Abstract
A study was conducted to evaluate the effects of level and source of fat in the diet of gestating beef cows on the postpartum performance of the dam and the progeny. Each year, 75 mature pregnant (183 ± 4.8 d until calving) Angus cows with similar BW (663 ± 21.5 kg) and BCS (2.6 ± 0.12; 1 to 5 scale) were randomly assigned to 1 of 15 outdoor pens. Each pen was assigned to 1 of 3 iso-caloric and iso-nitrogenous treatments: a low-fat diet (LF; 1.4 ± 0.12% EE) and two high-fat diets (HF; 3.3 ± 0.20% EE) including a canola seed- (CAN) or a flaxseed (FLX)-based pelleted feed. Diets were formulated to meet the requirements of pregnant beef cows and fed until calving. Data were analyzed as a randomized complete block design with contrasts for the effects of level (LF vs. HF) and source (CAN vs. FLX) of fat. No differences (P ≥ 0.21) were found for BW or calving to weaning ADG of cows. The average BCS during the first 42 d of lactation was greater (P<0.01) for LF compared with HF (2.63 vs. 2.51) with no difference (P = 0.35) for CAN vs. FLX cows. Subcutaneous fat thickness over the ribs was greater (P ≤ 0.01) for LF compared with that of HF cows at calving (5.7 vs. 4.3 mm) and at weaning (4.3 vs. 3.7 mm) with no difference (P ≥ 0.11) for CAN vs. FLX cows. Over the first 42 d of lactation, no difference (P ≥ 0.23) was observed for 12-h milk yield. Milk protein concentration was greater (P = 0.03) for CAN compared with FLX (3.11 vs. 3.01%) cows, whereas no difference (P ≥ 0.28) was observed for any other milk component. Milk fat from FLX cows had greater (P < 0.01) CLA and CLnA concentrations than that of CAN cows during the first 42 d of lactation. Pregnancy rate of HF cows tended (P = 0.07) to be greater than that of LF cows with no difference (P = 0.77) for CAN vs. FLX cows. Calves from HF cows were heavier (P ≤ 0.01) at birth (42.9 vs. 40.2 kg) than those from LF cows. From calving to weaning, ADG of calves born to CAN cows was greater (P = 0.03) that that of calves born to FLX cows (1.19 vs. 1.13 kg/d) with no difference (P = 0.18) for calves born to LF vs. HF cows. At slaughter, progeny of HF cows had greater (P ≤ 0.03) shrunk BW (605 vs. 579 kg) and HCW (355 vs. 339 kg) compared with those from LF cows with no difference (P ≥ 0.16) for progeny of CAN vs. FLX cows. These results show that feeding a HF diet over gestation results in heavier calves at birth and at slaughter, and superior calf gains from birth to slaughter as well as heavier carcasses, possibly due to a developmental programming effect.
Keywords: fat supplementation, fatty acid profile, gestating beef cows, postpartum effects, progeny performance
INTRODUCTION
Reproductive performance of cows and birth to weaning performance of their progeny have been shown to be influenced by nutrition of the dam during gestation (Hess et al., 2005; Funston et al., 2010). In the Great Plains of North America, the energy requirement for maintenance of gestating beef cows increases as they are often exposed to temperatures below their thermo-neutral zone (NRC, 2000). Therefore, providing dietary energy to pregnant beef cows in an efficient manner is a major nutritional goal in cow-calf operations to increase performance of both the dam and progeny.
In ruminants, fat inclusion up to 6% of the total DMI increases the energy density of high-forage diets without negative effects on animal performance (Palmquist, 1994; Hess et al., 2008). Also, feeding high-fat diets during late gestation has been shown to improve pregnancy rates in beef cows compared with diets with similar energy content (Bellows et al., 2001; Graham et al., 2001). Moreover, feeding a high-fat diet during gestation not only has a positive effect on performance of beef cows, but can also improve the performance of the offspring including heavier calves at birth (Añez-Osuna et al., 2019), reduced calf mortality at birth (Lammoglia et al., 1999), and from birth to weaning (Petit and Berthiaume, 2006).
Recent research has shown that the degree of improvement observed by increasing the level of fat in the diet of beef cows may depend on the type of fatty acids in the diet. For example, relative to diets high in saturated fatty acids (SFA), feeding diets high in polyunsaturated fatty acids (PUFA) to beef cows has resulted in greater pregnancy rates (Petit and Berthiaume, 2006; Lopes et al., 2009). Birth to weaning performance of the calf has also been improved by feeding diets high in PUFA to the dam over gestation, and to the calf during early life. For example, Garcia et al. (2014 and 2015) increased the concentration of linoleic (LA) and α-linolenic (ALA) acid in the diet of dairy cows over late gestation and in milk replacers fed to their calves for 30 d after calving. These authors reported improvements in calves’ birth to weaning ADG, immune response, and overall health by increasing the LA and ALA concentrations in the diets for both gestating cows and young calves.
Overall, feeding high-fat diets during gestation has been shown to benefit the reproductive performance of beef cows and the birth to weaning performance of the progeny. Moreover, the magnitude of improvement appears to depend on the degree of saturation of fatty acids included in the diet. However, few research studies have been conducted to evaluate the effects of level and source of fat fed to beef cows during gestation on the weaning to slaughter performance of their progeny. Therefore, the objective of this study was to evaluate the effects of level and source (monounsaturated vs. PUFA) of fat in the diet of gestating beef cows on their postpartum performance and on the birth to slaughter performance of their progeny.
MATERIALS AND METHODS
This paper is the second of two companion papers addressing the effects of level and source of fat in the diet of gestating beef cows. The first companion paper (Añez-Osuna et al., 2019) addressed the prepartum responses of the cow and birth weight of the progeny.
All animals were obtained from the main herd of the Western Beef Development Centre’s research ranch and cared for in accordance with the Canadian Council of Animal Care guidelines (CCAC, 2009). All experimental procedures were approved by University of Saskatchewan Animal Care Committee (Protocol No. 20090107).
Location
A 2-yr study (2015 to 2016 and 2016 to 2017 for years 1 and 2, respectively) was conducted at the Termuende Research Ranch of the Western Beef Development Centre (WBDC) near Lanigan (51°51′N, 105°02′W), Saskatchewan, Canada, and at the University of Saskatchewan Beef Cattle Research and Teaching Unit (BCRTU) located in Saskatoon (52°09′N, 106°36′W), Saskatchewan, Canada.
Dietary Treatments
Treatments consisted of 3 diets differing in level and source of fat that cows received during the last 2 trimesters of gestation. Details have been presented in the companion paper (Añez-Osuna et al., 2019). Briefly, on 24 October 2014 and 23 October 2015 (for years 1 and 2, respectively), 75 multiparous (≥3 calving) pregnant Angus cows were stratified by initial BW (662 ± 52.4 kg) and BCS, and divided into 15 homogenous groups (5 cows/group). Each group was randomly assigned to 1 of 15 outdoor research pens. Subsequently, each pen was randomly assigned to 1 of 3 replicated (n = 5) dietary treatments which consisted of a low-fat (LF) diet (1.4 ± 0.12% EE) and 2 high-fat (HF) diets (3.3 ± 0.20% EE). Mixed hay consisting of bromegrass (Bromus sp.) and alfalfa (Medicago sativa L.), barley straw, rolled barley grain, and 2 high-fat pelleted feeds were used as ingredients to formulate the treatment diets (Table 1). The 2 high-fat pellets were formulated using canola seed (CAN) as an enriched source of mono-unsaturated fatty acids (MUFA) or flaxseed (FLX) as an enriched source of PUFA. High-fat diets (CAN and FLX) were formulated to provide 300 g of fat/cow/d from pelleted feeds. Amounts fed were such that each pen received equal amounts of digestible energy (DE; 2.42 ± 0.11 Mcal/kg), CP (10.5 ± 0.4%), and total DM (12.9 ± 1.0 kg/cow/d). Diets were formulated to meet the DE and CP intake requirements of pregnant beef cows over the second and third trimesters of gestation for a projected 40-kg calf at birth according to NRC (2000). The amount fed to each pen was adjusted every 2 wk according to estimated day of gestation, weight gain, and changes in weather conditions. Diets were offered once daily as total mixed rations (TMR) using a mixer wagon with feeding starting at 0800 h. Bunks were cleaned every 2 wk due to accumulation of orts if needed. The same cows were used each year unless culled for injury or failure to conceive, in which case, similar replacements were obtained from the same herd. Each year, treatment diets were fed from the start of the second trimester of gestation until calving (183 ± 4.8 d).
Table 1.
Daily dry matter intake, nutrient, and fatty acid composition (average ± SD) of treatment diets fed to beef cows over gestation
| Treatments1 | |||
|---|---|---|---|
| Item | LF | CAN | FLX |
| DMI, kg/cow | |||
| Total | 12.9 ± 1.05 | 12.9 ± 0.98 | 12.8 ± 1.11 |
| Ingredient, % DM | |||
| Hay | 35.0 ± 2.84 | 28.1 ± 2.22 | 29.0 ± 2.73 |
| Barley straw | 37.3 ± 5.34 | 34.9 ± 7.17 | 33.3 ± 4.86 |
| Barley grain | 27.8 ± 5.94 | 8.23 ± 3.98 | 3.08 ± 3.19 |
| CAN pellet | – | 28.8 ± 3.71 | – |
| FLX pellet | – | – | 34.6 ± 6.15 |
| Nutrient,2 % DM | |||
| CP | 10.3 ± 0.50 | 10.6 ± 0.37 | 10.7 ± 0.42 |
| ADF | 39.4 ± 2.31 | 39.2 ± 3.44 | 38.8 ± 2.46 |
| NDF | 58.5 ± 1.93 | 58.4 ± 3.88 | 57.0 ± 3.85 |
| EE | 1.40 ± 0.12 | 3.31 ± 0.16 | 3.27 ± 0.23 |
| Calcium | 0.45 ± 0.06 | 0.52 ± 0.06 | 0.46 ± 0.09 |
| Phosphorus | 0.25 ± 0.02 | 0.25 ± 0.01 | 0.24 ± 0.01 |
| TDN2 | 54.0 ± 2.26 | 55.1 ± 2.90 | 55.4 ± 2.13 |
| NEm, Mcal/d | 1.09 ± 0.08 | 1.13 ± 0.10 | 1.14 ± 0.07 |
| NEg, Mcal/d | 0.53 ± 0.07 | 0.56 ± 0.09 | 0.57 ± 0.07 |
| Fatty acid,3 % of total | |||
| 16:0 | 28.2 ± 0.70 | 12.3 ± 1.36 | 11.9 ± 0.63 |
| 18:0 | 2.03 ± 0.16 | 2.09 ± 0.10 | 3.16 ± 0.13 |
| c9-18:1 | 16.7 ± 0.91 | 47.2 ± 1.21 | 19.1 ± 0.37 |
| c11-18:1 | 1.22 ± 0.05 | 3.57 ± 0.19 | 0.93 ± 0.03 |
| 18:2n-6 | 36.9 ± 0.70 | 22.5 ± 0.85 | 22.7 ± 0.98 |
| 18:3n-3 | 8.13 ± 0.50 | 7.76 ± 0.83 | 39.2 ± 1.34 |
| ∑SFA | 34.8 ± 0.81 | 17.0 ± 1.34 | 17.1 ± 0.84 |
| ∑MUFA | 20.1 ± 0.99 | 52.7 ± 1.32 | 21.0 ± 0.33 |
| ∑PUFA | 45.1 ± 1.02 | 30.3 ± 1.20 | 61.9 ± 0.74 |
1LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed-based pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
2CP = crude protein; ADF = acid detergent fiber; NDF = neutral detergent fiber; EE = ether extract; TDN = total digestible nutrients; NEm = net energy of maintenance; NEg = net energy of gain; TDN = calculated using the Pennsylvania-State equations (Adams, 1980); NEm and NEg = calculated using the NRC (2000) summative equation.
3∑SFA = sum of saturated fatty acids; ∑MUFA = sum of monounsaturated fatty acids; ∑PUFA = sum of polyunsaturated fatty acids.
Housing, Handling, and Feeding
All cow-calf pairs were managed in a single group and received equal management from calving until weaning. Within the first 48 h after birth, all calves were ear tagged and received injections (0.5 cc) of vitamins A and D (Vitamin AD3 Forte, Rafter 8 Products, Calgary, AB, Canada) and vitamin E plus selenium (Selon E Injection, Vetoquinol Canada Inc., Lavaltrie, QC, Canada). Bull calves were castrated within 48 h after birth using rubber bands. As cows calved, cow-calf pairs were moved to a common pen (120 × 77 m) equipped with water bowls and portable feed bunks. During the time spent in the common pen (53 ± 14 d postpartum), mixed hay (9.9 ± 0.4% CP, 51.1 ± 1.5% ADF, and 69.1 ± 0.5% NDF) comprised of smooth bromegrass (Bromus inermis L.), hybrid bromegrass (B. inermis × B. riparius), and alfalfa (Medicago sativa L.) was offered free choice, and cows were supplemented with 1.8 kg/cow/d of rolled barley grain (12.9 ± 1.3% CP, 13.5 ± 2.0% ADF, and 25.9 ± 3.3% NDF).
By mid-June of each year, all cows and calves were vaccinated with Vista Once SQ (Merck Animal Health, Kirkland, QC, Canada), Vision 7 with Spur (Merck Animal Health, Kirkland, QC, Canada), and Anthrax Spore Vaccine (Colorado Serum Company, Denver, CO). As well, all calves were implanted with Ralgro (Merck Animal Health, Kirkland, QC, Canada). Subsequently, cow-calf pairs were moved to cool-season pastures until weaning where cows grazed a mixture of smooth bromegrass (Bromus inermis L.), red fescue (Festuca rubra L.), and Kentucky bluegrass (Poa pratensis L.) pastures until weaning. During this period, cows were exposed to a 63-d breeding season (25:1 cows to bull ratio) starting on 2 July and 5 July for years 1 and 2, respectively. In both years, half sibling, registered Angus bulls were used as sires after passing a breeding soundness evaluation. All animals had ad libitum access to a 2:1 mineral [15.5% Ca, 7% P, 30 ppm Se, 20 ppm Co, 200 ppm I, 1500 ppm Cu, 5000 ppm Mn, 5000 ppm Zn, 1000 ppm Fe, 1.0 ppm F (max), 500 000 IU/kg vitamin A (min), 50000 IU/kg vitamin D (min), 2500 IU/kg vitamin E (min); Cargill Animal Nutrition, Manitoba, Canada] and cobalt-iodized salt [99.0% NaCl (min), 39.0% Na, 150 ppm I, 100 ppm Co; FeedRite Ltd., Humboldt, Saskatchewan, Canada] at all times.
