Abstract
Background
Lack of information about the dispersal of vector species barricade surveillance and control.
Aims
Therefore, the aim of the present study was to determine the species diversity of Culex mosquito's larvae in the coastal areas of the Persian Gulf.
Methods
Mosquito larvae were collected from six places in three main environmental categories: urban (UA), rural (RA) and uninhabited areas (UNA), using dipping technique. Four dips were taken from each breeding site (350 ml each). Larval investigation was conducted two times a month during the study period. Diversity studies were conducted separately for each category by calculating classic diversity indices.
Results
In total, 1369 specimens belonging to 10 different species were collected and identified, as follows: Culex hortensis, Cx. laticinctus, Cx. mimeticus, Cx. perexiguus, Cx. pipiens, Cx. modestus, Cx. sinaiticus, Cx. theileri, Cx. torrentium and Cx. tritaeniorhynchus. None of these mosquito species have been recorded previously in this region. Diversity analysis indicated higher species richness for RA (Margalef 1/26). The average diversity indices for the three environment types ranged from 1.50 to 1.64 for Shannon index and from 0.730 to 0.738 for Simpson index.
Conclusions
Biodiversity analysis indicated that species diversity in rural, urban and uninhabited areas is somewhat similar. Therefore, attention to all areas in vector control programs is essential.
Keywords: Culex, species diversity, mosquitoes, vector control
1. Introduction
Mosquitoes are the most important vectors of public health due to their roles in the transmission of diseases such as Malaria, Lymphatic filariasis, West Nile fever (WNV)‚ Zika, Sindbis fever‚ Dengue fever‚ and Encephalitis [1]–[4]. Among these mosquito-borne diseases‚ Malaria‚ West Nile‚ Sindbis‚ Dengue fever, and Dirofilariasis have been reported in Iran [2],[3],[5],[6]. According to a new study, the risk of Rift Valley fever in Iran is remarkable [7]. In addition, Zika and Dengue fever threatening the Persian Gulf region and southern Iran [8],[9] Dirofilaria immitis (causes heartworm disease) has been reported from Fars province [10]. In Iran, natural infection with third stage of D. immitis larvae was recorted in Culex theileri, this could be the main vector of this roundworm parasite [3]. Sero-survey of WNV in the equine populations in Iran indicated circulation of virus in southwestern provinces [11]. Culex pipiens reported as a bridge vector for WNV [12]. Therefore, due to the possibility of culicid mosquitoes to transmit vector-borne diseases, the necessity of the present study is highlighted. There is an urgent need to gather baseline data on species richness and diversity of mosquitoes so that their roles as vectors of various human and animal diseases may be better understood [13]. There is no information about the fauna and diversity of mosquitoes in the study area, but some species of mosquitoes including Cx. perexiguus, Cx. quinquefasciatus, Cx. sitiens, Cx. arbieeni, Cx. tritaeniorhynchus, Culiseta longiareolata and Ochlerotatus caspius have been recorded in south of Iran and Iranian islands in the Persian Gulf [2],[9]. Therefore, the main objective of the present study was to determine species diversity of medically important mosquitoes (Diptera, Culicini) in the coastal areas of the Persian Gulf.
2. Materials and methods
2.1. Study area
To develop the study, six sites were selected based on ease of access and topography in the different areas (urban areas (UA), rural areas (RA) and uninhabited area (no human populations) (UNA)) of the north of Bushehr province (50°31′1E, 29°34′45N), in the Southwest of Iran. Date palm and shrimp cultivation are two of the most important cultural activities in rural areas. Two sites for each of those areas were selected. During this period of study (January–December 2017), the average maximum and minimum temperatures were 48 °C and 1 °C in July and February, respectively (Table 1).
