Abstract
The present study used Escherichia coli lipopolysaccharide (LPS) to investigate whether maternal immune challenge during late gestation altered programming of the offspring hypothalamus and hypothalamic–pituitary–adrenal axis (HPAA). In addition, interactions of maternal diet, supplementation with fish oil (FO) or microalgae (AL), and complex vs. simple weaning diets were investigated. Briefly, Landrace × Yorkshire sows (N = 48) were randomly assigned to diets supplemented with FO, AL, or a standard gestation control diet (CON) from day 75 of gestation (gd 75) until parturition. On gd 112, half the sows from each dietary treatment were immune challenged with LPS (10 μg/kg BW) or saline as a control. At 21 d postpartum, the offspring were weaned, and half the animals from each maternal treatment were allocated to either a complex or simple weaning diet. At 28 d postpartum, the offspring’s hourly fever and 2-h cortisol responses to LPS immune challenge (40 μg/kg BW) were measured to assess hypothalamus and HPAA function. Results indicated that the maternal temperature of sows on the FO diet returned to baseline levels faster than sows on the AL and CON diets after LPS immune challenge (P < 0.05). In contrast, there was no difference in the maternal cortisol response across the dietary treatments (P > 0.10). Regardless of the dietary treatments, the maternal LPS immune challenge induced a greater cortisol response in male offspring (P = 0.05) and a greater fever response in female offspring (P = 0.03) when they were LPS immune challenged post-weaning. Male offspring from LPS-immune-challenged sows fed the FO and AL diets had a greater fever response than male offspring from the maternal CON diet group (P ≤ 0.05). Last, no effect of the complex or simple weaning diets was observed for the nursery pig cortisol or fever responses to LPS immune challenge. In conclusion, LPS immune challenge during late pregnancy altered responsiveness of the offspring hypothalamus and HPAA to this same microbial stressor, and a sex-specific response was influenced by maternal dietary supplementation with FO and AL.
Keywords: fish oil, hypothalamic–pituitary–adrenal axis, lipopolysaccharide, microalgae, nursery pig, weaning diet complexity
INTRODUCTION
Bacterial infection occurring during pregnancy can impair the normal development of the offspring (Veru et al., 2014; Wankhade et al., 2016). For swine, this can lead to welfare issues and lead to economic losses for producers. Bacterial lipopolysaccharide (LPS) endotoxin has been used to model infection in many animal species (Gayle et al., 2004; Fisher et al., 2010; Lay et al., 2011). The inflammatory cytokines secreted during LPS immune challenge provide a stress signal that activates the maternal hypothalamic–pituitary–adrenal axis (HPAA), resulting in increased maternal cortisol production. Maternal cortisol may alter the programming of the developing fetal HPAA, increasing offspring susceptibility to disease in later life (Karrow, 2006; French et al., 2013; Zijlmans et al., 2017). As omega-3 polyunsaturated fatty acids (n-3 PUFA) have potent anti-inflammatory properties (Luo et al., 2013), their inclusion in maternal diets may help protect the offspring from this type of maternal stress (Fisher et al., 2014).
Traditionally, producers have used complex diets, containing multiple animal-based or highly purified protein sources, to support the health of newly weaned pigs. However, a study found that a less costly simple diet did not negatively affect the growth of pigs (Skinner et al., 2014), although based on their immune status, animals appeared to be stressed (Levesque et al., 2013). The effect of simple vs. complex diets on HPAA function is unknown, and maternal supplementation with n-3 PUFA during pregnancy may help support the health of weaned pigs fed a simple diet. Therefore, this study used an LPS immune challenge to stress sows and offspring to test the hypotheses: 1) maternal LPS immune challenge alters programming of the prenatal HPAA, and maternal supplementation with fish oil (FO) or microalgae (AL) sources of n-3 PUFA helps protect against this, and 2) HPAA function is influenced by nursery diet quality, and maternal supplementation with FO and AL during late gestation may provide anti-inflammatory effects.
MATERIALS AND METHODS
This study was conducted between January and September 2016 at the University of Guelph Arkell Swine Research Station under the Animal Utilization Protocol #2492, which was approved by the University of Guelph animal care committee.
Sow Experimental Procedure
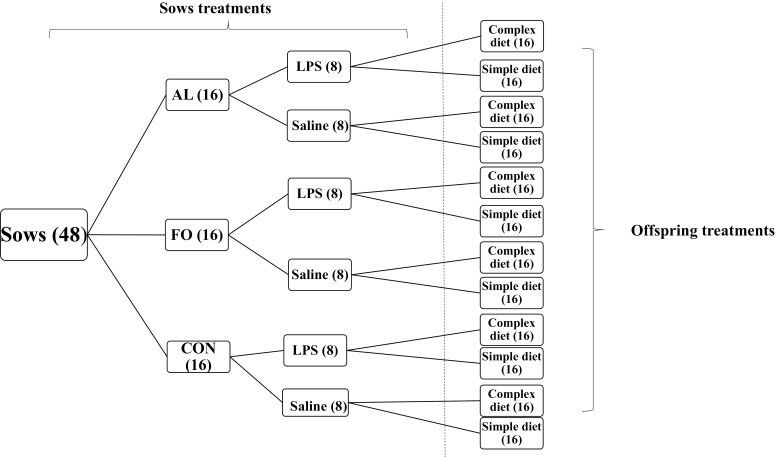
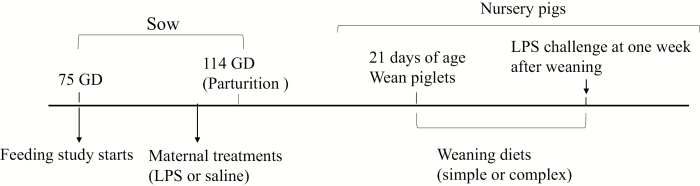
The sow-feeding trial was conducted as a block design, composed of 4 blocks in total with 12 sows (Landrace × Yorkshire) per block. Pregnant sows (parity 2 to 5) were selected based on breeding date, which allowed them to farrow in the same week. Sows were randomly allocated to 1 of 3 dietary treatment groups: 3.12% AL (Alltech Inc., Nicholasville, KY; n = 16), 3.1% FO (Grand Valley Frontier, Cambridge, ON, Canada; n = 16), or a typical gestation diet containing corn oil as a source of fat (CON; n = 16, Fig. 1). The AL and FO diets were matched according to docosahexaenoic acid (DHA) levels (Table 1), which was determined by a pilot study. Blood samples were collected from the retro-orbital sinus of sows at gestation d (gd) 74 (approximate gestation period is 114 d) for plasma fatty acids analysis. Sows were fed a daily ration of 2.5 kg of gestation diet from gd 75 until farrowing (Fig. 2). Experimental diets were formulated to meet the estimated nutrient requirements for sows in late gestation (NRC, 2012), and daily feed allowance was calculated based on metabolizable energy of experimental diets. Timing of dietary supplementation was chosen to ensure maternal n-3 PUFA enrichment, while also minimizing feed costs. After farrowing, the sows were fed a common diet to meet the estimated nutrient requirements for sows in lactation (NRC, 2012). Animals were fed in the morning, and water was available ad libitum. On gd 111, sows were transferred from gestation crates to farrowing crates and monitored for signs of farrowing. Because pigs follow a diurnal cortisol circadian response with relatively consistent cortisol levels around 0800 to 1100 h (Kranendonk et al., 2006a,b), blood samples were collected within this period on gd 111 to measure basal serum cortisol concentration and plasma n-3 PUFA enrichment; blood was collected in serum collection tubes (BD Vacutainer, Mississauga, ON, Canada) and plasma collection tubes containing sodium heparin 95 USP units (BD Vacutainer). On gd 112 at 0800 h, 2 sows per dietary treatment per block were injected i.m. with 10 μg/kg BW of LPS (Escherichia coli, serotype O55:B5, Sigma–Aldrich Co., St. Louis, MO) dissolved in 2 mL of sterile saline (0.9% NaCl solution, Vétoquinol N.-A. Inc., Princeville, QC, Canada), and the other 2 sows per dietary treatment per block were injected i.m. with 2 mL of sterile saline. The LPS challenge was performed in late gestation due to the rapid development of the HPAA at this time (Kanitz et al., 2006). The rectal temperature of each sow was monitored hourly over 6 h post-LPS immune challenge using a digital thermometer to assess the hypothalamic fever response. Blood samples (10 mL) were collected 2 h post-challenge in serum collection tubes, which was the peak cortisol response determined by Lee et al. (2019). Blood samples were allowed to clot for approximately 1 h. All blood samples were centrifuged at 1,000 × g for 25 min at room temperature. Plasma and serum were aliquoted into 2-mL vials and stored at −80 °C until further analysis could be conducted.
