Abstract
The thyme (Thymus vulgaris) essential oil was assessed as antibiotic growth promoter replacement in quail chick diet and in vitro test. In total, 250-d-old Japanese quail chicks (mixed sex) were allocated into 5 dietary treatments of 5 replications (6 females and 4 males in each cage with the size of 40× 90× 25 cm) under a completely randomized design. The dietary treatments were included the control diet, control diet without any additive, control diet plus 100 ppm flavophospholipol as an antibiotic growth promoter, control diet plus 200 ppm TVE, control diet plus 300 ppm TVE, and control diet plus 400 ppm T. vulgaris essential (TVE) oil. Feed intake, BW gain, feed conversion ratio (FCR), organs weight, morphology of intestine, serum lipids, and microbial population were measured on day 35. Lipid oxidation of stored muscle tissue was measured by TBARS test. GC–MS assay, DPPH method, and well diffusion method were evaluated for determination of components, antioxidant, and antimicrobial properties, respectively. FCR improved significantly in 400 ppm TVE compared with 200 and 300 ppm TVE (P < 0.05). The serum triglyceride decreased significantly in both sexes receiving 400 ppm TVE compared with control. Villi height increased significantly in duodenum accompanied by decreasing crypt dept at all TVE levels compared with control and antibiotic. The breast muscle tissue of quail fed on 300 and 400 ppm TVE reduced the rate of oxidation during refrigerated storage compared with control. Thymol was the main component (35.40%) of the thymus oil. The considerable antioxidant activity of TVE was identified by IC50 of 58.48 µg/mL. Moreover, zones of growth inhibition of Gram-positive bacteria and Escherichia coli were numerically greater in different doses of TVE than antibiotics. Therefore, The TVE is suitable alternative component for antibiotic growth promoters by dosing consideration. However, it is possible that antibiotic resistance would increase for these natural compounds along the time.
Keywords: antioxidant, morphology, performance, quail, serum lipids, thyme oil
Introduction
Application of synthetic antibiotics in poultry husbandry can result in increased antibiotic resistance bacteria. Hence, researchers are seeking new natural feed additive alternatives to improve growth performance, modulate microbial populations, and improve meat quality and animal health. Phytogenic essential oils are one of the natural feed additives that their main effects and modes of action on poultry production have been reviewed previously (Brenes and Roura, 2010). It has been reported that the type and application dosage of essential oils have the major influence on determining the performance parameter and nutrient availability on the birds (Chowdhury et al., 2018; Liu et al., 2018). In addition, it is claimed that phytogenic essential oils improved intestinal health indices in laying hens (Mousavi et al., 2018).
Among the plants, one of the large plant families is the Lamiaceae family, and the genus Thymus is part of this family. The most famous species of Thymus is vulgaris that commonly was called thyme (Stahl-Biskup and Saez, 2004). Chemical analyses have shown the presence of different compounds in the essential oil of this plant. These compounds are flavonoids, tannins, saponins, and phenols, and it is rich in the monoterpene thymol and its isomer carvacrol (Fachini-Queiroz et al., 2012; Walentowska and Flaczyk, 2013). This medical plant can display antimicrobial, antifungal, antioxidant, antiviral, anti-cancer, and anti-inflammatory activities due to the existence of thymol and carvacrol (Pina‐Vaz et al., 2004; Behnia et al., 2008; Cetinus et al., 2013; Ferreira et al., 2016; Nabavi et al., 2015).
The production of Japanese quail (Coturnix japonica) as a meat-type bird for human food has been popular in many countries (Jeke et al., 2018). The cholesterol value and skin fat is low in quail meat while it is rich in vitamins like folate, E, K, and B complex as well as other micronutrients (Manafi et al., 2016). This bird has been used as an animal model in numerous studies due to its short lifespan and high growth rate (Huss et al., 2008). The feeding requirement of Japanese quail is about 20 to 25 g/d and reaches to the weight of 140 to 180 g between 5 to 8 wk of age (Altine et al., 2016).
The purposes of this study was first to evaluate the effects of different dosage of thyme essential oil on quail physiology and nutrition, and second to compare this oil with antibiotics used in poultry farming as a natural alternative, and third to identify whether the thyme essential oil has antioxidant or antibacterial properties in vitro.
MATERIALS AND METHODS
The experimental protocols were approved by the Research Ethics Committee of the Shahid Bahonar University of Kerman, Iran (License number: 1396/137).
In Vivo Tests
Providing essential oil
Hydrodistillated T. vulgaris essential (TVE) oil was purchased from Barij essence Kashan factory in Iran.
Housing
This study was carried out in standard quail breeding buildings in Yazd agricultural and natural resources research center (Yazd, Iran). Two halls (A and B) were prepared for the trial. In first 15 d, chicks were kept in the hall A at 36 °C temperature and in the cages containing 40 W lamps, baby drinker, and chicken feeding tray. After day 15, chicks moved to hall B equipped with automatic linear feeder and nipple drinker with 32 °C temperature and 50% relative humidity. The hall temperature was dropped 2° weekly and maintained at 26 °C in last week. Quail were maintained on 24 h lighting regimen and had free access to water.
Birds, diets, and experimental design
A total of 250-d-old Japanese quail chicks (mixed sex) were allocated to 5 dietary treatments of 5 replications (6 females and 4 males in each cage with the size of 40× 90× 25 cm) under a completely randomized design. The dietary treatments were included the control diet, control diet without any additive, control diet plus 100 ppm flavophospholipol (Iranian Veterinary Pharmaceuticals Company, Iran, Tehran) as an antibiotic growth promoter, control diet plus 200 ppm TVE, control diet plus 300 ppm TVE, and control diet plus 400 ppm TVE. Corn–soy meal mash diets contained 2,900 kcal ME/kg and 22% CP were formulated using nutritional requirements of quail during growing and developing phases (Table 1 Rostagno et al., 2011). The essential oil was mixed with the required oil of the diets and added to control diet. Quail chicks were fed on experimental rations from the beginning of trial and were allowed ad libitum access to both feed and water throughout the study period of 35 d.
Table 1.
Feed ingredients and nutrient composition of basal diets during different production phases
| Ingredient, % DM | Grower and developer phases |
|---|---|
| Corn, yellow | 55.34 |
| Soybean meal | 39.21 |
| Soybean oil | 1.92 |
| Limestone | 1.21 |
| Dicalcium phosphate | 1.34 |
| Common salt | 0.41 |
| Vitamin premix1 | 0.25 |
| Mineral premix2 | 0.25 |
| DL-Methionine | 0.08 |
| Nutrient composition | |
| MEn, kcal/kg | 2,900 |
| CP, % | 22.00 |
| Calcium, % | 0.90 |
| Non-phytate P, % | 0.37 |
| Na, % | 0.17 |
| Methionine, % | 0.42 |
| Lysine, % | 1.19 |
| Threonine, % | 0.79 |
| Tryptophan, % | 0.21 |
| Arginine, % | 1.19 |
1Vitamin premix provided the followings per kilogram of diet: vitamin A, 12,000 IU; cholecalciferol, 5,000 IU; vitamin E, 45 IU; vitamin K3, 2.4 mg; thiamine, 2.6 mg; riboflavin, 6.6 mg; pantothenic acid, 25 mg; niacin, 55 mg; choline chloride, 500 mg; biotin, 0.1 mg; folic acid, 1.5 mg; pyridoxine 5.5 mg; vitamin B12, 0.015 mg; BHT, 1 mg.
2Mineral premix provide the followings per kilogram of diet: iron, 50 mg; zinc, 85 mg; manganese, 90 mg; iodine, 1 mg; copper, 10 mg; selenium, 0.25 mg.
Performance data collection
Birds and the rest of the feed per cage were weighed weekly and feed intake (FI), BW gain (BWG), and feed conversion ratio (FCR) per bird calculated weekly as well as the end of the trial (35 d). Mortalities were recorded daily to adjust performance data. Total FI, total BWG, and total FCR were reported as total performance.
