Autoimmune thyroid disease is caused by thyroid autoantibodies and may present with hypothyroidism, euthyroid, or hyperthyroidism (1). Thyroid antibodies include antibodies to thyroglobulin (Tg), thyroid peroxidase (TPO), thyroid stimulating hormone receptor (TSHR), sodium/iodide symporter (NIS), pendrin, and thyroid hormones (2). Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) are the two major types of autoimmune thyroid disease and share lymphocytic infiltration of the thyroid parenchyma and alteration of thyroid morphology and function (2). Many retrospective studies on surgical specimens over the last decade have shown a strong positive association between papillary thyroid cancer (PTC) risk and HT (3,4). Paradoxically, patients with PTC coexisting with HT had favorable clinical outcomes compared to those without HT (4). However, the role of GD in thyroid cancer development, growth and progression is still debated.
The tumor microenvironment surrounding cancer cells consists of fibroblasts, endothelial cells, pericytes, immune cells, and extracellular matrix components. Tumor-stroma crosstalk regulates tumor initiation and progression. Tumor-associated macrophages frequently encountered in thyroid cancer are highly heterogenous and plastic cells which can change function and express different cell phenotypes depending on the local microenvironment (5-8). Macrophages can be polarized to classically activated, anti-inflammatory macrophages (M1/kill-type) which exhibit antitumor activities. Alternatively, activated macrophages of the M2/repair type have anti-inflammatory and pro-tumoral activities and regulate wound healing under the influence of the microenvironment (1,5). Autoimmune thyroid disease reflects the loss of immunological self-tolerance and can provide a hostile immune microenvironment promoting tumor growth.
Imam et al. reported that the risk of developing thyroid cancer is relatively high in patients with euthyroid HT and relatively low in those with GD (1). They also observed the complementary actions of natural killer (NK) cells and macrophages in the background of different autoimmune thyroid diseases. The results were published in an article in the Journal for ImmunoTherapy of Cancer, January 2019 (1). The researchers studied whether the presence of HT and GD was associated with the incidence and clinical behavior of differentiated thyroid cancer (DTC). Among 2,633 patients undergoing thyroidectomies, 206 (7.8%), 576 (21.9%), and 1,851 (70.3%) had GD, HT, and non-autoimmune thyroid diseases, respectively. The incidence of DTC was significantly lower in patients with GD compared to those with HT and non-autoimmune thyroid diseases (7.8% vs. 44.4% and 32.4%, respectively). In the subgroup analysis of HT patients, patients with euthyroid HT had a higher incidence of DTC than those with hypothyroid HT (47.9% vs. 38.4%). DTC coexisting with GD showed less aggressive clinicopathologic features compared to tumors with HT and non-autoimmune thyroid disease. Low TPO antibody titers were associated with a higher risk for DTC in both the HT and GD groups.
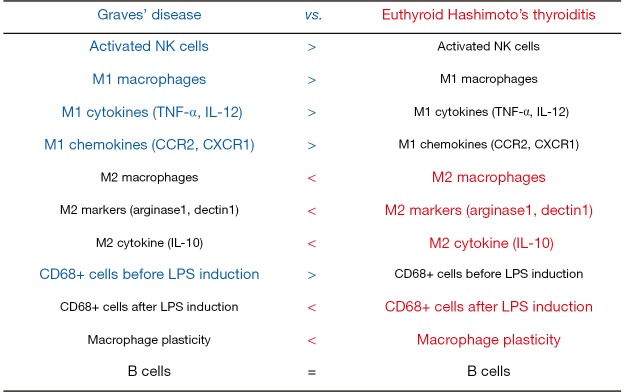
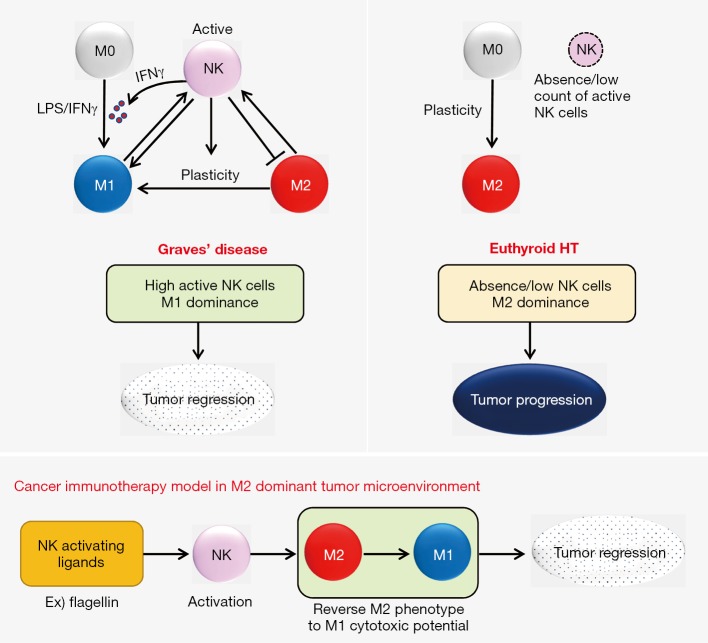
The researchers further investigated how the tumor microenvironment and cellular mechanisms modulated DTC in two groups of patients with GD and euthyroid HT with normal thyroid function (1). The immune microenvironment of DTC was more favorable to elimination in patients with GD than in those without GD. The study results are summarized in Figure 1. The proportion of tumor-infiltrating immune cells in the background of GD was high in active NK cells and M1 macrophages and low in M2 macrophages, whereas immune infiltrates in the background of euthyroid HT were low in NK cells and M1 macrophages and high in M2 macrophages. There was no difference in humoral immune responses between the GD and euthyroid HT groups. Macrophages in the presence of euthyroid HT exhibited high plasticity after induction with lipopolysaccharide (LPS) while LPS induction had no significant effect on the macrophages in the presence of GD. In autologous co-cultures of M1/M2 and resting/activated NK cells, NK cells reverted M2 macrophages to the M1 phenotype and M2 macrophages increased the expression of IFNγ by the NK cells (Figure 2). NK cells activated by M1 macrophages induced IFNγ production, which in turn IFNγ induced M1 polarization (9). The IFNγ-dominant tumor microenvironment favored the conversion of M2 macrophages to M1 cells. In the background of GD, activated NK cells induced a pro-inflammatory M1cell-dominant microenvironment (Figure 2). Euthyroid HT had a non-inflammatory M2 cell-dominant microenvironment with a high degree of macrophage plasticity induced by LPS stimulation.
