Abstract
Studies of language disorders have shaped our understanding of brain–language relationships over the last two centuries. This article provides a review of this research and how our thinking has changed over the years regarding how the brain processes language. In the 19th century, a series of famous case studies linked distinct speech and language functions to specific portions of the left hemisphere of the brain, regions that later came to be known as Broca’s and Wernicke’s areas. One hundred years later, the emergence of new brain imaging tools allowed for the visualization of brain injuries in vivo that ushered in a new era of brain-behavior research and greatly expanded our understanding of the neural processes of language. Toward the end of the 20th century, sophisticated neuroimaging approaches allowed for the visualization of both structural and functional brain activity associated with language processing in both healthy individuals and in those with language disturbance. More recently, language is thought to be mediated by a much broader expanse of neural networks that covers a large number of cortical and subcortical regions and their interconnecting fiber pathways. Injury to both grey and white matter has been seen to affect the complexities of language in unique ways that have altered how we think about brain–language relationships. The findings that support this paradigm shift are described here along with the methodologies that helped to discover them, with some final thoughts on future directions, techniques, and treatment interventions for those with communication impairments.
Keywords: Aphasia, acquired, Acquired language disorders, Neuroimaging, Neuropsychology, Neuroscience, Cognitive neuroscience
INTRODUCTION
Language is a skill that most of us take for granted. We use it automatically and, for the most part, effortlessly, and can enjoy participating in conversations without having to think about the rapid computations that occur in our brains as we speak. It allows us to have our own unique form of social interaction and facilitates our ability to solve complex problems. It allows us to write our ideas down on paper and read what others have said, even over distant locations or periods of time. Language is, in fact, a significant part of what makes us human.
When language is taken away from us—as can happen after a brain injury—we are forced to realize how valuable it is and how much we depend on it in our everyday lives. We begin to understand how complicated language is, the many different ways in which it can be affected, and how difficult it is to regain once it is lost. Thus, the study of language disorders after an injury to the brain has helped us to understand more about the building blocks of human language, how these change after injury, and hopefully, how we can use this information to assist those who have been afflicted.
In discussing language disorders, it is important to distinguish them from disorders of “speech”. For this review, the term “language” will refer to a core system that enables the user to assign or decode a symbol (word, sign, or other form of linguistic label) to an object or concept they want to convey or comprehend, and also to apply the appropriate grammatical rules to arrange or decipher phrases and sentences. In this definition, language is thus the “engine” of communication, without which the elaborated sharing of verbal information cannot take place. When language is disrupted in this way as a result of injury to the brain, we refer to this as “aphasia”. On the other hand, “speech” is defined here as the mechanism by which language is orally expressed and constitutes how an utterance is ultimately articulated. Some individuals may have a motor speech deficit that makes it difficult to produce words (e.g., some form of dysarthria) or to coordinate complex movements for articulation (i.e., apraxia of speech).
HISTORICAL STUDIES OF LANGUAGE DISORDERS AND THE BRAIN
Many textbooks suggest that the study of brain and language disorders began in the 19th century, but, in fact, the field has a long and scholarly past. The oldest records go back to the ancient Egyptians around 3000 B.C. with a papyrus described by Edwin Smith. In hieroglyphics, a head-injured man is described who became “speechless” when pressure was applied to the area of his injury. The ancient Greeks also wrote accounts of speech loss after brain injury and further associated these with a paralysis on the right side of the body (Corpus Hippocraticum).
Despite numerous intervening reports over the centuries, Pierre Paul Broca gets credit for first associating language with a specific region of the brain (Paul Broca, 1861b). While working as a surgeon at a long-term care institution outside of Paris, Broca encountered a patient who could only produce the utterance “tan, tan” each time he attempted to speak. At autopsy, Broca observed an area of softening in the inferior frontal gyrus. Several months later, Broca saw another patient with a similar disorder who also had damage to the inferior frontal gyrus. At that point, Broca concluded that “the integrity of the third frontal convolution (and perhaps of the second) seems indispensable to the exercise of the faculty of articulate language.” (Paul Broca, 1861a).
With additional cases, Broca realized the injury was always on the left side, thus establishing the lateralization of the disorder (Broca, 1865). This region later became known as Broca’s area, although for many years, numerous reports were published that contradicted Broca’s finding (see Dronkers, Plaisant, Iba-Zizen, & Cabanis, 2007). It was Broca’s impression that his patients understood everything that was said to them, which is why he objected to Trousseau coining the term “aphasia” (or loss of language; Broca, 1864). Nevertheless, the term prevailed and the disorder Broca described was later referred to as “Broca’s aphasia”.1
Thirteen years after Broca’s original publications, Carl Wernicke described two cases of a very different kind of aphasia that involved comprehension deficits and fluent speech without the production difficulties described by Broca (Wernicke, 1874). Such cases had been described before, but based on the autopsy results of one of these patients, Wernicke attributed the comprehension deficits to the posterior portion of the left superior temporal gyrus, a region that later came to be known as Wernicke’s area. Wernicke incorporated Broca’s findings with his own cases and proposed a novel model of language processing that included the notion that language functions could also be disrupted after injury to the connecting pathways (e.g., the arcuate fasciculus) that transferred information from Wernicke’s to Broca’s areas. Wernicke’s model of language, later modified by Lichtheim (1885) and renewed by Geschwind (1965), forms the basis of most models of language published in textbooks today.
This classic model was not always held in favor, however, as many additional models were proposed with the idea that aphasia was caused by a more generalized impairment (e.g., Freud, 1891; Goldstein, 1948; Head, 1926; Jackson, 1878, 1879; Lashley, 1950). The Russian neuropsychologist, Alexandre Luria, had the most detailed alternative account, describing different components or “factors” of cognitive functions that could be localized in the brain that, when damaged, led to constellations of different syndromes including aphasia. His work, compiled over many years of observations, included diagrams of the areas of the skull that were penetrated by gunshot wounds acquired during World War II (Luria, 1947, 1966). Luria’s many works form the model still used by neuropsychologists and speech pathologists throughout Russia today.
At the same time that Geschwind was modifying the Wernicke-Lichtheim model of aphasia in the mid-20th century, the fields of speech-language pathology, neuropsychology, and behavioral neurology were growing and changing the way that aphasia was described and treated. Several new language assessment tools were developed that enabled clinicians to assess the extent of the disorder so that diagnosis and treatment could be improved (Porch, 1967; Schuell, 1965). The Boston Diagnostic Aphasia Examination (BDAE; Goodglass & Kaplan, 1972) provided profiles for classifying the different types of aphasia, incorporating both clinical and linguistic features. The Western Aphasia Battery (WAB; Kertesz, 1982) later added a numeric scoring system to facilitate patient classification. To assess more specific deficits, specialized tests were developed, such as the Boston Naming Test, designed to examine naming deficits across a range of difficulty, further teasing out the nature of the disorder by examining the quality of the responses given (Kaplan, Goodglass, & Weintraub, 1983).
Linguistic analyses of language disorders also began to emerge around the middle of the 20th century, providing a new perspective on language organization. Zurif, Caramazza, and Meyerson (1972) made the important observation that patients with Broca’s aphasia do not, in fact, understand everything, but rather have difficulty understanding sentences containing complex grammatical constructions. By association, Broca’s area came to be known as a “center” for syntactic processing. Similar attempts at localization were investigated in other linguistic domains, such as morphology, lexical-semantics, phonetics, and phonology.
Around this time, the emergence of new neuroimaging techniques became key in defining the brain areas that might be ascribed to such language disorders, whether they were clinical diagnoses or linguistic descriptions. The early computerized tomographic (CT) scans of the 1970s had relatively low spatial resolution but were instrumental in delineating the location of brain injuries in vivo, unlike the autopsy studies or skull penetration research that had been the cornerstone of localizationist theories. The Wernicke-Lichtheim model was now being called back into question as investigators were finding that brain–behavior relationships such as the association between Broca’s area and Broca’s aphasia were not as clear as once thought (e.g., Mohr, 1976), nor were the boundaries of these classic brain regions well delineated (e.g., Bogen & Bogen, 1976).