Weighing and blood sampling
At final day of trial (35 d), feed was removed 3 h before slaughtering. One bird per cage (random sex) nearest to the average weight of same cage was selected to measure organs weight and also two birds (1 male and 1 female) from each cage were separately selected to measure serum lipids due to variance in serum lipid ranges between sexes. Each bird was exsanguinated by cutting the jugular vein, and 5 mL of blood samples were collected by small plastic tubes. Viscera were removed immediately; thereafter the weights of liver, heart, tights, carcass, breast, and gastrointestinal tract were taken. Carcass yield and relative weights of organs were calculated as a percentage of live BW. After transferring blood samples to the lab, they were centrifuged at 3,000 rpm for 10 min (HETTICH Rotafix32A, Germany) and sera stored at –20 °C until further analysis. Serum triglyceride and cholesterol concentrations were measured using a biochemical analyzer (Autolab, PM 4000, Auto analyzer, Medical System, Rome, Italy) according to the procedures recommended by producing company.
Tissue sampling
During visceral separation, 1 cm segments of the duodenum (proximal intestine), jejunum (before the Meckel’s diverticulum), and ileum (distal intestine) were excised, washed in physiological saline solution, and fixed in 10% buffered formalin. The tissue samples were later embedded in paraffin, and a 2-μm section of each sample was placed on a glass slide and stained with hematoxylin and eosin according to Baurhoo et al. (2007) procedure. Pictures of villus and crypts were obtained with a video camera (SPoT idea, Diagnostic Instrument Inc., Sterling Heights, MI), and then measurements were made by a computer using the Spot Basic (Diagnostic Instruments Inc., Sterling Heights, MI) imaging software.
Microbial population
The contents of the ileum were collected in the tubes and transferred to the laboratory for counting the microbial population (total coliforms and the lactic acid bacteria). The contents of ileum were diluted in normal saline. Plates containing MRS agar (deMan, Rogosa and Sharpe agar) were prepared for counting lactic acid producing bacteria and plates containing MacConkey agar were prepared for counting total coliforms. After bacteria culture, plates incubated for 24 ho in 37 °C temperature. All plates were counted during 24 h after incubation (Li, 1991).
TBARS assay
(TBARS) test is one of the most accurate tests to determine lipid oxidation in animal tissues. In general, this test expresses the concentration of malondialdehyde, which is a good indicator of oxidation. For measurement of lipid stability in quail storage muscle tissue, the quail breast muscle tissue (5 replicates) was defrosted at room temperature after 60 d storage in –22 °C and homogenized with phosphate buffer (0.1 molar, containing 5 mM EDTA, pH = 7) on liquid nitrogen. Then, 40 μL of homogenized breast tissue was added to 40 μL of 0.9% sodium chloride plus 40 μL distilled water and placed at 37 °C for 20 min. Then, the reaction was stopped by using 600 mL hydrochloric acid (0.8 molar, containing 12.5% trichloroacetic acid). In the next step, 780 mL 1% thiobarbituric acid were added to the solution, boiled for 20 min and cooled to 4 °C. The cooled solution was centrifuged at 1,500 × g for 20 min. Finally, the absorbance of the above solution was measured at 532 nm and the data were expressed in milligram malondialdehyde per kilogram (Subbarao et al., 1990; Botsoglou et al., 2003).
In Vitro Tests
GC–MS system
Three samples of thyme oil were injected to GC–MS system for determining the components. The GC–MS system was used for analysis of the essential oil was the CP 3800 model (Varian BV company) developed by Australia and equipped by a capillary column of DB-5 (30 m × 0.25 mm × 0.25 µm). The injector temperature equal to 240 °C and detector was 230 °C. Electron impact was 70 eV and helium was the carrier gas at a flow rate of 1.0 mL/min with split ratio of 1/20. Temperature was held at 60 °C and programmed at 3 °C/min to 240 °C and volume injected was 1 µL. Finally, for identifying the components of the oil, mass spectra of the components were compared with data of the computer library (Nist 62 lib database) and Kovats retention index (Adams, 2007).
Well diffusion assay
The bacteria selected for microbial test, including Gram-negative bacteria Escherichia coli (ATCC 25922), Salmonella typhimurium (ATCC 14028) and Gram-positive bacteria Staphylococcus aureus (PTCC 1337) and Bacillus cereus (PTCC 1015) were provided from the faculty of veterinary medicine, Shahid Bahonar University of Kerman, Iran. Pathogenic bacteria transferred from –20 °C to MHB (Mueller Hinton Broth) medium and were renewed after 24 h of incubation at 37 °C. The inoculums density was set to 0.5 McFarland standards (108 CFU/mL). 0.5 McFarland suspension of each bacterium inoculated on plates (60 mm in size) contained MHA (Muller Hinton Agar) by swab. The wells of 6 mm diameters were punched in agar surface and the amounts of 1,100, 2,200, 4,400, and 8,800 µg/mL of TVE added to the wells. Control plates were attached to ceftriaxone (CRO-30) and tetracycline (Te-30) antibiotic disks for each bacterium. Finally, after incubating plates at 37 °C for 18 h (Memmert incubator, Germany), inhibition zones diameters were measured in millimeter by a ruler. This experiment was repeated 3 times for each strain and mean values with standard deviations of inhibition zones were recorded as final results (Jorgensen and Turnidge, 2015).
Antioxidant activity determination
In this experiment, we used DPPH (2,2-diphenyl-1-picrylhydrazyl, Sigma-Aldrich, Germany) as free radical to test antioxidant activity of TVE on the basis of Brand-Williams et al., 1995. Further, beta-hydroxy toluene (BHT, Sigma-Aldrich, Germany) antioxidant potential was determined as a standard antioxidant. At first, 0.004% DPPH methanolic solution was prepared. Then, 50 µL of the oil different dilutions in methanol were added to test tubes contained 2.5 mL DPPH solution (final concentrations were 294, 147, 73.5, 36.7, 18.3, and 9 µg/mL). In parallel, there was a control to contain all reagents except the essential oil. After 60 min, data were read at a wavelength of 517 nm by a spectrophotometer (Biotek Instruments). The following equation was used for the calculation of the antioxidant activity:
where %I is the inhibition percentage of DPPH, “A blank” is the absorbance of DPPH with methanol at 517 nm and “A sample” is the absorbance of different TVE dilutions in 517 nm. Finally, the IC50 parameter was used to compare the activity of TVE with BHT standards (IC50 is the concentration of essential oil that inhibits 50% of free radicals).
Statistical Analysis
All the data obtained in this experiment were analyzed by one-way analysis of variance using ANOVA procedures of SAS statistical software (SAS, 1999). A completely randomized design was applied in the analysis of all traits. The experimental unit was a cage. Yij = μ + Aj + ei(j), where Yij is the observed value for a particular character, μ is the overall mean, Aj is the effect of A treatment level j, and ei(j) is the random error associated with the ijth recording. Data averages were compared by using Duncan’s multiple range tests at the P < 0.05 significant level.
RESULTS
In Vivo Tests
Performance traits (BWG, FI, and FCR)
The performance data of quail fed with different levels of TVE are illustrated in Table 2. FI increased significantly in T200 treatment compared with antibiotic (P < 0.05). However, different levels of TVE could not decrease FI than control or antibiotic. Birds fed with different levels of TVE revealed no remarkable BWG difference than control or antibiotic (P > 0.05). FCR improved significantly in 400 ppm TVE compared with 200 and 300 ppm TVE (P < 0.05) although it was not significant than control or antibiotic. The contrast between antibiotic and 200 ppm TVE was significant (P < 0.05).
Table 2.
Effect of different levels of thyme essential oil and antibiotic on total performance of quail during 35 d rearing1
| Treatments | FI2, g/d per bird | BWG2, g/d per bird | FCR2, g feed/g gain |
|---|---|---|---|
| Control | 18.40ab | 6.29 | 2.92ab |
| Antibiotic3 | 18.32b | 6.45 | 2.84b |
| T200 | 18.61a | 6.14 | 3.03a |
| T300 | 18.47ab | 6.20 | 2.98 a |
| T400 | 18.24b | 6.39 | 2.82b |
| P-value4 | 0.035 | 0.168 | 0.009 |
| SEM | 0. 089 | 0.107 | 0.045 |
| P-value for contrasts4 | |||
| Control vs. antibiotic | 0.951 | 0.785 | 0.623 |
| Control vs. T200 | 0.373 | 0.780 | 0.372 |
| Control vs. T300 | 0.962 | 0.945 | 0.829 |
| Control vs. T400 | 0.619 | 0.958 | 0.479 |
| Antibiotic vs. T200 | 0.116 | 0.191 | 0.028 |
| Antibiotic vs. T300 | 0.650 | 0.363 | 0.140 |
| Antibiotic vs. T400 | 0.950 | 0.990 | 0.997 |
1All data are average of 5 replicates.
