Abstract
The objective of this study was to determine the effects of increased AA and energy intake during late gestation on reproductive performance, milk composition, and metabolic and redox status of sows. A total of 118 Yorkshire sows (third through sixth parity) were randomly assigned to dietary treatments from day 90 of gestation until farrowing. Dietary treatments consisted of combinations of 2 standardized ileal digestible (SID) AA levels [14.7 or 20.6 g/d SID Lys, SID Lys and other AA met or exceeded the NRC (2012) recommendations] and 2 energy levels (28.24 or 33.78 MJ/d intake of NE) in a 2 × 2 factorial design. After parturition, all sows were fed a standard lactation diet. Blood samples were collected and analyzed for parameters on metabolism, redox status, and amino acid profile. The data were analyzed using the generalized linear mixed models to reveal the impact of dietary levels of energy, AA, and their interaction. Sows with increased intake of AA had greater BW gain (P < 0.01) during late gestation. Furthermore, the BW loss during lactation was increased in sows with increasing intake of energy (P < 0.05) or AA (P < 0.05). Sows fed high energy had higher total litter birth weights (20.2 kg vs. 18.4 kg, P < 0.05) and shorter duration of farrowing (261 min vs. 215 min, P < 0.05), compared with those fed low energy, which likely was due to higher (P < 0.05) plasma glucose and lower (P < 0.05) plasma lactate prior to parturition. High AA intake in late gestation increased the ADG of piglets during the following lactation (P < 0.05), and increased the concentrations of plasma urea, and the following AA: Lys, Met, Thr, Val, Ile, Leu, Phe, Asp, Ser, and Arg at farrowing (P < 0.05). In conclusion, the increased intake of energy increased total litter weight of newborns and shortened the farrowing duration, which likely was due to improved energy status at farrowing. Furthermore, sows with increased intake of AA led to higher growth rate of piglets during the following lactation, accompanying with the increasing levels of plasma urea and amino acids. Therefore, the higher energy intake in late gestation appeared to improve litter weight and farrowing duration, while higher AA intake may have positive effect on piglets performance in lactation.
Keywords: farrowing duration, nutrition, oxidative stress, parturition, sows
Introduction
Feeding during the late gestation is critical to the development and growth of the fetuses and maternal tissues such as placenta, uterus, mammary tissue, retention of maternal lipid, and protein (Samuel et al., 2012; Mallmann et al., 2019). Therefore, proper nutrition is important for the litter birth weight, mammary development, milk production, and metabolic status of sows, which in turn may improve the piglet survivability, piglet weaning weight, and minimize body mobilization of sows (Mosnier et al., 2010; Theil et al., 2014). The increase in the litter size over the last decades has reduced the individual piglet weight at birth (Foxcroft et al., 2006; Beaulieu et al., 2010), which suggests that the nutritional supply for the gestating sow needs to be improved.
Daily requirements of lysine and energy may be calculated factorially, and it was recently shown that lysine required by sows increased exponentially as gestation progressed, whereas the energy requirement remained rather constant for sows approaching farrowing (Feyera and Theil, 2017). Another recent study reported that increasing AA or/and energy in late gestation would increase the sow body condition, stillbirth rate, and litter performance (Gonçalves et al., 2016). Nevertheless, it is unknown whether increasing intake of energy and/or AA affects the metabolic and redox status, which in turn may influence the farrowing process, production, and composition of colostrum or milk (Theil et al., 2012). Moreover, a well-balanced feed composition is important to achieve maximal feed efficiency (Pedersen et al., 2019), and redox status could be important indicators for oxidation patterns in sows during the transition and lactation periods (Zhao et al., 2013).
Therefore, the objective of this study was to determine the effects of increased energy or/and AA during late gestation on sow performance, and blood metabolites used as markers for metabolic status, and redox status. It was hypothesized that increased energy or/and AA during late gestation would improve litter performance and farrowing process through altering metabolic pattern and redox status.
MATERIALS AND METHODS
This study was conducted at Tianfu Sow Farm, Giastar Group, Chengdu, China. The experiment followed the actual law of animal protection and was approved by the Animal Care and Use Committee of the Sichuan Agricultural University (DKY-B20121602), and was performed in accordance with the National Research Council’s Guideline for the Care and Use of Laboratory Animals.
Animals and Diets
At day 90 of gestation, a total of 118 multiparous Yorkshire sows (parity 3 to 6) with mean backfat thickness (17.6 ± 0.7 mm) and body weight (273.7 ± 4.7 kg) were selected and randomly assigned to 4 dietary treatments. The experimental diets were fed from the day 90 of gestation to farrowing. Dietary treatments consisted of the combinations of 2 standardized ileal digestible (SID) AA levels [14.7 or 20.6 g/d SID Lys, other SID AA were calculated according to their ratio to Lys indicated by NRC (2012), all SID AA levels met or exceeded the NRC (2012) recommendations] and 2 energy levels (28.24 or 33.76 MJ/d intake of NE) in a 2 × 2 factorial design; the details are shown in Table 1. The daily allowance of nutrients is shown in Table 2. The higher AA level was implemented by including more soybean meal, whereas higher energy was obtained by greater inclusion of soy oil in diet and increased daily feed supply (+0.2 kg/d). During day 90 to 110 of gestation, sows were housed in individual crates (1.8 × 0.8 m) with an autoloading feeding system and 2 nipple waters. All sows were fed individually 1 meal daily in the morning (between 0700 and 0800 h) and had ad libitum access to water. On day 110 of pregnancy, sows were moved to the farrowing room and kept in individual farrowing crates thereafter. After farrowing, sows were fed the same commercial lactation diet until day 21 of lactation and feed intake was increased by 1 kg/d from farrowing day to day 5 of lactation, to reach ad libitum feeding. The feed intake of each sow during lactation was recorded daily and average daily feed intake was calculated. Estrus detection was conducted twice daily (0900 and 1500 h) beginning 2 d after weaning using fence line contact with a boar. Sows were considered to be in estrus when they exhibited the standing reflex in the presence of the boar. The ambient temperature of sow house was maintained between 22 and 25 °C using draught fan and waterfall wall.
Table 1.
Ingredients and composition of gestation diets (as-fed basis)1,2
| NE-NAA | HE-NAA | NE-HAA | HE-HAA | |
|---|---|---|---|---|
| Ingredient, % (as-fed basis) | ||||
| Corn (CP 8.2%) | 42.20 | 48.32 | 39.52 | 47.01 |
| Barley (CP 11.2%) | 22.16 | 17.66 | 19.00 | 13.00 |
| Soybean meal (CP 43.4%) | 3.00 | 3.00 | 16.80 | 16.40 |
| Soybean hull (CP 11.7%) | 6.00 | 6.00 | 6.00 | 6.00 |
| Wheat bran (CP 16.4%) | 21.30 | 16.00 | 13.50 | 8.70 |
| Soy oil | 0.00 | 4.00 | 0.00 | 4.00 |
| L-Lys·HCl (98%) | 0.17 | 0.17 | 0.04 | 0.04 |
| L-Thr (98%) | 0.07 | 0.07 | 0.04 | 0.07 |
| DL-Met (98%) | 0.00 | 0.00 | 0.01 | 0.00 |
| L-Trp (98%) | 0.01 | 0.01 | 0.00 | 0.01 |
| NaCl | 0.40 | 0.38 | 0.40 | 0.38 |
| KCl | 0.50 | 0.47 | 0.50 | 0.47 |
| Dicalcium phosphate | 1.60 | 1.50 | 1.60 | 1.50 |
| Limestone | 2.00 | 1.88 | 2.00 | 1.88 |
| Choline chloride (50%) | 0.15 | 0.14 | 0.15 | 0.14 |
| Phytase | 0.04 | 0.04 | 0.04 | 0.04 |
| Vitamin and mineral premix3 | 0.40 | 0.38 | 0.40 | 0.38 |
| Nutrient levels | ||||
| NE (MJ/kg) | 9.41 | 11.26 | 9.41 | 11.26 |
| Crude protein, % | 11.6 | 10.8 | 15.6 | 14.6 |
| Crude fat, % | 2.8 | 6.7 | 2.5 | 6.5 |
| Crude Fiber, % | 5.6 | 5.2 | 5.5 | 5.1 |
| NDF, % | 16.1 | 15.3 | 16.0 | 14.9 |
| Available P, % | 0.4 | 0.4 | 0.4 | 0.4 |
| SID4 AA, % | ||||
| Lys | 0.49 | 0.46 | 0.69 | 0.64 |
| Met | 0.16 | 0.15 | 0.22 | 0.21 |
| Thr | 0.36 | 0.34 | 0.50 | 0.50 |
| Trp | 0.11 | 0.10 | 0.15 | 0.14 |
| Val | 0.43 | 0.40 | 0.62 | 0.58 |
1NE-NAA: dietary levels of energy and amino acids meet or exceed NRC (2012) and Nutrient Requirements of Swine, China (2018); HE-NAA: level of energy was 20% higher than NE-NAA; NE-HAA: level of AA was 40% higher than NE-NAA; HE-HAA: levels of both energy (+20%) and AA (+40%) were higher than NE-NAA.
