = white matter hyperintensity.
The results of the VLSM analysis are shown in Figure 2B. We found voxels with a significant association between the presence of white matter hyperintensities and depressive symptoms after correction for age, sex, total white matter hyperintensity volume and multiple testing. These significant voxels were almost exclusively located in the corticospinal tract, near the superior longitudinal fasciculus and the temporal part of the superior longitudinal fasciculus. The exact number of significant voxels in each white matter tract is provided in Table 2.
Table 2.
Results, voxel-based lesion–symptom mapping*
| Anatomic region† | Region size in voxels (n) | Tested voxels (n) | Significant voxels (n) |
|---|---|---|---|
| Forceps major | 22285 | 9537 | 0 |
| Forceps minor | 35840 | 5063 | 0 |
| Anterior thalamic radiation | 43203 | 13661 | 0 |
| Corticospinal tract | 27767 | 5975 | 15 |
| Cingulum | 13829 | 1309 | 0 |
| Parahippocampal white matter | 5234 | 0 | 0 |
| Inferior fronto-occipital fasciculus | 49378 | 24187 | 0 |
| Inferior longitudinal fasciculus | 37450 | 9955 | 0 |
| Superior longitudinal fasciculus | 59703 | 29336 | 1 |
| Superior longitudinal fasciculus, temporal part | 22910 | 12710 | 1 |
| Uncinate fasciculus | 15662 | 4371 | 0 |
Tested and significant voxels for each anatomic region, after correction for age, sex, total white matter hyperintensity volume and multiple testing by applying a false discovery rate.
Johns Hopkins University diffusion tensor imaging white matter atlas.31
Subsequent stratification for syndrome diagnosis showed no significant voxels for any subgroup.
Region-of-interest analyses
We used ROI analyses to determine whether white matter hyperintensity volumes in predefined white matter tracts were associated with depressive symptoms. Table 3 shows the association between total and regional white matter hyperintensity volumes and depressive symptoms.
Table 3.
Results, region-of-interest analyses*
| Anatomic region† | Model | Total sample (n = 680) | SCD (n = 168) | MCI (n = 164) | Dementia (n = 348) | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| β | p value | β | p value | β | p value | β | p value | ||
| Total WMH volume | Model 1 | −0.03 | 0.47 | ||||||
| Forceps major | Model 1 | −0.06 | 0.16 | ||||||
| Forceps minor | Model 1‡ | 0.05 | 0.20 | 0.16 | 0.04 | 0.04 | 0.63 | 0.03 | 0.66 |
| Model 2‡ | 0.26 | 0.02 | — | — | — | — | |||
| Anterior thalamic radiation | Model 1‡ | −0.01 | 0.85 | 0.05 | 0.55 | 0.07 | 0.45 | −0.07 | 0.24 |
| Corticospinal tract | Model 1 | 0.03 | 0.46 | ||||||
| Cingulum | Model 1 | −0.02 | 0.70 | ||||||
| Inferior fronto-occipital fasciculus | Model 1‡ | −0.05 | 0.23 | 0.05 | 0.53 | −0.02 | 0.78 | −0.10 | 0.06 |
| Inferior longitudinal fasciculus | Model 1‡ | −0.04 | 0.30 | 0.11 | 0.17 | −0.05 | 0.58 | −0.10 | 0.07 |
| Superior longitudinal fasciculus | Model 1 | −0.03 | 0.42 | ||||||
| Superior longitudinal fasciculus, temporal part | Model 1 | −0.03 | 0.46 | ||||||
| Uncinate fasciculus | Model 1 | −0.01 | 0.78 | ||||||
MCI = mild cognitive impairment; SCD = subjective cognitive decline; WMH = white matter hyperintensity.