Backgrounding
On 23 October 2015 and 24 October 2016 (for years 1 and 2, respectively), all calves were weaned, separated in 2 groups according to sex, and managed similarly until slaughter. At weaning, all calves were revaccinated with Vista Once SQ (Merck Animal Health, Kirkland, QC, Canada) and Vision 7 (Merck Animal Health, Kirkland, QC, Canada). Immediately after weaning, calves were housed (according to sex) in 2 large outdoor pens (90 × 43 m) equipped with portable feeding troughs and water bowls; and backgrounded over the course of the fall-winter (143 ± 1.0 d) at the WBDC research ranch. During this period, calves had free choice access to mixed hay (12.3 ± 3.0% CP, 41.0 ± 1.9% ADF, and 59.0 ± 1.7% NDF) comprised of smooth bromegrass (Bromus inermis L.), hybrid bromegrass (B. inermis × B. riparius), and alfalfa (Medicago sativa L.), and were supplemented daily with 1 kg/hd of a commercial (Blair’s Crop & Livestock Solutions, Nokomis, SK, Canada) pelleted feed (15.7 ± 0.4% CP, 12.5 ± 1.3% ADF, and 29.7 ± 0.9% NDF). Calves had ad libitum access to a 1:1 mineral [11.5% Ca, 10% P, 20 ppm Co, 200 ppm I, 2000 ppm Cu, 5000 ppm Mg, 5000 ppm Mn, 5000 ppm Zn, 4900 ppm Fe, 50 ppm F (max), 500 000 IU/kg vitamin A (min), 50000 IU/kg vitamin D (min), 2500 IU/kg vitamin E (min); Cargill Animal Nutrition, Manitoba, Canada] and cobalt-iodized salt [99.0% NaCl (min), 39.0% Na, 150 ppm I, 100 ppm Co; FeedRite Ltd., Humboldt, Saskatchewan, Canada] at all times. On 14 March 2016 and 15 March 2017 (for years 1 and 2, respectively), all calves were moved to the University of Saskatchewan BCRTU where they remained separated according to sex until slaughter. At the BCTRU, both sexes were randomly allotted 2 pens per sex (17 ± 2.3 hd/pen) with each pen (12 × 24 m) balanced for dietary treatment of the dam. Upon arrival to the BCRTU, all calves were vaccinated with Ultrabac 7/Somubac (Zoetis Canada Inc., Kirkland, QC, Canada), Bovi-Shield GOLD One Shot (Zoetis Canada Inc., Kirkland, QC, Canada), treated for external and internal parasites with Bimectin Pour-On (Bimedia-MTC Animal Health Inc., Cambridge, ON, Canada), and implanted with Ralgro (Merck Animal Health, Kirkland, QC, Canada). During the first 37 and 44 d (for years 1 and 2, respectively) at the BCRTU, calves continued to receive a high-forage backgrounding diet consisting of (DM basis) 52.2 ± 3.3% barley silage, 34.5 ± 3.6% rolled barley grain, 7.7 ± 0.7% canola meal, and 5.6 ± 0.4% mineral and vitamin supplement (9.0% CP, 9.2% Ca, 0.32% P, 1.6% Na, 0.28% Mg, 0.60% K, 0.12% S; 4.9 ppm Co, 185 ppm Cu, 16.6 ppm I, 84 ppm Fe, 500 mg Mn, 2.0 ppm Se, 558 ppm Zn, 550 ppm monensin; 40,000 IU vitamin A, 5,000 IU vitamin D, and 600 IU vitamin E per kg supplement), and formulated to provide (DM basis) 13.5 ± 0.5% CP, 1.56 ± 0.14 Mcal/kg NEm, and 0.96 ± 0.13 Mcal/kg NEg. This high-forage diet was offered ad libitum (5% carry over) as a TMR with feeding occurring once daily during the morning. The targeted dietary monensin concentration was 33 ppm (DM basis). The targeted end point of the backgrounding program was 400 kg of average shrunk BW (averaged across both steers and heifers).
Finishing
Following the backgrounding phase, calves were transitioned over 16 d to a high-grain finishing diet using a 5-step adaptation program. During the adaptation period, the diet composition was changed every 4 d in such a way that the barley silage and canola meal content in the diet were gradually decreased as barley grain was increased to formulated levels in the finishing diet. The finishing diet consisted of (DM basis) 10.8 ± 0.1% barley silage, 84.1 ± 0.1% rolled barley grain, and 5.1 ± 0.0% mineral and vitamin supplement (9.0% CP, 9.2% Ca, 0.32% P, 1.6% Na, 0.28% Mg, 0.60% K, 0.12% S; 4.9 ppm Co, 185 ppm Cu, 16.6 ppm I, 84 ppm Fe, 500 mg Mn, 2.0 ppm Se, 558 ppm Zn, 550 ppm monensin; 40,000 IU vitamin A, 5,000 IU vitamin D, and 600 IU vitamin E per kg supplement) and was formulated to provide 11.8 ± 0.4% CP, 1.85 ± 0.03 Mcal/kg NEm, and 1.22 ± 0.02 Mcal/kg NEg. This diet was fed over 76 and 87 d (for years 1 and 2, respectively) and offered ad libitum (5% carry over) as a TMR with feeding occurring once daily during the morning. Calves were reimplanted at 74 ± 5.7 d before slaughter with Revalor-S (Merck Animal Health, Kirkland, QC, Canada) for steer calves and Revalor-H (Merck Animal Health, Kirkland, QC, Canada) for heifer calves. The targeted end point of finishing was 595 kg of average shrunk BW (averaged across steers and heifers).
Data Collection
Feeds and DM intake.
All feed ingredients were sampled every 2 wk. Hay, rolled barley grain, and canola meal samples were dried in an air forced oven at 55 °C for 48 h, whereas barley silage samples were dried for 72 h. Dried samples were ground to pass a 1 mm screen (Thomas-Wiley Laboratory Mill Model 4; Thomas Scientific, Swedesboro, NJ) and stored at −20 °C until analysis.
Body weight.
Each cow was weighed over 2 consecutive days at calving (within 48 h after calving) and weaning. As well, cows used for partial milk yield estimation were weighed over 2 consecutive days at 21 and 42 d postpartum. Birth weight was recorded for all calves within the first 24 h after birth. Calves from cows used for partial milk yield estimation were weighed at 21 and 42 d of age after separation from the dam for 12 h. At weaning, as well as at the end of the backgrounding and finishing phases, all calves were weighed on 2 consecutive days. All calves were weighed once monthly throughout the backgrounding phase and every 2 wk throughout the finishing phase. The BW of cows and calves at weaning (WW) were adjusted to 180 d, and the BW of calves at the end of backgrounding were adjusted to 365 d as follows:
Body condition scoring and subcutaneous fat thickness.
Body condition scores for each cow were determined by the same experienced technician at calving, 21 and 42 d postpartum, and at weaning using the Scottish scale where 1 is the emaciated and 5 is the grossly fat (Lowman et al. 1976; Wildman et al. 1982). Ultrasound measurements of subcutaneous fat thickness (SCFT) over the third quarter of the longissimus dorsi (LD) muscle between the 12th and 13th ribs, and the rump area, at the interface of the biceps femoris and gluteus medius muscles, were determined on each cow at weaning using an Aloka SSD-500V ultrasound machine and an Aloka UST-5044 probe (3.5 MHz-17 cm; Aloka Inc., Wallingford, CT). Ultrasound images were collected and internal calipers of the ultrasound machine, calibrated to ±1 mm, were used to measure SCFT.
Milk yield and composition.
On days 21 and 42 of lactation, partial milk yields were estimated from the first 3 to 4 cows calving from each pen. Briefly, on the day before sampling, cows were separated from their calves at 1300 h and then rejoined at 1900 h, and calves were then allowed to suckle for 45 min to exhaust the milk from the mammary gland. Immediately after nursing, cows were separated from their calves. On the next morning, starting at 0700 h, 30 IU of oxytocin (OXY-20 NW, Rafter 8 Products, Calgary, AB, Canada) were administrated intravenously, and cows were milked from 2 diagonally opposite quarters using a portable milking machine (Deluxe Portable Pump, E-Zee Milking Equipment, Gordonville, PA). The milk yield reached from both quarters was weighed, times by 2, and used as an estimator of the total (4 quarters) 12-h partial milk yield. Immediately after collection, 2 milk samples were obtained from the milk collected from each cow. One 20-mL sample containing a preservative was refrigerated at 4 °C and sent for analysis within the next 72 h to the CanWest DHI Central Milk Testing Laboratory (Edmonton, AB, Canada). Another 40-mL sample was collected into a 50-mL sterile centrifuge tube (VWR International, Radnor, PA) and stored at −20 °C until analysis.
Blood serum collection.
Blood was collected from all cows at calving and from cows used for milk yield estimation at 21 and 42 d of lactation. Blood samples were collected from each cow via jugular venipuncture into 10-mL untreated vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). Blood samples were allowed to clot at room temperature for 30 min, centrifuged (2,000 × g at 4 °C for 15 min), and serum was harvested into 1.5-mL tubes (Eppendorf, GCS, New York, NY) and refrigerated at −20 °C until analysis.
Muscle and adipose tissue collection.
At birth and weaning, biopsy samples of the LD muscle were collected between the 12th and 13th ribs of the first 2 bull-calves born from each pen. Briefly, calves were restrained, hair was removed from the biopsy site, and 4 mL of a local anesthetic (lidocaine HCl 2%, Zoetis Canada Inc., Kirkland, QC) were administered. The biopsy site was then cleaned using 70% ethanol and a 2-cm incision was made using a sterile scalpel. A biopsy sample (approximately 1 g) was collected from the LD muscle using a 6-mm sterile biopsy punch (Integra Miltex, Integra LifeSciences Corp., Plainsboro NJ), washed with phosphate-buffered saline, placed into 1-mL sterile polypropylene cryogenic vial (Cryo.s, Greiner Bio-One North America, Inc., Monroe, NC), snap frozen in liquid N, and stored at −80 °C until analysis. Samples were used for RNA extraction and evaluation of the relative expression of growth, myogenic, and adipogenic genes through real-time PCR.
Adipose tissue (AT) samples (approximately 5 g) were obtained from the brisket of all steers and heifers at slaughter. Samples were placed into 60-mL sterile polyethylene bags (Fisher Scientific, Ottawa, ON), and stored at −20 °C until analysis. Samples were analyzed for fatty acid composition using gas chromatography.
Carcass traits.
Each year, all calves were slaughtered on the same day at a commercial processing plant (Cargill Foods, High River, AB, Canada) at an average shrunk BW of 595 ± 58 kg. Hot carcass weight (HCW) was determined immediately, and the carcasses were chilled for 24 h and evaluated using the Computer Vision Grading System (VBG 2000 e + v Technology GmbH, Oranienburg, Germany) for quality, and yield grade, longissimus dorsi muscle area (LMA), and marbling score according to the Canadian Beef Grading Agency (CBGA, 2009). The yield grade (YG) is a measure of the overall lean yield calculated from the LMA and fat depth and consists of Canada 1 = 59% or more; Canada 2 = 58–54%; and Canada 3 = 53% or less. Quality grades included A for beef carcasses with trace marbling (USDA Standard equivalence), AA for beef carcasses with slight marbling (USDA Select equivalence), AAA for beef carcasses with small to moderate marbling (USDA Choice equivalence), and prime for beef carcasses with slightly abundant or greater marbling (USDA Prime equivalence). Dressing % was calculated as a ratio of HCW to shrunk BW (96% of final BW).
Laboratory Analysis
Chemical analyses of feeds were performed by Cumberland Valley Analytical Services Inc. (Hagerstown, MD) and analyzed in duplicate according to the AOAC International (AOAC, 2012). Hay and barley silage samples were analyzed by near-infrared spectroscopy (NIRS) using a Foss NIRSystems 5000 (NIR Systems, Inc., Silver Spring, MD) for determination of DM, CP, ADF, NDF, ash, Ca, and P. Barley grain and canola meal samples were analyzed for DM by drying at 135 °C for 2 h (method 930.15; AOAC, 2012), CP (method 990.03; AOAC, 2012) using a Leco FP 528 Nitrogen Combustion Analyzer (Leco, St. Joseph, MI), ADF (method 973.18; AOAC, 2012), ash (method 942.05; AOAC, 2012), and Ca and P (method 985.01; AOAC, 2012). The method of Van Soest et al. (1991), with the addition of amylase and sodium sulfite, was used to determine NDF content. The Pennsylvania-State equations based on ADF were used to calculate the total digestible nutrient (TDN) values for all feeds (Adams, 1980). Digestible energy (DE), net energy of maintenance (NEm), and gain (NEg) were calculated according to NRC (2000).
Blood metabolites.
Serum samples were used to determine nonesterified fatty acids (NEFA) and β-hydroxybutyrate (BHBA) concentrations. Serum NEFA concentration was determined using the NEFA-HR (2) kit (Wako Diagnostics Corp., Richmond, VA) and absorbance was read on a spectrophotometer (Epoch 2, Biotek Instruments Inc., Winooski, VT, USA) at 550 nm. Serum BHBA concentration was determined through the enzymatic oxidation of BHBA to acetoacetate catalyzed by 3-hydroxybutrate dehydrogenase (Williamson et al., 1962). The associated reduction of NAD to NADH was determined photometrically at 340 nm using a microplate spectrophotometer (Epoch 2, Biotek Instruments Inc., Winooski, VT). The inter- and intra-plate assay CV was 7.8 ± 3.4% and 9.5 ± 2.7%, respectively.
Fatty acid extraction and gas chromatography.