Thus, by the early 1980s, improved descriptions of language were added to theoretical models of brain–behavior relationships, along with better assessment tools and new ways to view the brain after injury. The predominate theories were essentially serial processing models in which information passed from Wernicke’s area to Broca’s area by way of the fiber tract that connected them. The precise functions of these two areas was still under debate, a discussion that would continue well into the next decades.
LANGUAGE RESEARCH IN THE LATE 20TH CENTURY AND BEYOND
Neuroimaging
The biggest advance in understanding brain-language relationships and language disorders was triggered by remarkable advancements in computer technology and computer-based neuroimaging in the late 20th century, allowing researchers to visualize brain structures in three dimensions with high resolution and to monitor functional brain activity via blood flow and metabolism. These tools led to an eruption of innovative new ideas that expanded our understanding of the linguistic brain into a broader network with sub-networks and extensive interconnections. Below, we organize these discoveries in terms of the tools that were used with brain-injured individuals to investigate the brain basis of language.
Lesion-symptom mapping
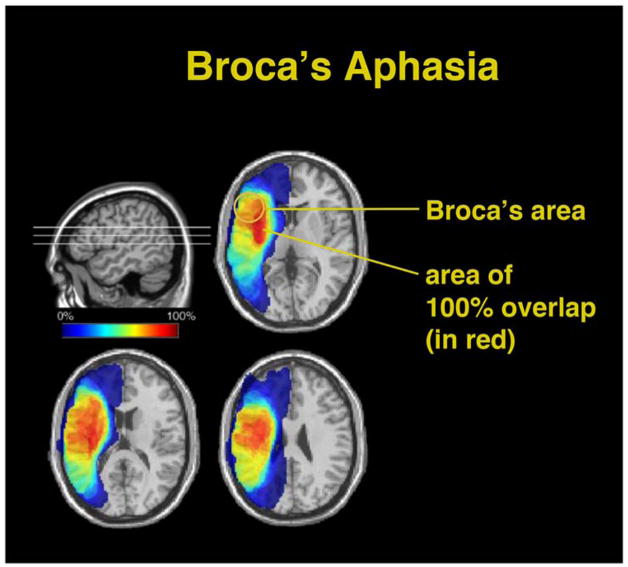
Using computerized reconstructions of patients’ lesions based on CT and MRI scans, we are now able to transform an individual patient’s imaging data into standard neuroimaging space, so that the lesions of large groups of patients can be directly compared. Reconstructed lesions of patients who share the same disorder or syndrome can be superimposed on each other to determine if a common area of overlap can be found. Generally, studies using these techniques have confirmed that aphasia syndromes do not localize to small, discrete regions of the brain such as Broca’s or Wernicke’s areas (e.g., Dronkers & Ludy, 1998; Kertesz, Harlock, & Coates, 1979; Naeser & Hayward, 1978). For example, patients with a chronic Broca’s aphasia (> 6 months) have lesions in Broca’s area only approximately 75% of the time (Dronkers & Baldo, 2010; see Figure 1). In fact, small lesions that affect only Broca’s area lead to a transient mutism that resolves in 3 to 6 weeks (Dronkers, Redfern, & Knight, 2000; Penfield & Roberts, 1959). This mutism certainly reflects a contribution of Broca’s area to speech production, but the rapid recovery indicates that Broca’s area is only one part of a more complicated system.
Fig. 1.
Overlapping of lesions in 36 individuals with Broca’s aphasia persisting longer than one year. The area of 100% overlap (shown in red) is large and encompasses structures beneath the pars opercularis and triangularis, the region collectively known as Broca’s area. Broca’s area is only lesioned in approximately 75% of these cases. (Adapted from Dronkers & Baldo, 2010.)
In fact, lesions producing long-lasting Broca’s aphasia are typically large and involve neighboring tissue of the inferior and middle frontal gyri, insula, basal ganglia, and surrounding white matter (see Figure 1). Injury to these structures each produce different symptoms that ultimately contribute to the overall disorder. For example, one symptom that co-exists with Broca’s aphasia is “apraxia of speech,” a problem in coordinating the different articulators necessary for speech production. Individuals diagnosed with apraxia of speech (regardless of aphasia type) have a common area of infarction in the superior pre-central gyrus of the insula (Dronkers, 1996).
Importantly, individuals without apraxia of speech have lesions that completely spare the superior pre-central gyrus of the insula, despite encompassing much of the same left hemisphere territory. Apraxia of speech can occur in isolation, but is a common occurrence in cases of Broca’s aphasia. Thus, lesions associated with Broca’s aphasia are not restricted to any one area; rather, the different isolated symptoms and their associated brain regions combine together to produce a persisting syndrome such as Broca’s aphasia.
Regarding Wernicke’s area and Wernicke’s aphasia, individuals with lesions constrained to Wernicke’s area also exhibit only a transient Wernicke’s aphasia that resolves relatively quickly. Individuals with a persisting Wernicke’s aphasia may have some involvement of Wernicke’s area, but a large critical area of overlap is in the posterior middle temporal gyrus, and particularly, the underlying white matter (Dronkers & Baldo, 2010; Dronkers, Redfern, & Ludy, 1995). The involvement of white matter is key to understanding the clinical presentation of Wernicke’s aphasia and will be addressed further below.
Voxel-based mapping methods
Recently, voxel-based morphometry (VBM; see Ashburner & Friston, 2000) and voxel-based lesion symptom mapping (VLSM; Bates et al., 2003) were developed to statistically relate brain changes to clinical symptoms in progressive illnesses and in stroke, respectively. Unlike previous lesion analysis techniques, VBM and VLSM allow for the analysis of continuous data across a range of patient performance and aphasia severity, rather than simply comparing a subset of patients with and without a particular syndrome or diagnosis. In this way, these techniques are statistically very powerful as they make use of all available data and calculate statistics to test the relationship of language (or other cognitive) symptoms to discrete brain regions within each voxel (or three-dimensional (3D) pixel). VLSM and VBM techniques have further refined our understanding of the roles that specific brain regions play in supporting particular speech and language processes (see Baldo, Wilson, & Dronkers, 2012; Mechelli, Price, Friston, & Ashburner, 2005, for reviews).
The use of VBM has been particularly useful in studying patients with language deficits associated with neurodegenerative disease. Recent work using VBM in primary progressive aphasia (PPA; Mesulam, 2001), a neurodegenerative disorder that initially affects language, has revealed at least three variants of PPA (semantic, non-fluent, and logopenic), each involving different brain regions and different language profiles (Gorno-Tempini et al., 2004). Other VBM analyses (e.g., Mummery, Shallice, & Price, 1999) have corroborated earlier pioneering clinic-pathological studies implicating the anterior temporal pole in the semantic variant, also known as semantic dementia (Hodges, Patterson, Oxbury, & Funnell, 1992), specifically in the processing of semantic memory.
Other regions that have been highlighted through the use of VLSM in aphasic stroke patients include the left anterior temporal lobe for semantic production (Mirman et al., 2015). This finding is consistent with the kind of naming errors observed in neurosurgical patients undergoing resection of the left anterior temporal lobe and in those with primary progressive aphasia with anterior lobe atrophy (see below). Another VLSM study conducted in neurosurgical patients sought to tease out the different contributions to naming within the temporal lobe (Wilson et al., 2015). Those with pending anterior temporal lobe resections were found to have significant naming deficits pre-surgery, but resections in the left posterior middle temporal gyrus and posterior ventral temporal areas were most predictive of naming deficits post-surgery. Of course, these temporal areas are connected to each other via several major fiber pathways and the contribution of these pathways in supporting semantic processing is still being unraveled.
