Abstract
The objective of this study was to assess how exposure to ergot alkaloids during 2 stages of gestation alters fetal growth, muscle fiber formation, and miRNA expression. Pregnant ewes (n = 36; BW = 83.26 ± 8.14 kg; 4/group; 9 groups) were used in a 2 × 2 factorial arrangement with 2 tall fescue seed treatments [endophyte-infected (E+) vs. endophyte-free (E−)] fed during 2 stages of gestation (MID, days 35 to 85 vs. LATE, days 86 to 133), which created 4 possible treatments (E−/E−, E+/E−, E−/E+, or E+/E+). Ewes were individually fed a total mixed ration containing E+ or E− fescue seed according to treatment assignment. Terminal surgeries were conducted on day 133 of gestation for the collection of fetal measurements and muscle samples. Data were analyzed as a 2 × 2 factorial with fescue treatment, stage of gestation, and 2-way interaction as fixed effects. Fetuses exposed to E+ seed during LATE gestation had reduced (P = 0.0020) fetal BW by 10% compared with E− fetuses; however, fetal body weight did not differ (P = 0.41) with E+ exposure during MID gestation. Fetuses from ewes fed E+ seed during MID and LATE gestation tended to have smaller (P = 0.058) kidney weights compared with E− fetuses. Liver weight was larger (P = 0.0069) in fetuses fed E− during LATE gestation compared with E+. Fetal brain weight did not differ by fescue treatment fed during MID (P = 0.36) or LATE (P = 0.40) gestation. The percentage of brain to empty body weight (EBW) was greater (P = 0.0048) in fetuses from ewes fed E+ fescue seed during LATE gestation, which is indicative of intrauterine growth restriction (IUGR). Primary muscle fiber number was lower (P = 0.0005) in semitendinosus (STN) of fetuses exposed to E+ during MID and/or LATE gestation compared with E−/E−. miRNA sequencing showed differential expression (P < 0.010) of 6 novel miRNAs including bta-miR-652_R+1, mdo-miR-22-3p, bta-miR-1277_R-1, ppy-miR-133a_L+1_1ss5TG, hsa-miR-129-1-3p, and ssc-miR-615 in fetal STN muscle. These miRNA are associated with glucose transport, insulin signaling, intracellular ATP, hypertension, or adipogenesis. This work supports the hypothesis that E+ tall fescue seed fed during late gestation reduces fetal weight and causes asymmetrical growth, which is indicative of IUGR. Changes in primary fiber number and miRNA of STN indicate that exposure to E+ fescue fed during MID and LATE gestation alters fetal muscle development that may affect postnatal muscle growth and meat quality.
Keywords: ergot alkaloids, fescue toxicosis, miRNA, muscle fiber, sheep
INTRODUCTION
Ergovaline and ergovalinine are the predominant (84% to 97%) ergot alkaloids produced by the endophyte (Epichloë coenophiala) that infects tall fescue [Lolium arundinaceum (Schreb.) Darbysh] (E+; Lyons et al., 1986) and are responsible for vasoconstrictive events observed in fescue toxicosis (Strickland et al., 2011; Foote et al., 2012). Ergovaline has also been shown to be a potent vasoconstrictor in the bovine umbilical and uterine arteries (Dyer, 1993) and ovine (Klotz et al., 2019) and reduces blood flow to developing placental tissues and fetuses. Placental weight is highly correlated with fetal birthweight in cases of induced placental dysfunction such as hyperthermia (Alexander and Williams, 1971; Galan et al., 2005), maternal undernutrition (Wallace, 1948), and uteroplacental embolism (Lang et al., 2000). Reduced birth weights have also been reported in offspring born to dams exposed to endophyte-infected tall fescue during gestation (Watson et al., 2004; Duckett et al., 2014a).
Prenatal muscle growth is due to hyperplasia of muscle fibers, which is complete prior to birth. Fahey et al. (2005b) reported that muscle fiber hyperplasia is complete by about 85 d in the sheep. The ratio of secondary to primary muscle fibers is reduced with intrauterine crowding in pigs, that is, runt pig (Aberle, 1984; Pardo et al., 2013), maternal undernutrition from days 28 to 78 in sheep (Zhu et al., 2004), and ergot alkaloid exposure during gestation in sheep (Duckett et al., 2014a). microRNA (miRNA) are small, noncoding RNA molecules that result in translational repression and gene silencing through binding of 3′ untranslated region of the mRNAs (Filipowicz et al., 2008) and have been shown to be involved in muscle development. Current literature supports a strong role for several miRNA in prenatal muscle development, but these have not been thoroughly examined in meat-producing animals. The objective of this study was to assess how feeding tall fescue seed containing ergot alkaloids during MID and/or LATE gestation alters fetal growth, muscle fiber type, and muscle miRNA expression.
MATERIALS AND METHODS
All animal experimental procedures were reviewed and approved by the Clemson University Institutional Animal Care and Use Committee (AUP 2014–081).
