Abstract
The thermal biology of ectotherms is often used to infer species' responses to changes in temperature. It is often proposed that temperate species are more cold-tolerant, less heat-tolerant, more plastic, have broader thermal performance curves (TPCs) and lower optimal temperatures when compared to tropical species. However, relatively little empirical work has provided support for this using large interspecific studies. In the present study, we measure thermal tolerance limits and thermal performance in 22 species of Drosophila that developed under common conditions. Specifically, we measure thermal tolerance (CTmin and CTmax) as well as the fitness components viability, developmental speed and fecundity at seven temperatures to construct TPCs for each of these species. For 10 of the species, we also measure thermal tolerance and thermal performance following developmental acclimation to three additional temperatures. Using these data, we test several fundamental hypotheses about the evolution and plasticity of heat and cold resistance and thermal performance. We find that cold tolerance (CTmin) varied between the species according to the environmental temperature in the habitat from which they originated. These data support the idea that the evolution of cold tolerance has allowed species to persist in colder environments. However, contrary to expectation, we find that optimal temperature (Topt) and the breadth of thermal performance (Tbreadth) are similar in temperate, widespread and tropical species and we also find that the plasticity of TPCs was constrained. We suggest that the temperature range for optimal thermal performance is either fixed or under selection by the more similar temperatures that prevail during growing seasons. As a consequence, we find that Topt and Tbreadth are of limited value for predicting past, present and future distributions of species.
This article is part of the theme issue ‘Physiological diversity, biodiversity patterns and global climate change: testing key hypotheses involving temperature and oxygen’.
Keywords: thermal performance curve, reaction norm, plasticity, Drosophila, life-history, fitness, thermal limits
1. Introduction
Temperature directly affects many biological processes, from enzymatic reactions to population growth [1–3], making environmental temperature an important abiotic determinant of fitness for most organisms [4]. The thermal biology of animals is often characterized from either thermal tolerance (the ability to survive short-term exposure to extreme temperatures) or thermal performance (quantified by measuring fitness-related traits over a range of temperatures) [4–6]. Thermal performance and thermal tolerance have both been used to predict patterns of species distribution and responses to environmental change [7–11]. However, the usefulness of these measures requires an understanding of the evolution and plasticity of thermal traits as well as a critical evaluation of how these traits vary among and within species.
It is often assumed that thermal tolerance and performance will vary predictably with the environment of terrestrial ectotherms [4,5,12]. As illustrated in figure 1a, temperate species that experience a greater range of temperatures throughout the year are predicted to tolerate colder temperatures, have a lower optimal temperature (Topt) and to maintain performance across a wider range of temperatures (a broader performance breadth, Tbreadth), compared to tropical species [4,13,14]. Empirical evidence for these predictions comes mainly from studies on tolerance of acute exposure to heat and cold stress [9,15], where temperature tolerance is measured by observing cessation of neuromuscular control and/or the onset of death [16]. These approaches have facilitated direct comparison across taxa and geographical ranges and large comparative studies and meta-analyses show that species tend to increase cold tolerance with distance from the equator [9,17,18]. Interestingly, upper thermal limits vary less across latitudes, which is consistent with less latitudinal variability in maximal environmental temperature [15,19,20] (figure 1b). Additionally, studies on ectotherms have found that tolerance of and performance at high temperatures are evolutionarily constrained in many species [19,21–23].
Figure 1.
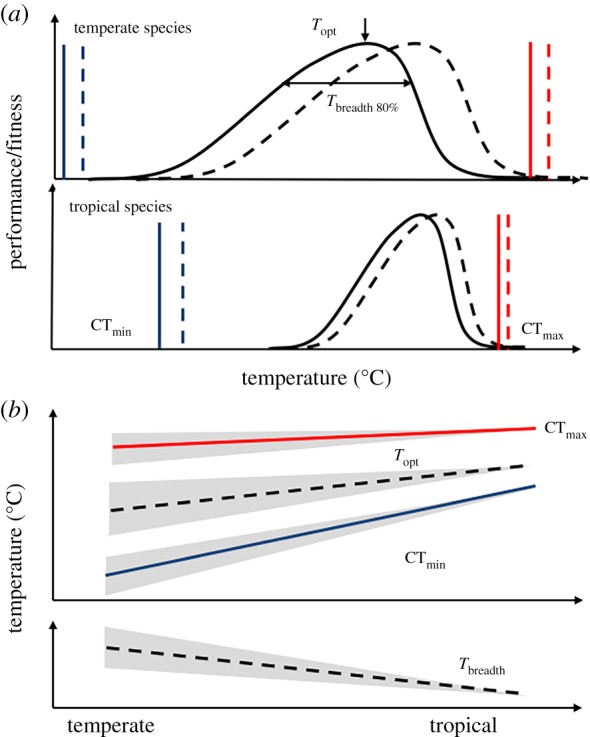
(a) Thermal performance curves (TPCs) can be characterized by an optimal temperature (Topt) and a thermal breadth (Tbreadth—defined here as the range where performance is above 80% of optimal). Thermal performance is also restricted within upper and lower tolerance limits (CTmax and CTmin, respectively) beyond which behaviour ceases. Thermal adaptation and/or acclimation can potentially shift the TPC and tolerance limits (dashed lines in (a), grey area in (b)) but the evolutionary and ecological patterns of such shifts have rarely been examined in a systematic and comparative manner. (b) Changes in CTmax that result from either acclimation or adaptation to an environmental temperature typically have no or a weak positive slope. In the case of CTmin, a strong positive slope is expected. Few studies have systematically investigated the role of acclimation/adaptation for Topt or Tbreadth but theory predicts that Topt should have a slope such that animals adapted, or acclimated (degree of plasticity represented as grey area around the slope) to warmer environments should have higher thermal optima (Topt) and a lower thermal breadth (Tbreadth). (Online version in colour.)
Thermal performance is a broad term that can be measured on many traits including metabolism, locomotion, growth rate and fecundity [6,24–28]. Because of the variety of traits and methods used to assess thermal performance, there are few directly comparable studies that can be used to investigate broad-scale patterns of adaptation in performance in terms of either thermal optima (Topt) or thermal breadth (Tbreadth) [6,29]. Moreover, it is often time- and resource-consuming to generate the data across the many experimental temperatures needed to generate high-quality thermal performance estimates—especially if more than one trait or one species is considered. The dearth of comparable studies may also reflect the practical challenges related to the generation of robust thermal performance curve (TPC) data. Despite little empirical evidence, it is generally assumed that Topt and Tbreadth will follow theoretically adaptive patterns across latitudinal and environmental gradients (figure 1).