Figure 1.
Experimental design for sows fed diets containing microalgae (AM), fish oil (FO), or a control diet (CON) and challenged with lipopolysaccharide (LPS) or saline as a control, and offspring fed complex or simple nursery diets. Sample size is indicated in the parentheses.
Table 1.
Ingredient composition of experimental sow diets
| AL1 | FO2 | CO3 | |
|---|---|---|---|
| Ingredient composition, % | |||
| Corn (NRC; 8.3% CP) | 74.28 | 74.06 | 77.06 |
| Corn oil | 1.55 | 1.79 | 1.89 |
| Fish oil4 | - | 3.10 | - |
| Algae5 | 3.12 | - | - |
| Soybean meal | 17.38 | 17.38 | 17.38 |
| l-Lysine | 0.07 | 0.07 | 0.07 |
| Salt | 0.40 | 0.40 | 0.40 |
| Limestone | 1.52 | 1.52 | 1.52 |
| Mono-cal phosphate | 1.17 | 1.17 | 1.17 |
| Vitamin/mineral premix6 | 0.50 | 0.50 | 0.50 |
| Vitamin E4 | 0.02 | 0.02 | 0.02 |
| Calculated nutrient content7 | |||
| ME, kcal/kg | 3097 | 3153 | 3090 |
| CP, % | 14.48 | 14.61 | 14.46 |
| SID8 lysine, % | 0.64 | 0.65 | 0.64 |
| SID methionine + cysteine, % | 0.43 | 0.44 | 0.43 |
| SID threonine, % | 0.45 | 0.46 | 0.45 |
| SID tryptophan, % | 0.13 | 0.13 | 0.13 |
| Analyzed nutrient content | |||
| DM, % | 86.98 | 88.17 | 87.53 |
| CP, % | 14.34 | 15.03 | 14.44 |
| Phosphorus, % | 0.57 | 0.58 | 0.54 |
| Sodium, % | 0.18 | 0.17 | 0.17 |
| Calcium, % | 0.81 | 0.78 | 0.88 |
| Potassium, % | 0.65 | 0.67 | 0.65 |
| Magnesium, % | 0.14 | 0.14 | 0.13 |
1AL = microalgae.
2FO = fish oil.
3CO = 1.89% corn oil control.
4Fish oil and Vitamin E provided by Grand Valley Fortifiers (Cambridge, ON, CA).
5Algae provided by Alltech Inc. (Nicholasville, KY) and supplied as dried biomass containing 15.8% CP, 70% CF, and 17% DHA.
6Supplied per kg of diet: vitamin A, 12,000 IU as retinyl acetate; vitamin D3, 1,200 IU as cholecalciferol; vitamin E, 48 IU as dl-α-tocopherol acetate; vitamin K, 3 mg as menadione; vitamin B12, 0.03 mg; pantothenic acid, 18 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2.4 mg; niacin, 30 mg; thiamin, 18 mg; pyridoxine, 1.8 mg; biotin, 200 µg; Cu, 18 mg as CuSO4·5H2O; Fe, 120 mg as FeSO4; Mn, 24 mg as MnSO4; Zn, 126 mg as ZnO; Se, 0.36 mg as FeSeO3; I, 0.6 mg as KI;DSM.
7Calculated on the basis of the NRC (2012) ingredient values.
8SID = standardized ileal digestible.
Figure 2.
Experimental design and timeline of the sow and nursery pig feeding trials. GD = gestation day; LPS = lipopolysaccharide.
Offspring Experimental Procedure
Forty-eight nursery pigs (Landrace × Yorkshire × Duroc) were selected from sows within each block (Fig. 1); 4 nursery pigs (2 females and 2 castrated males) were randomly selected from each sow. The nursery pigs were weaned at 21 d postpartum and were randomly allocated to 1 of 2 weaning diets (Fig. 1); complex or simple. These 2 diets were formulated to meet the estimated nutrient requirements for nursery pigs (NRC, 2012; Table 2); feed and water were provided ad libitum.
Table 2.
Ingredient formulation of experimental nursery pig diets
| Diet | Simple | Complex |
|---|---|---|
| Ingredient composition, % | ||
| Corn (NRC; 8.3% CP) | 45.28 | 32.57 |
| Wheat | 15.00 | 15.00 |
| Fat, animal/vegetable blend | 5.00 | 5.00 |
| Soybean meal | 30.00 | 10.00 |
| Soybean protein isolate | — | 9.30 |
| Whey | — | 20.00 |
| Blood plasma | — | 4.50 |
| l-Lysine | 0.37 | — |
| l-Methionine | 0.15 | 0.13 |
| l-Tryptophan | 0.02 | — |
| l-Threonine | 0.24 | 0.05 |
| Salt | 0.50 | — |
| Limestone | 1.30 | 0.89 |
| Monocalcium phosphate | 1.52 | 1.94 |
| Mineral/vitamin premix1 | 0.60 | 0.60 |
| Vitamin E2 | 0.02 | 0.02 |
| Calculated nutrient content3 | ||
| ME, kcal/kg | 3471 | 3552 |
| CP, % | 20.85 | 23.40 |
| SID4 lysine, % | 1.21 | 1.25 |
| SID Met + Cys, % | 1.20 | 0.79 |
| SID threonine, % | 0.48 | 0.86 |
| SID tryptophan, % | 0.74 | 0.29 |
| Analyzed nutrient content, % | ||
| DM | 87.08 | 88.05 |
| CP | 21.18 | 20.41 |
| Phosphorus | 0.63 | 0.74 |
| Calcium | 0.87 | 0.85 |
| Sodium | 0.18 | 0.31 |
| Potassium | 0.89 | 0.81 |
| Magnesium | 0.17 | 0.14 |
1Supplied per kg of diet: vitamin A, 12,000 IU as retinyl acetate; vitamin D3, 1,200 IU as cholecalciferol; vitamin E, 48 IU as dl-α-tocopherol acetate; vitamin K, 3 mg as menadione; vitamin B12, 0.03 mg; pantothenic acid, 18 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2.4 mg; niacin, 30 mg; thiamin, 18 mg; pyridoxine, 1.8 mg; biotin, 200 µg; Cu, 18 mg as CuSO4·5H2O; Fe, 120 mg as FeSO4; Mn, 24 mg as MnSO4; Zn, 126 mg as ZnO; Se, 0.36 mg as FeSeO3; I, 0.6 mg as KI;DSM.
