Abstract
A study was conducted to evaluate the effects of feeding an acidogenic diet with a low dietary cation-anion difference (DCAD) on acid-base balance, blood, milk, and urine Ca concentrations of sows during lactation. A total of 30 multiparous sows (parity: 4.5 ± 2.9, Smithfield Premium Genetic, Rose Hill, NC) were allotted to 1 of 2 dietary treatments: CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets). The lower DCAD was achieved by the addition of an acidogenic mineral. The DCAD was calculated as mEq (Na + K – Cl)/kg diet. Sows had a daily access to 2-kg feed from day 94 of gestation to parturition and ad libitum access to feed during lactation. Blood and urine pH and Ca, serum macrominerals, serum biochemistry, Ca-regulating hormones, and milk composition were measured. Sows in ACI had a lower (P < 0.05) blood pH than sows in CON at day 1 of lactation. Sows in ACI had a lower (P < 0.05) urine pH at day 108 of gestation, days 1, 9, and 18 of lactation compared with sows in CON. Sows in ACI had greater (P < 0.05) concentrations of serum total Ca at days 1 and 18 of lactation than sows in CON. There was a greater (P < 0.05) concentration of colostrum Ca in ACI than in CON. There was no difference in urine Ca concentration between treatments during lactation. Concentrations of parathyroid hormone and 1,25-dihydroxycholecalciferol were not different between treatments at either day 1 or 18 of lactation. Sows in ACI tended to have a smaller (P = 0.086) concentration of total alkaline phosphatase in serum at day 18 of lactation compared with sows in CON. At day 1 of lactation, the concentration of serum Cl in ACI was greater (P < 0.05) than that in CON. Feed intake, BW loss, and litter performance were not different between treatments. Collectively, feeding an acidogenic diet with a low DCAD to sows can induce a mild metabolic acidosis at farrowing, reduce the urine pH consistently, and increase serum total Ca and colostrum Ca concentrations during lactation but without altering the parathyroid hormone and 1,25-dihydroxycholecalciferol levels during lactation.
Keywords: acid-base equilibrium, calcium, dietary cation-anion difference, sow
INTRODUCTION
With continuous genetic selection, sows produce larger and heavier litters nowadays which causes an increased demand for lactation and therefore increased nutrient mobilization from maternal tissue (Kim et al., 2013). The requirement for Ca is greatest during late gestation and lactation when Ca is used to maintain fetal skeleton development and milk production (Miller et al., 1994; Close and Cole, 2001; NRC, 2012). Calcium is responsible for a series of physiological functions for sows. Deficiency of Ca or a low blood Ca concentration could result in prolonged farrowing, tetany, recumbency, posterior paralysis, and sudden death, all of which may increase the rate of unplanned culling in a sow herd (Kirk et al., 2005; Kenneth et al., 2012).
Calcium is the major mineral in the milk of sows. To maintain milk Ca concentration at an adequate level, maternal bone resorption commonly increases to compensate for the insufficient Ca intake (Mahan and Vallet, 1997). Secretion of Ca into milk can cause the reduction of Ca concentration in the extracellular fluid. Low Ca concentration triggers the secretion of parathyroid hormone (PTH) and formation of 1,25 dihydroxycholecalciferol (1,25-[OH]2D), which is responsible for increasing intestinal Ca absorption and Ca resorption from bone.
Manipulation of dietary cation-anion difference (DCAD) has impacts on blood acid-base metabolism and blood Ca concentration. The DCAD is defined as milliequivalents (mEq) of (Na + K – Cl) per kilogram of diet (Mongin, 1981; Patience et al., 1987). The Na, K, and Cl are considered to have the greatest effects on acid-base metabolism in swine (NRC, 2012). Therefore, the relationship between these 3 elements is commonly used to predict whether the diet will elicit an acidogenic or alkalinogenic response (Oetzel, 1991). Particularly for dairy cattle, an acidogenic diet can result in a low DCAD and can be very effective in reducing the incidence of hypocalcemia by inducing a metabolic acidosis and increasing Ca mobilization (Oetzel, 1991; Horst et al., 1997; DeGaris and Lean, 2008). One of the most recent publications in gestation sows also indicated that a low DCAD could increase Ca mobilization via increasing blood, urine, and fecal Ca concentration (Darriet et al., 2016). Consequently, it is hypothesized that lowering DCAD during lactation may alter the acid-base balance and increase blood and milk Ca concentration. The objective of this study was to determine whether lowering DCAD could alter the acid-base balance, blood, milk, and urine Ca concentration of lactating sows.
MATERIALS AND METHODS
A protocol for the use of animals in this study was approved by North Carolina State University Animal Care and Use Committee.
Animal and Experimental Diets
A total of 30 gestating sows (15 sows per treatment, Smithfield Premium Genetic, Rose Hill, NC) were blocked according to their parity and initial BW. Within blocks, sows were randomly allotted to 1 of 2 dietary treatments: CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets) in a randomized complete block design (DCAD = mEq [Na + K – Cl] per kilogram of diet). Therefore, the acidogenic diets were designed to provide a low DCAD. Four sows in each treatment were removed from the experiment due to pregnancy failure. Therefore, 22 multiparous sows with an average parity of 4.5 ± 2.9 and an average BW of 224.1 ± 38.7 kg successfully farrowed and remained in the study. Sows were fed 2-kg experimental diets from day 94 of gestation, 3-kg experimental diets from day 108 of gestation, and allowed ad libitum access to feed during lactation (18 d). The acidogenic diets were supplemented with 2.0 and 2.2% acidogenic mineral supplement (Cad-mate, Granco Minerals, Disputanta, VA) during gestation and lactation, respectively. The ingredient composition of experimental diets for CON and ACI was the same except for yellow corn, poultry fat, and acidogenic mineral supplement. The dietary Ca and P concentrations between treatments were not different. The experimental diets (Table 1) were corn-soybean meal based and formulated to meet or exceed the nutrient requirements recommended by NRC (2012). Sows were housed in gestation crates (2.0 × 0.6 m) at North Carolina State University Swine Educational Unit (Raleigh, NC) and were moved to farrowing crates (2.1 × 1.5 m) at day 106 of gestation. The temperature in farrowing rooms was maintained at a minimum of 20 °C. Heat lamps were provided to piglets on both sides of farrowing crates.
Table 1.
Composition of experimental diets (as-fed basis)
| Item | Dietary treatment1 | |||
|---|---|---|---|---|
| Gestation | Lactation | |||
| CON | ACI | CON | ACI | |
| Ingredient, % | ||||
| Corn, yellow dent | 81.30 | 78.30 | 70.10 | 66.40 |
| Soybean meal, dehulled | 13.85 | 13.85 | 23.50 | 23.50 |
| Cad-mate2 | 0.00 | 2.00 | 0.00 | 2.20 |
| L-Lys HCl | 0.00 | 0.00 | 0.16 | 0.16 |
| L-Thr | 0.00 | 0.00 | 0.01 | 0.01 |
| Poultry fat | 1.00 | 2.00 | 2.08 | 3.58 |
| Salt | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin premix3 | 0.04 | 0.04 | 0.04 | 0.04 |
| Mineral premix4 | 0.15 | 0.15 | 0.15 | 0.15 |
| Dicalcium phosphate | 2.05 | 2.05 | 2.38 | 2.38 |
| Limestone, ground | 1.11 | 1.11 | 1.08 | 1.08 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated composition5 | ||||
| DM, % | 89.67 | 90.02 | 89.94 | 90.34 |
| ME, Mcal/kg | 3.36 | 3.34 | 3.40 | 3.40 |
| CP, % | 13.33 | 13.08 | 17.14 | 16.83 |
| SID Lys, % | 0.54 | 0.54 | 0.91 | 0.90 |
| Ca, % | 0.94 | 0.94 | 1.03 | 1.03 |
| Total P, % | 0.70 | 0.69 | 0.80 | 0.79 |
| Available P, % | 0.43 | 0.43 | 0.51 | 0.50 |
| Mg, % | 0.14 | 0.26 | 0.16 | 0.29 |
| Na, % | 0.27 | 0.27 | 0.33 | 0.33 |
| K, % | 0.57 | 0.56 | 0.73 | 0.73 |
| S, % | 0.13 | 0.28 | 0.16 | 0.36 |
| Cl, % | 0.33 | 0.66 | 0.37 | 0.73 |
| DCAD-Eq1, mEq/kg6 | 170 | 74 | 226 | 124 |
| DCAD-Eq2, mEq/kg7 | 170 | −32 | 226 | 6 |
| Analyzed composition | ||||
| DM, % | 88.64 | 88.77 | 89.33 | 89.48 |
| CP, % | 12.50 | 13.38 | 16.52 | 16.71 |
| Ca, % | 0.97 | 0.87 | 1.12 | 1.10 |
| Total P, % | 0.73 | 0.66 | 0.93 | 0.81 |
| Mg, % | 0.12 | 0.22 | 0.15 | 0.25 |
| Na, % | 0.24 | 0.21 | 0.18 | 0.19 |
| K, % | 0.52 | 0.52 | 0.74 | 0.70 |
| S, % | 0.16 | 0.31 | 0.21 | 0.37 |
| Cl, % | 0.39 | 0.65 | 0.38 | 0.72 |
| DCAD-Eq1, mEq/kg6, 8 | 127 | 41 | 160 | 58 |
| DCAD-Eq2, mEq/kg7, 8 | 127 | −53 | 160 | −41 |
1Dietary cation-anion difference (DCAD) was expressed as milliequivalents (mEq) (Na + K – Cl) per kilogram diet. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets).