It is often suggested that species from highly fluctuating thermal environments (temperate and sub-tropical species) exhibit greater plasticity than species from stable environments [4,12,30,31]. Species from temperate environments are therefore assumed to have larger acclimation responses that should also be reflected in more plastic TPCs (figure 1). Tests of this hypothesis are, however, mostly limited to tolerance traits (CTmin/CTmax) and comparative studies often fail to find marked evolutionary differences in plasticity across latitudinal gradients ([32–37] but see [38,39]). Despite the lack of comparative data on TPCs of ectotherms, textbook examples often depict temperate species as having greater plasticity in addition to broad TPCs and lower optimal temperatures [4,40,41] (figure 1). These patterns are based largely on theoretical work predicting that environmental variation will drive the evolution of thermal acclimation to seasonal environments [4,12,13,42,43]. On the other hand, there are many traits linked to fitness and population growth (such as reproduction, growth and developmental success) that are only relevant for parts of the yearly cycle, particularly in species that occur farther from the equator. Since summer maximal temperatures vary less across latitude, it is possible that such performance traits vary less along the temperate to tropical axis [44].
In the present study, we address these questions by measuring thermal tolerance limits and thermal performance of several fitness components (developmental viability, development speed and adult fecundity) at seven test temperatures in 22 species of Drosophila reared at a common temperature. For 10 of these species, we also measure thermal limits and thermal performance following developmental acclimation to three additional developmental temperatures. Using the interspecific data, we are able to test three a priori assumptions: that tropical species from stable warm climates (i) are less cold tolerant; (ii) have a higher Topt; and (iii) have a lower Tbreadth compared to temperate species from colder and more variable thermal environments (compare patterns in figure 1a,b). Furthermore, by examining intraspecific patterns of plasticity within species reared at different temperatures, we examine the plasticity of TPCs and test if temperate species from variable environments are more plastic in thermal tolerance or thermal performance traits (grey shaded area in figure 1b).
2. Material and methods
(a). Experimental protocol
Thermal traits were measured in 22 species of Drosophila through assessment of thermal tolerance (CTmin and CTmax) as well as thermal performance in life-history traits (using data of three fitness traits: egg-laying capacity, egg-to-adult viability and developmental speed). The 22 species span the Drosophila phylogeny and include tropical, temperate, widespread, cold-adapted and xeric species (electronic supplementary material, table S1). Flies used in this experiment originated from several laboratory stocks (electronic supplementary material, table S1) but we have recently shown that there is no significant or systematic difference in thermal tolerance and life-history traits investigated in flies recently collected in the field and laboratory stocks, respectively [45]. To obtain data, we reared 15 species under common garden conditions: density-controlled, 19°C, 12 L : 12D, on standard fly media [46]. Data for an additional 10 species were extracted from a previous study [7] with almost identical rearing conditions (electronic supplementary material, table S1). Three of the species were included in both the new dataset and the dataset from Overgaard et al. [7]. Collectively, these data allowed us to explore evolutionary and ecological patterns in thermal tolerance and in thermal performance optima and breadth.
To investigate if/how thermal acclimation affects thermal tolerance and thermal performance, we explored the role of developmental thermal acclimation for 10 of the species (Drosophila birchii, D. immigrans, D. lutescens, D. melanogaster, D. mercatorum, D. montana, D. mojavensis, D. simulans, D. subobscura and, D. yakuba, highlighted in electronic supplementary material, table S1). Each of these species was reared at 15, 19, 23 and 27°C prior to assessments of thermal performance (fecundity, viability and developmental speed) or thermal tolerance (CTmin and CTmax). Because D. subobscura and D. montana originate from cooler climates, they were acclimated to 11, 15, 19 and 23°C, respectively.
(b). Rearing of experimental animals
To produce experimental flies, we allowed parental flies to oviposit on medium and subsequently placed the eggs in vials with 7 ml standard fly medium at a density of 40 eggs/vial (typically we set up 15 vials (600 eggs), per species/acclimation group). Upon emergence, the adult flies were transferred to fresh food bottles and tipped every third day until eggs and first instar larvae were observed in the bottle. The adults were then deemed to be reproductively mature and subsequently used for experimentation to estimate critical thermal limits (CTmin or CTmax) or to test developmental speed, viability and fecundity.
(c). Thermal performance (fitness traits)
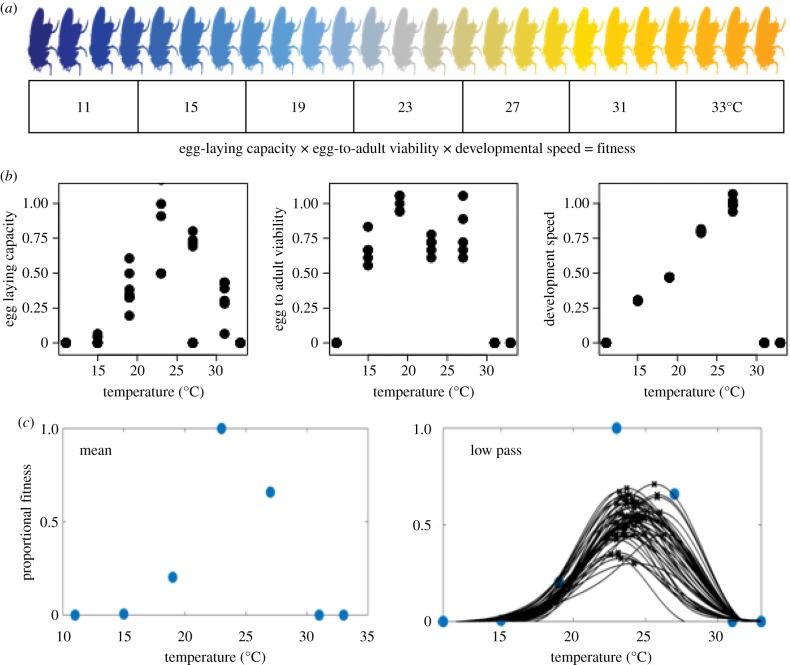
Viability and developmental speed were measured by transferring eggs laid by each species/acclimation combination to vials with 7 ml standard fly food (20 eggs per vial) and then placing the vials at one of the seven test temperatures (11, 15, 19, 23, 27, 31 and 33°C; figure 2). A minimum of five replicates per species/acclimation combination were placed at each of the test temperatures. The vials were scored daily for the number of adults that emerged to assess developmental speed (calculated as 1/time to emergence). The proportion of adults that emerged was used to determine egg-to-adult viability at each temperature (figure 2).