Experimental Design
Mature Suffolk ewes, naive to endophyte-infected tall fescue, were purchased in Northeast Iowa and transported to Clemson University 90 d prior to the start of the experiment. Ewes (n = 36; BW = 83.26 ± 8.14 kg; 4/group; 9 groups) that were confirmed pregnant by transrectal ultrasound at day 30 of gestation. Pregnant ewes were used in a 2 × 2 factorial arrangement with 2 fescue treatments [endophyte-infected (E+) vs. endophyte-free (E−)] fed during 2 stages of gestation (MID, days 35 to 85 and/or LATE, days 86 to 133), which created 4 possible treatments (E−/E−, E+/E−, E−/E+, or E+/E+). Endophyte-infected (E+; Black Magic turf-type tall fescue seed) and endophyte-free (E−; Bull turf-type tall fescue seed) seed were grown in Oregon and obtained from Caudill Seed Warehouse (Louisville, KY). Tall fescue seed was fed individually to supply 1.77 mg animal−1 d−1 of ergovaline and ergovalinine for E+ and the same weight of E− tall fescue seed was fed to supply 0 mg animal−1 d−1 of ergovaline and ergovalinine. Ergovaline and ergovalinine concentrations fed in this study were based on previous research and is detailed in Britt et al. (2019). Ewes were individually penned into stalls (1.8 × 0.5 × 0.91 m) at 0700 and individually fed their respective treatment diet for 90 min. After individual feeding, ewes were removed from stalls and kept in a group pen (10 to 12 hd/pen) with ad libitum access to water and sheep mineral (Purina Sheep Mineral, Land O’Lakes Inc., Arden Hills, MN) and with access to inside and outside areas devoid of forage or hay. The total mixed ration (TMR) composition was formulated to minimize sorting of the seed when mixed in the TMR and incorporated 25% cottonseed hulls as a source of roughage (McCann et al., 1990). Samples of seed and TMR were subjected to nutrient analyses, and rations were developed to meet NRC requirements for pregnant ewes with twins during early and late gestation (NRC, 2007). Immediately prior to feeding, fescue seed (E+ or E−) was added to individual TMR rations, mixed thoroughly, and fed according to treatment assignment. Ewes within each group were fed equal amounts of TMR and seed daily to maintain similar feed intake across all treatments. Feed intake and body weight changes during the study are reported in Britt et al. (2019). On day 133 of gestation, ewes underwent terminal surgery where fetuses were removed and euthanized. Additional information on experimental design, ewe parameters and placental development are available in Britt et al. (2019).
Fetal Sample Collection
Each fetus was towel dried, and fetal weight was collected. Crown-rump length, abdominal circumference, and thoracic circumference were measured. The fetus was exsanguinated, and the hide, head, feet, tail, and viscera were removed to collect weights. Carcass weight was measured and dressing percentage calculated. From the viscera, weights were collected on all organs and the total digestive tract. From the left side of the carcass, individual muscles [longissimus (LM), psoas major and minor (PM), gluteus medias (GM), biceps femoris (BF), semitendinosus (STN), semimembranosus and adductor (SM/AD), and quadriceps femoris (QF)] were collected and weighed. Adipose depots [subcutaneous, kidney, heart, mesenteric, omental, and internal (inside body cavity near kidneys but adhered to body wall) fat depots] were also collected when present and weighed. Brown adipose tissue (BAT) was collected from the scapular region, snap frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA extraction. From the right side of each fetus, all fat and muscle were removed for total body proximate composition analysis. Total fat-free lean muscle and total fat mass were calculated using raw dissection weights and proximate analysis results.
Quantitative Real-Time RT-PCR
A subset of fetal lambs was selected from E−/E− and E+/E+ treatments based on fetal weight representing the mean response of each treatment (n = 4 per treatment; n = 8 total) and was not from the same ewe but instead from 3 different ewes for each treatment to determine BAT presence. The presence of both uncoupling protein 1 (UCP1) and myogenic factor 5 (MYF5+) gene expression was used to confirm the presence of BAT in the fat samples from the scapular region. Gene expression analysis was conducted using quantitative real-time RT-PCR (qPCR) methods according to Duckett et al. (2009, 2014b). Briefly, total RNA was collected from snap-frozen tissue samples using Trizol reagent and the PureLink Mini RNA purification kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA) according to manufacturer specifications. RNA yield and quality was determined using the NanoDrop 1000 spectrophotometer (Thermo Scientific, Thermo Fisher, Waltham, MA). RNA was converted to cDNA using qScript cDNA SuperMix (Quanta Bio, Beverly, MA) according to the manufacturer instructions. qPCR was performed using an Eppendorf Realplex Mastercycler (Eppendorf AG, Hamburg, Germany) and Perfecta (Quanta Bio, Beverly, MA) SYBR green according to the manufacturer’s specifications. An initial hold of 2 min at 95 °C was followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. Primers were designed to span exon boundaries using Primer 3 software (Table 1). Glyceraldehyde 3-phosphate dehydrogenase, β-actin (bACT), thymus cell antigen 1, tubulin, and cyclophilin were tested for stability in each tissue using RefFinder (Xie et al., 2012) for the selection of a housekeeping gene. The most stable housekeeping gene was bACT for brown fat and was used for normalization. Results are expressed as relative abundance from the control (E−/E−).
Table 1.
Primer sequences used for RT-PCR
| Gene1 | Forward, 5′-3′ | Reverse, 5′-3′ | Efficiency |
|---|---|---|---|
| UCP1 | TGGGGATCTTTGCTAACCAG | ATGTTTTGCTTCCCCTTCCT | 0.91 |
| MYF5 | GATTCTCAGCCTGCAACTCC | ATTTTTGGTGCCTCCTTCCT | 1.03 |
| GAPDH | GGGTCATCATCTCTGCACCT | GGTCATAAGTCCCTCCACGA | 1.01 |
| ACTB | GGGCAGTGATCTCTTTCTGC | CTCTTCCAGCCTTCCTTCCT | 1.03 |
| TUB | CGAGAGCTGTGACTGTCTGC | GGCATGACGCTAAAGGTGTT | 1.02 |
| CYC | GGTCATCGGTCTCTTTGGAA | TCCATCACACGATGGAA | 1.01 |
| THY1 | GGGCACCACAGAGGAAGTTA | TCCTTGATCACACGATGGAA | 1.05 |
1UCP1 = uncoupling protein 1; MYF5 = myogenic factor 5; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; ACTB = beta-actin; TUB = tubulin; CYC = cyclophilin B protein; Thy1 = thymus cell antigen 1.