Results are presented as standardized β. This assumption-free region-of-interest analysis served to identify strategic white matter tracts in which white matter hyperintensity volume is correlated with depressive symptoms, independent of total white matter hyperintensity burden. The Geriatric Depression Scale, as measure of depressive symptoms, was standardized into a z-score. We excluded the tract parahippocampal white matter (Johns Hopkins University atlas31) from our analyses because of the limited white matter hyperintensity in this tract. We first entered age, sex, centre and syndrome diagnosis into a linear regression model (Model 1). If regional volumes showed a significant (p < 0.05) association in Model 1, we added the normalized total white matter hyperintensity volume to the model (Model 2). To check if associations between depressive symptoms and anatomic regions differed according to diagnostic group, we included interaction terms (dummy diagnosis × anatomic region) in the model. When we found an interaction between syndrome diagnosis and anatomic region (p < 0.10), we stratified the results for syndrome diagnosis, and the stβ is displayed for each diagnostic group separately. When no significant interaction was found, the interaction term was removed from the model and the overall β was reported.
Johns Hopkins University diffusion tensor imaging white matter atlas.31
Significant interaction term; subsequently stratified for syndrome diagnosis.
Neither total white matter hyperintensity volume nor regional white matter hyperintensity volume in specific tracts were related to depressive symptoms. We did find interactions between syndrome diagnosis and regional white matter hyperintensity volume in the forceps minor, the anterior thalamic radiation, the inferior fronto-occipital fasciculus and the inferior longitudinal fasciculus, suggesting that the association between depressive symptoms and regional white matter hyperintensity volume in these regions is different for subjective cognitive decline, mild cognitive impairment and dementia. Subsequent stratification for syndrome diagnosis showed that in patients with subjective cognitive decline, regional white matter hyperintensity in the forceps minor was associated with more depressive symptoms (β = 0.16; p < 0.05). Additional adjustment for normalized total white matter hyperintensity volume resulted in a slightly stronger association (β = 0.26; p < 0.05). We found no significant associations for mild cognitive impairment or dementia. Finally, we performed analyses with additional adjustment for antidepressant medication and MRI field strength and vendor, and the results were unchanged (data not shown).
Exploratory region-of-interest analyses in a subgroup of patients with cerebrospinal fluid biomarkers
We performed exploratory analyses in a subgroup of patients with cerebrospinal fluid biomarkers (n = 446; Appendix 1, Table S1). We found no significant interactions between amyloid status and white matter hyperintensity volume in relation to depressive symptoms in any region.
Discussion
This lesion–symptom mapping study on depressive symptoms indicates the corticospinal tract and forceps minor as strategic white matter tracts in which white matter hyperintensities were associated with depressive symptoms in a memory-clinic cohort of patients with vascular brain injury. The overall impact of white matter hyperintensities on these symptoms was modest, but their location appeared to be particularly important in patients with subjective cognitive decline.
The analyses used in this study (VLSM and ROI linear regression) resulted in different strategic white matter hyperintensity locations. We detected an association between regional white matter hyperintensity in the corticospinal tract and depressive symptoms only at the voxel level, and the number of significant voxels was limited (only 15 of 5975). With the ROI analyses at the regional level, we found no congruent correlation with the corticospinal tract but identified a modest association between the forceps minor and GDS only in the subgroup with subjective cognitive decline. The statistical power (due to more rigorous correction for multiple testing) for the VLSM analyses might have been insufficient. However, a main advantage of VLSM is its very high spatial resolution. Still, our results for the forceps minor were consistent with previous findings on the role of white matter hyperintensities in frontal and temporal locations in depression. 5,11 The lack of convergence of findings from the VLSM and ROI analyses may be because effect sizes are quite small. Moreover, the exploratory nature of the ROI analysis warrant replication of our findings and further investigation in other large memory-clinic cohorts with optimal lesion coverage.