Figure 2.
(a) For each species (and acclimation temperature), thermal performance was measured three ways across seven test temperatures (11, 15, 19, 23, 27, 31 and 33°C): as egg-to-adult viability, developmental speed (1/developmental time in days) and fecundity measured as egg-laying. Each of these trait measurements was replicated 5 to 15 times. Each replicate was standardized to the mean maximal value of a given trait across the seven test temperatures. We generated a proxy for fitness by taking the product of each of these traits. Specifically, we subsample replicates from each trait at each test temperature (see §2f). (c) Using these data, we fit a TPC with the low pass function and estimated Topt and Tbreadth (defined as the temperature range where the fitness estimate is above 80% of the maximum value). We repeated this procedure 50 times by randomly subsampling the data. This subsampling procedure mitigates the effects of spurious variability within and between species/acclimation temperatures and enables us to obtain estimates of variability in our Topt and Tbreadth estimates. (Online version in colour.)
Fecundity was measured by placing a single mated pair of adult flies (sorted with less than 5 min of CO2, 48 h prior to experimentation) into empty 35 ml plastic vials containing a small spoon filled with 1 ml of standard fly medium. For each of the test temperatures, we used a minimum of seven and a maximum of 15 replicates per species/acclimation combination. Flies were allowed to lay eggs for 48 h. After 24 h, spoons were replaced to prevent the medium from drying out at high temperatures and flies were assessed for survival daily. In cases where one member of the pair was dead, the replicate was removed from the final analysis.
(d). Thermal tolerance (CTmin/CTmax)
Twenty mated females from each species/acclimation combination were placed in individual 5 ml glass vials with lids. The vials were then submerged in a circulating water/glycol bath at 20°C and exposed to a slow temperature-ramp down or up (rate of 0.1°C per minute) for assessment of critical thermal minimum and maximum, respectively. The temperature at which all movement ceased was recorded as the tolerance limit (CTmin or CTmax) for that individual.
(e). Environmental data
We used published environmental values calculated from the mean environmental conditions experienced across the distribution range of a given species (electronic supplementary material, table S1 and [17] for details). For the analysis, we considered the following environmental traits: latitude, mean annual temperature, the temperature of the warmest quarter, the temperature of the coldest quarter, precipitation in the wettest quarter and precipitation in the driest quarter as these have previously been identified as relevant to thermal tolerance in ectotherms.
(f). Data analysis
Egg-to-adult viability, developmental speed and fecundity were measured at each of seven test temperatures (11, 15, 19, 23, 27, 31 and 33°C), after which each fitness component was standardized to the mean maximal value possible within a given species/acclimation temperature combination (i.e. if the highest mean viability across the seven test temperatures was 80%, then viability of the six other test temperatures was normalized to this value). In this way, all trait values were between 0 and 1 for each of the three traits. Using these normalized trait values, we computed a composite fitness measure by taking the product of all three (see [7] for details). Using this estimate of composite fitness, we fit the data to estimate the optimal temperature (Topt) and the breadth of thermal performance (Tbreadth), which represents the temperature interval in which composite fitness is greater than 80% of Topt (see figures 1 and 2 and electronic supplementary material, methods for further details).
For our estimate of Topt and Tbreadth, we took the normalized trait values for each of the test temperatures and up-sampled the data to a 0.1°C spacing, using simple linear interpolation. We then smoothed the result with a zero-phase low pass finite impulse response (FIR) filter (implemented in MATLAB 2016a, The MathWorks, Inc., Mass., USA). The result was a smooth estimate of the data, sampled at 4°C intervals and then optimally up-sampled to yield a frequency resolution of 0.1°C. This low pass fit entailed no a priori assumptions on the underlying shape of the data and comparisons to Gaussian and polynomial fits were qualitatively similar (see electronic supplementary material, figures S1 and S2). In order to obtain an estimate of variance in our estimates of Topt and Tbreadth and to mitigate effects of spurious data within and between species/acclimation temperature treatments, we performed this analysis repeatedly by bootstrapping the data in MATLAB, thereby subsampling the data 50 times. Specifically, we made each of the 50 estimates by randomly sampling half (rounded up—i.e. five replicates would be sampled three times) of the available replicates per trait per test temperature (electronic supplementary material, figure S2).
(g). Adaptation in thermal performance and thermal tolerance
With 22 species distributed across the Drosophila phylogeny that differed in their distribution and environmental sensitivities, we were able to investigate the relationship between thermal tolerance/performance and the environmental conditions experienced by different species in nature. To test for evolved differences across species, we analysed the data from animals reared at a common temperature (19°C) using a generalized linear mixed model approach with either CTmin, CTmax, Topt or Tbreadth as the response variable. We then performed formal model selection using all of the environmental variables (electronic supplementary material, table S3) as predictor variables. We treated species as a random effect. For thermal performance, we nested species within resampling iteration to account for pseudo-replication generated by resampling the data. These analyses were performed in R (v. 3.3.3) using the nlme package [47]. We did not find a significant phylogenetic signal in our data (see electronic supplementary material, methods) and thus did not include phylogenetic corrections in our analysis.
The composite fitness measure reported here is based on measurements where the egg/larvae are exposed chronically to the test temperature in the assessment of egg-to-adult viability and developmental speed. Such chronic treatments do, in themselves, represent an acclimation treatment. Therefore, we performed a similar analysis of Topt by fitting a performance curve using only the data of egg-laying capacity, which represents a more acute measure of thermal performance. This analysis resulted in qualitatively similar patterns to the analysis based on composite fitness (compare electronic supplementary material, tables S3 and S4 and compare figure 3 and electronic supplementary material, figure S5).
Figure 3.
