Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is the most prevalent disease of swine globally. Infection of weanling pigs with PRRSV leads to a complex immune response resulting in significant disease and decreased growth performance. Previous experimental evidence suggests that increasing concentrations of soybean meal in the diet of young pigs confer benefits in terms of growth performance and immune parameters. The objective of this experiment was to identify potential modes of action for this benefit, specifically the ability for soy-derived isoflavones (ISF) to confer immunological benefits to young pigs infected with PRRSV. Four dietary treatments differing in soy protein source (soy protein concentrate vs. enzyme-treated soybean meal) and ISF supplementation (none vs. 1,500 mg total ISF/kg) were fed; the control diet (CON) contained soy protein concentrate and no supplemental ISF. Weanling pigs (60 barrows, 21 d of age, 5.71 ± 0.44 kg) from a naturally Mycoplasma hyopneumoniae (Mh)–infected source herd were individually housed in disease containment chambers and provided ad libitum access to experimental diets for 7 d before receiving either a sham inoculation or a 9.28 × 103 50% tissue culture infective dose of PRRSV at 28 d of age (0 d postinoculation). A total of 5 experimental treatments included an uninfected group receiving the CON diet, plus four infected groups each receiving a different dietary treatment. Growth performance and rectal temperatures were recorded throughout the study, and blood was collected for quantification of serum PRRSV load, presence of anti-PRRSV antibodies, differential complete blood counts, cytokine concentrations, and T-cell immunophenotyping. Data were analyzed as a 2-way or 3-way ANOVA for all treatments including PRRSV-infected pigs, in addition to a single degree of freedom contrast to compare uninfected and infected pigs receiving the CON diet. PRRSV-infection reduced growth rate and efficiency compared with noninfected controls with minimal influences by ISF. Supplemental ISF reduced PRRSV-induced band neutrophilia and improved cytotoxic-to-helper T-cell ratios. These results suggest that ISF contribute to activation of adaptive immune system pathways and could benefit recovery from and clearance of PRRSV infections.
Keywords: disease, immune system, isoflavones, porcine reproductive and respiratory syndrome virus, soybean, swine
INTRODUCTION
Porcine reproductive and respiratory syndrome virus (PRRSV) continues to be one of the leading pathogens causing high economic impact on modern swine production. Previous studies estimated that production losses within breeding and growing swine herds to be more than US$650 million per year, costing producers US$3 to US$11 per pig infected (Holtkamp et al., 2013; Schweer et al., 2017). Controlling spread and eradication of PRRSV has remained a challenge due to ease of transmission between animals, heterogenic properties of the virus itself, and its ability to persist within an animal host and environment. Although vaccination protocols are available, their efficacies vary and often do not result in complete sterilizing immunity (Loving et al., 2015). For those reasons, nutritional intervention of or management strategies is of high interest for common diseases affecting livestock due to relatively low cost and ease of implementation. It has been demonstrated that increasing the level of soybean meal in the diet fed to pigs under immune stress reduces adverse effects of illness and may improve growth performance (Boyd et al., 2010; Rochell et al., 2015). However, it remains to be seen whether improved growth performance was due to differences in dietary amino acids or one or more other dietary components, though there appears to be evidence that differences are likely not due to differences in nutrient digestibility (Schweer et al., 2018). Isoflavones (ISF) are flavonoid compounds that are enriched in soybeans and possess anti-inflammatory and antioxidative properties (Smith and Dilger, 2018). With that in mind, the objective of this study was to determine whether soy-derived ISF, fed at dietary concentrations comparable to a commercial diet fed to weanling pigs, would demonstrate protective effects against a PRRSV-challenge. It was also of interest to determine whether soy protein source influenced the efficacy of dietary ISF modulating a disease challenge in weaning age pigs.
MATERIALS AND METHODS
The protocol for this experiment was approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee of the University of Illinois at Urbana – Champaign.
Animal Husbandry and Experimental Design
Sixty weanling barrows (5.71 ± 0.44 kg initial body weight) were obtained from a PRRS-negative, non-PRRS vaccinated commercial herd (1050 Cambro genetics; Carthage Veterinary Service, Ltd.) and housed in disease containment chambers in the Edward R. Madigan Laboratory at the University of Illinois (Urbana, IL) for 21 d (−7 to 14 d postinoculation, DPI) immediately after weaning at approximately 21 d of age. A single corridor of containment chambers was used, with access to 8 chambers each housing 4 individual pigs, and the total number of pigs was split into 2 separate cohorts (n = 28 and n = 32 in cohorts 1 and 2, respectively) that were conducted in successive months. Each chamber (3.34 m2 total floor space) was divided into 4 individual pens (0.84 m2 per pen) and each was equipped with 1 nipple waterer and 1 feeder.
Experimental diets were provided beginning at the time of allotment. Pigs were weighed upon arrival for allotment into 5 experimental treatment groups. Pigs were assigned to dietary treatments and allotted to containment chambers (blocks) based on body weight and litter so that weight distributions were similar within a chamber across all treatment groups. Litter of origin (14 litters total across the 2 cohorts) was taken into account, and pigs from each litter were stratified across treatment groups as evenly as possible. This allotment resulted in 12 pigs for each treatment group, with each chamber having 1 replicate pig per dietary treatment with the exception of the uninfected group (3 blocks total). One intramuscular injection of enrofloxacin (7.5 mg/kg BW; Baytril 100; Bayer, Shawnee Mission, KS) was administered on the day pigs arrived as a prophylactic measure against bacterial infections during transition to the new rearing environment. Pigs were provided their assigned experimental diet and allowed to adjust to housing conditions for 7 d prior to initiating inoculation procedures. Lights were maintained on a 12-h light cycle throughout the study, with light provided from 0600 to 1800 h in a thermostatically controlled environment with containment chamber temperatures set at 28–29 °C throughout the study.
As stated, 5 experimental treatments were used in this study, with 4 different diets and 2 states of infection. A 2 × 2 + 1 factorial arrangement of dietary soy protein sources (soy protein concentrate [SPC], Arcon AF, ADM, Decatur, IL vs. enzyme-treated soybean meal [ETSBM], HP300; Hamlet Protein, Findlay, OH) and supplemental ISF (none vs. Novasoy400; ADM, Decatur, IL) constituted the total of dietary treatments (Table 1). Isoflavones were added to the test diets at levels that would be typical for a commercially relevant corn-SBM diet fed to pigs with approximately 20% SBM inclusion. The control diet contained SPC as a protein source with no addition of soy ISF, and this diet was fed to both sham-inoculated and PRRSV-infected groups. Experimental diets were isocaloric and, with the exceptions of corn and protein source, identical in ingredient composition. Isoflavone and saponin concentrations of ingredients and experimental diets were quantified via HPLC at the USDA–ARS National Center for Agricultural Utilization Research (Peoria, IL) according to procedures of Berhow et al. (2006). Crude protein was determined by measuring nitrogen using a Leco analyzer (TruMac N, Leco Corp., St. Joseph, MI) standardized with EDTA and amino acid concentrations were determined at the University of Missouri Agricultural Experiment Station (Columbia, MO; Table 2) according to AOAC (2002) official methods [920.39 and 982.30 E(a, b, c), for crude protein and amino acid concentrations, respectively]. Gross energy of the experimental diets was determined using an adiabatic bomb calorimeter (Parr Instruments, Moline, IL), and DM (method 934.01, AOAC International, 2002) and OM were performed by determining percent ask (method 942.05, AOAC International, 2002) and subtracting from 100. Diets were analyzed for total dietary fiber according to Prosky et al. (1994), but no separation of soluble and insoluble fractions was made. Diets were formulated on a standardized ileal digestible (SID) amino acid basis with identical concentrations across all diets (Table 3). All diets met or exceeded NRC (2012) nutrient requirements for weanling pigs and analyzed dietary concentrations are presented in Table 4.
Table 1.
Experimental treatments
| Treatment | Dietary treatment | Infection status1 |
|---|---|---|
| Control | Soy protein concentrate | Uninfected |
| Control | Soy protein concentrate | PRRSV-infected |
| Control + ISF | Soy protein concentrate + ISF | PRRSV-infected |
| HP300 | HP300 | PRRSV-infected |
| HP300 + ISF | HP300 + ISF | PRRSV-infected |
ISF = soy isoflavones; PRRSV = porcine reproductive and respiratory syndrome virus.
1All pigs in second cohort were naturally coinfected with M. hyopneumoniae prior to the start of the study at the source farm.
Table 2.
Analyzed isoflavone, saponin, and amino acid concentrations of experimental ingredients (as-fed basis)
| Item | Ingredient | ||
|---|---|---|---|
| SPC1 | ETSBM2 | ISF3 | |
| Isoflavones, mg/kg | |||
| Total genistein | 0.00 | 908 | 177,317 |
| Total daidzein | 0.00 | 1,314 | 201,902 |
| Total glycitein | 0.00 | 196 | 22,983 |
| Total isoflavones | 0.00 | 2,417 | 402,203 |
| Total saponins, mg/kg | 1,313 | 3,656 | – |
| Total amino acids, % | |||
| Indispensable | |||
| Arginine | 4.89 | 3.91 | 0.12 |
| Histidine | 1.71 | 1.36 | 0.04 |
| Isoleucine | 3.29 | 2.54 | 0.06 |
| Leucine | 5.28 | 4.14 | 0.07 |
| Lysine | 4.30 | 3.29 | 0.06 |
| Methionine | 0.92 | 0.73 | 0.02 |
| Phenylalanine | 3.44 | 2.74 | 0.20 |
| Threonine | 2.55 | 2.09 | 0.05 |
| Tryptophan | 0.96 | 0.75 | 0.33 |
| Valine | 3.41 | 2.65 | 0.06 |
| Dispensable | |||
| Alanine | 2.84 | 2.34 | 0.07 |
| Aspartic acid | 7.43 | 5.97 | 0.20 |
| Cysteine | 0.89 | 0.72 | 0.07 |
| Glutamic acid | 12.41 | 9.80 | 0.34 |
| Glycine | 2.83 | 2.28 | 0.08 |
| Proline | 3.50 | 2.76 | 0.12 |
| Serine | 2.92 | 2.46 | 0.04 |
| Tyrosine | 2.32 | 1.95 | 0.17 |
1Soy protein concentrate (SPC) manufactured by traditional process to remove soluble sugars and reduce anti-nutritional factors; ADM Foods & Wellness, Decatur, IL.
2Enzyme-treated soybean meal (ETSBM) manufactured for piglet feed with a low content of antinutritional factors (trypsin inhibitors, antigens, and flatulent oligosaccharides); Hamlet Protein, Findlay, OH.