Fatty acid methyl esters (FAME) were prepared from milk fat and AT samples. Briefly, 25 mL of raw milk were centrifuged at 17,800 × g for 30 min at 4 °C. Subsequently, 1 g of the resulting cream (top layer) was collected and transferred into a 2-mL microcentrifuge tube, centrifuged at 19,300 × g for 20 min at 20 °C, and 40 mg of the top fat layer were weighed into a pyrex tube with a teflon lined screw cap. For AT samples, 40 mg were weighed into a pyrex tube with a teflon-lined screw cap and freeze-dried overnight to a constant weight. All milk fat and AT samples were methylated by base catalyzed methylation using 0.5 N sodium methoxide as detailed in Añez-Osuna et al. (2019). Fatty acid methyl esters obtained from milk fat and AT samples were analyzed using a Varian CP-3800 gas chromatograph (Varian Inc., Walnut Creek, CA) using the conditions described by Vahmani et al. (2017) and Kramer et al. (2008), except only one GC analysis using the 175 °C plateau temperature program was used (i.e., further analyses to separate minor isomers was not conducted). Fatty acids were identified using reference standard No. 603 from Nu-Chek Prep Inc. (Elysian, MN). Branched-chain FAME were identified using a GLC reference standard BC-Mix1 from Applied Science (State College, PA). The UC-59M standard from Nu-Chek Prep was used for conjugated linoleic acid (CLA) isomers. Polyunsaturated fatty acid biohydrogenation intermediates not included in the standard mixtures were identified by their retention times and elution orders as reported in literature (Cruz-Hernandez et al., 2004; Kramer et al., 2008) and this included recently identified Δ-9 desaturation products of trans-18:1 isomers (Vahmani et al., 2016). The FAME were quantified using chromatographic peak area and internal standard based calculations.
RNA extraction.
Total RNA was extracted with Trizol reagent. Longissimus dorsi muscle tissue samples (100 mg) were homogenized in 1 mL of Trizol reagent (Life Technologies, Inc., Burlington, ON, Canada) using the Precellys 24 Tissue Homogenizer with the Cryolys accessory (Bertin Technologies, Montignyle-Bretonneux, France) and 2-mL bead tubes (Precellys Hard tissue grinding MK28, Bertin Technologies, Rockville, MD). Homogenized samples were incubated for 5 min at room temperature to allow complete dissociation of nucleoprotein complexes. Samples were then centrifuged at 12,000 × g for 10 min at 4 °C and the supernatant was transferred to a 1.5-mL microcentrifuge tube. Chloroform was added (200 µL) and samples were shaken and incubated at room temperature for additional 2 to 3 min. Samples were centrifuged at 12,000 × g for 15 min at 4 °C. After centrifugation, the dissolved RNA was pipetted to a new 1.5-mL tube, and the RNA was precipitated with 500 µL of isopropyl alcohol. Samples were then incubated at room temperature for 10 min and centrifuged at 12,000 × g for 10 min at 4 °C. The supernatant was removed, and the RNA precipitate was washed with 75% ethanol. The RNA and ethanol were vortexed and centrifuged at 7,500 × g for 5 min at 4 °C. The RNA was then dissolved in nuclease-free H2O (Ambion, Foster City, CA). All total RNA samples were quantified using a spectrophotometer (ND-1000, NanoDrop, Wilmington, DE), were evaluated for RNA integrity (RIN) using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and stored at −80 °C until cDNA synthesis. The RIN value of RNA isolated from all samples was >7.
Real-time PCR.
For gene expression analysis, total RNA (1.5 µg) from each sample was reverse transcribed with the High Capacity cDNA reverse transcription kit (Life Technologies Inc., Carlsbad, CA) according to the manufacturer’s instructions. RNase inhibitor (Life Technologies Inc.) was also added to the reaction at a concentration of 2 U/μl. After reverse transcription, the cDNA was diluted to 1 ng/μl with nuclease-free H2O (Ambion). Real-Time PCR for gene expression analysis was performed in duplicate using 1 ng of cDNA in 96-well fast plates using the SYBR fast master mix ABI prism (D-Mark Biosciences) and the Step-One Plus Real-time PCR system (Life Technologies Inc.). A blank sample and a minus reverse transcriptase were added to control for nonspecific amplification. Relative standard curves, made from serial dilution of a pooled cDNA from all LD muscle samples and ranging from 20 to 0.02 ng, were used to determine the relative quantity of each sample. The Primer3 software and species-specific sequences found in GenBank were used for the design of primers (Table 2). Primers were designed to cover exon-exon junctions when possible and ran with an annealing/extension temperature in the real time-PCR reaction of 60 °C (Paradis et al., 2017). The amplification efficiency for each gene was determined using serial dilution of tissue-specific cDNA and was found to be 100 ± 10% for all genes (data not shown). Eukaryotic Translation Elongation Factor 1 Alpha 2 (EEF1A2), Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), Hydroxymethylbilane Synthase (HMBS), Ribosomal Protein L19 (RPL19), and Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein, Zeta Polypeptide (YWHAZ) were tested as endogenous control genes, and the best individual or combination of endogenous control was chosen using NormFinder (Andersen et al., 2004). As a result, GAPDH and RPL19 were used as the endogenous controls to correct for RNA extraction and reverse transcription efficiency in LD muscle samples at birth and weaning, respectively. The endogenous control genes were tested for any dietary treatment effect and were found to be stable confirming their usefulness as suitable endogenous controls.
Table 2.
Primer sequences and amplification conditions for gene expression measured by real-time PCR in longissimus dorsi muscle of male calves1
| Gene | Primer | Sequence | Product size (bp) |
|---|---|---|---|
| EEF1A2 | Fwd | 5′-AGTTCACGTCCCAGGTCATC-3′ | 149 |
| Rev | 5′-CTCCAACTTCTTGCCAGAGC-3′ | ||
| GAPDH | Fwd | 5′-TGACCCCTTCATTGACCTTC-3′ | 143 |
| Rev | 5′-GATCTCGCTCCTGGAAGATG-3′ | ||
| HMBS | Fwd | 5′-CTACTTCGCTGCATTGCTGA-3′ | 105 |
| Rev | 5′-CAGGTACAGTTGCCCATCCT-3′ | ||
| IGF1 | Fwd | 5′-GATGCTCTCCAGTTCGTGTG-3′ | 141 |
| Rev | 5′-CTCCAGCCTCCTCAGATCAC-3′ | ||
| IGF1R | Fwd | 5′-CAAAGGCAATCTGCTCATCA-3′ | 139 |
| Rev | 5′-CAGGAAGGACAAGGAGACCA-3′ | ||
| IGF2 | Fwd | 5′-CCAGCGATTAGAAGTGAGCC-3′ | 95 |
| Rev | 5′-AGACCTAGTGGGGCGGTC-3′ | ||
| IGF2R | Fwd | 5′-GCAATGCTAAGCTTTCGTATTACG-3′ | 188 |
| Rev | 5′-GGTGTACCACCGGAAGTTGTATG-3′ | ||
| LPL | Fwd | 5′-GTGACCGAATCTGTGGCTAAC-3′ | 251 |
| Rev | 5′-GGCACCCAACTCTCATACATT-3′ | ||
| MYOD1 | Fwd | 5′-GAACACTACAGCGGCGACTC-3′ | 121 |
| Rev | 5′-AGTAAGTGCGGTCGTAGCAG-3′ | ||
| MYOG | Fwd | 5′-CAGTGAATGCAGCTCCCATA-3′ | 164 |
| Rev | 5′-CGACATCCTCCACTGTGATG-3′ | ||
| PPARγ | Fwd | 5′-CGGTTTCAGAAGTGCCTT G-3′ | 137 |
| Rev | 5′-GGTCAGCAGACTCTGGGTTC-3′ | ||
| RPL19 | Fwd | 5′-ACCCCAATGAGACCAATGAA-3′ | 101 |
| Rev | 5′-ATGGACAGTCACAGGCTTCC-3′ | ||
| SCD | Fwd | 5′-ACCTGGCTGGTGAATAGTGC-3′ | 212 |
| Rev | 5′-AAGGTGTGGTGGTAGTTGTGG-3′ | ||
| YWHAZ | Fwd | 5′ -AGACGGAAGGTGCTGAGAAA-3′ | 123 |
| Rev | 5′-CGTTGGGGATCAAGAACTTT-3′ |
1GenBank accession numbers in Paradis et al. (2017).
Statistical Analysis
Postpartum data on 2 cows and their calves from the flax treatment (one in each year) were removed from the analysis due to death of the cow from natural causes unrelated to treatment during the breeding season. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). The average of each research pen where cows received their prepartum treatment diets represented the experimental unit. Data were analyzed as a Randomized complete block design (RCBD) using the Mixed procedure. The statistical model included the fixed effect of treatment and the random effect of year. The average number of days that cows were exposed to treatment diet (from start of the trial until calving), as well as the proportion of heifer calves within the original pen, was also included in the model as covariates. The effects of treatment, time, and their interaction were evaluated for milk parameters and animal BW measured at 21 and 42 d of lactation using a RCBD accounting for repeated measures where 8 covariance structures were tested. The covariance structure with the lowest Akaike’s and Bayesian information criterion (AIC and BIC) values was selected (Littell et al., 1998). The Glimmix procedure was used to analyze categorical data including BCS, pregnancy status, quality grade, and yield grade score. The Kenward–Roger option was used to estimate denominator degrees of freedom. Preplanned contrasts were used to determine the effects of level (LF vs. HF) and source (CAN vs. FLX) of fat. Significant differences were declared at P ≤ 0.05, and trends at P < 0.10.
RESULTS AND DISCUSSION
Postpartum Performance of Cows
Results on the performance of cows are presented in Tables 3 and 4. Level (P ≥ 0.72) and source (P ≥ 0.28) of dietary fat during gestation did not affect BW at calving (Table 3), or the average BW and ADG during the first 42 d of lactation (Table 4). Although there was no interaction (P = 0.18) between treatment and time, BW of cows decreased linearly (P < 0.01) throughout the first 42 d of lactation (Table 4). At calving, BCS of cows tended (P ≤ 0.09) to be greater for LF compared with HF cows (2.74 vs. 2.63) and for FLX compared with CAN (2.69 vs. 2.56) cows (Table 3). Greater BCS observed at calving in cows fed the LF diet over gestation is most likely a carryover effect of the greater BCS reported for the same group of cows at 23 ± 4.6 d prior to calving (Añez-Osuna et al., 2019). During the first 42 d of lactation, all treatments experienced a decrease (P < 0.01) in BCS from 2.7 to 2.4, and the average BCS of LF cows during the first 42 d of lactation was greater (P < 0.01) than that of HF cows (2.63 vs. 2.51) with no difference (P = 0.32) between CAN and FLX cows (Table 4). No effects (P ≥ 0.35) of level or source of dietary fat fed over gestation were observed on BCS change during the first 42 d of lactation.
Table 3.
Effects of level and source of fat in the diet of beef cows during gestation on their postpartum and reproductive performance
| Treatments1 | Contrasts2 | |||||
|---|---|---|---|---|---|---|
| Item | LF (n = 10) |
CAN (n = 10) |
FLX (n = 10) |
SEM | LF vs. HF | CAN vs FLX |
| At calving | ||||||
| BW, kg | 704 | 703 | 704 | 7.51 | 0.95 | 0.81 |
| BCS3 | 2.74 | 2.56 | 2.69 | 0.05 | 0.07 | 0.09 |
| Thin, % of cows | 1.6 | 9.8 | 0.8 | 4.98 | 0.63 | 0.09 |
| Optimal, % of cows | 95.8 | 86.9 | 98.4 | 4.29 | 0.90 | 0.08 |
| Over conditioned, % of cows | 2.6 | 3.3 | 0.8 | 1.44 | 0.98 | 0.98 |
| SCFT,4 mm | ||||||
| Rib | 5.7 | 3.9 | 4.6 | 0.30 | <0.01 | 0.11 |
| Rump | 6.4 | 4.9 | 5.7 | 0.40 | 0.02 | 0.12 |
| At weaning | ||||||
| Days postpartum, d | 183 | 184 | 182 | 1.51 | 0.81 | 0.34 |
| BW, kg | 680 | 685 | 681 | 8.09 | 0.58 | 0.49 |
| Cumulative ADG, kg/d | −0.13 | −0.10 | −0.15 | 0.07 | 0.76 | 0.21 |
| BW180, kg | 680 | 684 | 682 | 7.82 | 0.54 | 0.69 |
| BCS3 | 2.62 | 2.56 | 2.61 | 0.06 | 0.49 | 0.46 |
| Thin, % of cows | 2.8 | 0.0 | 2.4 | 2.23 | 0.98 | 0.99 |
| Optimal, % of cows | 96.2 | 100.0 | 95.4 | 2.63 | 0.97 | 0.97 |
| Over conditioned, % of cows | 1.0 | 0.0 | 2.2 | 1.82 | 0.98 | 0.98 |
| SCFT,4 mm | ||||||
| Rib | 4.3 | 3.4 | 3.9 | 0.43 | 0.01 | 0.11 |
| Change | −1.2 | −0.5 | −0.8 | 0.29 | 0.16 | 0.54 |
| Rump | 4.5 | 4.4 | 4.4 | 0.52 | 0.84 | 0.90 |
| Change | −2.0 | −0.5 | −1.5 | 0.42 | 0.05 | 0.14 |
| Reproductive performance | ||||||
| Pregnancy rate, % of cows | 85.3 | 96.0 | 94.7 | 4.01 | 0.07 | 0.77 |
| Calving rate, % of cows | 83.3 | 90.1 | 89.0 | 5.23 | 0.33 | 0.89 |
| First calving, Julian d | 106 | 104 | 105 | 2.62 | 0.33 | 0.68 |
| Last calving, Julian d | 126 | 134 | 127 | 4.43 | 0.45 | 0.26 |
| Calving span, d | 20 | 30 | 22 | 4.74 | 0.30 | 0.23 |
| Calving distribution | ||||||
| At 21 d, % of cows | 84.0 | 71.8 | 76.9 | 7.22 | 0.27 | 0.66 |
| At 42 d, % of cows | 95.9 | 94.2 | 95.7 | 3.61 | 0.82 | 0.79 |
| Average, Julian d | 14 | 16 | 14 | 2.00 | 0.55 | 0.43 |
| C-C,5 d | 366 | 369 | 367 | 2.28 | 0.56 | 0.50 |
1LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed-based pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
2HF = average of CAN and FLX.