Proximate Composition
Total lean samples from the right side of each fetus were chopped and mixed (Blixer3 Series D, Robot Coupe Inc., Ridgeland, MS), and a portion was removed for subsequent moisture content analysis. The remaining sample amount was frozen at −20 °C, lyophilized (VirTis, SP Scientific, Warminster, PA), mixed again (Blixer3), and then stored at −20 °C. Nitrogen content was analyzed in duplicate by the combustion method utilizing a Leco FP-2000 N analyzer (Leco Corp., St. Joseph, MI). Nitrogen amount was multiplied by 6.25 to determine the crude protein content. Moisture content was determined in triplicate by weight loss of the samples after drying for 24 h at 100 °C. Ash content was established by ashing samples for 8 h at 600 °C. An Ankom XT-15 Extractor (Ankom Technology, Macedon, NY), with hexane as a solvent, was used to determine total fat content from duplicate samples.
Muscle Fiber Histology
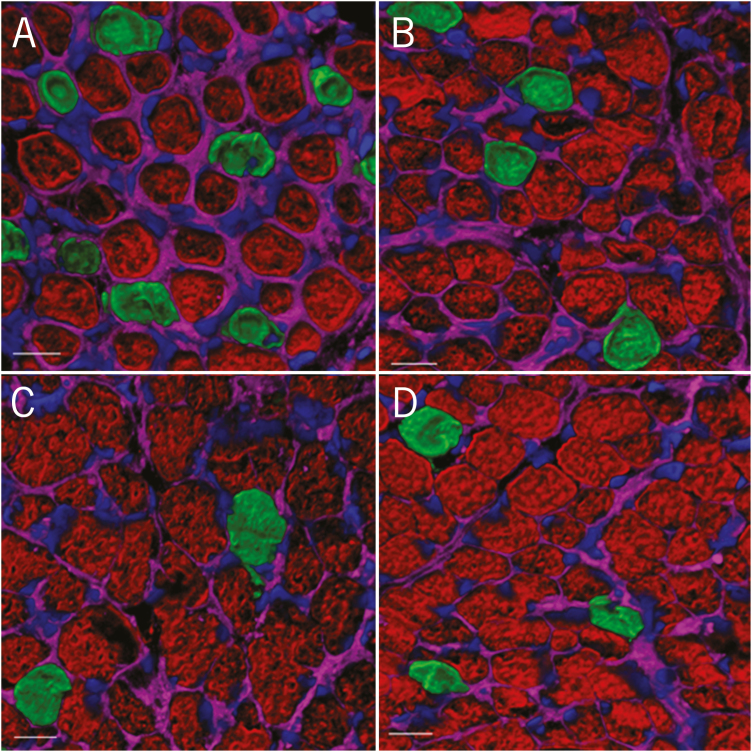
A subset of lambs (n = 16; 4 per treatment) was selected for muscle fiber histology based on fetal weight representing the mean response for each treatment and was not from the same ewe but instead from 3 different ewes for each treatment. Semitendinosus muscle was selected for muscle fiber histology examination due to ease of collection at slaughter and as a representative of hind limb muscles that were influenced by ergot alkaloid feeding in utero (Table 3). Semitendinosus muscle samples were collected from each lamb within 30 min of euthanasia. Muscle samples were placed in a form and covered with optimal cutting temperature solution (Fisher Scientific, Waltham, MA) and then flash frozen in liquid nitrogen. Muscle samples were stored at −80 °C until subsequent muscle histology. Muscle samples were cryosectioned at a thickness of 10 µm and stained using primary antibodies to myosin heavy chain (MHC)-fast (secondary, Type II fibers; Abcam, My-32) and MHC-slow (primary, Type I fibers; Hybridoma Bank, BA-F8). Two tissue sections per animal were subjected to immunofluorescence staining for muscle fiber typing. Briefly, secondary antibodies of goat anti-mouse IgG1 and IgG2b labeled with Alexa-Fluor 546 (red) and Alexa-Fluor 488 (green), respectively, were utilized for fluorescence. Sections were counterstained with DAPI (blue) and Alexa-Fluor 633 (magenta) wheat germ agglutinin at 7.5 µg/mL to label nuclei and muscle fibers (Kostrominova, 2011), respectively. Stained muscle sections were mounted in glycerol:PBS (1:1 vol/vol), and samples were imaged at 1.5× zoom using a Leica SPE confocal microscope (Leica Microsystems, Buffalo Grove, IL) equipped with a Leica ACS Apo 40× objective (numerical aperture = 1.15). Ten unique sample regions were imaged sequentially using a single photomultiplier tube (PMT) with excitation wavelengths of 405 nm (DAPI, blue), 488 nm (Alexa-Fluor 488, green), 532 nm (Alexa-Fluor 546, red), and 635 nm (Alexa-Fluor 633, magenta) and with emission wavelengths of 415 to 475 nm, 500 to 570 nm, 550 to 600 nm, and 640 to 700 nm, respectively. The number of primary (red) and secondary (green) was counted, and cross-sectional area of myofibers was measured using IMT iSolution Lite (version 9.4, IMT i-Solutions Inc., Vancouver, BC, Canada). Results were averaged for each lamb and subjected to statistical analyses as described below (Fig. 1).
Table 3.
