Abstract
A total of 2,156 weaned pigs (6.75 ± 0.11 kg BW) were used in a 42-d study to evaluate whether improvements in growth performance associated with super-dosing phytase can be explained by the complete dephosphorylation of phytate and liberation of inositol. Two phytase doses (0 and 2,500 FTU/kg) and 3 inositol concentrations (0%, 0.15%, and 0.30%) were combined to create 6 dietary treatments in a 2 × 3 factorial arrangement. Pigs were fed a 3-phase feeding program, with periods being 10, 10, and 22 d, respectively. Blood samples were collected on days 0, 21, and 42 from a subset of 48 pigs to analyze mineral and myo-inositol concentrations. During Phase 1, super-dosing phytase tended to improve ADG compared with pigs fed diets without phytase (P = 0.09). Increasing concentrations of inositol improved the efficiency of gain in pigs fed diets without phytase (1,022.1, 1,040.9, and 1,089.2 g/kg), but not diets with phytase (1,102.2, 1,087.2, and 1,076.2 g/kg), and this improvement was equivalent to that observed with super-dosing phytase in the absence of inositol (interaction, P = 0.015). During Phase 2, super-dosing phytase improved ADG (P = 0.001), resulting in heavier BW (P = 0.007). During Phase 3 and overall, inositol supplementation increased ADG and ADFI in a quadratic manner (P < 0.10), with the highest ADG and ADFI observed for pigs fed 0.15% of inositol. Super-dosing phytase increased serum Zn on day 21, but not on day 42 (interaction, P = 0.008), increased serum Cu (P = 0.01), but decreased serum Fe (P = 0.02). Plasma myo-inositol increased linearly from 66.9 to 97.1 and 113.2 nmol/mL with increasing inositol (P < 0.001). When plasma myo-inositol was analyzed within the subgroup of pigs fed diets without added inositol, super-dosing phytase increased plasma myo-inositol from 57.81 to 76.05 nmol/mL (0 and 2,500 FTU/kg, respectively; P = 0.05). Results demonstrate that exogenous inositol improved efficiency of gain in weaned pigs to the same level as that observed with super-dosing phytase, but this occurred only during the first 10 d of the nursery period. This suggests that the improvement in efficiency of growth when applying super-dosing phytase could be linked, in part, to complete dephosphorylation of phytate and liberation of myo-inositol, and that myo-inositol had a greater metabolic impact in piglets immediately after weaning. Consequently, myo-inositol may be a conditionally essential nutrient for young pigs during weaning stress, but further research is needed to prove this hypothesis.
Keywords: myo-inositol, phytase, weaned pigs
Introduction
Supplementation of phytase at a dose that exceeds the standard level used to release phytate-bound P is considered as super-dosing phytase (usually levels well over 500 FTU/kg and up to 2,500 FTU/kg). Recent studies have shown that super-dosing phytase improves growth performance of pigs compared with the standard phytase doses (Wilcock et al., 2014; Lee and Bedford, 2016; Moran et al., 2017). Theoretically, it is possible that high levels of phytase can dephytinize the diets, which implies degradation of insoluble antinutritional inositol hexaphosphoric acid (IP6) and subsequent generation of lower inositol phosphates (IP5-1) into inositol (Cowieson et al., 2011). Previous work in poultry (Karadas et al., 2010; Cowieson et al., 2013, 2015; Walk et al., 2014) suggests that part of the benefit of super-dosing phytase could be attributed to the generation of inositol from the destruction of phytate initiated by exogenous phytase (Adeola and Cowieson, 2011).
Inositol is a cyclic sugar alcohol that exists predominantly as myo-inositol or phosphatidylinositol in tissue and cells of mammals (Holub, 1986). Phosphatidylinositol is a cellular mediator of signal transduction and regulates metabolism and growth (Hawthorne and White, 1976). However, there is no known nutritional requirement of myo-inositol for human and animals, except for fish and gerbils (Holub, 1986; McDowell, 2000; Combs, 2012). Myo-inositol could play a role in improving growth performance in pigs, but its mode of action is still unclear and warrants further investigation. To date, there are no published studies that have evaluated the supplementation of exogenous inositol and super-dosing phytase to determine performance responses in pigs. Therefore, the objective of this study is to evaluate and compare the impact of inositol supplementation and exogenous phytase at super-dosing levels on performance and blood metabolites of nursery pigs housed under commercial conditions.
MATERIALS AND METHODS
Animals were treated humanely, and all practices and procedures used in these experiments were consistent with the Guide for the Care and Use of Animals in Agricultural Research and Teaching (FASS, 2010). The experiments were conducted under the supervision of licensed veterinarians.
Animals, Housing, and Experimental Design
The experiment was conducted in a commercial research facility owned and operated by Hanor Company (White Hall, IL). Research rooms occupy 25% of the 11,000 pig site. Two identical nursery rooms, equipped with an automated feeding system (Feedlogic Corporation, Wilmar, MN) that can blend, weigh, and record feed delivered to individual pens, were used in this trial. Pigs were weaned at approximately 22 d of age. A combined total of 2,156 barrows and gilts (Camborough sow × PIC TR-4 sire) with an average initial BW of 6.75 ± 0.11 kg were used in a 42-d study to evaluate and compare the impact of inositol and exogenous phytase at super-dosing levels on nursery pig performance, fecal scores, and blood metabolites. Pigs were received at night, placed in a total of 96 pens (22 pigs per pen), and given access to a common nursery starter diet for a total of 0.25 kg per pig (5.5 kg in each feeder) overnight. The following morning, feeders were cleared of the common diet and pigs were blocked by weight and sex and randomly assigned to 1 of 6 dietary treatments within weight block and sex (16 replicates per treatment). Treatment diets were fed immediately after pigs were placed in their assigned pens.