The vascular depression hypothesis proposes that white matter hyperintensities caused by cerebrovascular disease disrupt the frontostriatal–subcortical circuits and predispose people for late-life depression.32 Previous studies on white matter pathways and depressive symptoms have primarily employed diffusion tensor imaging. Most studies examined patients with major depressive disorder or late-life depression. A recent review on white matter alterations in emotional disorders (ranging from major depressive disorder to anxiety disorders and obsessive compulsive disorder) found reduced fractional anisotropy as a marker for white matter integrity in frontotemporal and frontoparietal white matter tracts compared with healthy controls.33 The largest clusters of reduced fractional anisotropy incorporated several white matter tracts, including the left forceps minor, the anterior thalamic radiation, the inferior fronto-occipital fasciculus and the uncinate fasciculus. A study in patients without dementia but with small-vessel disease found lower white matter integrity in patients with depressive symptoms, particularly in the prefrontal white matter tracts.34 In contrast, a previous study in a small group of patients with major depressive disorder found increased white matter integrity in the corticospinal tract compared with controls using tractography clustering methods.35 Previous research suggests that diffusion tensor imaging underestimates fractional anisotropy in regions where fasciculi cross. Because the corticospinal tract is located in an area with crossing fasciculi (i.e., the superior longitudinal fasciculus), these results measured with diffusion tensor imaging should be interpreted with caution.36 However, our lesion–symptom mapping analyses in a large cohort of memory-clinic patients (including an adjustment for multiple comparisons using FDR correction) also showed an association between the corticospinal tract and depressive symptoms. Our results provide further evidence for a potential role of the corticospinal tract in depressive symptoms. The corticospinal tract is a descending tract of the central nervous system, starting in the cortex and terminating in the spinal cord, and it is known to be involved in controlling movements of the limbs and trunk. It is possible that our findings for the corticospinal tract are related to psychomotor symptoms in depression. We know that depression comprises many combinations of clinical symptoms. Population-based and clinical (in patients with major depressive disorder) studies have investigated the presence of these depressive “subtypes” and suggest the reflection of specific neurobiological biomarkers in particular brain regions between the subtypes.37,38 In the present study, we had access only to the total GDS score. Future research with different measures for depressive symptoms is needed to identify the potential presence of depressive subtypes in a memory-clinic population.
Consistent with our previous study, we did not find an association between white matter hyperintensity and depressive symptoms in patients with dementia.10 However, our previous results of a higher propensity for depressive symptoms in patients with subjective cognitive decline and white matter hyperintensity is consistent with the present study, because we found an association between regional white matter hyperintensity in the forceps minor and depressive symptoms in patients with subjective cognitive decline. The forceps minor is a commissural fibre that connects the medial and lateral surfaces of both frontal lobes. It has previously been associated with executive dysfunction and reduced psychomotor speed in patients with vascular brain injury,15 core cognitive deficits in patients with late-life depression and vascular cognitive impairment. Studies in patients with subjective cognitive decline found subthreshold symptoms of depression and anxiety.39 Most patients with subjective cognitive decline do not necessarily meet the diagnostic criteria for a psychiatric condition such as major depressive disorder. Affective symptoms in subjective cognitive decline show increased risk of progression to mild cognitive impairment and dementia, suggesting the subthreshold symptoms of depression as a possible manifestation of preclinical Alzheimer disease in these people.40,41 Conversely, our analyses in a subgroup with cerebrospinal fluid biomarkers showed that the association between white matter hyperintensities and depressive symptoms is not influenced by Alzheimer disease pathology. To investigate whether factors other than white matter hyperintensity and Alzheimer disease pathology could explain these results, research in other cohorts is needed to provide more evidence. More complex multivariate models (e.g., Bayesian network analysis or multivariate lesion–symptom mapping) might also be of value.
Limitations
Among the limitations of this study is that we used the GDS as a measure of depressive symptoms. Cognitive issues in mild cognitive impairment and dementia may affect the diagnostic accuracy of the GDS.42 However, the design of the GDS with questions structured in a yes/no format makes it easy to use, even for patients with cognitive impairment. The level and severity of depressive symptoms in this study was relatively low, particularly in patients with dementia, but were consistent with previous studies in memory-clinic populations. 10,43 Still, this may have reduced the effect sizes and sensitivity to detect associations, despite the large sample size. Second, a relatively high number of patients with subjective cognitive decline used antidepressant medication (20%) compared with patients with mild cognitive impairment (11%) or dementia (12%). The antidepressant medication may have decreased the severity of depressive symptoms and led to lower scores on the GDS, potentially leading to an underestimation of the association between white matter hyperintensity location and depressive symptoms. However, the use of antidepressant medication will be more common in those with higher scores on the GDS, but additional analyses with adjustment for antidepressant medication showed similar results. Finally, the inclusion of our patients at tertiary referral centres and the exclusion of patients with cortical infarcts could limit the generalizability of our findings. On the other hand, the TRACE-VCI cohort is a large memory-clinic cohort of patients with a wide spectrum of vascular brain injury and different levels of cognitive impairment not limited to specific clinical diagnoses such as vascular dementia or Alzheimer disease. In addition, the use of data from different MRI scanners could have influenced the quality of the white matter hyperintensity segmentations and subsequent analyses. However, we have assessed the performance of our segmentation method and it showed no systematic errors across MRI scanners. Nevertheless, additional adjustment for field strength and vendor did not change our results. The use of different MRI scanners could also be seen as a strong point of our study, because it highlights the robustness of our approach and increases the generalizability of our results. Moreover, the large lesion coverage, particularly in the frontoparietal regions, allowed us to include a large number of white matter tracts, leading to greater accuracy and statistical power. We also performed 2 independent hypothesis-free statistical analyses (VLSM and ROI-based linear regression models).