Main effects of fescue seed treatment (endophyte-infected, E+ or endophyte-free, E−) and stage of gestation (MID, days 35 to 85 or LATE, days 86 to 133) on fetal size and organ weights at day 133 of gestation1
| Stage of gestation | MID | LATE | |||
|---|---|---|---|---|---|
| Fescue seed treatment | E− | E+ | E− | E+ | SE |
| Fetus, n | 28 | 31 | 29 | 30 | |
| Fetal body weight, g | 4297.1 | 4185.4 | 4465.5a | 4017.0b | 512.36 |
| Crown-rump length, cm | 51.12 | 50.92 | 54.55c | 53.57d | 2.63 |
| Thoracic circumference, cm | 35.07 | 34.98 | 35.79a | 34.26b | 1.71 |
| Abdominal circumference, cm | 31.06 | 29.90 | 31.36c | 29.61d | 3.80 |
| Carcass weight, g | 1953.1 | 1881.1 | 2024.5a | 1809.8b | 282.36 |
| Pelt, head, feet weight, g | 1409.0 | 1375.3 | 1453.1a | 1331.2b | 152.82 |
| Digesta, g | 680.1 | 648.4 | 699.9a | 628.6b | 123.08 |
| Individual organs, g | |||||
| Brain | 50.56 | 48.82 | 50.54 | 48.85 | 6.09 |
| Heart | 33.19 | 31.25 | 33.79c | 30.64d | 5.52 |
| Liver | 107.29 | 109.38 | 115.74a | 100.94b | 19.61 |
| Lungs | 148.21 | 149.91 | 149.43 | 148.69 | 22.71 |
| Thymus | 17.93 | 16.52 | 19.30a | 15.15b | 6.83 |
| Percent of EBW basis | |||||
| Carcass | 48.31 | 47.82 | 48.37 | 47.76 | 2.03 |
| Pelt, head, feet | 34.84 | 35.33 | 34.67 | 35.50 | 1.99 |
| Digesta | 7.25 | 7.06 | 7.36 | 6.94 | 1.33 |
| Individual organs, % EBW | |||||
| Brain | 2.46 | 2.56 | 2.34b | 2.67a | 0.42 |
| Heart | 0.82 | 0.80 | 0.80 | 0.82 | 0.090 |
| Liver | 5.16 | 5.52 | 5.43 | 5.25 | 0.67 |
| Lungs | 7.24 | 7.66 | 7.06b | 7.84a | 1.00 |
| Thymus | 0.83 | 0.84 | 0.88 | 0.78 | 0.31 |
abMeans in the same row with uncommon superscripts differ (P < 0.05).
cdMeans in the same row with uncommon superscripts differ (P < 0.10).
1The interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.15).
Figure 1.
Confocal laser scanning microscope images of semitendinosus muscle fibers. Cryosections were stained with antibodies to myosin heavy chain (MHC)-fast (red) to identify Type 2 muscle fibers and antibodies to MHC-slow (green) to identify Type 1 muscle fibers. Samples were also counterstained with DAPI to highlight the nucleus (blue) and wheat germ agglutinin (magenta) to decorate the extracellular matrix. Representative images from each treatment group [E−/E− (A), E+/E− (B), E−/E+ (C), and E+/E+ (D)] are shown here.
Sample Preparation for miRNA Sequencing
A subset of lambs (n = 12; 3 per treatment) was selected for miRNA sequencing based on fetal weight representing the mean response for each treatment and was not from the same ewe but instead from 3 different ewes for each treatment. The sequencing subset included 3 of the 4 samples that were used for muscle fiber histology. Muscle samples were collected from each lamb within 30 min of euthanasia. Samples were trimmed of any adipose or connective tissues, diced, frozen in liquid nitrogen, and stored at −80 °C until subsequent RNA extraction. Total cellular RNA was extracted using mirVANA miRNA Isolation Kit (Invitrogen, Thermo Fisher, Waltham, MA). RNA yield and quality were analyzed using a NanoDrop 1000 Spectrophotometer (Thermo Fisher). RNA integrity number (RIN) was determined using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). All samples had a RIN value of 7.0 or greater. RNA samples were shipped on dry ice to LCSciences (Houston, TX) for miRNA sequencing and data analyses.