Two phytase doses (0 and 2,500 FTU/kg) and 3 inositol inclusion levels (0%, 0.15%, and 0.30%) were combined to create 6 dietary treatments (Table 1) in a 2 × 3 factorial arrangement. Pigs were fed a 3-phase feeding program, with periods being 10, 10, and 22 d for Phases 1, 2, and 3, respectively. Phytase was not given nutrient release values in diet formulation for all diets to specifically determine the impact of super-dosing independently of P release. In accordance, dietary Ca and P were supplied in excess of NRC (2012) recommendations for control and phytase supplemented diets.
Table 1.
Composition of the experimental diets, as-fed basis1
| Feeding phase | |||
|---|---|---|---|
| Ingredient, % | 1 | 2 | 3 |
| Soybean meal, 47% CP | 25.00 | 25.00 | 32.05 |
| Whey permeate2 | 22.00 | 6.10 | — |
| Steamed ground oats | 20.00 | 7.50 | — |
| Corn | 10.50 | 38.72 | 46.61 |
| Corn distillers’ dried grains with solubles | 8.00 | 8.00 | 16.00 |
| Soy protein concentrate3 | 7.00 | 7.00 | — |
| Choice white grease | 3.50 | 3.55 | 1.90 |
| Limestone | 1.12 | 1.07 | 1.08 |
| Monocalcium phosphate | 0.67 | 1.14 | 1.00 |
| Zinc oxide | 0.35 | 0.28 | — |
| Lysine·HCl | 0.40 | 0.40 | 0.44 |
| Salt | 0.35 | 0.40 | 0.40 |
| Organic acid blend4 | 0.30 | 0.10 | 0.10 |
| dl-Methionine | 0.22 | 0.19 | 0.15 |
| Toxin binder5 | 0.20 | 0.20 | — |
| Vitamin and mineral premix6 | 0.15 | 0.15 | 0.15 |
| Pellet binder | 0.10 | 0.10 | — |
| l-Threonine | 0.10 | 0.09 | 0.11 |
| Choline chloride | 0.07 | 0.07 | — |
| Cu chloride | 0.03 | 0.03 | — |
| l-Tryptophan | 0.02 | 0.03 | 0.03 |
| Calculated composition | |||
| ME, Mcal/kg | 3.56 | 3.49 | 3.38 |
| CP, % | 24.12 | 23.53 | 23.92 |
| SID7 lysine, % | 1.46 | 1.43 | 1.40 |
| SID isoleucine, % | 0.93 | 0.89 | 0.86 |
| SID threonine, % | 0.86 | 0.84 | 0.84 |
| SID tryptophan, % | 0.26 | 0.27 | 0.25 |
| SID valine, % | 0.99 | 0.97 | 0.93 |
| Ca, % | 0.82 | 0.80 | 0.75 |
| Available P, % | 0.40 | 0.40 | 0.37 |
| Ca:available P, ratio | 2.07 | 2.03 | 2.08 |
| STTD8 Ca, % | 0.58 | 0.59 | 0.55 |
| STTD P, % | 0.43 | 0.40 | 0.36 |
| STTD Ca:STTD P, ratio | 1.37 | 1.47 | 1.54 |
1Six experimental diets were manufactured by first creating 4 dietary treatments with 0% or 0.30% of inositol with or without 2,500 FTU/kg of phytase for each Phase. Then, diets containing 0.15% of inositol were created on the farm by blending half of diets containing 0% and 0.30% of inositol with or without phytase, using an automated feeding system. The source of phytase was Quantum Blue (AB Vista, Marlborough, UK), and the source of inositol was inositol hexanicotinate (Zhucheng Haotian Pharmaceutical Co., Shandong, China). Analyzed phytase concentrations for Phases 1, 2, and 3 were 605 and 1,940, 632 and 2,120, and 361 and 1,643 FTU/kg, for 0 and 2,500 FTU/kg phytase treatments, respectively.
2Dairy Lac 80 (International ingredients Corp., Monett, MO).
3Soy protein product (ADM, Chicago, IL).
4Aviplus (Vetagro Inc., Chicago, IL).
5Flo-Bond (Brookside Agra, O’Fallon, IL).
6Supplied per kg of complete diet: 105 mg of zinc as zinc sulfate, 100 mg of iron as ferrous sulfate, 45 mg of manganese as manganous oxide, 15 mg of copper as copper sulfate, 0.7 mg of iodine as ethylenediaminedihydroiodide, 0.3 mg of selenium as sodium selenite, 9,923 IU of vitamin A, 1,654 IU of vitamin D3, 77 mg of vitamin E, 3.9 mg of vitamin K, 44.1 µg of vitamin B12, 9.9 mg of riboflavin, 33.1 mg of d-pantothenic acid, 55.1 mg of niacin, 3.3 mg of thiamin, 5.5 mg of pyridoxine, 992 µg of folic acid, and 276 µg of biotin.
7Standardized ileal digestible.
8Standardized total tract digestible.
Experimental diets were manufactured in a commercial feed mill (Greenfield, IL) owned and operated by Hanor Company. The phytase used was derived from an Escherichia coli 6-phytase expressed in Trichoderma reesei (Quantum Blue, AB Vista, Marlborough, UK), and the source of inositol was inositol FCC (97% purity, Zhucheng Haotian Pharmaceutical Co., Shandong, China). Diets were manufactured by first creating the 4 dietary treatments with 0% or 0.30% of inositol with or without phytase for each phase. Diets containing 0.15% of inositol were created on the farm using the automated feeding system by blending 50% of the diets containing 0% and 50% of the diets containing 0.30% of inositol within the 0 and 2,500 FTU/kg phytase diets.