3Novasoy 400 (ISF) has a guaranteed 40% minimum total isoflavone concentration (referred to as suppl. ISF in subsequent tables); ADM, Decatur, IL. Diets were formulated to contain ISF concentrations similar to those observed in typical weaning commercial diets containing SBM.
Table 3.
Ingredient and calculated composition of experimental diets (as-fed basis)
| Soy sourceSuppl. ISF | SPC | ETSBM | |||
|---|---|---|---|---|---|
| Item | No | Yes | No | Yes | |
| Ingredient, % | |||||
| Corn | 62.52 | 62.11 | 59.25 | 58.91 | |
| HP300 | 0.00 | 0.00 | 20.80 | 20.80 | |
| Arcon AF | 17.50 | 17.50 | 0.00 | 0.00 | |
| Dried whey | 12.00 | 12.00 | 12.00 | 12.00 | |
| Poultry byproduct meal1 | 4.00 | 4.00 | 4.00 | 4.00 | |
| Choice white grease | 1.50 | 1.50 | 1.50 | 1.50 | |
| Ground limestone | 0.60 | 0.60 | 0.60 | 0.60 | |
| Monocalcium phosphate | 0.20 | 0.20 | 0.20 | 0.20 | |
| Sodium chloride | 0.40 | 0.40 | 0.40 | 0.40 | |
| Vitamin and mineral premix2 | 0.30 | 0.30 | 0.30 | 0.30 | |
| Choline chloride | 0.07 | 0.07 | 0.07 | 0.07 | |
| L-Lys HCl | 0.43 | 0.43 | 0.49 | 0.49 | |
| DL-Met | 0.18 | 0.18 | 0.16 | 0.16 | |
| L-Trp | 0.07 | 0.07 | 0.05 | 0.05 | |
| L-Thr | 0.20 | 0.20 | 0.18 | 0.18 | |
| L-Val | 0.03 | 0.03 | 0.00 | 0.00 | |
| Novasoy400 | 0.00 | 0.41 | 0.00 | 0.34 | |
| Calculated composition | |||||
| ME, kcal/kg3 | 3,472 | 3,458 | 3,465 | 3,453 | |
| CP, %3 | 21.29 | 21.26 | 21.28 | 21.25 | |
| SID amino acids, %3 | |||||
| Lys | 1.34 | 1.34 | 1.34 | 1.34 | |
| Met + Cys | 0.73 | 0.73 | 0.74 | 0.74 | |
| Trp | 0.26 | 0.26 | 0.26 | 0.26 | |
| Thr | 0.86 | 0.86 | 0.86 | 0.86 | |
| Val | 0.88 | 0.88 | 0.88 | 0.88 | |
| Isoflavones, mg/kg | |||||
| Total genistein | 2 | 690 | 135 | 702 | |
| Total daidzein | 1 | 723 | 105 | 701 | |
| Total glycitein | 0 | 87 | 25 | 97 | |
| Total isoflavones | 3 | 1,500 | 265 | 1,500 | |
| Total saponins, mg/kg | 664 | 1,013 | 840 | 1,128 |
All pigs received allotted treatment diet upon starting −7 DPI. DPI = day post-inoculation; ISF = isoflavones; SID = standardized ileal digestible.
1Low ash pet-food-grade poultry byproduct meal, American Proteins, Inc., Hanceville, AL
2Vitamin-mineral premix (JBS United, Sheridan, IN) included the following per kilogram of complete diet: Vitamin A (retinyl acetate), 11,128 IU; Vitamin D3 (cholecalciferol), 2,204 IU; Vitamin E (dl-α tocopheryl acetate), 66 IU; Vitamin K (menadione nicotinamide bisulfite), 1.42 mg; Thiamine (thiamine mononitrate), 0.24 mg; Riboflavin, 6.58 mg; Pyridoxine (pyridoxine hydrochloride), 0.24 mg; Vitamin B12, 0.03 mg; d-Pantothenic acid (d-calcium pantothenate), 23.5 mg; Niacin (nicotinamide and nicotinic acid), 44 mg; Folic acid, 1.58 mg; Biotin, 0.44 mg; Cu (copper sulfate), 10 mg; Fe (iron sulfate), 125 mg; I (potassium iodate), 1.26 mg; Mn (manganese sulfate), 60 mg; Se (sodium selenite), 0.3 mg; and Zn (zinc oxide), 100 mg.
3Metablizable energy and standardized ileal digestible (SID) amino acid values were calculated using NRC (2012). Analyzed crude protein determined as CP = (N × 6.25).
Table 4.
Analyzed composition of experimental diets (as-fed basis)
| Soy sourceSuppl. ISF | SPC | ETSBM | |||
|---|---|---|---|---|---|
| Item | No | Yes | No | Yes | |
| Dry matter, % | 90.66 | 90.19 | 90.26 | 90.03 | |
| Organic matter, % | 94.60 | 94.42 | 94.30 | 94.19 | |
| Crude protein1, % | 21.29 | 21.26 | 21.28 | 21.25 | |
| Lactose, % | 8.75 | 8.75 | 8.75 | 8.75 | |
| Total dietary fiber, % | 11.89 | 11.83 | 11.83 | 11.79 | |
| Isoflavones, ppm | |||||
| Total geinistein | 52.0 | 757 | 291 | 947 | |
| Total daidzein | 0.00 | 625 | 358 | 969 | |
| Total glycitin | 34.0 | 69.0 | 48.0 | 125 | |
| Total isoflavones | 87.0 | 1,451 | 697 | 2,041 | |
| Total saponins, ppm | 1,336 | 1,316 | 1,382 | 1,252 | |
| Total amino acids, % | |||||
| Indispensable | |||||
| Arginine | 1.28 | 1.26 | 1.20 | 1.24 | |
| Histidine | 0.50 | 0.50 | 0.48 | 0.49 | |
| Isoleucine | 0.91 | 0.90 | 0.88 | 0.92 | |
| Leucine | 1.75 | 1.73 | 1.69 | 1.72 | |
| Lysine | 1.48 | 1.44 | 1.42 | 1.48 | |
| Methionine | 0.48 | 0.47 | 0.42 | 0.45 | |
| Phenylalanine | 0.97 | 0.95 | 0.94 | 0.97 | |
| Threonine | 0.99 | 0.96 | 0.91 | 0.97 | |
| Tryptophan | 0.31 | 0.30 | 0.28 | 0.30 | |
| Valine | 1.04 | 1.04 | 0.98 | 1.02 | |
| Dispensable | |||||
| Alanine | 1.06 | 1.04 | 1.00 | 1.04 | |
| Aspartic acid | 1.97 | 1.95 | 1.87 | 1.95 | |
| Cysteine | 0.30 | 0.30 | 0.29 | 0.29 | |
| Glutamic acid | 3.54 | 3.53 | 3.43 | 3.49 | |
| Glycine | 0.98 | 0.94 | 0.89 | 0.95 | |
| Proline | 1.22 | 1.19 | 1.14 | 1.19 | |
| Serine | 0.87 | 0.87 | 0.83 | 0.85 | |
| Tyrosine | 0.65 | 0.64 | 0.61 | 0.65 |
All pigs received allotted treatment diet upon starting −7 DPI. DPI = day postinoculation; ISF = isoflavones.
1Analyzed crude protein determined as % total nitrogen × 6.25.
Following a 1-wk adaptation period to experimental diets, blood was collected from each pig to establish 0 DPI baseline measurements and to ensure that all pigs were PRRSV-negative at study initiation. Immediately following blood collection, pigs were administered via intranasal inoculation either 2 mL of a 2% fetal bovine serum + phosphate-buffered saline solution (sham-control) or 9.3 × 103 50% tissue culture infective dose of PRRS virus (strain NADC20, courtesy of Dr. Federico Zuckermann, University of Illinois, Urbana, IL).
Although the model was designed to only utilize PRRSV as the immune challenge, the source herd for piglets used for this study tested positive for Mycobacterium hyopneumoniae (Mh) after completion of the first cohort and prior to delivery of pigs for the second cohort. We confirmed Mh status in individual pigs by qRT-PCR detection of the bacterium in lung tissue only from pigs included in the second cohort, but it is likely that pigs from the first cohort were also harboring the bacterium based on the length of time required for Mh to establish infection and present associated clinical signs (Maes et al. 2018). It should be noted that all but 2 pigs from the second cohort, including those not infected with PRRSV, tested positive for Mh specifically, though the 2 Mh-negative pigs did test positive for general Mycoplasma by qRT-PCR.
Growth Performance, Rectal Temperatures, and Blood Collection
Individual pig and feeder weights were recorded weekly throughout the study to allow for calculation of average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (gain to feed, G:F). Growth performance data are reported in reference to the inoculation schedule (−7 to 14 DPI). Rectal temperatures of all pigs were measured on 0, 3, 6, 8, 12, and 14 DPI using an over-the-counter, consumer-grade digital thermometer in the morning prior to any blood sample collection.
Blood (up to 8 mL total) was collected from the jugular vein of each pig into evacuated tubes (BD, Franklin Lakes, NJ) at 0, 3, 6, 12, and 14 DPI using a 21 gauge needle. Blood samples from 0 DPI were collected immediately before inoculation. Three milliliters of blood were collected into tubes containing EDTA as an anti-coagulant (i.e., whole blood), placed on ice, and submitted to the University of Illinois Veterinary Clinical Pathology Laboratory for analysis as described in the following section. Three milliliters of blood were collected into serum tubes, allowed to clot at room temperature, and centrifuged at 1,300 × g for 15 min at 20 °C. Serum was removed from centrifuged samples and stored in 0.5-mL aliquots at °80 °C pending subsequent analyses. On 12 DPI, a total of 8 mL of whole blood were collected from each animal, placed on ice, and used for isolation of peripheral blood mononuclear cells (PBMC) for immunophenotyping procedures as described below.