Conclusion
The present study provides further insight into the relationship between white matter hyperintensity location and depressive symptoms by performing a large-scale lesion–symptom mapping study in depressive symptoms. We have shown that the impact of white matter hyperintensity on depressive symptoms is modest, but appears to be dependent on the location of white matter hyperintensities, particularly in patients with subjective cognitive decline. Our results suggest different etiologies of depressive symptoms in a memory-clinic population with vascular brain injury. Changes in white matter tracts might underlie the occurrence of depressive symptoms in this population.
Acknowledgements
The TRACE-VCI study is supported by Vidi grant 91711384 and Vici Grant 918.16.616 from ZonMw, the Netherlands, Organisation for Health Research and Development, and grant 2010T073 from the Dutch Heart Association to G. Biessels. Research of the VUmc Alzheimer Centre is part of the neurodegeneration research program of Amsterdam Neuroscience. The VUmc Alzheimer Centre is supported by Alzheimer Nederland and Stichting VUmc Fonds. The clinical database structure was developed with funding from Stichting Dioraphte. A. Leeuwis and A. Hooghiemstra are appointed on a grant from The Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2012-06 Heart Brain Connection). F. Barkhof is supported by the NIHR Biomedical Research Centre at University College London Hospital.
Footnotes
Competing interests: N. Prins serves on the advisory board of Boehringer Ingelheim and Probiodrug, and on Abbvie’s DSMB M15-566 trial; has provided consultancy services for Sanofi, Takeda and Kyowa Kirin Pharmaceutical Development; receives research support from Alzheimer Nederland (project number WE.03-2012-02); and is the CEO and co-owner of Brain Research Centre, Amsterdam, the Netherlands. P. Scheltens has acquired grant support (for the institution) from GE Healthcare and Piramal; and in the past 2 years he has received consultancy/speaker fees (paid to the institution) from Medavante, Novartis, Probiodrug, Biogen, Roche, Toyama and EIP Pharma. F. Barkhof serves as a consultant for Biogen-Idec, Janssen Alzheimer Immunotherapy, Bayer-Schering, Merck-Serono, Roche, Novartis, Genzyme and Sanofi-aventis; has received sponsoring from EU-H2020, NWO, SMSR, TEVA, Novartis, Toshiba and Imi; and serves on the editorial boards of Radiology, Brain, Neuroradiology, MSJ and Neurology. Research programs of W.M. van der Flier have been funded by ZonMW, NWO, EU-FP7, Alzheimer Nederland, Cardiovasculair Onderzoek Nederland, Stichting Dioraphte, Gieskes-Strijbis fonds, Pasman Stichting, Boehringer Ingelheim, Piramal Imaging, Roche BV, Janssen Stellar, Biogen and Combinostics; all funding is paid to her institution. G. Biessels has been funded by the Dutch Heart Association, ZonMW, the Netherlands Organisation for Health Research and Development and European Union Horizon 2020. No other competing interests declared.