2Diets were fed from day 90 of gestation to farrowing. Corn and soybean meal were analyzed for total AA content before dietary formulation, and NRC (2012) SID digestibility values were used to calculate SID AA. Net energy of diets was calculated based on the databank of China Feed Ingredients (2018).
3Provided per kg of diet: Zn 100 mg, Cu 30 mg, Fe 100 mg; Mn 40 mg, I 0.25 mg, Se 0.25 mg, Hy.D 0.07 mg, VA 2 mg, VB6 14 mg, VE 30 mg, VC 100 mg, biotin 0.1 mg, folic acid 2.5 mg, carnitine 46mg, organic chromium 0.3 mg.
4SID = standardized ileal digestible.
Table 2.
The daily allowance of nutrients1
| NE-NAA | HE-NAA | NE-HAA | HE-HAA | |
|---|---|---|---|---|
| Feed allowance prior to parturition, kg/d | 3.00 | 3.20 | 3.00 | 3.20 |
| NE, MJ/d | 28.24 | 33.78 | 28.24 | 33.78 |
| Crude protein, g/d | 349.5 | 344.7 | 469.1 | 468.4 |
| Crude fat, g/d | 84.0 | 214.6 | 75.8 | 206.8 |
| Crude fiber, g/d | 169.2 | 166.2 | 166.3 | 163.7 |
| Ca, g/d | 36.2 | 36.2 | 37.3 | 37.4 |
| Available P, g/d | 12.3 | 12.1 | 12.8 | 12.6 |
| SID AA, g/d | ||||
| Lys | 14.7 | 14.7 | 20.6 | 20.6 |
| Met | 4.8 | 4.9 | 6.7 | 6.6 |
| Thr | 10.8 | 11.0 | 15.0 | 16.1 |
| Trp | 3.3 | 3.1 | 4.4 | 4.6 |
| Val | 13.0 | 12.9 | 18.5 | 18.5 |
1NE-NAA: dietary levels of energy and amino acids meet or exceed NRC (2012) and Nutrient Requirements of Swine, China (2018); HE-NAA: level of energy was 20% higher than NE-NAA; NE-HAA: level of AA was 40% higher than NE-NAA; HE-HAA: levels of both energy (+20%) and AA (+40%) were higher than NE-NAA.
Data Collection and Sampling
The individual sow body weight was measured in the afternoon (1600 h) on day 90, 110 of gestation and day 21 of lactation using a specially designed floor scale, while sow body weight at day 2 of lactation was estimated by deduction of total litter weights of piglets and placenta from BW of sows at day 110 of gestation. Meanwhile, backfat thickness was measured ultrasonically (Renco Lean-Meater, USA) at the level of the 10th rib on each side, 65 mm from the midline. Besides, farrowing duration (min.), total number of piglets born, including the number of piglets born alive, number of stillborn, and number of mummified fetuses, were recorded. The percentage of intrauterine growth-restricted pigs (IUGR, BW less than 1 kg), stillborn and mummified pigs in each treatment was calculated. In 51 farrowings (n = 10 to 15 per treatment), all piglets were weighed at birth and 24 h after the onset of parturition for calculation of BW-24 h,then all piglets within treatment were cross-fostered and weighed each week for calculating piglet ADG, mean weight, and litter weight at weaning. Preweaning mortality (%) was calculated by dividing the total number of piglets in each treatment by the number of dead piglets after cross-foster.
Blood samples (10 mL) were collected from sows 4 h after feedings on day 90, 110 of gestation and at weaning, as well as 1 h after onset of farrowing (n = 10 to 15 sows per treatment), in heparinized tubes and plasma was harvested by centrifuging at 4 °C for 15 min (3,000 × g) and stored at −20 °C for later analysis. The colostrum samples were collected within 1 h after onset of farrowing and milk was collected at day 7 of lactation after oxytocin injection (n = 10 to 15 sows per treatment).
Colostrum Intake and Milk Composition
Colostrum intake
The estimation of the colostrum intake for individual piglets was based on the following equation described by Theil et al. (2014): CI = ─106 + 2.26 × WG + 200 × BWB + 0.111 ×D ─ 1,414 × WG/D + 0.0182 × WG/BWB,
where CI is colostrum intake (g), WG is weight gain of the individual piglet from birth to 24 h after first born piglet (g), BWB is BW of individual piglet at birth (kg), and D is the duration of colostrum intake (min) for individual piglet, that is the time difference (min.) between birth time and 24 h after first born piglet. We included 14, 10, 12, and 14 sows for estimating colostrum intake by NE-NAA, HE-NAA, NE-HAA, and HE-HAA, respectively.
Composition of colostrum and milk
Frozen samples (n = 10 to 15) were thawed at 4 °C, and then 18 mL of each sample was used for milk composition analysis. The fat, protein, lactose, DM, and urea nitrogen content of colostrum and milk were measured using a milk composition analyzer (Milkoscan FT, Denmark).
Metabolic Parameters
The plasma concentrations of urea, total protein (TP), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl aminotransferase (GGT), lactate, triglyceride (TG), NEFA, and glucose (GLU) were measured by automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan) through corresponding commercial kits (Sichuan Maker Biotechnology Inc., Chengdu, China). There was less than 5% variation of intra-assay and interassay coefficients for each assay. Plasma amino acids were analyzed as described by Peng et al. (2016). There was less than 5% variation between intra-assay and interassay coefficients for each assay.
Measurement of the Antioxidant Parameters in the Serum
Plasma samples were used to measure malondialdehyde (MDA), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and protein carbonyl using specific assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The plasma concentration of MDA was measured using thiobarbituric acid method described by Hou et al. (2017). The sample was incubated with MDA standard in water bath at 95 °C and reacted with thiobarbituric acid, and then the sample and MDA standards were extracted through alcohol and finally the sample and MDA standards were measured for absorbance at 532 nm spectrophotometrically. The concentration of plasma T-SOD was measured according to the method described by Hu et al. (2018b). T-SOD has specifically ability to inhibit the free radicals of superoxide anion, thereby reducing the production of nitrite, and nitrite can be measured spectrophotometrically. The absorbance was measured at 550 nm and the concentration of plasma T-SOD was calculated by reduced nitrite. The concentration of plasma GSH-Px was determined according to the method described by Zhang et al. (2008) and the activity was expressed by the rate of oxidation of GSH, and the absorbance of the plasma sample and the standard were measured at 412 nm spectrophotometrically. The concentration of plasma protein carbonyl was determined according to the method described by Pialoux et al. (2009), total protein content of the plasma sample needs to be determined initially, and then protein carbonyl of the plasma sample or standard derived to dinitrobenzene (DNP) was detected with anti-DNP antibody followed by incubation with a secondary antibody, and finally the absorbance was measured at 412 nm.