Blood and Sera Measurements
At 0, 3, 6, and 14 DPI, a multiparameter, automated hematology analyzer (CELL-DYN 3700, Abbott Laboratories, Abbott Park, IL) was used to determine the total and differential cell counts of whole blood by the University of Illinois Veterinary Clinical Pathology Laboratory. Serum from the same experimental time points was submitted to the University of Illinois Veterinary Diagnostic Laboratory to be analyzed for PRRSV load at 0, 3, 6, and 14 DPI by qRT-PCR using Z-PRRSV Multiplex reagents (Tetracore, Rockville, MD) and identical extraction conditions and a single quantity of starting RNA material for all experimental samples. Both positive and “no-template” controls were run along the experimental samples. The assay used a single-tubed method based on fluorogenic probe hydrolysis (TaqMan, Applied Biosystems, Foster City, CA). Viral load was expressed as cycle threshold (Ct) values, where a higher Ct value represents a lower amount of PRRSV mRNA. Additionally, a PRRSV antibody screen by ELISA was performed on qRT-PCR-positive samples collected on 6 and 14 DPI for detection of anti-PRRSV antibodies. The results for this assay are expressed as a sample to positive ratio (S/P ratio) that represents the following relationship: [sample mean × (mean of optical absorbance) − negative control mean]/(positive control mean − negative control mean). An S/P ratio greater than 0.4 is considered a positive result and the larger the number, the more anti-PRRSV antibodies that are present. Finally, serum concentrations of cytokines tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin-1 beta (IL-1β), interleukin-10 (IL-10), interleukin-8 (IL-8), interferon alpha (IFNα), and interleukin-4 (IL-4) were also measured at 0, 3, 6, and 14 DPI with the Swine Cytokine Magnetic 7-Plex Panel according to manufacturer instructions (Novex by Life Technologies, Frederick, MD).
T-Cell Immunophenotyping
Whole blood collected on 12 DPI was used for isolation of PBMC for a T-cell immunophenotyping procedure using flow cytometry. In brief, PBMC were isolated by placing individual whole blood samples over a density gradient (SepMate 15 [IVD] and Lymphoprep, StemCell Technologies, Cambridge, MA) and centrifuging at 1,200 × g for 25 min at 20 °C. Isolated PBMC were then washed with phosphate buffer saline containing 2% fetal bovine serum before being counted using the Moxi Z Mini Automated Cell Counter (ORFLO Technologies, Ketchum, ID). Individual samples were prepared with 1.0 × 106 cells and labeled with external fluorescent antibodies against cell surface markers CD3 (PE-Cy7 Mouse Anti-Pig CD3ε, BD Pharmingen, San Jose, CA), CD4 (Alexa Fluor Mouse Anti-Pig CD4a, BD Pharmingen, San Jose, CA), and CD8 (PE Mouse Anti-Pig CD8b, BD Pharmingen, San Jose, CA). Following application of external antibodies, cells were permeabilized using the BD Perm/Wash Buffer (BD Biosciences, San Jose, CA) and fixed using 4% paraformaldehyde solution. The following day, cells were labeled with an intracellular fluorescent antibody against IFN-γ (PerCP5.5 Mouse Anti-Pig IFN-γ, BD Pharmingen, San Jose, CA). Prepared, labeled cells were evaluated at the University of Illinois Flow Cytometry Facility using a BD LSR II Flow Cytometry Analyzer (BD Biosciences, San Jose, CA) and flow cytometer outputs were analyzed using FCS Express 5 Plus (De Novo Software, Glendale, CA).
Statistical Analyses
Statistical analysis was dependent on whether outcomes were measured at a single time point or at multiple time points for the same subject. Apart from growth performance outcomes, individual pig was considered the experimental unit, with 12 replicate pens or pigs for each of the 5 experimental treatments. A total of 4 different diets were fed to PRRSV-infected pigs, whereas a single group of uninfected pigs received only the control diet. Thus, the 2- or 3-way analyses of variance described below involve effects between diets and within PRRSV-infected pigs, whereas a separate analysis was used to compare uninfected and infected groups receiving the control diet. In all cases, interaction means are presented in data tables, whereas significant main effect means are presented in data figures.
For all single time-point outcomes, a 2-way ANOVA was conducted using the MIXED procedure of SAS 9.3 (SAS Institute, Inc., Cary, NC) with factors including the dietary soy source (SPC vs. ETSBM) and addition of supplemental soy ISF (no vs. yes). Comparison of uninfected and infected groups each fed the control diet was made using a single degree of freedom contrast between these experimental treatments.
With regard to repeated measures outcomes, a 3-way ANOVA was conducted for all outcomes involving samples collected from the same subject at multiple time points with factors including the dietary soy source (SPC vs. ETSBM), addition of supplemental soy ISF (no vs. yes), and time (DPI). Repeated measures were sliced by DPI for main effect and interaction means and those P-values are included in data tables. No 2-way or 3-way interactive effects involving DPI were significant, so these effects were removed from the statistical model. Comparison of uninfected and infected groups each fed the control diet was made using a single degree of freedom contrast between these experimental treatments. In all cases, outliers were identified as having an absolute Studentized residual value of 3 or greater and significance was accepted with at P ≤ 0.05.
RESULTS
Growth Performance
Growth performance results are shown in Table 5. Within the PRRSV-infected groups, no main or interactive effects were noted during the preinoculation period (P > 0.05; −7 to 0 DPI). However, significant decreases (P < 0.01) in ADG were observed between uninfected and infected groups fed the control diet throughout the postinoculation period, thereby signifying a successful PRRSV-infection. During the infective period, there were main effects of ISF supplementation on G:F between 6 and 14 DPI and main effects of soy source on ADG and G:F over the entire 2-wk infection period (P < 0.05; 0 to 14 DPI). From 6 to 14 DPI, pigs receiving the ETSBM + ISF diet experienced less efficient growth compared with pigs receiving only ETSBM (173 vs. 484 g/kg, respectively; P < 0.05). Over the 2-wk infection period, pigs receiving the ETSBM diet maintained a higher ADG compared with pigs fed the Control diet, regardless of ISF supplementation (219 vs. 137 g/d, respectively; P < 0.05). Additionally, pigs receiving ETSBM, regardless of ISF supplementation, had greater feed efficiency compared with pigs receiving the Control + ISF diet over the 2-wk infection period (P < 0.05). Overall, PRRSV-infection reduced ADG and G:F during the 2-wk infection period (0 to 14 DPI) resulting in a lower final body weight (P < 0.0001) for PRRSV-infected pigs compared with uninfected pigs fed the Control diet.
Table 5.
Effects of dietary soy isoflavones level and porcine reproductive and respiratory virus (PRRSV) infection on growth performance of weanling pigs
| Item | Uninfected | PRRSV-infected | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected vs. Infected Control | Main effects2 | Interaction | ||||||||
| Control1 | Control1 | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy source | ISF | Soy source × ISF | |||
| BW, kg | ||||||||||
| initial | 5.72 | 5.74 | 5.57 | 5.73 | 5.70 | 0.43 | 0.872 | 0.887 | 0.814 | 0.992 |
| final | 12.12 | 8.65* | 7.89 | 9.28 | 8.91 | 0.45 | <0.001 | 0.057 | 0.196 | 0.151 |
| −7 to 0 DPI3 | ||||||||||
| ADG, g/d | 161 | 127 | 160 | 155 | 173 | 20.9 | 0.287 | 0.321 | 0.214 | 0.727 |
| ADFI, g/d4 | 677 | 632* | 654 | 604 | 649 | 21.6 | 0.042 | 0.453 | 0.137 | 0.621 |
| G:F, g/kg4 | 264 | 214 | 240 | 259 | 275 | 35.1 | 0.300 | 0.272 | 0.559 | 0.878 |
| 0 to 6 DPI | ||||||||||
| ADG, g/d | 275 | 103* | 83.2 | 126 | 107 | 35.2 | 0.001 | 0.362 | 0.441 | 0.965 |
| ADFI, g/d4 | 1,236 | 1,381 | 1,188 | 1,128 | 1,403 | 257.0 | 0.295 | 0.885 | 0.756 | 0.084 |
| G:F, g/kg4 | 248 | 75.5* | 99.2 | 120 | 93.6 | 37.1 | <0.001 | 0.551 | 0.977 | 0.443 |
| 6 to 14 DPI | ||||||||||
| ADG, g/d | 460 | 190* | 156 | 270 | 219 | 46.7 | 0.0002 | 0.099 | 0.317 | 0.838 |
| ADFI, g/d4 | 728 | 489* | 423 | 549 | 450 | 149.8 | 0.080 | 0.633 | 0.442 | 0.845 |
| G:F, g/kg4 | 526 | 437 | 268 | 484 | 173 | 99.5 | 0.384 | 0.803 | 0.020 | 0.463 |
| 0 to 14 DPI3 | ||||||||||
| ADG, g/d | 380 | 137* | 103 | 219 | 165 | 39.5 | <0.001 | 0.015 | 0.125 | 0.712 |
| ADFI, g/d4 | 775 | 648 | 558 | 629 | 601 | 186.6 | 0.229 | 0.888 | 0.485 | 0.708 |
| G:F, g/kg4 | 566 | 250* | 171 | 378 | 361 | 59.8 | 0.001 | 0.009 | 0.413 | 0.598 |
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
Values represent least square means of 6 to 12 pigs. All pigs received allotted treatment diet starting −7 DPI. DPI = days postinoculation; ISF = isoflavones.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day post-inoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
3Feed intake data and G:F values for −7 to 0 DPI from the first cohort of pigs (n = 28) were omitted due to a mechanical error with the feeders that led to incorrect feed intake measurements.
4ADFI was omitted if there was a net weight loss over measured period; G:F data were omitted if calculated value was less than 0 g/kg or greater than 1,000 g/kg.
Rectal Temperatures
Rectal temperature data were collected frequently over the entire infection period (0 to 14 DPI; Table 6). Infection with PRRSV increased (P < 0.05) rectal temperatures of pigs at 0, 3, 6, and 8 DPI, but there was no influence (P > 0.05) of dietary soy protein source or ISF supplementation on this outcome.
Table 6.