2Vitamin E provided by Grand Valley Fortifiers (Cambridge, ON, CA).
3Calculated on the basis of the NRC (2012) ingredient values.
4SID = standardized ileal digestible.
At 27 d postpartum, blood samples (10 mL) were collected from the nursery pigs between 0800 and 1000 h before LPS challenge for measurement of basal serum cortisol and plasma PUFA concentrations. At 28 d postpartum, between 0800 and 1000 h, 48 pigs were injected i.m. with 40 μg/kg BW of LPS. Rectal temperature was monitored hourly for 2 h post-LPS immune challenge to assess the hypothalamic fever response. Blood samples were collected before and 2 h post-LPS challenge, processed as described earlier, and plasma and serum were aliquoted into 2-mL vials and stored at −80 °C for fatty acid and cortisol analyses (Table 3). After the 2-h blood collection, nursery pigs were euthanized using 0.3 mL/kg pentobarbital administered by cardiac puncture (Table 3).
Table 3.
DHA, EPA, total n-3, and ratio of n-6/n-3 in sow diets supplemented with AL, FO, or CON
| Maternal diet | |||
|---|---|---|---|
| Fatty acids | AL1 | FO2 | CON3 |
| DHA4, mg/g | 3.76 | 3.16 | 0.03 |
| EPA5, mg/g | 0.19 | 3.49 | 0.08 |
| Total n-36, mg/g | 5.43 | 10.21 | 1.70 |
| n-6/n-37 | 4.76 | 2.70 | 14.29 |
1AL = microalgae.
2FO = fish oil.
3CON = control.
4DHA = docosahexaenoic acid.
5EPA = eicosapentaenoic acid.
6Total n-3 = total omega-3 polyunsaturated fatty acids.
7n-6/n-3 = ratio of omega-6 to omega-3 polyunsaturated fatty acids.
Cortisol Enzyme Immunoassay
Cortisol analysis was conducted by ELISA for serum samples obtained before and 2 h post-LPS immune challenge according to the manufacturer’s instructions (K003-H1W, Arbor Assays, Ann Arbor, MI). All samples were run in triplicate, and a standard curve was included on each plate. The interassay CV was 6.6%.
PUFA Analysis
The plasma samples of sows and nursery pigs were sent to the Lipid Analytic Laboratories (Guelph, ON, Canada) for PUFA analysis. Total lipids were extracted from 200 μL of sample using the Folch method (Folch et al., 1956). The serum phospholipid fraction was separated from the neutral lipids by thin-layer chromatography. The fatty acid methyl esters were prepared from the isolated phospholipid fraction and analyzed on a Varian 3400 gas–liquid chromatograph (Palo Alto, CA) with a 60-m DB-23 capillary column (0.32 mm internal diameter, FID detection). Fatty acid standards and mixtures thereof (Nu Chek Prep, Elysian, MN) were used to ensure quantitative and qualitative accuracy and recovery.
Statistical Analysis
Statistical analysis was conducted using PROC GLIMMIX of SAS version 9.4. The sow and nursery pig studies were arranged as a completely randomized blocked design (Fig. 1). Sow fever response was analyzed using a repeated-measures analysis, and the statistical model included the fixed effects of maternal diet (AL, FO, or CON), maternal LPS status (LPS or saline) and time, as well as their interactions, and included block as a random variable. The repeated-measures analysis used an ar(1) covariance structure. Sow cortisol response was analyzed using multiple means comparisons; the statistical model included the fixed effects of maternal gestation diet and maternal LPS status as well as their interactions, included the random effect of block, and used basal cortisol concentrations as a covariate. Nursery pig fever response as analyzed using a multiple means comparison and included the fixed effects of maternal diet, maternal LPS status, offspring nursery diet (simple or complex) and sex (m or f), time, and their interactions, and included the random effects of block, litter, and pen. Nursery pig cortisol response was analyzed using a multiple means comparison. The statistical model included the fixed effects of maternal diet, maternal LPS status, nursery diet and sex, as well as their interactions, and included the random effects of block, litter, and pen and used basal cortisol as a covariate. Multiple means comparison was also used to analyze data from fatty acid analysis using the fixed effect of maternal diet. Where fixed effects were not found to be significant, they were subsequently removed from the statistical model. A Tukey–Kramer adjustment was used for all analyses with 2 or more comparisons. Significant differences were reported where P ≤ 0.05, and trends were reported where 0.05 < P < 0.10.
Prior to statistical analyses, initial Pearson correlation coefficients were determined to investigate correlations among litter size, birth weight, and survival rate with serum cortisol levels and temperature. This analysis revealed that neither LPS nor maternal dietary treatment influenced litter size, birth weight, and survival rate compared with control groups. Therefore, these parameters were not included as covariates in the formal statistical analyses. In addition, no effects of maternal diet or maternal LPS status were observed for sow reproductive traits including gestation length, litter size, number of stillborn piglets, litter weight at birth, number of piglets weaned, and average daily gain during lactation.
RESULTS
PUFA Analysis
Total n-3 PUFA, DHA, and eicosapentaenoic acid (EPA) concentrations were enriched in the plasma of sows fed either FO or AL during the feeding period (Table 4). The total plasma n-3 PUFA levels of sows from the FO treatment were greater than those from sows in the AL treatment, and total plasma n-3 PUFA levels of AL sows were greater than CON sows (P < 0.05). Sow plasma DHA levels were significantly different across the diets, even though the DHA concentration was matched between AL and FO diets. The plasma DHA was greatest in the AL sows, intermediate in the FO sows, and lowest in the CON sows (P < 0.01). Plasma EPA levels in the FO sows were greater than sows from the AL and CON dietary treatments (P < 0.01), but there was no difference between the AL and CON diet sows (P > 0.10). The highest-to-lowest plasma n-6:n-3 PUFA ratio was found to be CON, AL, and then FO sows, respectively (P < 0.05).
Table 4.
DHA, EPA, total n-3, and ratio of n-6/n-3 in plasma from sows fed diets supplemented with AL, FO, or CON
| Maternal diet (n = 4 per treatment) | SEM1 | P-value2 | |||
|---|---|---|---|---|---|
| Fatty acids | AL3 | FO4 | CON5 | ||
| DHA6, mg/100 mL | 3.94a | 2.93b | Not detected | 0.11 | <0.0001 |
| EPA7, mg/100 mL | 0.94b | 3.00a | 0.16b | 0.32 | <0.0001 |
| Total n-38, mg/100 mL | 6.96b | 12.46a | 2.12c | 0.43 | <0.0001 |
| n-6/n-39 | 6.12b | 3.00c | 20.24a | 0.79 | <0.0001 |
a–cDiffering letters across rows indicate significant differences among treatments (P < 0.05).
1SEM = maximum value of the standard error of the means.
2 P-value for the main effect of dietary treatment.
3AL = microalgae.
4FO = fish oil.
5CON = control.
6DHA = docosahexaenoic acid.
7EPA = eicosapentaenoic acid.
8Total n-3 = total omega-3 polyunsaturated fatty acids.
9n-3/n-6 = ratio of total omega-3 polyunsaturated fatty acid to total omega-6 polyunsaturated fatty acids.
