Acute myeloid leukaemia (AML) is a severe systemic malignancy originated from self-renewing transformed immature myeloid precursors. AML can often be a fatal disease since transformed cells operate highly effective machinery which allows them to protect themselves against induction of programmed cells death and also to suppress anti-cancer activity of natural killer (NK) and cytotoxic T cells (CTCs) thus evading immune attack [4, 6]. These biochemical networks act as a “multilayer protection cover” for AML cells since even if they are attacked by the immune cells, their killing becomes problematic due to effectiveness of anti-apoptotic machinery. Therefore, it is crucially important to understand the mechanisms underlying functional activity of anti-apoptotic biochemical networks operated by AML cells in order to select optimal targets and design new drugs for successful and efficient therapy.
In the study published in this issue of EBioMedicine, Ruvolo and colleagues have demonstrated that two biochemical cell survival pathways controlled by galectin-3 (a beta-galactoside-binding protein) and cluster of differentiation (CD) 74 (a signalling receptor and a chaperone for major histocompatibility complex type II proteins which are involved in antigen presentation) are cross-linked in a biochemical protein network, which is associated with poor survival of AML patients [7]. Importantly, this invaluable clinical study was performed using samples obtained from a large number of AML patients (511 in total) with newly diagnosed disease when malignant cells normally display high pro-survival and immune evasion activities. The study reports that galectin-3 expression is prognostic for poor survival rate of AML patients. Survival rate of AML patients with galectin-3 positive malignant cells was even lower when CD74-dependent signalling networks (in particular, the one involving transmembrane receptor known as CD44) were active. Intriguingly, the study reported galectin-3 to act as a negative regulator of the protein phosphatase 2A (PP2A), the enzyme involved in deactivation of several intracellular signalling cascades (see below). It was shown that galectin-3 affects regulatory (B) subunit of this enzyme (the enzyme has three subunits – structural (A), regulatory (B) and catalytic (C), [7]).
This work is of great value for both basic and clinical AML research since it is not just a report of cross-links between biochemical networks but also a solid clinical verification of the evidence obtained in basic in vitro, ex vivo and in vivo studies, which can now be put together in order to identify the best targets for complex anti-AML therapy.
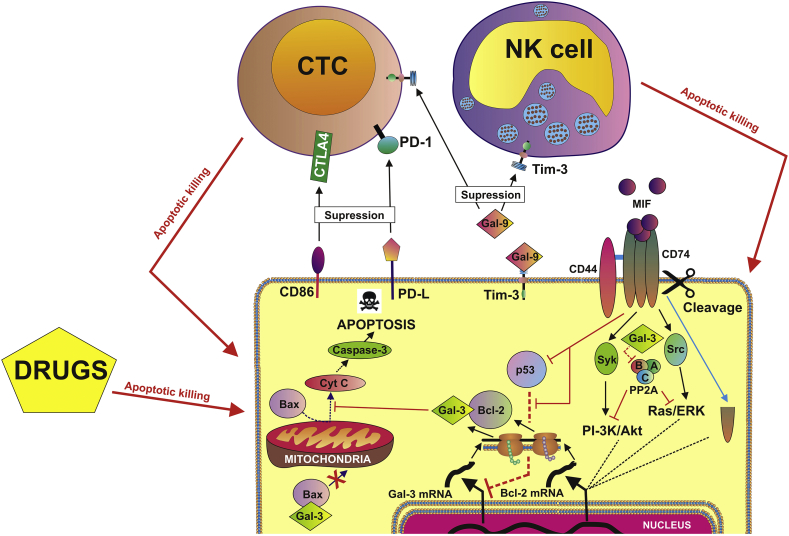
AML cells operate several pathways which allow them to disable cytotoxic lymphoid cells (NK and CTCs; summarised in the Fig. 1). This includes implication of CD86-CTLA4 (cytotoxic T-lymphocyte-associated protein 4) as well as ligands of programmed cell death protein (PD-L)-PD1 pathways [10]. Furthermore, AML cells operate Tim-3 (T-cell immunoglobulin and mucin-domain containing protein 3)-galectin-9 (another member of galectin family of proteins, [4]) secretory pathway which impairs anti-AML activities of both NK cells and CTCs. However, if AML cell is attacked by cytotoxic lymphoid cells or subjected to action of endogenous (e. g. pro-apoptotic cytokines) or exogenous (e. g. drugs) inducers of apoptosis, it manages to survive with the help of galectin-3 and CD74/CD44 signalling networks which appear to be connected [7].
