Abstract
We investigated the binding of IgE and different types of allergen–IgE complexes to CD23-expressing human B cells. We performed the experiments using chimeric Bip 1 (CB1), a chimeric humanized IgE specific for the major birch allergen, Bet v 1, together with monomeric and oligomeric forms of recombinant Bet v 1 (rBet v 1), and Bet v 1-specific IgG antibodies. In this model IgE binding to CD23 was independent of variations in antibody affinities towards monomeric and oligomeric Bet v 1 as demonstrated by plasmon surface resonance. CB1 alone or in the form of small immune complexes consisting of one molecule of CB1 plus allergen, showed comparable binding to CD23 on B cells. Using anti-IgE antibody probes discriminating CD23-bound from CD23-unbound IgE, it is demonstrated that in large immune complexes obtained with oligomeric Bet v 1 or by super-crosslinking of small immune complexes with Bet v 1-specific IgG, anti-IgE staining of B cells increased. This increase of staining was due to the presence of IgE antibodies in the immune complexes that were not directly engaged in CD23 binding, and thus available for IgE detection. Our study thus reveals that CD23 can bind in a comparable manner to free IgE and IgE–allergen complexes of different size and composition, which may also include allergen-specific IgG. The interplay of free IgE with IgE–allergen immune complexes of different sizes and composition with CD23 binding represents a mechanism for the modulation of CD23-mediated immune responses such as IgE-facilitated allergen presentation in allergic diseases.
Keywords: allergy, allergen, CD23, IgE, immune complexes, super-crosslinking
The low-affinity receptor for IgE, FcεRII, also termed CD23, is a 45 kDa transmembrane glycoprotein belonging to the C-type lectin protein family, which is expressed on B cells, T cells, Langerhans cells, monocytes, eosinophils, neutrophils, macrophages and platelets.1 CD23 expression on B cells and macrophages is higher in allergic patients compared to non-allergic persons,2,3 and increases upon allergen exposure as it occurs for example during the pollen season.4
Allergen-specific immunotherapy (SIT) reduces CD23 expression on B cells indicating its clinical relevance.4,5 Binding of allergens via IgE to CD23 on antigen presenting cells, strongly augments allergen-specific T cell proliferation and the synthesis of pro-inflammatory cytokines.6,7 Successful SIT induces allergen-specific IgG antibodies that inhibit IgE-facilitated allergen presentation, and thus T cell activation and cytokine production.8 This mechanism may therefore be important for the reduction of allergen-specific T cell activity during SIT.9 Allergen-induced T cell proliferation can be also inhibited with anti-CD23 antibodies, which therefore, have been suggested for therapy of allergic diseases.10 Accordingly, the extent of the inhibition of IgE-facilitated allergen presentation via CD23 by SIT-induced allergen-specific IgG as measured by the facilitated allergen binding assay has been proposed as a possible surrogate marker for SIT.11,12
In this study, we established a molecular model to investigate modes of IgE binding to CD23 using defined molecular tools, that is, a human monoclonal IgE specific for the major birch pollen allergen, Bet v 1, chimeric Bip 1 (CB1), recombinant monomeric and oligomeric Bet v 1 as well as Bet v 1-specific IgG recognizing epitopes distinct from those bound by CB1. These four modes were, 1: binding of isolated monomeric CB1 to CD23, 2: binding of CD23 to CB1 bound to recombinant Bet v 1 (rBet v 1) monomer, 3: binding of CD23 to CB1 bound to a oligomeric form of rBet v 1 containing several binding sites for IgE and 4: binding of CD23 to CB1–Bet v 1–IgG immune complexes. In the latter two binding modes, larger immune complexes were formed on the cell surface, which consisted of IgE directly bound to CD23 and IgE that was only part of the immune complexes without direct interaction to CD23.