Figure 1.
Summary of the results from Imam et al.’s 2019 study.
Figure 2.
A model of cross-talk between natural killer (NK) cells and macrophages in thyroid cancers coexisting with Graves’ disease and euthyroid Hashimoto’s thyroiditis (HT). In thyroid cancers with Graves’ disease, activated NK cells and M1 macrophages induce tumor regression. In thyroid cancers with euthyroid HT, M2-dominant macrophages and NK cell deficiency contribute to tumor progression. Activation of NK cells polarizes M2 macrophages into an M1 phenotype in a M2-dominant tumor microenvironment. The principles of NK cell-based immunotherapy can be applied to patients with incurable thyroid cancer.
The researchers showed that activated NK cells induced M1 macrophage polarization and down-regulated M2 macrophages and were cytotoxic to cancer cells. They proposed a model of cancer immunotherapy for thyroid cancer with an M2 macrophage-dominant microenvironment. Activation of NK cells by using TLR ligands such as flagellin could disrupt the balance of macrophages toward the M2 phenotypes in cancers (Figure 2). Activated NK cells reversed the M2 phenotype to the M1cytotoxic phenotype, which resulted in tumor regression. As anaplastic thyroid cancer cells are densely intermingled with ramified M2 macrophages with long and thin cytoplasmic processes (10,11), anaplastic thyroid cancer may be a candidate for such therapeutic approaches.
Genomic studies have classified PTCs into RAS-like and BRAF-like tumors based on their gene expression profiles (8,12). RAS-like and BRAF-like tumors were further subdivided into immunoreactive and immunodeficient types by immune-related functional gene sets (8). Immunoreactive BRAF-like thyroid tumors had higher levels of gene expression of M2 macrophages and showed worse clinical outcome than RAS-like and immunodeficient BRAF-like tumors (8).
In conclusion, the authors of this article bring us the recent advances in understanding the tumor microenvironment and immunity in thyroid cancer. The heterogeneity of the immune responses in the thyroid tumor microenvironment influences cancer development and suppression, and its outcome. Thus, increasing awareness of the changes in the microenvironment of thyroid cancer could help clinicians make decisions about more precise personalized treatments and predictions for prognoses. Immunotherapies targeted to cross-talk between macrophages and NK cells may be affordable for patients with incurable thyroid cancers.
Acknowledgments
Funding: This research was supported by a grant (2017R1D1A1B03029597) from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT.
Provenance: This is an invited article commissioned by the Section Editor De-Tao Yin (Department of Thyroid Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Imam S, Dar P, Paparodis R, et al. Nature of coexisting thyroid autoimmune disease determines success or failure of tumor immunity in thyroid cancer. J Immunother Cancer 2019;7:3. 10.1186/s40425-018-0483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco JS, Amaya-Amaya J, Anaya M. Thyroid disease and autoimmune diseases. Autoimmunity: From Bench to Bedside. Bogota, Colombia: El Rosario University Press, 2013. [PubMed] [Google Scholar]
- 3.Boi F, Pani F, Mariotti S. Thyroid Autoimmunity and Thyroid Cancer: Review Focused on Cytological Studies. Eur Thyroid J 2017;6:178-86. 10.1159/000468928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai X, Xia Y, Zhang B, et al. A meta-analysis of Hashimoto's thyroiditis and papillary thyroid carcinoma risk. Oncotarget 2017;8:62414-24. 10.18632/oncotarget.18620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723-37. 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018;233:6425-40. 10.1002/jcp.26429 [DOI] [PubMed] [Google Scholar]
- 7.Galdiero MR, Varricchi G, Marone G. The immune network in thyroid cancer. Oncoimmunology 2016;5:e1168556. 10.1080/2162402X.2016.1168556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim K, Jeon S, Kim TM, et al. Immune Gene Signature Delineates a Subclass of Papillary Thyroid Cancer with Unfavorable Clinical Outcomes. Cancers (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14-20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caillou B, Talbot M, Weyemi U, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PloS One 2011;6:e22567. 10.1371/journal.pone.0022567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DI, Kim E, Kim YA, et al. Macrophage Densities Correlated with CXC Chemokine Receptor 4 Expression and Related with Poor Survival in Anaplastic Thyroid Cancer. Endocrinol Metab (Seoul) 2016;31:469-75. 10.3803/EnM.2016.31.3.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676-90. 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]