Table 1. Ambient temperature and total rainfall in the north of Bushehr, January–December 2017.
| Months | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
| Minimum temperature (°C) | 5 | 1 | 10 | 14 | 22 | 24 | 28 | 27 | 24 | 15 | 10 | 5 |
| Maximum temperature | 27 | 26 | 30 | 38 | 45 | 47 | 48 | 46 | 42 | 39 | 34 | 30 |
| Mean temperature | 16 | 15 | 21.4 | 28.7 | 33.9 | 36.2 | 36.8 | 37.1 | 34.7 | 30.6 | 22.5 | 17.8 |
| Total rainfall (mm) | 8.2 | 29.2 | 28.8 | 0.1 | 5 | 0 | 0 | 0 | 0 | 0 | 34.2 | 2 |
2.2. Sampling methods and taxonomic identification
In order to study the ecology of mosquitoes, sampling was carried out by dipping technique with a metal dipper for collecting larvae. Four dips were taken from each breeding site (350 ml each). Larval investigation was conducted two times a month during the study period.
All the samples were brought to the laboratory of the Entomology Department, Tehran University of Medical Sciences, Iran. The mosquito larvae were preserved in 75% ethanol and the microscopic slides were prepared using the chloral gum mounting. Microscope was used for the taxonomic study and identification, up to the species level using taxonomic keys available in literature [14].
2.3. Biodiversity and statistical analysis
Diversity studies (alpha diversity) were conducted separately for each category (rural, urban and uninhabited areas) by calculating classic diversity indices like Margalef's (S − 1)/lnN [S = total number of species and N = total number of individuals) and Simpson's indexes (λ = 1 − ∑pi2, where pi = ni/N [ni is number of individuals of taxon i]) [15]. Dominance = 1 − Simpson index. Ranges from 0 (all taxa are equally present) to 1 (one taxon dominates the community completely). Shannon diversity index (H′ = −[Σ(pi lnpi)]) is commonly used to characterize species diversity in a community, accounting for both abundance and evenness of the species present [15]. Equitability index measures the evenness with which individuals are divided among the taxa present.
Two indices (Jaccard's and Whittaker's indices) were used for similarity and dissimilarity between habitats [2],[15]. Data were analyzed using PAST software version 3.14 (Paleontological Statistics Software Package). Non-parametric tests were used to evaluate differences in species abundances and weather-related variables between months. Because of our data were not normally distributed. The Pearson's correlation test was used to measure of the strength of the association between the variables.
3. Results
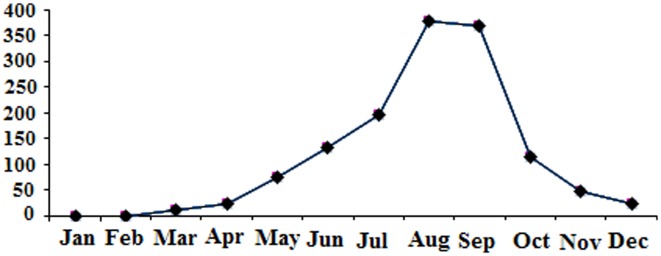
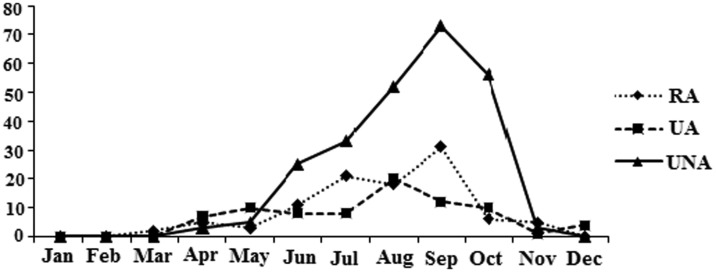
A total of 1369 specimens belonging to 10 different mosquito species were collected and identified. These were: Cx. hortensis Ficalbi, Cx. laticinctus Edwards, Cx. mimeticus Noe, Cx. perexiguus Theobald, Cx. pipiens Linnaeus, Cx. modestus Ficalbi, Cx. sinaiticus Kirkpatrick, Cx. theileri Theobald Cx. torrentium Martini and Cx. tritaeniorhynchus Giles. In the dipping collection, Cx. theileri (28.0%), Cx. pipiens (26.1%), Cx. tritaeniorhynchus (23.1%) were predominated, respectively. The greatest number of mosquitoes were collected from RA (723 specimens) and the lowest in the UA (209 specimens) (Table 2). Monthly variation in abundance of mosquitoes is provided (Figure 1). The highest number of mosquitoes were collected in August (379) and September (370 specimens), while the lowest were in January (0 specimen) and February (3 specimens). Depending on the month, difference was not significant in mosquito abundances according to Kruskal-Wallis test (P = 0.44). Analysis with Kruskal-Wallis and Mann-Whitney tests showed that, difference between environments (UA, RA and UNA) and abundance of mosquitoes was not significant (P = 0.33). Monthly abundance of dominant mosquito species (Cx. theileri) at three habitat types is also provided (Figure 2). Overall, in the present study there was a significant positive relationships between mean temperatures and abundance of mosquitoes (r = 0.75, P = 0.005). In contrast, abundance of mosquitoes decreased with increasing precipitation (r = −0.42, P = 0.16).