2FI = feed intake; BWG = BW gain; FCR = feed conversion ratio.
3Antibiotic is 100 ppm flavophospholipol. T200, T300, and T400 indicate the levels of 200, 300, and 400 ppm of Thymus vulgaris essential oil.
4Significance level (P < 0.05) by 95% confidence interval.
abDifferent superscripts indicate significant differences (P < 0.05) within the same column.
Organs weight
In this study, different levels of TVE and antibiotic had no marked effect on the relative weight of the liver, heart, carcass, breast, thighs and gastrointestinal tract (Table 3). No differences were observed when organs weight data were compared between treatments (P > 0.05).
Table 3.
Effect of different levels of thyme essential oil and antibiotic on carcass characteristics in 35-d aged quail (expressed as % of live BW)1
| Treatments | Liver | Heart | Carcass | Breast | Tights | Gastrointestinal tract3 |
|---|---|---|---|---|---|---|
| Control | 2.25 | 0.80 | 59.39 | 29.97 | 20.75 | 8.28 |
| Antibiotic2 | 2.00 | 0.85 | 61.44 | 29.71 | 20.89 | 8.73 |
| T200 | 2.07 | 0.84 | 62.02 | 30.74 | 21.10 | 7.39 |
| T300 | 2.41 | 0.84 | 59.03 | 29.24 | 20.75 | 8.88 |
| T400 | 2.56 | 0.82 | 58.66 | 27.81 | 20.30 | 8.00 |
| P-value4 | 0.701 | 0.858 | 0.671 | 0.546 | 0.701 | 0.786 |
| SEM | 0.352 | 0.041 | 2.203 | 0.834 | 0.659 | 0.663 |
| P-value for contrasts4 | ||||||
| Control vs. antibiotic | 0.977 | 0.864 | 0.945 | 0.999 | 0.999 | 0.996 |
| Control vs. T200 | 0.993 | 0.915 | 0.876 | 0.980 | 0.997 | 0.950 |
| Control vs. T300 | 0.996 | 0.891 | 0.999 | 0.985 | 1.000 | 0.987 |
| Control vs. T400 | 0.957 | 0.986 | 0.998 | 0.554 | 0.992 | 0.999 |
| Antibiotic vs. T200 | 0.999 | 0.999 | 0.999 | 0.945 | 0.999 | 0.815 |
| Antibiotic vs. T300 | 0.880 | 1.000 | 0.905 | 0.997 | 0.999 | 0.999 |
| Antibiotic vs. T400 | 0.718 | 0.989 | 0.852 | 0.665 | 0.978 | 0.976 |
1All data are average of 5 replicates.
2Antibiotic is 100 ppm flavophospholipol. T200, T300, and T400 indicate the levels of 200, 300, and 400 ppm of Thymus vulgaris essential oil.
3Gastrointestinal tract includes the total weight of the crop, gizzard, caecum, and intestines.
4Significance level (P < 0.05) by 95% confidence interval.
Serum lipids
Changes in the pattern of serum lipids were evaluated separately in male and female quail at the age of 35 due to difference in lipids ranges between sexes (Table 4). The level of triglyceride in male birds decreased significantly in T400 treatment compared with control and antibiotic (P < 0.05). In females, the amount of triglyceride in treatments containing 300 and 400 ppm TVE decreased significantly compared with control (P < 0.05) but not to antibiotic. Significant difference (P < 0.05) was observed when female triglyceride was compared between control and T400 treatments. However, blood cholesterol level was not affected by treatments in both sexes.
Table 4.
Effect of different levels of thyme essential oil and antibiotic on triglyceride and cholesterol (mg/dL) based on the sex of quail in 35 d1
| Triglyceride | Cholesterol | |||
|---|---|---|---|---|
| Treatments | Male | Female | Male | Female |
| Control | 255.70a | 1,564.0a | 215.93 | 268.07 |
| Antibiotic2 | 243.33ab | 1,161.1b | 188.60 | 257.73 |
| T200 | 170.07abc | 1,245.7ab | 189.10 | 316.50 |
| T300 | 156.27bc | 1,091.7b | 179.33 | 298.67 |
| T400 | 143.93c | 999.0b | 185.90 | 309.00 |
| P-value3 | 0.039 | 0.031 | 0.509 | 0.186 |
| SEM | 23.060 | 92.18 | 12.980 | 16.180 |
| P-value for contrasts3 | ||||
| Control vs. antibiotic | 0.997 | 0.128 | 0.703 | 0.994 |
| Control vs. T200 | 0.229 | 0.285 | 0.716 | 0.408 |
| Control vs. T300 | 0.135 | 0.063 | 0.461 | 0.774 |
| Control vs. T400 | 0.082 | 0.024 | 0.631 | 0.557 |
| Antibiotic vs. T200 | 0.355 | 0.977 | 1.000 | 0.246 |
| Antibiotic vs. T300 | 0.218 | 0.989 | 0.991 | 0.557 |
| Antibiotic vs. T400 | 0.135 | 0.814 | 0.999 | 0.358 |
1All data are average of 5 replicates.
2Antibiotic is 100 ppm flavophospholipol. T200, T300, and T400 indicate the levels of 200, 300, and 400 ppm of Thymus vulgaris essential oil.
3Significance level (P < 0.05) by 95% confidence interval.
abc Different superscripts indicate significant differences (P < 0.05) within the same column.
Morphology of intestine tissue
Table 5 presents the effects of different levels of TVE and antibiotic on the morphology of duodenum, jejunum, and ileum cells. The villi height, villi width, and the crypt depth of the intestine cells were influenced by the experimental treatments. The villi height and width increased in treatments contained different levels of TVE and antibiotics when compared with the control. There was a significant increase in duodenum and jejunum villi height (P < 0.0001) in treatments containing different levels of TVE compared with control and antibiotic. However, villi height in ileum showed more fluctuation between treatments so that it increased significantly only in T200 and T300 treatments (P < 0.05). The duodenum, jejunum, and ileum villi width were also affected by experimental treatments so that in duodenum a significant decrease in villi width were observed in the treatments containing different levels of TVE. The greatest villi width in ileum related to 300 ppm TVE which differed significantly with control an T200 groups (P < 0.05). The crypt dept showed only significant differences in the duodenum (P < 0.0001). The lowest crypt dept was related to the treatment contained 200 ppm TVE which was significantly lower than antibiotic and control treatments. The crypt dept in jejunum and ileum did not show any significant difference.
Table 5.