2Cad-mate (Granco Minerals, Disputanta, VA) is an acidogenic mineral supplement.
3The vitamin premix provided the following per kilogram of complete diet: 6613.8 IU of vitamin A as vitamin A acetate; 992.0 IU of vitamin D3; 19.8 IU of vitamin E; 2.64 mg of vitamin K as menadione sodium bisulfate; 0.03 mg of vitamin B12; 4.63 mg of riboflavin; 18.52 mg of D-pantothenic acid as calcium pantothenate; 26.46 mg of niacin; 0.66 mg of biotin.
4Mineral premix provided the following per kilogram of complete diet: 39.6 mg of Mn as manganous oxide; 165 mg of Fe as ferrous sulfate; 165 mg of Zn as zinc sulfate; 25.15 mg of Cu as copper sulfate; 0.30 mg of I as ethylenediamine dihydroiodide; and 0.30 mg of Se as sodium selenite.
5Calculated composition was based on NRC (2012).
6DCAD was calculated as mEq (Na + K – Cl) per kilogram diet.
7DCAD was calculated as mEq (Na + K – Cl – S) per kilogram diet. Only S from SO42− (from mineral supplement) was considered in this equation.
8DCAD was based on the analyzed mineral composition.
Litter size and the number of liveborn, stillborn, and mummified piglets were recorded within 24 h of parturition. Cross-fostering (CF) was done to adjust the litter size to 9.8 ± 0.2 piglets within 48 h after parturition in both treatments. Piglets were only allowed to be cross-fostered within the same treatment. Sows were weighed at days 94 and 108 of gestation, at days 1, 9, and 18 of lactation to calculate the BW loss. During lactation, the feed was provided twice (0630 and 1300 h daily) after the feed residue was collected and weighed at 0600 h. The feed intake of sows during lactation was calculated. Piglets were weighed at 1, 9, and 18 d of age to calculate the piglet and litter weight gain. Piglet survivability in each litter was calculated at day 18 of lactation.
Sampling and Analysis
Dry matter, CP, Ca, total P, Mg, Na, K, and S in feed samples were analyzed by North Carolina Department of Agriculture Feed and Forage Laboratory (Food and Drug Protection Division, Raleigh, NC). Feed samples were analyzed for moisture (method 930.15; AOAC, 2003) and CP by combustion (method 999.03; AOAC, 2003). To quantify the concentrations of Ca, total P, Mg, Na, K, and S in feed, 0.5 g of homogenized feed was digested in a microwave digestion system (MARS 6, CEM Corp., Matthews, NC) and analyzed using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Optima 5300 DV, Perkin Elmer Inc., Waltham, MA) with 3 replicate readings taken (method 958.01, AOAC, 2003). The concentration of Cl was analyzed by a commercial laboratory (Dairy One, Ithaca, NY). In detail, 0.5-g dried ground feed samples were extracted for 15 min in a 50 mL 0.1 equivalent per liter of HNO3 solution, followed by the potentiometric titration with AgNO3 using the Brinkman Metrohm 716 Titrino Titration Unit (Metrohm Ltd., C-H-9101, Herisau, Switzerland) as described by Cantliffe et al. (1970). The instrument for analyzing Cl was calibrated with 1,000 mg/kg of Cl standard solution with a CV within 5%. Standard samples from the Association of American Feed Control Officials were used for calibration. The DCAD of both control and acidogenic diets were also calculated according to the analyzed compositions of Na, K, Cl, and S (Table 1).
DCAD Calculation
The DCAD calculated from the analyzed mineral composition was smaller when compared with that calculated from mineral profiles in the formulation (Table 1). The analyzed composition showed that the diets were more acidic in general than what was calculated. Even slight changes in Na or K or Cl could affect DCAD calculation. Possibly, there were minor differences between mineral profiles and composition of minerals in ingredients used. Sometimes S is also included in the DCAD equation because S is a negatively fixed ion with high ionic strength, processing 60% of the acidifying strength of Cl (Tucker et al., 1991). The acidifying strength of S is mostly from SO42− provided by mineral supplements instead of the sulfur amino acid in swine. Therefore, the DCAD was 170 and 226 mEq/kg for the control diets and −32 and 6 mEq/kg for the acidogenic diets during gestation and lactation, respectively, if another DCAD equation (DCAD = mEq [Na + K – Cl – S] per kilogram diet) was used.
Blood samples from the jugular vein were collected 2 h after feeding, at days 93 and 108 of gestation, days 1, 9, and 18 of lactation (0900 to 1000 h). Blood samples were collected in S-Monovette tube (Sarstedt, Newton, NC) and centrifuged at 3,000 × g for 15 min at 4 °C to obtain serum. Urine samples (approximately 50 mL) were collected at midstream when sows first urinated in the early morning (0630 to 0800 h). At least 30 mL of colostrum samples were collected within 24 h of parturition. Thirty milliliters of milk samples were collected on day 18 of lactation. Colostrum and milk samples (approximately 40 mL) were obtained from all functioned mammary glands after injecting 0.5 mL of oxytocin into the ear vein. Samples of urine, serum, colostrum, and milk were stored in −80 °C until further analysis.
Blood and urine pH was measured by a pH meter (Accumet AB15, Fisher Scientific, Dubuque, IA) at room temperature immediately after the samples were obtained according to DeRouchey et al. (2003) and Razzaghi et al. (2012). The pH meter was standardized and calibrated by every 30 samples with standard buffers at pH 4, 7, and 10. Blood pH was adjusted to a common body temperature of 39 °C as described by Patience et al. (1987), using the factor of 0.0147 per °C (Severinghaus et al., 1956). At the same time, urinary specific gravity was measured by a refractometer (Handheld Refractometer, Fisher Scientific, Dubuque, IA) as a parameter for the density of urine.