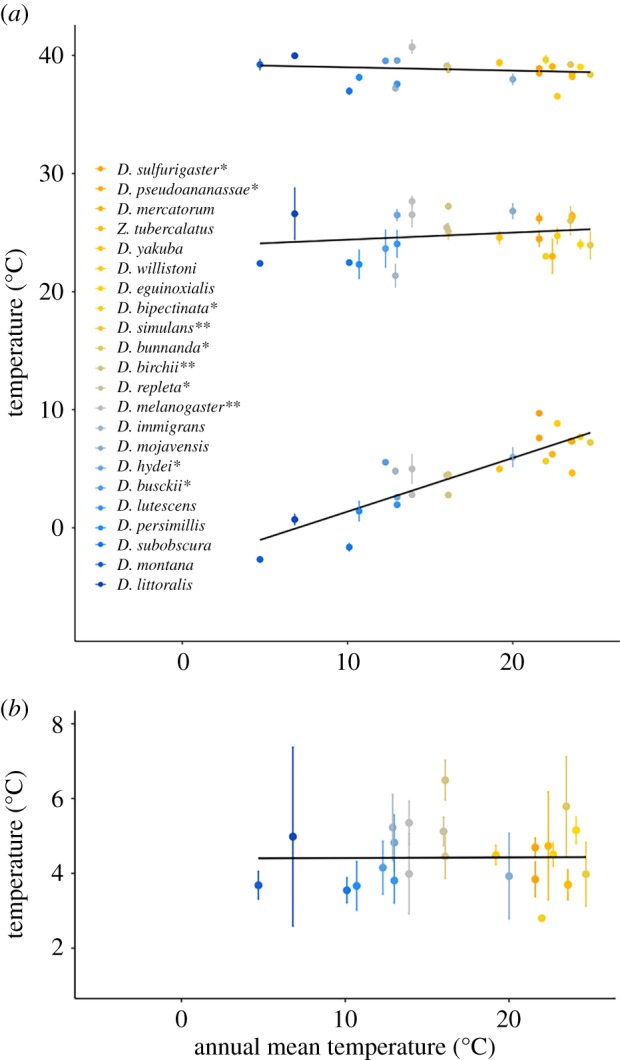
(a) The thermal limits (CTmin and CTmax) and the calculated thermal optimum (Topt) for all 22 species reared at 19/20°C as a function of annual mean temperature, showing that only CTmin exhibits a strong correlation to environmental temperature. The colour of each symbol corresponds to the mean absolute latitude of that species' range (orange for low and blue for high latitude). * indicates species included from [7] and ** indicates species included in both studies. Z. tubercalatus, Zaprionus tubercalatus. (b) The calculated breadth of thermal performance (Tbreadth) as a function of annual mean temperature shows no relationship with annual mean temperature. (Online version in colour.)
(h). Acclimation of thermal performance and thermal tolerance
For 10 species, we investigated if/how developmental acclimation affected CTmin, CTmax, Topt and Tbreadth. For this analysis, we used a generalized linear mixed model approach with either CTmin, CTmax, Topt or Tbreadth as the response variable and with species as a random effect. We nested species and acclimation within bootstrap iteration to account for pseudo-replication generated by resampling the data. We then performed formal model selection using acclimation and all of the relevant environmental variables (electronic supplementary material, table S3) as predictor variables. Additionally, we performed a similar analysis of Topt using only the data of egg-laying capacity, which revealed more short-term effects of developmental acclimation temperature (electronic supplementary material, table S4 and figure S6).
3. Results
(a). Adaptation in thermal performance and thermal tolerance
Thermal performance was quantified as both the temperature where the highest performance was obtained (Topt; figure 3a) and as the temperature range where high performance (>80% of Topt) was maintained (Tbreadth; figure 3b). Model selection suggested that mean temperature of the warmest quarter explained the highest amount of observed variance in Topt (F1,1690 = 0.034, p = 0.17) but the interspecific variance in Topt was not significantly explained by any of the climatic variables (electronic supplementary material, table S3). Tbreadth exhibited more variation within species than Topt, and similar to Topt, we failed to find significant relations with any of the climatic variables. The environmental variable that explained the highest amount of variance in Tbreadth was precipitation in the driest quarter (F1,1690 = 1.77, p = 0.09).
For thermal tolerance, model selection showed that CTmin increased significantly with the mean temperature of the coldest quarter (F1,296 = 32.97, p = 0.0007) and the explanatory power of the model was not improved by the inclusion of any of the other climatic variables (electronic supplementary material, table S3). CTmax showed no relationship to any of the climatic variables. The best model explaining variance in CTmax included the mean temperature of the warmest quarter as the sole predictor variable, but this was not significant (F1,313 = 0.03, p = 0.86). For all traits, we found that annual mean temperature represented the second-best model (for ease of comparison, we have chosen to plot all the traits against annual mean temperature in figure 3, electronic supplementary material, table S3).
(b). Acclimation of thermal performance and thermal tolerance
We considered developmental acclimation in 10 of the investigated species and did not find Topt to be correlated to acclimation temperature (F1,1690 = 0.47, p = 0.49, figure 4a). There was, however, a positive association between Topt and mean temperature of the warmest quarter in this smaller subset of species (F1,1690 = 10.21, p = 0.01, table 1). Tbreadth decreased significantly with acclimation temperature, suggesting that exposure to higher temperatures serves to decrease thermal breadth (F1,1690 = 23.97, p < 0.001, figure 4b). However, this pattern in Tbreadth was not clear at the single species level as none of the 10 species showed a significant directional response in Tbreadth (figure 4b).
Figure 4.
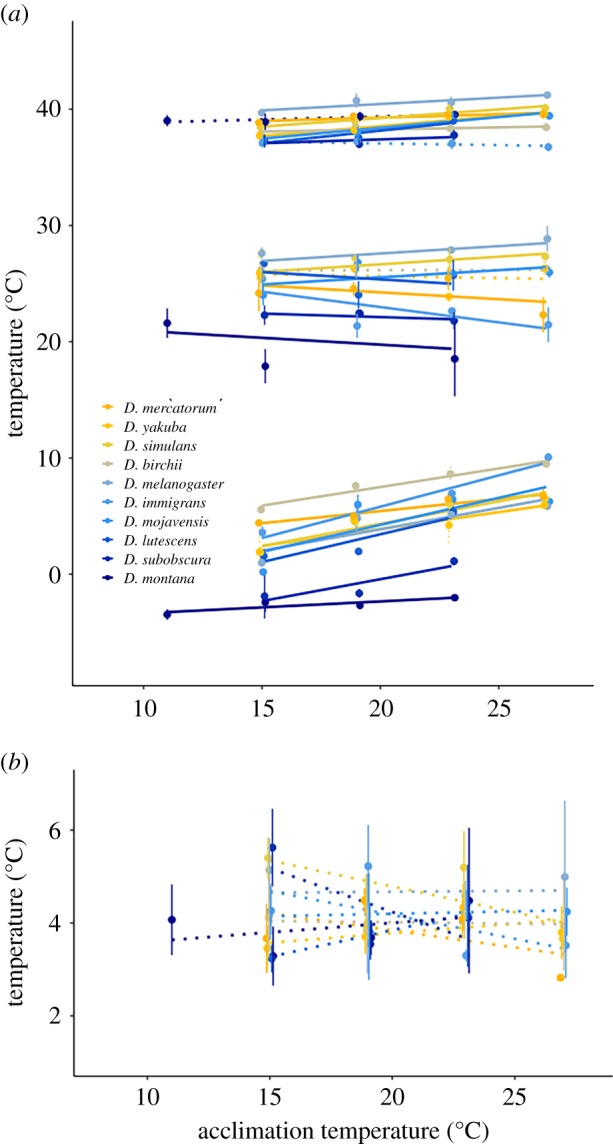
(a) The thermal limits (CTmin and CTmax) and the calculated thermal optima (Topt) for the 10 species reared at additional temperatures shown as a function of rearing temperature. The colour corresponds to the mean absolute latitude of that species’ range (orange for low and blue for high latitude), with solid lines representing a significant slope and dashed lines representing a non-significant slope. (b) The calculated breadth of thermal performance (Tbreadth) as a function of acclimation temperature.