Results
Dose-dependent binding of monomeric monoclonal IgE to CD23 on B cells
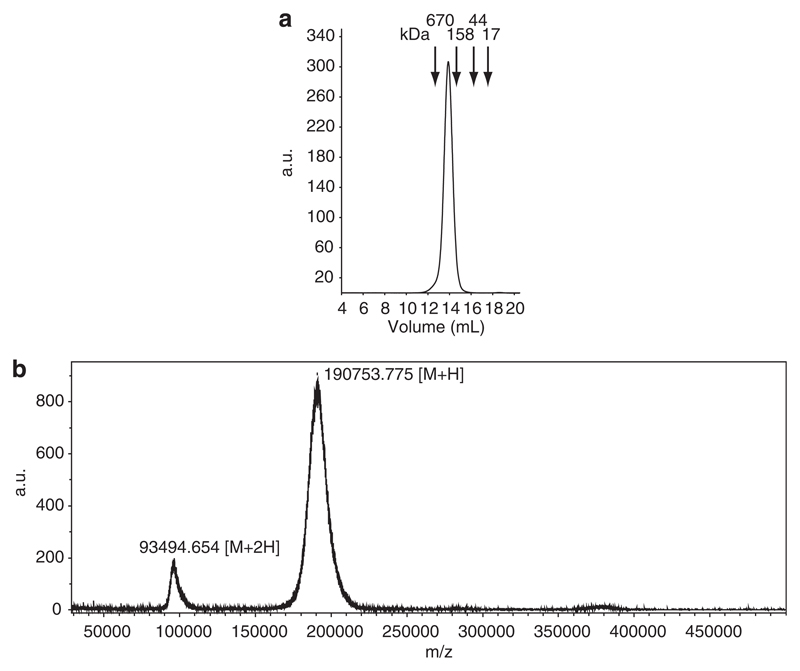
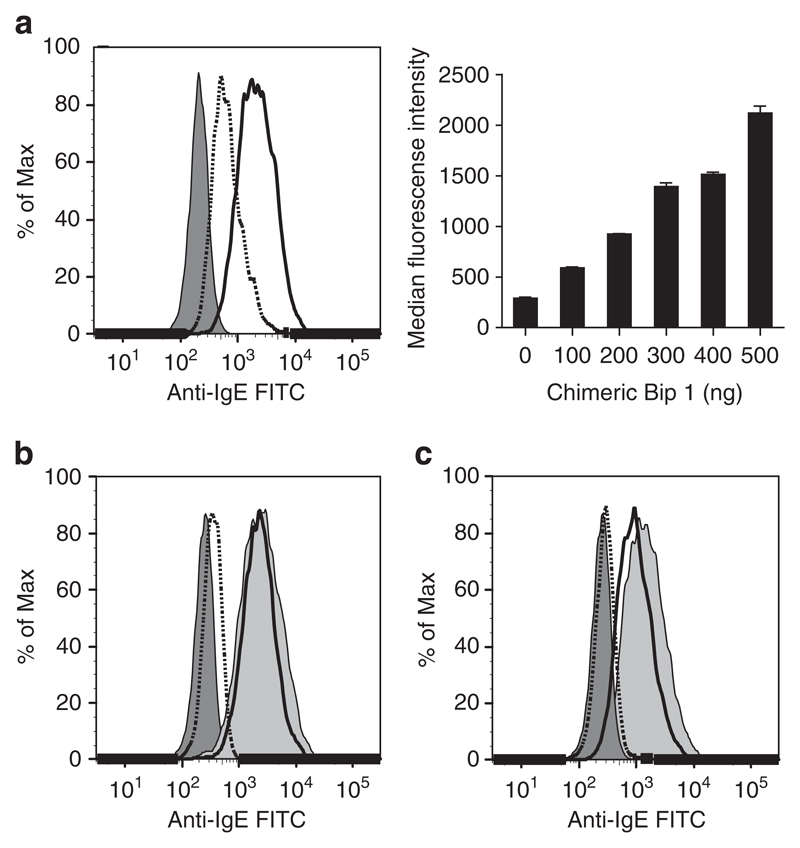
Figure 1a shows that the purified fraction of CB1 contains a single molecular species that elutes as a single peak corresponding to 190 kDa. This result was confirmed by mass spectrometry showing that the molecular mass of CB1 is exactly 190753 Da (Figure 1b). The second peak (that is, 93494.65 Da) corresponds to half of the molecule’s mass and represents a double-charged species (that is, M2H+) of the same protein. Binding experiments with freshly purified CB1 and a monoclonal anti-human IgE, which can recognize CD23-bound IgE demonstrate a dose-dependent and specific binding of monomeric IgE to CD23 on B cells, even in the absence of a specific allergen or ligands for IgE (Figure 2a). The binding of CB1 to CD23 could be inhibited with an anti-CD23 antibody blocking the IgE interaction with CD23 that was added to the cells before addition of CB1 (Figure 2b). It was also blocked by pre-incubation of CB1 with an anti-IgE antibody recognizing the CD23 binding site of IgE (Figure 2c).
Figure 1.
Size exclusion chromatography and MALDI-TOF analysis of chimeric Bip 1 antibody (CB1). (a) Size exclusion chromatography of CB1. Molecular masses of standards (kDa) are indicated on top, elution volumes are shown on the x-axis and the y-axis displays the adsorption at 280 nm as arbitrary units. (b) Molecular weight of CB1 by MALDI-TOF mass spectrometry. The x-axis shows the mass/charge (m/z) values and the y-axis the signal intensity.
Figure 2.