Serum samples at days 1 and 18 of lactation were sent to a commercial laboratory (Antech Diagnostics, Cary, NC) for biochemistry assays and mineral analysis. Concentrations of total protein, albumin, globulin, total alkaline phosphatase, creatinine, blood urea N, Ca, and P were determined by photometric assays (chemistry-immuno analyzer, AU640e, Olympus American Inc., Center Valley, PA) according to Chaytor et al. (2011) and Popovic et al. (2008). In detail, the chemistry-immuno analyzer is an open system using a multiwavelength diffraction grating spectrophotometric method. Serum samples for each test were diluted at 5 to 100 folds before the analysis. Regents for each test were bar coded, liquid, and ready to use. The following methods were used by the chemistry-immuno analyzer: total protein by the biuret method; albumin by the modified bromocresol green method; total alkaline phosphatase by the p-nitrophenyl phosphate method; creatinine by the modified Jaffe kinetic method; and blood urea N by the urease glutamate dehydrogenase method. Serum total Ca and P were determined by the arsenazo III method (Palade and Vergara, 1983) and the phosphomolybdate method (method 962.02, AOAC, 2003) according to Walk et al. (2013). The concentrations of serum Na, K, and Cl were determined by an ion selective electrodes method. The CV for each test was within 5%. Concentrations of serum Mg, urine Ca, and milk Ca were determined by a flame atomic absorption spectroscopy (AA-6701F, Shimadzu Scientific Instruments, Kyoto, Japan) as described by Armstrong et al. (2000). To determinate the concentrations of Mg and Ca, samples of serum, urine, and milk were digested with 5% HNO3 and were deionized with 0.1% LaCl3 and 0.2% KCl solutions. Concentrations of Mg and Ca were analyzed in duplicate. If the analyzed concentrations of Ca and Mg had a CV greater than 5%, the samples were analyzed again. Calibration was done by every 10 samples.
Concentrations of PTH and 1,25-(OH)2D in serum were determined by ELISA kits (Porcine Intact PTH ELISA kit, Immutopics International LLC, San Clemente, CA; 1,25-dihydroxy vitamin D EIA, IDS Ltd., Tyne and Wear, UK, respectively) according to the manufacturer’s instructions. The sensitivity of the porcine intact PTH assay, as determined by 16 duplicate determinations of the 0 pg/mL standard, was 1 pg/mL. The sensitivity of 1,25-(OH)2D assay, defined as the concentration corresponding to the mean minus 2 standard deviations of 20 replicates of the 0 calibrator, was 6 pmol/L.
Concentrations of fat, lactose, protein, and solids-nonfat in colostrum and milk were analyzed by mid-infrared spectrophotometric technique using a Milkoscan 4000 (Foss Electric, Hillerød, Denmark) at Virginia Tech United Federation of Dairy Herd Information Association Laboratory (Blacksburg, VA) as described by Garst et al. (1999). The instrument for milk composition analysis was regularly calibrated by using standard samples (composition is known) to ensure the concentrations of components of milk within a CV of 3.5% and a standard deviation smaller than 0.04.
Statistical Analysis
All data except liveborn, stillborn, mummified, and weaning litter size met the assumptions of normality and homogeneous variance, which were checked by using the UNIVARIATE procedure of SAS (SAS Inst. Inc., Cary, NC). As a result, data of liveborn, stillborn, mummified, and weaning litter size were analyzed with the GLIMMIX procedure of SAS. The rest data were analyzed using the MIXED procedure of SAS following a randomized complete block design. Initial BW and parity were considered as the blocking variables. Initial BW was considered as a random effect; dietary treatments and parity were considered as fixed effects. Data sets of blood and urinary pH at day 108 of gestation, days 1, 9, and 18 of lactation were analyzed with either initial blood or urine pH as a covariate (Verbeke and Molenberghs, 1997; Littell et al., 2000) because these initial values were different between treatment groups. The individual sow was considered as the experimental unit. Probability values less than or equaling 0.05 were considered statistically significant and between 0.05 and 0.10 as trends. During analysis, data greater than (75th Percentile + 1.5 × Interquartile Range) and smaller than (25th Percentile – 1.5 × Interquartile Range) were considered as outliers and excluded from analysis according to LeBlanc (2004).
RESULTS
Concentrations of Ca, PTH, and 1,25-(OH)2D
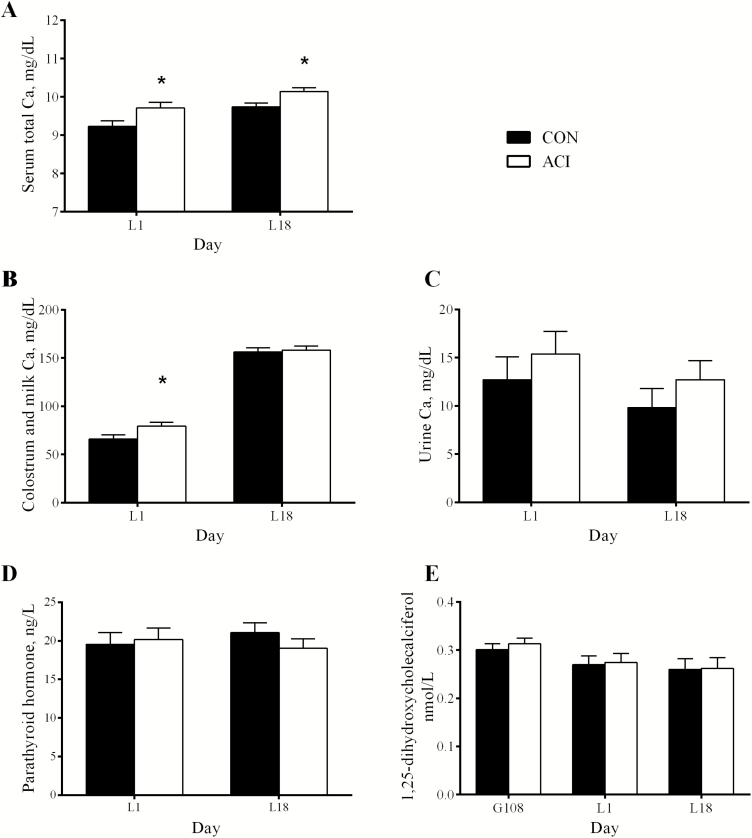
At day 1 of lactation, concentrations of serum total Ca and colostrum Ca of sows in ACI were greater (P < 0.05) when compared with those in CON (Figure 1). At day 18 of lactation, the concentration of serum total Ca was greater (P < 0.05) in ACI than in CON. The concentration of milk Ca was not different between treatments. The concentration of urine Ca did not differ between treatments at either day 1 or 18 of lactation. Concentrations of PTH and 1,25-(OH)2D were not different between treatments at either day 1 or 18 of lactation.
Figure 1.
Effect of dietary cation-anion difference (DCAD, [Na + K – Cl]) on serum total Ca (A), colostrum and milk Ca (B), and urine Ca concentrations (C), parathyroid hormone (D), and 1,25-dihydroxycholecalciferol concentrations (E) in gestating and lactating sow. Values were provided as least square mean and standard error, n = 11. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets). *Denotes difference (P < 0.05) between CON and ACI.
Serum Macrominerals
At day 1 of lactation, the concentration of serum Mg in ACI tended to be greater (P = 0.097) when compared with that in CON (Table 2). Concentrations of Na, K, and total P in serum were not different between treatments at day 1 of lactation; however, the concentration of serum Cl in ACI was greater (P < 0.05) than that in CON. At day 18 of lactation, the concentration of serum Mg in ACI tended to be greater (P = 0.087) than that in CON, whereas the concentration of serum Cl was not different between treatments.
Table 2.
Serum macrominerals composition
| Item | Dietary treatment1 | SEM | P-value | |
|---|---|---|---|---|
| CON | ACI | |||
| No. of sows | 11 | 11 | ||
| Serum, day 1 of lactation | ||||
| Na, mEq/L | 143.64 | 145.36 | 0.90 | 0.191 |
| K, mEq/L | 4.78 | 4.87 | 0.14 | 0.653 |
| Total Ca, mg/dL | 9.23 | 9.71 | 0.15 | 0.031 |
| Mg, mg/Dl | 1.87 | 1.98 | 0.04 | 0.097 |
| P, mg/dL | 7.31 | 7.21 | 0.26 | 0.804 |
| Cl, mEq/L | 101.82 | 104.73 | 0.79 | 0.017 |
| Na/K ratio | 30.18 | 30.09 | 0.88 | 0.943 |
| Serum, day 18 of lactation | ||||
| Na, mEq/L | 141.64 | 141.00 | 0.52 | 0.401 |
| K, mEq/L | 5.42 | 5.25 | 0.11 | 0.300 |
| Total Ca, mg/dL | 9.74 | 10.14 | 0.10 | 0.012 |
| Mg, mg/dL | 2.21 | 2.40 | 0.07 | 0.087 |
| P, mg/dL | 6.45 | 6.18 | 0.20 | 0.361 |
| Cl, mEq/L | 103.09 | 102.82 | 0.65 | 0.769 |
| Na/K ratio | 26.18 | 27.00 | 0.60 | 0.348 |
1Dietary cation-anion difference (DCAD) was expressed as milliequivalents (mEq) (Na + K – Cl) per kilogram diet. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets).