Sample Collection
Pig weight and feed disappearance were measured by pen on days 0, 10, 20, and 42 to calculate ADG, ADFI, and G:F. Fecal consistency was evaluated and fecal scoring was initiated on day 1 post-placement for 10 d. The fecal consistency was evaluated visually by pen, by the 1 trained individual. The score was not intended to be a measure of the quantity of scours within a pen, but the consistency of scouring noted for each individual pen. The evaluator was blinded to dietary treatments. The fecal scoring system consisted of the following: 0 = normal feces; 1 = soft feces; 2 = fluid feces; and 3 = completely liquid, projectile feces (wet hindquarters).
A subset of 48 barrows from 48 pens (8 pigs per treatment) were selected to collect blood samples. One barrow in each pen was selected randomly and tagged with individual identification to collect blood samples on days 0 (before offering dietary treatments), 21, and 42. Blood samples were collected from the jugular vein by venipuncture using a glass royal blue vacuum tube suitable for trace element testing (BD Vacutainer, Franklin Lakes, NJ) for serum collection and a green vacuum tube with heparin (BD Vacutainer) for plasma collection. After blood collection, the samples were centrifuged at 1,000 × g for 15 min, and serum and plasma were aliquoted and placed into vials for storage at −20 °C for analysis.
Sick pigs that showed lethargy or anorexia were medically treated and removed from the pen. An extra pen for each dietary treatment was reserved for sick pigs. Pigs that were removed were tagged with individual identification and placed in the appropriate treatment pen. Removed pigs were maintained on their original dietary treatment until the end of the study and included in the final analyses.
Chemical Analyses
Serum samples were analyzed for mineral panel (P, K, Ca, Mg, Zn, S, Cu, and Fe). Mineral analysis was determined by inductively coupled plasma with an optical emission spectrophotometer (Spectro Arcos FHS16, Ametek; Kieve, Germany). Plasma samples were analyzed for myo-inositol concentration by HPLC using a Dionex DX600 HPLC system as described by Laird (2016).
Statistical Analyses
Data were analyzed using SAS (SAS Inst. Inc., Cary, NC) as a randomized complete block design with a 2 × 3 factorial arrangement of treatments. Growth performance data were analyzed using the GLM procedure using pen as the experimental unit. The model included block, inositol level, phytase concentrations, and the interaction of inositol level and phytase concentration as fixed effects. Orthogonal contrast comparisons were conducted to determine linear and quadratic effects of inositol levels. The concentration of minerals in serum, plasma myo-inositol, and fecal score data were analyzed by the MIXED procedure as repeated measures over time on each experimental unit (pig). Concentrations of minerals and inositol measured on day 0 were used as a covariate when significant (P < 0.05). The model included inositol level, phytase concentration, the day of measurement, and their interactions as fixed effects and pig as random effect. Least square means were reported and differences were considered statistically significant at P ≤ 0.05 with tendencies at 0.05 < P ≤ 0.10.
RESULTS
Pigs grew normally throughout the experimental period, and during the study, only 7 pigs died representing a mortality of 0.27%. During Phase 1, diets with the super-dosing level of phytase tended to improve ADG compared with pigs fed diets without phytase (277 vs. 267 g/d, respectively; P = 0.09; Table 2). An interaction between inositol and phytase was observed for feed efficiency during Phase 1, showing that increasing levels of inositol improved the G:F ratio in pigs fed diets without phytase, but did not affect G:F in pigs with the super-dosing level of phytase (P = 0.015).
Table 2.
Effect of super-dosing phytase and inositol supplementation on growth performance of nursery pigs1
| 0 FTU/kg phytase | 2,500 FTU/kg phytase | P-values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inositol, % | 0 | 0.15 | 0.30 | 0 | 0.15 | 0.30 | SEM | Phytase | Inositol | Inositol |
| Linear | Quadratic | |||||||||
| BW, kg | ||||||||||
| 0 d | 6.73 | 6.70 | 6.73 | 6.71 | 6.72 | 6.71 | 0.06 | 0.894 | 0.986 | 0.830 |
| 10 d | 9.40 | 9.37 | 9.46 | 9.51 | 9.52 | 9.50 | 0.10 | 0.218 | 0.785 | 0.813 |
| 20 d | 14.47 | 14.41 | 14.57 | 14.85 | 14.84 | 14.69 | 0.14 | 0.007 | 0.834 | 0.885 |
| 42 d | 28.60 | 28.72 | 28.52 | 28.66 | 29.15 | 28.64 | 0.26 | 0.326 | 0.829 | 0.148 |
| ADG, g | ||||||||||
| 0 to 10 d | 266.4 | 267.1 | 268.8 | 277.2 | 276.8 | 275.6 | 6.51 | 0.091 | 0.949 | 0.992 |
| 11 to 20 d | 460.8 | 457.5 | 465.3 | 484.7 | 483.8 | 471.1 | 6.41 | 0.001 | 0.475 | 0.976 |
| 21 to 42 d2 | 673.2 | 680.3 | 663.9 | 657.8 | 681.3 | 664.6 | 7.74 | 0.473 | 0.870 | 0.021 |
| 0 to 42 d | 520.6 | 522.9 | 517.1 | 521.1 | 532.8 | 520.6 | 5.44 | 0.301 | 0.706 | 0.097 |
| ADFI, g | ||||||||||
| 0 to 10 d | 261.5 | 258.0 | 246.7 | 252.8 | 256.1 | 257.5 | 5.77 | 0.985 | 0.382 | 0.634 |
| 11 to 20 d3 | 589.3c | 600.5bc | 612.2ab | 627.1a | 626.0a | 607.6bc | 7.00 | 0.001 | 0.805 | 0.499 |
| 21 to 42 d | 1013.5 | 1018.8 | 999.7 | 992.6 | 1026.1 | 1000.8 | 12.05 | 0.671 | 0.813 | 0.052 |
| 0 to 42 d | 723.1 | 727.0 | 717.7 | 719.3 | 737.0 | 719.5 | 7.79 | 0.673 | 0.730 | 0.080 |
| G:F, g/kg | ||||||||||
| 0 to 10 d4 | 1,022.1c | 1,039.9bc | 1,089.2a | 1,102.2a | 1,087.2a | 1,076.2ab | 15.94 | 0.004 | 0.198 | 0.525 |
| 11 to 20 d | 782.3 | 762.4 | 762.4 | 774.4 | 773.9 | 777.7 | 7.83 | 0.327 | 0.289 | 0.381 |
| 21 to 42 d | 665.6 | 669.2 | 665.5 | 663.4 | 664.8 | 664.6 | 4.07 | 0.455 | 0.889 | 0.530 |
| 0 to 42 d | 721.0 | 720.6 | 721.8 | 725.1 | 723.6 | 724.4 | 3.29 | 0.234 | 0.991 | 0.738 |
a-cValues within a row with different superscripts differ (P ≤ 0.05).