miRNA Transcriptome
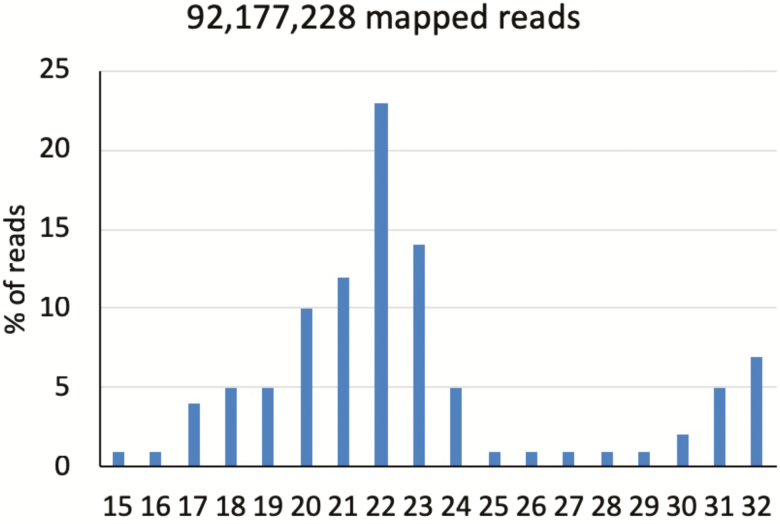
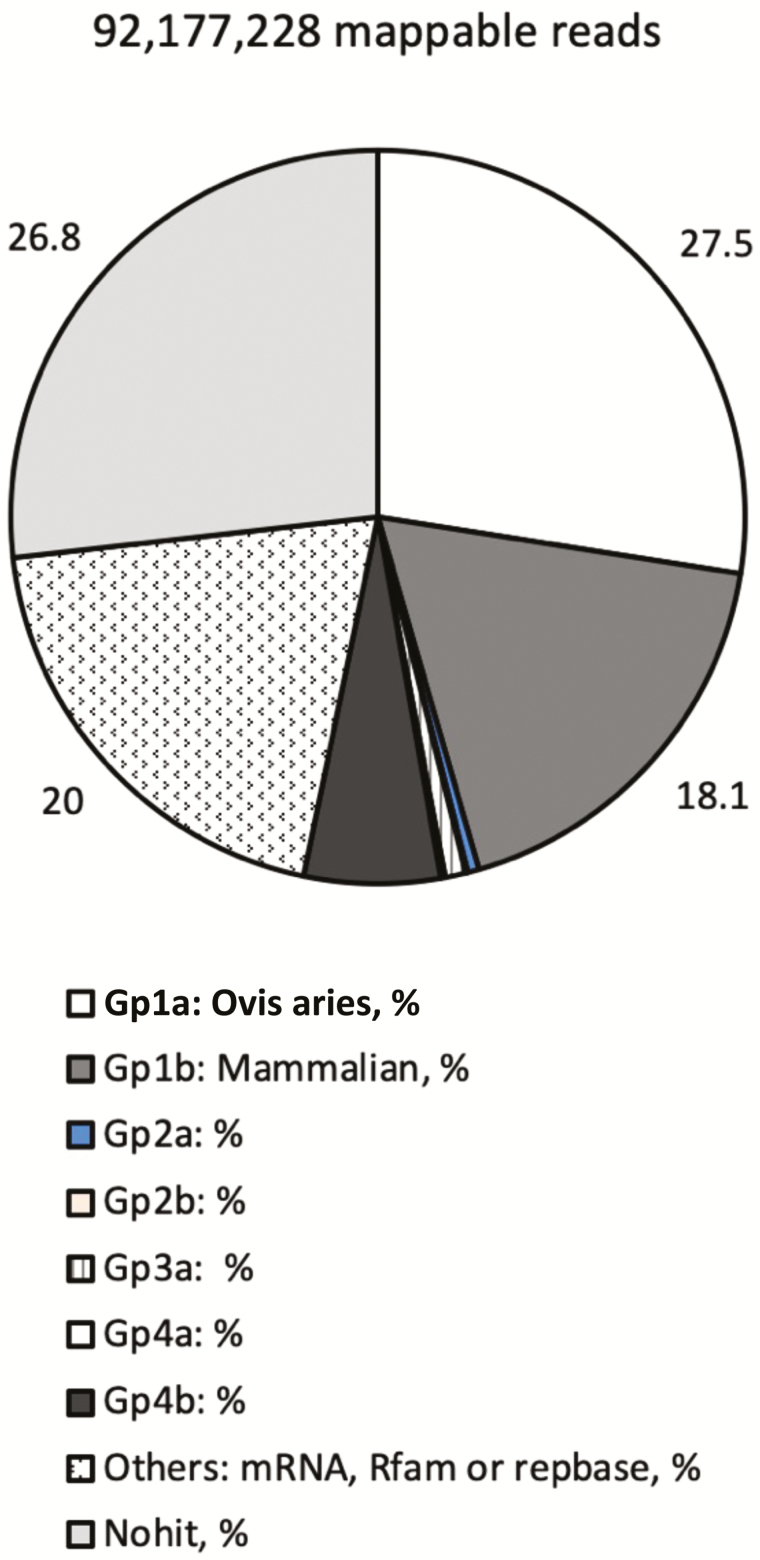
The RNA samples were processed to generate a cDNA library, which was utilized for sequencing. Sequencing generated a raw read count of 113, 525, and 743. The 3′ adaptor sequences were trimmed from the reads. Reads without a 3′ adaptor (5.2%) and reads less than 15 nucleotides (6.3%) or greater than 32 nucleotides (7.1%) were discarded. The remaining reads (81.2%) were utilized for mapping. This generated an average of 7,681,436 mappable reads per sample with 98% of reads having a Phred score (a prediction of the probability of an incorrect base-call) greater than 36. The following analysis was carried out by aligning reads to know miRNA sequences available in miRbase v21.0 followed by mapping to the ovine genome (ftp://ftp.ensembl.org/pub/release83/fasta/ovia_aries/dna/Ovis_aries.Oar_v3.1. dna.toplevel.fa.gz) as well as the genomes of other mammalian species. The results of database mapping can be seen in Fig. 5. Mappable reads were mapped to pre-miRNA (miRNAs) sequences available in miRbase for both the ovine (oar) genome and the genomes of additional mammalian species for the discovery of novel miRNA. Twenty-seven and a half percent of reads mapped discretely to miRNAs that aligned to the ovine genome (gpa1) and 18.1% mapped to miRNAs that aligned to genomes of Mammalia species (gp1b). The additional Mammalia species included, but were not limited to, bovine (bta), swine (ssc), orangutan (ppy), opossum (mdo), and human (hsa). Reads for miRNAs that were not mappable were annotated to ovine and mammalian genomes. This identified extended sequences that potentially form hairpin structures (gp2a; 0.5% of reads) and those that do not (gp2b; 0.1% of reads). Reads that went unmapped to the ovine and mammalian genomes were then mapped to mature miRNA sequences (gp3a; 0.9% of reads). Although 12,000 reads remained unmapped (gp3b), this accounted for 0.00% of total reads. Reads that went unmapped to miRNAs in miRbase were mapped to mRNA, Rfam (http://rfam.xfam.org/), and Repbase (https://www.girinst.org/repbase/) databases. Reads that mapped to mRNA, Rfam, or Repbase represented 20% of the total mappable reads and are denoted as “other.” Reads unmapped to mRNA, Rfam, or Repbase were mapped to the ovine genome and were identified as those likely to form hairpins (gp4a; 0.2% of reads) and those that were not (gp4b; 6.0%). Reads that did not map to miRNAs in miRbase, mRNA, Rfam, Repbase, or the ovine genome were considered “nohit” and represented 26.8% of reads. This analysis leads to the identification of 208 known ovine miRNA (gp1a) and 676 miRNA known to other species but novel to ovine (gp1b). An additional 4,280 unique miRNA were predicted.