3Thin = % of cows with a BCS < 2.5; Optimal = % of cows with a 2.5 ≤ BCS ≤ 3; Over conditioned = % of cows with BCS > 3.
4SCFT = subcutaneous fat thickness measured by ultrasound.
5C-C = calving to calving interval.
Table 4.
Effects of level and source of fat in the diet beef cows during gestation, time and treatment × time interaction on performance, partial milk yield, and milk composition during the first 42 d of lactation
| Treatment1 | Days relative to calving | P-value2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | LF (n = 10) |
CAN (n = 10) |
FLX (n = 10) |
SEM | 0 | 21 | 42 | SEM | LF vs. HF | CAN vs. FLX | D | trt × d |
| Performance of cows3 | ||||||||||||
| BW, kg | 694 | 691 | 701 | 13.7 | 705 | 695 | 686 | 12.9 | 0.72 | 0.28 | <0.01 | 0.18 |
| Cumulative ADG, kg/d | −0.49 | −0.53 | −0.46 | 0.44 | – | −0.54 | −0.45 | 0.44 | 0.93 | 0.63 | 0.21 | 0.54 |
| BCS | 2.63 | 2.49 | 2.53 | 0.07 | 2.66 | 2.56 | 2.44 | 0.07 | <0.01 | 0.32 | <0.01 | 0.75 |
| Change | −0.20 | −0.14 | −0.15 | 0.09 | – | −0.10 | −0.22 | 0.08 | 0.35 | 0.92 | 0.04 | 0.68 |
| Thin, % of cows | 3.7 | 16.7 | 6.7 | 8.34 | 2.9 | 6.2 | 21.6 | 9.51 | 0.04 | 0.09 | <0.01 | 0.84 |
| Optimal, % of cows | 96.3 | 83.3 | 93.3 | 8.31 | 97.1 | 93.8 | 78.4 | 8.86 | 0.07 | 0.06 | <0.01 | 0.65 |
| Over conditioned, % of cows | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | 0.0 | – | – | – | – | – |
| NEFA, µEq/L | 1,043 | 1,032 | 1,014 | 92.0 | 1,049 | 1,038 | 1,002 | 91.9 | 0.73 | 0.78 | 0.75 | 0.28 |
| BHBA, mg/dL | 11.3 | 10.8 | 11.4 | 0.64 | 10.7 | 11.3 | 11.4 | 0.59 | 0.75 | 0.31 | 0.24 | 0.39 |
| Milk parameters4 | ||||||||||||
| 12-h milk yield, kg | 5.9 | 6.2 | 5.8 | 0.43 | – | 5.8 | 6.1 | 0.39 | 0.74 | 0.23 | 0.14 | 0.44 |
| Fat, % | 3.50 | 3.51 | 3.66 | 0.17 | – | 3.24 | 3.87 | 0.12 | 0.70 | 0.54 | <0.01 | 0.97 |
| Fat yield, g | 217 | 224 | 214 | 18.9 | – | 193 | 243 | 15.7 | 0.91 | 0.66 | <0.01 | 0.78 |
| Protein, % | 3.07 | 3.11 | 3.01 | 0.07 | – | 3.22 | 2.90 | 0.07 | 0.82 | 0.03 | <0.01 | 0.81 |
| Protein yield, g | 183 | 193 | 171 | 16.6 | – | 187 | 177 | 15.7 | 0.91 | 0.08 | 0.23 | 0.54 |
| Lactose, % | 4.63 | 4.57 | 4.64 | 0.04 | – | 4.53 | 4.70 | 0.03 | 0.63 | 0.28 | <0.01 | 0.23 |
| Lactose yield, g | 273 | 286 | 264 | 20.0 | – | 262 | 287 | 17.9 | 0.88 | 0.25 | 0.01 | 0.83 |
| Total solids, % | 12.2 | 12.2 | 12.3 | 0.20 | – | 12.0 | 12.5 | 0.16 | 0.78 | 0.67 | <0.01 | 0.89 |
| Total solids yield, g | 818 | 890 | 796 | 62.4 | – | 706 | 963 | 49.5 | 0.75 | 0.31 | <0.01 | 0.56 |
| Milk energy, Mcal/kg | 0.68 | 0.68 | 0.69 | 0.02 | – | 0.66 | 0.70 | 0.01 | 0.72 | 0.57 | <0.01 | 0.97 |
| Milk energy yield, Mcal | 4.09 | 4.26 | 3.97 | 0.29 | – | 3.85 | 4.37 | 0.24 | 0.94 | 0.37 | <0.01 | 0.69 |
| MUN, mg/dL | 11.6 | 11.2 | 11.2 | 2.37 | – | 11.7 | 11.0 | 2.35 | 0.42 | 0.99 | 0.07 | 0.73 |
1LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed–based pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
2HF = average of CAN and FLX.
3Thin = % of cows with a BCS < 2.5; Optimal = % of cows with a 2.5 ≤ BCS ≤ 3; Over conditioned = % of cows with BCS > 3; NEFA = nonesterified fatty acids; BHBA = β-hydroxy butyrate.
412-h milk yield = milk yield estimated for 4 quarters over 12 h. Milk energy yield (Mcal) was calculated from milk composition (NRC, 2000) based on a predicted four-quarter 12-h yield. MUN = milk urea nitrogen.
There was no effect of level (P ≥ 0.54) or source (P ≥ 0.21) of dietary fat fed during gestation on dam calving to weaning ADG, BW at weaning, or on BW adjusted to 180 d postcalving (Table 3). Also, BCS of cows at weaning and the change in BCS from calving to weaning were not affected (P ≥ 0.28) by treatment. These results are consistent with those of Banta et al. (2011) who found no effect on BW, BW change, or BCS at weaning of multiparous beef cows receiving no supplement or fed either a high-linoleic or high-oleic supplement during mid to late gestation.
Although no differences in BCS were observed at weaning, when measured using ultrasound at the rib and rump location, the level of dietary fat fed during gestation affected body fat reserves over the period from calving to weaning. At calving, the SCFT from LF cows was greater (P ≤ 0.02) at both the rib (5.7 vs. 4.3 mm) and rump (6.4 vs. 5.3 mm) locations than those of HF cows, whereas the SCFT of CAN cows was not different (P ≥ 0.11) from FLX cows at the rib (3.9 vs. 4.6 mm) or rump (4.9 vs. 5.7 mm) locations (Table 3). At weaning, the rib SCFT was still greater (P = 0.01) for LF compared with HF cows and still not different (P = 0.11) for CAN compared with FLX cows. However, cows fed the LF diet during gestation had greater (P = 0.05) reduction in the SCFT at the rump (−2.0 vs. −1.0 mm) location from calving to weaning than those fed the HF diets, whereas no difference (P = 0.14) was observed between cows fed the CAN and FLX diets (Table 4). The reason for the greater loss in subcutaneous fat of LF cows could be attributed to a possible reduction in energy intake through a negative feedback effect of leptin. According to Murdoch et al. (2005), cattle with greater adipose tissue depots have a greater concentration of circulating leptin that might decrease the energy intake and the subsequent amount of body fat.
Blood Metabolites
Results for NEFA and BHBA concentrations in serum of cows during the first 42 d of lactation are shown in Table 4. No effects of treatment (P ≥ 0.31) were observed with serum NEFA and BHBA concentrations averaging of 1,029 ± 263 µEq/L and 11.2 ± 1.8 mg/dL (respectively) across treatments. The serum NEFA concentration of cows during the first 42 d of lactation was greater than the 490 and 526 µEq/L reported (490 and 526 µEq/L) by Bellows et al. (2001) during the prebreeding period of primiparous beef heifers fed a low- or a high-fat diet, 68 d prior to calving. The higher levels observed in the present study can be attributed to factors unrelated to treatment such as stress from handling and blood sampling (Bowden, 1971; Swanson and Morrow-Tesch, 2001). No effects of time or treatment × time interaction were observed for serum NEFA or BHBA concentrations from calving to 42 d of lactation. This lack of effect on serum NEFA and BHBA concentrations during the first 42 d of lactation is consistent with the fact that cows had similar ADG and average BW over the same period, as mentioned previously. Also, the lack of responses for serum NEFA or BHBA concentration during the first 42 d of lactation are likely due to the fact that all cows were fed the same diet after calving.
Milk Yield and Composition
The results for 12-h milk yield and milk composition during the first 42 d of lactation are shown in Table 4. The estimated 12-h milk yield during the first 42 d of lactation averaged 6.0 ± 1.0 kg across treatments and was not affected by either the level (P ≥ 0.74) or source (P ≥ 0.23) of dietary fat fed over gestation. This lack of response to prepartum fat supplementation is consistent with findings reported by Banta et al. (2011) who reported that supplementing beef cows during mid- to late-gestation with either a low- or two high-fat supplements had no effect on milk yield measured in early lactation.
The average across treatments for most milk components (3.6 ± 0.7% fat, 3.1 ± 0.2% protein, 4.6 ± 0.2% lactose, and 12.3 ± 0.7% total solids) during the first 42 d of lactation were similar to those reported by Rodrigues et al. (2014) for Angus and Angus-cross beef cows at the beginning of lactation (18 to 58 d of lactation). Milk fat, lactose, total solids, milk energy, and milk urea nitrogen (MUN) concentrations were not affected by either the level (P ≥ 0.63) or source (P ≥ 0.28) of dietary fat during gestation. No effects (P ≥ 0.25) were observed on milk fat, lactose, total solids, and energy yield. These results are in agreement with Alexander et al. (2002) and Banta et al. (2011) who reported no effect on milk fat, urea N, or solids during early lactation after prepartum supplementation of beef cows with supplements high in oleic or linolenic acid. However, in the present study, milk protein concentration was greater (P = 0.03) for CAN compared with FLX cows (3.11 vs. 3.01%), but no difference (P = 0.82) was found between LF and HF cows. Similarly, the estimated 12-h milk protein yield of CAN cows (193 g) tended (P = 0.08) to be greater than that of FLX cows (171 g). The reason for this greater level of protein in milk from CAN cows might be due to an increased availability of essential amino acids (EAA) such as methionine and lysine. It is known that the first 2 limiting EAA for milk production are methionine and lysine, and contents of these 2 EAA are greater in canola seed compared with flaxseed (Sosulski and Sarwar, 1973; Lee et al., 1995; Schwab and Broderick, 2017). Moreover, studies conducted using humans have shown that albumin can capture the excess of dietary EAA and transport them to other tissues for protein synthesis (De Feo et al., 1992). Across treatments, the concentrations of milk fat, lactose, total solids, and energy increased (P ≤ 0.01) from 21 to 42 d of lactation, whereas MUN concentration tended (P = 0.07) to decrease and milk protein concentration decreased (P < 0.01) from 21 to 42 d of lactation. No treatment × time effect (P ≥ 0.23) was observed for any of the milk parameters measured.
Milk Fatty Acid Profile
Results for milk fatty acid profile during the first 42 d of lactation are shown in Tables 5 and 6. Even though dietary treatments were ceased at calving and all cows received the same diet throughout the lactation period, differences were observed in milk fatty acid profile throughout the first 42 d of lactation. Cows fed the FLX diet during gestation had greater (P < 0.01) total PUFA, CLA, CLnA, and AD concentrations in milk fat (40.6 mg/g), whereas the total MUFA, BCFA, and SFA were not affected by either level (P = 0.86) or source (P = 0.65) of dietary fat fed over gestation. This is contrary to findings reported by Alexander et al. (2002) where supplementing fat prepartum to primiparous beef cows did not affect the fatty acid profile of milk collected at 30, 60, and 90 d of lactation. However, these authors only offered 115 g/d of supplemental fat in the form of high-fat range supplement over a 62-d prepartum period, whereas in the present study, cows received 300 g/d of fat from a pelleted feed over 183 d prepartum.
Table 5.