Blood pH, Urine pH, and Urinary Specific Gravity
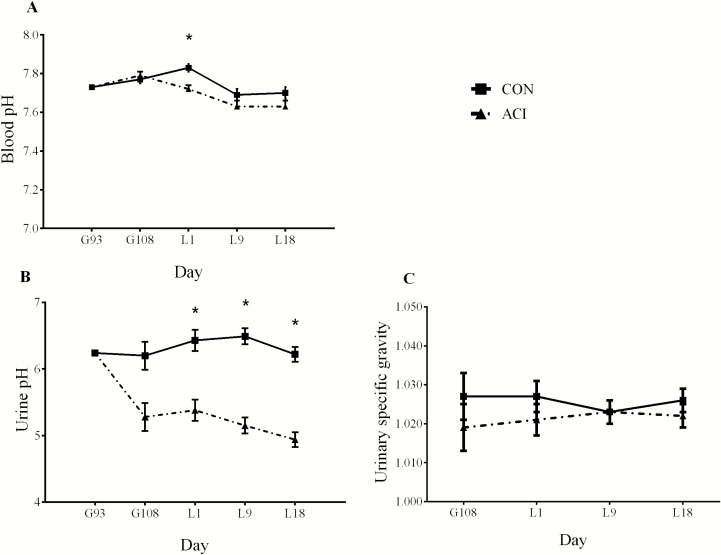
At day 94 of gestation, initial blood pH or urine pH was used as a covariate for pH data analysis (Figure 2). Urine pH in ACI was smaller (P < 0.05) at day 108 of gestation, days 1, 9, and 18 of lactation than that in CON. Blood pH in ACI was smaller (P < 0.05) than that in CON at day 1 of lactation. Blood pH (range from 7.30 to 7.48) was not different between treatments at day 108 of lactation, days 9, and 18 of lactation. Urinary specific gravity was not different between treatments throughout the experimental period.
Figure 2.
Effect of dietary cation-anion difference (DCAD, [Na + K – Cl]) on blood pH (A), urine pH (B), and urinary specific gravity (C) in gestating and lactating sow. Values were provided as least square mean and standard error, n = 11. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets). *Denotes difference (P < 0.05) between CON and ACI.
Blood Biochemistry Assays
At day 1 of lactation, the concentration of serum total protein in ACI tended to be greater (P = 0.085) than that in CON (Table 3). The concentration of serum albumin in ACI was greater (P < 0.05) than that in CON. The concentrations of serum total alkaline phosphatase and creatinine did not differ between treatments at day 1 of lactation. At day 18 of lactation, the concentration of serum total protein was not different between treatments. The concentration of serum albumin was greater (P < 0.05) in ACI when compared with that in CON at day 18 of lactation. Both concentrations of serum total alkaline phosphatase and creatinine tended to be smaller (P = 0.086 and 0.080, respectively) in ACI that those in CON at day 18 of lactation. Concentrations of serum globulin and blood urea N were not different between treatments at either day 1 or 18 of lactation.
Table 3.
Blood biochemistry assays of sows at parturition and weaning
| Item | Dietary treatment1 | SEM | P-value | |
|---|---|---|---|---|
| CON | ACI | |||
| No. of sows | 11 | 11 | ||
| Serum, day 1 of lactation | ||||
| Total protein, g/dL | 6.23 | 6.52 | 0.11 | 0.085 |
| Albumin, g/dL | 3.40 | 3.67 | 0.06 | 0.004 |
| Globulin, g/dL | 2.83 | 2.85 | 0.10 | 0.898 |
| Total alkaline phosphatase, U/L | 46.66 | 44.73 | 3.54 | 0.703 |
| Creatinine, mg/dL | 2.16 | 2.01 | 0.10 | 0.293 |
| Blood urea N, mg/dL | 9.91 | 9.00 | 0.26 | 0.264 |
| Serum, day 18 of lactation | ||||
| Total protein, g/dL | 7.17 | 7.31 | 0.18 | 0.591 |
| Albumin, g/dL | 3.55 | 3.77 | 0.07 | 0.042 |
| Globulin, g/dL | 3.63 | 3.54 | 0.20 | 0.753 |
| Total alkaline phosphatase, U/L | 63.80 | 43.27 | 8.20 | 0.086 |
| Creatinine, mg/dL | 1.60 | 1.43 | 0.07 | 0.080 |
| Blood urea N, mg/dL | 14.45 | 12.73 | 0.92 | 0.198 |
1Dietary cation-anion difference (DCAD) was expressed as milliequivalents (mEq) (Na + K – Cl) per kilogram diet. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets).
Colostrum and Milk Analysis
The compositions of colostrum and milk at either day 9 or 18 of lactation including fat, lactose, protein, and solids-nonfat were not different between treatments (Table 4).
Table 4.
Colostrum and milk compositions in lactating sows1
|
Item |
Dietary treatment2 | SEM | P-value | |
|---|---|---|---|---|
| CON | ACI | |||
| No. of sows | 11 | 11 | ||
| Colostrum | ||||
| Fat, % | 7.28 | 7.09 | 0.60 | 0.837 |
| Protein, % | 8.70 | 8.15 | 0.79 | 0.631 |
| Solids-nonfat, % | 15.33 | 15.07 | 0.71 | 0.807 |
| Lactose, % | 4.91 | 5.04 | 0.16 | 0.556 |
| Ca, mg/dL | 66.12 | 79.34 | 4.12 | 0.047 |
| Milk, day 9 of lactation | ||||
| Fat, % | 7.05 | 6.92 | 0.42 | 0.827 |
| Protein, % | 4.90 | 4.80 | 0.11 | 0.512 |
| Solids-nonfat, % | 13.28 | 13.11 | 0.33 | 0.736 |
| Lactose, % | 6.50 | 6.44 | 0.15 | 0.782 |
| Milk, day 18 of lactation | ||||
| Fat, % | 6.85 | 6.18 | 0.32 | 0.157 |
| Protein, % | 4.61 | 4.77 | 0.12 | 0.323 |
| Solids-nonfat, % | 12.96 | 13.07 | 0.11 | 0.505 |
| Lactose, % | 6.63 | 6.57 | 0.05 | 0.473 |
| Ca, mg/dL | 156.26 | 158.05 | 4.42 | 0.783 |
1Wet weight basis.
2Dietary cation-anion difference (DCAD) was expressed as milliequivalents (mEq) (Na + K – Cl) per kilogram diet. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets).
Sow and Piglet Performance
This study was not aimed to evaluate production traits of sows and piglets. However, performance data are provided to indicate their body condition. Initial BW of sows at day 94 of lactation did not differ between treatments (Table 5). Weight gain of sows at day 108 of gestation was not different between treatments. At day 1 of lactation, sow BW, litter weight, and piglet birth weight were not different between treatments. Numbers of live-born and stillborn piglets were not different between treatments. The number of piglets mummified in ACI was greater (P < 0.05) than that in CON. During days 1 to 9 of lactation, weight loss and ADFI of sows, litter size, and litter weight gain were not different between treatments. Piglet weight gain and piglet survivability did not differ between treatments at day 9 of lactation. Weight loss and ADFI from days 1 to 18 of lactation were not different between treatments. Litter weight gain, piglet weight gain, and piglet survivability in ACI were not different from those in CON from days 1 to 18 of lactation.