Figure 5.
Effect of feeding endophyte-infected (E+) or endophyte-free (E−) tall fescue seed to ewes during MID (d 35–85) and/or LATE (d 86–133) gestation on fetal semitendinosus muscle miRNA distribution of read length.
Statistical Analysis
Data were analyzed as a 2 × 2 factorial using the Mixed procedure of SAS (SAS 9.3, SAS Inst. Inc., Cary, NC) with fescue treatment, stage of gestation, and 2-way interaction as fixed effects in the model. Group (block) was included as a random variable in the model. Lamb was the experimental unit for all analyses (Hoffman et al., 2016a). For muscle histology, the model included section and image as random effects in the model. Fetal lamb number (single, twin, and triplet) was included as a covariate when significant (P < 0.05). Fetal lamb sex was evaluated as a covariate, but was not significant (P > 0.05) for any variable and therefore not included in the final model. Least squares means were generated and adjusted using Tukey for all pairwise comparisons. Significance was determined at P < 0.05 with trends at P < 0.10. Differential gene expression analysis for all miRNA data was provided by LCSciences.
RESULTS AND DISCUSSION
Fetal Measures
Information on the number of ewes and fetuses are presented in Table 2. Fetal number per ewe (P > 0.28) and fetal sex (P > 0.27) did not differ among fescue treatments fed during MID or LATE gestation. The interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.54). Overall, lambing rate would have been 180% in this experiment, which is within the normal range for mature Suffolk ewes (Notter, 2000).
Table 2.
Main effects of fescue seed treatment (endophyte-infected, E+ or endophyte-free, E−) and stage of gestation (MID, day 35 to 85 or LATE, days 86 to 133) on fetal number and sex at day 133 of gestation1
| Stage of gestation | MID | LATE | |||
|---|---|---|---|---|---|
| Fescue seed treatment | E− | E+ | E− | E+ | SE |
| Number of pregnant ewes | 16 | 16 | 16 | 16 | |
| Number of fetuses | 28 | 31 | 29 | 30 | |
| Fetal number/ewe | 1.75 | 1.94 | 1.81 | 1.88 | 0.55 |
| Fetal sex2 | 1.50 | 1.52 | 1.43 | 1.59 | 0.49 |
1The interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.54).
2Fetal sex: 1 = male, 2 = female.
For fetal size and organs (Table 3), the interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.15) with the exception of fetal kidney weight (P = 0.058) and percentage (P = 0.035), pancreas weight (P = 0.036) and percentage (P = 0.042), and spleen weight (P = 0.077) and percentage (P = 0.036). Feeding E− fescue seed during MID gestation did not alter (P > 0.21) fetal BW or body measures. Fetuses from ewes fed E+ seed during LATE gestation (E+/E+ and E−/E+) seed had smaller (P = 0.0013) fetal weight at day 133 of gestation by an average of 10% compared with E−. Fetuses from ewes fed E+ seed during LATE gestation also had smaller thoracic circumference (P = 0.0016), carcass weight (P = 0.0065), pelt/head/feet weight (P = 0.0044), and digesta (P = 0.035) weights compared with E−. Crown-rump length and abdominal circumference tended (P = 0.066 and 0.091, respectively) to be smaller for fetuses from ewes fed E+ fescue seed during LATE gestation compared with E−. When expressed on an EBW basis, the percentage of carcass, pelt/head/feet, or digesta did not differ (P > 0.14) by fescue seed treatment fed at MID or LATE gestation. The reduction in body weight and dimensions for fetuses exposed to E+ fescue seed during LATE gestation are indicative of intrauterine growth restriction (IUGR). Previous studies in both sheep (Duckett et al., 2014a) and cattle (Watson et al., 2004) also reported reduced lamb and calf birth weights at term due to ergot alkaloid exposure during gestation. Duckett et al. (2014a) noted a 36% reduction in lamb birth weight, and Watson et al. (2004) reported a 15% reduction in calf birth weight. In contrast, Caldwell et al. (2013) and Shoup et al. (2016) did not find differences in calf birth weight when cows grazed E+ tall fescue during gestation compared with nontoxic, novel endophyte-infected tall fescue pastures. These studies differ from the current study in that pregnancies were carried to term and fetuses were not harvested prior to parturition. Due to shortened gestation length observed in previous study with E+ fescue seed (Duckett et al., 2014a), this study was designed to collect fetuses at the same gestational age. A consequence of hyperthermia-induced IUGR in sheep has been noted as a reduction in the umbilical blood flow and an increase in the umbilical artery Doppler velocimetry index in addition to decreased fetal weight (Galan et al., 2005). Klotz et al. (2019) found that both uterine and umbilical arteries collected from ewes in this study were vasoactive in the presence of ergot alkaloids but that the umbilical artery had a much greater response. Rattray et al. (1974) documented that over 80% of fetal growth occurs in twin-bearing ewes during the last trimester of gestation and thus ergot alkaloid exposure during late gestation appears to have the greatest impact (−10%) on fetal growth.