Effects of level and source of fat in the diet of beef cows during gestation, time and treatment × time interaction on milk fat polyunsaturated fatty acid profiles during the first 42 d of lactation
| Treatment1 | Days | P-value 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid, % of total | LF (n = 10) |
CAN (n = 10) |
FLX (n = 10) |
SEM | 21 | 42 | SEM | LF vs. HF | CAN vs. FLX | d | trt × d |
| ∑PUFA | 2.93 | 2.90 | 2.93 | 0.16 | 3.06 | 2.78 | 0.16 | 0.88 | 0.65 | <0.01 | 0.72 |
| ∑n-3 | 1.19 | 1.20 | 1.30 | 0.07 | 1.38 | 1.08 | 0.07 | 0.23 | 0.09 | <0.01 | 0.95 |
| 18:3n-3 | 0.82 | 0.82 | 0.89 | 0.06 | 0.94 | 0.74 | 0.05 | 0.39 | 0.22 | <0.01 | 0.94 |
| 20:3 + 20:4 + 20:5n-3 | 0.17 | 0.16 | 0.19 | 0.01 | 0.20 | 0.14 | 0.01 | 0.49 | < 0.01 | <0.01 | 0.90 |
| 22:5 + 22:6n-3 | 0.20 | 0.22 | 0.23 | 0.02 | 0.23 | 0.20 | 0.02 | <0.01 | 0.07 | <0.01 | 0.16 |
| ∑n-6 | 1.74 | 1.71 | 1.63 | 0.11 | 1.68 | 1.70 | 0.11 | 0.10 | 0.13 | 0.45 | 0.82 |
| 18:2n-6 | 1.25 | 1.24 | 1.23 | 0.11 | 1.19 | 1.29 | 0.11 | 0.63 | 0.82 | <0.01 | 0.93 |
| 18:3n-6 | 0.04 | 0.04 | 0.03 | 0.00 | 0.04 | 0.04 | 0.00 | <0.01 | 0.01 | 0.17 | 0.24 |
| 20:2 + 20:3 + 20:4n-6 | 0.37 | 0.35 | 0.30 | 0.01 | 0.36 | 0.31 | 0.01 | <0.01 | <0.01 | <0.01 | 0.02 |
| 22:2 + 22:4n-6 | 0.08 | 0.08 | 0.08 | 0.00 | 0.09 | 0.07 | 0.00 | 0.85 | 0.03 | <0.01 | 0.22 |
| ∑CLnA | 0.07 | 0.08 | 0.11 | 0.01 | 0.09 | 0.08 | 0.01 | <0.01 | <0.01 | 0.03 | 0.48 |
| c9,t11,t15-18:3 | 0.04 | 0.03 | 0.05 | 0.00 | 0.04 | 0.04 | 0.00 | <0.01 | <0.01 | 0.68 | 0.70 |
| c9,t11,c15-18:3 | 0.04 | 0.04 | 0.06 | 0.00 | 0.05 | 0.04 | 0.00 | 0.02 | 0.01 | <0.01 | 0.53 |
| ∑AD | 0.37 | 0.40 | 0.58 | 0.09 | 0.43 | 0.47 | 0.09 | <0.01 | <0.01 | <0.01 | 0.07 |
| c9,t14- + c9,t13-18:2 | 0.11 | 0.14 | 0.20 | 0.02 | 0.14 | 0.16 | 0.02 | <0.01 | <0.01 | <0.01 | 0.04 |
| c9,t15-18:2 | 0.05 | 0.06 | 0.08 | 0.01 | 0.05 | 0.07 | 0.01 | <0.01 | <0.01 | <0.01 | 0.02 |
| t11,c15-18:2 | 0.14 | 0.13 | 0.21 | 0.03 | 0.18 | 0.15 | 0.03 | <0.01 | <0.01 | 0.03 | 0.55 |
| ∑CLA | 0.65 | 0.69 | 0.88 | 0.08 | 0.72 | 0.77 | 0.08 | <0.01 | <0.01 | <0.01 | 0.42 |
| c9,t11- + t7,c9-18:2 | 0.55 | 0.60 | 0.76 | 0.07 | 0.61 | 0.67 | 0.07 | <0.01 | <0.01 | <0.01 | 0.62 |
| c11,t13-18:2 | 0.04 | 0.04 | 0.05 | 0.00 | 0.04 | 0.04 | 0.00 | 0.89 | 0.15 | 0.32 | 0.43 |
| t11,c13- + c11,c11-18:2 | 0.02 | 0.02 | 0.04 | 0.01 | 0.03 | 0.03 | 0.01 | <0.01 | <0.01 | <0.01 | 0.41 |
| t,t-CLA | 0.03 | 0.03 | 0.03 | 0.00 | 0.03 | 0.03 | 0.00 | <0.01 | <0.01 | 0.38 | 0.78 |
| n-6:n-3 ratio | 1.53 | 1.50 | 1.29 | 0.04 | 1.29 | 1.59 | 0.04 | <0.01 | <0.01 | <0.01 | 0.98 |
| Total, mg/g | 35.9 | 36.5 | 40.6 | 2.74 | 38.9 | 36.4 | 2.72 | <0.01 | <0.01 | <0.01 | 0.43 |
1LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed based–pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
2HF = average of CAN and FLX.
c = cis; t = trans; ΣPUFA = sum of polyunsaturated fatty acids (Σn-6 + Σn-3); Σn-3 = sum of n-3 fatty acids; Σn-6 = sum of n-6 fatty acids; ΣCLnA = sum of conjugated linolenic acids; ΣAD = sum of atypical dienes; ΣCLA = sum of conjugated linoleic acids. Total = ΣPUFA + ΣCLnA + ΣAD + ΣCLA.
Table 6.
Effects of level and source of fat in the diet of beef cows during gestation, time and treatment × time interaction on milk fat monounsaturated, branched-chain, and saturated fatty acid profiles during the first 42 d of lactation
| Treatment1 | Days | P-value2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid, % of total | LF (n = 10) |
CAN (n = 10) |
FLX (n = 10) |
SEM | 21 | 42 | SEM | LF vs. HF | CAN vs. FLX | d | trt × d |
| ∑MUFA | 31.9 | 31.4 | 31.7 | 3.03 | 29.0 | 34.3 | 3.02 | 0.68 | 0.75 | <0.01 | 0.96 |
| ∑c-MUFA | 29.9 | 28.9 | 29.0 | 2.84 | 26.5 | 32.0 | 2.82 | 0.20 | 0.94 | <0.01 | 0.97 |
| c9-14:1 | 0.75 | 0.69 | 0.72 | 0.08 | 0.68 | 0.76 | 0.08 | 0.22 | 0.38 | <0.01 | 0.65 |
| c7- + c9- + c11-16:1 | 2.43 | 2.04 | 2.08 | 0.06 | 1.95 | 2.42 | 0.05 | <0.01 | 0.54 | <0.01 | 0.49 |
| c5- + c7- + c9-17:1 | 0.69 | 0.59 | 0.61 | 0.06 | 0.57 | 0.69 | 0.06 | <0.01 | 0.54 | <0.01 | 0.82 |
| c9- + c10-18:1 | 24.6 | 24.3 | 24.2 | 2.19 | 22.1 | 26.6 | 2.17 | 0.56 | 0.91 | <0.01 | 0.95 |
| c11-18:1 | 0.85 | 0.78 | 0.74 | 0.07 | 0.71 | 0.87 | 0.07 | <0.01 | 0.17 | <0.01 | 0.75 |
| c12- + c13- + c15-18:1 | 0.25 | 0.27 | 0.36 | 0.03 | 0.28 | 0.31 | 0.03 | <0.01 | < 0.01 | <0.01 | 0.62 |
| ∑t-MUFA | 2.00 | 2.53 | 2.73 | 0.32 | 2.49 | 2.34 | 0.32 | <0.01 | 0.03 | <0.01 | 0.56 |
| t9- + t10- + t11- + t12-16:1 | 0.07 | 0.09 | 0.12 | 0.02 | 0.10 | 0.10 | 0.02 | <0.01 | < 0.01 | 0.77 | 0.39 |
| t6-18:1 | 0.09 | 0.13 | 0.16 | 0.01 | 0.12 | 0.13 | 0.01 | <0.01 | < 0.01 | 0.01 | 0.04 |
| t9-18:1 | 0.19 | 0.24 | 0.22 | 0.02 | 0.21 | 0.22 | 0.02 | <0.01 | < 0.01 | 0.07 | 0.14 |
| t10-18:1 | 0.12 | 0.17 | 0.16 | 0.01 | 0.15 | 0.15 | 0.01 | <0.01 | 0.11 | 0.56 | 0.53 |
| t11-18:1 | 0.98 | 1.15 | 1.30 | 0.14 | 1.21 | 1.07 | 0.14 | <0.01 | < 0.01 | <0.01 | 0.92 |
| t12-18:1 | 0.10 | 0.16 | 0.17 | 0.02 | 0.14 | 0.15 | 0.02 | <0.01 | 0.08 | 0.01 | 0.16 |
| ∑BCFA | 3.91 | 3.87 | 3.81 | 0.06 | 3.92 | 3.81 | 0.04 | 0.40 | 0.49 | 0.02 | 0.12 |
| ∑SFA | 59.9 | 60.3 | 59.7 | 3.39 | 62.4 | 57.5 | 3.38 | 0.90 | 0.44 | <0.01 | 0.99 |
| 4:0 + 6:0 + 8:0 | 3.35 | 3.35 | 3.39 | 0.26 | 3.62 | 3.10 | 0.26 | 0.77 | 0.67 | <0.01 | 0.41 |
| 10:0 | 2.69 | 2.68 | 2.75 | 0.35 | 2.93 | 2.49 | 0.35 | 0.77 | 0.53 | <0.01 | 0.81 |
| 12:0 | 2.95 | 2.95 | 3.03 | 0.40 | 3.24 | 2.71 | 0.40 | 0.78 | 0.55 | <0.01 | 0.88 |
| 14:0 | 9.45 | 9.51 | 9.62 | 0.98 | 10.1 | 8.96 | 0.97 | 0.72 | 0.71 | <0.01 | 0.98 |
| 16:0 | 28.6 | 28.1 | 27.7 | 1.82 | 28.8 | 27.5 | 1.80 | 0.11 | 0.40 | <0.01 | 0.94 |
| 18:0 | 8.99 | 9.84 | 9.35 | 0.53 | 9.82 | 8.96 | 0.51 | 0.02 | 0.09 | <0.01 | 0.84 |
| 19:0 + 20:0 + 22:0 + 23:0 + 24:0 | 0.61 | 0.64 | 0.61 | 0.02 | 0.65 | 0.60 | 0.02 | 0.40 | 0.20 | <0.01 | 0.28 |
| Total, mg/g | 857 | 856 | 860 | 20.1 | 865 | 851 | 20.0 | 0.86 | 0.65 | 0.05 | 0.82 |
1LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed–based pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
2HF = average of CAN and FLX.
c = cis; t = trans; ΣMUFA = sum of monounsaturated fatty acids; Σc-MUFA = sum of cis-monounsaturated fatty acids; Σt-MUFA = sum of trans-18:1 isomers; ΣBCFA = sum of branched chain fatty acids; ΣSFA =sum of saturated fatty acids. Total = ΣPUFA + ΣCLnA + ΣAD + ΣCLA.
Total concentration of PUFA (n-3 + n-6) in milk fat was not affected by either the level (P = 0.88) or source (P = 0.65) of dietary fat (Table 5). However, the n-6:n-3 ratio was lower (P < 0.01) for FLX compared with CAN (1.29 vs. 1.50) cows and lower (P < 0.01) for HF compared with LF cows (1.40 vs. 1.53). Total concentration of n-3 fatty acids in milk fat tended to be greater (P = 0.09) for FLX compared with CAN (1.30 vs. 1.20%) but not different (P < 0.23) between HF and LF (1.25 vs. 1.19%) cows, whereas the total concentration of n-6 fatty acids was not affected by the level (P = 0.10) or source (P = 0.13) of dietary fat fed over gestation. Similarly, Mattos et al. (2004) reported an increase in total n-3 concentration in milk fat of dairy cows receiving a diet high in fish oil (PUFA) compared with a diet high in olive oil (MUFA) during the periparturient period. Among n-3 and n-6 fatty acids measured, the concentrations of 18:3n-3 and 18:2n-6 in milk fat during the first 42 d of lactation were not affected by either level (P ≥ 0.39) or source (P ≥ 0.22) of dietary fat fed during gestation with an average across treatments of 0.84% and 1.24% for 18:3n-3 and 18:2n-6, respectively. Time had a significant effect on the concentration of most PUFA determined in milk fat during the first 42 d of lactation. The total concentration of PUFA and n-3 fatty acids decreased (P < 0.01) with time, whereas the total concentration of n-6 fatty acids remained without change (P = 0.45) through the first 42 d of lactation.
Total concentration of conjugated linolenic (CLnA) and linoleic (CLA) acids, and atypical dienes (AD) were greater (P < 0.01) in milk fat of FLX cows than those of CAN cows, and greater (P < 0.01) in HF than in LF cows. The greater concentration of CLnA, AD, and CLA in milk fat of FLX cows is possibly due to mobilized adipose tissue with similar fatty acid composition. In our companion study (Añez-Osuna et al., 2019), the analysis of subcutaneous adipose tissue of cows at 23 d prior to calving showed that concentrations of CLnA, CLA, and AD were greater in cows fed the FLX diet during gestation. Since all treatments had a loss in BCS and had high levels of serum NEFA concentration during the first 42 d of lactation as mentioned previously, the 18 carbon fatty acids present in milk fat most likely originated from circulating fatty acids mobilized from adipose tissue. This is consistent with the fact that all 18 carbon fatty acids in milk are derived from circulating plasma lipids (Shingfield et al., 2010). Time had a significant effect on the concentrations of conjugated fatty acids determined in milk fat during the first 42 d of lactation. The total concentration of CLnA, AD, and CLA increased (P < 0.01). Also, a tendency (P = 0.07) for a treatment × time effect was observed for total concentration of AD in milk fat. The total concentration of AD in milk fat of FLX cows tended (P = 0.09) to be greater at 21 d than those of CAN and LF cows and further increased by 42 d while remaining steady for CAN and LF cows.
Total concentration of SFA, BCFA, MUFA, and cis-MUFA isomers in milk fat during the first 42 d of lactation were not affected by level (P ≥ 0.40) or source (P ≥ 0.44) of dietary fat fed over gestation (Table 6). However, the total concentration of trans-MUFA was greater (P < 0.01) for HF compared with LF cows at 21 d (2.63 vs. 2.00%) and greater (P = 0.03) for FLX compared with CAN cows (2.73 vs. 2.53%). For the SFA, only the concentration of stearic acid (18:0) in milk fat was affected by the level of dietary fat fed over gestation. The concentration of 18:0 in milk of HF cows was greater (P = 0.02) than that of LF cows (9.60 vs. 8.99%) and tended (P = 0.09) to be greater for CAN compared with FLX cows (9.84 vs. 9.35%), and likely relates to differences in complete biohydrogenation of 18 carbon PUFA. Among cis-MUFA isomers, the total concentrations of cis12-18:1, cis13-18:1, and cis15-18:1 were greater (P < 0.01) for HF cows compared with LF cows (0.32 vs. 0.25%), whereas cis11-18:1 was the only 18 carbon MUFA whose concentration in milk was greater (P < 0.01) in LF compared with HF cows (0.85% vs. 0.76%). This is also consistent with 18 carbon fatty acids in milk arising from circulating lipids as a result of adipose tissue mobilization. The concentration of cis11-18:1 was greater in adipose tissue of LF compared with HF cows at the end of gestation (Añez-Osuna et al., 2019). However, the concentration of oleic acid (cis9-18:1) in milk fat was not affected by the level (P = 0.56) or source (P = 0.91) of fat. This lack of treatment effect on the concentration of cis9-18:1 in milk is most likely due to a greater rate of desaturation of 18:0 in LF compared with HF cows. Even though greater amounts of 18:0 likely reached the mammary gland of HF cows due to adipose tissue mobilization as mentioned previously, the activity of Δ9-desaturase was most likely decreased in HF cows. The inhibition of Δ9-desaturase activity due to greater PUFA concentration has been reported by Chilliard et al. (2000). Time had a significant effect on the concentration of most MUFA and SFA in milk fat. The total concentration of MUFA and cis-MUFA isomers in milk fat increased (P < 0.01) from 21 to 42 d of lactation, whereas the total concentration of trans-MUFA isomers, BCFA, and SFA in milk fat decreased (P ≤ 0.02) from 21 to 42 d of lactation.