VLSM can also be used to reveal combinations of regions that support more complex language functions. For example, this technique was used to examine the left hemisphere regions necessary for the comprehension of different sentence types, ranging from simple declarative sentences (e.g., “The girl is sitting”) to more complex grammatical structures (e.g., “It’s the clown that the girl chases”; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004). Patients listened to sentences and were asked to choose the picture that best depicted the stimulus they heard. The VLSM analyses revealed five different brain regions that were important for language comprehension, but each one was associated with a different type of deficit. Lesions to the left posterior middle temporal gyrus and underlying white matter caused the most severe comprehension problems, with difficulty in understanding even the simplest sentence forms (see Figure 2a).
Fig. 2.
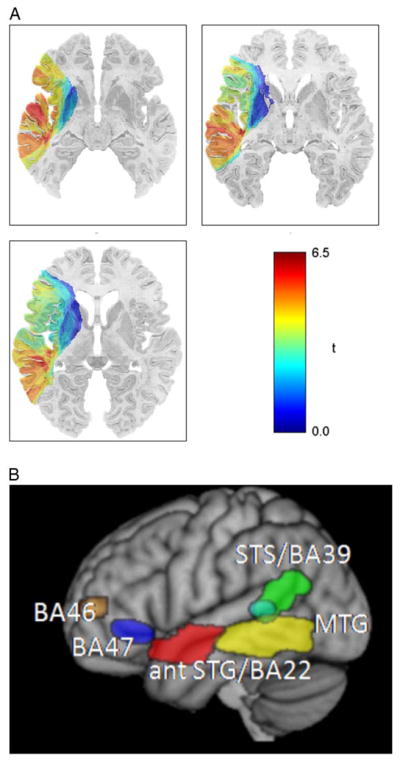
(A) VLSM plot showing positive t-values (in red) in the posterior middle temporal gyrus (pMTG), obtained by comparing patients with and without lesions at each voxel on the CYCLE-R sentence comprehension measure. Aphasic individuals with lesions in the pMTG had difficulty understanding even the simplest sentence forms. (B) Composite of all regions found to be significant on the CYCLE-R sentence comprehension measures, shown in MNI space. As the sentences became more syntactically complex, these additional regions were implicated. (Adapted from Dronkers et al., 2004, and Turken & Dronkers, 2011.)
As the sentences became more complex, numerous other processes and supporting brain regions were implicated (see Figure 2b). These results showed that many left hemisphere regions outside classical Wernicke’s area are involved in the comprehension of language, with each area becoming important depending on the complexity and demands of the comprehension task.
Recent papers have further refined these voxel-based mapping techniques by showing the importance of using non-parametric permutation-based statistics, incorporating potentially confounding covariates such as lesion volume and chronicity, accounting for limited coverage across the entire brain, using multivariate statistics, and reporting statistical maxima rather than center-of-mass results (e.g., Inoue, Madhyastha, Rudrauf, Mehta, & Grabowski, 2014; Mah, Husain, Rees, & Nachev, 2014; Rorden & Karnath, 2004; Wilson et al., 2015).
Furthermore, these techniques can be combined with white matter analysis techniques to identify both critical grey and white matter correlates of language (e.g., Baldo, Katseff, & Dronkers, 2012; Mirman et al., 2015). While functional imaging techniques reveal broad territories involved in language (see below), lesion-symptom mapping techniques such as VLSM identify brain regions that are clearly necessary for normal language functioning, for without them, patients cannot successfully communicate.
Diffusion MRI
Another significant advancement has been the use of MR diffusion imaging (dMRI) to investigate white matter connections and their role in language as well as patterns of language recovery. dMRI calculates the magnitude and direction of water diffusion for each voxel and is based on the premise that water diffuses more rapidly along the axis of large fiber bundles as compared to grey matter and cerebrospinal fluid (Alexander, Lee, Lazar, & Field, 2007). In brain injury, this information can be used to quantify microstructural tissue properties that reflect damage to these pathways, which can then be correlated with behavioral deficits.
Tractography further uses diffusion data to reconstruct white matter pathways that can then be examined for size, length, or structural integrity (Catani, Howard, Pajevic, & Jones, 2002). Reconstruction of fiber pathways based on newer acquisition strategies (e.g., high angular resolution diffusion-weighted imaging, HARDI) and more complex models (e.g., constrained spherical deconvolution, CSD) are better able to resolve such problems as fibers that cross within voxels (Tournier, Mori, & Leemans, 2011) and can provide appropriate tract-specific measurements of tract integrity (Dell’Acqua, Simmons, Williams, & Catani, 2013).
Overall, tractography based on these advanced models as well as those using the classic diffusion tensor method enables researchers to gain insights into pathways connecting cerebral language areas and their functional contributions (for reviews, see Bajada, Lambon Ralph, & Cloutman, 2015 and Dick, Bernal, & Tremblay, 2014). This revived hodological framework offers an important methodological advancement, as now DTI can reveal, in vivo, how damage to specific fiber tracts are responsible for particular deficits (for a more in-depth discussion see Catani & Mesulam, 2008).
Studies using DTI techniques and various types of tractography in patients with acquired brain damage have shown that tracts, such as the arcuate fasciculus (AF), contribute to distinct language production behaviors (Ivanova et al., 2016), particularly mapping sounds with articulatory (motor) stereotypes (Breier, Hasan, Zhang, Men, & Papanicolaou, 2008; Kümmerer et al., 2013), and the processing of complex syntax (Grossman et al., 2013; Wilson et al., 2011). Temporal tracts, such as the inferior longitudinal fasciculus (ILF), inferior frontal-occipital fasciculus (IFOF), and potentially also the uncinate fasciculus (UF) and middle longitudinal fasciculus, have been shown to be mainly involved in language comprehension (Ivanova et al., 2016; Kümmerer et al., 2013).
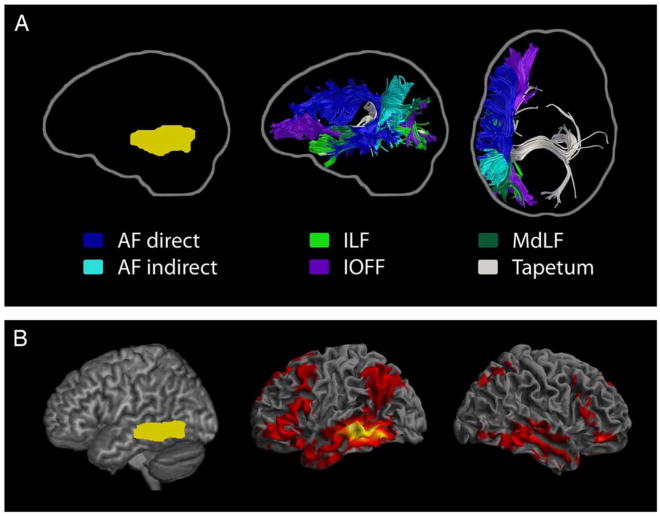
In particular, the white matter underlying the left posterior middle temporal gyrus contains branches of all the above-named tracts as well as the inter-temporal fibers of the corpus callosum (tapetum; Turken & Dronkers, 2011; see Figure 3a). Lesions beneath the left posterior middle temporal gyrus disrupt extensive connections to other areas of the brain, possibly explaining why patients with injuries to these fiber tracts have such severe and persisting language comprehension disorders.
Fig. 3.
(A) Streamline tractography of the major pathways associated with the left posterior middle temporal gyrus (pMTG; shown left in yellow). Results are shown from a neurologically-normal participant in sagittal and axial views and reflect the extensive connectivity affected by a lesion to the left pMTG. The direct and indirect segments of the arcuate fasciculus, the inferior occipito-frontal fasciculus, the middle longitudinal fasciculus, the inferior longitudinal fasciculus, and transcallosal projections, consistent with the tapetum, are shown. (B) Resting state functional connectivity profile of the left pMTG (shown left), warped to MNI stereotaxic space. The red regions in the middle and right panels correlate highly with the left pMTG. (Adapted from Turken & Dronkers, 2011.)