Binding of monomeric CB1 to CD23. (a) FACS histogram left panel, EBV-B cells were incubated with CB1 at the amount of 100 ng (dotted line), 500 ng (solid line) or phosphate buffered saline (PBS) (dark gray filled). Right panel, bar graphs show dose-dependent (x-axis: ng) increase in binding (y-axis: median fluorescence intensity) of CB1 to CD23 on EBV-B cells. Results are displayed as means±s.d.’s (b) FACS histogram of cells, which had been pre-incubated with anti-CD23 antibody (dotted line), matched isotype control antibody (solid line) before addition of CB1. Cells incubated with PBS (dark gray filled) or CB1 without pre-treatment (light gray filled). (c) Histogram of cells exposed to CB1 that was pre-incubated with anti-human IgE (dotted line) or a matched isotype control (solid line). Controls (PBS and CB1 without pretreatment) are as in (b).
Anti-IgE staining increases upon binding of larger immune complexes to CD23
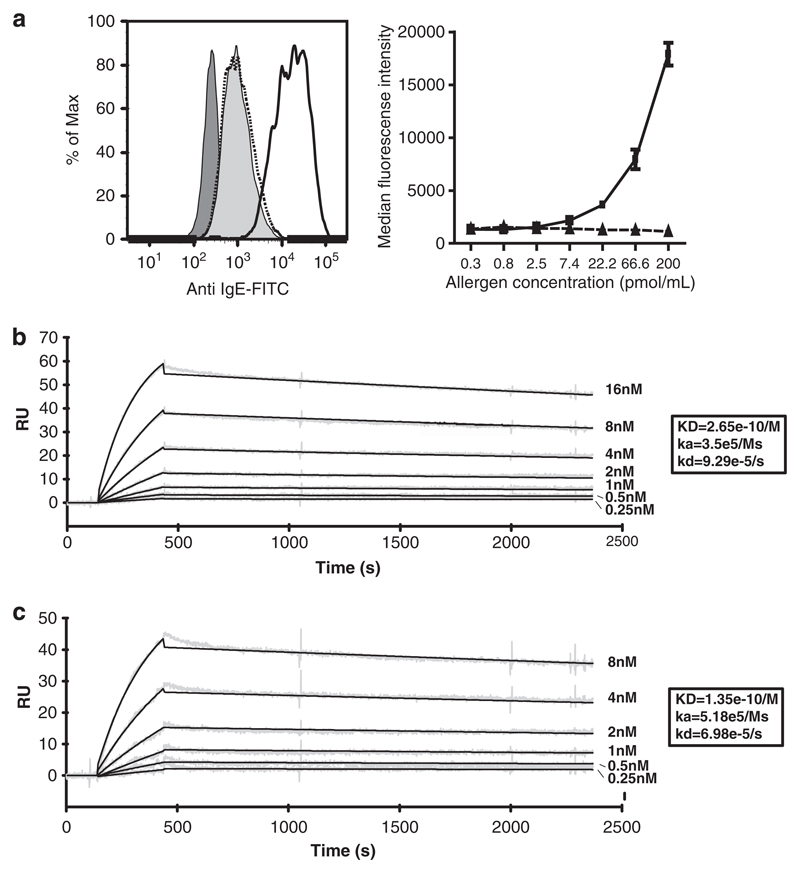
Immune complexes consisting of monomeric CB1 alone or monomeric Bet v 1 in complex with CB1 showed no differences regarding anti-IgE staining of B cells (Figure 3a, left panel). As IgE was always in excess over allergen in this experiment, anti-IgE staining did not increase when the concentration of Bet v 1 in the immune complexes was increased from 0.3 to 200 pmol ml−1 (Figure 3a, right panel).
Figure 3.
(a) Increased anti-IgE staining after binding of larger immune complexes to CD23. Left panel shows FACS histograms of anti-IgE-stained EBV-B cells incubated with CB1–Bet v 1 immune complexes (dotted line) or CB1–Bet v 1 trimer immune complexes (solid line). As controls, cells were either incubated with PBS (dark gray filled), or monomeric IgE without pre-treatment (light gray filled). Right panel shows the dose dependency of anti-IgE staining for CB1–Bet v 1 trimer (solid lines) or CB1–Bet v 1 (dotted line) immune complexes. Results are displayed as means of duplicate measurements ± s.d.s. Kinetic analysis of the binding of monomeric Bet v 1 (b) or Bet v 1 trimer (c) to CB1 using the BIAcore system. CB1 was injected at two-fold increasing concentrations into the flow cells containing immobilized antigens. Recorded (gray) and calculated (black) curves were superimposed. Resonance units are displayed at the y-axes and and the time (s) at the x-axes. The calculated KD, ka and kd values were noted.
However, when we generated again under conditions of IgE-excess immune complexes using CB1 and a oligomeric form of rBet v 1 (that is, rBet v 1 trimer, containing several CB1 binding sites), we noted much stronger anti-IgE staining of B cells compared to that of B cells incubated with complexes containing monomeric Bet v 1 (Figure 3a). The intensity of anti-IgE staining strongly increased with the concentration of rBet v 1 oligomer in the immune complexes.