Fig. 1.
Anti-apoptotic and immune evasion machineries operated by human AML cells. Galectins 3 and 9 are abbreviated as Gal-3 or Gal-9 respectively.
From recent reports, we know that galectin-3 is capable to protect mitochondria against defunctionalisation mainly via direct interaction with proteins from Bcl (B cell lymphoma) -2 family including anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins [5,6,9]. As a result of these interactions, Bcl-2 protects mitochondria and Bax loses its capability to cause mitochondrial dysfunction resulting in cytochrome c release followed by caspase-3/caspase cascade activation and apoptosis (Fig. 1). Importantly, galectin-3 has NWGR (Asn-Trp-Gly-Arg) motif specific to Bcl-2 family proteins which is necessary for homo- or heterodimerisation of these proteins and thus is capable of interacting with them [5,9]. Other galectin family members (for example galectin-9), despite their ability to interact with defunctionalised mitochondria in malignant cells [8], do not have this motif and thus do not display such an activity.
CD74 and CD44 receptor alliance, activated by the ligand called macrophage migration inhibitory factor (MIF), is capable of activating Ras/ERK (extracellular signal-regulating kinase) and phosphatidylinositol-3 kinase (PI-3 K)/Akt pathways [1, 7]). Also, active CD74 can undergo intermembrane proteolytic cleavage and activate its intracellular domain which displays functional activity. All these pathways activate transcription factors thus leading to expression of various genes, one of which is Bcl-2 ([1], Fig. 1).
Tumour suppressor protein p53 was reported to block expression of galectin-3 by repressing transcription of its gene [2]. However, CD74/CD44 activated by MIF, cause suppression of p53 activity [3] thus making galectin-3 expression possible (Fig. 1). On the other hand, PP2A, the enzyme mentioned above, targets crucial components of PI-3 K/Akt and Ras/ERK pathways leading to loss of their activities. Ruvolo and Co-Authors have shown that galectin-3 downregulates PP2A activity and thus prevents de-activation of AML cell survival pathways ([7], Fig. 1).
So, in addition to its novelty, the work of Ruvolo and colleagues provides excellent clinical verification of recently acquired basic knowledge. Furthermore, the study clearly demonstrates that galectin-3 and CD74 are promising targets for anti-AML therapy.
Disclosure
The authors declared no conflicts of interest.
References
- 1.Bucala R., Shachar I. The integral role of CD74 in antigen presentation, MIF signal transduction, and B cell survival and homeostasis. Mini Rev Med Chem. 2014;14:1132–1138. doi: 10.2174/1389557515666150203144111. [DOI] [PubMed] [Google Scholar]; Bucala, R., and Shachar, I. (2014). The integral role of CD74 in antigen presentation, MIF signal transduction, and B cell survival and homeostasis. Mini Reviews in Medicinal Chemistry 14, 1132-1138. [DOI] [PubMed]
- 2.Cecchinelli B., Lavra L., Rinaldo C., Iacovelli S., Gurtner A., Gasbarri A. Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol Cell Biol. 2006;26:4746–4757. doi: 10.1128/MCB.00959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cecchinelli, B., Lavra, L., Rinaldo, C., Iacovelli, S., Gurtner, A., Gasbarri, A., Ulivieri, A., Del Prete, F., Trovato, M., Piaggio, G., et al. (2006). Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol Cell Biol 26, 4746-4757. [DOI] [PMC free article] [PubMed]