Such findings provide anatomical support for models of language processing that have suggested that a dorsal stream (i.e., above the Sylvian fissure) is critical for mapping speech sounds to articulation and a more ventral (i.e., temporal lobe) stream participates in mapping sound to meaning (see Bornkessel-Schlesewsky, Schlesewsky, Small, & Rauschecker, 2015; Hickok & Poeppel, 2007; Saur et al., 2008). The dorsal stream in these models would be supported primarily by the AF, responsible for the serial processing of information involved in phonological and syntactic processing and the language output system. The ventral stream would be supported by temporal lobe tracts such as the IFOF, ILF, and UF, involved in parallel processing of linguistic input critical for the lexical-semantic system.
More recent analyses of diffusion imaging data from aphasic individuals examining the functional significance of tract segments (rather than whole tracts) revealed a more complex picture, with portions of the dorsal AF and ventral ILF and IFOF associated with deficits in both language production and comprehension (Ivanova et al., 2016). As studies using diffusion imaging in brain-injured individuals evolve, the field will continue its examination of the role of fiber pathways in the processing of language.
Functional neuroimaging
While structural imaging techniques are used to evaluate the physical abnormalities of the injured brain, functional brain imaging emerged in the later 20th century as a way to study ongoing activity in both normal and atypical brains. Measurements of metabolic activity and blood flow began to contribute to the study of speech and language mechanisms, initially using tools such as positron emission tomography (PET) and single photon emission computerized tomography (SPECT). Here, a radioactive tracer is used to track glucose metabolism or blood flow, both of which are correlated with active brain tissue. In aphasia, changes have been found in both lesioned and non-lesioned brain regions, suggesting a broader dysfunctional network in aphasia (Metter et al., 1986), particularly for subcortical lesions (Démonet, Puel, Celsis, & Cardebat, 1991).
After PET, functional neuroimaging, particularly functional MRI (fMRI), soon became the pervasive method of investigating brain-behavior relationships in the healthy human brain, by measuring the amount of blood flow to regions of the brain while the participant executes a particular task. Meta-analyses of the first generation of PET and fMRI studies revealed a broad network of language areas in the left hemisphere in the neurologically normal brain that are active during language processing, including the middle and inferior temporal gyri, fusiform and angular gyri, and left prefrontal areas (e.g., Binder et al., 1997; Price, 2000). Other, unexpected findings generated from functional imaging studies included the observation of activation in the cerebellum (see Mariën et al., 2014 for a review), and the right hemisphere during linguistic and verbal cognitive tasks (Vigneau et al., 2011).
In the study of language disorders, PET and fMRI have been applied more recently to identify areas of under- and over-activity following a brain injury and how those changes relate to language deficits and recovery (Perani et al., 2003; Saur et al., 2006; Weiller et al., 1995). With respect to recovery, some studies demonstrate that right hemisphere compensation is responsible for improvement in language over time (e.g., Rosen et al., 2000), while others show perilesional activity or involvement of other undamaged areas in the lesioned hemisphere (e.g., Fridriksson, Richardson, Fillmore, & Cai, 2012). Most studies reveal a mixture of these patterns (for reviews, see Crinion & Leff, 2007; Thompson & den Ouden, 2008). Very likely, the degree of contralateral compensation and involvement of domain-general areas depends on the linguistic impact of the areas that have been injured (Geranmayeh, Brownsett, & Wise, 2014; Heiss, Kessler, Thiel, Ghaemi, & Karbe, 1999).
In addition to measurements of blood flow, event-related potentials (ERPs), extracted from electroencephalograms (EEGs), were introduced as a way of measuring the time course of language processing, rather than just its location (Kutas & Hillyard, 1980). In patients, electrophysiological measurements are also used in electrocorticography (ECoG) where electrode grids are placed on the exposed surface of the brain during neurosurgery to record seizure activity and identify epileptic foci. These direct measurements have revealed novel findings such as the representation of distinct phonetic features within the superior temporal gyrus (Mesgarani, Cheung, Johnson, & Chang, 2014) and the temporal dynamics of speech production (Flinker et al., 2015). Increasingly, depth electrodes are being used to record epileptic activity in areas not easily accessible to the neurosurgeon. In such cases, the hippocampus has been found to provide an ongoing semantic context for incoming words, adding the hippocampus to the list of regions actively involved in language processing (Piai et al., 2016).
Resting state fMRI
While task-based fMRI studies dominated the literature in the latter part of the 20th century, several unforeseen observations emerged. One of these was the observation that even during so-called “baseline” or resting tasks, when no experimental input is provided and no explicit output is required, the brain still exhibited well-coordinated and organized activity (Raichle et al., 2001). Analysis of this “resting state” activity allowed for the extraction of different networks of areas that co-activate (i.e., areas demonstrating synchronous, spontaneous low-frequency blood flow fluctuations), providing insight into the functional connectivity of the brain (Cordes et al., 2000; Smith et al., 2013).
In the normal brain, the resting-state language network has been shown to encompass not only classical language processing regions, such as Broca’s and Wernicke’s areas, but also adjoining left prefrontal, temporal and parietal regions, including some subcortical structures, such as the caudate bilaterally and the left putamen/globus pallidus and subthalamic nuclei (Muller & Meyer, 2014; Smith et al., 2013; Tomasi & Volkow, 2012). Turken and Dronkers (2011) used resting state fMRI (rsfMRI) to identify a frontal-parietal-temporal language comprehension network that was based on left hemisphere brain regions known to be affected in individuals with aphasia. This network also included right hemisphere regions, supporting the importance of right hemisphere mechanisms in language comprehension, as well (see Figure 3b).
To date, there is only a handful of rsfMRI studies involving individuals with aphasia. Overall, they reveal abnormalities in multiple resting-state networks including the language network, demonstrating reduced functional connectivity in networks in the left hemisphere and between left hemisphere networks and right hemisphere regions (Nair et al., 2015; van Hees et al., 2014a). Also, there seems to be an association between a decrease in functional connectivity in language-cognition resting-state brain networks and residual language abilities (Nair et al., 2015; Zhu et al., 2014, but see Yang et al., 2016). While these studies are clearly preliminary, they do provide another piece of evidence that language is processed by a non-modular, highly integrated and yet vastly plastic neural system, that encompasses regions well beyond the classical language areas.
As with any technique, neuroimaging analyses of brain-injured patients have their limitations. First and foremost is that injury to one area of the brain almost certainly affects other regions as well. Hypoperfusion, structural and functional disconnections to other areas, and changes within the attentional system are some of the indirect effects associated with brain injuries, even focal ones (e.g., Ivanova et al., 2016; Jarso et al., 2013; Nadeau & Crosson, 1997; Turken & Dronkers, 2011). These factors are not typically seen on standard clinical images, and additional measures need to be performed to account for such effects.
Other Advances
Other Forms of Language Disorders
The exploration of language disorders in other languages has added to our knowledge of how language can be affected by brain injuries. Through cross-linguistic research, we have seen the effects of brain injury in numerous different languages and have learned that aphasia syndromes can present quite differently depending on the typology of the language. For example, Turkish-speaking individuals with Broca’s aphasia do not produce agrammatic speech because this richly inflected language contains words with numerous prefixes and suffixes that cannot be omitted without changing the meaning the word (Bates, Wulfeck, & MacWhinney, 1991).
Nevertheless, certain “neurolinguistic universals” also exist, such as dissociations in action and object naming, even in languages that do not carry special grammatical markings (e.g., Chinese; Bates, Chen, Tzeng, Li, & Opie, 1991). Signed languages, like oral languages, is similarly affected by brain injury. Based on findings in deaf individuals with aphasia, the core components of signed languages show the same brain–language patterns seen in hearing individuals, even though the modality is different (Poizner, Klima, & Bellugi, 1990). Similarly, the study of aphasia in multilingual individuals has taught us that, while lexical items (or even writing systems) of a speaker’s different languages can be differentially affected, deficits in the core systems of language (e.g., morphosyntax, phonology) parallel those in monolinguals (Paradis, 2004).