1Values represent least square means of 16 pens with 22 pigs per pen.
2Main effect of inositol (P = 0.06).
3Interaction of phytase × inositol (P = 0.010).
4Interaction of phytase × inositol (P = 0.015).
During Phase 2, pigs fed diets with the super-dosing level of phytase had increased ADG compared with pigs fed diets without phytase (480 vs. 461 g/d, respectively; P = 0.001), which resulted in heavier pigs at the end of this phase (P = 0.007). The ADFI increased as inositol supplementation increased in the absence of phytase, but this was not the case when pigs were fed diets with the super-dosing level of phytase (P = 0.01, phytase and inositol interaction).
During Phase 3 and the overall nursery period, inositol supplementation tended to increase ADG and ADFI in a quadratic manner (P < 0.10). The highest ADG and ADFI were observed for pigs fed diets with 0.15% of inositol. Fecal score evaluated during the first 10 d of the experiment was unaffected by dietary treatments (P > 0.05). The observed scour score was between 0 (normal feces) and 1 (soft feces) of the scoring system.
Serum Minerals and Plasma Myo-inositol
Interactions between inositol, phytase, and day of measurement were not significant (3-way interaction; P > 0.46) for any of the minerals measured in serum. An interaction between inositol concentration and day of measurement was observed for serum concentrations of P, K, Ca, Mg, and S (P < 0.05; Table 3). Inositol concentrations did not affect the serum concentration of P, K, Ca, Mg, and S when measured on day 21; however, on day 42, pigs supplemented with 0.15% of inositol had low serum concentrations of these 4 minerals. An interaction between super-dosing phytase and day of measurement was observed for the serum concentration of Zn (P = 0.008; Table 4). On day 21, super-dosing phytase increased the serum concentration of Zn, but this was not the case on day 42. Pigs fed diets with the super-dosing level of phytase had a greater concentration of serum Cu than pigs fed diets without phytase (2.06 vs. 1.82 mg/L, respectively; P = 0.01). In contrast, pigs fed diets with super-dosing phytase had lower concentrations of serum Fe than pigs fed diets without phytase (0.72 vs.1.15 mg/L, respectively; P = 0.02).
Table 3.
Effect of inositol supplementation and day of measurement on serum mineral concentrations of nursery pigs1
| Day 21 | Day 42 | SEM | |||||
|---|---|---|---|---|---|---|---|
| Inositol, % | 0 | 0.15 | 0.30 | 0 | 0.15 | 0.30 | |
| Mineral concentration, mg/L | |||||||
| P2 | 133.6ab | 144.6a | 138.9a | 136.2a | 122.0b | 135.5ab | 5.10 |
| K3 | 269.2a | 280.7a | 280.5a | 280.9a | 234.1b | 283.8a | 9.85 |
| Ca4 | 100.4a | 107.1a | 102.8a | 101.0a | 87.8b | 101.8a | 4.07 |
| Mg5 | 23.5ab | 25.6a | 23.9a | 24.9a | 20.9b | 23.2ab | 1.03 |
| Zn | 1.35 | 1.34 | 1.35 | 1.30 | 1.17 | 1.32 | 0.06 |
| S6 | 77.3a | 82.3a | 77.9a | 65.6b | 59.8c | 64.9b | 2.29 |
| Cu | 2.02 | 2.07 | 1.96 | 1.99 | 1.72 | 1.92 | 0.09 |
| Fe | 1.22 | 0.95 | 0.98 | 0.80 | 0.86 | 0.83 | 0.20 |
a-cValues within a row with different superscripts differ (P ≤ 0.05).
1Values are least square means of 16 pigs, representing main effects of inositol of a 2 × 3 factorial arrangement with 0 or 2,500 FTU/kg of phytase and 0, 0.15, or 0.30% inositol. The interaction between phytase and inositol was not significant.
2Interaction of inositol × day (P = 0.04).
3Interaction of inositol × day (P = 0.006).
4Interaction of inositol × day (P = 0.03).
5Interaction of inositol × day (P = 0.005).
6Interaction of inositol × day (P = 0.04).
Table 4.