Effects of dietary soy isoflavones level and porcine reproductive and respiratory virus (PRRSV) infection on daily rectal temperatures (°C) of weanling pigs
| DPI | Uninfected | PRRSV-infected | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected vs. infected control | Main effects2 | Interaction | ||||||||
| Control1 | Control | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy Source | ISF | Soy Source × ISF | |||
| 0 | 39.36 | 39.86* | 39.91 | 39.69 | 39.65 | 0.28 | 0.036 | 0.228 | 0.971 | 0.681 |
| 3 | 39.48 | 40.07* | 39.92 | 40.35 | 40.11 | 0.27 | 0.012 | 0.197 | 0.266 | 0.400 |
| 6 | 39.65 | 40.12 | 40.42 | 40.47 | 40.18 | 0.27 | 0.051 | 0.748 | 0.965 | 0.399 |
| 8 | 39.58 | 40.36* | 40.69 | 40.50 | 40.62 | 0.27 | 0.001 | 0.848 | 0.205 | 0.562 |
| 12 | 40.01 | 40.24 | 40.68 | 40.50 | 40.49 | 0.27 | 0.340 | 0.855 | 0.247 | 0.417 |
| 14 | 39.88 | 40.29 | 40.21 | 40.29 | 40.40 | 0.27 | 0.091 | 0.630 | 0.949 | 0.922 |
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
Values represent least square means of 10 to 12 pigs. All pigs received allotted treatment diet starting −7 DPI. DPI = days postinoculation; ISF = isoflavones; WBC = white blood cells; NEU = neutrophils; LYM = lymphocytes; MONO = monocytes.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day postinoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
Serum Viral Load and Anti-PRRSV Antibody Presence
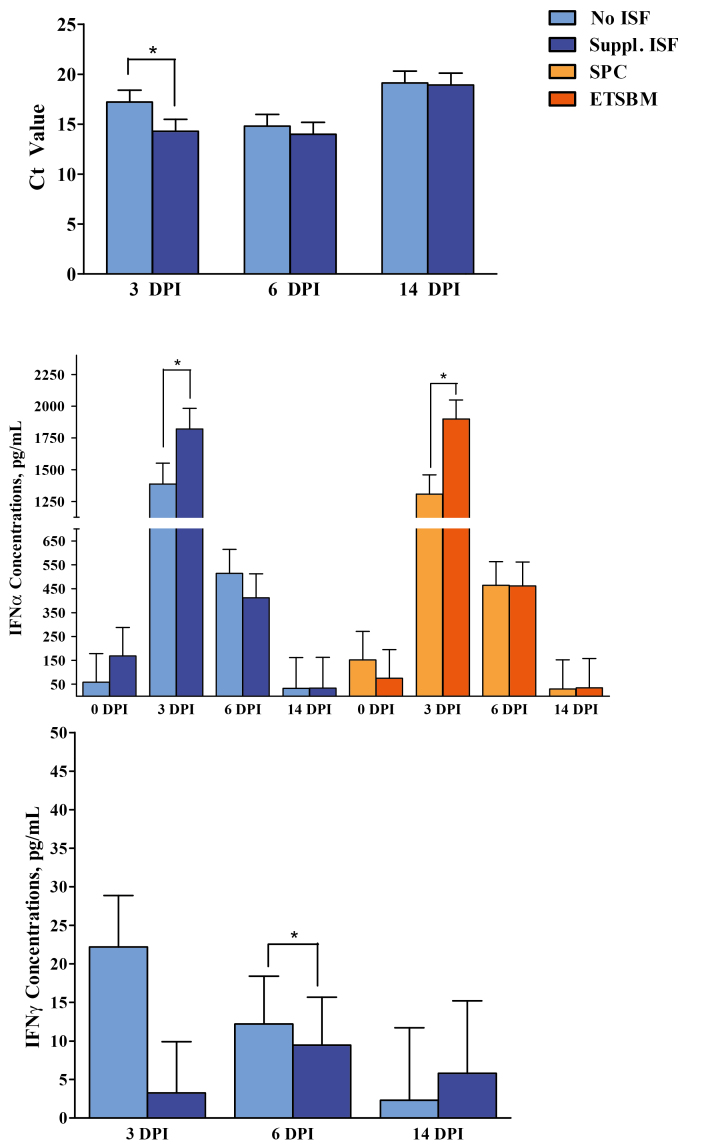
Results for serum viral load and presence of anti-PRRSV antibody assays can be found in Table 7. Analysis of serum by qRT-PCR indicated that all pigs were PRRSV-negative at 0 DPI. At 3, 6, and 14 DPI, all pigs in the uninfected group remained free of PRRSV as shown by negative qRT-PCR results, whereas all pigs inoculated with PRRSV tested positive for PRRSV mRNA. Among the PRRSV-infected groups, a main effect of ISF was observed at 3 DPI with Ct values, the number PCR cycles required to detect presence the viral genome, being lower (i.e., more viral mRNA copies present) in pigs receiving supplemental ISF than those pigs unsupplemented (14.27 vs. 17.19, respectively; P < 0.05; Figure 1). Additionally, there was an interaction main effect observed at 3 DPI with pigs fed the ETSBM + ISF diet having lower Ct values compared with pigs in all other groups (P < 0.05).
Table 7.
Effects of dietary soy isoflavones level and porcine reproductive and respiratory virus (PRRSV) infection on serum viral load and presence of anti-PRRSV antibodies in weanling pigs
| Item | Uninfected | PRRSV-infected | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected vs. infected control | Main effects2 | Interaction | ||||||||
| Control1 | Control1 | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy Source | ISF | Soy Source × ISF | |||
| Ct value3 | ||||||||||
| 0 DPI | ND | ND | ND | ND | ND | 1.52 | 0.812 | 0.968 | 0.968 | 0.999 |
| 3 DPI | ND | 15.57*b | 16.44b | 18.81b | 12.10a | 1.41 | <0.001 | 0.592 | 0.005 | 0.0001 |
| 6 DPI | ND | 14.12* | 15.39 | 15.37 | 12.66 | 1.41 | <0.001 | 0.522 | 0.430 | 0.166 |
| 14 DPI | ND | 18.75* | 19.09 | 19.51 | 18.69 | 1.45 | <0.001 | 0.860 | 0.795 | 0.948 |
| ELISA S/P ratio4 | ||||||||||
| 6 DPI | – | 0.087 | 0.070 | 0.040 | 0.045 | 0.10 | – | 0.661 | 0.935 | 0.974 |
| 14 DPI | – | 1.565 | 1.736 | 1.475 | 1.440 | 0.171 | – | 0.027 | 0.412 | 0.080 |
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
abMeans without common superscript letter do differ at P ≤ 0.05.
Values represent least square means of 10 to 12 pigs. All pigs received allotted treatment diet starting −7 DPI. DPI = days post-inoculation; ISF = isoflavones; ELISA = enzyme-linked immunosorbent assay; ND = not detected.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day post-inoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
3Cycle threshold (Ct) values represent the mean number of PCR cycles required to detect the presence of PRRSV mRNA. A higher Ct value indicates less PRRSV DNA in the serum. No PRRSV DNA was detected in the serum of any uninfected pigs.
4IDEXX-ELISA S/P Ratio of 0.4 or greater is considered positive result. IDEXX ELISA assays were not performed on samples confirmed negative by qRT-PCR.
Figure 1.
Main effect of isoflavone supplementation on the concentration of PRRSV antigen in serum, main effects of both soy source and isoflavone supplementation on serum interferon-alpha cytokine concentrations, and main effects of isoflavone supplementation on serum interferon-gamma cytokine concentrations. PRRSV = porcine reproductive and respiratory syndrome virus; ISF = isoflavones; SPC = soy protein concentrate; ETSBM = enzyme-treated soybean meal; DPI = days postinoculation; IFNα = interferon-alpha; IFNγ = interferon-gamma. Values represent least square means of 20 to 24 pigs, and error bars indicate standard error of the mean. Cycle threshold (Ct) values represent the mean number of PCR cycles required to detect the presence of PRRSV mRNA. A higher Ct value indicates less PRRSV DNA in the serum. An asterisk (*) denotes a difference (P < 0.05) due to soy protein source or isoflavone supplementation status at a given time point.
The presence of anti-PRRSV antibodies was also assessed on qRT-PCR-positive samples collected on 6 and 14 DPI by IDEXX-ELISA (Table 7). As a reminder, an S/P ratio greater than 0.4 is considered a positive result and the larger the number, the more anti-PRRSV antibodies that are present. Although there were no main or interactive effects observed at 6 DPI, a main effect of soy source was observed on 14 DPI (P < 0.05). Pigs receiving the control diets had greater concentrations of anti-PRRSV antibodies than those receiving the ETSBM diets, regardless of ISF supplementation (1.649 vs. 1.458, respectively; P < 0.05). Please note that this assay was for total anti-PRRSV antibody presence and was not specific for neutralizing antibody types.
Red Blood Cell Measurements
Results for red blood cell measurements can be found in Table 8. Between noninfected and infected pigs, PRRSV-infection resulted in reductions of red blood cell counts (RBC, × 106 cells/mL), hemoglobin concentrations (HGB, g/dL), hematocrit (HCT, %), mean corpsucular volume (MCV, fl), and mean corpuscular hemoglobin (MCH, pg) at 14 DPI (P < 0.05), but no other time points were affected. No main effects of soy protein source or ISF supplementation were observed for any red blood cell parameters. The only significant interaction effect between soy source and ISF supplementation was for mean corpuscular hemoglobin concentration (MCHC, g/dL) at 14 DPI. PRRSV-infected pigs receiving the ETSBM + ISF diet had lower MCHC compared with all other infected groups (P < 0.05).
Table 8.