Table 1.
Summary of mixed effects models for the evolution and acclimation of all four traits. The models with the lowest AIC values are shown in italics, additional models are the second best and directly relate to the annual mean temperatures as depicted in figures 3 and 4. AMT, annual mean temperature.
| model | est | s.e. | F-value | p-value |
|---|---|---|---|---|
| for evolution of thermal traits | ||||
| best models Topt | ||||
| optimum∼mean temp of the warmest | 0.155 | 0.108 | 2.038 | 0.167 |
| optimum∼AMT | 0.060 | 0.062 | 0.939 | 0.343 |
| best models Tbreadth | ||||
| Tb∼precip dry | 0.021 | 0.012 | 3.117 | 0.0907 |
| Tb∼AMT | 0.002 | 0.029 | 0.004 | 0.9506 |
| best models CTmin | ||||
| CTmin∼mean temp of the coldest | 0.284 | 0.049 | 32.970 | 0.0001 |
| CTmin∼AMT | 0.430 | 0.065 | 43.778 | <0.0001 |
| best models CTmax | ||||
| CTmax∼mean temp of the warmest | −0.015 | 0.080 | 0.034 | 0.8576 |
| CTmax∼AMT | 0.007 | 0.045 | 0.022 | 0.8839 |
| for plasticity of thermal traits | ||||
| best models Topt | ||||
| optimum∼acclitemp + mean temp of the warmest | ||||
| acclitemp | −0.006 | 0.008 | 0.467 | 0.4944 |
| mean temp of the warmest | 0.533 | 0.167 | 10.206 | 0.0127 |
| optimum∼acclitemp | −0.006 | 0.008 | 0.492 | 0.483 |
| best models Tbreadth | ||||
| optimum∼acclitemp | −0.024 | 0.005 | 23.972 | <0.0001 |
| best models CTmin | ||||
| CTmin∼AccTemp + mean temp of the coldest | ||||
| AccTemp | 0.314 | 0.009 | 1325.018 | <0.0001 |
| mean temp of the coldest | 0.240 | 0.082 | 8.512 | 0.019 |
| CTmin∼AccTemp + AMT | ||||
| AccTemp | 0.314 | 0.009 | 1326.229 | <0.0001 |
| AMT | 0.372 | 0.108 | 11.909 | 0.009 |
| best models CTmax | ||||
| CTmax∼AccTemp | 0.092 | 0.005 | 347.670 | <0.0001 |
For thermal tolerance traits, we found that CTmin increased with both acclimation temperature (F1,824 = 1326.54, p < 0.001) and mean temperature of the coldest quarter (F1,824 = 8.51, p = 0.019). The effect of acclimation temperature on CTmax was smaller but still significant (F1,872 = 347.56, p < 0.001). Additionally, we analysed if there was any relation between environmental variables and acclimation for any of the four traits (Topt, Tbreadth, CTmin or CTmax). Acclimation potential was calculated from the slope of the linear fits of trait values against acclimation temperature, where steep slopes indicate high plasticity. When acclimation potentials were analysed across species we found no relationship with the environmental origin, i.e. species from cold (temperate) climates did not have higher plasticity than those originating from warm (tropical) climates (electronic supplementary material, figure S4).
4. Discussion
How organisms cope with thermal variation and extreme temperature exposure is a central research topic in ecophysiology and evolutionary biology. Insects such as Drosophila spp. are often used as models and different measures of performance at different temperatures are used to explore adaptation, explain patterns of distribution and predict responses to climate change [7,9,11,19,48]. Measures obtained from such studies on thermal tolerance and performance include the ability to survive extreme temperatures (CTmin or CTmax), as well as physiological and behavioural measures of different life-history traits (characterized by Topt and Tbreadth). Numerous comparative studies have tested for an association between temperature tolerance and species' distributions. These studies typically provide support for the association between cold tolerance and species distributions (and a weak association with heat tolerance) [9,15,17–19,49]. The relationship between thermal performance measures (Topt and Tbreadth) and species distribution is less apparent. This is partially owing to the lack of large comparative datasets where the thermal performance of different species is examined under comparable conditions. Nevertheless, theory predicts that TPCs should differ considerably between tropical and temperate species, as outlined in figure 1 [4,5,12,13]. To test these predictions, the present study examined patterns of thermal performance and tolerance in 22 Drosophila species using a common garden design. Specifically, we tested the hypotheses that Topt, CTmin and CTmax are lower in species inhabiting cooler climates while thermal tolerance curves are broader (increased Tbreadth) in cold-adapted species. Further, we tested the hypothesis that developmental thermal acclimation can shift Topt, Tbreadth, CTmin and CTmax and that high latitude species from cooler climates are characterized by larger plasticity of these traits as such species have adapted to greater variability in environmental temperatures.
We find, in accordance with several earlier studies of ectotherms, that upper thermal limits (CTmax) are not significantly correlated with the average environmental temperature of the species' origin, but that cold tolerance (CTmin) is strongly correlated with the environmental temperatures that characterize the species’ geographical ranges [15,18,19,49,50]. Earlier studies including ca 100 species of Drosophila also failed to find a simple association between heat tolerance and annual mean temperature [19], but previous studies, including Kellermann et al. [19], do reveal a relationship between tolerance and the warmest environmental temperatures in Drosophila living in dry habitats. It is therefore possible that the inclusion of more xeric species in the present study would have resulted in a similar finding [19,51,52]. We observed considerably larger variation in CTmin between species and CTmin was strongly correlated to temperature (table 1, figure 2a). This result is consistent with several earlier studies of insects in general, and Drosophila specifically, which all find a strong relationship between cold tolerance and distribution range of species [9,15,18,53]. Thus, tropical species from relatively stable and warm thermal environments are considerably less cold tolerant than their temperate congeners.