At 27 d postpartum, PUFAs remained enriched in the nursery pigs’ plasma (Table 5). Total plasma n-3 PUFA levels of nursery pigs from the maternal CON treatment were lower than nursery pigs from the maternal AL and FO treatments (P < 0.05); however, there was no difference between nursery pigs from the maternal AL and FO treatments (P > 0.10). The plasma n-6:n-3 PUFA ratio of both AL and FO nursery pigs was lower than the CON nursery pigs (P < 0.05), and there was no difference between nursery pigs from the maternal AL and FO treatments (P > 0.10). Plasma DHA levels in nursery pigs from the maternal AL and FO treatments were greater than levels in the CON nursery pigs (P < 0.05); again, no differences between nursery pigs from the maternal AL and FO diets were observed (P > 0.10). Plasma EPA levels in nursery pigs were not different across the maternal dietary treatments (P > 0.10). Lastly, there were no significant effects of sex or maternal LPS status shown for PUFA concentration in the nursery pigs’ plasma.
Table 5.
DHA, EPA, total n-3, and ratio of n-6/n-3 in plasma from sows fed diets supplemented with AL, FO, or CON
| Maternal diets (n = 16 per treatment) | SEM1 | P-value2 | |||
|---|---|---|---|---|---|
| Fatty acids | AL3 | FO4 | CON5 | ||
| DHA6, mg/100 mL | 4.93a | 4.93a | 2.73b | 0.46 | 0.01 |
| EPA7, mg/100 mL | 0.73 | 0.54 | 0.34 | 0.15 | 0.17 |
| Total n-38, mg/100 mL | 8.08a | 8.10a | 5.34b | 0.61 | 0.002 |
| n-6/n-39 | 7.21b | 6.50b | 9.74a | 0.73 | 0.0003 |
a,bDiffering letters across rows indicate significant differences among treatments (P < 0.05).
1SEM = maximum value of the standard error of the means.
2 P-value for the main effect of dietary treatment.
3AL = microalgae.
4FO = fish oil.
5CON = control.
6DHA = docosahexaenoic acid.
7EPA = eicosapentaenoic acid.
8Total n-3 = total omega-3 polyunsaturated fatty acids.
9n-3/n-6 = ratio of total omega-3 polyunsaturated fatty acid to total omega-6 polyunsaturated fatty acids.
Fever Response to LPS Immune Challenge
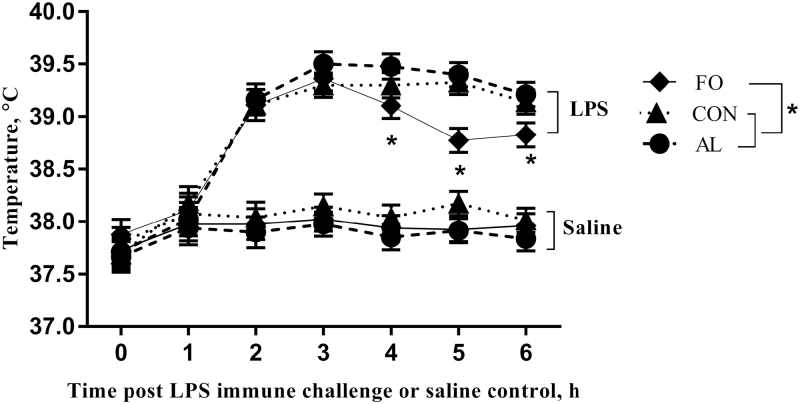
The rectal temperature of LPS immune challenged sows increased over time compared with the saline control sows (P < 0.05, Fig. 3). Sow temperatures increased 1 h post-LPS immune challenge and reached a peak around 3 h post-LPS immune challenge. At 4 h post-LPS immune challenge, the temperature of LPS immune challenged sows fed the FO diet was lower than the sows fed the AL (P = 0.03), and at 5 h post-LPS immune challenge, it was lower than the sows fed either the AL (P < 0.01) or CON diets (P < 0.01). Also, at 6 h post-LPS immune challenge, the temperature of the LPS immune challenged sows fed the FO diet remained lower than the sows fed either the AL diet (P = 0.02) or CON diets (P = 0.05).
Figure 3.
The rectal temperature response of sows fed diets supplemented with microalgae (AL), fish oil (FO), or a control diet (CON) and immune challenged with either 10 µg/kg BW lipopolysaccharide (LPS), i.m., or saline as a control on gestation day 112. Results presented as LSM ± SEM, n = 8 per treatment. Significant differences (P < 0.05) are denoted with an asterisk.
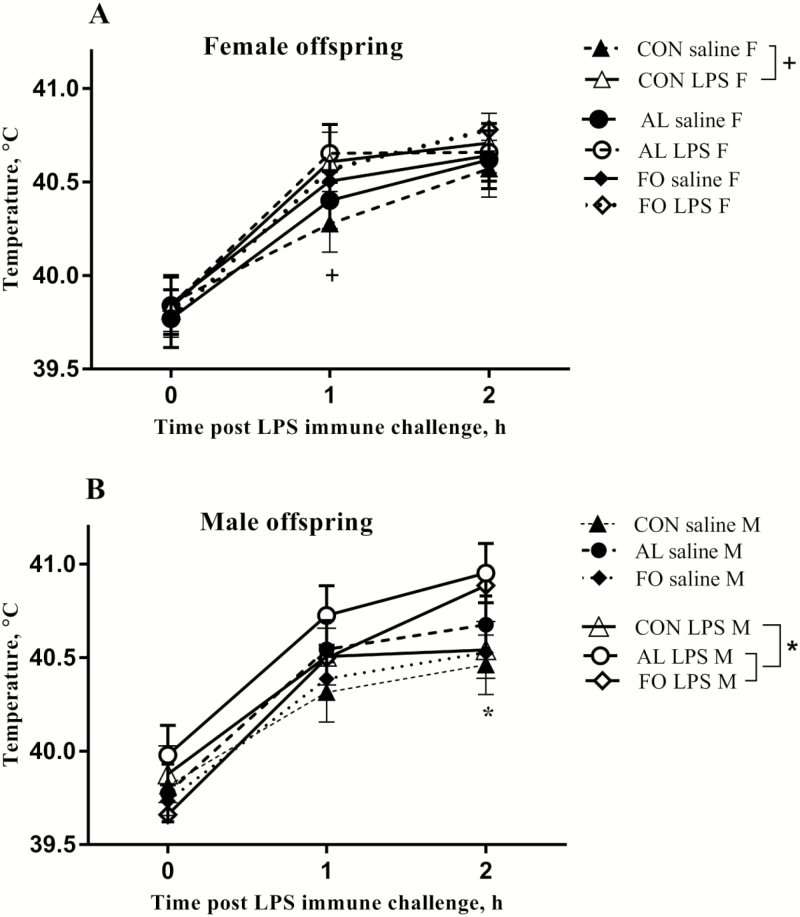
The rectal temperature of all LPS immune challenged nursery pigs increased over time (P < 0.05, Fig. 4), and a significant maternal LPS × sex interaction was found for the fever response of nursery pigs 1 h post-LPS immune challenge. Regardless of the maternal dietary treatment, the rectal temperature of female nursery pigs from sows that were LPS immune challenged was greater than that of female nursery pigs from sows that received saline (P = 0.03). Significant interactions among maternal LPS × maternal diets × sex were also found; at 1 h post-LPS immune challenge, the temperature of female nursery pigs from sows fed the CON diet that were LPS immune challenged was slightly greater than female nursery pigs from sows feed the CON diet that received the saline control (P = 0.07, Fig. 4A). A similar effect of LPS was not observed in female offspring from either the FO or AL treatments. In the male offspring, before LPS immune challenge, the temperature of FO male nursery pigs from sows that were previously LPS immune challenged was slightly lower than both the AL male (P = 0.08, Fig. 4B) and CON male nursery pigs (P = 0.07, Fig. 4B) from sows that were also LPS immune challenged. The rectal temperatures from male offspring from sows fed the CON diet that received LPS were lower than the temperatures of pigs from sows fed FO (P = 0.05) and AL (P = 0.02) that were also LPS challenged (Fig. 4B). Lastly, there were no significant effects of simple or complex nursery diets on fever response of nursery pigs.