Statistical Analysis
In this study, sows were regarded as the experimental unit and piglet data was reported as a mean for the litter. All data were checked for outliers and normality before being analyzed. During experiment, 2 sows from NE-HAA and 2 sows from HE-HAA group were culled because over 6 stillborn pigs within litter. Also, 2 sows from NE-HAA group were culled because their farrowing duration was over 10 h and agalactia. These culled sows were not used for data statistics on reproductive performance and sample collections. Reproductive performance, milk composition, and blood biochemistry were analyzed using the 2 × 2 factor procedure in the GLM model of SAS 9.4 (SAS Institute., Cary, NC). Moreover, odds of stillborn, mummified, and preweaning death of piglets were fitted using the GLIMMIX procedure. The results were expressed as the mean and SEM. Correlation analysis between reproductive performance and blood parameters of sows were analyzed by CORR procedure of SAS and results are represented by Pearson correlations (r) and level of significance (P). Mean differences were regarded statistically significant when probability values were less than 0.05, and a tendency was considered when probability values were less than 0.10.
RESULTS
Sows Body Condition
The increased BW gain was observed in sows fed high energy (+2.24 kg in average, P = 0.056) or high AA (+3.27 kg in average, P = 0.009) during late gestation. In contrast, sows had a greater BW loss during lactation when fed high energy (P = 0.016) or high AA (P = 0.035) during late gestation, respectively (Table 3).
Table 3.
Effects of the increased energy and AA intake during late gestation on body weight, backfat thickness and reproductive performance of sows1
| Energy | AA | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| N | H | N | H | Energy | AA | Energy × AA | ||
| BW, kg (day 90 gestation) | 274.13 | 273.25 | 276.46 | 270.92 | 4.02 | 0.83 | 0.24 | 0.60 |
| BW gain, kg (day 90–110 of gestation) | 17.80 | 20.04 | 17.28 | 20.55 | 1.17 | 0.06 | 0.01 | 0.71 |
| Lacation BW loss, kg (day 2–21) | 21.18 | 25.68 | 20.83 | 26.03 | 2.29 | 0.02 | 0.04 | 0.51 |
| Backfat thickness, mm | ||||||||
| Day 90 of gestation | 16.67 | 16.52 | 16.50 | 16.69 | 0.79 | 0.84 | 0.82 | 0.67 |
| Day 90 to 110 of gestation | 2.20 | 2.53 | 1.96 | 2.76 | 0.38 | 0.40 | 0.06 | 0.53 |
| Lactation loss (day 2–21) | 4.70 | 4.79 | 4.55 | 4.94 | 0.43 | 0.82 | 0.46 | 0.57 |
| Duration of farrowing, min | 261.41 | 215.41 | 220.11 | 256.71 | 16.07 | 0.04 | 0.08 | 0.97 |
| Total piglets born, no. | 14.44 | 15.20 | 14.78 | 14.86 | 0.76 | 0.25 | 0.88 | 0.19 |
| Piglets born alive, no. | 13.71 | 14.34 | 14.04 | 14.01 | 0.73 | 0.35 | 0.97 | 0.36 |
| Born alive, % | 94.92 | 94.38 | 94.96 | 94.34 | 1.40 | 0.28 | 0.27 | 0.22 |
| Stillborn, % | 3.37 | 3.10 | 3.31 | 3.16 | 1.16 | 0.44 | 0.32 | 0.14 |
| Mummified fetuses, % | 1.72 | 2.52 | 1.74 | 2.51 | 0.74 | 0.37 | 0.50 | 0.94 |
| Total born | ||||||||
| Litter birth weight, kg | 18.38 | 20.16 | 18.51 | 20.04 | 0.88 | 0.04 | 0.09 | 0.35 |
| Piglet birth weight, kg | 1.27 | 1.33 | 1.25 | 1.35 | 0.04 | 0.46 | 0.71 | 0.25 |
| Born alive | ||||||||
| Litter birth weight, kg | 17.82 | 19.35 | 17.89 | 19.28 | 0.89 | 0.07 | 0.12 | 0.57 |
| Piglet birth weight, kg | 1.30 | 1.35 | 1.28 | 1.38 | 0.03 | 0.46 | 0.68 | 0.23 |
| IUGR2, % | 7.08 | 6.56 | 7.09 | 6.56 | 1.73 | 0.97 | 0.98 | 0.80 |
| ADFI, kg/d | 5.26 | 5.22 | 5.27 | 5.21 | 0.02 | 0.34 | 0.27 | 0.66 |
| Wean-to-estrus interval, d | 5.04 | 5.06 | 5.11 | 4.98 | 0.25 | 0.96 | 0.65 | 0.96 |
1Energy-N: 28.24 MJ/d intake of NE, Energy-H: 33.78 MJ/d intake of NE. AA-N: 14.7 g/d SID Lys, AA-H: 20.6 g/d SID Lys.
2IUGR means intrauterine growth-restricted pigs with birth weight less than one kilogram.
Reproductive Performance and Litter Growth
Sows with high energy intake had a shorter duration of farrowing (P < 0.05). The average litter birth weight was greater in sows fed high energy (P < 0.05) and tended to be greater in sows fed high AA (P = 0.089). Moreover, litter birth weight for live born piglets tended to be increased in sows fed high energy (P = 0.072; Table 3). Piglets ADG during lactation was greater (P = 0.035) and weaning weight tended to be greater (P = 0.084) in sows fed high AA during late gestation (Table 4).
Table 4.
Effects of the increased energy and AA intake during late gestation on litter performance during lactation1
| Energy | AA | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| N | H | N | H | Energy | AA | Energy × AA | ||
| Litter size after cross-foster, no. | 11.52 | 12.06 | 11.64 | 11.94 | 0.30 | 0.20 | 0.71 | 0.81 |
| Litter size at weaning, no. | 9.99 | 10.47 | 10.15 | 10.32 | 0.38 | 0.47 | 0.99 | 0.17 |
| Preweaning mortality, % | 13.29 | 13.18 | 12.88 | 13.60 | 3.12 | 0.23 | 0.59 | 0.44 |
| Piglet weight after cross-foster, kg | 1.49 | 1.42 | 1.47 | 1.44 | 0.06 | 0.16 | 0.62 | 0.43 |
| Piglet ADG, g/d | ||||||||
| Week 1 | 188 | 184 | 183 | 189 | 13 | 0.34 | 0.63 | 0.83 |
| Week 2 | 233 | 237 | 227 | 243 | 14 | 0.97 | 0.46 | 0.41 |
| Week 3 | 268 | 286 | 257 | 297 | 22 | 0.57 | 0.12 | 0.55 |
| Day 1–21 | 238 | 239 | 228 | 248 | 10 | 0.97 | 0.04 | 0.36 |
| Litter weight at weaning, kg | 65.05 | 66.42 | 64.80 | 66.67 | 3.45 | 0.76 | 0.54 | 0.10 |
| Piglet mean weight at weaning, kg | 6.46 | 6.44 | 6.27 | 6.63 | 0.24 | 0.83 | 0.08 | 0.32 |
| BW Gain-24h1, g | 78.75 | 83.83 | 74.55 | 88.04 | 12.02 | 0.69 | 0.26 | 0.75 |
1Energy-N: 28.24 MJ/d intake of NE, Energy-H: 33.78 MJ/d intake of NE. AA-N: 14.7 g/d SID Lys, AA-H: 20.6 g/d SID Lys.