Effect of different levels of thyme essential oil and antibiotic on intestine morphology of quail in 35 d1
| Villi height, μm | Villi width, μm | Crypt dept, µm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum |
| Control | 993.3c | 806.0c | 750.0b | 93.3a | 86.6ab | 85.3bc | 84.0a | 74.6 | 68.6 |
| Antibiotic2 | 1,007.3c | 864.0b | 808.6ab | 94.6a | 89.3b | 89.3a | 84.0a | 77.3 | 68.0 |
| T200 | 1,051.3b | 904.6a | 848.6a | 84.0b | 87.3ab | 84.6b | 56.0c | 72.6 | 62.6 |
| T300 | 1,059.3ab | 918.0a | 870.6a | 86.0b | 90.6ab | 90.6a | 62.0b | 78.0 | 68.0 |
| T400 | 1,074.6a | 924.6a | 758.0b | 88.0b | 91.3a | 88.0ab | 60.0b | 74.6 | 66.0 |
| P-value3 | <.0001 | <.0001 | 0.023 | 0.002 | 0.089 | 0.011 | <.0001 | 0.185 | 0.291 |
| SEM | 4.61 | 6.74 | 21.65 | 1.31 | 1.06 | 0.93 | 1.00 | 1.36 | 1.77 |
| P-value for contrasts3 | |||||||||
| Control vs. antibiotic | 0.396 | 0.002 | 0.496 | 0.968 | 0.565 | 0.137 | 1.000 | 0.754 | 0.999 |
| Control vs. T200 | 0.0001 | <.0001 | 0.107 | 0.010 | 0.994 | 0.991 | <.0001 | 0.891 | 0.300 |
| Control vs. T300 | <.0001 | <.0001 | 0.041 | 0.041 | 0.221 | 0.035 | <.0001 | 0.587 | 0.999 |
| Control vs. T400 | <.0001 | <.0001 | 0.999 | 0.171 | 0.126 | 0.447 | <.0001 | 1.000 | 0.881 |
| Antibiotic vs. T200 | 0.001 | 0.026 | 0.787 | 0.171 | 0.777 | 0.070 | <.0001 | 0.294 | 0.401 |
| Antibiotic vs. T300 | 0.0003 | 0.004 | 0.447 | 0.015 | 0.934 | 0.899 | <.0001 | 0.998 | 1.000 |
| Antibiotic vs. T400 | <.0001 | 0.001 | 0.622 | 0.067 | 0.777 | 0.899 | <.0001 | 0.754 | 0.953 |
1All data are average of 5 replicates.
2Antibiotic is 100 ppm flavophospholipol. T200, T300, and T400 indicate the levels of 200, 300, and 400 ppm of Thymus vulgaris essential oil.
3Significance level (P < 0.05) by 95% confidence interval.
abc Different superscripts indicate significant differences (P < 0.05) within the same column.
Microbial population
In this study, the number of lactobacilli and total coliforms were not significantly affected by experimental treatments (Table 6). However, 400 ppm TVE was better in increasing lactobacilli related to other treatments numerically. Some edible additives derived from herbal products have direct or indirect effect on digestive tract microflora. Although birds take little nutritional advantage from intestinal microflora compared with other species, quail fed on the diets contained different levels of TVE showed lower coliforms compared to birds fed with antibiotic (P > 0.05). No differences were observed when lactobacilli and total coliforms data were compared between the treatments.
Table 6.
Effect of different levels of thyme essential oil and antibiotic on intestine microbial population of quail in 35 d (mean log 10 cfu Coliform/g sample)1
| Treatments2 | Lactobacilli | Coliforms |
|---|---|---|
| Control | 6.70 | 5.20 |
| Antibiotic | 7.07 | 5.49 |
| T200 | 7.00 | 5.38 |
| T300 | 6.46 | 5.21 |
| T400 | 7.67 | 5.12 |
| P-value3 | 0.74 | 0.53 |
| SEM | 0.294 | 0.368 |
| P-value 3 for contrasts | ||
| Control vs. antibiotic | 0.963 | 0.770 |
| Control vs. T200 | 0.981 | 0.999 |
| Control vs. T300 | 0.993 | 1.000 |
| Control vs. T400 | 0.917 | 0.999 |
| Antibiotic vs. T200 | 1.000 | 0.569 |
| Antibiotic vs. T300 | 0.836 | 0.652 |
| Antibiotic vs. T400 | 0.999 | 0.560 |
1All data are average of 5 replicates.
2Antibiotic is 100 ppm flavophospholipol. T200, T300, and T400 indicate the levels of 200, 300, and 400 ppm of Thymus vulgaris essential oil.
3Significance level (P < 0.05) by 95% confidence interval.
Lipid oxidation in tissue
The amount of lipid oxidation in breast muscle of quail is shown in Table 7 by TBARS factor. The greatest amount of TBARS produced in quail breast muscle tissue was related to control treatment, which was significantly different from antibiotic, T300, and T400 treatments (P < 0.05).
Table 7.
Lipid oxidation (Thiobarbituric acid reactive substances, TBARS, mg malondialdeyde/kg) of quail breast muscle tissue after 60 d storage at –22 °C1
| Treatments | TBARS |
|---|---|
| Control | 0.37a |
| Antibiotic2 | 0.12b |
| T200 | 0.23ab |
| T300 | 0.10b |
| T400 | 0. 10b |
| P-value3 | 0.028 |
| SEM | 0.179 |
| P-value for contrasts3 | |
| Control vs. antibiotic | 0.034 |
| Control vs. T200 | 0.216 |
| Control vs. T300 | 0.021 |
| Control vs. T400 | 0.022 |
| Antibiotic vs. T200 | 0.332 |
| Antibiotic vs. T300 | 0.827 |
| Antibiotic vs. T400 | 0.834 |
1All data are average of 5 replicates.
2Antibiotic is 100 ppm flavophospholipol. T200, T300, and T400 indicate the levels of 200, 300, and 400 ppm of Thymus vulgaris essential oil.
3Significance level (P < 0.05) by 95% confidence interval.
abDifferent superscripts indicate significant differences (P < 0.05) within the same column.
In Vitro Results
TVE components
The composition of TVE used in this experiment is shown in Table 8. In TVE, 29 components were identified by GC/–MS technique, which represented about 97.5% of the total detected constituents. The main constituents of TVE were thymol (35.40%), durenol (31.09%), p-cymene (6.70%), and carvacrol (3.30%) which embraced 76.50% of the essential oil and the rest of the oil consisted of some trace products.
Table 8.
Thymus vulgaris essential oil compounds
| Compounds | % Total1 | compounds | % Total |
|---|---|---|---|
| Thymol | 35.40 | Bicyclo[3.1.0] hexane, 4-methyl-1-(1-methylethyl)-, didehydro deriv. | 0.29 |
| Durenol | 31.09 | α-Terpineol | 0.28 |
| P‐Cymene | 6.70 | 4-Terpinenol | 0.28 |
| 3-Benzylsulfonyl-2,6,6-trimethylbicyclo(3.1.1)heptane | 4.58 | (−)-Spathulenol | 0.26 |
| Carvacrol | 3.34 | Caryophyllene oxide | 0.25 |
| 3-Carene | 2.99 | (+)-Gamma-gurjunene | 0.24 |
| Caryophyllene | 2.09 | Cineole | 0.19 |
| Propioin | 1.62 | Isoborneol | 0.09 |
| Bicyclo[2.2.1]heptan-2-ol, 1,3,3-trimethyl-, acetate, (1S-exo)- | 1.62 | Apiol | 0.08 |
| 2-Carene | 1.40 | Espatulenol | 0.05 |
| Benzene, 1-methoxy-4-methyl-2-(1-methylethyl)- | 1.32 | Isopropyl myristate | 0.05 |
| Thymol, acetate | 1.22 | (S)-(−)-Limonene | 0.05 |
| Caryophyllene | 0.95 | Isoaromadendrene epoxide | 0.04 |
| Methoxy-p-cymene | 0.50 | Unknown | 0.01 |
| Eucalyptol | 0.45 |
1Relative percentage obtained from peak area.
TVE antioxidant properties
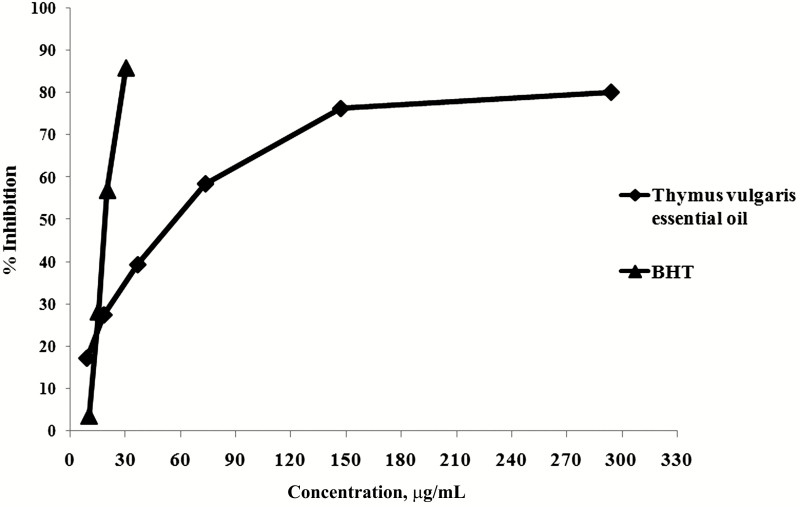
As shown in Table 9, IC50 of TVE was 58.48 µg/mL while IC50 of BHT was 20.28 µg/mL. These results were clearly revealed in Figure 1 and showed that by increasing the concentration of essential oil, inhibition percentage of free radicals increased logarithmically while in BHT increased linearly. However BHT indicated greater response relative to its concentration than thyme oil selected levels. TVE had weaker free radical scavenging capacity compared with BHT (IC50 of 58.48 vs. 20.28 µg/mL).