Figure 4.
Rectal temperature in (A) female (F) and (B) male (M) offspring challenged with 40 µg/kg lipopolysaccharide (LPS) from sows fed diets supplemented with microalgae (AL), fish oil (FO), or a control diet (CON) and challenged with either 10 µg/kg LPS or saline as a control. Results are presented as LSM ± SEM, n = 16 per treatment. Significant differences (P < 0.05) are denoted with an asterisk. Trends (0.05 < P < 0.1) are denoted with a plus sign.
Cortisol Response to LPS Immune Challenge
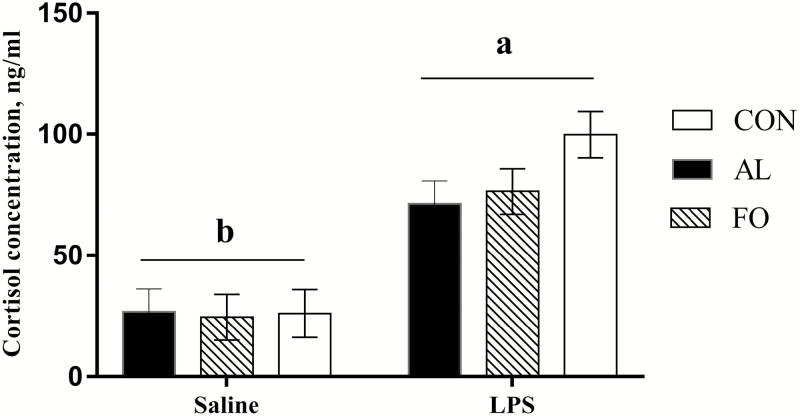
Regardless of the maternal diets, the serum cortisol concentration of the LPS immune challenged sows was increased over time when compared with the saline control sows (P < 0.01, Fig. 5). The serum cortisol concentration of sows in the CON treatment 2 h post-LPS immune challenge was slightly greater than corresponding sows in the AL and FO treatments; however, differences were not statistically significant (P > 0.10, Fig. 5).
Figure 5.
Sow serum cortisol concentrations 2 h post-immune challenge with lipopolysaccharide (LPS; 10 µg/kg BW, administered i.m.) or saline in sows fed diets supplemented with microalgae (AL), fish oil (FO), or a control diet (CON). Results are presented as LSM ± SEM, n = 8 per treatment. a,bDiffering letters above the bars denote significant differences (P < 0.05).
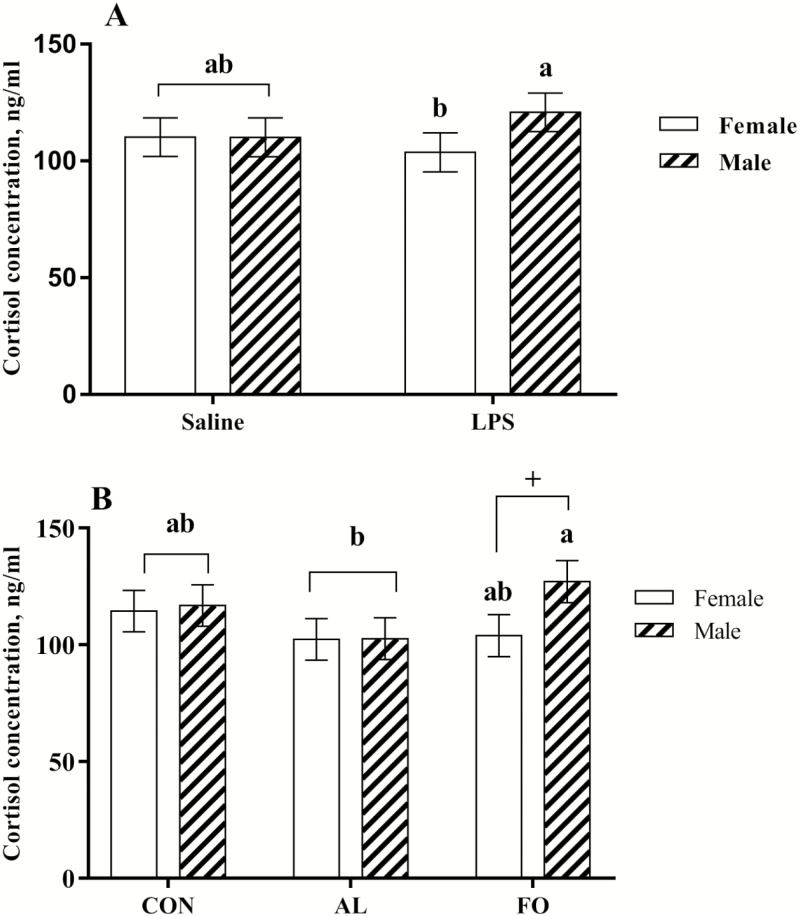
All LPS immune challenged nursery pigs also responded with an increase in cortisol concentration 2 h post-LPS immune challenge (P < 0.05). A significant maternal LPS × sex interaction was found; regardless of the maternal dietary treatments, male nursery pigs born to the LPS immune challenged sows had greater cortisol concentrations than female nursery pigs 2 h post-LPS immune challenge (P = 0.05, Fig. 6A). A significant maternal diet × sex interaction was also found for the nursery pig cortisol concentration 2 h post-LPS immune challenge. Regardless of maternal LPS status, the cortisol concentration of FO male nursery pigs was slightly greater than that of FO female nursery pigs 2 h post-LPS immune challenge (P = 0.07, Fig. 6B) and greater than the AL male nursery pigs (P = 0.04, Fig. 6B). However, there was no significant interaction among maternal diet × maternal LPS × sex. Finally, there was no significant difference in the cortisol response of nursery pigs fed the simple diets vs. complex diets.
Figure 6.
Serum cortisol concentrations in nursery pigs 2 h post-immune challenge with lipopolysaccharide (LPS; 40 µg/kg BW, administered i.m.). (A) Serum cortisol concentration of female and male nursery pigs from sows challenged with 10 µg/kg LPS in late gestation or saline as a control, n = 48 per treatment. (B) Serum cortisol concentration of female and male nursery pigs from sows fed diets supplemented with microalgae (AL), fish oil (FO), or a control diet (CON), n = 32 per treatment. Results are presented as LSM ± SEM. a,bDiffering letters above bars denote significant differences (P < 0.05).