As mentioned earlier, studies in individuals with primary progressive aphasia (PPA) add to our understanding of brain-language relationships. In particular, the semantic variant of PPA, which affects the ventral and lateral temporal lobes, causes a unique language profile affecting the naming and recognition of objects and persons (particularly low-frequency items), while the processing of grammatical information remains intact. Single word comprehension is also impaired, although the patient can repeat words and sentences without difficulty. This profile is not seen in vascular aphasia and is thought to reflect deficits in semantic memory, since the recognition deficit is consistent across all input modalities (Hodges et al., 1992; Snowden, Goulding, & Neary, 1989; Warrington, 1975).
Data from neurosurgical patients have also contributed in numerous ways. By stimulating various brain regions and subcortical connections during awake surgery, neurosurgeons can record disruptions in language thus avoiding resecting critical structures (Penfield & Roberts, 1959). Early work by Ojemann and colleagues demonstrated great individual variability in language localization, not only in classical language areas, but also in the anterior temporal and parietal lobes, thus questioning classical models.
Recently, the importance of subcortical connections was revealed with electrostimulation of the AF and IFOF, yielding speech arrest and naming difficulty with paraphasic errors (for reviews, see Chang, Raygor, & Berger, 2015; Duffau, 2014). Intraoperative mapping of neurosurgical patients has also revealed that patterns of recovery differ depending on the regions removed (Sanai, Mirzadeh, & Berger, 2008). (Ojemann, Ojemann, Lettich, & Berger, 1989). Surgical resections in temporal lobe epilepsy have also pointed to the importance of the temporal pole in supporting semantic functions (Lambon Ralph, Ehsan, Baker, & Rogers, 2012).
Linguistic approaches
In linguistic approaches to studying language disorders, the focus has shifted from adhering to a strict aphasia classification approach, to providing a detailed account of observed speech and language deficits and determining the level of breakdown within existing models of language processing (e.g., Friederici & Singer, 2015; Hagoort & Indefrey, 2014; Kay, Lesser, & Coltheart, 1996). For instance, some contemporary aphasia batteries have abandoned the aphasia classification scheme altogether (e.g., Kay, Coltheart, & Lesser, 1992; Howard, Swinburn, & Porter, 2010) and rather focus on detecting specific language deficits (e.g., Bastiaanse, Edwards, Mass, & Rispens, 2003; Cho-Reyes & Thompson, 2012).
Similarly, most current treatment studies are less focused on aphasia type or fluent/non-fluent distinctions, but rather look to observed patterns of specific linguistic deficits. These are often accompanied by measurements of neurophysiological or structural changes that accompany language gains (van Hees et al., 2014a; 2014b). Some linguistic approaches complement clinical speech-language pathology practice in which the benefits of intense treatment, group therapy, and new techniques are being investigated, sometimes in conjunction with an analysis of accompanying neural changes (e.g., Schlaug, Marchina, & Norton, 2009). While most studies have evaluated group effects, the ultimate goal is to integrate research into clinical practice to devise treatments that benefit the individual patient and their particular communication problems.
Another important contribution to research on language after brain injury concerns whether different semantic categories are selectively affected in some patients. Examples of such category-specific deficits include difficulty with word classes such as nouns versus verbs (Caramazza & Hillis, 1991) and proper versus common names (Semenza, 2009). Other studies have reported category-specific deficits in domains such as living versus non-living things (Warrington & Shallice, 1984) suggesting a more conceptual basis for these distinctions. Some authors have suggested that semantic dissociations could be related more to basic sensory-motor dimensions such as the motoric features of action names (as represented by verbs), sensory characteristics of objects (as with nouns), or the integration of visual and sensory information (as with living things) (Gainotti, Silveri, Daniel, & Giustolisi, 1995).
In fact, manipulability can account for patient deficits in naming both actions and objects, particularly when the lesion involves the motor hand cortex (Arévalo et al., 2007). This may follow for other sensorimotor domains, as well, accounting for such deficits as errors in the naming of musical instruments after lesions to left auditory cortices (Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004). These findings remind us that the brain regions involved in naming deficits span both the left and right hemispheres and well outside of the classical brain regions (Damasio et al., 2004).
Other studies in the linguistic domain include the exploration of the neural basis of such constructs as morphology, syntax, and phonology. While morphology is the system of how words are put together, syntax is the set of rules that governs how words are put together to make phrases or sentences. In recent work, aphasiologists have gone from thinking of Broca’s area as the sole location underlying syntactic processing to accepting that numerous brain regions contribute to the understanding of language, although these may each contribute in different ways, some linguistic, some non-linguistic (e.g., Dick et al., 2001; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Hickok & Poeppel, 2007; Tyler et al., 2011).
A broader distribution of areas has also been found for phonology, the sound system of language. Phonological errors in naming have been related to pre-motor cortex, pre- and postcentral gyri, and the supramarginal gyrus (Schwartz, Faseyitan, Kim, & Coslett, 2012), as well as the precentral gyrus of the insula and the arcuate and superior longitudinal fasciculi (Bates et al., 2003; Dronkers et al., 2007; Dronkers, 1996), depending on the nature of the error made (Bates et al., 2003; Dronkers et al., 2007; Dronkers, 1996; Schwartz et al., 2012).
Finally, successful communication relies on both hemispheres of the brain, not just the left. The more pragmatic aspects of language, those that provide additional context such as stress, intonation, and facial expressions, also contribute greatly to the meaning of an utterance. These tend to be more affected after right hemisphere injury, leading to impairments in social cues that complement the morphosyntactic, semantic, and phonological contributions of the left hemisphere. Other factors such as attention, emotion, insight, and the organization of information, can greatly affect how well a person interacts with their language partner.
Interaction between language and cognition
Although early aphasiologists sometimes spoke of aphasia as a loss of memory for words, the fields of language and memory diverged by the middle of the 20th century (but see Luria, 1966). More recent findings, however, have renewed an interest in the close relationship between language and other aspects of cognition. Examples of this include the famous case of H.M., who was treated for epilepsy with a bilateral temporal lobectomy that left him severely amnesic. Early research on H.M. focused on the dissociations he exhibited between implicit versus explicit memory, but later testing revealed that his deficits were not confined to the realm of memory, but also included deficits in language (MacKay, Stewart, & Burke, 1998).
Other studies emerged in the literature showing a strong relationship between language and cognition in aphasic individuals. Specifically, the degree of aphasia severity predicted impaired performance on supposedly non-verbal tasks such as problem-solving and executive functioning (Baldo, Paulraj, Curran, & Dronkers, 2015). Working memory was found to be related to language abilities, particularly comprehension, in aphasic individuals (Ivanova, Dragoy, Kuptsova, Ulicheva, & Laurinavichyute, 2015; Sung et al., 2009), and Riès and colleagues have shown the effects of cognitive control demands on word selection (Riès, Karzmark, Navarrete, Knight, & Dronkers, 2015). Such new findings strongly suggest that language cannot be studied in isolation, as it has strong connections with other brain networks supporting higher-level cognition. However, such interdependence between language and cognition does not mean that people with aphasia cannot “think” or have lost their intelligence. Even those who are most severely affected by aphasia have thoughts and ideas, can carry on with daily activities, can draw and paint, solve fundamental problems, and are most certainly are aware of their condition. The problem in aphasia is not with thought; it lies more in the ability to verbally mediate and communicate their thoughts and ideas.
Finally, we have learned that the acquired skills of reading and writing are partially dependent on the language network but also involve a unique set of critical brain regions. In the case of reading, a “visual word form area” has been identified in left posterior ventral temporo-occipital cortex that appears to be critical for single-word reading (Dehaene, 2009). Writing, on the other hand, has been alternately associated with left parietal cortex as well as left ventral temporal cortex (Lorch, 2013). Reading and writing impairments are often observed in individuals with aphasia but can also occur in isolation (alexia and agraphia, respectively). These “pure” forms of reading and writing impairment suggest a unique contribution of neural systems, distinct from the general language network.