Effect of super-dosing phytase and day of measurement on serum mineral concentrations of nursery pigs1
| Day 21 | Day 42 | ||||
|---|---|---|---|---|---|
| Phytase, FTU/kg | 0 | 2,500 | 0 | 2,500 | SEM |
| Mineral concentration, mg/L | |||||
| P | 137.6 | 140.4 | 135.6 | 126.9 | 4.12 |
| K | 280.2 | 273.4 | 273.7 | 258.8 | 8.04 |
| Ca | 103.7 | 103.1 | 100.3 | 93.4 | 3.32 |
| Mg | 24.5 | 24.2 | 23.5 | 22.5 | 0.84 |
| Zn2 | 1.25b | 1.45a | 1.30b | 1.23b | 0.05 |
| S | 81.2 | 77.1 | 65.5 | 61.5 | 1.87 |
| Cu3 | 1.9 | 2.2 | 1.8 | 2.0 | 0.07 |
| Fe4 | 1.2 | 0.9 | 1.1 | 0.6 | 0.16 |
abValues within a row with different superscripts differ (P ≤ 0.05).
1Values are least square means of 16 pigs, representing main effects of phytase of a 2 × 3 factorial arrangement with 0 or 2,500 FTU/kg of phytase and 0, 0.15, or 0.30% inositol. The interaction between phytase and inositol was not significant.
2Interaction of phytase × day (P = 0.008).
3Main effect of phytase (P = 0.01).
4Main effect of phytase (P = 0.02).
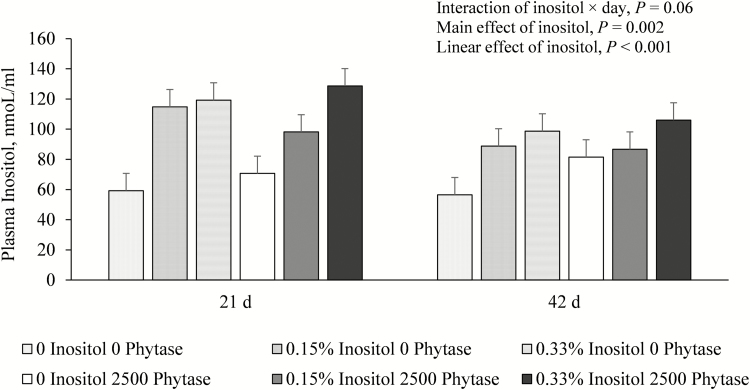
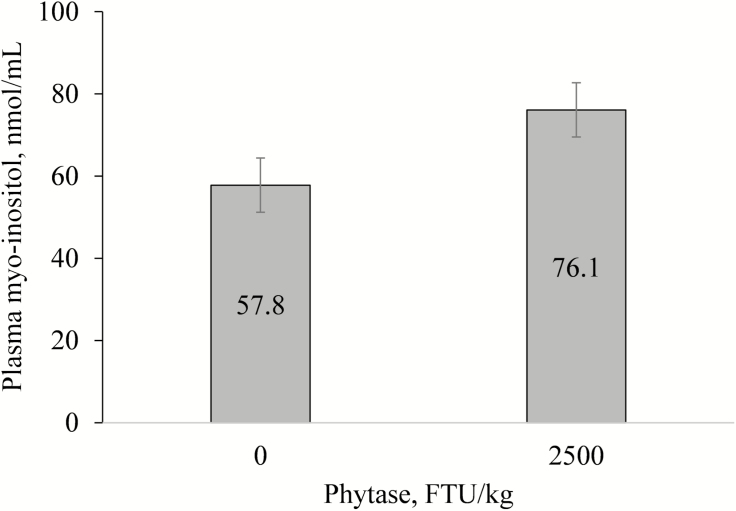
Increasing levels of supplemental inositol in the diets significantly increased the plasma myo-inositol concentration in a linear manner (P < 0.001; Fig. 1). When plasma myo-inositol concentration was analyzed within the subgroup of pigs fed control diets (without added inositol), super-dosing phytase increased plasma myo-inositol concentration (57.81 vs. 76.05 nmol/mL for 0 vs. 2,500 FTU/kg phytase, respectively; P = 0.05; Fig. 2).
Figure 1.
Effect of inositol and phytase supplementation on plasma myo-inositol levels on days 21 and 42. Bars represent least square means of 8 pigs. Interaction of inositol × phytase × day, P = 0.73; interaction of inositol × phytase, P = 0.38; interaction of phytase × day, P = 0.37; main effect of phytase, P = 0.49; and main effect of day, P = 0.01.
Figure 2.
Comparison of plasma myo-inositol between control treatment (0 FTU/kg phytase and 0% inositol) and super-dosing phytase (2,500 FTU/kg and 0% inositol). Value represents least squared means of 8 pigs. Phytase effect, P = 0.05.
Discussion
This study investigated the potential effects of super-dosing phytase on the performance of weaned pigs and whether those effects are related to the release of inositol from the phytate molecule. The inclusion rates of exogenous inositol in the dietary treatments (0.15% and 0.30%) were selected based on the estimated concentration of inositol that can be released by high doses of phytase in conventional pig and poultry diets (Cowieson et al., 2013, 2016). Therefore, it is hypothesized that similar performance responses can be expected between pigs fed diets with inositol in the absence of phytase, compared with pigs fed diets without inositol in the presence of a super-dosing level of phytase. This response was observed during the first 10 d immediately after weaning for feed efficiency, during which increasing levels of inositol improved the G:F ratio in pigs fed diets without supplemental phytase, and this improvement was equivalent to the improvement observed with super-dosing phytase. Supplementation of inositol to the diet with added phytase did not further improve feed efficiency. Another effect of inositol was observed during the final feeding phase in the nursery, where inositol tended to improve ADG and the greatest ADG was observed for pigs fed diets with 0.15% of inositol. However, the improvement in ADG during this phase was not associated with an improvement in feed efficiency.