DISCUSSION
The present study used an LPS immune challenge to simulate a bacterial infection during pregnancy to test the hypothesis that bacterial-induced stress can alter programming of the fetal pig’s HPAA to determine the efficacy of maternal dietary supplement with FO or AL for limiting the effects of maternal stress on offspring HPAA function and to investigate the role of nursery diet protein quality on offspring HPAA function. Maternal LPS immune challenge elicited both a fever and cortisol response in all challenged sows. FO dietary supplementation significantly attenuated the sow fever response when compared with the AL and CON diets. This finding was similar to those previously observed in nursery pigs (Carroll et al., 2003), guinea pigs (Pomposelli et al., 1989), humans (Cooper et al., 1993; Michaeli et al., 2007), and sheep (Fisher et al., 2014), where fish products attenuated the fever response to inflammatory stress challenges. The fever suppression in sows fed the FO diet may be attributed to the plasma EPA levels; previously, rabbits supplemented with pure EPA had an attenuated fever response to immune challenge with poly (I:C; Davidson et al., 2013). In contrast, sows fed the AL diet had greater plasma DHA levels compared with sows from the other dietary treatments, but did not have a reduced fever response. This effect was also observed previously, where nursery pigs fed diets supplemented with FO had a decreased fever response to LPS immune challenge compared with nursery pigs fed a diet supplemented with AL (Lee et al., 2019). Similarly, Chase et al. (2015) reported that DHA treatment did not reduce the production of the fever-associated cytokines, tumor necrosis factor alpha (TNF-α) and IL-1β, in human infant whole blood cells stimulated with LPS. Overall, this suggests that DHA may not play a significant role in regulating fever response as EPA does.
Both FO and AL diets appeared to reduce the sow cortisol response to LPS immune challenge in the present study, although this effect was not statistically significant. This is consistent with a previous study in pregnant ewes, where peak cortisol concentrations measured 4 h post-LPS immune challenge were not significantly affected by diets supplemented with fishmeal or soybean meal (Stryker et al., 2013). In contrast, Liu et al. (2013) and Gaines et al. (2003) found that FO supplementation in nursery pig diets reduced cortisol concentrations at 2 h post-LPS immune challenge (100 µg/kg BW; Liu et al., 2013) and up to 5 h post-LPS challenge (150 µg/kg BW; Gaines et al., 2003) compared with a control group. In humans, dietary FO supplementation has also been shown to lower cortisol concentrations 4 h and 6 h post-LPS immune challenge (2 ng/kg BW; Michaeli et al., 2007). These inconsistencies may be attributed to several possibilities, including physiologic differences between nursery pigs and sows, differences in LPS dose and route of exposure, differences in sample size, and differing sampling time points. It is also possible that the cortisol response to LPS immune challenge is different between pregnant and nonpregnant animals; Kabaroff et al. (2006) have shown that pregnant ewes had higher cortisol concentrations following LPS challenge compared with the nonpregnant ewes (Kabaroff et al., 2006).
It is interesting to note that maternal FO supplementation reduced the fever response in sows without affecting the cortisol response. This same response was previously observed in nursery pigs fed diets containing FO (Lee et al., 2019). This may be attributed in part to activation of the sympathoadrenal medullary axis or through rapid and direct stimulation of cortisol production in the adrenal gland (Bornstein and Chrousos, 1999). LPS can directly stimulate cortisol production from the adrenal glands in an ATCH-independent manner (Vakharia and Hinson, 2005). Gene expression within the adrenal gland of these sows may further elucidate the mechanisms involved in the fever and cortisol responses in sows fed the FO diet.
To our knowledge, this study is the first to show sex differences, and the combined influence of maternal diets and LPS immune challenge, on the offspring fever response in a swine model. The fever response in female offspring appeared to be more sensitive to maternal LPS status and maternal cortisol production than that of male offspring. Female offspring from sows challenged with LPS had greater fever response compared with female offspring from sows receiving saline as a control; these differences were not observed in the male offspring. Similarly, a previous study by de Groot et al. (2007) reported that glucocorticoid administration to sows in late gestation induced a greater fever response to LPS (2.2 μg/kg BW, i.v.) in female offspring compared with female offspring from nonchallenged sows. In addition, Collier et al. (2011) demonstrated that maternal restraint stress in sows during late gestation led to a greater production of the fever-inducing cytokine TNF-α in female offspring that were later LPS immune challenge (25 μg/kg BW, i.v.) compared with male offspring.
A lower rectal temperature response in the female offspring from sows fed the CON diet that received saline was observed compared with offspring from sows on the same diet challenged with LPS. This suggests maternal AL and FO supplementation reduces the effect of LPS challenge on the female offspring fever response to LPS. In male offspring, a decrease in rectal temperature response was observed in offspring from sows fed the CON diet and challenged with LPS compared with those from sows fed their AL or FO that were also challenged with LPS. This finding was contrary to expectations, with the combination of maternal AL and FO supplementation and maternal LPS challenge increasing the fever response to LPS in the male offspring. This increase in fever magnitude in male offspring may indicate a more rapid resolution of the fever response compared with offspring from sows fed the CON diet. A similar finding was observed by Lee et al. (2019) when nursery pigs were fed diets supplemented with FO and challenged with LPS.
Contrary to the observed fever response, the male offspring cortisol response following LPS challenge appeared to be more sensitive to maternal LPS exposure than female offspring. Regardless of the maternal diets, male offspring from sows that were LPS immune challenged showed significantly greater cortisol concentrations than female offspring. A similar response was observed by Fisher et al. (2014), where male offspring from ewes that were challenged with LPS had increased cortisol responses to an exogenous ACTH challenge compared with females. Contrarily, McCormick et al. (1995) observed a greater corticosterone response to stress in female rats from maternally stressed dams compared with females from non-stressed dams; no differences were observed in the male offspring. These inconsistencies among different studies may be attributed to differences in species or differences in stress challenges. However, Fisher et al. (2014) found that although male offspring from maternally stressed ewes appeared more sensitive to stress after weaning, these differences disappeared following an LPS challenge several months later, indicating that the effects of maternal stress on offspring stress response may be of a transient nature.
In this study, male offspring were castrated shortly after birth. It has been well characterized that instances of stress in early life may affect the degree to which an animal responds to subsequent stressors (van Bodegom et al., 2017). It is, therefore, possible that castration in early life may be a factor in the increased cortisol response in male offspring compared with female offspring. However, Merlot et al. (2013) did not observe differences in cortisol concentrations between castrated and intact male pigs. The effects of neonatal castration on subsequent HPAA response to stressors such as LPS; therefore, require further investigation and may further explain the results presently obtained.
Due to the age of the piglets (28 d of age), it is not likely that the differences observed between male and female offspring are a result of differing concentrations of sex hormones. Pigs reach sexual maturity around 150 to 220 d of age (Soede et al., 2011), and serum concentrations of estrogen and testosterone are quite low in early life (Hughes and Varley, 1980). The differences observed between the male and female cortisol response may also be attributed to differential cytokine concentrations; studies have shown that in humans, males have heightened cytokine production in response to LPS compared with females (Moxley et al., 2002; Kim-Fine et al., 2012). Further analysis of cytokines, as well as the transcriptome and/or epigenome in these pigs, may provide more insight into the observed differences in HPAA responses between male and female offspring.
Lastly, no significant effects of complex vs. simple weaning diets on the offspring’s fever and cortisol responses to LPS immune challenge were observed. Simple weaning diets have been reported to alter the production of proinflammatory cytokines in ileum and jejunum (Lee et al., 2016), although this may affect offspring gut health rather than HPAA function. It is possible that simple or complex nursery diets may affect the HPAA response to stressors with increased time on feed; in the present study, offspring were fed either a complex or simple nursery diet for only one week after weaning.