Table 9.
Antioxidant activity of Thymus vulgaris essential oil1
| Essential oil | T. vulgaris, µg/mL | BHT2, µg/mL |
|---|---|---|
| IC503 | 58.48 | 20.28 |
| STD | 0.039 | 0.97 |
1Data are average of triplicates.
2BHT= beta-hydroxy toluene.
3IC50 = the concentration of essential oil that inhibits 50% of free radicals.
Figure 1.
Comparison of % inhibition of free radicals by Thymus vulgaris essential oil and BHT. All data are average of triplicates. BHT = β-hydroxy toluene.
TVE antimicrobial properties
Table 10 shows antibacterial variables of TVE by the well diffusion method that is compared with standard antibiotics. As seen in the data of well diffusion, TVE could inhibit the growth of all bacteria, especially Gram-positive bacteria in all dosage. All the bacteria were removed from the plates contained 4,400 and 8,800 µg/mL of TVE so that the diameter of inhibition zone was more than 60 mm. However, the least inhibition of TVE was related to the plates contained S. typhimuriumin with 1,100 µg/mL TVE.
Table 10.
Zones of growth inhibition (mm) showing antibacterial activity of Thymus vulgaris essential oil against 4 bacterial strains assessed by well diffusion method1
| Compound | Amount, µg/mL | Gram (−) bacteria | Gram (+) bacteria | ||
|---|---|---|---|---|---|
| S. typhimurium (ATCC 14028) | E. coli (ATCC 25922) | B. cereus (PTCC1015) | S. aureus (PTCC 1337) | ||
| T. vulgaris | 1,100 | 10 ± 0.0 | 28 ± 2.8 | 12 ± 0.0 | 50 ± 0.0 |
| 2,200 | 12 ± 0.0 | 30 ± 0.0 | 24 ± 2.8 | 56 ± 2.8 | |
| 4,400 | 60 < | 60 < | 60 < | 60 < | |
| 8,800 | 60 < | 60 < | 60 < | 60 < | |
| Positive controls | disk code | ||||
| Tetracycline | Te-302 | 15 ± 1.4 | 20 ± 0.0 | 24 ± 1.4 | 25 ± 4.2 |
| Cephtriaxone | CRO-303 | 24 ± 2.8 | 8 ± 0.0 | 10 ± 1.4 | 37 ± 0.0 |
1Plate diameter = 60 mm; All data are average of triplicate ± SD.
2Te-30 = disks contained 30 μg tetracycline.
3CRO-30 = disks contained 30 μg cephtriaxone.
Discussion
The effects of thyme and its active compound thymol on poultry performance parameters have been reported in current research publications and variable results observed (Hoffman-Pennesi and Wu, 2010; Khaksar et al., 2012; Mehdipour et al., 2014; Oceľová et al., 2018). This variability may be due to the basal diet formulation, environment condition, the application dosage of the TVE, and the type of the selected bird (broiler, hen layer, quail, etc.). For example, performance parameters in broilers received 0.01%, 0.05%, 0.1% (w/w) thyme essential oils were not influenced significantly (Oceľová et al., 2018), while the quail fed on 1 g/kg of thyme oil showed greater BWG related to control (Khaksar et al., 2012). Similar to our results, the effects of 60, 100, and 200 mg/kg thymol and carvacrol combination were examined on broiler chicks and revealed that FI reduced linearly, while the birds received 200 mg/kg of this combination received the greatest BWG and feed efficiency (Hashemipour et al., 2013b). By the way, the TVE used in this experiment was low in carvacrol content.
According to Brenes and Roura (2010), essential oils stimulate oronasal sensing and digestive conditioning. The phenolic terpene content in thyme oil may give the diets a taste that was disagreeable to chicks during the early weeks (Cross et al., 2003). Nevertheless, compared with mammals, birds have lower taste bud numbers and fewer taste receptor genes, thus, they have most likely lower taste acuity (Roura et al., 2013). Therefore, reducing feed intake in 400 ppm TVE could not be most likely related to the taste of the essential oil. It seems that the positive effects of thyme on performance may be, in part, attributed to the content of thymol and its effects on improvement of feed consumption efficiency. It is pointed that thymol absorb intensively in the initial sections of the digestive tract (Oceľová et al., 2018). Hereon, it is reported evidences of antioxidant and antimicrobial properties (Botsoglou et al., 2002, Jang et al., 2007), development of nutrients digestibility (Hernandez et al., 2004), and stimulation of digestive enzymes secretion (Jang et al., 2007) in response to substances contained thymol and carvacrol. Therefore, in our experiment 400 ppm TVE may be involved in improving birds’ performance by reducing feed intake in response to efficacy of thymol on quail’s health.
Similarly to our findings, thyme essential oil did not influence significantly quail carcass characteristics in the reports of Denli et al. (2004). Nevertheless, it was reported that the percentages of breast and carcass in Japanese quail fed on thyme essential oil were greater than control (Khaksar et al., 2012).
Hypolipidemic effects of some medical plants, their essential oils, or their main components have been reported in several papers (Akbari et al., 2014; El-Ghousein and Al-Beitawi, 2009; Khaksar et al., 2012, Mehri et al., 2015). A study on male broilers fed on a high-viscosity diet supplemented with thymol + carvacrol revealed that the concentration of cholesterol decreased significantly (P < 0.05) in broilers received 200 mg/kg thymol + carvacrol though triglyceride did not change (Hashemipour et al., 2013a). However, essential oils or their effective compounds have the ability to decrease the plasma cholesterol and triglyceride levels (Edris, 2007). This event may be due to inhibition of enzymes involved in synthesis of these lipids by monoterpenes (Goldstein and Brown, 1990; Elson, 1995). It has been suggested that the hypocholesterolemic effect of essential oils is due to compounds in essential oil that have the ability to inhibit hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity, a key regulatory enzyme in cholesterol synthesis (Yu et al., 1994). However, according to our results, Khattak et al. (2013) observed no differences between the treatments when cholesterol values were compared.
Different parts of small intestine are the areas for absorption of ingested feed and the structure of them is related to their function. So, changes in feed and feed additives may affect the morphology of intestine different parts. In this case, Mohiti-Asli and Ghanaatparast-Rashti (2018) compared the effects of a combined phytogenic feed additive with oregano essential oil on intestinal morphology in broilers. They reported that broilers fed 300 ppm individual oregano essential oil in their diet had larger villi height, villi surface area, villi height to crypt dept ratio, and lower crypt dept in jejunum than those fed either control diet or combined phytogenic feed additive. Also, similar to our findings, Hashemipour et al. (2013b) reported that the inclusion of 100 and 200 ppm thymol and carvacrol increased villi height, surface area, and villi height to crypt dept ratio of jejunum and ileum in broilers. Villi development provided greater absorption surface for availability of nutrients (Awad et al., 2008). It is well known that development of nutrient absorption occurs by increasing the villi height and decreasing crypt depth. Therefore, thyme, essential oil may increase the availability of nutrients by affecting the height, width and crypt dept of the intestine villi. In the current study, 200, 300, and 400 ppm TVE reduced villi width in duodenum of quail. In contrast, the results of a study on rats showed that the villi width improved significantly in duodenum and jejunum of the rats fed thyme volatile oil (Sepehri Moghadam et al., 2014). However, crypt dept did not significantly influence by the treatments in jejunum and ileum but only in duodenum. Based on these results, it seems that supplementation of different levels of TVE in quail feed due to its thymol content has improved intestinal morphology. Accordingly, Du et al. (2016) reported that in the pathogen challenged birds, thymol and carvacrol increased significantly the villi height to crypt depth ratio compared with the basal diet.