Table 2. Composition and localities of the culicini mosquitoes collected in the north of Bushehr, January–December 2017 (RA; rural areas, UA; urban areas, UNA; uninhabited area).
| Species | RA | UA | UNA | Total (%) |
| Cx. theileri | 104 | 80 | 200 | 384 (28.0%) |
| Cx. pipiens | 283 | 30 | 44 | 357 (26.1%) |
| Cx. tritaeniorhynchus | 215 | 58 | 43 | 316 (23.1%) |
| Cx. modestus | 8 | 25 | - | 33 (2.4%) |
| Cx. sinaiticus | 19 | - | 36 | 55 (4.0%) |
| Cx. perexiguus | 48 | - | 68 | 116 (8.5%) |
| Cx. mimeticus | 28 | 11 | 26 | 65 (4.7%) |
| Cx. hortensis | - | - | 17 | 17 (1.2%) |
| Cx. laticinctus | 8 | - | - | 8 (0.6%) |
| Cx. torrentium | 10 | 5 | 3 | 18 (1.3%) |
Figure 1. Monthly variation in abundance of all mosquito species collected in all areas of the study, January–December 2017.
Figure 2. Monthly abundance of dominant mosquito species (Cx. theileri) at three habitat types (RA; rural areas, UA; urban areas, UNA; uninhabited area).
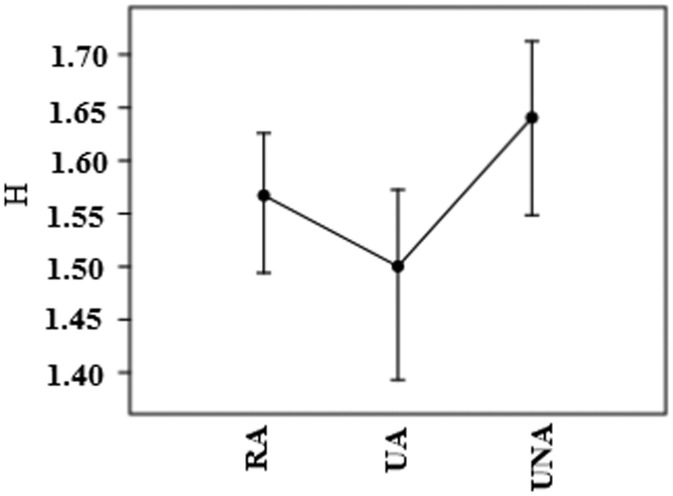
A greater species richness was found in RA (M = 1/21), while UA has the lowest (M = 0.93). The average diversity indices for the three environment types ranged from 1.50 to 1.64 for Shannon index and from 0.730 to 0.738 for Simpson index. Shannon index was highest in UNA and lowest in UA. While, Simpson index in UA was highest (Figure 3). The T-test showed no statistically significant difference between Shannon index (t = 1.17, p = 0.24) and Simpson index (t = 0.39, p = 0.69) in RA and UA. Similarly, there was also no statistically significant difference between Shannon index (t = −1.36, p = 0.17) and Simpson index (t = 0.20, p = 0.83) in RA and UNA. But, there was a significant difference between Shannon index in UNA and UA (t = −2.26, p = 0.024). However, difference between Simpson index was not significant in these areas (t = −0.15, p = 0.87). Greater evenness was observed in UA, because the most dominant species do not show such a strong influence as in the two other environments. Where, Cx. theileri in UNA and Cx. pipiens, Cx. theileri and Cx. tritaeniorhynchus in RA were dominant and shows a strong influence.