Effects of dietary soy isoflavone levels and porcine reproductive and respiratory virus (PRRSV) infection on red blood cell measures in weanling pigs
| Item | Uninfected | PRRSV-infected | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected vs. Infected Control | Main effects2 | Interaction | ||||||||
| Control1 | Control1 | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy Source | ISF | Soy Source × ISF | |||
| RBC, × 106/µL | ||||||||||
| 0 DPI | 6.53 | 6.66 | 6.84 | 6.49 | 6.81 | 0.180 | 0.624 | 0.456 | 0.099 | 0.305 |
| 3 DPI | 6.20 | 6.00 | 6.14 | 5.90 | 6.29 | 0.180 | 0.419 | 0.862 | 0.066 | 0.234 |
| 6 DPI | 5.97 | 6.05 | 6.17 | 5.87 | 6.00 | 0.175 | 0.778 | 0.220 | 0.375 | 0.502 |
| 14 DPI | 5.85 | 5.31* | 5.58 | 5.25 | 5.43 | 0.187 | 0.050 | 0.519 | 0.129 | 0.436 |
| HGB, g/dL | ||||||||||
| 0 DPI | 12.3 | 11.9 | 12.5 | 12.2 | 12.5 | 0.341 | 0.552 | 0.750 | 0.190 | 0.588 |
| 3 DPI | 11.5 | 10.6 | 11.1 | 10.8 | 11.4 | 0.341 | 0.119 | 0.472 | 0.070 | 0.268 |
| 6 DPI | 10.9 | 10.4 | 10.9 | 10.5 | 10.8 | 0.326 | 0.386 | 0.888 | 0.205 | 0.633 |
| 14 DPI | 11.0 | 9.0* | 9.5 | 9.1 | 9.5 | 0.358 | 0.002 | 0.874 | 0.156 | 0.540 |
| HCT, % | ||||||||||
| 0 DPI | 35.1 | 37.4 | 39.1 | 37.9 | 39.1 | 0.976 | 0.294 | 0.882 | 0.172 | 0.591 |
| 3 DPI | 35.7 | 33.4 | 34.9 | 33.8 | 35.6 | 0.976 | 0.272 | 0.537 | 0.081 | 0.314 |
| 6 DPI | 34.0 | 32.9 | 34.4 | 33.1 | 33.7 | 0.930 | 0.635 | 0.722 | 0.301 | 0.708 |
| 14 DPI | 34.7 | 28.5* | 30.3 | 28.8 | 30.0 | 1.03 | 0.010 | 0.893 | 0.116 | 0.442 |
| MCV, fl | ||||||||||
| 0 DPI | 58.6 | 56.2 | 57.2 | 58.5 | 57.5 | 1.91 | 0.281 | 0.257 | 0.937 | 0.579 |
| 3 DPI | 57.9 | 55.7 | 56.9 | 57.3 | 56.7 | 1.75 | 0.300 | 0.500 | 0.801 | 0.742 |
| 6 DPI | 57.4 | 54.6 | 55.8 | 56.4 | 56.1 | 1.72 | 0.209 | 0.346 | 0.712 | 0.706 |
| 14 DPI | 59.6 | 53.7* | 54.2 | 55.0 | 55.3 | 1.79 | 0.013 | 0.292 | 0.700 | 0.719 |
| MCH, pg | ||||||||||
| 0 DPI | 18.6 | 17.9 | 18.3 | 18.8 | 18.4 | 0.662 | 0.331 | 0.224 | 0.961 | 0.498 |
| 3 DPI | 18.4 | 17.7 | 18.1 | 18.3 | 18.1 | 0.650 | 0.374 | 0.451 | 0.698 | 0.717 |
| 6 DPI | 18.2 | 17.3 | 17.7 | 17.9 | 18.0 | 0.650 | 0.219 | 0.283 | 0.510 | 0.632 |
| 14 DPI | 18.8 | 17.0* | 17.1 | 17.4 | 17.5 | 0.675 | 0.037 | 0.312 | 0.775 | 0.762 |
| MCHC, g/dL | ||||||||||
| 0 DPI | 31.8 | 31.6 | 32.0 | 32.0 | 32.0 | 0.734 | 0.721 | 0.842 | 0.871 | 0.988 |
| 3 DPI | 31.7 | 31.6b | 31.9b | 31.9b | 29.3a | 0.734 | 0.943 | 0.092 | 0.096 | 0.024 |
| 6 DPI | 31.7 | 31.5 | 31.8 | 31.6 | 32.0 | 0.700 | 0.561 | 0.836 | 0.655 | 0.968 |
| 14 DPI | 31.5 | 31.6 | 31.6 | 31.6 | 31.7 | 0.774 | 0.662 | 0.920 | 0.991 | 0.999 |
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
abMeans without common superscript letter differ at P ≤ 0.05.
Values represent least square means of 10 to 12 pigs. All pigs received allotted treatment diet starting −7 DPI. DPI = days post-inoculation; ISF = isoflavones; RBC = red blood cells; HGB = hemoglobin; HCT = packed cell volume; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day postinoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
Leukocyte Measurements
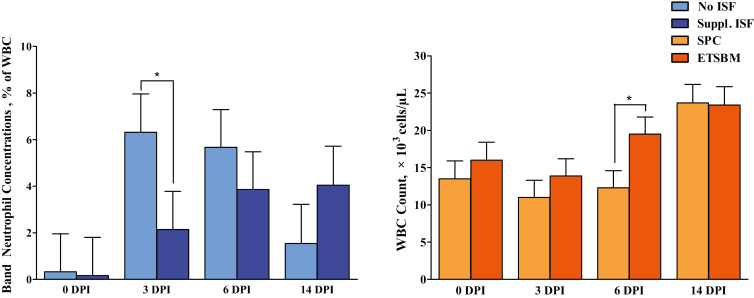
Results for leukocyte measurements are shown in Table 9. Regarding relative proportions of individual white blood cell (WBC) populations, PRRSV-infection resulted in leukopenia at 6 and 14 DPI, neutrophilia at 6 and 14 DPI, greater circulatory concentrations of band cells (i.e., circulating immature neutrophils; BAND) at 3 and 6 DPI, and lymphopenia at 3, 6, and 14 DPI when comparing noninfected and infected control pigs (P < 0.05). Infected control pigs also exhibited greater monocyte populations than noninfected controls, though this was only observed at 0 DPI (P < 0.05). There were main effects of ISF supplementation for band cells. Animals receiving supplemental ISF had lower (P < 0.05) concentrations of circulatory band cells, though this effect was only observed at 3 DPI (2.14% vs. 6.32%, respectively; Figure 2). There were main effects of soy source for total WBC counts and eosinophil concentrations. Animals receiving the ETSBM diet without supplemental ISF had greater (P < 0.05) WBC counts than all other treatments at 6 DPI, but this effect was not maintained at any other time point (19.47 × 103 cells vs. 12.32 × 103 cells, respectively; Figure 2). Additionally, pigs fed the ETSBM diet had lower (P < 0.05) circulatory eosinophil concentrations compared with pigs receiving the Control diet at 3 DPI, with pigs receiving the Control + ISF having the greatest (1.17% vs. 3.03%, respectively). There were interaction effects for WBC counts and eosinophils. Within infected groups, pigs receiving the ETSBM + ISF diet experienced the least severe (P < 0.05) leukopenia at 6 DPI (i.e., smaller reduction in WBC counts) compared with all other treatments. Additionally, as previously discussed for the main effect of soy source, pigs receiving the Control + ISF treatment had greater (P < 0.05) eosinophil concentrations than both ETSBM treatments, regardless of ISF supplementation.
Table 9.
Effects of dietary soy isoflavones level and porcine reproductive and respiratory virus (PRRSV) infection on differential leukocyte counts and relative population proportions in weanling pigs
| Item | Uninfected | PRRSV-infected | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected vs. infected control | Main effects2 | Interaction | ||||||||
| Control1 | Control1 | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy Source | ISF | Soy Source × ISF | |||
| WBC, × 103/µL | ||||||||||
| 0 DPI | 15.2 | 13.3 | 13.4 | 14.8 | 17.2 | 3.39 | 0.359 | 0.447 | 0.719 | 0.826 |
| 3 DPI | 16.1 | 12.2 | 9.83 | 14.2 | 13.7 | 3.39 | 0.061 | 0.360 | 0.670 | 0.789 |
| 6 DPI | 16.4 | 11.8*a | 12.7a | 24.4b | 14.5a | 3.24 | 0.031 | 0.026 | 0.153 | 0.018 |
| 14 DPI | 16.6 | 23.1* | 24.5 | 23.2 | 23.5 | 3.57 | 0.006 | 0.929 | 0.787 | 0.990 |
| NEU, % of WBC | ||||||||||
| 0 DPI | 49.1 | 45.3 | 38.9 | 47.5 | 40.5 | 4.64 | 0.522 | 0.671 | 0.137 | 0.492 |
| 3 DPI | 46.3 | 56.5 | 57.1 | 57.8 | 55.1 | 4.64 | 0.076 | 0.938 | 0.811 | 0.977 |
| 6 DPI | 38.7 | 51.5* | 54.2 | 59.1 | 47.4 | 4.42 | 0.030 | 0.345 | 0.301 | 0.281 |
| 14 DPI | 35.7 | 49.9* | 43.2 | 46.9 | 39.4 | 4.89 | 0.027 | 0.466 | 0.134 | 0.340 |
| BAND, % of WBC | ||||||||||
| 0 DPI | 0.00 | 0.135 | 0.00 | 0.550 | 0.333 | 2.06 | 0.858 | 0.810 | 0.924 | 0.995 |
| 3 DPI | 1.10 | 6.00* | 1.10 | 6.63 | 3.18 | 2.06 | 0.038 | 0.412 | 0.012 | 0.073 |
| 6 DPI | 0.304 | 6.11* | 2.45 | 5.25 | 5.27 | 1.99 | 0.016 | 0.542 | 0.267 | 0.422 |
| 14 DPI | 0.087 | 1.27 | 4.44 | 1.77 | 3.63 | 2.14 | 0.648 | 0.922 | 0.157 | 0.546 |
| LYM, % of WBC | ||||||||||
| 0 DPI | 47.1 | 45.7 | 51.3 | 45.3 | 51.7 | 4.44 | 0.780 | 0.973 | 0.149 | 0.546 |
| 3 DPI | 47.1 | 31.1* | 35.8 | 31.0 | 37.5 | 4.43 | 0.002 | 0.851 | 0.179 | 0.588 |
| 6 DPI | 52.9 | 32.2* | 32.8 | 27.7 | 38.1 | 4.24 | 0.0001 | 0.905 | 0.176 | 0.348 |
| 14 DPI | 51.7 | 39.5* | 41.9 | 45.0 | 47.7 | 4.67 | 0.032 | 0.219 | 0.592 | 0.588 |
| MONO, % of WBC | ||||||||||
| 0 DPI | 2.77 | 7.09* | 7.19 | 5.56 | 6.00 | 1.22 | 0.016 | 0.229 | 0.842 | 0.379 |
| 3 DPI | 4.47 | 3.25 | 2.10 | 2.98 | 2.62 | 1.22 | 0.472 | 0.911 | 0.508 | 0.905 |
| 6 DPI | 6.06 | 6.50 | 7.53 | 5.42 | 5.62 | 1.17 | 0.804 | 0.181 | 0.594 | 0.530 |
| 14 DPI | 4.73 | 4.81 | 5.86 | 3.70 | 5.07 | 1.28 | 0.963 | 0.454 | 0.307 | 0.679 |
| EOSIN, % of WBC | ||||||||||
| 0 DPI | 1.19 | 1.63 | 2.11 | 1.14 | 1.42 | 0.979 | 0.644 | 0.446 | 0.591 | 0.830 |
| 3 DPI | 0.993 | 2.25ab | 3.80b | 1.04a | 1.31a | 0.979 | 0.171 | 0.013 | 0.218 | 0.047 |
| 6 DPI | 1.79 | 3.06 | 2.49 | 2.25 | 3.40 | 0.951 | 0.176 | 0.934 | 0.677 | 0.669 |
| 14 DPI | 2.95 | 4.07 | 4.15 | 2.19 | 3.61 | 1.01 | 0.272 | 0.119 | 0.358 | 0.280 |
| BASO, % of WBC | ||||||||||
| 0 DPI | 0.200 | 0.100 | 0.207 | 0.00 | 0.083 | 0.240 | 0.826 | 0.631 | 0.682 | 0.942 |
| 3 DPI | 0.100 | 0.917 | 0.300 | 0.364 | 0.182 | 0.240 | 0.064 | 0.144 | 0.083 | 0.095 |
| 6 DPI | 0.200 | 0.636 | 0.455 | 0.250 | 0.182 | 0.636 | 0.327 | 0.147 | 0.581 | 0.482 |
| 14 DPI | 0.500 | 0.400 | 0.261 | 0.294 | 0.558 | 0.252 | 0.836 | 0.695 | 0.798 | 0.814 |
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
abMeans without common superscript letter differ at P ≤ 0.05.