Our own experience in studying the neural mechanisms of speech and language disorders has taught us that language is neither exclusively localized nor broadly distributed in the brain; it is a combination of both. While input or output mechanisms such as speech perception and articulation may show consistent patterns of localization, the more complex process of language involves numerous brain regions that work together to support language. Regions of the left temporal lobe (temporal pole, posterior middle temporal gyrus, and underlying white matter) contribute to the knowledge of things in our world as well as what we call them, although such information may be diffusely represented, depending on their relationships to other words, concepts, or motor and sensory functions.
For the processing of complex grammatical information, we see impairments after left frontal injury that include Brodmann’s area 47 and other regions that support executive functions, such as working memory, suggesting that these regions help with the manipulation of information that helps us to understand “who did what to whom” and to produce such constructions correctly. Thus, language also requires the contribution of areas that are more domain-general and support other functions besides just language, regions that become involved when language processing requires assistance from other cognitive systems. This includes functions such as attention, memory, perception, and pragmatics, some of which also rely on the right cerebral hemisphere.
Finally, we cannot forget the labyrinth of axonal connections that bind these structures together, providing the highways that allow the dynamics of language to happen. Thus, language does not just depend on two brain areas and one fiber tract, but is instead the result of a large network made up of constellations of neurons and neural events that contribute, as needed, to the task at hand.
FUTURE POSSIBILITIES
Predicting the future is an impossible challenge, but there are several promising approaches that will likely carry us into the next few decades. Viewing the brain as a “connectome” and language disorders as “disconnectomes” is one way of pursuing a network approach. This includes the recognition that there are both local networks and more broadly distributed ones that combine in different ways to support distinct language and cognitive functions. Thinking more about the remote effects of brain lesions as well as how grey and white matter structures mature across the lifespan will also help us to understand how these networks are developed and how they interact. Perhaps the classification of cortical areas according to their connections will become a more fruitful endeavor than thinking about the reverse relationship, as we currently do.
Megascience is certainly on the horizon with “big data” from large, multicenter studies enabling robust multivariate analyses that can be used to evaluate brain–behavior relationships on a large scale. The “Big Brain” project is already providing microscopic information on a 3D neuroanatomical reference brain, to assist with human brain mapping endeavors such as receptor mapping, fiber tracking, and gene expression (Amunts et al., 2013). Artificial intelligence models will improve with additional data, and the modeling of virtual lesions may also help us understand the effects of brain injury without depending on unfortunate accidents of nature. Techniques that offer high temporal resolution (ECoG, ERPs, and magnetoencephalography) will help settle the question of whether language operates in a serial or distributed manner and which components of language come online at which points in time.
For those working with brain-injured individuals, our greatest hope is that we will see major advances in therapies to facilitate neural recovery in the coming years. This will require a better understanding of the mechanisms of recovery as well as the development of new therapies and neural prostheses. For instance, transcranial stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (Hamilton, Chrysikou, & Coslett, 2011) have been used enhance language functions in both healthy and brain-injured individuals (Marangolo et al., 2013; Naeser et al., 2005; Schlaug, Marchina, & Wan, 2011).
The integration of research from the cellular level to the cognitive level is critical for understanding the neurobiology of recovery, such as the role of astrocytes in learning and recovery, and the chemical interactions that may spark new innovations in pharmaceutical interventions. The genetic and environmental influences on brain changes, as well as other factors (e.g., gender, handedness, motivation, intensity of therapy), have been shown to have differential impacts on outcome, and ongoing work is being done to understand how these different factors interact to predict recovery in each individual (Code, 2001).
We anticipate that future studies will continue to provide further insights into the restoration of damaged fiber tracts, the formation of novel connections, and the recruitment of other pathways not previously involved in linguistic processing. Preliminary studies using stem cells in stroke patients have also produced encouraging results with respect to both safety and tolerability of the treatment, as well as some promising improvements in motor functioning (Kalladka et al., 2016; Steinberg et al., 2016). Whether such improvements are related to the intervention or to spontaneous recovery still needs to be fully evaluated in larger, randomized clinical trials. Hopefully, stem cell studies will soon have an impact on the study of language recovery.
In the area of prosthetics, a few centers have begun to develop brain-machine interfaces to allow paralyzed individuals the ability to control mechanical devices by simply thinking. Aflalo et al. (2015) implanted quadriplegic individuals with a small grid over the parietal cortex that allows them to volitionally move a prosthetic arm. Such advances may translate to the language domain and help the speech- or language-disordered person to communicate again.
As we ponder the future, it is tempting to imagine that we might soon see the fine details of network activity in real time, and watch the process of recovery as it unfolds. Continued advances in neuroimaging will certainly assist with this progress, as will newer techniques we have not yet imagined. Still, we must keep in mind that revisiting past works and past models also has its place in new research. Finally, while all the new technologies at our disposal are tempting us to go further into the uncharted territories of the brain, it is important to remember that nothing can replace careful clinical assessments, and that our ultimate goal is to improve the quality of life for those with language disabilities.
As we move from the past into the future, we have arrived at the understanding that language is an extremely complex system that requires an extensive and interactive network of brain regions and the fibers them connect them. The areas of the brain that support language are far more extensive than Broca or Wernicke could ever have imagined, and the many fiber pathways that connect cortical regions may play an even more important role in language than previously thought. It is our hope that further advances in understanding the “Brain–Language Connectome” will provide us with the tools for understanding how language is processed in the normal brain, and for assisting our patients in their recovery from brain injury.
Acknowledgments
This work was supported in part by the U.S. Department of Veterans Affairs, Office of Research & Development CSR&D and R&D Programs and NIH/NIDCD grant 1 R01 DC016345. The contents reported within do not represent the views of the Department of Veterans Affairs or the United States Government. This article was also prepared within the framework of the Basic Research Program at the National Research University Higher School of Economics and supported within the framework of a subsidy by the Russian Academic Excellence Project “5-100”. We are grateful to the members of the Aphasia Center - And Turken, Carl Ludy, Krista Schendel, Brian Curran, Tim Herron, XJ Kang, Allison Zhong, Natalie Kacinek, Jet Vonk, and Janet Patterson - for their insightful comments and lively discussions on this topic. Special thanks go to our research participants who took the time to teach us all about language disorders. The authors state no conflicts of interest.
Footnotes
Today, it is known that most individuals with Broca’s aphasia have both language and motor speech deficits.