Similar results have been observed by Zyla et al. (2004), who reported improvements in BW gain in broilers fed diets supplemented with 0.1% inositol from days 1 to 21. Pirgozliev et al. (2007) reported enhanced BW gain in broilers fed P-deficient diets supplemented with 0.25% inositol from days 7 to 17. In contrast, Cowieson et al. (2013) observed that supplementation of 0.15% of inositol in broilers had a negative impact on BW and feed conversion rate during the starter phase (days 1 to 10), but a positive effect on BW and feed conversion rate in the finisher phase (days 21 to 42). Sommerfeld et al. (2018) reported improved G:F when free inositol was supplemented to broilers. The positive effect of inositol and phytase on feed efficiency during the first 10 days in the present study suggests that inositol had a greater metabolic impact in piglets immediately after weaning, which is well known as the most stressful period during the life of the pig. In tissues and cells of mammals, inositol exists predominantly as myo-inositol (isomeric form of inositol) or phosphatidylinositol (myo-inositol bound covalently to phospholipid; Holub, 1986; Michell, 2008). Phosphatidylinositol is a cellular mediator of signal transduction and regulates metabolism and growth (Hawthorne and White, 1976). Phosphatidylinositol has a major role in maintaining phospholipid structure in cellular membranes which could support the integrity and function of epithelial cells in the gastrointestinal tract of newly weaned piglets. Moreover, phosphatidylinositol regulates cellular processes such as amylase secretion, insulin release, and gluconeogenesis (McDowell, 2000), which could contribute to the improvement in performance immediately after weaning.
Positive effects of super-dosing phytase on growth performance of nursery pigs were observed in this study as indicated by improved the ADG during Phases 1 and 2, resulting in heavier pigs at the end of Phase 2. Pigs used in this study received diets adequate in nutrients. Likewise, several studies have reported improvements in growth performance of pigs when fed diets adequate in nutrients and supplemented with high doses of phytase (Kies et al., 2006; Wilcock et al., 2014; Moran et al., 2017), suggesting that the effects of super-dosing phytase are beyond the provision of extra nutrients.
Phytate is a strong chelator of divalent cations, forming the strongest to the weakest mineral-phytin complex with Zn2+, Cu2+, Ni2+, Co2+, Mn2+, Ca2+, and Fe2+, respectively (Cheryan, 1980; Persson et al., 1998). The formation of these complexes reduces the solubility and digestibility of these minerals in the intestine of pigs. Therefore, the addition of phytase not only can release P, but can also release other minerals from phytate, making them more available. Serum mineral concentrations can be used as an indicator of the mineral status of animals (Walk et al., 2013). Thus, the present study evaluated the effects of phytase and inositol on the mineral status of pigs. The initial serum concentration of minerals (at day 0) averaged 130.8, 212.2, 70.5, 25.3, 1.6, 68.4, 2.3, and 0.4 mg/L for P, K, Ca, Mg, Zn, S, Cu, and Fe, respectively, and the initial serum concentrations of these minerals were not statistically different across treatments. Previous studies in pigs (Adeola et al., 1995; Gebert et al., 1999; Walk et al., 2013) and broiler chickens (Sebastian et al., 1996) have reported that supplementation of phytase increases serum concentrations of P, Ca, Mg, Fe, Zn, and Cu. In the present study, serum concentrations of Zn and Cu increased when pigs were fed super-dosing levels of phytase. In contrast, super-dosing phytase reduced the serum concentration of Fe. According to Persson et al. (1998), phytate has the highest binding affinity for Zn and Cu; thus, higher serum levels of Zn and Cu observed when phytase was supplemented suggest that phytase was effective in liberating Zn and Cu from phytate making them more available. The reduction in the serum levels of Fe by super-dosing phytase is not clear and disagrees with previous findings (Stahl et al., 1999; Laird, 2016). According to Stahl et al. (1999), exogenous phytase (1,200 U/kg diet) effectively released phytate-bound Fe and increased the hemoglobin concentrations in piglets.
Serum concentrations of P, K, Ca, Mg, and S were reduced when pigs were supplemented with 0.15% of inositol, but only on day 42. The reason for this response is not clear. On the other hand, serum concentrations of these minerals were not affected by super-dosing phytase. It is known that P absorption is reduced when diets do not have a proper Ca to P ratio, with wide ratios reducing P absorption mainly when P levels are close to the requirement. Both Ca and P were supplemented above the requirements suggested by the NRC (2012), and the Ca to available P ratio in the dietary treatments was 2:1, which is within the recommended ratios (2:1 and 3:1; Crenshaw, 2001). Furthermore, the lack of effects of phytase on serum Ca and P concentrations could be explained by the tight regulation of Ca and P to maintain homeostasis within the body compartments of the animal (Crenshaw, 2001). Murry et al. (1997) suggested that supplemental phytase increased serum P concentrations in pig fed P-deficient diets, but this effect was limited when diets were adequate in P as was the case in the present study. Similar to our results, Pagano et al. (2007) and Kerr et al. (2010) did not observe effects of supplemental phytase on serum concentrations of P. Also, Laird (2016) did not detect differences in serum concentrations of Ca, P, K, and Mg in pigs fed P adequate diets supplemented with 2,000 FTU/kg.