Figure 3. Shannon index for each environmental category (UA, RA and UNA).
4. Discussion
In the present investigation, 10 species of mosquitoes were identified. Among them there are some major vectors of human and animal diseases such as Cx. pipiens (Usutu virus, WNV), Cx. theileri (Dirofilariasis, Rift Valley fever), Cx. perexiguus (Sindbis fever, West Nile fever), Cx. tritaeniorhynchus (Japanese encephalitis, Rift Valley fever, Sindbis fever, West Nile fever) [3],[9],[16].
Culex theileri (28.0%) was appeared from March to December to make a peak with 135 individuals in September, when the mean temperature was 34.7 °C. According to a study in Fars Province, Cx. theileri reached its peak in June (n = 311) [17].This finding suggests that the oviposition activity of Cx. theileri is limited by the temperatures. It was the most abundant species in urban areas, this is in disagreement with its dispersal on the western region of Spain [18]. Monthly activities of this species in Mohr and Darab counties (Fars Province) were reported from April to September [2]. The present study does not provides similar findings.
Culex theileri is found in the most parts of the world. This mosquito has been recorded in all provinces of Iran and has involvement in the transmission of Dirofilaria immitis nematode [3]. In the study of ecology of mosquitoes in Fars, Cx. theileri preferred habitats with permanent water and without plants [17]. This finding is not similar to the present study.
Culex pipiens was the most frequent species in rural areas, whereas Cx. tritaeniorhynchus came in the second order. This is in disagreement with its dispersal on the western region of Spain, where, it was the most abundant species in in urban environments [18].
According to Margalef index, RA show the highest diversity. The reason for this might be due to ecological and climatological factors. In the present study, Shannon and equitability indices shows that in RA, species such as Cx. tritaeniorhynchus and Cx. pipiens intensely dominate the rest of species present in the community. Something similar occurs in the case of UNA, where Cx. theileri develop a strong influence. Finally, greater evenness degree can be observed in UA, because the most dominant species do not show such a strong influence as in the two other cases. There is very little data on the biodiversity in mosquito populations in Iran. In the present study, the maximum value for Shannon's index was 1.64. According to a study in Brazil, the maximum Shannon's index was 2.16 [19]. In another research in Iran, the maximum value for Shannon's index was 1.7 [2]. Normally, the Shannon index in real ecological units ranges between 1.5 and 3.5, therefore, a smaller amount makes it difficult to interpret actual species diversity [20].
We think that these differences in previous reports are due to the differences in the climate, sample size and data analysis method. Diversity indices are different from one season to another, consequently a diversity index result needs to be compared to other results of the same area [19]. Biodiversity studies have been ignored in this area, so comparison of results in the future should be made more precise.
5. Conclusion
In our research, some possible vectors of medical and veterinary importance were identified. In this area of study, mosquitoes were more active in August and September. Biodiversity analysis indicated that species diversity in rural, urban and uninhabited areas is somewhat similar. Therefore, attention to all areas in vector control programs is essential.
Acknowledgments
We would like to thank from the “Clinical Research Development Center of Baqiyatallah hospital” for their kindly cooperation. Also special thanks to Baqiyatallah University of Medical Sciences for their financial support (Project No.: 95-10-001069).