Weights of the brain (P = 0.18) and lungs (P = 0.84) did not differ among fescue treatments fed at different stages of gestation. Feeding E+ seed during LATE gestation (E+/E+ and E−/E+) resulted in fetuses with smaller heart (P = 0.036), liver (P = 0.0069), and thymus (P = 0.027) weights compared with E− fetuses. On an EBW basis, E+ fetuses had larger brain (P = 0.0048) and lung (P = 0.0053) percentages than E−. Fetuses with a smaller birth weight also had asymmetric growth, evidenced by having significantly larger brain to body weight ratio. During conditions of IUGR, fetal brain growth will be conserved at the expense of muscle, bone, and fat development, leading to larger brain mass as a percentage of body weight (Forbes et al., 1977; Rabin et al., 1994). In this study, brain weight did not differ with E+ fescue exposure even though fetal weight was reduced, and therefore, when expressed on a body weight basis, fetuses exposed to E+ fescue during LATE gestation had heavier brain weight as a percentage of EBW compared to E−. These results agree with others who reported that maternal food restriction during late gestation (Redmer et al., 2012) or throughout gestation (Lopez-Tello et al., 2017) caused asymmetrical growth in fetuses. The increased brain:fetal weight ratio is indicative of IUGR and demonstrates that exposure to E+ fescue during LATE gestation (days 85 to 133) causes IUGR in sheep.
Fetal kidney weight tended to be higher (P = 0.058) for E−/E− compared with all other E+ fescue treatments (Table 4). On an EBW basis, the percentage of kidney was lower (P = 0.024) for E+/E− compared with E−/E−. Nephrogenesis in the sheep begins prior to day 50 of gestation, peaks by day 80, and ends around day 120 (Gimonet et al., 1998). The timeline for fetal kidney development may explain why E+ fescue exposure regardless of stage of gestation reduced fetal kidney weight. Pancreas weight was lower (P = 0.036) for E+/E+ fetuses compared with all other treatments. On an EBW basis, the percentage of pancreas was lower (P = 0.042) for E+/E+ compared with E+/E−. Spleen weight tended to be lower (P = 0.077) for E+/E+ compared with E−/E− or E+/E−. On an EBW basis, the percentage of spleen remained smaller (P = 0.036) for E+/E+ and E−/E− fetuses compared with E+/E−. Similarly, Duckett et al. (2014a) noted similar reductions in kidney and spleen weights at birth in lambs from dams fed E+ fescue seed during gestation (day 35 to parturition). Camacho et al. (2017) also reported smaller pancreas mass in hyperthermia-induced IUGR fetuses that lead to alterations in glucose utilization and insulin sensitivity at 3 d of age.
Table 4.
Simple effects of fescue seed treatment (endophyte-infected, E+ or endophyte-free, E−) by stage of gestation (MID, days 35 to 85 or LATE, days 86 to 133) on fetal kidney, pancreas, and spleen1
| Fescue seed/stage of gestation | E−/E− | E+/E− | E−/E+ | E+/E+ | SE |
|---|---|---|---|---|---|
| Fetus, n | 14 | 15 | 14 | 16 | |
| Kidneys, g | 22.42a | 19.44b | 18.69b | 19.14b | 3.32 |
| Kidneys, % EBW | 0.52a | 0.47b | 0.49ab | 0.51ab | 0.064 |
| Pancreas, g | 3.61a | 3.87a | 3.50a | 2.69b | 0.94 |
| Pancreas, % EBW | 0.16ab | 0.19a | 0.18ab | 0.15b | 0.047 |
| Spleen, g | 5.89c | 6.59c | 5.45cd | 4.62d | 1.60 |
| Spleen, % EBW | 0.26b | 0.32a | 0.28ab | 0.25b | 0.071 |
abMeans in the same row with uncommon superscripts differ (P < 0.05).
cdMeans in the same row with uncommon superscripts differ (P < 0.10).
1The interaction between fescue seed treatment and stage of gestation was significant (P < 0.10).
Fat depots, on a weight or EBW basis, were not influenced (P > 0.43) by fescue seed treatment fed during MID or LATE gestation (Table 5). Brown adipose tissue is vital to newborn lambs as a source of heat via nonshivering thermogenesis (Smith and Horwitz, 1969) and originates from myogenic lineage (Seale et al., 2008). Low abundance of BAT or a failure to express UCP1 may lead to hypothermia in newborn lambs (Clarke et al., 1996). Therefore, we collected scapular adipose depots to confirm BAT and measure UCP1 abundance. Scapular adipose depots were confirmed to be BAT by the presence of both UCP1 (Symonds, 2013) and MYF5 (Seale et al., 2008). However, there was no difference (P > 0.75) in the abundance of UCP1 and MYF5 by fescue treatment in the BAT (data not shown). Pope et al. (2012) noted that at day 80 of gestation there was no UCP1 activity in fetal adipose tissue but that UCP1 activity level peaked by day 140 to birth. Additional research would be needed to evaluate BAT mass and UCP1 abundance closer to parturition to determine if fescue treatment alters BAT and influences lamb survival at birth.
Table 5.