Values represent least square means of 10 to 12 pigs. All pigs received allotted treatment diet starting −7 DPI. DPI = days postinoculation; ISF = isoflavones; WBC = white blood cells; NEU = neutrophils; BAND = immature neutrophils aka. band cells; LYM = lymphocytes; MONO = monocytes; EOSIN = eosinophils; BASO = basophils.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day postinoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
Figure 2.
Main effect of isoflavone supplementation on the relative concentrations of band neutrophils in whole blood and main effect of soy source on total while blood cell counts in peripheral whole blood. ISF = isoflavones; SPC = soy protein concentrate; ETSBM = enzyme-treated soybean meal; DPI = days postinoculation; WBC = white blood cells. Values represent least square means of 20 to 24 pigs, and error bars indicate standard error of the mean. An asterisk (*) denotes a difference (P < 0.05) due to soy protein source or isoflavone supplementation status at a given time point.
Serum Cytokine Concentrations
Serum cytokine concentrations, quantified using a porcine-specific multiplexed ELISA, are shown in Table 10. Due to large numbers of samples with low or absent detection, cytokines IL-1β, IL-8, and IL-4 were omitted from analysis. PRRSV-infected pigs had higher circulating concentrations of IFNα and TNFα at 3 and 14 DPI, respectively, compared with uninfected controls. No other cytokines or time points were influenced by infection status.
Table 10.
Effects of dietary soy isoflavones level and porcine reproductive and respiratory virus (PRRSV) infection on serum cytokine concentrations (pg/mL) in weanling pigs
| Item, pg/mL | Uninfected | PRRSV-infected | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected vs. infected control | Main effects2 | Interaction | ||||||||
| Control1 | Control1 | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy source | ISF | Soy Source × ISF | |||
| TNFα | ||||||||||
| 0 DPI | 128 | 33.2 | 38.7 | 39.6 | 50.0 | 181 | 0.088 | 0.953 | 0.958 | 0.999 |
| 3 DPI | 72.7 | 89.7 | 120 | 85.1 | 104 | 92.9 | 0.665 | 0.897 | 0.786 | 0.992 |
| 6 DPI | 58.7 | 75.1 | 140 | 59.9 | 73.4 | 98.8 | 0.676 | 0.634 | 0.666 | 0.929 |
| 14 DPI | 52.2 | 152* | 113 | 187 | 353 | 92.9 | 0.017 | 0.094 | 0.429 | 0.160 |
| IFNα | ||||||||||
| 0 DPI | 18.6 | 101 | 202 | 16.4 | 134 | 164 | 0.722 | 0.645 | 0.509 | 0.882 |
| 3 DPI | 19.3 | 1,132*a | 1,483ab | 1,643bc | 2,156c | 232 | <0.001 | 0.005 | 0.039 | 0.005 |
| 6 DPI | 142 | 501 | 428 | 529 | 396 | 147 | 0.118 | 0.990 | 0.465 | 0.900 |
| 14 DPI | 5.31 | 44.6 | 17.1 | 21.6 | 50.2 | 190 | 0.930 | 0.976 | 0.998 | 0.999 |
| IFNγ | ||||||||||
| 0 DPI | BDL | BDL | BDL | BDL | BDL | – | – | – | – | – |
| 3 DPI | BDL | 39.4a | 3.39b | 4.96b | 3.10b | 10.1 | – | 0.069 | 0.048 | 0.026 |
| 6 DPI | BDL | 12.2 | 8.91 | 12.2 | 10.0 | 9.31 | – | 0.950 | 0.746 | 0.989 |
| 14 DPI | 2.88 | 2.58 | 2.00 | 2.04 | 9.62 | 14.2 | 0.996 | 0.772 | 0.775 | 0.963 |
| IL-10 | ||||||||||
| 0 DPI | BDL | BDL | 12.8 | BDL | BDL | 39.6 | – | – | – | – |
| 3 DPI | BDL | 26.2 | 23.7 | 33.8 | 24.9 | 19.8 | – | 0.755 | 0.810 | 0.977 |
| 6 DPI | BDL | 27.1 | 42.1 | 33.5 | 31.3 | 22.9 | – | 0.914 | 0.759 | 0.959 |
| 14 DPI | BDL | BDL | 33.6 | BDL | 154 | 28.0 | – | – | – | – |
a-cMeans without common superscript letter differ at P ≤ 0.05.
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
Values represent least square means of 10 to 12 pigs. All pigs received allotted treatment diet starting −7 DPI. DPI = days postinoculation; ISF = isoflavones; TNFα = tumor necrosis factor alpha; IFNα = interferon alpha; IFNγ = interferon gamma; IL-10 = interleukin 10; BDL = below detectable limits.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day post-inoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
There were main effects for both soy source and ISF supplementation and interaction effects for IFNα concentration at 3 DPI (Figure 1). Pigs that received the ETSBM + ISF diet expressed the greatest (P < 0.05) concentrations of IFNα when compared with pigs fed the Control diet, regardless of ISF supplementation. Additionally, there were interaction and main effects for ISF supplementation on IFNγ at 3 DPI (Figure 1). Pigs receiving the Control diet without supplemental ISF concentrations at 3 DPI had greater (P < 0.05) IFNγ than all other treatments, though this effect was not observed at any other time point.
T-Cell Immunophenotyping
Results for immunophenotyping analysis of PBMC using flow cytometry can be found in Table 11. Relative proportions of total T-cells (i.e., positive for the CD3 cell-surface marker) and helper T-cells (i.e., positive for the CD3 and CD4 cell-surface markers) did not differ between uninfected and infected control animals. However, PRRSV-infection increased (P < 0.05) the relative proportion of cytotoxic T-cells (i.e., positive for the CD3 and CD8 cell-surface markers) in the total lymphocyte population when compared with uninfected pigs receiving the control diet. PRRSV-infection also increased (P < 0.05) the proportion of dual-positive T-cells (i.e., positive for the CD3, CD4, and CD8 cell-surface markers), but showed a reduction in the percentage of dual-positive T-cells with active antiviral effector pathways (i.e., dual-positive T-cells also expressing intracellular maker interferon-gamma).
Table 11.
Effects of dietary soy isoflavones level and porcine reproductive and respiratory virus (PRRSV) infection on peripheral blood T cell immunophenotypes of weanling pigs
| Item | Uninfected | PRRSV-infected | SEM | Uninfected vs. Infected Control | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Main effects2 | Interaction | |||||||||
| Control1 | Control1 | Control + ISF1 | ETSBM1 | ETSBM + ISF1 | Soy Source | ISF | Soy Source × ISF | |||
| T-Cell, % (CD3+)3 |
55.30 | 53.21 | 53.01 | 53.89 | 59.02 | 6.66 | 0.610 | 0.393 | 0.539 | 0.434 |
| Helper T-Cell, % (CD3+CD4+)4 |
22.03 | 20.18 | 23.09 | 18.03 | 26.41 | 2.13 | 0.463 | 0.783 | 0.011 | 0.202 |
| Cytotoxic T-Cell, % (CD3+CD8+)4 |
12.44 | 22.78* | 22.43 | 22.71 | 19.20 | 3.21 | 0.003 | 0.527 | 0.459 | 0.506 |
| Dual-Positive T-Cell, % (CD3+CD4+CD8+)4 | 4.30 | 6.66* | 6.32 | 6.15 | 5.13 | 1.19 | 0.022 | 0.287 | 0.406 | 0.603 |
| Dual-Positive T-Cell Expressing IFNγ, % (CD3+CD4+CD8+IFNγ+)4 | 90.10 | 79.72* | 79.25 | 77.94 | 83.41 | 4.06 | 0.001 | 0.594 | 0.292 | 0.253 |
| Dual-Positive T-Cell Expressing IFNγ MFI (CD3+CD4+CD8+IFNγ+)5 |
764.1 | 501.0* | 515.4 | 490.6 | 577.3 | 88.5 | 0.002 | 0.511 | 0.212 | 0.397 |
| CD4:CD8 ratio | 2.15 | 1.06* | 1.17 | 0.918 | 1.53 | 0.176 | 0.001 | 0.535 | 0.049 | 0.159 |
*Difference (P ≤ 0.05) between uninfected and infected groups fed the control diet.
Values represent least square means of 10 to 12 pigs with collection of blood occurring at 12 DPI. All pigs received allotted treatment diet starting −7 DPI. DPI = days postinoculation; ISF = isoflavones.
1The following diet names have been assigned to a respective experimental diet groups: Control: soy protein concentrate + no supplemented ISF, fed to both uninfected and PRRSV-infected control pigs; Control + ISF: soy protein concentrate + supplemented ISF, fed to PRRSV-infected pigs only; ETSBM: ETSBM + no supplemented ISF, fed to PRRSV-infected pigs only; ETSBM + ISF: ETSBM + supplemented ISF, fed to PRRSV-infected pigs only.
2Main effect of day post-inoculation (DPI); no 2- or 3-way interactive effects involving DPI were significant, so they were not included in the statistical model.
3Percent of total lymphocytes that are positive for cell-surface marker CD3.
4Percent of CD3-positive lymphocytes that are also positive for cell-surface markers CD4, CD8, CD4/CD8, or CD4/CD8 and intercellular IFN γ.
5Median fluorescence intensity (MFI) is a measure of the fluorescence intensity in a fluorescence channel being measured. It provides an alternative measurement to percent positive for comparison of cell populations between individuals. It is less sensitive to outliers, which is important for very small cell populations like the dual-positive T-cells.