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/s0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- 2.Keshavarzi D, Soltani Z, Ebrahimi M, et al. Monthly prevalence and diversity of mosquitoes (Diptera; Culicidae) in Fars Province, Southern Iran. Asian Pac J Trop Dis. 2017;7:112–120. [Google Scholar]
- 3.Azari-Hamidian S, Yaghoobi-Ershadi M, Javadian E, et al. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 2009;23:111–121. doi: 10.1111/j.1365-2915.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 4.Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal. PloS one. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinikar S, Ghiasi SM, Moradi A, et al. Laboratory Detection Facility of Dengue Fever (DF) in Iran: The First Imported Case. Int J Infect Dis. 2010;8:1–2. [Google Scholar]
- 6.Khoobdel M, Vatandoost H, Abaei MR. Arthropod borne diseases in imposed war during 1980–88. Irannian J Arthropod-Born Dis. 2008;2:24–32. [Google Scholar]
- 7.Fakour S, Naserabadi S, Ahmadi E. The first positive serological study on rift valley fever in ruminants of Iran. J Vector Borne Dis. 2017;54:348–352. doi: 10.4103/0972-9062.225840. [DOI] [PubMed] [Google Scholar]
- 8.Khoobdel M, JonaidiJafari N, Izadi M. Is the Zica threatening the Iran and others Middle East countries? Iranian J Mil Med. 2016;17:187–190. [Persian] [Google Scholar]
- 9.Khoobdel M, Azari-Hamidian S, Hanafi-Bojd AA. Mosquito fauna (Diptera: Culicidae) of the Iranian islands in the Persian Gulf II. Greater Tonb, Lesser Tonb and Kish Islands. J Nat Hist. 2012;46:1939–1945. [Google Scholar]
- 10.Jafari S, Khaksar Z. Prevalence of Dirofilaria immitis in dogs of Fars province of Iran. J Appl Anim Res. 1996;9:27–31. [Google Scholar]
- 11.Ahmadnejad F, Otarod V, Fallah M, et al. Spread of West Nile virus in Iran: a cross-sectional serosurvey in equines, 2008–2009. Epidemiol Infect. 2011;139:1587–1593. doi: 10.1017/S0950268811000173. [DOI] [PubMed] [Google Scholar]
- 12.Hamer GL, Kitron UD, Brawn JD, et al. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Guedes DR, Paiva MH, Donato MM, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6:e69. doi: 10.1038/emi.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azari-Hamidian S, Harbach RE. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2009;2078:1–33. [Google Scholar]
- 15.Magurran AE. Ecological diversity and its measurement. 1 Ed. Dordrecht: Springer; 1988. Why diversity? pp. 1–5. [Google Scholar]
- 16.Zakhia R, Mousson L, Vazeille M, et al. Experimental transmission of West Nile Virus and Rift Valley Fever Virus by Culex pipiens from Lebanon. PLOS Negl Trop Dis. 2018;12:e0005983. doi: 10.1371/journal.pntd.0005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soltani Z, Keshavarzi D, Ebrahimi M, et al. The fauna and active season of mosquitoes in west of Fars Province, Southwest of Iran. Arch Razi Inst. 2017;72:203–238. doi: 10.22092/ari.2017.111603. [DOI] [PubMed] [Google Scholar]
- 18.Bravo-Barriga D, Gomes B, Almeida AP, et al. The mosquito fauna of the western region of Spain with emphasis on ecological factors and the characterization of Culex pipiens forms. J Vector Ecol. 2017;42:136–147. doi: 10.1111/jvec.12248. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira-de-Freitas V, França RM, Bartholomay LC, et al. Contribution to the Biodiversity Assessment of Mosquitoes (Diptera: Culicidae) in the Atlantic Forest in Santa Catarina, Brazil. J Med Entomol. 2017;54:368–376. doi: 10.1093/jme/tjw196. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald G. Biogeography: introduction to space, time and life, 1 Ed. New York: John Wiley and Sons; 2002. pp. 420–428. [Google Scholar]