Table 5.
Effects of dietary cation-anion difference (DCAD) on performance of sows and litters
| Item | Dietary treatment1 | SEM | P-value | |
|---|---|---|---|---|
| CON | ACI | |||
| No. of sows | 11 | 11 | ||
| Sow BW (day 94 of gestation), kg | 224.4 | 223.8 | 12.0 | 0.973 |
| Parity | 4.0 | 3.7 | 0.9 | 0.835 |
| Day 108 of gestation | ||||
| BW gain, kg | 5.8 | 7.4 | 1.1 | 0.355 |
| Day 1 of lactation | ||||
| BW, kg | 222.7 | 222.4 | 11.0 | 0.986 |
| Litter weight, kg | 14.9 | 15.9 | 0.4 | 0.345 |
| Piglet birth weight, kg | 1.46 | 1.52 | 0.04 | 0.346 |
| Litter size after CF, piglet | 9.8 | 9.7 | 0.2 | 0.697 |
| Liveborn | 11.7 | 10.2 | 0.8 | 0.100 |
| Stillborn | 0.6 | 0.4 | 0.5 | 0.778 |
| Mummified | 0.0 | 0.7 | 0.2 | 0.027 |
| Day 9 of lactation | ||||
| BW loss, kg | 12.0 | 6.5 | 2.7 | 0.168 |
| Litter size, piglet | 9.5 | 9.3 | 0.2 | 0.502 |
| ADFI, kg | 7.4 | 7.0 | 0.7 | 0.256 |
| Litter weight gain, kg | 15.5 | 14.7 | 0.5 | 0.526 |
| Piglet weight gain, kg | 1.61 | 1.59 | 0.08 | 0.845 |
| Piglet survivability, % | 93.0 | 89.9 | 2.7 | 0.333 |
| Day 18 of lactation | ||||
| BW loss during lactation, kg | 14.3 | 9.1 | 3.3 | 0.284 |
| ADFI during lactation, kg | 7.7 | 7.4 | 0.5 | 0.543 |
| Litter size, piglet | 9.5 | 9.3 | 0.2 | 0.436 |
| Litter weight gain, kg | 39.5 | 36.9 | 1.7 | 0.284 |
| Piglet weight gain, kg | 4.11 | 3.98 | 0.17 | 0.618 |
| Piglet survivability, % | 93.0 | 89.9 | 2.7 | 0.450 |
| Ca daily intake, g/d2 | 86.4 | 81.6 | 3.8 | 0.390 |
1Dietary cation-anion difference was expressed as milliequivalents (mEq) (Na + K – Cl) per kilogram diet. Two dietary treatments were CON (control diets were corn-soybean meal based with a calculated DCAD of 170 and 226 mEq/kg during late gestation and lactation, respectively) or ACI (acidogenic diets had a DCAD 100 mEq/kg lower than the control diets).
2Ca daily intake was calculated as the analyzed Ca concentration timed the ADFI during lactation.
DISCUSSION
Calcium requirements during late gestation and lactation increase in proportion to the need of fetal growth and the levels of milk production (Miller et al., 1994; Close and Cole, 2001; NRC, 2012). Sows can mobilize Ca from bone tissues such as hydroxyapatite crystals in order to maintain the milk Ca concentration (Mahan and Vallet, 1997). In this study, an acidogenic diet was fed to sows in ACI from late gestation until the end of lactation. Acidogenic mineral supplementation has been commonly used to acidify diet and as an approach to reduce the incidence of milk fever in dairy cows (Horst et al., 1997; Goff and Horst., 1998; DeGaris et al., 2008). Previous studies (Block, 1984; Fredeen et al., 1988; Goff et al., 1991) in ruminants have demonstrated that feeding an acidogenic diet can cause metabolic acidosis and help cows to maintain blood Ca concentration through parturition.
Measuring urine pH is considered as a sensitive method for assessing the acid-base balance of extracellular fluid (Seifi et al., 2004). Commonly, sows maintain the urine and blood pH at a normal range from 6 to 7.5 and 7.25 to 7.45, respectively. This study showed that urine pH (range 6.48 to 5.06) and blood pH (range 7.48 to 7.37) were decreased by a low DCAD. Darriet et al. (2016) reported similar results about blood and urine pH when DCAD (calculated as Na + K − Cl − S) was reduced from 33 to −216 mEq/kg. The decrease in urine and blood pH in response to a low DCAD is caused by the increased strong anions (S and Cl) intake, which increases hydrogen ions in extracellular fluid. In this study, increased dietary Cl has resulted in an enhanced serum Cl concentration. The urine pH of sows fed an acidogenic diet was relatively lower in this study compared with that in other studies (Tucker et al. 1988; Budde and Crenshaw, 2003; Darriet et al., 2016), and was similar to what DeRouchey et al. (2003) found. The relatively low urine pH observed in this experiment when compared with that in these literature could possibly be caused by the different urine collection methods and variability in measurement environments such as temperatures which could affect urine pH (Rosenthal, 1948).
The decreased urine and blood pH demonstrated that the sows fed an acidogenic diet were undergoing a mild metabolic acidosis. The pattern of pH changes showed that the decrease in urine pH in this study was greater initially and subsequently became smaller when sows were fed an acidogenic diet continuously. The blood pH was reduced at day 1 of lactation and was not affected during the rest period of lactation by a low DCAD. It is well recognized that maintaining the intracellular and extracellular pH in a normal physiological range is essential to keep the protein such as enzymes, transporters, and receptors functioning properly. The pattern of changes in both urine and blood pH could be partially due to the buffering effects of Ca and phosphate when hydroxyapatite crystals are broken down to resist the strong ion difference (Roche et al., 2007). On the other hand, increasing renal excretion of hydrogen ions, Cl, SO4, and NH4 and regulating blood gas within normal physiological ranges also help us to resist the strong ion difference, as suggested by previous literature (Patience and Chaplin, 1997; DeRouchey et al., 2003; Darriet et al., 2016).
However, urinary specific gravity was not changed by lowering DCAD in this study. Urinary specific gravity is calculated as the ratio of the density of a urine specimen to the density of water (Osborne and Stevens, 1999). Decreased or increased urinary specific gravity can be caused by abnormal renal function and different hydration status (Simerville, 2005). The insignificant result of urinary specific gravity may suggest that lowering DCAD by 100 mEq/kg did not impair the renal function or influence hydration status of sows.
In this study, the serum total Ca was mildly elevated when sows were fed an acidogenic diet, which agrees to the result of other studies (Goff et al., 1991; Budde and Crenshaw, 2003; Razzaghi et al., 2012). The serum total Ca includes 3 fractions, which are ionized (44%), complexed (10%), and protein-bound Ca (46%) (Goldstein, 1990). It is speculated that the increased serum total Ca occurred with the increased 3 fractions of Ca in the same ratio, resulting in a constant proportion of Ca (Goldstein, 1990). Among the protein-bound Ca, about 81% of it binds to albumin and about 19% of it binds to globulin (Moore, 1970). In this case, decreased blood pH is associated with the increased disassociation of Ca from albumin and an increased ionized Ca concentration (Loken et al., 1960; Agnes et al., 1993). Previous studies (Oetzel et al., 1988; DeRouchey et al., 2003; Darriet et al., 2016) also demonstrated that ionized Ca was increased by a low DCAD. Even ionized Ca was not measured in this study, serum albumin and total Ca were increased by a low DCAD. No hypocalcemia sows were observed in this study, as serum total Ca concentration for all experimental sows only ranged from 8.3 to 10.3 mg/dL, which could still fall into a relative safe margin (2.2 to 2.6 mmol/L or 8.5 to 10.5 mg/dL; Swenson, 1984; Kim et al., 2009; Nitovski et al., 2011). Hypocalcemia in sows is described with symptoms of muscle weakness and posterior paralysis when there is a great milk production (Durrell, 1942; Jennings, 1985; NRC, 1998). The possible reason could be that the lactation of this study was terminated early (day 18 of lactation). Hypocalcemia could be critical especially for sows with extensive lactation, considering there are increased milk yield and milk Ca concentration at late lactation (Pond and Maner, 1974; Miller et al., 1994).