Antimicrobial activity is recognized as one of the most important beneficial effects of essential oils, although their precise antimicrobial mechanism is still not fully understood. A large number of in vitro studies have shown that essential oils containing thymol and carvacrol exhibited antimicrobial activity against intestinal microbes such as Clostridium perfringens, S typhimurium, and E. coli (Helander et al., 1998; Hammer et al., 1999). The antimicrobial effect of essential oils is due to their lipophilic properties, which make them easily enter the bacterial and release cell membrane compounds to the external environment (Helander et al., 1998). On the other hand, it seems that the effects of essential oils on gut microflora are not stable, although the essential oils are generally recognized as antimicrobial agents. Therefore, the antimicrobial activity of essential oils in poultry is supposed to be influenced by the basal diet and environmental conditions (Jang et al., 2007).
Poultry meat due to containing high concentrations of unsaturated fatty acids with multiple bands is especially susceptible to oxidative degradation (Luna et al., 2010). It has been reported that the essential oils contain phenolic compounds have increasing regenerative capacity, since phenolic compounds prevent oxidation by disabling free fatty radicals and proxy radicals (Huange et al., 2011). Similar to our results, Luna et al. (2010) reported that TBARS of chicken thigh meat samples in control group were larger than the groups fed with 342 mg/kg thymol and carvacrol. In addition, it was reported that the thyme oil, similar to β-hydroxy anisole and β-hydroxy toluene, decreased TBARS in the samples (Saricoban and Tahsin Yilmaz, 2014). In this study, the antioxidant activity of thyme oil may be related to thymol content. In this case, Yanishlieva et al. (1999) proposed that thymol is a more effective and more active antioxidant than carvacrol, because thymol has greater steric hindrance of the phenolic group than carvacrol.
Thymol is a natural monoterpene phenol derivative of p-cymene, C10H14O, found in oil of thyme and some other species (Austgulen et al., 1987). The thyme essential oil components found here were approximately homogenous with the results of the most studies. For example, Boskovic et al. (2015) showed that thymol, p-cymene, linalool, γ-terpinene, and 1,8-cineole were the most dominant compounds of thyme essential oil (50.48%, 24.79%, 4.69%, 4.14%, and 4.35% of the oil, respectively). Additionally, the results of other previous studies revealed that thymol was the most common components of T. vulgaris oil (Lee et al., 2005; Ferreira et al., 2016; Foe et al., 2016; Moghaddaszadeh-Ardebili, 2016; Mohammed et al., 2016; Szczepanik et al., 2012). In contrast, Quesada et al. (2016) used a chemotype of TVE with low odor intensity that did not have any thymol and had a very low content of linalool with the major component of 1,8 cineole. This incompatibility also approved by another study that used T. vulgaris oil of Eastern Morocco (Imelouane et al., 2009). Therefore, the analysis obtained here showed that our purchased TVE got from a chemotype was rich in thymol and durenol.
In a study, Lee et al. (2005) evaluated antioxidant activity of T. vulgaris compounds and found that all chemicals in thyme exhibited dose-dependent inhibitory activity. They found that among the chemicals were identified in the extracts of thyme, the exhibition of potent antioxidant activities was related to thymol, carvacrol (isothymol) and eugenol. It seems that the power of the antioxidant activity of TVE in this study is related to high thymol content of TVE. It is proved that phenolic components like thymol could act as an antioxidant due to high reaction with peroxyl radicals. They deposed of free radicals by hydrogen atom transfer (Amorati et al., 2013). TVE had weaker free radical scavenging capacity compared with BHT. However, BHT is a pure powder without any additional ingredients, while natural essential oils are mixtures of several components, the different types of antioxidants or oxidizable terpenoid components. In fact, the overall performance of thyme oil as antioxidant depending on the complex interplay among components and the oxidizable material, the exact essential oil composition and experimental conditions, and synergistic or antagonistic behavior (Amorati et al., 2013).
It is proved that the effect of essential oils on Gram-positive bacteria is relatively substantial (Hyldgaard et al., 2012). In addition, it is exhibited that phenolic compounds like thymol are able to disintegrate the outer membrane of Gram-negative bacteria and increase the permeability of the cytoplasmic membrane to ATP (Lambert et al., 2001). In accordance with our results, thyme essential oil could inhibit E. coli and S. typhimurium growth by the microdilution method (Boskovic et al., 2015). Also, thyme essential oil efficiently killed Salmonella in suspension and prevented biofilms formation (Miladi et al., 2016). Compared with antibiotics (tetracycline and cephtriaxone), TVE acted weaker in removing or inhibiting of bacteria at low concentrations. In fact, the antibacterial effects of TVE may mostly be related to the thymol content, since in many studies it is proved that this substance could inhibit bacterial growth (Trombetta et al., 2005; Mathela et al., 2010; Wattanasatcha et al., 2012).
This study approved antimicrobial and antioxidant properties of TVE oil in vitro and evaluated this oil as feed additive in quail’s nutrition for improving performance, organs weight, serum lipids, intestinal morphology, microbial population, and lipid oxidation in storage meat. Comparisons were made between TVE and synthetic substances. Based on the results obtained here, the main component of this essential oil was thymol that may be the main reason for advantages of this essential oil. In addition, although some gut health variables were affected by the treatments, the response for animal growth and efficiency was not found. The effect of TVE on performance was dose dependant. So, TVE in high doses may have the potential to improve quail performance by lowering FI. Also, TVE decreased serum triglycerides dose dependently. The decreased TBARS in storage muscle tissue of quail fed with TVE showed that TVE could reduce the rate of oxidation of quail’s muscle tissue during refrigerated storage. TVE could compete with synthetic substances in all aspects in high dose. Therefore, by dosing consideration, TVE is a suitable alternative for synthetic substances especially antibiotic growth promoters without having any adverse effect on quail’s health. However, it should be noted that these compounds are antibiotics with both short- and long-term effects. So, it is possible that antibiotic resistance would increase for these natural compounds along the time.
Conflict of interest statement. None declared.