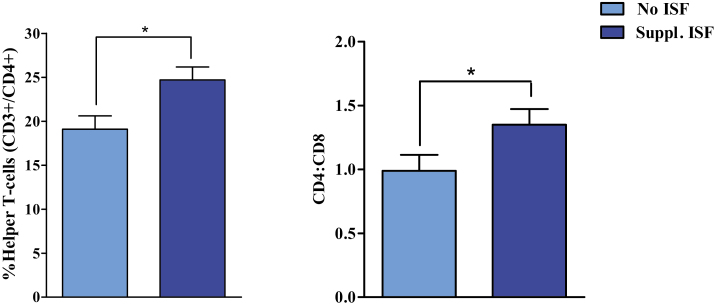
Among PRRSV-infected pigs, there was a main effect (P = 0.011) of ISF supplementation on the relative proportion of helper T-cell populations, with ISF supplementation increasing (P < 0.05) the proportion of helper T-cells (Figure 3). PRRSV-infected pigs receiving the ETSBM + ISF diet maintained the largest proportion of helper T-cells (26.4%), which was higher than pigs receiving the control or ETSBM diets without supplemental ISF. Pigs receiving the Control + ISF diet maintained the second largest proportion, which was not different from other treatment groups. Across all parameters, there were no main effects of soy protein source or interaction between soy source and ISF supplementation.
Figure 3.
Main effects of isoflavone supplementation on the proportion of Helper T-cells (CD3+/CD4+) and Helper:Cytotoxic T-cell ratios (CD4:CD8) in peripheral whole blood. ISF = isoflavones. Values represent least square means of 20 to 24 pigs, and error bars indicate standard error of the mean. An asterisk (*) denotes a difference (P < 0.05) due to isoflavone supplementation status for a given outcome.
DISCUSSION
Isoflavones are bioactive components that have been shown to have antiviral activity and are found in high concentrations in soybeans and soybean-derived feedstuffs (Wang and Murphy, 1996). This activity has been demonstrated against PRRSV specifically in live animal studies previously (Greiner et al., 2001a; Greiner et al., 2001b). However, these studies were unable to identify the effects of soy ISF when present in normal concentrations and proportions found naturally in soybean meal (SBM) without confounding effects of differing amino acid concentrations or sources in the diet. For that reason, one of our aims was to determine effects of industry relevant concentrations of soy ISF in diets otherwise devoid of these compounds and balanced for SID amino acid concentrations on the response of pigs to disease challenge. The two protein sources used for this study were a soy protein concentrate (Arcon AF, ADM, Decatur, IL) and enzyme-treated soybean meal (HP300, Hamlet Protein, Findlay, OH), both of which are soy protein sources that have undergone additional processing beyond that of SBM. Soybean meal, while an affordable protein option, is often limited in weanling pig diets for the first few weeks postweaning due to the immaturity of the weanling pig’s digestive capacity and presence of antinutritional factors such as oligosaccharides that can exacerbate gastrointestinal distress in young pigs (Stein, 2002). However, additional processing of SBM to either SPC or ETSBM can remove some of these antinutritional factors by solubilizing and removing residual carbohydrate fractions (Peisker, 2001). Soy protein concentrate (SPC) typically contains larger residual carbohydrate fractions than soy protein isolate (SPI), but both will have a greater proportions of protein than SBM (Shurtleff and Aoyagi, 2016). It is important to note that during the manufacturing of SPC and SPI, much of the estrogenic and antigenic factors such as soy ISF are also removed, which allowed us to develop basal diets with minimal to no ISF present (residual ISF concentrations. Using protein sources largely devoid of ISF ensured that we could supplement soy-derived ISF directly using a highly enriched source to reach concentrations near that of what is normally observed in SBM at typical inclusion levels for a nursery swine diet (USDA-ARS, 2016).
In general, our disease challenge model reduced ADG and growth efficiency of infected individuals, indicating that a successful infection with PRRSV was established. This was also supported by increased rectal temperatures in our infected pigs over that of our noninfected controls throughout the postinfective period, which is expected during acute PRRSV infections. Reductions in average daily gain supported previous findings of foundational PRRSV-challenge studies performed by our laboratory and others, though we observed a more severe reduction in performance than expected (Greiner et al., 2000; Rochell et al., 2015), likely due to differences in PRRSV strain virulence between studies. Additionally, the presence of an unintended Mh coinfection may have further diverted available energy sources away from body weight gain in our pigs, exacerbating the negative effects of our PRRSV intervention. To the contrary, we did not observe reductions in feed intake following PRRSV-inoculation, which was not in agreement with the findings by Greiner et al. who observed an approximate 22% decrease in ADFI from the 4 d immediately preceding inoculation to days 4 to 8 postinoculation (Greiner et al., 2000). When determining potential causes for this difference, we should note that there was observable feed wasting by the pigs as they transitioned onto their pelleted diet postweaning. With that in mind, an improved G:F ratio was observed over the entire growth period from 0 to 14 DPI for pigs fed ETSBM as a protein source. This could be due to the additional processing that ETSBM undergoes as a soy protein product, resulting in greater removal of residual antinutritional factors (e.g., raffinose and stachyose) that may contribute to higher digestibility values of the total mixed diet (Peisker M, 2001).
Aside from reduction in growth performance, other common indicators of infection status in swine respiratory models are serum viral load and circulating inflammatory cytokine concentrations. In previous PRRSV challenge models, PRRSV infection caused rapid increases in serum viral concentrations when pigs were experimentally inoculated at 29 d of age, peaking at 4 DPI (Greiner et al., 2000). In subsequent studies by the same group evaluating the effects of individual soy ISF supplementation, similar results were observed with serum viral concentrations peaking at 4 DPI (Greiner et al., 2001a; Greiner et al., 2001b). When weaning age pigs were supplemented with soy genistein alone, the primary ISF found in soybeans, a linear reduction of serum PRRS viral concentrations was observed (Greiner et al., 2001a). However, when supplemented with daidzein alone, the second most prevalent ISF found in soybeans, no effects on serum PRRS viral concentrations were observed, suggesting that other ISF fractions or interactions between ISF fractions possess more antiviral activity (Greiner et al., 2001b; Dia et al., 2008).
Although no significant main effects for soy source were observed in our study, ISF supplementation increased serum PRRS viral concentrations on 3 DPI. A strong interaction between soy source and ISF supplementation was observed, which was most evident in our ETSBM + ISF treatment group that maintained the highest serum PRRS viral concentrations (i.e., lowest Ct values) at both 3 and 6 DPI. In a previous study from our laboratory, concentration of SBM (17.5% vs. 29.0%) in the diet had no influence on Ct values until 14 DPI with pigs fed higher concentrations of SBM having lower serum PRRS viral concentrations (Rochell et al., 2015). Importantly, the methods used to detect serum PRRSV viral antigen differ between the studies discussed above; studies by Greiner et al. utilized a cytopathic effect assay, an indirect viral detection assay involving serial dilutions of serum and viral clearing results of the assay to calculate the virus concentration (Reed and Muench, 1938; Greiner et al., 2000; Greiner et al., 2001a; Greiner et al., 2001b). Rochell et al. (2015) utilized detection of PRRS viral mRNA via real-time PCR, a direct viral detection assay and the same assay utilized in our present study.
Understanding the differences in effects on serum PRRS viral concentrations across previous studies and ours is difficult, mainly due to lack of in vivo studies evaluating antiviral properties of ISF. In vitro, genistein in particular exhibits high inhibitory activity against viruses. Against human rotavirus, viral infectivity of cultured macrophages (MA-104 cell line) exposed to genistein or a mixture of ISF was reduced by 33% to 72%. Mixtures where genistein was absent lost these reductions of viral infectivity, demonstrating its importance for mediating viral infections (Andres et al., 2007). Against herpes simplex virus types-1 and -2 (HSV-1 and HSV-2), genistein moderately inhibited cytopathic effects, which are structural changes to the host cell that occur when they become infected by a virus resulting in subsequent cell death (Lyu et al., 2005). As stated above, measuring CPE was how Greiner et al. detected the presence of PRRS virus in serum and observed significant reductions with genistein supplementation utilizing that assay. Therefore, reasoning for why Rochell et al. and our study did not see similar reductions in serum PRRS viral concentrations may likely be due to our use of real-time PCR vs. a CPE assay, which may be influenced by the presence of free, bio-activated genistein in circulating serum collected for the assay instead of direct effects on the virus itself in vivo. Differences in PRRSV strain and severity of established infection (i.e., presence or absence of dual-infection and strain virulence factors) may have also affected the ability of ISF to influence the response to PRRSV infections across these studies.
Regarding inflammatory cytokines as an indicator of infection status, previous studies involving PRRSV and ISF mainly focused on IFN-γ, a key pro-inflammatory cytokine released during viral infections. Concentrations of IFN-γ in the current study were well-aligned with what have been observed previously in our laboratory (Rochell et al., 2015), yet we observed here an unremarkable IFN-γ response to dietary ISF supplementation. In the study conducted by Greiner et al. (2000), PRRSV infection alone caused an increase in serum IFN-γ concentrations, peaking at 4 DPI. These researchers suggested that the increase was due to IFN-γ production by cytotoxic T-cells and natural killer cells in response to the virus in order to support clearance of PRRSV by tissue resident macrophages. Subsequent PRRSV-challenge studies by the same group suggested a quadratic reduction of serum IFN-γ concentrations in response to genistein supplementation (Greiner et al., 2001a). Alternatively, daidzein supplementation at 200 or 800 ppm (but not 400 ppm) caused increased serum IFN-γ concentrations at peak viremia (4 DPI) (Greiner et al., 2001b). Researchers speculated that the reductions for genistein alone may be related to its influence on intracellular signaling pathways. Such pathways on which ISF may elicit inhibitory action are mainly initiated by tyrosine-specific kinases, which are also targets for a variety of virus types though this is not the case for PRRSV. However, inhibition of these kinases is likely a primary mechanism for reducing viral infectivity by ISF (Akiyama et al., 1987). In addition to tyrosine kinase mediation, genistein administered at low concentrations (0.5–5 µM) may stimulate natural killer cell activity in vitro, which could increase the rate of pathogen clearance (Zhang et al., 1999). Within our study, minimal effects on IFN-γ concentrations were observed, suggesting that ISF at the concentrations provided in study only mildly influenced cellular pathways involved in IFN-γ production.
Under the influence of inflammatory cytokines, erythrocyte and leukocyte production and systemic concentrations are also affected by disease status. By 14 DPI, PRRSV-infection decreased RBC counts and elicited reductions in associated parameters (e.g., HGB, HCT, MCV, and MCH). There is evidence that PRRSV-infection and virulence factors associated with specific strains contribute to the onset of anemia with varying severity (Halbur et al., 2002). Although previous research suggests that diets containing higher concentrations of SBM had improved hematocrit values and tendency for greater hemoglobin concentrations, both of which are used as clinical indicators of anemia severity, our findings do not suggest that soy source or ISF supplementation plays a factor in those pathways (Rochell et al., 2015). Regarding differential WBC counts, PRRSV-infection caused leukopenia detectable starting at 6 DPI, which is consistent with previous PRRSV studies (Halbur et al., 2002; Toepfer-Berg et al., 2004; Liu et al., 2013). For our study, ISF supplementation within soy protein source decreased relative band neutrophil (i.e., immature neutrophil) populations during the acute phase of infection. This could suggest that ISF reduces the stimulation of aggressive pro-inflammatory pathways in the early immune response to PRRSV, which may confer benefits to the overall health and recovery of the pig.