Lowering DCAD increased the colostrum Ca concentration. Previous studies consistently found that Ca excretion in other body fluid such as urine was increased by a low DCAD (Lemann et al., 1967; Goff and Horst, 1998; Budde and Crenshaw, 2003; Razzaghi et al., 2012). Calcium excretion in milk increases as the period of lactation processes (Pond and Maner, 1974; Miller et al., 1994). The concentration range of milk Ca agrees to what other studies reported that Ca concentration in colostrum is about 87 mg/dL and Ca concentration in milk at the end of lactation increases to about 180 mg/dL (Kent et al., 1998). Even though there was a 20% increase in colostrum Ca of ACI sows, concentrations of nutrients such as protein, lactose, and fat in colostrum and milk at both days 9 and 18 of lactation were not different between treatments. The lack of difference in those nutrients could be one of the reasons for the result that no difference in litter performance was observed between treatments.
Even serum total Ca was increased by a low DCAD in this study, the levels of Ca-regulating hormones, such as 1,25-(OH)2D and PTH, were not increased by a low DCAD. The possible reason for the insignificant result of PTH could be that metabolic acidosis augments the effects of PTH on mobilizing Ca instead of increasing the concentration of PTH (Beck and Webster, 1976; Goff et al., 1991; Horst et al., 1997). Both metabolic acidosis and PTH have additive effects on the net efflux of Ca from bone and on bone cell function (Bushinsky and Nilsson, 1995). Metabolic acidosis inhibits the activity of osteoblast which is responsible for the bone matrix mineralization and increases the activity of osteoclast which is responsible for the bone resorption (Bushinsky, 1994; Bushinsky and Nilsson, 1995; Brandao-Burch et al., 2005). The decreased serum total alkaline phosphatase (a biochemical marker of bone mineralization) in sows fed an acidogenic diet also suggested that there was a decreased activity of osteoblast with less bone mineralization (Orimo, 2010). Likewise, the increased serum Mg concentration in ACI sows is consistent with our speculation about increased bone resorption, as the osteoclast breaks down bone tissue and releases both Ca and Mg into the extracellular fluid such as blood and milk.
This study was not aimed to evaluate production traits of sows and piglets. However, performance data are provided to indicate their body condition. There was no difference in the performance of sows between CON and ACI. Serum creatinine concentration and blood urea N were not different between treatments, which is consistent with the performance data such as backfat and body weight loss. Because creatinine is an index of muscle catabolism (Schutte et al., 1981; Nelssen et al., 1985) and blood urea N is an index of N excretion (Kohn et al., 2005). These results are in agreement to the results of other studies (Dove and Haydon, 1994; DeRouchey et al., 2003), in which the DCAD at gestation and lactation were adjusted from 250 to130 mEq/kg or from 500 to 0 mEq/kg. A low DCAD could negatively affect the feed intake of dairy cows (Oetzel and Barmore, 1993); however, the reduction in feed intake was not observed in lactating sows fed diets with a low DCAD range from 0 to 130 mEq/kg (current study; Dove and Haydon, 1994; DeRouchey et al., 2003). Roux (2008) noticed a reduction in ADFI during late gestation; however, no reduction in feed intake during lactation was observed when a low DCAD was applied. The nonsignificant results of performance between treatments could be either due to the insufficient statistical power for sow performance data or explained by that DCAD in this study was within the safe margin (Patience et al., 1987).
A low DCAD may potentially reduce or prevent the incidence of urinary tract infections by inducing an acidic environment in the urinary tract. Urinary tract infections occur when bacteria, predominantly Escherichia (E.) coli, proliferate at the opening of the vulva, vagina, and urethra, then spread up to the bladder and even kidney (Carr and Walton, 1993; Ronald, 2002). Acidic urine is possible to reduce the E. coli proliferation as it colonizes well in a neutral environment of the urinary tract (Abdul-Raouf et al., 1993; Presser et al., 1997). In addition, Tillon and Madec (1984) and Sanz et al. (2007) considered the lesions in the urinary tract as the primary cause (more than 50%) of sudden death or culling of sows. Further studies are required to determine the effects of DCAD on the microbial composition of urinary tract, incidence of urinary tract infections, culling rate, and reproductive performance in a larger sow herd.
In conclusion, feeding an acidogenic diet with a low DCAD induced a mild metabolic acidosis at farrowing, and reduced the urine pH consistently during the lactation. A low DCAD increased concentrations of serum and colostrum Ca of lactating sows without changing 1,25-(OH)2D and PTH levels.
LITERATURE CITED
- Abdul-Raouf U. M., Beuchat L. R., and Ammar M. S.. 1993. Survival and growth of escherichia coli O157:H7 in ground, roasted beef as affected by ph, acidulants, and temperature. Appl. Environ. Microbiol. 59:2364–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnes F., Sartorelli P., Bisso M. C., and Dominoni S.. 1993. Ionized calcium in calf serum: relation to total serum calcium, albumin, total protein and ph. Zentralbl. Veterinarmed. A 40:605–608. doi: 10.1111/j.1439-0442.1993.tb00673.x [DOI] [PubMed] [Google Scholar]
- AOAC 2003. Official methods of analysis of AOAC International. Metals and other elements in plants and pet foods, inductively coupled plasma spectroscopic method. 17th ed.Assoc. Off. Anal. Chem., Gaithersburg, MD. [Google Scholar]
- Armstrong T. A., Spears J. W., Crenshaw T. D., and Nielsen F. H.. 2000. Boron supplementation of a semipurified diet for weanling pigs improves feed efficiency and bone strength characteristics and alters plasma lipid metabolites. J. Nutr. 130:2575–2581. doi: 10.1093/jn/130.10.2575. [DOI] [PubMed] [Google Scholar]
- Beck N., and Webster S. K.. 1976. Effects of acute metabolic acidosis on parathyroid hormone action and calcium mobilization. Am. J. Physiol. 230:127–131. doi:10.1152/ajplegacy.1976.230.1.127. [DOI] [PubMed] [Google Scholar]
- Block E. 1984. Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. J. Dairy Sci. 67:2939–2948. doi: 10.3168/jds.S0022-0302(84)81657-4. [DOI] [PubMed] [Google Scholar]
- Brandao-Burch A., Utting J. C., Orriss I. R., and Arnett T. R.. 2005. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif. Tissue Int. 77:167–174. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- Budde R. A., and Crenshaw T. D.. 2003. Chronic metabolic acid load induced by changes in dietary electrolyte balance increased chloride retention but did not compromise bone in growing swine. J. Anim. Sci. 81:197–208. doi:/2003.811197x [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A. 1994. Acidosis and bone. Miner. Electrolyte Metab. 20:40–52. [PubMed] [Google Scholar]
- Bushinsky D. A., and Nilsson E. L.. 1995. Additive effects of acidosis and parathyroid hormone on mouse osteoblastic and osteoclastic function. Am. J. Physiol. 269(6 Pt 1):C1364–C1370. doi: 10.1152/ajpcell.1995.269.6.C1364. [DOI] [PubMed] [Google Scholar]