Reproductive Performance
Reproductive performance parameters of cows are shown in Table 3. Cows fed the HF diets over gestation tended (P = 0.07) to have greater pregnancy rates than cows fed the LF diet (95.4% vs. 85.3%). This is consistent with other studies where beef cows supplemented with fat prepartum exhibited improved pregnancy rates (Lammoglia et al., 1997; Bellows et al., 2001; Grings et al., 2001). According to Hess et al. (2005), feeding fat to beef cows at least 60 d before calving results in improved pregnancy rates. No difference (P = 0.77) was observed in pregnancy rates of cows fed the CAN and FLX diet (96.0% vs. 94.7%). Level or source of dietary fat over gestation had no effect on calving span (P ≥ 0.23), calving distribution (P ≥ 0.27), or calving to calving interval (P = 0.50). In contrast, although a numerical difference in calving rates (6–7%) was still observed in favor of cows fed the HF diets during gestation, no effect (P = 0.33) of level of dietary fat during gestation could be declared.
The lack of effect of the source of fat (CAN vs. FLX) fed over gestation on reproductive performance is likely the result of the rumen biohydrogenation of dietary n-6 and n-3 fatty acids. According to Santos et al. (2008), n-6 and n-3 PUFA have the greatest effect on improving the reproductive performance of cattle. However, the rumen biohydrogenation process changes the configuration of n-6 and n-3 PUFA, thus reducing their effectiveness in improving the reproductive performance (Staples et al., 2002; Santos et al., 2013).
Performance of the Progeny
Results for performance of the progeny are shown in Table 7. Cows fed HF diets during gestation had heavier (P < 0.01) calves (42.9 kg) at birth compared with those from LF (40.2 kg) cows, whereas no difference (P = 0.24) was found between calves from CAN (42.4 kg) and FLX (43.3 kg) cows. Despite being exposed to similar amounts of dietary energy and protein over the last 2 trimesters of gestation, calves born to cows fed HF diets most likely had greater fetal growth due to an increase in placental nutrient uptake. In rodents, it has been reported (Jones et al., 2009) that feeding a high-fat diet to the dam during gestation has resulted in an increase in protein expression of glucose transporter 1 (GLUT1) and sodium-coupled neutral amino acid transporter 2 (SNAT2) in microvillous plasma membranes of isolated placentas. During the first 42 d after birth, no effects of either level (P = 0.55) or source (P = 0.74) of dietary fat fed over gestation were observed on the cumulative ADG of calves. This is consistent with Garcia et al. (2014) who reported no effects on ADG during the first 30 d for Holstein calves born to cows fed a low-fat or 1 of 2 high-fat diets over gestation. This lack of difference in ADG of calves during the first 42 d of lactation can be attributed to the fact that cows had similar ADG and milk yield during the same period, as mentioned previously. However, in the present study, the average BW during the first 42 d of calves from HF cows was greater (P < 0.01) than that of LF cows (65.9 vs. 61.8 kg), whereas no difference (P = 0.55) was observed between calves born from CAN and FLX cows (65.9 vs. 65.8 kg). This difference in average BW over the first 42 d between calves from HF and LF cows is most likely a reflection of the difference observed in birth weight between these 2 treatments.
Table 7.
Effects of level and source of fat in the diet prepartum of beef cows on the performance of their progeny
| Treatments2 | Contrasts3 | |||||
|---|---|---|---|---|---|---|
| Item1 | LF (n = 10) |
CAN (n = 10) |
FLX (n = 10) |
SEM | LF vs. HF | CAN vs. FLX |
| At birth | ||||||
| Date, Julian d | 114 | 113 | 115 | 1.65 | 0.87 | 0.52 |
| BW, kg | 40.2 | 42.4 | 43.3 | 1.08 | <0.01 | 0.24 |
| First 42 d | ||||||
| BW, kg | 61.8 | 65.9 | 65.8 | 1.22 | <0.01 | 0.94 |
| Cumulative ADG, kg/d | 1.04 | 1.06 | 1.07 | 0.05 | 0.55 | 0.74 |
| At weaning | ||||||
| Age, d | 183 | 184 | 183 | 1.85 | 0.71 | 0.52 |
| BW, kg | 248 | 260 | 251 | 8.39 | 0.05 | 0.05 |
| Cumulative ADG, kg/d | 1.13 | 1.19 | 1.13 | 0.05 | 0.18 | 0.03 |
| BW180, kg | 244 | 256 | 247 | 9.19 | <0.05 | <0.05 |
| Backgrounding period | ||||||
| Final BW, kg | 411 | 433 | 420 | 5.59 | 0.02 | 0.14 |
| Backgrounding ADG, kg/d | 0.91 | 0.97 | 0.95 | 0.02 | 0.07 | 0.46 |
| Cumulative ADG, kg/d | 1.02 | 1.08 | 1.04 | 0.02 | 0.07 | 0.14 |
| BW365, kg | 413 | 437 | 423 | 7.94 | 0.03 | 0.15 |
| Finishing period | ||||||
| Final BW, kg | 603 | 639 | 620 | 9.88 | 0.03 | 0.21 |
| Finishing ADG, kg/d | 1.90 | 2.04 | 1.97 | 0.06 | 0.16 | 0.43 |
| Cumulative ADG, kg/d | 1.22 | 1.29 | 1.24 | 0.02 | <0.05 | 0.16 |
| Shrunk BW, kg | 579 | 614 | 595 | 9.61 | 0.03 | 0.19 |
| At slaughter | ||||||
| Age, d | 463 | 465 | 463 | 10.4 | 0.55 | 0.30 |
| HCW, kg | 339 | 361 | 349 | 5.51 | 0.02 | 0.16 |
| Dressing, % | 58.5 | 58.8 | 58.7 | 0.27 | 0.48 | 0.77 |
| Quality grade, % | ||||||
| AAA | 78.6 | 78.7 | 84.2 | 12.9 | 0.68 | 0.54 |
| AA | 16.8 | 17.6 | 9.7 | 14.8 | 0.57 | 0.29 |
| A | 0.0 | 0.0 | 0.0 | – | – | – |
| Yield grade, % | ||||||
| 3 | 49.6 | 53.6 | 46.9 | 20.7 | 0.95 | 0.60 |
| 2 | 32.6 | 38.9 | 40.6 | 18.6 | 0.46 | 0.89 |
| 1 | 14.2 | 5.3 | 10.6 | 4.99 | 0.25 | 0.41 |
| LMA, cm2 | 84.5 | 85.2 | 83.2 | 1.23 | 0.86 | 0.23 |
| Fat thickness, mm | 12.7 | 13.7 | 12.7 | 1.52 | 0.51 | 0.37 |
| Marbling score | 444 | 452 | 443 | 10.7 | 0.78 | 0.61 |
1Cumulative ADG = average daily gain from birth; BW180 = body weight adjusted to 180 d; BW365 = body weight adjusted to 365 d; HCW = hot carcass weight; LMA = longissimus muscle area.
2LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed–based pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
3HF = average of CAN and FLX.
At weaning, cumulative ADG of calves born to CAN cows was greater (P = 0.03) than that of calves from FLX cows (1.19 vs. 1.13 kg/d), with no difference (P = 0.18) between calves from LF and HF cows (1.13 vs. 1.16 kg/d). Consequently, the weaning BW of calves born to CAN cows tended (P = 0.05) to be greater than that of calves born to FLX cows (260 vs. 251 kg), and the weaning BW of calves from HF cows also tended (P = 0.05) to be greater than that of calves from LF cows (256 vs. 248 kg). These tendencies became significant (P = 0.049) when weaning BW was adjusted to 180 d.
The reason for the superior performance from birth to weaning of calves from CAN cows compared with those from FLX cows is not clear. A possible explanation could be the greater milk protein concentration and yield observed in CAN cows during the first 42 d of lactation. Daley et al. (1987) reported a significant positive correlation (r = 0.39) between milk protein yield of B. taurus and B. Taurus × B. indicus cows and the preweaning weight of their calves. However, whether this difference in milk protein was maintained after 42 d remains unknown. Another possible explanation could be a decrease in milk fat synthesis due to the greater concentration of CLA observed in milk fat from FLX cows. Milk fat concentration and yield have been decreased by including flaxseed in the diet of dairy cows (Cortes et al., 2010). This decrease in milk fat has been attributed to reductions in the expression of genes that encode for enzymes associated with lipid synthesis in the mammary gland tissue after being exposed to CLA isomers (Baumgard et al., 2002; Peterson et al., 2002). Whether the difference in CLA concentration in milk fat between FLX and CAN cows remained after 42 d of lactation is unknown. However, as mentioned previously, mobilized adipose tissue was likely the reason for the greater CLA concentration in milk fat of FLX cows, and this tissue mobilization was most likely increased after 42 d since cows were approaching peak milk yield. Peak of lactation has been established between 56 and 70 d of lactation for Angus and Angus-cross beef cows (Rodrigues et al., 2014).
At the end of the backgrounding phase, calves from HF cows tended (P = 0.07) to have greater cumulative (1.06 vs. 1.02 kg/d) and backgrounding (0.96 vs. 0.91 kg/d) ADG than calves from LF cows, whereas no difference (P ≥ 0.14) was observed in cumulative (1.08 vs. 1.04 kg/d) or backgrounding (0.97 vs. 0.95 kg/d) ADG between calves born to CAN and FLX cows. As a result, the difference observed in weaning BW between calves from CAN and FLX cows disappeared after the backgrounding phase, whereas calves from HF cows remained heavier than calves from LF cows. At the end of the backgrounding phase, calves born to HF cows had greater (P ≤ 0.03) BW (427 vs. 411 kg) and 365-d adjusted BW (430 vs. 413 kg) compared with those from LF cows, whereas no difference (P ≥ 0.14) was observed between calves from CAN and FLX cows. The differences observed in performance from birth to the end of backgrounding of calves from LF compared with those from HF cows suggest a developmental programming effect from the level of fat fed over gestation. The possible lower uptake and transport of nutrients by the placenta of cows fed the LF diet could not only have resulted in lighter calves at birth (as indicated previously), but also could have diminished the postnatal growth and development of the progeny of LF cows compared with that of HF cows. According to Nathanielsz (2006), growth and development of fetal organs can be negatively affected when exposed to nutrient-restricted environments. Consequently, the postnatal performance of the offspring may be reduced (Greenwood et al., 2017).
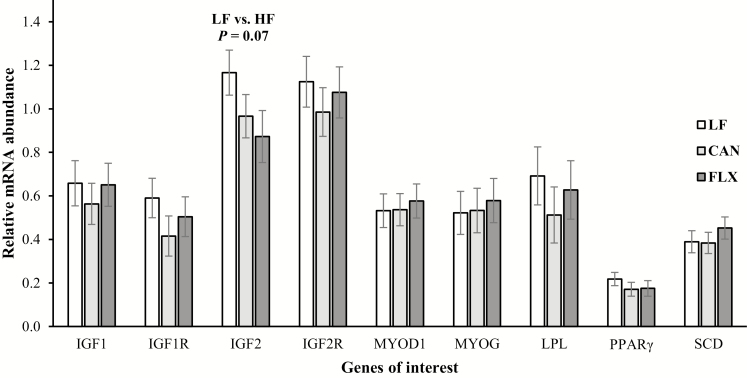
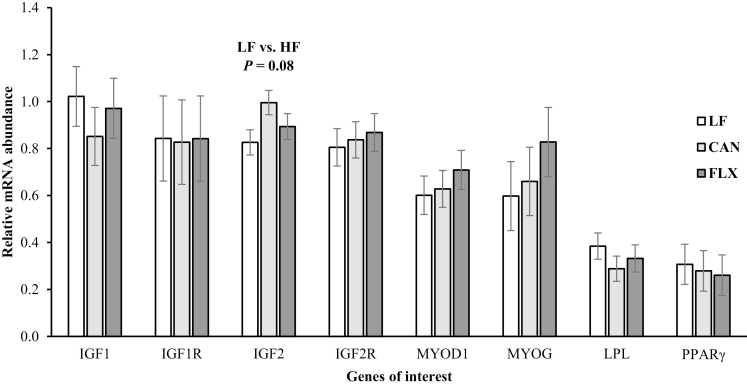
During the finishing phase, no effects of either level (P = 0.16) or source (P = 0.43) of dietary fat fed over gestation were observed for finishing ADG of calves. However, the cumulative ADG from birth to finishing was greater (P = 0.045) for calves born to cows fed the HF diets relative to those born to cows fed the LF diet (1.27 vs. 1.22 kg/d), whereas no difference (P = 0.16) was observed between calves from CAN and FLX cows (1.29 vs. 1.24 kg/d). Therefore, calves born to HF cows had greater (P = 0.03) final (630 vs. 603 kg) and shrunk (604 vs. 579 kg) BW at the end of the finishing phase than calves born to LF cows, whereas no difference (P ≥ 0.20) was observed between calves from CAN and FLX cows. At slaughter, the HCW of calves born to HF cows was 16 kg greater (P = 0.02) than that of calves born to LF cows (355 vs. 339 kg), whereas no difference was observed between calves from CAN and FLX cows. No effects of level (P ≥ 0.25) or source (P ≥ 0.16) of dietary fat fed during gestation to pregnant cows were observed for slaughter dressing percent, quality and yield grade, ribeye area, fat thickness, or marbling score of the progeny (Table 7). As well, no major effects were observed on fatty acid composition of subcutaneous adipose tissue of the progeny at slaughter (Table 8). Among all the evaluated fatty acids, only the concentrations of 20:3n-6 and 20:4n-6 in adipose tissue of calves from LF cows were greater (P ≤ 0.04) than calves from HF cows. This greater concentration of 20:3n-6 and 20:4n-6 is likely a carryover effect from lactation since the sum of thes fatty acids (20:2n-6, 20:3n-6, and 20:4n-6) was also greater (P < 0.01) in milk fat from LF cows compared with HF cows (Table 5).
Table 8.