References
- Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, … Andersen R. Decoding motor imagery from the posterior parietal cortex of a tetraplecig human. Science. 2015;348(6237):906–910. doi: 10.7910/DVN/GJDUTV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Lepage C, Borgeat L, Mohlberg H, Dickscheid T, Rousseau M, … Evans A. BigBrain: An ultrahigh-resolution 3D human brain model. Science. 2013;21:1472–1475. doi: 10.1126/science.1235381. [DOI] [PubMed] [Google Scholar]
- Arévalo A, Perani D, Cappa S, Butler A, Bates E, Dronkers N. Action and object processing in aphasia: From nouns and verbs to the effect of manipulability [corrected] [published erratum appears in Brain Lang 2007;102:284] Brain & Language. 2007;100(1):79–94. doi: 10.1016/j.bandl.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. NeuroImage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bajada CJ, Lambon Ralph MA, Cloutman LL. Transport for Language South of the Sylvian Fissure: The routes and history of the main tracts and stations in the ventral language network. Cortex. 2015;69:141–151. doi: 10.1016/j.cortex.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26:338–354. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Paulraj SR, Curran BC, Dronkers NF. Impaired reasoning and problem-solving in individuals with language impairment due to aphasia or language delay. Frontiers in Psychology. 2015;6:1–14. doi: 10.3389/fpsyg.2015.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Wilson SM, Dronkers NF. Uncovering the neural substrates of language: A voxel-based lesion symptom mapping approach. Advances in the Neural Substrates of Language: Toward a Synthesis of Basic Science and Clinical Research. 2012:1–18. doi: 10.1002/9781118432501.ch28. [DOI]
- Bastiaanse R, Edwards S, Mass E, Rispens J. Assessing comprehension and production of verbs and sentences: The Verb and Sentence Test (VAST) Aphasiology. 2003;17(1):49–73. doi: 10.1080/729254890. [DOI] [Google Scholar]
- Bates E, Chen S, Tzeng O, Li P, Opie M. The noun-verb problem in Chinese aphasia. Brain & Language. 1991;41:203–233. doi: 10.1016/0093-934x(91)90153-r. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bates E, Wulfeck B, MacWhinney B. Cross-linguistic research in aphasia: An overview. Brain and Language. 1991;41(2):123–148. doi: 10.1016/0093-934X(91)90149-U. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen JE, Bogen GM. Wernicke’s region-Where is it? Annals of the New York Academy of Sciences. 1976;280:834–843. doi: 10.1111/j.1749-6632.1976.tb25546.x. [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M, Small SL, Rauschecker JP. Neurobiological roots of language in primate audition: Common computational properties. Trends in Cognitive Sciences. 2015;19(3):142–150. doi: 10.1016/j.tics.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR American Journal of Neuroradiology. 2008;29(3):483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Nouvelle observation d’aphémie produite par une lésion de la troisième circonvolution frontale. Bulletins de La Société D’anatomie (Paris), 2e Serie. 1861a;6:398–407. [Google Scholar]
- Broca P. Perte de la parole: Ramollissement chronique et destruction partielle du lobe anterieur gauche du cerveau. Bulletins de La Societe D’anthropologie, 1re Serie. 1861b;2:235–238. [Google Scholar]
- Broca P. Sur les mots aphemie, aphasie et aphrasie; Lettre a M. le Professeur Trousseau. Gazette Des Hopitaux. 1864 Janvier23 [Google Scholar]
- Broca P. Sur le siege de la faculte du langage articule. Bulletin de La Societe d’Anthropologie. 1865;6:337–393. [Google Scholar]
- Caramazza A, Hillis AE. Lexical organization of nouns and verbs in the brain. Nature. 1991;349(6312):788–790. doi: 10.1038/349788a0. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam MM. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: An overview for neurosurgeons. Journal of Neurosurgery. 2015;122:250–261. doi: 10.3171/2014.10.JNS132647. [DOI] [PubMed] [Google Scholar]
- Cho-Reyes S, Thompson CK. Verb and sentence production and comprehension in aphasia: Northwestern Assessment of Verbs and Sentences (NAVS) Aphasiology. 2012;26(10):1250–1277. doi: 10.1080/02687038.2012.693584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code C. Multifactorial processes in recovery from aphasia: Developing the foundations for a multileveled framework. Brain and Language. 2001;77(1):25–44. doi: 10.1006/brln.2000.2420. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, … Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR American Journal of Neuroradiology. 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Leff AP. Recovery and treatment of aphasia after stroke: Functional imaging studies. Current Opinion in Neurology. 2007;20(6):667–673. doi: 10.1097/WCO.0b013e3282f1c6fa. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Reading in the brain: The new science of how we read. New York, NY: Penguin Books; 2009. [Google Scholar]
- Dell’Acqua F, Simmons A, Williams SCR, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Human Brain Mapping. 2013;34(10):2464–2483. doi: 10.1002/hbm.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démonet JF, Puel M, Celsis P, Cardebat D. “Subcortical” aphasia: Some proposed pathophysiological mechanisms and their rCBF correlates revealed by SPECT. Journal of Neurolinguistics. 1991;6(3):319–344. doi: 10.1016/0911-6044(91)90025-E. [DOI] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. The language connectome: New pathways, new concepts. The Neuroscientist. 2014;20(5):453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Dick F, Bates E, Wulfeck B, Utman J, Dronkers NF, Gernsbacher M. Language deficits, localization and grammar: Evidence for a distributive model of language breakdown in aphasics and normals. Psychological Review. 2001;108(4):759–788. doi: 10.1037/0033-295x.108.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Baldo JV. Language: Aphasia. Encyclopedia of Neuroscience. 2010:343–348. doi: 10.1016/B978-008045046-9.01876-3. [DOI]
- Dronkers NF, Ludy CA. Brain lesion analysis in clinical research. In: Stemmer B, Whitaker HA, editors. Handbook of neurolinguistics. San Diego, CA: Academic Press; 1998. pp. 173–187. [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: High resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130(Pt 5):1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Redfern BB, Knight RT. The neural architecture of language disorders. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge: The MIT Press; 2000. pp. 949–958. [Google Scholar]
- Dronkers NF, Redfern BB, Ludy CA. Lesion localization in chronic Wernicke’s aphasia. Brain and Language. 1995;51(1):62–65. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duffau H. The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/j.cortex.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. Redefining the role of Broca’s area in speech. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):2871–2875. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. In: On aphasia. Stengel E, translator and editor. New York: International University Press; 1891. [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. NeuroImage. 2012;60(2):854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Singer W. Grounding language processing on basic neurophysiological principles. Trends in Cognitive Sciences. 2015;19:329–338. doi: 10.1016/j.tics.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Silveri MC, Daniel A, Giustolisi L. Neuroanatomical correlates of category-specific semantic disorders: A critical survey. Memory. 1995;3(3–4):247–263. doi: 10.1080/09658219508253153. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SLE, Wise RJS. Task-induced brain activity in aphasic stroke patients: What is driving recovery? Brain. 2014;137(Pt 10):2632–2648. doi: 10.1093/brain/awu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Goldstein K. Language and language disturbances: Aphasic symptom complexes and their significance for medicine and theory of language. New York: Grune & Stratton; 1948. [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, … Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Powers J, Ash S, McMillan C, Burkholder L, Irwin D, Trojanowski JQ. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain and Language. 2013;127(2):106–120. doi: 10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P. The neurobiology of language beyond single words. Annual Review of Neuroscience. 2014;37:347–362. doi: 10.1146/annurev-neuro-071013-013847. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and Language. 2011;118(1–2):40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head H. Aphasia and kindred disorders of speech. New York: Macmillan; 1926. No Title. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annals of Neurology. 1999;45(4):430–438. doi: 10.1002/1531-8249(199904)45:4<430::AID-ANA3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews. Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Swinburn K, Porter G. Putting the CAT out: What the comprehensive aphasia test has to offer. Aphasiology. 2010;24(1):56–74. doi: 10.1080/02687030802453202. [DOI] [Google Scholar]
- Inoue K, Madhyastha T, Rudrauf D, Mehta S, Grabowski T. What affects detectability of lesion-deficit relationships in lesion studies? NeuroImage: Clinical. 2014;6:388–397. doi: 10.1016/j.nicl.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova MV, Isaev DY, Dragoy OV, Akinina YS, Petrushevskiy AG, Fedina ON, … Dronkers NF. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex. 2016;85:165–181. doi: 10.1016/j.cortex.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Ivanova MV, Dragoy OV, Kuptsova SV, Ulicheva AS, Laurinavichyute AK. The contribution of working memory to language comprehension: Differential effect of aphasia type. Aphasiology. 2015;29:645–664. doi: 10.1080/02687038.2014.975182. [DOI] [Google Scholar]
- Jackson JH. On affectations of speech from diseases of the brain. I. Brain. 1878;1:304–330. [Google Scholar]
- Jackson JH. On affectations of speech from diseases of the brain II. Brain. 1879;2:323–356. [Google Scholar]