2BW Gain-24h means body weight gain during the 24 h after birth.
Milk Composition
Sows fed high energy had higher concentrations of fat in colostrum (P = 0.047). However, there was a decreased fat concentration (P = 0.048), and concomitantly increased lactose concentration (P = 0.03) in milk at day 7 of sows fed high energy in late gestation (Table 5).
Table 5.
Effects of the increased energy and AA intake during late gestation on colostrum intake and milk composition1
| Energy | AA | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| N | H | N | H | Energy | AA | Energy × AA | ||
| Colostrum intake, g | 319.61 | 304.00 | 312.12 | 311.49 | 13.66 | 0.89 | 0.34 | 0.78 |
| Colostrum | ||||||||
| Fat, % | 4.27 | 5.20 | 4.74 | 4.73 | 0.46 | 0.047 | 0.90 | 0.87 |
| Protein, % | 19.62 | 20.30 | 19.50 | 20.41 | 1.51 | 0.82 | 0.90 | 0.38 |
| Lactose, % | 2.66 | 2.96 | 2.93 | 2.70 | 0.22 | 0.21 | 0.32 | 0.90 |
| Total solid, % | 27.00 | 28.65 | 27.86 | 27.79 | 1.63 | 0.38 | 0.98 | 0.46 |
| Urea nitrogen, mg/dL | 28.70 | 29.98 | 28.49 | 30.19 | 1.45 | 0.62 | 0.96 | 0.53 |
| Milk, 7 d | ||||||||
| Fat, % | 9.33 | 7.90 | 8.59 | 8.64 | 0.59 | 0.048 | 0.79 | 0.35 |
| Protein, % | 6.07 | 6.04 | 6.09 | 6.02 | 0.18 | 0.81 | 0.92 | 0.30 |
| Lactose, % | 5.39 | 6.05 | 5.73 | 5.72 | 0.23 | 0.03 | 0.92 | 0.52 |
| Total solid, % | 21.48 | 20.69 | 21.09 | 21.07 | 0.74 | 0.31 | 0.80 | 0.20 |
| Urea nitrogen, mg/dL | 27.03 | 25.67 | 26.16 | 26.54 | 1.11 | 0.26 | 0.57 | 0.19 |
1Energy-N: 28.24 MJ/d intake of NE, Energy-H: 33.78 MJ/d intake of NE. AA-N: 14.7 g/d SID Lys, AA-H: 20.6 g/d SID Lys.
Metabolic Parameters
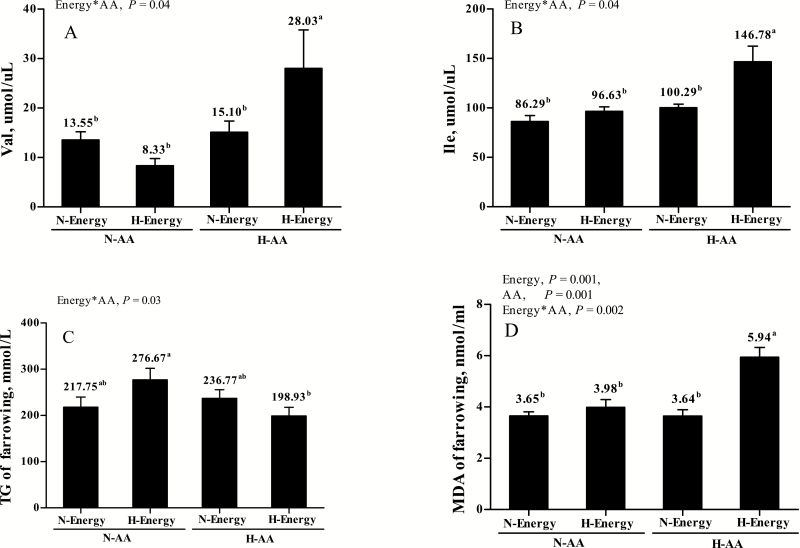
Sows fed high AA had increased (P < 0.05) plasma concentration of urea on day 110 and at farrowing. Consistently, markedly increased (P < 0.05) plasma concentrations of Lys, Met, Thr, Val, Ile, Leu, Phe, Asp, Ser, and Arg were observed at farrowing in sows with high intake of AA. In contrast, only 3 amino acids in plasma (Trp, Ile, and Phe) were increased in sows fed high energy (P < 0.05; Table 6). On day 110 of gestation, sows fed high energy had greater (P < 0.05) plasma concentration of glucose, tended to have greater (P = 0.061) plasma concentration of NEFA, and had lower (P < 0.05) plasma lactate (Table 7). Moreover, sows fed high energy had markedly lower ratio of urea to NEFA at both farrowing and weaning (P < 0.05; Table 7). Besides, the AA × energy interaction (P < 0.05) affected plasma concentrations of Val and Ile, as well as TG (Fig. 1).
Table 6.
Effect of the increased energy and AA intake on plasma AA concentrations at farrowing1
| Energy | AA | SEM | P value | |||||
|---|---|---|---|---|---|---|---|---|
| N | H | N | H | Energy | AA | Energy × AA | ||
| Essential amino acids, µmol/µL | ||||||||
| Ile | 93.29 | 121.71 | 91.46 | 123.54 | 7.42 | 0.008 | 0.002 | 0.04 |
| Leu | 88.18 | 108.41 | 83.72 | 112.88 | 8.56 | 0.06 | 0.006 | 0.37 |
| Lys | 105.86 | 121.75 | 92.83 | 134.79 | 14.60 | 0.40 | 0.02 | 0.33 |
| Met | 38.16 | 46.49 | 35.59 | 49.05 | 4.77 | 0.20 | 0.04 | 0.27 |
| Phe | 84.22 | 101.59 | 84.01 | 101.80 | 5.56 | 0.01 | 0.01 | 0.23 |
| Ser | 102.00 | 103.10 | 88.68 | 116.42 | 9.85 | 0.93 | 0.02 | 0.73 |
| Trp | 60.57 | 77.40 | 66.35 | 71.62 | 6.37 | 0.02 | 0.51 | 0.43 |
| Thr | 125.84 | 125.95 | 103.94 | 147.85 | 13.33 | 0.84 | 0.009 | 0.65 |
| Val | 14.33 | 18.18 | 10.94 | 21.57 | 3.27 | 0.60 | 0.04 | 0.04 |
| Nonessential amino acids, µmol/µL | ||||||||
| Asp | 12.30 | 11.44 | 9.76 | 13.98 | 1.34 | 0.40 | 0.02 | 0.87 |
| Ala | 589.74 | 550.63 | 520.39 | 619.98 | 66.60 | 0.65 | 0.18 | 0.65 |
| Arg | 67.91 | 77.03 | 60.12 | 84.82 | 8.76 | 0.44 | 0.03 | 0.09 |
| Glu | 161.04 | 171.52 | 150.84 | 181.71 | 18.82 | 0.76 | 0.23 | 0.27 |
| Gly | 520.49 | 536.96 | 470.16 | 587.28 | 54.94 | 0.87 | 0.07 | 0.70 |
| His | 58.21 | 68.28 | 56.21 | 70.28 | 6.22 | 0.19 | 0.07 | 0.29 |
| Pro | 251.61 | 224.16 | 221.58 | 254.19 | 22.02 | 0.40 | 0.15 | 0.41 |
| Tyr | 68.09 | 82.07 | 66.40 | 83.76 | 7.97 | 0.14 | 0.07 | 0.41 |
1Energy-N: 28.24 MJ/d intake of NE, Energy-H: 33.78 MJ/d intake of NE. AA-N: 14.7 g/d SID Lys, AA-H: 20.6 g/d SID Lys.