ACKNOWLEDGMENT
The authors appreciate Veterinary Medicine Department, Shahid Bahonar University of Kerman (Iran) for providing laboratory facilities.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
LITERATURE CITED
- Adams R. P. 2007. Identification of essential oil components by gas chromatography/mass spectroscopy, 4th ed. Carol Stream, IL: Allured Publishing Corporation. [Google Scholar]
- Akbari M., and Torki M.. 2014. Effects of dietary chromium picolinate and peppermint essential oil on growth performance and blood biochemical parameters of broiler chicks reared under heat stress conditions. Int. J. Biometeorol. 58:1383–1391. doi: 10.1007/s00484-013-0740-1 [DOI] [PubMed] [Google Scholar]
- Ali M. N., Hassan M. S., and Abd El-Ghany F. A.. 2007. Effect of strain, type of natural antioxidant and sulphate ion on productive, physiological and hatching performance of native laying hens. Int. J. Poult. Sci. 6(8):539–554. doi: 10.3923/ijps.2007.539.554 [DOI] [Google Scholar]
- Altine S., Sabo M. N., Muhammad N., Abubakar A., Saulawa L. A.. 2016. Basic nutrient requirements of the domestic quails under tropical conditions: a review. World Sci. News. 49(2):223–235. [Google Scholar]
- Amorati R., Foti M. C., and Valgimigli L.. 2013. Antioxidant activity of essential oils. J. Agric. Food Chem. 61:10835–10847. doi: 10.1021/jf403496k [DOI] [PubMed] [Google Scholar]
- Austgulen L. T., Solheim E., and Scheline R. R.. 1987. Metabolism in rats of p-cymene derivatives: carvacrol and thymol. Pharmacol. Toxicol. 61:98–102. doi:10.1111/ j.1600-0773.1987.tb01783.x [DOI] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., and Böhm J.. 2008. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing enterococcus faecium and oligosaccharides. Int. J. Mol. Sci. 9:2205–2216. doi: 10.3390/ijms9112205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurhoo B., Phillip L., and Ruiz-Feria C. A.. 2007. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 86:1070–1078. doi: 10.1093/ps/86.6.1070 [DOI] [PubMed] [Google Scholar]
- Behnia M., Haghighi A., Komeylizadeh H., Tabaei S. J., and Abadi A.. 2008. Inhibitory effects of iranian Thymus vulgaris extracts on in vitro growth of entamoeba histolytica. Korean J. Parasitol. 46:153–156. doi: 10.3347/kjp.2008.46.3.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskovic M., Zdravkovic N., Ivanovic J., Janjic J., Djordjevic J., Starcevic M., and Baltic M. Z.. 2015. Antimicrobial activity of thyme (Thymus vulgaris) and oregano (Origanum vulgare) essential oils against some food-borne microorganisms. Procedia Food Sci. 5:18–21. doi: 10.1016/j.profoo.2015.09.005 [DOI] [Google Scholar]
- Botsoglou N. A., Christaki E., Fletouris D. J., Florou-Paneri P., and Spais A. B.. 2002. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 62:259–265. doi: 10.1016/S0309-1740(01)00256-X [DOI] [PubMed] [Google Scholar]
- Botsoglou N. A., Govaris A., Botsoglou E. N., Grigoropoulou S. H., and Papageorgiou G.. 2003. Antioxidant activity of dietary oregano essential oil and alpha-tocopheryl acetate supplementation in long-term frozen stored turkey meat. J. Agric. Food Chem. 51:2930–2936. doi: 10.1021/jf021034o [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M. E., and Berset C.. 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28:25–30. doi: 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- Brenes A., and Roura E.. 2010. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 158(1 to 2):1–14. doi: 10.1016/j.anifeedsci.2010.03.007 [DOI] [Google Scholar]
- Cetinus E., Temiz T., Ergul M., Altun A., Cetinus S., and Kaya T.. 2013. Thyme essential oil inhibits proliferation of DLD-1 colorectal cancer cells through antioxidant effect. Cumhuriyet Med. J. 35(1):14–24. http://dergipark.gov.tr/223/issue/4224/56199 [Google Scholar]
- Chowdhury S., Mandal G. P., and Patra A. K.. 2018. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilization, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed Sci. Technol. 236:86–97. doi: 10.1016/j.anifeedsci.2017.12.002 [DOI] [Google Scholar]
- Cross D. E., Svoboda K., Mcdevitt R. M., and Acamovic T.. 2003. The performance of chickens fed diets with or without thyme oil and enzymes. Br. Poult. Sci. 44:18–19. doi: 10.1080/713655293 [DOI] [Google Scholar]
- Denli M., Okan F., and Uluocak A. N.. 2004. Effect of dietary supplementation of herb essential oils on the growth performance, carcass and intestinal characteristics of quail (Coturnix coturnix japonica). S. Afr. J. Anim. Sci. 34(3):174–179. http://www.sasas.co.za/Sajas.html [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., and Guo Y.. 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 7:19. doi: 10.1186/s40104-016-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edris A. E. 2007. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother. Res. 21:308–323. doi: 10.1002/ptr.2072 [DOI] [PubMed] [Google Scholar]
- El-Ghousein S. S., and Al-Beitawi N. A.. 2009. The effect of feeding of crushed thyme (Thymus vulgaris L.) on growth, blood constituents, gastrointestinal tract, and carcass characteristics of broiler chickens. J. Poult. Sci. 46(2):100–104. doi: 10.2141/jpsa.46.100 [DOI] [Google Scholar]
- Elson C. E. 1995. Suppression of mevalonate pathway activities by dietary isoprenoids: protective roles in cancer and cardiovascular disease. J. Nutr. 125(6 Suppl):1666S–1672S. doi: 10.1093/jn/125.suppl_6.1666S [DOI] [PubMed] [Google Scholar]
- Fachini-Queiroz F. C., Kummer R., Estevão-Silva C. F., Carvalho M. D., Cunha J. M., Grespan R., Bersani-Amado C. A., and Cuman R. K.. 2012. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. Essential oil, on the inflammatory response. Evid. Based Complement. Alternat. Med. 2012:657026. doi: 10.1155/2012/657026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L. E., Benincasa B. I., Fachin A. L., França S. C., Contini S. S. H. T., Chagas A. C. S., and Beleboni R. O.. 2016. Thymus vulgaris L. essential oil and its main component thymol: anthelmintic effects against haemonchus contortus from sheep. Vet. Parasitol. 228:70–76. doi: 10.1016/j.vetpar.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Foe F. M. C., Nyegue M. A., Kamdem S. L., Essama R. H., and Etoa F.. 2016. Chemical composition, antioxidant effects and antimicrobial activities of some spices’ essential oils on food pathogenic bacteria. Afr. J. Biotechnol. 15(16):649–656. doi: 10.5897/AJB2015.15070 [DOI] [Google Scholar]
- Goldstein J. L., and Brown M. S.. 1990. Regulation of the mevalonate pathway. Nature 343:425–430. doi: 10.1038/343425a0 [DOI] [PubMed] [Google Scholar]
- Hammer K. A., Carson C. F., and Riley T. V.. 1999. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 86:985–990.doi: 10.1046/j.1365-2672.1999.00780.x [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., and Khaksar V.. 2013a. Effects of carboxy methyl cellulose and thymol + carvacrol on performance, digesta viscosity and some blood metabolites of broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 98:672–679. doi: 10.1111/jpn.12121 [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Raji A., and Van Krimpen M. M.. 2013b. Effect of thymol+ carvacrol by next enhance 150® on intestinal development of broiler chickens fed CMC containing diet. Iran J. Appl. Anim. Sci. 3:567–576. www.ijas.ir [Google Scholar]
- Helander I. M., Alakomi H. L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E. J., Gorris L. G. M., and VonWright A.. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 46:3590–3595. doi: 10.1021/jf980154m [DOI] [Google Scholar]
- Hernández F., Madrid J., García V., Orengo J., and Megías M. D.. 2004. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 83:169–174. doi: 10.1093/ps/83.2.169 [DOI] [PubMed] [Google Scholar]
- Hoffman-Pennesi D., and Wu C.. 2010. The effect of thymol and thyme oil feed supplementation on growth performance, serum antioxidant levels, and cecal Salmonella population in broilers. J. Appl. Poult. Res. 19(4):432–443. doi: 10.3382/japr.2009-00141 [DOI] [Google Scholar]
- Huange B., Jingesheng H., Xiaoquan B., Hong Z., Xincheng Y., and Youwei W.. 2011. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 87:46–53. doi: 10.1016/j.meatsci.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Huss D., Poynter G., and Lansford R.. 2008. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab anim. 37(11):513. doi: 10.1038/laban1108-513 [DOI] [PubMed] [Google Scholar]