Regarding other measures of inflammatory status, immunophenotyping results by flow cytometry indicated that PRRSV in the presence of a Mh co-infection resulted in shifts of peripheral effector T-cell populations. Effector T-cells have specific and complementary effector functions that aid in the clearance of pathogens, but alterations of proportions of these cells may indicate disruptions in immune system function. In the human medical field, the ratio of effector helper T-cell and cytotoxic T-cell populations has been utilized as a measure of immune system status and presence of immunosuppression. Although not well characterized among healthy individuals, the ratio of CD3+ helper T-cells to cytotoxic T-cells (CD4:CD8 or helper/suppressor or cytotoxic) typically ranges between 1.5 and 2.0 with a higher ratio value indicating better immune system status. An inverse of this ratio can result by targeted cell death of helper T-cells, population expansion of cytotoxic T-cells, or a combination thereof (Mcbride and Striker, 2017). In humans, this ratio tends to decrease as they age, a phenomenon referred to as “immunosenesence,” and humans of older age groups with inverted CD4:CD8 ratios had higher mortality rates than those with normal ratios (Wikby et al., 2005).
For the pigs in this study, PRRSV-infection appeared to inverse the CD4:CD8 ratio by increasing the proportion of cytotoxic T-cells in the periphery. For PRRSV-infected pigs fed the Control diet, infection resulted in a reduction of the CD4:CD8 ratio by half (2.15 vs. 1.06 for PRRSV-infected vs. noninfected animals receiving the Control diet, respectively). Within our PRRSV-infected pigs and independent of soy protein source, consumption of ISF increased the CD4:CD8 T-cell ratio from 0.99 to 1.35 (Figure 3). We also observed an increased proportion of peripheral helper T-cells, which partially restored the CD4:CD8 ratio to be closer to that observed in noninfected control animals. Although there is a lack of information on whether this relationship between helper and cytotoxic T-cells is also observed in swine, it could suggest that increased efforts to better understand implications of altered ratios of these effector cell populations on susceptibility to disease in growing pigs may be beneficial.
IMPLICATIONS
When isolating the impact of ISF on disease-challenged pigs, there appears to be nonbeneficial regarding growth performance. However, adaptive immune responses appear to be altered beneficially when disease-challenged growing pigs consume ISF, which may elicit benefits during the recovery period in terms of compensatory growth. For these reasons, studying the effect of soy ISF throughout the grow–finish period is warranted.
LITERATURE CITED
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., and Fukami Y.. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592–5595. PMID: 3106339. [PubMed] [Google Scholar]
- Andres A., Donovan S. M., Kuhlenschmidt T. B., and Kuhlenschmidt M. S.. 2007. Isoflavones at concentrations present in soy infant formula inhibit rotavirus infection in vitro. J. Nutr. 137:2068–2073. doi: 10.1093/jn/137.9.2068. [DOI] [PubMed] [Google Scholar]
- Berhow M. A., Kong S. B., Vermillion K. E., and Duval S. M.. 2006. Complete quantification of group A and group B soyasaponins in soybeans. J. Agric. Food Chem. 54:2035–2044. doi: 10.1021/jf053072o. [DOI] [PubMed] [Google Scholar]
- Boyd R. D., Johnston M. E., and Zier-rush C.. 2010. Soybean meal level modulates the adverse effect of high immune stress on growth and feed efficiency in growing pigs. Proc. MN Nutr. Conf. 71:167–174. [Google Scholar]
- Dia V. P., Berhow M. A., and Gonzalez De Mejia E.. 2008. Bowman-birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J. Agric. Food Chem. 56:11707–11717. doi: 10.1021/jf802475z. [DOI] [PubMed] [Google Scholar]
- Greiner L. L., Stahly T. S., and Stabel T. J.. 2000. Quantitative relationship of systemic virus concentration on growth and immune response in pigs. J. Anim. Sci. 78:2690– 2695. doi: 10.2527/2000.78102690x [DOI] [PubMed] [Google Scholar]
- Greiner L. L., Stahly T. S., and Stabel T. J.. 2001a. The effect of dietary soy genistein on pig growth and viral replication during a viral challenge. J. Anim. Sci. 79:1272–1279. doi: 10.2527/2001.7951272x [DOI] [PubMed] [Google Scholar]
- Greiner L. L., Stahly T. S., and Stabel T. J.. 2001b. The effect of dietary soy daidzein on pig growth and viral replication during a viral challenge. J. Anim. Sci. 79:3113–3119. doi: 10.2527/2001.79123113x [DOI] [PubMed] [Google Scholar]
- Halbur R. G., Pallarés F. J., Rathje J. A., Evans R., Hagemoser W. A., Paul P. S., and Meng X. J.. 2002. Effects of different US isolates of porcine reproductive and respiratory syndrome virus (PRRSV) on blood and bone marrow parameters of experimentally infected pigs. Vet. Rec. 151:344–348. doi:10.1136/vr.151.12.344. [DOI] [PubMed] [Google Scholar]
- Holtkamp D. J., Kliebenstein J. B., Neumann E. J., Zimmerman J. J., Rotto H. F., Yoder T. K., Wang C., Yeske P. E., Mowrer C. L., and Haley C. A.. 2013. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Heal. Prod. 21:72–84. doi: 10.2460/javma.2005.227.385 [DOI] [Google Scholar]
- Liu Y., Che T. M., Song M., Lee J. J., Almeida J. A., Bravo D., Van Alstine W. G., and Pettigrew J. E.. 2013. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 91:5668–5679. doi: 10.2527/jas.2013-6495. [DOI] [PubMed] [Google Scholar]
- Loving C. L., Osorio F. A., Murtaugh M. P., and Zuckermann F. A.. 2015. Innate and adaptive immunity against porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 167:1–14. doi: 10.1016/j.vetimm.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S.-Y., Rhim J.-Y., and Park W.-B.. 2005. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 28:1293–1301. doi: 10.1024/0301-1526.34.4.281a [DOI] [PubMed] [Google Scholar]
- Maes D., Sibila M., Kuhnert P., Segalés J., Haesebrouck F., and Pieters M.. 2018. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound. Emerg. Dis. 65(Suppl. 1): 110–124. https://doi.org/ 10.1111/tbed.12677 [DOI] [PubMed] [Google Scholar]
- Mcbride J. A., and Striker R.. 2017. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 13:1–7. doi: 10.1371/journal.ppat.1006624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisker M. 2001. Manufacturing of soy protein concentrate for animal nutrition. In: Brufau J. (ed). Feed manufacturing in the Mediterranean region. Improving safety: from feed to food. Cahiers Options Méditerranéennes, Reus, Spain: p. 103–107. [Google Scholar]
- Prosky L., Asp N. G., Schweizer T. F., Devries J. W., and Furda I.. 1994. Determination of insoluble and soluble dietary fiber in foods and food-products – Collaborative study. J. AOAC Int. 75:360–367. PMID: 8012222. [PubMed] [Google Scholar]
- Reed L. J., and Muench H.. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497. [Google Scholar]
- Rochell S. J., Alexander L. S., Rocha G. C., Van Alstine W. G., Boyd R. D., Pettigrew J. E., and Dilger R. N.. 2015. Effects of dietary soybean meal concentration on growth and immune response of pigs infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 93:2987–2997. doi: 10.2527/jas.2014-8462. [DOI] [PubMed] [Google Scholar]
- Schweer W. P., Patience J. F., Burrough E. R., Kerr B. J., and Gabler N. K.. 2018. Impact of PRRSV infection and dietary soybean meal on ileal amino acid digestibility and endogenous amino acid losses in growing pigs. J. Anim. Sci. 96:1846–1859. doi: 10.1093/jas/sky093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweer W., Schwartz K., Patience J. F., Karriker L., Sparks C., Weaver M., Fitzsimmons M., Burkey T. E., and Gabler N. K.. 2017. Porcine Reproductive and Respiratory Syndrome virus reduces feed efficiency, digestibility, and lean tissue accretion in grow-finish pigs. Transl. Anim. Sci. 1:480–488. doi: 10.2527/tas2017.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff W., and Aoyagi A.. 2016. History of modern soy protein ingredients -isolates, concentrates, and textured soy protein products (1911–2016): extensively annotated bibliography and sourcebook. Soy Info Center, Lafayette, CA: http://www.soyinfocenter.com/pdf/190/Pro1.pdf (Accessed 4 January 2019.) [Google Scholar]
- Smith B. N., and Dilger R. N.. 2018. Immunomodulatory potential of dietary soybean-derived isoflavones and saponins in pigs. J. Anim. Sci. 96:1288–1304. doi: 10.1093/jas/sky036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H. H. 2002. Experience of feeding pigs without antibiotics: a european perspective. Anim. Biotechnol. 13:85–95. doi: 10.1081/ABIO-120005772. [DOI] [PubMed] [Google Scholar]
- Toepfer-Berg T. L., Escobar J., Van Alstine W. G., Baker D. H., Salak-Johnson J., and Johnson R. W.. 2004. Vitamin E supplementation does not mitigate the acute morbidity effects of porcine reproductive and respiratory syndrome virus in nursery pigs. J. Anim. Sci. 82:1942–1951. doi: 10.2527/2004.8271942x. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture – Agricultural Research Service (USDA-ARS), Nutrient Data Laboratory 2016. USDA National Nutrient Database for Standard Reference, Release 28. Version Current: September 2015, slightly revised May 2016. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/ [Google Scholar]
- Wang H.-J., and Murphy P. A.. 1996. Mass balance study of isoflavones during soybean processing. J. Agric. Food Chem. 44:2377–2383. doi: 10.1021/jf950535p [DOI] [Google Scholar]
- Wikby A., Ferguson F., Forsey R., Thompson J., Strindhall J., Löfgren S., Nilsson B. O., Ernerudh J., Pawelec G., and Johansson B.. 2005. An immune risk phenotype, cognitive impairment, and survival in very late life: Impact of allostatic load in Swedish octogenarian and nonagenarian humans. J. Gerontol. – Ser. A. 60:556–565. doi: 10.1093/gerona/60.5.556 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Song T. T., Cunnick J. E., Murphy P. A., and Hendrich S.. 1999. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J. Nutr. 129:399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]