Table 7.
Effect of the increased energy and AA intake during late gestation on plasma metabolites during peripartum period1
| Energy | AA | SEM | P value | |||||
|---|---|---|---|---|---|---|---|---|
| N | H | N | H | Energy | AA | Energy × AA | ||
| Glucose, mmol/L | ||||||||
| Day 90 | 3.30 | 3.44 | 3.46 | 3.28 | 0.33 | 0.69 | 0.60 | 0.81 |
| Day 110 | 2.70 | 3.97 | 3.34 | 3.33 | 0.37 | 0.003 | 0.93 | 0.39 |
| Farrowing | 3.52 | 3.03 | 3.41 | 3.13 | 0.46 | 0.34 | 0.46 | 0.48 |
| Weaning | 4.51 | 4.56 | 4.02 | 5.05 | 0.34 | 0.89 | 0.10 | 0.69 |
| Lactate, mmol/L | ||||||||
| Day 90 | 2.57 | 2.61 | 2.48 | 2.70 | 0.24 | 0.88 | 0.39 | 0.90 |
| Day 110 | 2.83 | 2.42 | 2.62 | 2.62 | 0.17 | 0.046 | 0.93 | 0.10 |
| Farrowing | 2.47 | 2.46 | 2.35 | 2.58 | 0.19 | 0.85 | 0.19 | 0.29 |
| Weaning | 2.20 | 1.88 | 2.44 | 1.65 | 0.24 | 0.07 | 0.02 | 0.73 |
| NEFA, µmol/L | ||||||||
| Day 90 | 75.76 | 90.50 | 84.69 | 81.57 | 13.36 | 0.24 | 0.81 | 0.54 |
| Day 110 | 89.77 | 137.80 | 119.42 | 108.16 | 24.89 | 0.06 | 0.71 | 0.91 |
| Farrowing | 721.10 | 882.52 | 828.38 | 775.24 | 92.05 | 0.12 | 0.74 | 0.09 |
| Weaning | 64.64 | 79.15 | 75.28 | 68.51 | 17.45 | 0.87 | 0.81 | 0.35 |
| Urea, mmol/L | ||||||||
| Day 90 | 4.40 | 4.31 | 4.29 | 4.42 | 0.19 | 0.65 | 0.47 | 0.62 |
| Day 110 | 4.16 | 3.90 | 3.79 | 4.27 | 0.21 | 0.17 | 0.03 | 0.18 |
| Farrowing | 3.22 | 3.23 | 2.84 | 3.61 | 0.25 | 0.96 | 0.002 | 0.12 |
| Weaning | 6.60 | 6.30 | 6.18 | 6.72 | 0.44 | 0.18 | 0.13 | 0.24 |
| Urea/NEFA | ||||||||
| Day 90 | 58.08 | 47.62 | 50.66 | 54.19 | 0.08 | 0.65 | 0.71 | 0.55 |
| Day 110 | 46.34 | 28.30 | 31.74 | 39.48 | 0.05 | 0.55 | 0.06 | 0.50 |
| Farrowing | 4.47 | 3.66 | 3.43 | 4.66 | 0.32 | 0.007 | 0.31 | 0.91 |
| Weaning | 102.10 | 79.60 | 82.09 | 98.09 | 0.86 | 0.02 | 0.87 | 0.89 |
| TG 1, µmol/L | ||||||||
| Day 90 | 345.97 | 329.57 | 338.78 | 336.76 | 23.65 | 0.471 | 0.97 | 0.34 |
| Day 110 | 481.59 | 525.55 | 502.04 | 505.10 | 47.08 | 0.40 | 0.92 | 0.60 |
| Farrowing | 227.26 | 237.80 | 247.21 | 217.85 | 21.15 | 0.85 | 0.22 | 0.03 |
| Weaning | 368.68 | 411.90 | 435.35 | 345.23 | 116.51 | 0.39 | 0.53 | 0.48 |
| TP 2 , g/L | ||||||||
| Day 90 | 83.25 | 83.95 | 83.99 | 83.21 | 1.89 | 0.71 | 0.68 | 0.82 |
| Day 110 | 75.30 | 79.47 | 76.60 | 78.17 | 1.96 | 0.44 | 0.38 | 0.89 |
| Farrowing | 75.20 | 74.20 | 73.94 | 75.46 | 2.36 | 0.66 | 0.55 | 0.89 |
| Weaning | 80.92 | 80.16 | 82.72 | 78.36 | 2.25 | 0.98 | 0.11 | 0.67 |
1Energy-N: 28.24 MJ/d intake of NE, Energy-H: 33.78 MJ/d intake of NE. AA-N: 14.7 g/d SID Lys, AA-H: 20.6 g/d SID Lys.
2TG, triglyceride.
3TP, total protein.
Figure 1.
The interactive effects of energy and AA levels on plasma metabolites at farrowing.
Hepatic and Redox Status
Sows fed high energy during late gestation had lower (P = 0.04) plasma concentration of ALB, whereas sows fed high AA during late gestation had higher (P = 0.003) plasma concentration of ALB at farrowing (Table 8). For redox status, plasma concentration of MDA was increased (P < 0.01) in sows fed high energy or high AA, and also an AA × energy interaction (P = 0.002) was identified for plasma concentration of MDA, which was highest in sows with HE-HAA (Fig. 1).
Table 8.