Increased anti-IgE staining is not due to different affinities of IgE to monomeric or oligomeric forms of Bet v 1
We next investigated if the increase in IgE-staining of B cells observed with immune complexes consisting of CB1 and oligomeric Bet v 1 was related to differences in binding affinities of CB1 to rBet v 1 monomer versus rBet v 1 trimer using surface plasmon resonance. The kinetics of the interaction was determined from the calculated rates of complex formation (ka) and dissociation (kd). The ka and kd of both monomeric and oligomeric Bet v 1 to CB1 were in a very similar range (~5 × 105 Ms and 8 × 10−5 s−1, respectively) (Figures 3b and c). Based on the binding curves, the interaction kinetics reflects rapid association between CB1 and Bet v 1 (monomer or trimer) and relatively slow disassociation. The strength of binding or interaction affinity (KD) was calculated as the ratio between the kinetic rate constants (kd/ka) and was found to be high (that is, 10−10 M) for both monomeric and oligomeric rBet v 1 (Figures 3b and c).
Super-crosslinking of allergen IgE immune complexes with polyclonal IgG increases anti-IgE staining of CD23-bearing B cells
As the affinity of CB1 for monomeric rBet v 1 and oligomeric Bet v 1 was similar, increased anti-IgE staining with oligomeric Bet v 1–IgE complexes may be explained by the fact that rBet v 1 trimer can simultaneously bind several IgE molecules, and thus bring them to the cells. This situation mimics the binding of polyclonal IgE recognizing several epitopes on allergens.
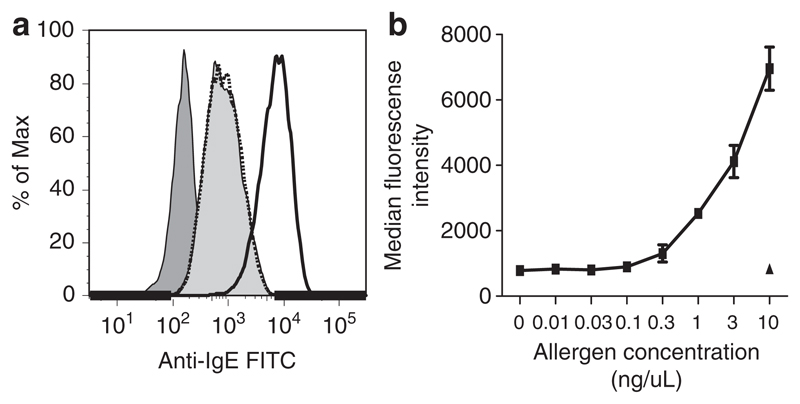
Another possibility for generating larger immune complexes would be the presence of polyclonal allergen-specific IgG antibodies that do not block IgE binding to the allergen and can, therefore, super-crosslink IgE–allergen immune complexes. In fact, when we crosslinked CB1–Bet v 1 immune complexes with anti-Bet v 1 IgG antibodies a strong increase of anti-IgE staining compared to incubation with CB1 alone was found (Figure 4a). In the presence of crosslinking rabbit anti-Bet v 1 IgG, anti-IgE staining of B cells increased strongly with increasing Bet v 1 concentration (Figure 4b). When an unrelated allergen (that is grass pollen allergen Phl p 5) was used at the highest concentration, anti-IgE staining remained at the same level as with CB1 alone (Figure 4).
Figure 4.
(a) FACS histograms of anti-IgE-stained EBV-B cells incubated with CB1–Bet v 1 immune complexes crosslinked with rabbit anti-Bet v 1 IgG (solid line), CB1 alone (light gray filled), CB1 with Phl p 5 plus rabbit anti-Bet v 1 IgG (dotted line) or PBS alone (dark gray filled). (b) dose dependency of anti-IgE staining when the concentration of Bet v 1 (filled squares) in the immune complexes with CB1 and rabbit anti-Bet v 1 IgG is increased. The filled triangle marks the intensity of anti-IgE staining for the highest concentration of Phl p 5. Results are displayed as means of duplicate measurements ±s.d.’s.
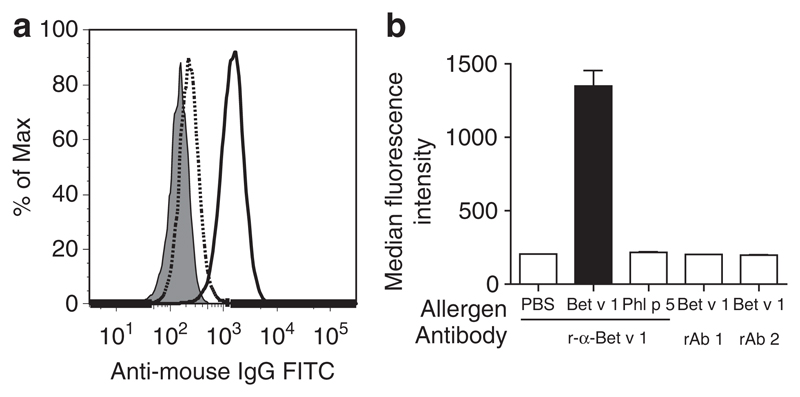
Detection of IgE that is not directly engaged in receptor binding in complexes obtained by super-crosslinking of allergen–IgE immune complexes with polyclonal IgG
We next sought to investigate whether all of the IgE present in larger immune complexes is engaged in binding to CD23 on the B cells. For this purpose, we incubated B cells with immune complexes formed using CB1 and monomeric Bet v 1 followed by crosslinking with rabbit anti-Bet v 1 IgG antibodies. Next, mouse monoclonal anti-IgE antibodies that recognize the CD23-binding site of IgE, and hence discriminate between free IgE and CD23-bound were added, and anti-IgE labeling of cells was detected by FACS.