Effects of level and source of fat in the diet prepartum of beef cows on the fatty acid profiles in subcutaneous adipose tissue of their progeny at slaughter
| Treatments2 | Contrasts3 | |||||
|---|---|---|---|---|---|---|
| Fatty acid,1 % of total | LF (n = 10) |
CAN (n = 10) |
FLX (n = 10) |
SEM | LF vs. HF | CAN vs. FLX |
| ∑PUFA | 1.11 | 1.09 | 1.11 | 0.08 | 0.84 | 0.74 |
| ∑n-3 | 0.26 | 0.27 | 0.27 | 0.02 | 0.75 | 0.86 |
| 18:3n-3 | 0.23 | 0.23 | 0.23 | 0.02 | 0.57 | 0.95 |
| 22:5n-3 | 0.04 | 0.03 | 0.04 | 0.00 | 0.32 | 0.51 |
| ∑n-6 | 0.85 | 0.82 | 0.84 | 0.08 | 0.66 | 0.71 |
| 18:2n-6 | 0.75 | 0.74 | 0.75 | 0.07 | 0.90 | 0.87 |
| 20:3n-6 | 0.05 | 0.04 | 0.05 | 0.00 | 0.04 | 0.07 |
| 20:4n-6 | 0.05 | 0.04 | 0.05 | 0.01 | 0.02 | 0.20 |
| ∑CLnA | 0.04 | 0.04 | 0.04 | 0.00 | 0.90 | 0.16 |
| c9,t11,t15-18:3 | 0.01 | 0.01 | 0.01 | 0.00 | 0.42 | 0.78 |
| c9,t11,c15-18:3 | 0.03 | 0.02 | 0.03 | 0.00 | 0.50 | 0.08 |
| ∑AD | 0.41 | 0.42 | 0.41 | 0.05 | 0.79 | 0.53 |
| c9,t14- + c9,t13-18:2 | 0.18 | 0.18 | 0.18 | 0.02 | 0.91 | 0.83 |
| c9,t15-18:2 | 0.09 | 0.09 | 0.09 | 0.01 | 0.95 | 0.73 |
| ∑CLA | 0.48 | 0.51 | 0.51 | 0.06 | 0.34 | 0.96 |
| c9,t11- + t7,c9-18:2 | 0.45 | 0.47 | 0.47 | 0.05 | 0.39 | 0.96 |
| t11,c13-18:2 | 0.02 | 0.02 | 0.02 | 0.00 | 0.86 | 0.35 |
| t,t-CLA | 0.01 | 0.02 | 0.02 | 0.01 | 0.19 | 0.30 |
| ∑MUFA | 59.8 | 59.9 | 60.3 | 0.49 | 0.58 | 0.60 |
| ∑c-MUFA | 57.8 | 57.9 | 58.4 | 0.58 | 0.56 | 0.54 |
| c9-14:1 | 1.31 | 1.24 | 1.21 | 0.10 | 0.35 | 0.75 |
| c9-16:1 | 5.75 | 5.58 | 5.44 | 0.23 | 0.25 | 0.57 |
| c9-17:1 | 1.82 | 1.75 | 1.75 | 0.06 | 0.20 | 0.96 |
| c9-18:1 | 44.0 | 44.5 | 45.2 | 0.74 | 0.15 | 0.32 |
| c11-18:1 | 2.83 | 2.78 | 2.78 | 0.08 | 0.54 | 0.95 |
| ∑t-MUFA | 2.02 | 2.01 | 1.91 | 0.11 | 0.63 | 0.49 |
| t6-18:1 | 0.12 | 0.12 | 0.11 | 0.01 | 0.40 | 0.50 |
| t9-18:1 | 0.20 | 0.20 | 0.20 | 0.01 | 0.90 | 0.82 |
| t10-18:1 | 1.01 | 0.98 | 0.88 | 0.08 | 0.44 | 0.40 |
| t11-18:1 | 0.31 | 0.33 | 0.33 | 0.05 | 0.25 | 0.98 |
| ∑BCFA | 1.15 | 1.18 | 1.17 | 0.04 | 0.65 | 0.79 |
| ∑SFA | 37.0 | 36.8 | 36.5 | 0.44 | 0.51 | 0.59 |
| 14:0 | 2.83 | 2.72 | 2.61 | 0.11 | 0.08 | 0.32 |
| 16:0 | 24.1 | 24.0 | 23.6 | 0.53 | 0.39 | 0.29 |
| 17:0 | 1.50 | 1.47 | 1.47 | 0.04 | 0.65 | 0.98 |
| 18:0 | 7.80 | 7.88 | 8.03 | 0.37 | 0.64 | 0.69 |
| 20:0 | 0.04 | 0.05 | 0.05 | 0.00 | 0.69 | 0.90 |
1 c = cis; t = trans; ΣPUFA = sum of polyunsaturated fatty acids (Σn-6 + Σn-3); Σn-3 = sum of n-3 fatty acids; Σn-6 = sum of n-6 fatty acids; ΣCLnA = sum of conjugated linolenic acids; ΣAD = sum of atypical dienes; ΣCLA = sum of conjugated linoleic acids; ∑MUFA = sum of monounsaturated fatty acids; ∑c-MUFA = sum of cis-MUFA fatty acids; ∑t-MUFA = sum of trans-MUFA fatty acids; ∑BCFA = sum of branched chain fatty acids; ∑SFA = sum of monounsaturated fatty acids.
2LF = cows fed a low-fat diet; CAN = cows fed a high-fat diet including a canola seed–based pelleted feed; FLX = cows fed a high-fat diet including a flaxseed-based pelleted feed.
3HF = average of CAN and FLX.
From weaning to slaughter, calves were separated in 2 large groups according to sex. Consequently, it was not possible to statistically analyze the DMI and G:F of calves during the postweaning period. Therefore, the reason for the superior performance of calves born to cows fed the HF diets over gestation is unclear. A possible greater synthesis of adipose tissue related hormones due to a greater body fat mass of calves born to HF cows might help explain their increased performance. Although no statistical difference was observed between calves from HF and LF cows in subcutaneous or intramuscular fat measured via camera grading at slaughter, the total amount of body fat including visceral fat may have been greater in calves born to HF cows. Studies using rodent (Guo and Jen, 1995) and swine (Quiniou et al., 2008) models have shown that feeding a high-fat diet during gestation increases the total body fat content of the progeny. Adipose tissue can act as a highly active endocrine organ where steroid hormones (including estrogen) are synthesized (Kershaw and Flier, 2004). Estrogen dosing of beef cattle has resulted in improved ADG and F:G (Beconi et al., 1995; Cleale et al., 2013). This improvement in performance has been attributed to an increase in the secretion frequency of pituitary GH and its concentration in serum (Grigsby and Trenkle, 1986; Plouzek and Trenkle, 1991), and to an increase in circulating and muscle expression of IGF1 (Dayton and White, 2014).
Gene Expression
At birth (Figure 1), the IGF2 mRNA abundance in LD muscle of male calves tended (P = 0.07) to be greater for those born to LF cows compared with those born to HF cows. Previous studies have found that nutrient restriction during gestation resulted in an increased mRNA expression of IGF2 in skeletal muscle of sheep (Brameld et al., 2000) and beef cattle (Paradis et al., 2017) fetuses. Although in the present study LF cows were not nutrient restricted during the last 2 trimesters of gestation, LF fetuses were most likely exposed to lower plane of nutrition compared with HF fetuses due to a reduced placental uptake of nutrients, as explained previously. This could help us to explain the upregulated expression of IGF2 in LD muscle at birth of calves from LF cows compared with those from HF cows. Moreover, Brameld et al. (2000) and Maltin (2008) suggest that fetuses exposed to lower levels of nutrients would experience an early and accelerated increase in IGF2 expression in skeletal muscle, and this would lead to a greater fiber-type specification rather than secondary myogenesis. This possible lower number of secondary muscle fibers could help us to explain the diminished growth observed in calves from LF cows relative to those from HF cows. However, this hypothesis needs to be substantiated and more research is required. No effects of level or source of dietary fat during gestation were observed in the expression of any other gene evaluated in LD muscle of calves at birth.
Figure 1.
mRNA abundance for insulin-like growth factors, and myogenesis- and adipogenesis-related genes, in longissimus dorsi muscle from calf at birth exposed to a low-fat or 2 high-fat diet in utero during the 2nd and 3rd trimesters of gestation.
Contrary to findings at birth, the IGF2 mRNA abundance in LD muscle of male calves at weaning (Figure 2) tended (P = 0.08) to be lower for those born to LF cows compared with those born to HF cows. The reason for this trend for IGF2 expression in LD muscle of calves from HF cows at weaning is not clear. According to Schiaffino et al. (2013), hypertrophy and fusion of myosatellite cells are the most common mechanism for postnatal muscle growth, and both IGF1 and IGF2 are upregulated in skeletal muscle undergoing this process (Charge and Rudnicki, 2004). However, the role of IGF1 in muscle growth (hypertrophy) is significantly greater than that of IGF2 (Schiaffino et al., 2013). Further research is needed to establish the reason for the upregulation of IGF2 in LD muscle at weaning from calves born to HF cows.
Figure 2.
mRNA abundance for insulin-like growth factors, and myogenesis- and adipogenesis-related genes, in longissimus dorsi muscle from calf at weaning exposed to a low-fat or 2 high-fat diet in utero during the 2nd and 3rd trimesters of gestation.
CONCLUSIONS
The level and source of fat in the diet of gestating beef cows during the last 2 trimesters of gestation did not affect their BW or ADG from calving to weaning. However, cows fed the LF diet over gestation had greater BCS at calving and experienced a greater loss in adipose tissue from calving to weaning. Also, feeding the FLX diet over gestation increased the total concentration (mg/g) of PUFA, CLnA, CLA, and AD during the first 42 d of lactation. Pregnancy rate of cows fed the HF diets over gestation tended to be greater at the end of the breeding season compared with those fed the LF diet.
Compared with the LF diet, feeding the HF diets over gestation resulted in heavier calves at birth, greater calf performance from birth to slaughter, and superior shrunk BW and HCW of the progeny at slaughter. The reason for this difference in performance between the progeny of HF and LF cows is likely due to a developmental programming effect as the result of a possible greater placenta nutrient uptake and transport. However, feeding the FLX diet during gestation diminished the birth to weaning performance of the progeny possibly due to a negative effect of the increased levels of CLA isomers in milk fat on milk fat yield.
In conclusion, these data suggest that feeding beef cows a high-fat diet over gestation results in heavier progeny, superior postnatal performance, and greater HCW at slaughter, which indicates the possibility of improving the performance of beef cattle through a developmental programing effect. However, more research is needed in order to establish the physiological mechanisms involved.
Conflict of interest statement. None declared.
Footnotes
The authors wish to acknowledge the following funding organizations: the Saskatchewan Agriculture Development Fund; the Natural Sciences and Engineering Research Council of Canada; and West Central Pelleting, Ltd., Wilkie, SK.
LITERATURE CITED
- Adams R. S. 1980. Penn State forage testing service revised regression equations. Dairy Sci. Ext. Memo DSE-90-56. Pennsylvania State University, PA. [Google Scholar]
- Alexander B. M., Hess B. W., Hixon D. L., Garrett B. L., Rule D. C., McFarland M., Bottger J. D., Moss G. E., and Simms D. D.. 2002. Influence of prepartum fat supplementation on subsequent beef cow reproduction and calf performance. Prof. Anim. Sci. 18:351–357. doi: 10.15232/S1080-7446(15)31545-X [DOI] [Google Scholar]
- Andersen C. L., Jensen J. L., and Ørntoft T. F.. 2004. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496 [DOI] [PubMed] [Google Scholar]
- Añez-Osuna F., Penner G. B., Campbell J., Dugan M. E. R., Fitzsimmons C. J., Jefferson P. G., Lardner H. A, and McKinnon J. J.. 2019. Level and source of fat in the diet of gestating beef cows: I. Effects on the prepartum performance of the dam and birth weight of the progeny. J. Anim. Sci. 97:3103–3119. doi: 10.1093/jas/skz171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC International 2012. Official Methods of Analysis. 19th ed. AOAC Int., Gaithersburg, MD. [Google Scholar]
- Banta J. P., Lalman D. L., Owens F. N., Krehbiel C. R., and Wettemann R. P.. 2011. Effects of prepartum supplementation of linoleic and mid-oleic sunflower seed on cow performance, cow reproduction, and calf performance from birth through slaughter, and effects on intake and digestion in steers. J. Anim. Sci. 89:3718–3727. doi: 10.2527/jas.2011-3937 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., Matitashvili E., Corl B. A., Dwyer D. A., and Bauman D. E.. 2002. Trans-10, cis-12 conjugated linoleic acid decreases lipogenic rates and expression of genes involved in milk lipid synthesis in dairy cows. J. Dairy Sci. 85:2155–2163. doi: 10.3168/jds.S0022-0302(02)74294-X [DOI] [PubMed] [Google Scholar]
- Beconi M. G., Howard M. D., Forbes T. D., Muntifering R. B., Bradley N. W., and Ford M. J.. 1995. Growth and subsequent feedlot performance of estradiol-implanted vs nonimplanted steers grazing fall-accumulated endophyte-infested or low-endophyte tall fescue. J. Anim. Sci. 73:1576–1584. doi: 10.2527/1995.7361576x [DOI] [PubMed] [Google Scholar]
- Bellows R. A., Grings E. E., Simms D. D., Geary T. W., and Bergman J. W.. 2001. Effects of feeding supplemental fat during gestation to first-calf beef heifers. Prof. Anim. Sci. 17:81–89. doi:10.15232/S1080-7446(15)31602–8 [Google Scholar]
- Bowden D. M. 1971. Non-esterified fatty acids and ketone bodies in blood as indicators of nutritional status in ruminants: a review. Can. J. Anim. Sci. 51:1–13. [Google Scholar]
- Brameld J. M., Mostyn A., Dandrea J., Stephenson T. J., Dawson J. M., Buttery P. J., and Symonds M. E.. 2000. Maternal nutrition alters the expression of insulin-like growth factors in fetal sheep liver and skeletal muscle. J. Endocrinol. 167:429–437. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care 2009. CCAC guidelines on: the care and use of farm animals in research, teaching and testing. Canadian Council on Animal Care; Ottawa, ON, Canada. [Google Scholar]
- CBGA (Canadian Beef Grading Agency) 2009. Canadian beef grading system http://beefgradingagency.ca. Accessed August 2018.