Effects of the increased energy and AA intake during late gestation on hepatic function and redox status-related parameters of sows during the peripartum period1
| Energy | AA | SEM | P value | |||||
|---|---|---|---|---|---|---|---|---|
| N | H | N | H | Energy | AA | Energy × AA | ||
| ALB 2 , g/L | ||||||||
| Day 90 | 41.66 | 41.12 | 41.04 | 41.74 | 1.11 | 0.65 | 0.56 | 0.99 |
| Day 110 | 37.00 | 37.95 | 37.04 | 37.92 | 1.05 | 0.35 | 0.40 | 0.59 |
| Farrowing | 39.63 | 37.17 | 36.48 | 40.32 | 1.08 | 0.04 | 0.003 | 0.89 |
| Weaning | 37.44 | 37.93 | 37.94 | 37.43 | 1.26 | 0.76 | 0.73 | 0.96 |
| ALT 3 , U/L | ||||||||
| Day 90 | 59.39 | 62.58 | 60.73 | 61.24 | 4.38 | 0.46 | 0.91 | 0.79 |
| Day 110 | 56.73 | 62.83 | 61.65 | 57.91 | 3.77 | 0.12 | 0.38 | 0.83 |
| Farrowing | 55.39 | 55.76 | 55.48 | 55.68 | 3.00 | 0.90 | 0.94 | 0.99 |
| Weaning | 62.57 | 75.63 | 67.58 | 70.62 | 8.26 | 0.20 | 0.86 | 0.71 |
| AST 4 , U/L | ||||||||
| Day 90 | 37.60 | 36.95 | 38.26 | 36.29 | 2.96 | 0.82 | 0.51 | 0.95 |
| Day 110 | 31.95 | 30.61 | 30.58 | 31.98 | 2.52 | 0.65 | 0.61 | 0.57 |
| Farrowing | 46.47 | 44.94 | 45.70 | 45.71 | 5.64 | 0.88 | 0.95 | 0.47 |
| Weaning | 35.25 | 35.85 | 33.45 | 37.65 | 4.00 | 0.70 | 0.31 | 0.85 |
| GGT 5 , U/L | ||||||||
| Day 90 | 31.81 | 32.58 | 33.38 | 31.01 | 2.89 | 0.77 | 0.41 | 0.28 |
| Day 110 | 33.97 | 35.40 | 36.04 | 33.33 | 3.83 | 0.71 | 0.50 | 0.97 |
| Farrowing | 37.69 | 36.09 | 37.50 | 36.29 | 3.33 | 0.73 | 0.68 | 0.50 |
| Weaning | 33.86 | 31.08 | 35.09 | 29.85 | 3.53 | 0.15 | 0.15 | 0.31 |
| MDA 6 , nmol/ml | ||||||||
| Day 90 | 3.57 | 3.94 | 3.94 | 3.57 | 0.50 | 0.49 | 0.48 | 0.70 |
| Day 110 | 3.65 | 4.96 | 3.82 | 4.79 | 0.28 | <0.001 | 0.001 | 0.002 |
| Farrowing | 4.06 | 4.26 | 3.69 | 4.62 | 0.55 | 0.73 | 0.12 | 0.052 |
| T-SOD 7 , nmol/ml | ||||||||
| Day 90 | 71.66 | 74.19 | 72.92 | 72.93 | 2.71 | 0.95 | 0.40 | 0.12 |
| Day 110 | 60.45 | 56.93 | 60.18 | 57.20 | 3.62 | 0.40 | 0.49 | 0.65 |
| Farrowing | 58.77 | 58.00 | 60.00 | 56.77 | 5.30 | 0.89 | 0.57 | 0.39 |
| Protein carbonyl, nmol/mg | ||||||||
| Day 90 | 0.93 | 0.93 | 0.92 | 0.94 | 0.03 | 0.91 | 0.56 | 0.85 |
| Day 110 | 1.11 | 1.09 | 1.11 | 1.10 | 0.04 | 0.53 | 0.81 | 0.08 |
| Farrowing | 1.01 | 1.03 | 0.99 | 1.05 | 0.03 | 0.78 | 0.06 | 0.27 |
| GSH-Px 8 , U/L | ||||||||
| Day 90 | 1.35 | 1.34 | 1.33 | 1.36 | 0.10 | 0.95 | 0.83 | 0.71 |
| Day 110 | 1.10 | 1.15 | 1.14 | 1.11 | 0.15 | 0.77 | 0.89 | 0.81 |
| Farrowing | 1.20 | 1.12 | 1.20 | 1.12 | 0.11 | 0.48 | 0.51 | 0.89 |
1Energy-N: 28.24 MJ/d intake of NE, Energy-H: 33.78 MJ/d intake of NE. AA-N: 14.7 g/d SID Lys, AA-H: 20.6 g/d SID Lys.
2ALB, albumin.
3ALT, alanine aminotransferase.
4AST, aspartate aminotransferase.
5GGT, glutamyl aminotransferase.
6MDA, malondialdehyde.
7T-SOD, total superoxide dismutase.
8GSH-Px, glutathione peroxidase.
Correlation Between Reproductive Performance and Blood Parameters
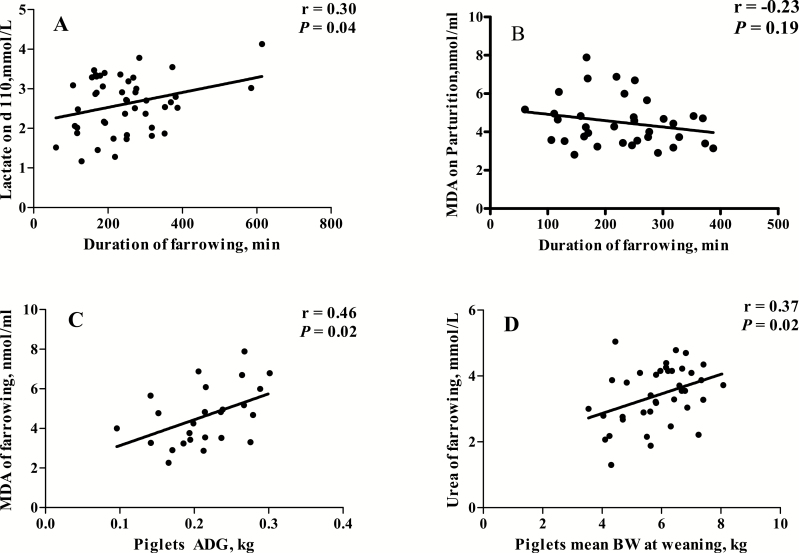
Correlation analysis was performed to evaluate the potential link between reproductive performance and blood parameters. The lactate concentration on day 110 was positively correlated with duration of farrowing (P < 0.05). The urea concentration at farrowing was positively correlated with piglets mean BW at weaning (P = 0.02). Additionally, the MDA concentration at farrowing was positively correlated with piglets ADG (P = 0.02) (Fig. 2).
Figure 2.
Correlation between reproductive traits and blood parameters of sows.
Discussion
The increase in the litter size due to the selection for hyperprolific genotypes has caused reduction in individual piglet birth weight during the last few decades (Town et al., 2005; Campos et al., 2012; Amdi et al., 2013). Therefore, the nutritional requirements of gestating sows during late gestation needs to be reassessed. Several studies have shown that the increased intake of nutrients during late gestation had a positive effect on the piglet birth weight (Yang et al., 2009; Ren et al., 2017). The present study aimed to study effects of increasing energy or/and AA during late gestation on reproductive performance, milk composition, metabolic, and redox status of hyperprolific sows.
Fetal Growth and BW Changes in Late Gestation
The increased energy intake resulted in the higher litter birth weights of either total born or born alive piglets, which is consistent with previous study by Gonçalves et al. (2016) and Ren et al. (2017). Moreover, the birth weight of live pigs was increased up to 50 or 100 g by increasing the intake of energy or AA, respectively. It has been reported that the pigs with higher birth weight had higher colostrum intake (Amdi et al., 2013) and survival rate (Quesnel, 2011). In addition, the increased energy or AA intake lead to heavier body weight of sows at 110 d of gestation, suggesting the elevated deposition of fat and muscles in late gestation. Similarly, the increased body weight gain was observed in sows by increasing feed intake in late gestation (Mallmann et al., 2018). Meanwhile, sows with increased body weight gain in late gestation lost more body weight during lactation, which had been reported either in first parity or multiparous sows (Kim et al., 2016; Ren et al., 2018). Although feed intake during lactation was not markedly differ between groups, the increasing growth rate of piglets would require more milk production, which means more body mobilization from sows, thus the profound body weight loss was observed in sows with increasing AA intake.
Farrowing Duration and Related Metabolism
The farrowing duration was markedly lower in sows fed high energy, indicating more energy was supplied for farrowing process. In line with that, Feyera and Theil (2017) showed that farrowing duration and stillbirth rate of hyperprolific sows were increased when sows were depleted of energy. Inconsistently, however, the increased intake of NE at 28.24 MJ/d had been shown to increase stillborn rate, and it has been ascribed to the heavier birth weight (Gonçalves et al., 2016). In this study, feeding high energy diet lead to higher level of glucose and NEFA on day 110 of gestation, suggesting more energy is available at onset of farrowing. On one hand, the increased blood level of NEFA may be resulting from more fat intake, because 4% soy oil was added in diet to increase energy level. On the other hand, the higher level of NEFA may indicate the greater use of energy substrate deprived from fat, which is physiologically prioritized around farrowing (Boyd and Kensinger, 1998). Nevertheless, the NEFA should enter the citric acid cycle as acetyl-CoA, and react with oxaloacetate that is directly supplied by glucose and AA (Decaluwe et al., 2014). In this study, therefore, the increased ratio of urea to NEFA in sows fed increased AA suggest more amino acids were used for energy by deamination in liver, which could be not beneficial for normal metabolism. Furthermore, sows fed low energy diet appeared to suffer from hypoglycemia, regarding the glucose level was only 2.7mmol/L in average, which may partly explain why there was longer farrowing duration by feeding low energy diet.