Thermal performance is inherently more complex to measure and analyse than thermal limits, partly because it is difficult to identify which traits are the most appropriate to explore when assessing the relation between temperature and fitness (or population growth potential) (reviewed in [6]). There are relatively few large-scale empirical studies of TPCs because of their time-consuming nature, but directly comparable datasets are necessary in order to test theoretical expectations of thermal performance. The present study is, to our knowledge, the largest common garden examination of intraspecific patterns in Topt or Tbreadth in insects. We chose to measure and combine three aspects of fitness: rate of egg production, egg-to-adult viability and developmental speed. Each of these traits can be argued to capture major aspects of fitness (population growth potential) and by using the product of the three traits we aimed to provide a composite trait related to species fitness [7]. The use of fitness-related traits, and our composite trait in particular, offers a direct measure of population growth potential in contrast to traits such as locomotor performance or feeding rate that are connected to fitness more indirectly (i.e. feeding and locomotion are included in traits such as developmental rate and reproduction, but this is not necessarily true the other way round). There was variation within and among species in both Topt and Tbreadth, suggesting that these trait parameters do evolve; however, this variation was not correlated to any of the climatic or phylogenetic variables investigated (table 1, figure 3a; electronic supplementary material, table S2). Importantly, we do not find that temperate species have a lower Topt than tropical species, and we do not observe increased Tbreadth in temperate or widespread species as compared to tropical or restricted species. These results are in sharp contrast to the widely accepted theoretical construct which suggests that there are marked and directional patterns in both TPC shape, TPC temperature range and TPC plasticity among ectotherms (figure 1, [4,13,14]). The lack of empirical evidence for directional patterns in TPCs in the present study raises the question of whether these ‘expected’ patterns are valid representations of TPC's from species with marked differences in environmental origin. This was also questioned in two recent meta-analyses by Sørensen et al. [29] and Tüzün & Stoks [54], who explored inter- and intraspecific patterns of thermal performance among insects and other ectothermic animals. Both of these studies found some evidence of positive correlations between Topt and environmental temperature, but in both cases, this relation was weak and only explained a small fraction of the variance in Topt observed among and within species (see also discussion below).
To investigate if plasticity in TPCs is related to rearing temperature, we examined the plasticity of TPCs in 10 of the 22 species with the hypothesis that cold acclimation would shift the TPC (Topt and Tbreadth) to lower temperatures and with the additional expectation that temperate species are more plastic than tropical species from stable thermal environments. We found no support for either of these hypotheses. Developmental temperature shifted cold tolerance limits (CTmin) by approximately 0.4°C per °C of acclimation, whereas heat tolerance limits (CTmax) shifted by approximately 0.1°C per°C of change in acclimation temperature. This is consistent with previous reports of acclimation responses in thermal tolerance limits of Drosophila [34,37,55]. In contrast to the thermal tolerance limits, we observed no clear change in Topt with acclimation (table 1). There was seemingly an overall significant decrease in Tbreadth with increasing acclimation temperature, but this was very small (0.24°C shrinking of Tbreadth for a 10°C shift in acclimation temperature, table 1) and not significant for any of the 10 species when analysed individually. Furthermore, there was no relationship between the degree of plasticity in either tolerance (CTmin and CTmax) or performance (Topt and Tbreadth) traits when the species were ranked according to annual mean temperature (figure 4a; electronic supplementary material, figure S4). This lack of pattern in plasticity is consistent both when analysing our measure of composite fitness (that includes long-term exposure to the test temperatures) and when using fecundity alone (electronic supplementary material, figure S5). Accordingly, we do not find any support suggesting that temperate species have higher plasticity than tropical species. Our finding of similar plasticity among species from the temperate and tropical origin is also consistent with earlier reports from Drosophila [34,37,55,56] and is emerging as a general pattern in ectothermic animals [35], although some have found a weak but positive association between plasticity and latitude in a large meta-analysis of ectotherms [36]. Thus, we conclude that our empirical findings offer no support for the idea that distance from the equator, degree of seasonality or thermal safety margin (calculated as the difference between upper thermal limit and maximum environmental temperature) correlate with the degree of plasticity, as has been suggested elsewhere [12,13,57].
Our results contradict expectations as neither CTmax, TPCs nor plasticity of TPCs vary with latitude or thermal characteristics of origin in ways predicted from theoretical studies. This lack of empirical support raises the question of how this discrepancy can be explained. One possible explanation could relate to a putative evolutionary ‘limit’, or at least an inertial hurdle, to the evolution of CTmax and Topt [58–60]. Specifically, a lack of genetic variation may prevent adaptation and plasticity of physiological tolerance traits [61–64]. A second reason might be owing to behavioural thermoregulation causing species from different latitudes to experience more similar thermal regimes as they may show a similar preference for particular microhabitats (i.e. seeking cooler refuges in warm environments and warm refuges in cool environments) [62]. It is also possible that the theory of TPCs is valid, but that we are simply analysing the wrong ‘fitness-related traits', which compromises our conclusions. Traits related to growth and reproduction are generally more important during the summer/growth season [44], when temperatures differ less between temperate and tropical species. The species investigated in our study, for example, may experience quite similar selection pressures when it comes to the thermal performance of development and reproduction. By contrast, we do actually find marked differences in tolerance traits (CTmin) that separate the species according to the environmental origin. Perhaps this is also true for other forms of tolerance traits (i.e. immune function, desiccation tolerance, starvation tolerance at low/high temperature). It is not possible for us to discern if the theoretical patterns of TPCs are ‘wrong’ or if we are simply measuring the ‘wrong’ traits. We note, however, that most other fitness curves are also based on growth-related traits and we argue that analysis of such fitness curves should be used cautiously in modelling as they are unlikely to reveal how past, current and future climates can influence species distribution.