To determine whether the positive effects of super-dosing phytase were associated with the release of inositol, plasma myo-inositol concentrations were determined. On day 0, the circulating concentrations of myo-inositol averaged 57.2 nmol/mL, and it was equal among treatments. Plasma myo-inositol was clearly increased by inositol supplementation, increasing from 66.9 to 97.1 and 113.2 nmol/mL when adding 0%, 0.15%, and 0.30% of inositol, respectively. This coincided with an improvement in feed efficiency in Phase 1 of the nursery period. When plasma myo-inositol concentration was analyzed within the subgroup of pigs fed control diets without added inositol, super-dosing phytase increased plasma myo-inositol concentration from 57.8 to 76.1 nmol/mL compared with diets without phytase. This suggests that super-dosing phytase is liberating inositol, but not to the same extent that can be achieved with the direct supplementation of exogenous inositol. These results are in agreement with Laird et al. (2016), who demonstrated that super-dosing phytase (2,000 to 8,000 FTU/kg) improved portal and peripheral serum myo-inositol concentrations in weaner, grower, and finisher pigs. Holloway et al. (2016) reported that increasing levels of phytase reduced the concentration of phytate (IP6) and IP5 and increased the concentration of IP4, IP3, and inositol in ileal digesta when up to 2,500 FTU/kg of phytase was fed, suggesting intestinal degradation of phytate. In 2 experiments with broilers conducted by Cowieson et al. (2015), plasma myo-inositol increased from 47, 56, 62, and 65 mg/L when feeding 0, 1,000, 2,000, and 3,000 phytase units in Exp. 1 and from 49, 61, and 63 mg/L when feeding 0, 1,000, and 2,000 phytase units in Exp. 2. Sommerfeld et al. (2018) reported increased inositol concentrations in plasma, crop, and ileum in broilers supplemented with free inositol, and this was also observed when phytase was supplemented. The role of myo-inositol is still unclear in monogastric nutrition. Myo-inositol is not an essential nutrient in mammals because it can be synthesized from glucose-6-phosphate. Myo-inositol has been suggested to simulate the insulin signaling pathway, stimulating protein synthesis, which can explain improvements in growth performance associated with increase circulating levels of myo-inositol (Croze and Soulage, 2013).
In conclusion, the results suggest that supplementation of exogenous inositol improved feed efficiency (in diets without phytase) in weaned pigs, and this improvement was equivalent to the improvement observed with super-dosing phytase (2,500 FTU/kg) in the absence of inositol, but this occurred only during the first 10 d of the nursery period. This suggests that the improvement in growth performance when applying super-dosing phytase could, in part, be linked to IP6 breakdown and myo-inositol production and that myo-inositol had a greater metabolic impact in piglets immediately after weaning, which is well known as the most stressful period during the life of the pig. Consequently, it should be considered that myo-inositol may be a conditionally essential nutrient for young pigs during stress periods, but further research is needed to prove this hypothesis.
Footnotes
Partial funding was provided by AB Vista, Marlborough, UK. Appreciation is expressed to The Hanor Company (Franklin, KY) for providing access to their facilities and their staff for assistance in conducting the studies.
LITERATURE CITED
- Adeola O., and Cowieson A. J.. 2011. Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi: 10.2527/jas.2010-3715 [DOI] [PubMed] [Google Scholar]
- Adeola O., Lawrence B. V., Sutton A. L., and Cline T. R.. 1995. Phytase-induced changes in mineral utilization in zinc-supplemented diets for pigs. J. Anim. Sci. 73:3384–3391. doi: 10.2527/1995.73113384x [DOI] [PubMed] [Google Scholar]
- Cheryan M. 1980. Phytic acid interactions in food systems. Crit. Rev. Food Sci. Nutr. 13:297–335. doi: 10.1080/10408398009527293 [DOI] [PubMed] [Google Scholar]
- Combs G. F. 2012. The vitamins: Fundamental aspects in nutrition and health. 4th ed. Academic Press, San Diego, CA: p. 410–414. [Google Scholar]
- Cowieson A. J., Aureli R., and Guggenbuhl P.. 2015. Possible involvement of myo-inositol in the physiological response of broilers to high doses of microbial phytase. Anim. Prod. Sci. 55:710–719. doi: 10.1071/AN14044 [DOI] [Google Scholar]
- Cowieson A. J., Ptak A., Mackowiak P., Sassek M., Pruszynska-Oszmalek E., Zyla K., Swiatkiewicz S., Kaczmarek S., and Józefiak D.. 2013. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 92:2124–2134. doi: 10.3382/ps.2013-03140 [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., Ruckebusch J. P., Knap I., and Guggenbuhl P.. 2016. Phytate-free nutrition: a new paradigm in monogastric animal production. Anim. Feed Sci. Technol. 222:180–189. doi: 10.1016/j.anifeedsci.2016.10.016 [DOI] [Google Scholar]
- Cowieson A. J., Wilcock P., and Bedford M. R.. 2011. Super-dosing effects of phytase in poultry and other monogastrics. Worlds Poult. Sci. J. 67:225–236. doi: 10.1017/S0043933911000250 [DOI] [Google Scholar]
- Crenshaw T. D. 2001. Calcium, phosphorus, vitamin D and vitamin K in swine nutrition. In: Southern L., Lewis A. J., editors, Swine nutrition. CRC Press, Boca Raton, FL: p. 188–212. [Google Scholar]
- Croze M. L., and Soulage C. O.. 2013. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 95:1811–1827. doi: 10.1016/j.biochi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- FASS 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Fed. Anim. Sci. Soc., Champaign, IL. [Google Scholar]