Even with the anti-IgE antibody, which does not recognize CD23-bound IgE, we observed an increase in the anti-IgE staining when CB1–Bet v 1 immune complexes that had been super-crosslinked with rabbit IgG anti-Bet v 1 were bound to the B cells (Figure 5a). This increase in staining must therefore result from the presence of CB1 in the immune complexes that are not directly engaged in receptor binding (Figure 5). When rabbit IgG antibodies without specificity for Bet v 1 were used in the interaction (rAb 1 and rAb2), the anti-IgE staining was comparable to that found for CB1 alone (Figure 5b).
Figure 5.
(a) Visualization of CB1 that is not bound to CD23 in CB1–Bet v 1–rabbit anti-Bet v 1 immune complexes. FACS histogram of EBV-B cells loaded with CB1–Bet v 1–rabbit anti-Bet v 1 immune complexes with an anti-human IgE which does not recognize CD23-bound IgE (solid line). Cells were also incubated with PBS plus CB1 plus rabbit anti-Bet v 1 (dark gray filled), or CB1 plus Phl p 5 plus rabbit anti-Bet v 1 (dotted line) as controls. (b), bar graphs showing binding of CB1 to CD23 on EBV-B cells when complexed with different allergen-antibody combinations as shown on the x-axis. Results are displayed as means of duplicate measurements ±s.d.’s.
Discussion
In this study, a molecular model system for the binding of human IgE and allergen–IgE immune complexes to B cell-bound CD23 was established. In order to study responses for a clinically relevant allergen, a human monoclonal IgE antibody specific for the major birch pollen allergen Bet v 1, CB1, was expressed and purified as a strictly monomeric species by affinity and size exclusion chromatography. CB1 showed a highly specific and dose-dependent binding to CD23 on B cells, which could be blocked with anti-CD23 or with a monoclonal anti-human IgE antibody recognizing the binding site for CD23. Immune complexes consisting of CB1 and a monomeric form of rBet v 1 (that is, small immune complexes) showed an identical binding to CD23 as was observed with CB1 alone. Strong increases of IgE staining on the surface of CD23-bearing B cells were found when IgE–allergen immune complexes were formed using a oligomeric form of rBet v 1, which contains several binding sites for CB1 (that is, large immune complexes) or when ‘small’ immune complexes were super-crosslinked with polyclonal IgG antibodies, which recognize epitopes different from those recognized by CB1. The stronger anti-IgE staining of larger immune complexes was thus owing to the fact that besides CD23-bound IgE they also contained IgE which was not directly engaged in CD23 binding, and thus was available for anti-IgE detection with antibodies directed to the CD23 binding site of IgE.
Both binding modes may be of clinical relevance. In allergic patients, the formation of larger immune complexes occurs in the presence of polyclonal IgE, which is capable of recognizing and binding different allergen epitopes, thus efficiently targeting them to effector cells. In regards to mast cells and basophils it has been observed that the intensity of degranulation increases with the number of IgE binding sites on allergens.13,14 Increased IgE-allergen binding to CD23-bearing B cells will likely cause increases in the downstream events such antigen presentation and T cell activation,15,16 and thus lead to elevated release of cytokines2,17,18 and of pro-inflammatory mediators.19–21 Using a different approach, that is, monoclonal IgE antibodies recognizing different epitopes with different affinity, similar findings were reported recently.22 However, the latter study could not unambiguously demonstrate that increases of IgE binding to CD23 can be obtained by purely oligoclonal recognition of epitopes that are recognized with the same affinity as the authors used antibodies recognizing different epitopes and with different affinities. In our study we used one monoclonal antibody that recognized the same epitope on monomeric and oligomeric Bet v 1 with similar binding affinity and we, thus, can attribute the increased binding to CD23 to multivalent recognition of the allergen.