The present study represents the largest common garden examination of thermal tolerance and performance in insects to our knowledge. The results support the idea that the thermal tolerance limits (primarily CTmin) have evolved in response to extreme environmental conditions and that they act to limit range expansion. Moreover, those differences are reflected in species distribution, while we find no clear patterns related to optimal thermal performance. This leads us to conclude that TPCs are not particularly useful for predicting current or future species distributions. Specifically, there was no association with latitude or climatic characteristics of species distributions in thermal performance (Topt and Tbreadth) based on population growth parameters. The absence of clear shifts along environmental gradients in thermal performance among Drosophila species calls to question if this is also the case for other ectothermic animals and when testing thermal performance in the field. We argue that there should be a continued search for thermal performance traits that do differ markedly between species from different environmental origin. Identifying such traits will reveal great insights into the evolutionary adaptations that have allowed some species to occur new habitats.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Kirsten Kromand, Trine Bech Søgaard and Annemarie Højmark for help in the fly labs.
Data accessibility
Data (trait data and climatic data files) available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1t38n12 [65].
Authors' Contributions
Data were collected and analyzed by H.J.M. K.B. fitted and compared performance curves. V.K. led the phylogenetic analysis. All authors contributed to writing and editing and approved the final manuscript.
Competing interests
There are no conflicts of interest with respect to this manuscript or the reported data.
Funding
We thank the Villum Fonden (J.O., H.J.M.), the Danish Council for Independent Research (DFF-4002-00036—J.O.; DFF-8021-00014B—T.N.K.) and Aarhus University Research Foundation (J.G.S.-AUFF-E-2015-FLS-8-72), for supporting this work.
References
- 1.Schulte PM, Healy TM, Fangue NA. 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702. ( 10.1093/icb/icr097) [DOI] [PubMed] [Google Scholar]
- 2.Frazier MR, Huey RB, Berrigan D. 2006. Thermodynamics constrains the evolution of insect population growth rates: ‘warmer is better’. Integr. Comp. Biol. 168, 512–520. ( 10.1086/506977) [DOI] [PubMed] [Google Scholar]
- 3.Cossins AR, Bowler K. 1987. Rate compensations and capacity adaptations. In Temperature biology of animals, pp. 155–203. Dordrecht, Netherlands: Springer. [Google Scholar]
- 4.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Huey RB, Kingsolver JG. 1989. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 4, 131–135. ( 10.1016/0169-5347(89)90211-5) [DOI] [PubMed] [Google Scholar]
- 6.Sinclair BJ, et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372–1385. ( 10.1111/ele.12686) [DOI] [PubMed] [Google Scholar]
- 7.Overgaard J, Kearney MR, Hoffmann AA. 2014. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Glob. Chang. Biol. 20, 1738–1750. ( 10.1111/gcb.12521) [DOI] [PubMed] [Google Scholar]
- 8.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2, 686 ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 9.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B 267, 739–745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley LB, Kingsolver JG. 2012. The demographic impacts of shifts in climate means and extremes on alpine butterflies. Funct. Ecol. 26, 969–977. ( 10.1111/j.1365-2435.2012.01969.x) [DOI] [Google Scholar]
- 11.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 13.Gilchrist GW. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270. ( 10.1086/285797) [DOI] [Google Scholar]
- 14.Payne NL, Smith JA. 2017. An alternative explanation for global trends in thermal tolerance. Ecol. Lett. 20, 70–77. ( 10.1111/ele.12707) [DOI] [PubMed] [Google Scholar]
- 15.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574. ( 10.1139/z97-783) [DOI] [Google Scholar]
- 17.Kellermann V, Loeschcke V, Hoffmann AA, Kristensen TN, Fløjgaard C, David JR, Svenning J, Overgaard J. 2012. Phylogenetic constraints in key functional traits behind species' climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution 66, 3377–3389. ( 10.1111/j.1558-5646.2012.01685.x) [DOI] [PubMed] [Google Scholar]
- 18.Kimura MT. 2004. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 140, 442–449. ( 10.1007/s00442-004-1605-4) [DOI] [PubMed] [Google Scholar]
- 19.Kellermann V, Overgaard J, Hoffmann AA, Fløjgaard C, Svenning J-C, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16 228–16 233. ( 10.1073/pnas.1207553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann A, Chown S, Clusella-Trullas S, Fox C. 2012. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934–949. ( 10.1111/j.1365-2435.2012.02036.x) [DOI] [Google Scholar]
- 21.García-Robledo C, Kuprewicz EK, Staines CL, Erwin TL, Kress WJ. 2016. Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proc. Natl Acad. Sci. USA 113, 680–685. ( 10.1073/pnas.1507681113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 23.Kristensen TN, Overgaard J, Lassen J, Hoffmann AA, Sgrò C. 2015. Low evolutionary potential for egg-to-adult viability in Drosophila melanogaster at high temperatures. Evolution 69, 803–814. ( 10.1111/evo.12617) [DOI] [PubMed] [Google Scholar]
- 24.Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. 2010. Can mechanism inform species’ distribution models? Ecol. Lett. 13, 1041–1054. ( 10.1111/j.1461-0248.2010.01479.x) [DOI] [PubMed] [Google Scholar]
- 25.Diamond SE, Frame AM, Martin RA, Buckley LB. 2011. Species' traits predict phenological responses to climate change in butterflies. Ecology 92, 1005–1012. ( 10.1890/10-1594.1) [DOI] [PubMed] [Google Scholar]
- 26.Hertz PE, Huey RB, Nevo E. 1983. Homage to Santa-Anita: thermal sensitivity of sprint speed in agamid lizards. Evolution 37, 1075–1084. ( 10.2307/2408420) [DOI] [PubMed] [Google Scholar]
- 27.Kingsolver JG, Huey RB. 2008. Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268. [Google Scholar]
- 28.Schulte PM. 2015. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866. ( 10.1242/jeb.118851) [DOI] [PubMed] [Google Scholar]
- 29.Sørensen JG, White CR, Duffy GA, Chown SL. 2018. A widespread thermodynamic effect, but maintenance of biological rates through space across life's major domains. Proc. R. Soc. B 285, 20181775 ( 10.1098/rspb.2018.1775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. [Google Scholar]
- 31.Van Tienderen PH. 1991. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution 45, 1317–1331. ( 10.1111/j.1558-5646.1991.tb02638.x) [DOI] [PubMed] [Google Scholar]