Contributors: A. Leeuwis, N. Weaver, H. Kuijf, W. van der Flier and G. Biessels designed the study. A. Leeuwis, N. Weaver, L. Exalto, H. Kuijf, N. Prins, P. Scheltens, F. Barkhof and G. Biessels acquired the data, which A. Leeuwis, N. Weaver, J.M. Biesbroek, A. Hooghiemstra, N. Prins, P. Scheltens, F. Barkhof, W. van der Flier and G. Biessels analyzed. A. Leeuwis and N. Weaver wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Members of the TRACE-VCI study group: M.R. Benedictus, J. Bremer, W.M. van der Flier, A.E. Leeuwis, J. Leijenaar, N.D. Prins, P. Scheltens, B.M. Tijms (Alzheimer Centre and Department of Neurology, Amsterdam UMC, location VU University Medical Centre, Amsterdam, The Netherlands); F. Barkhof, M.P. Wattjes (Department of Radiology and Nuclear Medicine, Amsterdam UMC, location VU University Medical Centre, Amsterdam, The Netherlands); C.E. Teunissen (Department of Clinical Chemistry, Amsterdam UMC, location VU University Medical Centre, Amsterdam, The Netherlands); T. Koene (Department of Medical Psychology, Amsterdam UMC, location VU University Medical Centre, Amsterdam, The Netherlands); E. van den Berg, H. van den Brink, G.J. Biessels, J.M.F. Boomsma, L.G. Exalto, D.A. Ferro, C.J.M. Frijns, O. Groeneveld, R. Heinen, S.M. Heringa, L.J. Kappelle, Y.D. Reijmer, J. Verwer, N.A. Weaver (Department of Neurology, University Medical Centre Utrecht, Utrecht, The Netherlands); J. de Bresser, H.J. Kuijf (Department of Radiology/Image Sciences Institute, University Medical Centre Utrecht, Utrecht, The Netherlands); H.L. Koek (Department of Geriatrics, University Medical Centre Utrecht, Utrecht, The Netherlands); J.M.F. Boomsma, H.M. Boss, H.C. Weinstein (Department of Neurology, Onze Lieve Vrouwe Gasthuis (OLVG) West, Amsterdam, The Netherlands); and F. Barkhof (Department of Radiology, National Institute for Health Research (NIHR) and University College London Hospitals NHS Foundation Trust (UCLH) Biomedical research center, London, United Kingdom).
References
- 1.World health statistics 2007. Geneva: World Health Organization; 2007. Available: www.who.int/whosis/whostat/2007/en/ [Google Scholar]
- 2.van Agtmaal MJM, Houben AJHM, Pouwer F, et al. Association of microvascular dysfunction with late-life depression. JAMA Psychiatry. 2017;74:729. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anor CJ, O’Connor S, Saund A, et al. Neuropsychiatric symptoms in Alzheimer disease, vascular dementia, and mixed dementia. Neurodegener Dis. 2017;17:127–34. doi: 10.1159/000455127. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Leonards CO, Sterzer P, et al. White matter lesions and depression: a systematic review and meta-analysis. J Psychiatr Res. 2014;56:56–64. doi: 10.1016/j.jpsychires.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS, Meyers BS, Young RC, et al. “Vascular depression” hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan KRR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 7.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexopoulos GS. Role of executive function in late-life depression. J Clin Psychiatry. 2003;64:18–23. [PubMed] [Google Scholar]
- 9.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2007;79:619–24. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 10.Leeuwis AE, Prins ND, Hooghiemstra AM, et al. Microbleeds are associated with depressive symptoms in Alzheimer’s disease. Alzheimer’s Dement (Amst) 2018;10:112–20. doi: 10.1016/j.dadm.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan MS, O’Brien JT, Firbank MJ, et al. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADrosoph Inf Serv study. Int J Geriatr Psychiatry. 2006;21:983–9. doi: 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- 12.Soennesyn H, Oppedal K, Greve OJ, et al. White matter hyperintensities and the course of depressive symptoms in elderly people with mild dementia. Dement Geriatr Cogn Dis Extra. 2012;2:97–111. doi: 10.1159/000335497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalby RB, Frandsen J, Chakravarty MM, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res. 2010;184:38–48. doi: 10.1016/j.pscychresns.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–32. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biesbroek JM, Weaver NA, Hilal S, et al. Impact of strategically located white matter hyperintensities on cognition in memory clinic patients with small vessel disease. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0166261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duering M, Zieren N, Hervé D, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011;134:2366–75. doi: 10.1093/brain/awr169. [DOI] [PubMed] [Google Scholar]