- Cantliffe D. J., Macdonald G. E., and Peck N. H.. 1970. The potentiometric determination of nitrate and chloride in plant tissue. New York Food Life Sci. Bull. 1:1–7. [Google Scholar]
- Carr J., and Walton J. R.. 1993. Bacterial flora of the urinary tract of pigs associated with cystitis and pyelonephritis. Vet. Rec. 132:575–577. doi: 10.1136/vr.132.23.575 [DOI] [PubMed] [Google Scholar]
- Chaytor A. C., See M. T., Hansen J. A., de Souza A. L., Middleton T. F., and Kim S. W.. 2011. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 89:124–135. doi: 10.2527/jas.2010-3005. [DOI] [PubMed] [Google Scholar]
- Close W. H., and Cole D. J. A.. 2001. The pre-breeding gilt: Minerals. In: Close W. H., and Cole D. J., editors, Nutrition of sows and boars. Nottingham University Press, Nottingham, United Kingdom: p. 9–27. doi: 10.1016/s0377-8401(00)00217-0 [DOI] [Google Scholar]
- Darriet C., Axe D. E., and Crenshaw T. D.. 2016. Acidogenic mineral additions increased Ca mobilization in prepartum sows. J. Anim. Sci. 95:212–225. doi:10.2527/jas.2016.0859 [DOI] [PubMed] [Google Scholar]
- DeGaris P. J., and Lean I. J.. 2008. Milk fever in dairy cows: a review of pathophysiology and control principles. Vet. J. 176:58–69. doi: 10.1016/j.tvjl.2007.12.029. [DOI] [PubMed] [Google Scholar]
- DeRouchey J. M., Hancock J. D., Hines R. H., Cummings K. R., Lee D. J., Maloney C. A., Dean D. W., Park J. S., and Cao H.. 2003. Effects of dietary electrolyte balance on the chemistry of blood and urine in lactating sows and sow litter performance. J. Anim. Sci. 81:3067–3074. doi: 10.2527/2003.81123067x. [DOI] [PubMed] [Google Scholar]
- Dove C. R., and Haydon K. D.. 1994. The effect of various diet nutrient densities and electrolyte balances on sow and litter performance during two seasons of the year. J. Anim. Sci. 72:1101–1106. doi:/1994.7251101x [DOI] [PubMed] [Google Scholar]
- Durrell W. B. 1942. Hypocalcaemia in sow. Can. J. Comp. Med. Vet. Sci. 6:305–306. [PMC free article] [PubMed] [Google Scholar]
- Fredeen A. H., DePeters E. J., and Baldwin R. L.. 1988. Characterization of acid-base disturbances and effects on calcium and phosphorus balances of dietary fixed ions in pregnant or lactating does. J. Anim. Sci. 66:159–173. doi: 10.2134/jas1988.661159x [DOI] [PubMed] [Google Scholar]
- Garst A. S., Ball S. F., Williams B. L., Wood C. M., Knight J. W., Moll H. D., Aardema C. H., and Gwazdauskas F. C.. 1999. Influence of pig substitution on milk yield, litter weights, and milk composition of machine milked sows. J. Anim. Sci. 77:1624–1630. doi:/1999.7771624x [DOI] [PubMed] [Google Scholar]
- Goff J. P., and Horst R. L.. 1998. Use of hydrochloric acid as a source of anions for prevention of milk fever. J. Dairy Sci. 81:2874–2880. doi: 10.3168/jds.S0022-0302(98)75847-3. [DOI] [PubMed] [Google Scholar]
- Goff J. P., Horst R. L., Mueller F. J., Miller J. K., Kiess G. A., and Dowlen H. H.. 1991. Addition of chloride to a prepartal diet high in cations increases 1,25-dihydroxyvitamin D response to hypocalcemia preventing milk fever. J. Dairy Sci. 74:3863–3871. doi: 10.3168/jds.S0022-0302(91)78579-2 [DOI] [PubMed] [Google Scholar]
- Goldstein D. A. 1990. Serum calcium. In Walker H. K., Hall W. D., and Hurst J. W., editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Butterworth Publishers, Stoneham, MA: p. 677–679. [PubMed] [Google Scholar]
- Haydon K. D., West J. W., and McCarter M. N.. 1990. Effect of dietary electrolyte balance on performance and blood parameters of growing-finishing swine fed in high ambient temperatures. J. Anim. Sci. 68:2400–2406. doi:/1990.6882400x [DOI] [PubMed] [Google Scholar]
- Horst R. L., Goff J. P., Reinhardt T. A., and Buxton D. R.. 1997. Strategies for preventing milk fever in dairy cattle. J. Dairy Sci. 80:1269–1280. doi: 10.3168/jds.S0022-0302(97)76056-9. [DOI] [PubMed] [Google Scholar]
- Jennings D. S. 1985. Hypocalcaemia in sows. Pig J. 14:47–51. [Google Scholar]
- Kenneth S., D’Allaire S., Drolet R., and Abell C.. 2012. Longevity in breeding animals. In: Zimmerman J. J., Karriker L. A., Ramirex A., Schwartz K. J., and Stevenson G. W., editors. Diseases of swine. John Wiley and Sons, Inc., Oxford, United Kingdom: p. 50–59. [Google Scholar]
- Kent J. C., Arthur P. G., and Hartmann P. E.. 1998. Citrate, calcium, phosphate and magnesium in sows’ milk at initiation of lactation. J. Dairy Res. 65:55–68. doi: 10.1017/S0022029997002574 [DOI] [PubMed] [Google Scholar]
- Kim S., Park J. W., Kim D., Kim D., Lee I. H., and Jon S.. 2009. Bioinspired colorimetric detection of calcium(II) ions in serum using calsequestrin-functionalized gold nanoparticles. Angew. Chem. Int. Ed. Engl. 48:4138–4141. doi: 10.1002/anie.200900071. [DOI] [PubMed] [Google Scholar]
- Kim S. W., Weaver A. C., Shen Y. B., and Zhao Y.. 2013. Improving efficiency of sow productivity: nutrition and health. J. Anim. Sci. Biotechnol. 4:26. doi: 10.1186/2049-1891-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R. K., Svensmark B., Ellegaard L. P., and Jensen H. E.. 2005. Locomotive disorders associated with sow mortality in danish pig herds. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 52:423–428. doi: 10.1111/j.1439-0442.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- Kohn R. A., Dinneen M. M., and Russek-Cohen E.. 2005. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 83:879–889. doi: 10.2527/2005.834879x. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. C. 2004. Exploratory data analysis: using graphs and statistics to understand data. In LeBlanc D. C., editor, Statistics, concepts and applications for science. Jone and Bartlett Publishers, Boston. MA: p. 48–49. [Google Scholar]
- Lemann J., Litzow J. R., and Lennon E. J.. 1967. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J. Clin. Invest. 46:1318–1328. doi: 10.1172/JCI105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R. C., Pendergast J., and Natarajan R.. 2000. Modelling covariance structure in the analysis of repeated measures data. Stat. Med. 19:1793–1819. doi:10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.3.co;2-q [DOI] [PubMed] [Google Scholar]
- Loken H. F., Havel R. J., Gordan G. S., and Whittington S. L.. 1960. Ultracentrifugal analysis of protein-bound and free calcium in human serum. J. Biol. Chem. 235:3654–3658. [PubMed] [Google Scholar]
- Mahan D. C., and Vallet J. L.. 1997. Vitamin and mineral transfer during fetal development and the early postnatal period in pigs. J. Anim. Sci. 75:2731–2738. doi:/1997.75102731xdoi:/1997.75102731x [DOI] [PubMed] [Google Scholar]
- Miller M. B., Hartsock T. G., Erez B., Douglass L., and Alston-Mills B.. 1994. Effect of dietary calcium concentrations during gestation and lactation in the sow on milk composition and litter growth. J. Anim. Sci. 72:1315–1319. doi:/1994.7251315x [DOI] [PubMed] [Google Scholar]
- Mongin P. 1981. Recent advances in dietary anion-cation balance: applications in poultry. Proc. Nutr. Soc. 40:285–294. doi: 10.1079/pns19810045 [DOI] [PubMed] [Google Scholar]