It was also observed that male offspring from sows fed the FO diet had a significantly greater cortisol concentration than males from sows fed the AL diet. It is worth noting that the n-3 PUFA concentrations in nursery pig plasma remained enriched at 28 d postpartum before the LPS immune challenge was carried out. At this time, DHA, but not EPA, concentrations in the plasma of nursery pigs from the maternal FO and AL treatment groups remained greater than concentrations measured in the offspring from the maternal CON group. Given that n-3 PUFAs have potent anti-inflammatory properties, a potential carryover effect on the offspring cortisol and fever responses to LPS immune challenge may have occurred in the present study; this will be an important consideration if mechanisms of HPAA programming are investigated in future experiments. Clouard et al. (2015) also reported a similar outcome, where DHA levels in the brain were increased in pigs at 4 weeks of age from sows fed diets supplemented with FO in gestation and lactation, compared pigs from sows fed a control diet (Clouard et al., 2015). Clouard et al. (2015) further reported that DHA levels were not significantly different among nursery pigs at 14 wk of age (Clouard et al., 2015). Therefore, in future studies, it may be beneficial to investigate the HPAA response in pigs at a later life stage when maternal n-3 PUFA carryover has disappeared.
Finally, the results obtained in offspring from sows fed the AL diet may in part be attributed to other nutrients or compounds found in the AL supplement, including antioxidants, minerals, vitamins, carotenes, beta-glucans, and possible antinutritive factors (Chen and Huang, 2010; Yaakob et al., 2014; Norambuena et al., 2015). These compounds may influence the activities of HPAA during stress response (Yaakob et al., 2014). For example, antioxidants were found to compensate the oxidative stress response, such as by neutralizing reactive oxygen species (Spiers et al., 2015). Future studies should, therefore, investigate the role of these biomolecules and their contribution to the observed results between the AL and FO treatments.
Overall, this study provided insight into the effects of maternal LPS-induced alteration and maternal dietary supplementation with FO or AL on the fever and cortisol responses of offspring during LPS immune challenge. The combined influence of maternal LPS-induced alteration and maternal n-3 PUFAs dietary supplementation was observed in a sex-specific pattern of the fever response in offspring. In addition, there was no evidence to suggest that feeding a simple nursery diet adversely affected offspring health based on the physiological endpoints measured in this study. Until demonstrated otherwise, using simple diets to feed nursery pigs may be a cost-effective management strategy. Future analysis including cytokine profiling and tissue specific gene expression may help to tease out how maternal LPS and maternal dietary n-3 PUFA supplementation alter the programming of the fetal HPAA.
Footnotes
The authors thank the staff at the Arkell research station as well as Julia Zhu and Douglas Wey at the University of Guelph for their assistance throughout this trial. The authors also thank Grand Valley Fortifiers and Alltech Inc. for supplying the FO and AL, respectively. Financial support for this project was provided by Alltech Inc. (#052363), the Natural Sciences and Engineering Research Council (#401018), and the Ontario Ministry of Agriculture, Food and Rural Affairs.
LITERATURE CITED
- Bornstein S. R., and Chrousos G. P.. 1999. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J. Clin. Endocrinol. Metab. 84:1729–1736. doi: 10.1210/jcem.84.5.5631 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Gaines A. M., Spencer J. D., Allee G. L., Kattesh H. G., Roberts M. P., and Zannelli M. E.. 2003. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs. I. Effects on the immune axis when fed diets containing spray-dried plasma. Domest. Anim. Endocrinol. 24:341–351. [DOI] [PubMed] [Google Scholar]
- Chase H. P., Boulware D., Rodriguez H., Donaldson D., Chritton S., Rafkin-Mervis L., Krischer J., Skyler J. S., and Clare-Salzler M.; Type 1 Diabetes TrialNet Nutritional Intervention to Prevent (NIP) Type 1 Diabetes Study Group 2015. Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes. Pediatr. Diabetes 16:271–279. doi: 10.1111/pedi.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., and Huang X. F.. 2010. Beta-glucan may play an important role in algae hypocholesterolemia effect. Eur. J. Nutr. 49:63–64. doi: 10.1007/s00394-009-0079-x [DOI] [PubMed] [Google Scholar]
- Clouard C., Souza A. S., Gerrits W. J., Hovenier R., Lammers A., and Bolhuis J. E.. 2015. Maternal fish oil supplementation affects the social behavior, brain fatty acid profile, and sickness response of piglets. J. Nutr. 145:2176–2184. doi: 10.3945/jn.115.214650 [DOI] [PubMed] [Google Scholar]
- Collier C. T., Williams P. N., Carroll J. A., Welsh T. H. Jr, and Laurenz J. C.. 2011. Effect of maternal restraint stress during gestation on temporal lipopolysaccharide-induced neuroendocrine and immune responses of progeny. Domest. Anim. Endocrinol. 40:40–50. doi: 10.1016/j.domaniend.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. L., Gibbons L., Horan M. A., Little R. A., and Rothwell N. J.. 1993. Effect of dietary fish oil supplementation on fever and cytokine production in human volunteers. Clin. Nutr. 12:321–328. [DOI] [PubMed] [Google Scholar]
- Davidson J., Higgs W., and Rotondo D.. 2013. Eicosapentaenoic acid suppression of systemic inflammatory responses and inverse up-regulation of 15-deoxyδ(12,14) prostaglandin J2 production. Br. J. Pharmacol. 169:1130–1139. doi: 10.1111/bph.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J., Kranendonk G., Fillerup M., Hopster H., Boersma W., Hodgson D., van Reenen K., and Taverne M.. 2007. Response to LPS in female offspring from sows treated with cortisol during pregnancy. Physiol. Behav. 90:612–618. doi: 10.1016/j.physbeh.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Fisher R. E., Karrow N. A., Quinton M., Finegan E. J., Miller S. P., Atkinson J. L., and Boermans H. J.. 2010. Endotoxin exposure during late pregnancy alters ovine offspring febrile and hypothalamic–pituitary–adrenal axis responsiveness later in life. Stress 13:334–342. doi: 10.3109/10253891003663762 [DOI] [PubMed] [Google Scholar]
- Fisher R. E., Or’Rashid M., Quinton M., AlZahal O., Boermans H. J., McBride B. W., and Karrow N. A.. 2014. Maternal supplementation with fishmeal protects against late gestation endotoxin-induced fetal programming of the ovine hypothalamic-pituitary-adrenal axis. J. Dev. Orig. Health Dis. 5:206–213. doi: 10.1017/S2040174414000191 [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., and Slone Stanley G. H.. 1956. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–510. doi: 10.1016/j.ultrasmedbio.2011.03.005 [DOI] [PubMed] [Google Scholar]
- French S. S., Chester E. M., and Demas G. E.. 2013. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol. Behav. 119:175–184. doi: 10.1016/j.physbeh.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Gaines A. M., Carroll J. A., Yi G. F., Allee G. L., and Zannelli M. E.. 2003. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs. Domest. Anim. Endocrinol. 24:353–365. [DOI] [PubMed] [Google Scholar]
- Gayle D. A., Beloosesky R., Desai M., Amidi F., Nuñez S. E., and Ross M. G.. 2004. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286:R1024–R1029. doi: 10.1152/ajpregu.00664.2003 [DOI] [PubMed] [Google Scholar]