The second mode of increased binding of immune complexes to CD23 by super-crosslinking with polyclonal allergen-specific IgG may occur in allergic patients, who also produce allergen-specific IgG that recognize epitopes on allergens distinct from those recognized by IgE, and hence can bind simultaneously to the allergen. It has been shown that such IgG antibodies occur in allergic patients even as an unwanted effect of SIT, and that such antibodies can enhance effector cell activation and allergic inflammation.23,24 On the other hand, it has been shown that the induction of allergen-specific IgG antibodies that block IgE binding to allergens is an important mechanism of successful SIT,25,26 and can downregulate the activity of allergen-specific T cells and allergic inflammation via inhibition of IgE-facilitated allergen presentation. Several studies suggest that the induction of ‘misdirected’ allergen-specific IgG antibodies that do not block IgE binding to the allergen is possible.23,24 If such ‘misdirected’ IgG is induced during SIT it may augment the binding of IgE–allergen immune complexes to CD23, and thus may even increase allergic inflammation.23 The latter mechanism may be one possible factor contributing to the failure of SIT.
Our study demonstrates different modes of binding of IgE alone and of differently sized and composed allergen–IgE immune complexes to CD23, which may modulate the allergic immune response. Besides the well-established competition of blocking IgG for IgE-facilitated allergen presentation, which downregulates allergic inflammation,8,9,11,12 we provide evidence for two modes of increased binding of IgE–allergen immune complexes to CD23 on B cells that may contribute to augmentation of allergic inflammation, and thus may be also of clinical importance.
Methods
Chimeric Bip 1, monomeric and oligomeric rBet v 1 and Epstein-Barr virus-B cells
The monoclonal human IgE specific for the major birch pollen allergen, Bet v 1, designated CB1, was engineered by grafting the mouse fragment antigen-binding region from the antibody Bip 1 onto the human IgE fragment crystallizable.27 Monomeric rBet v 1 and rBet v 1 trimer, resembling an oligomeric form of Bet v 1, were expressed in Escherichia coli and purified as described.28,29 rBet v 1 trimer but not rBet v 1 monomer contains several binding sites for CB1 and crosslinks CB1 when bound to FcεRI on basophils or mast cells and induces mediator release.13,30 Rabbit anti-Bet v 1 antibodies were obtained as described.13,31 The EBV-transformed B cells were a gift from Dr Barbara Bohle, Department of Pathophysiology and Allergy Research, Medical University of Vienna.
Size exclusion chromatography and mass spectrometric analysis of monomeric CB1
CB1 was obtained as described13,27 and subjected to an additional purification step using size exclusion chromatography to obtain a monomeric preparation. Size exclusion chromatography was performed on a Superose 6 FPLC column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The MALDI mass spectrum of CB1 was acquired in a linear mode with MALDI ToF (Microflex, Bruker, Billerica, MA, USA).
FACS-based analysis of IgE binding to CD23 on B cells
EBV-transformed B cells (105 cells per condition) were incubated with monomeric CB1 for 1 h at 4 °C. Following a washing step, the cells were blocked with blocking buffer (phosphate buffered saline containing 0.1% sodium azide, 0.5% bovine serum albumin and 20% AB serum (Lonza, Walkersville, MD, USA)) for 30 min at 4 °C. CB1 binding to CD23 was revealed by staining with FITC-labeled anti-human IgE antibody (KPL, Gaithersburg, MD, USA) using a flow cytometer (BD FACS Canto, BD Biosciences, Rockville, MD, USA). Data was analyzed using Flowjo (Tree Star, Inc., Ashland, OR, USA). Live cells were gated using forward-sideward scatter and 7-AAD (Sigma, St Louis, MO, USA) discrimination.
For inhibition of CB1 binding to B cells using anti-CD23 antibodies, the EBV-B cells were pre-incubated with 5 μg anti-human CD23 (DakoCytomation, Glostrup, Denmark) for 1 h at 4 °C, and washed prior to the addition of CB1. Equal amounts of a mouse IgG1 antibody (anti-His6-Tag, Dianova, Germany) were used as isotype control. CB1 binding was determined using FITC-labeled anti-IgE antibodies as described above.
Inhibition with anti-human IgE was done by incubating CB1 with a mouse monoclonal anti-human IgE antibody (BD Pharmingen, San Diego, CA, USA) recognizing the CD23 binding site of human IgE, for 1 h at 37 °C, before addition to B cells. CB1 binding to B cells was analyzed by staining with anti-IgE as described above. A mouse monoclonal anti-human IgG (BD Pharmingen) was used as isotype control.
Binding of IgE immune complexes to CD23 on B cells
Monomeric CB1 was incubated with increasing concentrations of monomeric rBet v 1 or rBet v 1 trimer for 1 h at 37 °C. The complexes were then exposed to B cells for 1 h at 4 °C. Following a wash and blocking step, bound CB1 was detected by FACS as described above. In another set of experiments, immune complexes were first formed between monomeric CB1 and Bet v 1 by incubation at 37 °C for 1 h, then incubated with B cells, followed by super-crosslinking with rabbit anti-Bet v 1 IgG antibodies. Bound CB1 was detected with FITC-labeled anti-human IgE antibodies. For control purposes, experiments were performed with CB1 that was either pre-incubated with a Bet v 1-unrelated allergen (that is, grass pollen allergen Phl p 5) or phosphate buffered saline.