- Hyldgaard M., Mygind T., and Meyer R. L.. 2012. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 3:12. doi: 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imelouane B., Amhamdi H., Wathelet J. P., Ankit M., Khedid K., and El Bachiri A.. 2009. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int. J. Agric. Biol. 11(2):205–208. [Google Scholar]
- Jang I. S., Ko Y. H., Kang S. Y., and Lee C. Y.. 2007. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 134(3–4): 304–315. doi: 10.1016/j.anifeedsci.2006.06.009 [DOI] [Google Scholar]
- Jeke A., Phiri C., Chitindingu K., Taru P.. 2018. Ethnomedicinal use and pharmacological potential of Japanese quail (Coturnix coturnix japonica) bird’s meat and eggs, and its potential implications on wild quail conservation in Zimbabwe: a review. Cogent. Food Agric. 4(1):1–12. doi: 10.1080/23311932.2018.1507305 [DOI] [Google Scholar]
- Jorgensen J. H., and Turnidge J. D.. 2015. Susceptibility test methods: dilution and disk diffusion methods. In: Manual of clinical microbiology., 11th ed. American Society of Microbiology, pp. 1253–1273. doi:10.1128/9781555817381 [Google Scholar]
- Khaksar V., Van Krimpen M., Hashemipour H., and Pilevar M.. 2012. Effects of thyme essential oil on performance, some blood parameters and ileal microflora of Japanese quail. J. Poult. Sci. 49(2):106–110. doi: 10.2141/jpsa.011089 [DOI] [Google Scholar]
- Khattak F. A., Ronchi P., Castelli N., Sparks 2013. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 93:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R. J., Skandamis P. N., Coote P. J., and Nychas G. J.. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91:453–462. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Umano K., Shibamoto T., and Lee K. G.. 2005. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 91(1):131–137. doi: 10.1016/j.foodchem.2004.05.056 [DOI] [Google Scholar]
- Li Y. L. 1991. Culture medium manual. Changchun, China: Jilin Science and Technology Press. [Google Scholar]
- Liu S., Song M., Yun W., Lee J., Lee C. Kwak W. Han N. Kim H., and Cho J.. 2018. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. (Berl.). 102:1257–1265. doi: 10.1111/jpn.12944 [DOI] [PubMed] [Google Scholar]
- Luna A., Lábaque M. C., Zygadlo J. A., and Marin R. H.. 2010. Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult. Sci. 89:366–370. doi: 10.3382/ps.2009-00130 [DOI] [PubMed] [Google Scholar]
- Manafi M., Hedayati M., and Khalaji S.. 2016. Effectiveness of phytogenic feed additive as alternative to bacitracin methylene disalicylate on hematological parameters, intestinal histomorphology and microbial population and production performance of japanese quails. Asian-Australas. J. Anim. Sci. 29:1300–1308. doi: 10.5713/ajas.16.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathela C. S., Singh K. K., and Gupta V. K.. 2010. Synthesis and in vitro antibacterial activity of thymol and carvacrol derivatives. Acta Pol. Pharm. 67:375–380. [PubMed] [Google Scholar]
- Mehdipour Z., Afsharmanesh M., and Sami M.. 2014. Effects of supplemental thyme extract (Thymus vulgaris L.) on growth performance, intestinal microbial populations, and meat quality in Japanese quails. Comp. Clin. Pathol. 23(5):1503–1508. doi: 10.1007/s00580-013-1813-6 [DOI] [Google Scholar]
- Mehri M., Sabaghi V., and Bagherzadeh-Kasmani F.. 2015. Mentha piperita (peppermint) in growing Japanese quails’ diet: serum biochemistry, meat quality, humoral immunity. Anim. Feed Sci. Technol. 206:57–66. doi: 10.1016/j.anifeedsci.2015.05.022 [DOI] [Google Scholar]
- Miladi H., Mili D., Ben Slama R., Zouari S., Ammar E., and Bakhrouf A.. 2016. Antibiofilm formation and anti-adhesive property of three mediterranean essential oils against a foodborne pathogen salmonella strain. Microb. Pathog. 93:22–31. doi: 10.1016/j.micpath.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Moghaddaszadeh-Ardebili S. 2016. The communication between chemical composition and supportive effects of Thymus vulgaris on immune system. Ann. Res. Antioxid. 1(2):e21. [Google Scholar]
- Mohammed Z., Akhtar M. S., Said S., Weli A. M., Al-Sabahi J. N., Al-Riyami Q., and AlAbri A. A.. 2016. Composition of essential oil of Thymus vulgaris grown in Oman. J. Essent. Oil-Bear Plants. 19(2):475–478. doi: 10.1080/0972060X.2014.977569 [DOI] [Google Scholar]
- Mohiti-Asli M., and Ghanaatparast-Rashti M.. 2018. Comparing the effects of a combined phytogenic feed additive with an individual essential oil of oregano on intestinal morphology and microflora in broilers. J. Appl. Anim. Res. 46(1):184–189. doi: 10.1080/09712119.2017.1284074 [DOI] [Google Scholar]
- Mousavi A., Mahdavi A. H., Riasi A., and Soltani-Ghombavani M.. 2018. Efficacy of essential oils combination on performance, ileal bacterial counts, intestinal histology and immunocompetence of laying hens fed alternative lipid sources. J. Anim. Physiol. Anim. Nutr. (Berl.). 102:1245–1256. doi: 10.1111/jpn.12942. [DOI] [PubMed] [Google Scholar]
- Nabavi S. M., Marchese A., Izadi M., Curti V., Daglia M., and Nabavi S. F.. 2015. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 173:339–347. doi: 10.1016/j.foodchem.2014.10.042 [DOI] [PubMed] [Google Scholar]
- Oceľová V., Chizzola R., Battelli G., Pisarcikova J., Faix S., Gai F., and Placha I.. 2018. Thymol in the intestinal tract of broiler chickens after sustained administration of thyme essential oil in feed. J. Anim. Physiol. Anim. Nutr. 103(1):204–209. doi: 10.1111/jpn.12995 [DOI] [PubMed] [Google Scholar]
- Pina‐Vaz C., Goncalves Rodrigues A., Pinto E., Costa‐de‐Oliveira S., Tavares C., Salgueiro L., and Martinez‐de‐Oliveira J.. 2004. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 18(1):73–78. doi:10.1111/j.1468-3083.2004.00886.x [DOI] [PubMed] [Google Scholar]
- Quesada J., Sendra E., Navarro C., and Sayas-Barbera E.. 2016. Antimicrobial active packaging including chitosan films with Thymus vulgaris L. essential oil for ready-to-eat meat. Foods. 5(3):57. doi: 10.3390/foods5030057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H. S., Albino L. F. T., Donzele J. L., Gomes P. C., Oliveira R. T., Lopes D. C., and Euclides R. F.. 2011. Brazilian tables for poultry and swine. Composition of feedstuffs and nutritional requirements, 3rd ed. Brazil: UFV Viçosa. [Google Scholar]
- Roura E., Baldwin M. W., and Klasing K. C.. 2013. The avian taste system: potential implications in poultry nutrition. Anim. Feed Sci. Technol. 180(1–4):1–9. doi: 10.1016/j.anifeedsci.2012.11.001 [DOI] [Google Scholar]
- Sariçoban C., and Yilmaz M. T.. 2014. Effect of thyme/cumin essential oils and butylated hydroxyl anisole/butylated hydroxyl toluene on physicochemical properties and oxidative/microbial stability of chicken patties. Poult. Sci. 93:456–463. doi: 10.3382/ps.2013-03477 [DOI] [PubMed] [Google Scholar]
- SAS 1999. SAS statistics user’s guide. Statistical analytical system, 5th Rev. ed. Cary, NC: SAS Institute Inc. [Google Scholar]
- Sepehri Moghadam H., Emadi M., and Molashahi E.. 2014. Effect of the essential oil of thyme on intestinal morphology in Rat. J. Herbal Drugs (JHD). 5(1):39–43. http://www.jhd.iaushk.ac.ir [Google Scholar]
- Stahl-Biskup E., and Saez F.. 2004. Thyme. Handbook of herbs and spices, vol. 2Cambridge, England: CRC Press, Wood head Publishing Limited. [Google Scholar]
- Subbarao K. V., Richardson J. S., and Ang L. C.. 1990. Autopsy samples of alzheimer’s cortex show increased peroxidation in vitro. J. Neurochem. 55:342–345. doi:10.1111/j.1471-4159.1990.tb08858.x [DOI] [PubMed] [Google Scholar]
- Szczepanik M., Zawitowska B., and Szumny A.. 2012. Insecticidal activities of Thymus vulgaris essential oil and its components (thymol and carvacrol) against larvae of lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopathy. 30(1):129–142. [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M. G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., and Bisignano G.. 2005. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentowska J., and Flaczyk J. F.. 2013. Thyme essential oil for antimicrobial protection of natural textiles. Int. Biodeterior. Biodegr. 84:407–411. [Google Scholar]
- Wattanasatcha A., Rengpipat S., and Wanichwecharungruang S.. 2012. Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 434:360–365. doi: 10.1016/j.ijpharm.2012.06.017 [DOI] [PubMed] [Google Scholar]
- Yanishlieva N. V., Marinova E. M., Gordon M. H., and Raneva V. G.. 1999. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 64:59–66. doi:10.1016/S0308-8146(98)00086-7 [Google Scholar]
- Yu S. G., Abuirmeileh N. M., Qureshi A. A., Elson C. E.. 1994. Dietary beta ionone suppresses hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. J. Agric. Food Chem. 42:1493–1496. doi:10.1021/jf00043a019 [Google Scholar]