The shorter farrowing duration by feeding high energy diet could be reflected by the lower level of lactate at day 110 of gestation, which had been associated with the muscle contractions before and during farrowing (Mosnier et al., 2010). In contrast, the higher level of lactate together with longer farrowing duration indicate insufficient supply of oxygen, may be as a consequence of fatigue. In addition, sows fed high AA diet tended to increase farrowing duration, which is consistent with previous study by Tydlitát et al.(2008), demonstrating the higher intake of crude protein in late gestation prolonged farrowing duration, high protein intake may increase heat increment and more AA utilized for deamination in liver, which could be not beneficial for energy metabolism.
Colostrum Production and Composition
The colostrum intake and weight gain of piglets during the colostrum period did not markedly differ across groups. Consistent with previous study (Jackson et al., 1995), the fat content in colostrum was increased by increasing energy intake, which may be associated with the higher levels of plasma NEFA at day 110 of farrowing, because NEFA is precursor for colostral fat produced by the mammary gland (King, 2000).
Litter Performance and Milk Composition During Lactation
In this study, the heavier sows by increasing energy and/or AA intake in late gestation would have more body weight loss during lactation, which is supported by the previous results (Shelton et al., 2009; Ren et al., 2017). In addition, it is intriguing to observe the increased piglet ADG during the lactation period in response to increasing maternal intake of AA in late gestation. We postulated the faster growth of piglets may result from either suckling more milk, or the greater birth weight. On one hand, because the development of mammary gland may be enhanced in sows with increasing intake of AA, as indicated by the markedly higher levels of systematic amino acids, particularly Val, Ile, Leu, and Arg, which may stimulate the proliferation of mammary epithelial cells and in turn milk production (Lei et al., 2012; Rezaei et al., 2016; Holanda et al., 2018). On the other hand, the relatively higher birth weight would lead to higher postnatal growth through improving colostrum intake (Amdi et al., 2013) and muscular development et al. (De Vos et al., 2014; Hu et al., 2018a). Furthermore, it is unknown why the lower content of fat, but higher lactose appeared in milk at day 7 when sows were fed high energy in late gestation. These findings suggest that intrinsic sow factors around farrowing (e.g., energy status) have an impact on the subsequent lactation performance.
The hepatic status prepartum is related to the colostrum production and foetal growth (Loisel et al., 2014). The increased plasma level of albumin at farrowing when feeding high AA in late gestation suggest that the better liver function, because the systematic level of albumin is generally reduced in cows due to the deletion of albumin by inflammatory response during the peripartal period (Trevisi et al., 2010). In contrast, the higher level of albumin indicated a better liver function and milk production of sows (Zhou et al., 2016). In this study, therefore, the increased AA in late gestation may supply sufficient AA for synthesis of albumin in liver, and for the requirement of inflammatory process during peripartal period, which could be beneficial for subsequent milk production and piglet growth.
Implications
The sows with increased intake of energy had increased litter weight of newborns and shorter duration of farrowing, which likely was associated with improved energy status at farrowing. However, sows fed with high dietary AA leaded to higher growth rate of piglets during lactation, which may be ascribed to the numerical greater birth weight and/or to improved mammary development.
Footnotes
This work was supported by the National Key R&D Program of China (NO. 2018YFD0501000), Overseas Expertise Introduction Project for Discipline Innovation (111 Project, No. D17015) and National Natural Science Foundation of China (NO. 31872372). Author disclosures: L.Q.C., L.H., C.W., Q.X., Q.Z., X.P., Z.F.F., Y.L., S.Y.X., B.F., J.L., J.Y.T., P.K.T., and D.W. had no conflicts of interest.
These authors contributed equally to this work.
LITERATURE CITED
- Amdi C., Krogh U., Flummer C., Oksbjerg N. and Theil P. K.. 2013. Intrauterine growth restricted piglets defined by their head shape ingest insufficient amounts of colostrum. J Anim Sci. 91: 5605–5613. doi:10.2527/jas.2013-6824 [DOI] [PubMed] [Google Scholar]
- Beaulieu A. D., Aalhus J. L., Williams N. H., and Patience J. F.. 2010. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 88:2767–2778. doi: 10.2527/jas.2009-2222 [DOI] [PubMed] [Google Scholar]
- Boyd R. D., and Kensinger R. S.. 1998. Metabolic precursors for milk synthesis. Wageningen Pers, Wageningen, The Netherlands. [Google Scholar]
- Campos P. H., Silva B. A., Donzele J. L., Oliveira R. F., and Knol E. F.. 2012. Effects of sow nutrition during gestation on within-litter birth weight variation: A review. Animal 6:797–806. doi: 10.1017/S1751731111002242 [DOI] [PubMed] [Google Scholar]
- De Vos M., Che L., Huygelen V., Willemen S., Michiels J., Van Cruchten S., and Van Ginneken C.. 2014. Nutritional interventions to prevent and rear low-birthweight piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 98:609–619. doi: 10.1111/jpn.12133 [DOI] [PubMed] [Google Scholar]
- Decaluwé R., Maes D., Cools A., Wuyts B., De Smet S., Marescau B., De Deyn P. P., and Janssens G. P.. 2014. Effect of peripartal feeding strategy on colostrum yield and composition in sows. J. Anim. Sci. 92:3557–3567. doi: 10.2527/jas.2014-7612 [DOI] [PubMed] [Google Scholar]
- Feyera T., and Theil P. K.. 2017. Energy and lysine requirements and balances of sows during transition and lactation: A factorial approach. Livestock Science. 201:50–57. doi:10.1016/j.livsci.2017.05.001. [Google Scholar]
- Foxcroft G. R., Dixon W. T., Novak S., Putman C. T., S. C. Town, and M. D. Vinsky. 2006. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 84 (Suppl):E105–E112. doi:10.2527/2006.8413_supplE105x [DOI] [PubMed] [Google Scholar]
- Gonçalves M. A., Gourley K. M., Dritz S. S., Tokach M. D., Bello N. M., DeRouchey J. M., Woodworth J. C., and Goodband R. D.. 2016. Effects of amino acids and energy intake during late gestation of high-performing gilts and sows on litter and reproductive performance under commercial conditions. J. Anim. Sci. 94:1993–2003. doi: 10.2527/jas.2015-0087 [DOI] [PubMed] [Google Scholar]
- Holanda D. M., Marcolla C. S., Guimar S. E. F., Neves M. M., Hausman G. J., Duarte M. S., M. L. T. Abreu, and Saraiva A.. 2019. Dietary L-arginine supplementation increased mammary gland vascularity of lactating sows. Animal; 13:790–798. doi:10.1017/S1751731118002069. [DOI] [PubMed] [Google Scholar]
- Hou X., Wang T., Ahmad H., and Xu Z.. 2017. Ameliorative effect of ampelopsin on LPS-induced acute phase response in piglets. J. Funct. Foods. 35:489–498. doi:10.1016/j.jff.2017.05.044 [Google Scholar]