- Chargé S. B., and Rudnicki M. A.. 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chilliard Y., Ferlay A., Mansbridge R. M., and Doreau M.. 2000. Ruminant milk fat plasticity: nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 49:181–205. doi.10.1051/animres:2000117 [Google Scholar]
- Cleale R. M., Amodie D., Bechtol D. T., Drouillard J. S., Edmonds J. D., Edmonds M., Hunsaker B. D., Kraft L. A., Lawrence T. E., Rulli R. D., et al. 2013. Effects of estradiol benzoate and trenbolone acetate, alone or in combination at dose levels present in synovex choice, on performance by feedlot heifers. J. Anim. Sci. 91:970–977. doi: 10.2527/jas.2012-5214 [DOI] [PubMed] [Google Scholar]
- Côrtes C., da Silva-Kazama D. C., Kazama R., Gagnon N., Benchaar C., Santos G. T., Zeoula L. M., and Petit H. V.. 2010. Milk composition, milk fatty acid profile, digestion, and ruminal fermentation in dairy cows fed whole flaxseed and calcium salts of flaxseed oil. J. Dairy Sci. 93:3146–3157. doi: 10.3168/jds.2009-2905 [DOI] [PubMed] [Google Scholar]
- Cruz-Hernandez C., Deng Z., Zhou J., Hill A. R., Yurawecz M. P., Delmonte P., Mossoba M. M., Dugan M. E., and Kramer J. K.. 2004. Methods for analysis of conjugated linoleic acids and trans-18:1 isomers in dairy fats by using a combination of gas chromatography, silver-ion thin-layer chromatography/gas chromatography, and silver-ion liquid chromatography. J. AOAC Int. 87:545–562. [PubMed] [Google Scholar]
- Daley D. R., McCuskey A., and Bailey C. M.. 1987. Composition and yield of milk from beef-type bos taurus and bos indicus X bos taurus dams. J. Anim. Sci. 64:373–384. doi: 10.2527/jas1987.642373x [DOI] [PubMed] [Google Scholar]
- Dayton W. R., and White M. E.. 2014. Meat Science And Muscle Biology Symposium–role of satellite cells in anabolic steroid-induced muscle growth in feedlot steers. J. Anim. Sci. 92:30–38. doi: 10.2527/jas.2013-7077 [DOI] [PubMed] [Google Scholar]
- De Feo P., Horber F. F., and Haymond M. W.. 1992. Meal stimulation of albumin synthesis: a significant contributor to whole body protein synthesis in humans. Am. J. Physiol. 263(4 Pt 1):E794–E799. doi: 10.1152/ajpendo.1992.263.4.E794 [DOI] [PubMed] [Google Scholar]
- Funston R. N., Larson D. M., and Vonnahme K. A.. 2010. Effects of maternal nutrition on conceptus growth and offspring performance: implications for beef cattle production. J. Anim. Sci. 88(Suppl): E205–E215. doi:10.2527/jas.2009–2351 [DOI] [PubMed] [Google Scholar]
- Garcia M., Greco L. F., Favoreto M. G., Marsola R. S., Wang D., Shin J. H., Block E., Thatcher W. W., Santos J. E., and Staples C. R.. 2014. Effect of supplementing essential fatty acids to pregnant nonlactating holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 97:5045–5064. doi: 10.3168/jds.2013-7473 [DOI] [PubMed] [Google Scholar]
- Garcia M., Shin J. H., Schlaefli A., Greco L. F., Maunsell F. P., Santos J. E. P., Staples C. R., and Thatcher W. W.. 2015. Increasing intake of essential fatty acids from milk replacer benefits performance, immune responses, and health of preweaned Holstein calves. J. Dairy Sci. 98:458–477. doi:10.3168/jds.2014–8384 [DOI] [PubMed] [Google Scholar]
- Graham K. K., Bader J. F., Patterson D. J., Kerley M. S., and Zumbrunnen C. N.. 2001. Supplementing whole soybeans prepartum increases first service conception rate in postpartum suckled beef cows. J. Anim. Sci. 79 (Suppl. 2): 106 (Abstr.). [Google Scholar]
- Greenwood P., Clayton E., and Bell A.. 2017. Developmental programming and beef production. Anim. Front. 7:38–47. doi: 10.2527/af.2017-0127 [DOI] [Google Scholar]
- Grigsby M. E., and Trenkle A.. 1986. Plasma growth hormone, insulin, glucocorticoids and thyroid hormones in large, medium and small breeds of steers with and without an estradiol implant. Domest. Anim. Endocrinol. 3:261–267. doi: 10.1016/0739-7240(86)90024-X [DOI] [Google Scholar]
- Grings E. E., Short R. E., Blummel M., MacNeil M. D., and Bellows R. A.. 2001. Prepartum supplementation with protein or fat and protein for grazing cows in three seasons of calving. Proc. West. Sec. Amer. Soc. Anim. Sci. 52:501–504. [Google Scholar]
- Guo F., and Jen K. L.. 1995. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol. Behav. 57:681–686. [DOI] [PubMed] [Google Scholar]
- Hess B. W., Lake S. L., Scholljegerdes E. J., Weston T. R., Nayigihugu V., Molle J. D. C., and Moss G. E.. 2005. Nutritional controls of beef cow reproduction. J. Anim. Sci. 83:E90–E106. doi: 10.2527/2005.8313_supplE90x [DOI] [Google Scholar]
- Hess B. W., Moss G. E., and Rule D. C.. 2008. A decade of developments in the area of fat supplementation research with beef cattle and sheep. J. Anim. Sci. 86:E188–E204. doi: 10.2527/jas.2007-0546 [DOI] [PubMed] [Google Scholar]
- Jones H. N., Woollett L. A., Barbour N., Prasad P. D., Powell T. L., and Jansson T.. 2009. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. Faseb J. 23:271–278. doi: 10.1096/fj.08-116889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. K., Hernandez M., Cruz-Hernandez C., Kraft J., and Dugan M. E.. 2008. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids. 43:259–273. doi: 10.1007/s11745-007-3143-4 [DOI] [PubMed] [Google Scholar]
- Kershaw E. E., and Flier J. S.. 2004. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89:2548–2556. doi: 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- Lammoglia M. A., Bellows R. A., Grings E. E., and Bergman J. W.. 1999. Effects of prepartum supplementary fat and muscle hypertrophy genotype on cold tolerance in newborn calves. J. Anim. Sci. 77:2227–2233. doi: 10.2527/1999.7782227x [DOI] [PubMed] [Google Scholar]
- Lammoglia M. A., Bellows R. A., Grings E. E., Bergman J. W., Short R. E., and MacNeil M. D.. 1997. Effects of dietary fat composition and content, breed and calf sex on birth weight, dystocia, calf vigor and postpartum reproduction of first calf beef heifers. Proc. West. Sec. Amer. Soc. Anim. Sci. 48:81–84. [Google Scholar]
- Lee K. H., Qi G. H., and Sim J. S.. 1995. Metabolizable energy and amino acid availability of full-fat seeds, meals, and oils of flax and canola. Poult. Sci. 74:1341–1348. doi: 10.3382/ps.0741341 [DOI] [PubMed] [Google Scholar]
- Littell R. C., Henry P. R., and Ammerman C. B.. 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi: 10.2527/1998.7641216x [DOI] [PubMed] [Google Scholar]
- Lopes C. N., Scarpa A. B., Cappellozza B. I., Cooke R. F., and Vasconcelos J. L.. 2009. Effects of rumen-protected polyunsaturated fatty acid supplementation on reproductive performance of bos indicus beef cows. J. Anim. Sci. 87:3935–3943. doi: 10.2527/jas.2009-2201 [DOI] [PubMed] [Google Scholar]
- Lowman B. G., Scott N. A., and Sommerville S. H.. 1976. Condition scoring for cattle. East of Scotland College of Agriculture Bulletin No. 6. Edinburgh School of Agriculture, Edinburgh. [Google Scholar]
- Maltin C. A. 2008. Muscle development and obesity: is there a relationship? Organogenesis 4:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos R., Staples C. R., Arteche A., Wiltbank M. C., Diaz F. J., Jenkins T. C., and Thatcher W. W.. 2004. The effects of feeding fish oil on uterine secretion of PGF2ALPHA, milk composition, and metabolic status of periparturient holstein cows. J. Dairy Sci. 87:921–932. doi: 10.3168/jds.S0022-0302(04)73236-1 [DOI] [PubMed] [Google Scholar]
- Murdoch G. K., Okine E. K., Dixon W. T., Nkrumah J. D., Basarab J. A., and Christopherson R. J.. 2005. Growth. In: Dijkstra J.et al. , editors, Quantitative aspects of ruminant digestion and metabolism. CABI Publishing, Cambridge, MA: p. 489–521. [Google Scholar]
- Nathanielsz P. W. 2006. Animal models that elucidate basic principles of the developmental origins of adult diseases. Ilar J. 47:73–82. doi: 10.1093/ilar.47.1.73 [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) 2000. Nutrient requirements of beef cattle. 7th revised ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Palmquist D. L. 1994. The role of dietary fats in efficient of ruminants. J. Nutr. 124:1377S–1382S. doi: 10.1093/jn/124.suppl_8.1377S [DOI] [PubMed] [Google Scholar]
- Paradis F., Wood K. M., Swanson K. C., Miller S. P., McBride B. W., and Fitzsimmons C.. 2017. Maternal nutrient restriction in mid-to-late gestation influences fetal mrna expression in muscle tissues in beef cattle. BMC Genomics 18:632. doi: 10.1186/s12864-017-4051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. G., Matitashvili E. A., and Bauman D. E.. 2002. Diet-induced milk fat depression is characterized by increased milk fat content of t10, c12 CLA and corresponding reductions in lipogenic gene expression. FASEB J. 16:A232 (Abstr.). [Google Scholar]
- Petit H. V. and Berthiaume R.. 2006. Effect of feeding different sources of fat during gestation and lactation on reproduction of beef cows and calf performance. Can. J. Anim. Sci. 86:235–243. doi: 10.4141/A05-086 [DOI] [Google Scholar]
- Plouzek C. A., and Trenkle A.. 1991. Growth hormone parameters at four ages in intact and castrated male and female cattle. Domest. Anim. Endocrinol. 8:63–72. [DOI] [PubMed] [Google Scholar]
- Quiniou N., Richard S., Mourot J., and Etienne M.. 2008. Effect of dietary fat or starch supply during gestation and/or lactation on the performance of sows, piglets’ survival and on the performance of progeny after weaning. Animal 2:1633–1644. doi: 10.1017/S1751731108002991 [DOI] [PubMed] [Google Scholar]
- Rodrigues P. F., Menezes L. M., Azambuja R. C., Suñé R. W., Barbosa Silveira I. D., and Cardoso F. F.. 2014. Milk yield and composition from angus and angus-cross beef cows raised in southern brazil. J. Anim. Sci. 92:2668–2676. doi: 10.2527/jas.2013-7055 [DOI] [PubMed] [Google Scholar]
- Santos J. E., Bilby T. R., Thatcher W. W., Staples C. R., and Silvestre F. T.. 2008. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod. Domest. Anim. 43 (Suppl 2):23–30. doi: 10.1111/j.1439-0531.2008.01139.x [DOI] [PubMed] [Google Scholar]
- Santos J.E., Greco L.F., Garcia M., Thatcher W.W., and Staples C.R.. 2013. The role of specific fatty acids on dairy cattle performance and fertility. Florida Ruminant Nutrition Symposium, University of Florida, Gainesville, FL: p. 74–88. [Google Scholar]
- Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., and Sandri M.. 2013. Mechanisms regulating skeletal muscle growth and atrophy. Febs J. 280:4294–4314. doi: 10.1111/febs.12253 [DOI] [PubMed] [Google Scholar]
- Schwab C. G., and Broderick G. A.. 2017. A 100-year review: protein and amino acid nutrition in dairy cows. J. Dairy Sci. 100:10094–10112. doi: 10.3168/jds.2017-13320 [DOI] [PubMed] [Google Scholar]
- Shingfield K. J., Bernard L., Leroux C., and Chilliard Y.. 2010. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 4:1140–1166. doi: 10.1017/S1751731110000510 [DOI] [PubMed] [Google Scholar]
- Sosulski F. W., and Sarwar G.. 1973. Amino acid composition of oilseed meals and protein isolates. Can. Inst. Food Sci. Technol. J. 6:1–5. doi:10.1016/S0315-5463(73)73954–7 [Google Scholar]
- Staples C. R., Mattos R., and Thatcher W. W.. 2002. Feeding fatty acids to dairy cows for fertility. Proc. Departments of Poultry Husbandry, Animal Husbandry, and Biochemistry and Nutrition, New York State College of Agriculture, and the Graduate School of Nutrition, Cornell University, Ithaca, NY: p. 229. [Google Scholar]
- Swanson J. C., and Morrow-Tesch J.. 2001. Cattle transport: historical, research, and future perspectives. J. Anim. Sci. 79(suppl.): E102–E109. doi: 10.2527/jas2001.79E-SupplE102x [DOI] [Google Scholar]
- Vahmani P., Aalhus J. L., Rolland D. C., McAllister T. A., Prieto N., Block H. C., Proctor S. D., Guan L. L., and Dugan M. E. R.. 2017. Sequential feeding of lipid supplement enriches beef adipose tissues with 18:3n-3 biohydrogenation intermediates. Lipids 52:641–649. doi: 10.1007/s11745-017-4259-9 [DOI] [PubMed] [Google Scholar]
- Vahmani P., Rolland D. C., Gzyl K. E., and Dugan M. E. R.. 2016. Non‐conjugated cis/trans 18: 2 in beef fat are mainly Δ‐9 desaturation products of trans‐18: 1 isomers. Lipids. 51:1427–1433. doi: 10.1007/s11745-016-4207-0 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Wildman E. E., Jones G. M., Wagner P. E., Boman R. L., Troutt H. F. Jr., and Lesch T. N.. 1982. A dairy cow body condition scoring system and its relationship to selected production characteristics. J. Dairy Sci. 65:495–501. doi:10.3168/jds.S0022-0302(82)82223–6. [Google Scholar]
- Williamson D. H., Mellanby J., and Krebs H. A.. 1962. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem. J. 82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]