A monoclonal mouse anti-human IgE (BD Pharmingen) recognizing the CD23 binding site of IgE was used to stain IgE antibodies in CD23-bound complexes that were not engaged in receptor binding. In these experiments mouse anti-human IgE was detected with anti-mouse IgG FITC (BD Bioscienes) for 30 min at 4 °C. Controls in these experiments were mixtures of CB1 with Phl p 5 or with phosphate buffered saline. Rabbit anti-Bet v 1 IgG antibodies were replaced with two rabbit IgG antibodies without specificity for Bet v 1 in control experiments.
Surface plasmon resonance-based assessment of affinity and interaction kinetics between monomeric or oligomeric Bet v 1 and CB1
Surface plasmon resonance measurements were performed at 25 °C on a C1 sensor chip using Biacore 2000 (GE Healthcare Biacore AB, Uppsala, Sweden). For ligand immobilization on the chips, the sensor chip surfaces in two different flow cells were activated by injection of a 1:1 mixture of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysuccinimide, followed by injection of monomeric Bet v 1 in the first cell and Bet v 1 oligomer in the second flow cell. An unrelated allergen (Phl p 5) was injected in the reference flow cell. All proteins were injected at a concentration of 20 μg ml−1. To determine association- (ka) and dissociation rate constants (kd), CB1 was diluted in HBS-EP running buffer (0.01 M HEPES, 0.15 M NaCl, 3 mm EDTA, 0.005% v/v surfactant P20, pH 7.4) and injected at two-fold dilution steps, starting from 0.25 nm to 16 nm, in random order into the flow cells. After a dissociation time of 30 min, the surface was regenerated by injection of 10 mm glycine-HCl, pH 2. Results were confirmed by repeating the experiment under the same conditions, and results were evaluated using BIAEvaluation 3.2 (GE Healthcare Biacore AB).
Acknowledgements
This study was supported by the allergy research program F46 of the Austrian Science Fund, FWF, project grants F4604, 4605, 4613 and in part by the Christian Doppler Research Association, Vienna, Austria and Biomay, Vienna, Austria.
References
- 1.Delespesse G, Suter U, Mossalayi D, Bettler B, Sarfati M, Hofstetter H, et al. Expression, structure, and function of the CD23 antigen. Adv Immunol. 1991;49:149–191. doi: 10.1016/s0065-2776(08)60776-2. [DOI] [PubMed] [Google Scholar]
- 2.Corominas M, Mestre M, Bas J, Verdaguer J, Valls A, Romeu A, et al. CD23 expression on B-lymphocytes and its modulation by cytokines in allergic patients. Clin Exp Allergy. 1993;23:612–617. doi: 10.1111/j.1365-2222.1993.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams J, Johnson S, Mascali JJ, Smith H, Rosenwasser LJ, Borish L. Regulation of low affinity IgE receptor (CD23) expression on mononuclear phagocytes in normal and asthmatic subjects. J Immunol. 1992;149:2823–2829. [PubMed] [Google Scholar]
- 4.Hakansson L, Heinrich C, Rak S, Venge P. Activation of B-lymphocytes during pollen season. Effect of immunotherapy. Clin Exp Allergy. 1998;28:791–798. doi: 10.1046/j.1365-2222.1998.00295.x. [DOI] [PubMed] [Google Scholar]
- 5.Jung CM, Prinz JC, Rieber EP, Ring J. A reduction in allergen-induced Fc epsilon R2/CD23 expression on peripheral B cells correlates with successful hyposensitization in grass pollinosis. J Allergy Clin Immunol. 1995;95:77–87. doi: 10.1016/s0091-6749(95)70155-9. [DOI] [PubMed] [Google Scholar]
- 6.Pirron U, Schlunck T, Prinz JC, Rieber EP. IgE-dependent antigen focusing by human B lymphocytes is mediated by the low-affinity receptor for IgE. Eur J Immunol. 1990;20:1547–1551. doi: 10.1002/eji.1830200721. [DOI] [PubMed] [Google Scholar]
- 7.Mudde GC, Bheekha R, Bruijnzeel-Koomen CA. Consequences of IgE/CD23-mediated antigen presentation in allergy. Immunol Today. 1995;16:380–383. doi: 10.1016/0167-5699(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 8.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–2952. [PubMed] [Google Scholar]
- 9.van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–129. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 10.Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. J Allergy Clin Immunol. 2003;112:563–570. doi: 10.1016/s0091-6749(03)01861-x. [DOI] [PubMed] [Google Scholar]
- 11.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–922. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 12.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, et al. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–79. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellge G, Laffer S, Mierke C, Vrtala S, Hoffmann MW, Klempnauer J, et al. Development of an in vitro system for the study of allergens and allergen-specific immunoglobulin E and immunoglobulin G: Fcepsilon receptor I supercross-linking is a possible new mechanism of immunoglobulin G-dependent enhancement of type I allergic reactions. Clin Exp Allergy. 2005;35:774–781. doi: 10.1111/j.1365-2222.2005.02248.x. [DOI] [PubMed] [Google Scholar]