- Jarso S, Li M, Faria A, Davis C, Leigh R, Sebastian R, … Hillis AE. Distinct mechanisms and timing of language recovery after stroke. Cognitive Neuropsychology. 2013;30(7–8):454–475. doi: 10.1080/02643294.2013.875467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, … Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. The Lancet. 2016;388(10046):787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic assessments of language processing in aphasia (PALPA) Lawrence Erlbaum; 1992. [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic assessments of language processing in aphasia (PALPA): An introduction. Aphasiology. 1996;10(2):159–180. doi: 10.1080/02687039608248403. [DOI] [Google Scholar]
- Kertesz A. Western aphasia battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain and Language. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, … Saur D. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136(Pt 2):619–629. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;11:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Ehsan S, Baker GA, Rogers TT. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain. 2012;135(Pt 1):242–258. doi: 10.1093/brain/awr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley K. In search of the engram. Society of Experimental Biology Symposium. 1950;4:454–482. [Google Scholar]
- Lichtheim L. On aphasia. Brain. 1885;7:433–484. [Google Scholar]
- Lorch M. Written language production disorders: Historical and recent perspectives. Current Neurology and Neuroscience Reports. 2013;13:369. doi: 10.1007/s11910-013-0369-9. [DOI] [PubMed] [Google Scholar]
- Luria AR. Traumatic aphasia. The Hague; 1947. Reprinted in translation, Mouton, 1970. [Google Scholar]
- Luria AR. Higher cortical functions in man. New York: Basic Books; 1966. [Google Scholar]
- MacKay DG, Stewart R, Burke DM. H.M. Revisited: Relations between language comprehension, memory, and the hippocampal system. Journal of Cognitive Neuroscience. 1998;10(3):377–394. doi: 10.1162/089892998562807. [DOI] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137(9):2522–2531. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Calpagnano MA, Campana S, Razzano C, Caltagirone C, Marini A. tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience. 2013;7:539. doi: 10.3389/fnhum.2013.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, … Ziegler W. Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum. 2014;13(3):386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry applications of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1:1–9. doi: 10.2174/1573405054038726. [DOI] [Google Scholar]
- Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343(6174):1006–1010. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Annals of Neurology. 2001;49(4):425–432. doi: 10.1002/ana.91. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Jackson C, Kempler D, Riege WH, Hanson WR, Mazziotta JC, Phelps ME. Left hemisphere intracerebral hemorrhages studied by (F-18)- fluorodeoxyglucose PET. Neurology. 1986;36(9):1155–1162. doi: 10.1212/wnl.36.9.1155. [DOI] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, Schwartz MF. Neural organization of spoken language revealed by lesion–symptom mapping. Nature Communications. 2015;6:6762. doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP. Broca’s area and Broca’s aphasia. In: Whitaker H, editor. Studies in neurolinguistics. Vol. 1. New York: Academic Press; 1976. pp. 201–233. [Google Scholar]
- Muller AM, Meyer M. Language in the brain at rest: New insights from resting state data and graph theoretical analysis. Frontiers in Human Neuroscience. 2014;8:228. doi: 10.3389/fnhum.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Shallice T, Price CJ. Dual-process model in semantic priming: A functional imaging perspective. NeuroImage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, Crosson B. Subcortical aphasia. Brain and Cognition. 1997;58:355–402. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Hayward RW. Naeser 1978 - Lesion localization in aphasia with cranial computed tomography and the Boston Diagnostic Aphasia Exam. Neurology. 1978;28:545–551. doi: 10.1212/wnl.28.6.545. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, … Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: An open-protocol study. Brain and Language. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nair VA, Young BM, La C, Reiter P, Nadkarni TN, Song J, … Prabhakaran V. Functional connectivity changes in the language network during stroke recovery. Annals of Clinical and Translational Neurology. 2015;2(2):185–195. doi: 10.1002/acn3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. Journal of Neurosurgery. 1989;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Paradis M. A neurolinguistic theory of bilingualism. Amsterdam/Philadelphia: John Benjamins; 2004. [Google Scholar]
- Penfield W, Roberts L. Speech and brain mechanisms. Princeton, NJ: Princeton University Press; 1959. [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, … Fazio F. A fMRI study of word retrieval in aphasia. Brain and Language. 2003;85(3):357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Piai V, Anderson KL, Lin JJ, Dewar C, Parvizi J, Dronkers NF, Knight RT. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proceedings of the National Academy of Sciences of the United States of America. 2016;4:11366–11371. doi: 10.1073/pnas.1603312113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poizner H, Klima E, Bellugi U. What the hands reveal about the brain. Cambridge, MA: The MIT Press; 1990. Retrieved from https://mitpress.mit.edu/authors/howard-poizner. [Google Scholar]
- Porch BE. Porch index of communicative ability. Palo Alto, CA: Consulting Psychologists Press; 1967. [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional imaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riès SK, Karzmark CR, Navarrete E, Knight RT, Dronkers NF. Specifying the role of the left prefrontal cortex in word selection. Brain and Language. 2015;149:135–147. doi: 10.1016/j.bandl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, … Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. New England Journal of Medicine. 2008;358(1):18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher W, Schnell S, Ku D, Vry M, Umarova R, … Rijntjes M. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Annals of the New York Academy of Sciences. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Wan CY. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychology Review. 2011;21(3):288–301. doi: 10.1007/s11065-011-9181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuell H. Differential diagnosis of aphasia with the Minnesota Test. Minneapolis: University of Minnesota Press; 1965. [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135(12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza C. The neuropsychology of proper names. Mind and Language. 2009;24(4):347–369. doi: 10.1111/j.1468-0017.2009.01366.x. [DOI] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, … Van Essen DC. Functional connectomics from resting-state fMRI. Trends in Cognitive Sciences. 2013;17(12):666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J, Goulding P, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2(3):167–182. https://doi.org/MCI-Converted#101;Thesis_references-Converted#324. [Google Scholar]
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, … Schwartz NE. Clinical outcomes of transplanted modified bone marrow–derived mesenchymal stem cells in stroke. Stroke. 2016;47(7):1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JE, McNeil MR, Pratt SR, Dickey MW, Hula WD, Szuminsky NJ, Doyle PJ. Verbal working memory and its relationship to sentence-level reading and listening comprehension in persons with aphasia. Aphasiology. 2009;23(7–8):1040–1052. doi: 10.1080/02687030802592884. [DOI] [Google Scholar]
- Thompson CK, den Ouden DB. Neuroimaging and recovery of language in aphasia. Current Neurology and Neuroscience Reports. 2008;8(6):475–483. doi: 10.1007/s11910-008-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry. 2012;17(8):841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J, Mori S, Leemans a. Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine. 2011;65(6):1532–1556. doi: 10.1002/mrm.22924.Diffusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Randall B, Wright P, Devereux BJ, Zhuang J, … Stamatakis EA. Left inferior frontal cortex and syntax: Function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134(Pt 2):415–431. doi: 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees S, Mcmahon K, Angwin A, de Zubicaray G, Read S, Copland DA. A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Human Brain Mapping. 2014a;35(8):3919–3931. doi: 10.1002/hbm.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabilitation and Neural Repair. 2014b;28(4):325–334. doi: 10.1177/1545968313508654. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Jobard G, Petit L, Crivello F, … Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? NeuroImage. 2011;54(1):577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. The Quarterly Journal of Experimental Psychology. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–54. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Müller S, Bier D, … Diener HC. Recovery from wernicke’s aphasia: A positron emission tomographic study. Annals of Neurology. 1995;37(6):723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische Symptomencomplex. Breslau: Kohn and Weigert; 1874. [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, … Gorno-Tempini ML. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72(2):397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Lam D, Babiak MC, Perry DW, Shih T, Hess CP, … Chang EF. Transient aphasias after left hemisphere resective surgery. Journal of Neurosurgery. 2015;862:1–13. doi: 10.3171/2015.4.JNS141962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Li J, Li Y, Li R, Pang Y, Yao D, … Chen H. Altered intrinsic regional activity and interregional functional connectivity in post-stroke aphasia. Scientific Reports. 2016;6:24803. doi: 10.1038/srep24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Chang J, Freeman S, Tan Z, Xiao J, Gao Y, Kong J. Changes of functional connectivity in the left frontoparietal network following aphasic stroke. Frontiers in Behavioral Neuroscience. 2014;8:167. doi: 10.3389/fnbeh.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurif EB, Caramazza A, Meyerson R. Grammatical judgements of agrammatic aphasics. Neuropsychologia. 1972;10:405–417. doi: 10.1016/0028-3932(72)90003-6. [DOI] [PubMed] [Google Scholar]