- Hughes P. E., and Varley M. A.. 1980. Reproduction in the pig. Butterworth & Co. (Publ.) Ltd., London, UK. [Google Scholar]
- Kabaroff L., Boermans H., and Karrow N. A.. 2006. Changes in ovine maternal temperature, and serum cortisol and interleukin-6 concentrations after challenge with Escherichia coli lipopolysaccharide during pregnancy and early lactation. J. Anim. Sci. 84:2083–2088. doi: 10.2527/jas.2005-625 [DOI] [PubMed] [Google Scholar]
- Kanitz E., Otten W., and Tuchscherer M.. 2006. Changes in endocrine and neurochemical profiles in neonatal pigs prenatally exposed to increased maternal cortisol. J. Endocrinol. 191:207–220. doi: 10.1677/joe.1.06868 [DOI] [PubMed] [Google Scholar]
- Karrow N. A. 2006. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: lessons learned from the model inflammagen, lipopolysaccharide. Brain. Behav. Immun. 20:144–158. doi: 10.1016/j.bbi.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Kim-Fine S., Regnault T. R., Lee J. S., Gimbel S. A., Greenspoon J. A., Fairbairn J., Summers K., and de Vrijer B.. 2012. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J. Matern. Fetal. Neonatal Med. 25:2470–2474. doi: 10.3109/14767058.2012.684165 [DOI] [PubMed] [Google Scholar]
- Kranendonk G., Hopster H., Fillerup M., Ekkel E. D., Mulder E. J. H., and Taverne M. A. M.. 2006a. Cortisol administration to pregnant sows affects novelty-induced locomotion, aggressive behaviour, and blunts gender differences in their offspring. Horm. Behav. 49:663–672. doi: 10.1016/j.yhbeh.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Kranendonk G., Hopster H., Fillerup M., Ekkel E. D., Mulder E. J., Wiegant V. M., and Taverne M. A.. 2006b. Lower birth weight and attenuated adrenocortical response to ACTH in offspring from sows that orally received cortisol during gestation. Domest. Anim. Endocrinol. 30:218–238. doi: 10.1016/j.domaniend.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Lay D. C., Jr, Kattesh H. G., Cunnick J. E., Daniels M. J., Kranendonk G., McMunn K. A., Toscano M. J., and Roberts M. P.. 2011. Effect of prenatal stress on subsequent response to mixing stress and a lipopolysaccharide challenge in pigs. J. Anim. Sci. 89:1787–1794. doi: 10.2527/jas.2010-3612 [DOI] [PubMed] [Google Scholar]
- Lee I. K., Kye Y. C., Kim G., Kim H. W., Gu M. J., Umboh J., Maaruf K., Kim S. W., and Yun C. H.. 2016. Stress, nutrition, and intestinal immune responses in pigs – A review. Asian-Australas. J. Anim. Sci. 29:1075–1082. doi: 10.5713/ajas.16.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., You L., Oh S.-Y., Li Z., Code A., Zhu C., Fisher-Heffernan R., Regnault T., De Lange C., Huber L.-A., and Karrow N.. 2019. Health benefits of supplementing nursery pig diets with microalgae or fish oil. Animals 9:80. doi: 10.3390/ani9030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque C. L., Miller E., Zhu J., and de Lange K.. 2013. Simple assessment of piglet robustness in relation to nursery diet quality and feeding antibiotics. J. Anim. Sci. 91:30. [Google Scholar]
- Liu Y., Chen F., Li Q., Odle J., Lin X., Zhu H., Pi D., Hou Y., Hong Y., and Shi H.. 2013. Fish oil alleviates activation of the hypothalamic-pituitary-adrenal axis associated with inhibition of TLR4 and NOD signaling pathways in weaned piglets after a lipopolysaccharide challenge. J. Nutr. 143:1799–1807. doi: 10.3945/jn.113.179960 [DOI] [PubMed] [Google Scholar]
- Luo J., Huang F., Xiao C., Fang Z., Peng J., and Jiang S.. 2013. Responses of growth performance and proinflammatory cytokines expression to fish oil supplementation in lactation sows’ and/or weaned piglets’ diets. Biomed Res. Int. 2013:905918. doi: 10.1155/2013/905918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C. M., Smythe J. W., Sharma S., and Meaney M. J.. 1995. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res. Dev. Brain Res. 84:55–61. [DOI] [PubMed] [Google Scholar]
- Merlot E., Thomas F., and Prunier A.. 2013. Comparison of immune and health markers in intact and neonatally castrated male pigs. Vet. Rec. 173:317. doi: 10.1136/vr.101667 [DOI] [PubMed] [Google Scholar]
- Michaeli B., Berger M. M., Revelly J. P., Tappy L., and Chioléro R.. 2007. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin. Nutr. 26:70–77. doi: 10.1016/j.clnu.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Moxley G., Posthuma D., Carlson P., Estrada E., Han J., Benson L. L., and Neale M. C.. 2002. Sexual dimorphism in innate immunity. Arthritis Rheum. 46:250–258. doi:10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- Norambuena F., Hermon K., Skrzypczyk V., Emery J. A., Sharon Y., Beard A., and Turchini G. M.. 2015. Algae in fish feed: Performances and fatty acid metabolism in juvenile Atlantic salmon. PLoS ONE 10:e0124042. doi: 10.1371/journal.pone.0124042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed. National Academies Press, Washington, DC. [Google Scholar]
- Pomposelli J. J., Mascioli E. A., Bistrian B. R., Lopes S. M., and Blackburn G. L.. 1989. Attenuation of the febrile response in guinea pigs by fish oil enriched diets. J. Parenter. Enteral Nutr. 13:136–140. doi: 10.1177/0148607189013002136 [DOI] [PubMed] [Google Scholar]
- Skinner L. D., Levesque C. L., Wey D., Rudar M., Zhu J., Hooda S., and de Lange C. F.. 2014. Impact of nursery feeding program on subsequent growth performance, carcass quality, meat quality, and physical and chemical body composition of growing-finishing pigs. J. Anim. Sci. 92:1044–1054. doi: 10.2527/jas.2013-6743 [DOI] [PubMed] [Google Scholar]
- Soede N. M., Langendijk P., and Kemp B.. 2011. Reproductive cycles in pigs. Anim. Reprod. Sci. 124:251–258. doi: 10.1016/j.anireprosci.2011.02.025 [DOI] [PubMed] [Google Scholar]
- Spiers J. G., Chen H. J., Sernia C., and Lavidis N. A.. 2015. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 8:456. doi: 10.3389/fnins.2014.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker J. A., Fisher R., You Q., Or-Rashid M. M., Boermans H. J., Quinton M., McBride B. W., and Karrow N. A.. 2013. Effects of dietary fish meal and soybean meal on the ovine innate and acquired immune response during pregnancy and lactation. Animal 7:151–159. doi: 10.1017/S175173111200136X [DOI] [PubMed] [Google Scholar]
- Vakharia K., and Hinson J. P.. 2005. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology 146:1398–1402. doi: 10.1210/en.2004-0882 [DOI] [PubMed] [Google Scholar]
- van Bodegom M., Homberg J. R., and Henckens M. J. A. G.. 2017. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front. Cell. Neurosci. 11:87. doi: 10.3389/fncel.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veru F., Laplante D. P., Luheshi G., and King S.. 2014. Prenatal maternal stress exposure and immune function in the offspring. Stress 17:133–148. doi: 10.3109/10253890.2013.876404 [DOI] [PubMed] [Google Scholar]
- Wankhade U. D., Thakali K. M., and Shankar K.. 2016. Persistent influence of maternal obesity on offspring health: mechanisms from animal models and clinical studies. Mol. Cell. Endocrinol. 435:7–19. doi: 10.1016/j.mce.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Yaakob Z., Ali E., Zainal A., Mohamad M., and Takriff M. S.. 2014. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. (Thessalon) 21:6. doi: 10.1186/2241-5793-21-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M. A. C., Beijers R., Riksen-Walraven M. J., and de Weerth C.. 2017. Maternal late pregnancy anxiety and stress is associated with children’s health: A longitudinal study. Stress 20:495–504. doi: 10.1080/10253890.2017.1348497 [DOI] [PubMed] [Google Scholar]