- 14.Gieras A, Focke-Tejkl M, Ball T, Verdino P, Hartl A, Thalhamer J, et al. Molecular determinants of allergen-induced effector cell degranulation. J Allergy Clin Immunol. 2007;119:384–390. doi: 10.1016/j.jaci.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijden FL, van Neerven RJ, Kapsenberg ML. Relationship between facilitated allergen presentation and the presence of allergen-specific IgE in serum of atopic patients. Clin Exp Immunol. 1995;99:289–293. doi: 10.1111/j.1365-2249.1995.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Heijden FL, Joost van Neerven RJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150:3643–3650. [PubMed] [Google Scholar]
- 17.Ezeamuzie CI, Al-Attiyah R, Shihab PK, Al-Radwan R. Low-affinity IgE receptor (FcepsilonRII)-mediated activation of human monocytes by both monomeric IgE and IgE/anti-IgE immune complex. Int Immunopharmacol. 2009;9:1110–1114. doi: 10.1016/j.intimp.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Gosset P, Tillie-Leblond I, Oudin S, Parmentier O, Wallaert B, Joseph M, et al. Production of chemokines and proinflammatory and antiinflammatory cytokines by human alveolar macrophages activated by IgE receptors. J Allergy Clin Immunol. 1999;103:289–297. doi: 10.1016/s0091-6749(99)70504-x. [DOI] [PubMed] [Google Scholar]
- 19.Paul-Eugene N, Kolb JP, Abadie A, Gordon J, Delespesse G, Sarfati M, et al. Ligation of CD23 triggers cAMP generation and release of inflammatory mediators in human monocytes. J Immunol. 1992;149:3066–3071. [PubMed] [Google Scholar]
- 20.Becherel PA, Mossalayi MD, Ouaaz F, Le Goff L, Dugas B, Paul-Eugene N, et al. Involvement of cyclic AMP and nitric oxide in immunoglobulin E-dependent activation of Fc epsilon RII/CD23+ normal human keratinocytes. J Clin Invest. 1994;93:2275–2279. doi: 10.1172/JCI117227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palaniyandi S, Tomei E, Li Z, Conrad DH, Zhu X. CD23-dependent transcytosis of IgE and immune complex across the polarized human respiratory epithelial cells. J Immunol. 2011;186:3484–3496. doi: 10.4049/jimmunol.1002146. [DOI] [PubMed] [Google Scholar]
- 22.Holm J, Willumsen N, Wurtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J Allergy Clin Immunol. 2011;127:1029–1037. doi: 10.1016/j.jaci.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 23.Visco V, Dolecek C, Denepoux S, Le Mao J, Guret C, Rousset F, et al. Human IgG monoclonal antibodies that modulate the binding of specific IgE to birch pollen Bet v 1. J Immunol. 1996;157:956–962. [PubMed] [Google Scholar]
- 24.Flicker S, Steinberger P, Eibensteiner PB, Lebecque S, Kraft D, Valenta R. Molecular characterization of a human immunoglobulin G4 antibody specific for the major birch pollen allergen, Bet v 1. Clin Exp Allergy. 2008;38:365–373. doi: 10.1111/j.1365-2222.2007.02883.x. [DOI] [PubMed] [Google Scholar]
- 25.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 26.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 27.Laffer S, Hogbom E, Roux KH, Sperr WR, Valent P, Bankl HC, et al. A molecular model of type I allergy: identification and characterization of a nonanaphylactic anti-human IgE antibody fragment that blocks the IgE-FcepsilonRI interaction and reacts with receptor-bound IgE. J Allergy Clin Immunol. 2001;108:409–416. doi: 10.1067/mai.2001.117593. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann-Sommergruber K, Susani M, Ferreira F, Jertschin P, Ahorn H, Steiner R, et al. High-level expression and purification of the major birch pollen allergen, Bet v 1. Protein Expr Purif. 1997;9:33–39. doi: 10.1006/prep.1996.0671. [DOI] [PubMed] [Google Scholar]
- 29.Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. Faseb J. 2001;15:2045–2047. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- 30.Campana R, Vrtala S, Maderegger B, Dall’Antonia Y, Zafred D, Blatt K, et al. Altered IgE epitope presentation: a model for hypoallergenic activity revealed for Bet v 1 trimer. Mol Immunol. 2011;48:431–441. doi: 10.1016/j.molimm.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrtala S, Akdis CA, Budak F, Akdis M, Blaser K, Kraft D, et al. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J Immunol. 2000;165:6653–6659. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]