- Hu L., F. Han, Chen L., Peng X., Chen D. W., Wu D., Che L. Q. and Zhang K.Y.. 2018a. High nutrient intake during the early postnatal period accelerates skeletal muscle fiber growth and maturity in intrauterine growth restricted pigs. Genes and Nutrition. 11:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L. Hu, Peng X., Qin L. L., Wang R., Fang Z. F., Y. Lin, Xu S. Y., Feng B., Wu D., and Che L. Q.. 2018b. Dietary nucleotides supplementation during the suckling period improves the antioxidative ability of neonates with intrauterine growth retardation when using a pig model. RSC Advances. 8:16152–16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. R., Hurley W. L., Easter R. A., Jensen A. H., and Odle J.. 1995. Effects of induced or delayed parturition and supplemental dietary fat on colostrum and milk composition in sows. J. Anim. Sci. 73:1906–1913.doi:10.2527/1995.7371906x [DOI] [PubMed] [Google Scholar]
- Kim J. S., Yang X., and Baidoo S. K.. 2016. Relationship between body weight of primiparous sows during late gestation and subsequent reproductive efficiency over six parities. Asian-Australas. J. Anim. Sci. 29:768–774. doi: 10.5713/ajas.15.0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. H. 2000. Factors that influence milk production in well-fed sows. J Anim Sci. 78:19–25. doi:10.2527/2000. 78suppl_319x [DOI] [PubMed] [Google Scholar]
- Lei J., Feng D., Zhang Y., Dahanayaka S., Li X., Yao K., Wang J., Wu Z., Dai Z., and Wu G.. 2012. Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43:2179–2189. doi: 10.1007/s00726-012-1302-2 [DOI] [PubMed] [Google Scholar]
- Loisel F., Farmer C., Ramaekers P., and Quesnel H.. 2014. Colostrum yield and piglet growth during lactation are related to gilt metabolic and hepatic status prepartum. J. Anim. Sci. 92:2931–2941. doi: 10.2527/jas.2013-7472 [DOI] [PubMed] [Google Scholar]
- Mallmann A. L., Betiolo F. B., Camilloti E., Mellagi A. P. G., Ulguim R. R., Wentz I., Bernardi M. L., Gonçalves M. A. D., Kummer R., and Bortolozzo F. P.. 2018. Two different feeding levels during late gestation in gilts and sows under commercial conditions: Impact on piglet birth weight and female reproductive performance. J. Anim. Sci. 96:4209–4219. doi: 10.1093/jas/sky297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann A. L., Camilotti E., Fagundes D. P., Vier C. E., Mellagi A. P. G., Ulguim R. R., Bernardi M. L., Orlando U. A. D., Gonçalves M. A. D., Kummer R., et al. 2019. Impact of feed intake during late gestation on piglet birth weight and reproductive performance: A dose-response study performed in gilts. J. Anim. Sci. 97:1262–1272. doi: 10.1093/jas/skz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier E., Etienne M., Ramaekers P., and Père M. C.. 2010. The metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livestock Science. 127:127–136. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington, DC: National Academy Press. [Google Scholar]
- Pedersen T. F., Chang C. Y., Trottier N. L., Bruun T. S., and Theil P. K.. 2019. Effect of dietary protein intake on energy utilization and feed efficiency of lactating sows. J. Anim. Sci. 97:779–793. doi: 10.1093/jas/sky462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Hu L., Liu Y., Yan C., Fang Z. F., Lin Y., Xu S. Y., Li J., Wu C. M., Chen D. W., et al. 2016. Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal 10:1812–1820. doi: 10.1017/S1751731116000999 [DOI] [PubMed] [Google Scholar]
- Pialoux V., Mounier R., Rock E., A. Mazur, Chmitt L., Richalet J., Robach P., Coudert J., and Fellmann N.. 2009. Effects of acute hypoxic exposure on prooxidant/antioxidant balance in elite endurance athletes. Int J Sports Med. 30:87–93. doi:10.1055/s-0028-1103284 [DOI] [PubMed] [Google Scholar]
- Quesnel H. 2011. Colostrum production by sows: Variability of colostrum yield and immunoglobulin G concentrations. Animal 5:1546–1553. doi: 10.1017/S175173111100070X [DOI] [PubMed] [Google Scholar]
- Ren P., Yang X. J., Kim J. S., Menon D., and Baidoo S. K.. 2017. Effect of different feeding levels during three short periods of gestation on sow and litter performance over two reproductive cycles. Anim. Reprod. Sci. 177:42–55. doi: 10.1016/j.anireprosci.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Ren P., Yang X. J., Railton R., Jendza J., Anil L., and Baidoo S. K.. 2018. Effects of different levels of feed intake during four short periods of gestation and housing systems on sows and litter performance. Anim. Reprod. Sci. 188:21–34. doi: 10.1016/j.anireprosci.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Rezaei R., Wu Z., Hou Y., Bazer F. W., and Wu G.. 2016. Amino acids and mammary gland development: Nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 7:20. doi: 10.1186/s40104-016-0078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel R. S., Moehn S., Pencharz P. B., and Ball R. O.. 2012. Dietary lysine requirement of sows increases in late gestation. J. Anim. Sci. 90:4896–4904. doi: 10.2527/jas.2011-4583 [DOI] [PubMed] [Google Scholar]
- Shelton N. W., Neill C. R., Derouchey J. M., Tokach M. D., Goodband R. D., and Nelssen J. L.. 2009. Effects of increasing feeding level during late gestation on sow and litter performance. Kansas Agricultural Experiment Station Research Reports; 10:38–50. doi:10.4148/2378-5977.6780. [Google Scholar]
- Theil P. K., Lauridsen C., and Quesnel H.. 2014. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 8:1021–1030. doi: 10.1017/S1751731114000950 [DOI] [PubMed] [Google Scholar]
- Theil P. K., Nielsen M. O., Sørensen M. T., and Lauridsen C.. 2012. Lactation, milk and suckling. Nutritional physiology of pigs. Danish Pig Research Centre, Copenhagen: p. 1–47. [Google Scholar]
- Town S. C., Patterson J. L., Pereira C. Z., Gourley G., and Foxcroft G. R.. 2005. Embryonic and fetal development in a commercial dam-line genotype. Anim. Reprod. Sci. 85:301–316. doi: 10.1016/j.anireprosci.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Trevisi E., Zecconi A., Bertoni G., and Piccinini R.. 2010. Blood and milk immune and inflammatory profiles in periparturient dairy cows showing a different liver activity index. J. Dairy Res. 77:310–317. doi: 10.1017/S0022029910000178 [DOI] [PubMed] [Google Scholar]
- Tydlitát D., Vinkler A., and Czanderlová L.. 2008. Influence of crude protein intake on the duration of delivery and litter size in sows. ACTA VET BRNO. 77:25–30. doi:10.1016/j.jff.2017.05.044 [Google Scholar]
- Yang Y. X., Heo S., Jin Z., Yun J. H., Choi J. Y., Yoon S. Y., Park M. S., Yang B. K., and Chae B. J.. 2009. Effects of lysine intake during late gestation and lactation on blood metabolites, hormones, milk composition and reproductive performance in primiparous and multiparous sows. Anim Reprod Sci. 112:199–214. [DOI] [PubMed] [Google Scholar]
- Zhang X.-D., Zhu Y.-F., Cai L.-S., and Wu T.-X.. 2008. Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture. 280:136–139. doi:10.1016/j.aquaculture.2008.05.010 [Google Scholar]
- Zhao Y., Flowers W. L., Saraiva A., Yeum K. J., and Kim S. W.. 2013. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J. Anim. Sci. 91:5848–5858. doi: 10.2527/jas.2013-6388 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Loor J. J., Piccioli-Cappelli F., Librandi F., Lobley G. E., and Trevisi E.. 2016. Circulating amino acids in blood plasma during the peripartal period in dairy cows with different liver functionality index. J. Dairy Sci. 99:2257–2267. doi: 10.3168/jds.2015-9805 [DOI] [PubMed] [Google Scholar]