- 3.De R., Sarkar S., Mazumder S., Debsharma S., Siddiqui A.A., Saha S.J. Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74-NF-kappaB signaling. J Biol Chem. 2018;293:19740–19760. doi: 10.1074/jbc.RA118.003935. [DOI] [PMC free article] [PubMed] [Google Scholar]; De, R., Sarkar, S., Mazumder, S., Debsharma, S., Siddiqui, A.A., Saha, S.J., Banerjee, C., Nag, S., Saha, D., Pramanik, S., et al. (2018). Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74-NF-kappaB signaling. J Biol Chem 293, 19740-19760. [DOI] [PMC free article] [PubMed]
- 4.Goncalves Silva I., Yasinska I.M., Sakhnevych S.S., Fiedler W., Wellbrock J., Bardelli M. The Tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid Leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Goncalves Silva, I., Yasinska, I.M., Sakhnevych, S.S., Fiedler, W., Wellbrock, J., Bardelli, M., Varani, L., Hussain, R., Siligardi, G., Ceccone, G., et al. (2017). The Tim-3-galectin-9 Secretory Pathway is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 22, 44-57. [DOI] [PMC free article] [PubMed]
- 5.Harazono Y., Kho D.H., Balan V., Nakajima K., Zhang T., Hogan V. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget. 2014;5:9992–10001. doi: 10.18632/oncotarget.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]; Harazono, Y., Kho, D.H., Balan, V., Nakajima, K., Zhang, T., Hogan, V., and Raz, A. (2014). Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget 5, 9992-10001. [DOI] [PMC free article] [PubMed]
- 6.Ruvolo P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863:427–437. doi: 10.1016/j.bbamcr.2015.08.008. [DOI] [PubMed] [Google Scholar]; Ruvolo, P.P. (2016). Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta 1863, 427-437. [DOI] [PubMed]
- 7.Ruvolo P.P., Hu C.W., Qiu Y., Ruvolo V.R., Go R.L., Hubner S.E. LGALS3 is connected to CD74 in a previously unknown protein network that is associated with poor survival in patients with AML. EBioMedicine. 2019 doi: 10.1016/j.ebiom.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ruvolo, P.P., Hu, C.W., Qiu, Y., Ruvolo, V.R., Go, R.L., Hubner, S.E., Coombes, K.R., Andreeff, M., Qutub, A.A., and Kornblau, S.M. (2019). LGALS3 is connected to CD74 in a previously unknown protein network that is associated with poor survival in patients with AML. EBioMedicine, doi: 10.1016/j.ebiom.2019.05.025 [DOI] [PMC free article] [PubMed]
- 8.Sakhnevych S.S., Yasisnka I.M., Fasler-Kan E., Sumbayev V.V. Mitochondrial defunctionalization supresses Tim-3-Galectin-9 secretory pathway in human colorectal Cancer cells and thus can possibly affect tumor immune escape. Front Pharmacol. 2019;10:342. doi: 10.3389/fphar.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sakhnevych, S. S. Yasisnka, I. M., Fasler-Kan, E. and Sumbayev, V. V. (2019) Mitochondrial Defunctionalization Supresses Tim-3-Galectin-9 Secretory Pathway in Human Colorectal Cancer Cells and Thus Can Possibly Affect Tumor Immune Escape. Front Pharmacol 10, 342 [DOI] [PMC free article] [PubMed]
- 9.Yang R.Y., Hsu D.K., Liu F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang, R.Y., Hsu, D.K., and Liu, F.T. (1996). Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA 93, 6737-6742. [DOI] [PMC free article] [PubMed]
- 10.Yasinska I.M., Goncalves Silva I., Sakhnevych S., Gibbs B.F., Raap U., Fasler-Kan E. Biochemical mechanisms implemented by human acute myeloid leukemia cells to suppress host immune surveillance. Cell Mol Immunol. 2018;15:989–991. doi: 10.1038/s41423-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yasinska, I.M., Goncalves Silva, I., Sakhnevych, S., Gibbs, B.F., Raap, U., Fasler-Kan, E., and Sumbayev, V.V. (2018). Biochemical mechanisms implemented by human acute myeloid leukemia cells to suppress host immune surveillance. Cell Mol Immunol 15, 989-991. [DOI] [PMC free article] [PubMed]