- Gebert S., Bee G., Pfirter H. P., and Wenk C.. 1999. Phytase and vitamin E in the feed of growing pigs: influence on growth, mineral digestibility and fatty acids in digesta. J. Anim. Physiol. Anim. Nutr. 81:9–19. doi: 10.1046/j.1439-0396.1999.811187.x [DOI] [Google Scholar]
- Hawthorne J. N., and White D. A.. 1976. Myo-inositol lipids. In: Munson P. L., Diczfalusy E., Glover J., Olson R. E., editors, Vitamins and hormones. vol. 33 Academic Press, New York. p. 529–573. [DOI] [PubMed] [Google Scholar]
- Holloway C. L., Boyd R. D., Walk C. L., and Patience J. F.. 2016. Impact of super-dosing phytase in diets fed to 40 kg, 60 kg and 80 kg pigs on phytate catabolism. J. Anim. Sci. 94(Suppl. 2):112–113(Abstr.) doi: 10.2527/msasas2016-238 [DOI] [Google Scholar]
- Holub B. J. 1986. Metabolism and function of myo-inositol and inositol phospholipids. Annu. Rev. Nutr. 6:563–597. doi: 10.1146/annurev.nu.06.070186.003023 [DOI] [PubMed] [Google Scholar]
- Karadas F., Pirgozliev V., Pappas A. C., Acamovic T., and Bedford M. R.. 2010. Effects of different dietary phytase activities on the concentration of antioxidants in the liver of growing broilers. J. Anim. Physiol. Anim. Nutr. (Berl) 94:519–526. doi: 10.1111/j.1439-0396.2009.00938.x [DOI] [PubMed] [Google Scholar]
- Kerr B. J., Weber T. E., Miller P. S., and Southern L. L.. 2010. Effect of phytase on apparent total tract digestibility of phosphorus in corn-soybean meal diets fed to finishing pigs. J. Anim. Sci. 88:238–247. doi: 10.2527/jas.2009-2146 [DOI] [PubMed] [Google Scholar]
- Kies A. K., Kemme P. A., Sebek L. B., van Diepen J. T., and Jongbloed A. W.. 2006. Effect of graded doses and a high dose of microbial phytase on the digestibility of various minerals in weaner pigs. J. Anim. Sci. 84:1169–1175. doi: 10.2527/2006.8451169x [DOI] [PubMed] [Google Scholar]
- Laird S. 2016. The effects of super-dosing phytase in the growing pig. PhD Diss. Univ. of Leeds, Leeds, UK. [Google Scholar]
- Lee S. A., and Bedford M. R.. 2016. Inositol – An effective growth promotor? Worlds. Poult. Sci. J. 72:1–17. doi: 10.1017/S0043933916000660 [DOI] [Google Scholar]
- McDowell L. R. 2000. Vitamins in animal and human nutrition. 2nd ed. Iowa State University Press, Ames, IA; p. 660–666. [Google Scholar]
- Michell R. H. 2008. Inositol derivatives: evolution and functions. Nat. Rev. Mol. Cell Biol. 9:151–161. doi: 10.1038/nrm2334 [DOI] [PubMed] [Google Scholar]
- Moran K., Boyd R. D., Zier-Rush C., Wilcock P., Bajjalieh N., and van Heugten E.. 2017. Effects of high inclusion of soybean meal and a phytase superdose on growth performance of weaned pigs housed under the rigors of commercial conditions. J. Anim. Sci. 95:5455–5465. doi: 10.2527/jas2017.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry A. C., Lewis R. D., and Amos H. E.. 1997. The effect of microbial phytase in a pearl millet-soybean meal diet on apparent digestibility and retention of nutrients, serum mineral concentration, and bone mineral density of nursery pigs. J. Anim. Sci. 75:1284–1291. doi: 10.2527/1997.7551284x [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pagano A. R., Yasuda K., Roneker K. R., Crenshaw T. D., and Lei X. G.. 2007. Supplemental Escherichia coli phytase and strontium enhance bone strength of young pigs fed a phosphorus-adequate diet. J. Nutr. 137:1795–1801. doi: 10.1093/jn/137.7.1795 [DOI] [PubMed] [Google Scholar]
- Persson H., Turk M., Nyman M., and Sandberg A.-S.. 1998. Binding of Cu, Zn, and Cd to inositol tri, tetra, penta and hexaphophates. J. Agric. Food Chem. 8561:3194–3200. doi: 10.1021/jf971055w [DOI] [Google Scholar]
- Pirgozliev V., Allymehr M., Sarwar S., Acamovic T., and Bedford M. R.. 2007. The effect of dietary inositol on performance and mucin excretion when fed to chickens. Brit. Poultr. Abstr. 3:4–5. [Google Scholar]
- Sebastian S., Touchburn S. P., Chavez E. R., and Lague P. C.. 1996. The effects of supplemental microbial phytase on the performance and utilization of dietary calcium, phosphorus, copper, and zinc in broiler chickens fed corn-soybean diets. Poult. Sci. 75:729–736. doi: 10.3382/ps.0750729 [DOI] [PubMed] [Google Scholar]
- Sommerfeld V., Künzel S., Schollenberger M., Kühn I., and Rodehutscord M.. 2018. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 97:920–929. doi: 10.3382/ps/pex390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C. H., Han Y. M., Roneker K. R., House W. A., and Lei X. G.. 1999. Phytase improves iron bioavailability for hemoglobin synthesis in young pigs. J. Anim. Sci. 77:2135–2142. doi: 10.2527/1999.7782135x [DOI] [PubMed] [Google Scholar]
- Walk C. L., Santos T. T., and Bedford M. R.. 2014. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 93:1172–1177. doi: 10.3382/ps.2013-03571 [DOI] [PubMed] [Google Scholar]
- Walk C. L., Srinongkote S., and Wilcock P.. 2013. Influence of a microbial phytase and zinc oxide on young pig growth performance and serum minerals. J. Anim. Sci. 91:286–291. doi: 10.2527/jas.2012-5430 [DOI] [PubMed] [Google Scholar]
- Wilcock P. C., Bradley L., Chewning J. J., and Walk C. L.. 2014. The effect of superdosing phytase on inositol and phytate concentration in the gastrointestinal tract and its effect on pig performance. J. Anim. Sci. 92 (E-Suppl. 2):383(Abstr.) [Google Scholar]
- Zyla K., Mika M., Stodolak B., Wikiera A., Koreleski J., and Swiatkiewicz S.. 2004. Towards complete dephosphorylation and total conversion of phytates in poultry feeds. Poult. Sci. 83:1175–1186. doi: 10.1093/ps/83.7.1175 [DOI] [PubMed] [Google Scholar]