- Moore E. W. 1970. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J. Clin. Invest. 49:318–334. doi: 10.1172/JCI106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelssen J. L., Lewis A. J., Peo E. R. Jr, and Crenshaw J. D.. 1985. Effect of dietary energy intake during lactation on performance of primiparous sows and their litters. J. Anim. Sci. 61:1164–1171. doi: 10.2134/jas1985.6151164x [DOI] [PubMed] [Google Scholar]
- Nitovski A., Milenković M., Jotanović S. R., Milanović V. S., Radovic B. V., Grčak D. T., and Grčak M.. 2011. Level of glucose, calcium and phosphorus in blood serum of the first litter sows with normal and disturbed lactation. Biotechnol. Anim. Husb. 27:843–852. doi: 10.2298/BAH1103843N [DOI] [Google Scholar]
- NRC 1998. Nutrient requirements of swine. 10th ed. Natl. Acad. Press, Washington, DC. doi: 10.17226/6016 [DOI] [Google Scholar]
- NRC 2012. Nutrient Requirements of Swine. 11th ed. Natl. Acad. Press, Washington, DC. doi: 10.17226/13298 [DOI] [Google Scholar]
- Oetzel G. R. 1991. Meta-analysis of nutritional risk factors for milk fever in dairy cattle. J. Dairy Sci. 74:3900–3912. doi: 10.3168/jds.S0022-0302(91)78583-4. [DOI] [PubMed] [Google Scholar]
- Oetzel G. R., and Barmore J. A.. 1993. Intake of a concentrate mixture containing various anionic salts fed to pregnant, nonlactating dairy cows. J. Dairy Sci. 76:1617–1623. doi: 10.3168/jds.S0022-0302(93)77495-0 [DOI] [Google Scholar]
- Oetzel G. R., Olson J. D., Curtis C. R., and Fettman M. J.. 1988. Ammonium chloride and ammonium sulfate for prevention of parturient paresis in dairy cows. J. Dairy Sci. 71:3302–3309. doi: 10.3168/jds.S0022-0302(88)79935-X. [DOI] [PubMed] [Google Scholar]
- Orimo H. 2010. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 77:4–12. doi: 10.1272/jnms.77.4 [DOI] [PubMed] [Google Scholar]
- Osborne C. A., and Stevens J. B.. 1999. Urine specific gravity, refractive index, or osmolality: which one would you choose. In: Osborne C. A., and Stevens J. B., editors. Urinalysis: a clinical guide to compassionate patient care. Bayer Corporation, Leverkusen, Germany: p. 73–85. [Google Scholar]
- Palade P., and Vergara J.. 1983. Stoichiometries of arsenazo III-ca complexes. Biophys. J. 43:355–369. doi: 10.1016/S0006-3495(83)84359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience J. F., Austic R. E., and Boyd R. D.. 1987. Effect of dietary electrolyte balance on growth and acid-base status in swine. J. Anim. Sci. 64:457–466. doi: 10.2134/jas1987.642457x [DOI] [PubMed] [Google Scholar]
- Patience J. F., and Chaplin R. K.. 1997. The relationship among dietary undetermined anion, acid-base balance, and nutrient metabolism in swine. J. Anim. Sci. 75:2445–2452. doi:/1997.7592445x [DOI] [PubMed] [Google Scholar]
- Pond W. G., and Maner J. H.. 1974. Lactation. In: Pond W. G., editor, Swine production in temperate and tropical environments. W. H. Freeman and Co, San Francisco, CA: p. 153–161. [Google Scholar]
- Popovic N. T., Srebocan E. R., Coz-Rakovac M., Hacmanjek I., Strunjak-Perovic, and Jadan M.. 2008. Short communication blood biochemistry of captive Atlantic bluefin tuna Thunnus thynnus farmed in the Adriatic Sea. J. Appl. Ichthyol. 24:614–616. doi: 10.1111/j.1439-0426.2008.01108.x. [DOI] [Google Scholar]
- Presser K. A., Ratkowsky D. A., and Ross T.. 1997. Modelling the growth rate of escherichia coli as a function of ph and lactic acid concentration. Appl. Environ. Microbiol. 63:2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaghi A., Aliarabi H., Tabatabaei M. M., Saki A. A., Valizadeh R., and Zamani P.. 2012. Effect of dietary cation-anion difference during prepartum and postpartum periods on performance, blood and urine minerals status of holstein dairy cow. Asian-Australas. J. Anim. Sci. 25:486–495. doi: 10.5713/ajas.2011.11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J. R., Dalley D. E., and O’Mara F. P.. 2007. Effect of a metabolically created systemic acidosis on calcium homeostasis and the diurnal variation in urine ph in the non-lactating pregnant dairy cow. J. Dairy Res. 74:34–39. doi: 10.1017/S0022029906002123. [DOI] [PubMed] [Google Scholar]
- Ronald A. 2002. The etiology of urinary tract infection: traditional and emerging pathogens. Dis. Mon. 49:71–82. doi: 10.1067/mda.2003.8 [DOI] [PubMed] [Google Scholar]
- Rosenthal T. B. 1948. The effect of temperature on the ph of blood and plasma in vitro. J. Biol. Chem. 173:25–30. [PubMed] [Google Scholar]
- Roux M. L., Johnston S. L., Lirette R. D., Bidner T. D., Southern L. L., and Jardon P. W.. 2008. The effect of diets varying in dietary cation-anion difference fed in late gestation and in lactation on sow productivity. Prof. Anim. Sci. 24:149–155. doi: 10.15232/S1080-7446(15)30829-9 [DOI] [Google Scholar]
- Sanz M., Roberts J. D., Perfumo C. J., Alvarez R. M., Donovan T., and Almond G. W.. 2007. Assessment of sow mortality in a large herd. J. Swine Health Prod. 15:30–36. [Google Scholar]
- Schutte J. E., Longhurst J. C., Gaffney F. A., Bastian B. C., and Blomqvist C. G.. 1981. Total plasma creatinine: an accurate measure of total striated muscle mass. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 51:762–766. doi: 10.1152/jappl.1981.51.3.762. [DOI] [PubMed] [Google Scholar]
- Seifi H. A., Mohri M., and Kalamati Zadeh J.. 2004. Use of pre-partum urine ph to predict the risk of milk fever in dairy cows. Vet. J. 167:281–285. doi: 10.1016/S1090-0233(03)00114-X. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W., Stupfel M., and Bradley A. F.. 1956. Accuracy of blood pH and PCO2 determinations. J. Appl. Physiol. 9:189–196. doi: 10.1097/00132586-195802000-00003 [DOI] [PubMed] [Google Scholar]
- Simerville J. A., Maxted W. C., and Pahira J. J.. 2005. Urinalysis: a comprehensive review. Am. Fam. Physician 71:1153–1162. [PubMed] [Google Scholar]
- Swenson M. J. 1984. Physiological properties and cellular and chemical constituents of blood. In: Swenson M. J., editor, Dukes’ physiology of domestic animals. 10th ed. Comstock Publishing Associates, Cornell University Press, London, United Kingdom: p. 15–40. [Google Scholar]
- Tillon J. P., and Madec F.. 1984. Diseases affecting confined sows. Data from epidemiological observations. Ann. Rech. Vet. 15:195–199. [PubMed] [Google Scholar]
- Tucker W. B., Harrison G. A., and Hemken R. W.. 1988. Influence of dietary cation-anion balance on milk, blood, urine, and rumen fluid in lactating dairy cattle. J. Dairy Sci. 71:346–354. doi:10.3168/jds.S0022-0302(88)79563–6 [DOI] [PubMed] [Google Scholar]
- Tucker W. B., Hogue J. F., Waterman D. F., Swenson T. S., Xin Z., Hemken R. W., Jackson J. A., Adams G. D., and Spicer L. J.. 1991. Role of sulfur and chloride in the dietary cation-anion balance equation for lactating dairy cattle. J. Anim. Sci. 69:1205–1213. doi:/1991.6931205x [DOI] [PubMed] [Google Scholar]
- Verbeke G., and Molenberghs G.. 1997. Linear mixed models and missing data. In: Verbeke G., Molenberghs G., editors, Linear mixed models in practice: A SAS-Oriented Approach, 1997 ed. Springer-Verlag, New York: p. 160–172. doi: 10.1007/978-1-4612-2294-1 [DOI] [Google Scholar]
- Walk C. L., Srinongkote S., and Wilcock P.. 2013. Influence of a microbial phytase and zinc oxide on young pig growth performance and serum minerals. J. Anim. Sci. 91:286–291. doi: 10.2527/jas.2012-5430. [DOI] [PubMed] [Google Scholar]