- 32.Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. 2008. Phylogenetic patterns of species loss in Thoreau's woods are driven by climate change. Proc. Natl Acad. Sci. USA 105, 17 029–17 033. ( 10.1073/pnas.0806446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labra A, Pienaar J, Hansen TF. 2009. Evolution of thermal physiology in Liolaemus lizards: adaptation, phylogenetic inertia, and niche tracking. Am. Nat. 174, 204–220. ( 10.1086/600088) [DOI] [PubMed] [Google Scholar]
- 34.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 35.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Chang. 5, 61 ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 37.Sørensen JG, Kristensen TN, Overgaard J. 2016. Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: is it important for keeping up with climate change? Curr. Opin. Insect Sci. 17, 98–104. ( 10.1016/j.cois.2016.08.003) [DOI] [PubMed] [Google Scholar]
- 38.Naya DE, Catalán T, Artacho P, Gaitán-Espitia JD, Nespolo RF. 2011. Exploring the functional association between physiological plasticity, climatic variability, and geographical latitude: lessons from land snails. Evol. Ecol. Res. 13, 647–659. [Google Scholar]
- 39.Markle TM, Kozak KH. 2018. Low acclimation capacity of narrow-ranging thermal specialists exposes susceptibility to global climate change. Ecol. Evol. 8, 4644–4656. ( 10.1002/ece3.4006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreteau B, Morin J-P, Gibert P, Pétavy G, Pla É, David JR. 1997. Evolutionary changes of nonlinear reaction norms according to thermal adaptation: a comparison of two Drosophila species. Comptes Rendus l'Académie des Sci. III-Sciences la Vie 320, 833–841. ( 10.1016/S0764-4469(97)85020-2) [DOI] [PubMed] [Google Scholar]
- 41.Morin JP, Moreteau B, Pétavy G, David JR. 1999. Divergence of reaction norms of size characters between tropical and temperate populations of Drosophila melanogaster and D. simulans. J. Evol. Biol. 12, 329–339. ( 10.1046/j.1420-9101.1999.00038.x) [DOI] [Google Scholar]
- 42.Sultan SE, Spencer HG. 2002. Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271–283. ( 10.1086/341015) [DOI] [PubMed] [Google Scholar]
- 43.Levins R. 1969. Thermal acclimation and heat resistance in Drosophila species. Am. Nat. 103, 483–499. ( 10.1086/282616) [DOI] [Google Scholar]
- 44.Chown SL, Sinclair BJ, Leinaas HP, Gaston KJ. 2004. Hemispheric asymmetries in biodiversity—a serious matter for ecology. PLoS Biol. 2, e406 ( 10.1371/journal.pbio.0020406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLean HJ, Kristensen TN, Sørensen JG, Overgaard J. 2018. Laboratory maintenance does not alter ecological and physiological patterns among species: a Drosophila case study. J. Evol. Biol. 31, 530–542. ( 10.1111/jeb.13241) [DOI] [PubMed] [Google Scholar]
- 46.Kristensen TN, et al. 2015. Fitness components of Drosophila melanogaster developed on a standard laboratory diet or a typical natural food source. Insect Sci. 23, 771–779. ( 10.1111/1744-7917.12239) [DOI] [PubMed] [Google Scholar]
- 47.R Core Team. 2014. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/.
- 48.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 49.Nyamukondiwa C, Terblanche JS, Marshall KE, Sinclair BJ. 2011. Basal cold but not heat tolerance constrains plasticity among Drosophila species (Diptera: Drosophilidae). J. Evol. Biol. 24, 1927–1938. ( 10.1111/j.1420-9101.2011.02324.x) [DOI] [PubMed] [Google Scholar]
- 50.Kellermann V, van Heerwaarden B, Sgro CM, Hoffmann AA.. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246. ( 10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 51.Stratman R, Markow TA. 1998. Resistance to thermal stress in desert Drosophila. Funct. Ecol. 12, 965–970. ( 10.1046/j.1365-2435.1998.00270.x) [DOI] [Google Scholar]
- 52.Gibbs AG. 2002. Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 133, 781–789. ( 10.1016/S1095-6433(02)00208-8) [DOI] [PubMed] [Google Scholar]
- 53.Andersen JL, Manenti T, Sørensen JG, MacMillan HA, Loeschcke V, Overgaard J. 2015. How to assess Drosophila cold tolerance: chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol. 29, 55–65. ( 10.1111/1365-2435.12310) [DOI] [Google Scholar]
- 54.Tüzün N, Stoks R. 2018. Pathways to fitness: carry-over effects of late hatching and urbanisation on lifetime mating success. Oikos 127, 949–955. ( 10.1111/oik.05033) [DOI] [Google Scholar]
- 55.Schou MF, Mouridsen MB, Sørensen JG, Loeschcke V. 2017. Linear reaction norms of thermal limits in Drosophila: predictable plasticity in cold but not in heat tolerance. Funct. Ecol. 31, 934–945. ( 10.1111/1365-2435.12782) [DOI] [Google Scholar]
- 56.Kellermann V, Sgrò CM. 2018. Evidence for lower plasticity in CTMAX at warmer developmental temperatures. J. Evol. Biol. 31, 1300–1312. ( 10.1111/jeb.13303) [DOI] [PubMed] [Google Scholar]
- 57.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 58.Huey RB, Hertz PE, Sinervo B. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 161, 357–366. ( 10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 59.van Heerwaarden B, Kellermann V, Sgrò C.. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 60.Hangartner S, Hoffmann AA. 2015. Evolutionary potential of multiple measures of upper thermal tolerance in Drosophila melanogaster. Funct. Ecol. 30, 442–452. ( 10.1111/1365-2435.12499) [DOI] [Google Scholar]
- 61.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dillon ME, Wang G, Garrity PA, Huey RB. 2009. Thermal preference in Drosophila. J. Therm. Biol. 34, 109–119. ( 10.1016/j.jtherbio.2008.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckley LB, Ehrenberger JC, Angilletta MJ. 2015. Thermoregulatory behavior limits local adaptation of thermal niches and confers sensitivity to climate change. Funct. Ecol. 29, 1038–1047. ( 10.1111/1365-2435.12406) [DOI] [Google Scholar]
- 64.Gunderson AR, Leal M. 2015. Patterns of thermal constraint on ectotherm activity. Am. Nat. 185, 653–664. ( 10.1086/680849) [DOI] [PubMed] [Google Scholar]
- 65.MacLean HJ, Sørensen JG, Kristensen TN, Loeschcke V, Beedholm K, Kellermann V, Overgaard J. 2019. Data from: Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Dryad Digital Repository ( 10.5061/dryad.1t38n12) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- MacLean HJ, Sørensen JG, Kristensen TN, Loeschcke V, Beedholm K, Kellermann V, Overgaard J. 2019. Data from: Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Dryad Digital Repository ( 10.5061/dryad.1t38n12) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data (trait data and climatic data files) available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1t38n12 [65].