- 17.Duering M, Gesierich B, Seiler S, et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology. 2014:1946–50. doi: 10.1212/WNL.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boomsma J, Exalto L, Barkhof F, et al. Vascular cognitive impairment in a memory clinic population: rationale and design of the “Utrecht-Amsterdam clinical features and prognosis in vascular cognitive impairment” (TRACE-VCI) study. J Med Internet Res Protoc. 2017;6:e60. doi: 10.2196/resprot.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 20.Lewczuk P, Kornhuber J, Wiltfang J. The German Competence Net Dementias: standard operating procedures for the neurochemical dementia diagnostics. J Neural Transm. 2006;113:1075–80. doi: 10.1007/s00702-006-0511-9. [DOI] [PubMed] [Google Scholar]
- 21.Duits FH, Teunissen CE, Bouwman FH, et al. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–23. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenwijk MD, Pouwels PJW, Daams M, et al. Accurate white matter lesion segmentation by k nearest neighbor classification with tissue type priors (kNN-TTPs) NeuroImage Clin. 2013;3:462–9. doi: 10.1016/j.nicl.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuijf H. Image processing techniques for quantification and assessment of brain MRI. Utrecht, the Netherlands: Utrecht University; 2013. [Google Scholar]
- 25.Ritter F, Boskamp T, Homeyer A, et al. Medical image analysis. IEEE Pulse. 2011;2:60–70. doi: 10.1109/MPUL.2011.942929. [DOI] [PubMed] [Google Scholar]
- 26.Fonov V, Evans A, McKinstry RC, et al. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. [Google Scholar]
- 27.Klein S, Staring M, Murphy K, et al. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 28.Rorden C, Bonilha L, Fridriksson J, et al. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–65. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rorden C, Karnath H, Bonilha L. Improving lesion–symptom mapping. J Cogn Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 30.Sperber C, Karnath HO. Impact of correction factors in human brain lesion-behavior inference. Hum Brain Mapp. 2017;38:1692–701. doi: 10.1002/hbm.23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–47. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneed JR, Culang-Reinlieb ME. The vascular depression hypothesis: an update. Am J Geriatr Psychiatry. 2011;19:99–103. doi: 10.1097/jgp.0b013e318202fc8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins LM, Barba A, Campbell M, Lamar M, et al. Shared white matter alterations across emotional disorders: a voxel-based metaanalysis of fractional anisotropy. NeuroImage Clin. 2016;12:1022–34. doi: 10.1016/j.nicl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Uden IWM, Tuladhar AM, de Laat KF, et al. White matter integrity and depressive symptoms in cerebral small vessel disease: the RUN DMC study. Am J Geriatr Psychiatry. 2015;23:525–35. doi: 10.1016/j.jagp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Sacchet MD, Prasad G, Foland-Ross LC, et al. Structural abnormality of the corticospinal tract in major depressive disorder. Biol Mood Anxiety Disord. 2014;4:1–10. doi: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douaud G, Jbabdi S, Behrens TEJ, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage. 2011;55:880–90. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tully PJ, Debette S, Mazoyer B, et al. White matter lesions are associated with specific depressive symptom trajectories among incident depression and dementia populations: three-city Dijon MRI study. Am J Geriatr Psychiatry. 2017;25:1311–21. doi: 10.1016/j.jagp.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Lamers F, Beekman ATF, Van Hemert AM, et al. Six-year longitudinal course and outcomes of subtypes of depression. Br J Psychiatry. 2016;208:62–8. doi: 10.1192/bjp.bp.114.153098. [DOI] [PubMed] [Google Scholar]
- 39.Hill NL, Mogle J, Wion R, et al. Subjective cognitive impairment and affective symptoms: a systematic review. Gerontologist. 2016;56:e109–27. doi: 10.1093/geront/gnw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donovan NJ, Locascio JJ, Marshall GA, et al. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry. 2018;175:530–7. doi: 10.1176/appi.ajp.2017.17040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–81. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Craen AJM, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry. 2003;18:63–6. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 43.Mccutcheon ST, Han D, Troncoso J, Koliatsos VE, et al. Clinicopathological correlates of depression in early Alzheimer’s disease in the NACC. Int J Geriatr Psychiatry. 2016;30:1301–11. doi: 10.1002/gps.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]