Main effects of fescue seed treatment (endophyte-infected, E+ or endophyte-free, E−) and stage of gestation (MID, days 35 to 85 or LATE, days 86 to 133) on fetal fat depots and individual muscles at day 133 of gestation1
| Stage of gestation | MID | LATE | |||
|---|---|---|---|---|---|
| Fescue seed treatment | E− | E+ | E− | E+ | SE |
| Fetus, n | 28 | 31 | 29 | 30 | |
| Fat depots, g | |||||
| Heart fat | 3.39 | 3.40 | 3.64 | 3.16 | 1.97 |
| Internal fat | 5.51 | 5.51 | 5.78 | 5.24 | 2.93 |
| Kidney fat | 19.27 | 18.33 | 19.86 | 17.74 | 4.66 |
| Omental fat | 5.30 | 5.25 | 5.55 | 5.01 | 1.64 |
| Subcutaneous fat | 1.51 | 0.71 | 1.24 | 0.98 | 2.31 |
| Mesenteric fat | |||||
| Fat depots, % of EBW | |||||
| Heart fat | 0.17 | 0.18 | 0.17 | 0.17 | 0.094 |
| Internal fat | 0.26 | 0.28 | 0.26 | 0.29 | 0.16 |
| Kidney fat | 0.93 | 0.94 | 0.92 | 0.95 | 0.23 |
| Omental fat | 0.24 | 0.27 | 0.24 | 0.26 | 0.073 |
| Subcutaneous fat | 0.077 | 0.043 | 0.058 | 0.061 | 0.12 |
| Mesenteric fat | 0.19 | 0.19 | 0.18 | 0.20 | 0.106 |
| Individual muscles, g | |||||
| Biceps femoris | 26.65 | 26.54 | 28.08a | 25.11b | 4.54 |
| Gluteus medius | 15.81 | 15.59 | 16.56c | 14.85d | 3.28 |
| Longissimus | 50.90 | 50.51 | 53.61a | 47.80b | 7.92 |
| Psoas major + minor | 13.33 | 12.60 | 14.02a | 11.90b | 3.17 |
| Quadriceps femoris | 36.12 | 35.35 | 38.60a | 32.86b | 7.54 |
| Semimembranosus + adductor | 39.10 | 37.90 | 42.30a | 34.69b | 6.81 |
| Semitendinosus | 9.01 | 8.84 | 9.69a | 8.16b | 2.48 |
| Individual muscles, % of EBW | |||||
| Biceps femoris | 1.32 | 1.34 | 1.35 | 1.31 | 0.16 |
| Gluteus medius | 0.78 | 0.79 | 0.79 | 0.78 | 0.11 |
| Longissimus | 2.54 | 2.56 | 2.58 | 2.52 | 0.24 |
| Psoas major + minor | 0.66 | 0.64 | 0.67 | 0.63 | 0.14 |
| Quadriceps femoris | 1.78 | 1.79 | 1.84 | 1.73 | 0.25 |
| Semimembranosus + adductor | 1.94 | 1.92 | 2.03a | 1.83b | 0.23 |
| Semitendinosus | 0.44 | 0.45 | 0.46 | 0.43 | 0.095 |
abMeans in the same row with uncommon superscripts differ (P < 0.05).
cdMeans in the same row with uncommon superscripts differ (P < 0.10).
1The interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.15).
The interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.34) for muscle mass, percentage, or body composition. Feeding E− fescue seed during MID gestation did not alter (P > 0.21) fetal muscle mass, percentage, or body composition. Weights of all individual muscles excised were lighter (P < 0.05) in fetuses exposed to E+ fescue seed during LATE gestation compare with E− (Table 5). These 9 muscles represent about 60% of total muscle weight and make a major contribution to the retail value in finished lamb carcasses. Semimembranosus and adductor weight as a percent of EBW was greater (P = 0.044) for E−/E− fetuses than E−/E+ and E+/E+. Other muscle weights as percent of EBW did not differ (P > 0.30) among fescue treatments. A consequence of IUGR has been noted as a reduction in the amount of hindlimb muscle as a percent of the total body weight due to reduced protein synthesis and accretion rates (Rozance et al., 2018). Yates et al. (2016) discovered that muscle fiber size in both STN and BF was reduced in IUGR fetuses and that could explain the overall reduction in lean muscle mass. In addition, Duckett et al. (2014a) noted that neonatal lambs from ewes fed E+ fescue during gestation had reduced hindlimb muscle (ST, GM, SM, QF, and BF) weights that when normalized to EBW were not different from E− lambs.
Total fat-free lean weight was lighter (P = 0.002) by 15.3% for E+/E+ than to E−/E− and E+/E− fetuses (Table 6). Total bone weight was heavier (P = 0.011) for E− fetuses than those of E+ exposed fetuses during LATE gestation. Total fat (subcutaneous, intermuscular, and intramuscular) weight did not differ (P > 0.45) among treatments. Duckett et al. (2014a) also reported lower total muscle weights for lambs at birth born to ewes fed E+ fescue seed from day 35 to parturition. On an EBW basis, there were no differences (P > 0.51) in total fat-free lean, bone, or fat content of the carcass. There were also no differences (P > 0.32) in proximate composition (moisture, crude protein, total lipid, crude lipid, glycogen, and ash content) of the lean muscle from fetuses based on fescue seed treatment. A reduction in muscle mass at birth and an increase in central fat deposition later in life are characteristics of IUGR fetuses (Gale et al., 2001; Sayer et al., 2004).
Table 6.
Main effects of fescue seed treatment (endophyte-infected, E+ or endophyte-free, E−) and stage of gestation (MID, days 35 to 85 or LATE, days 86 to 133) on fetal body composition at day 133 of gestation1
| Stage of gestation | MID | LATE | |||
|---|---|---|---|---|---|
| Fescue seed treatment | E− | E+ | E− | E+ | SE |
| Carcass composition (right side), g | |||||
| Total fat-free lean | 314.51 | 310.21 | 338.32a | 286.39b | 64.53 |
| Bone | 669.27 | 658.38 | 695.85a | 631.80b | 99.53 |
| Total fat | 11.19 | 9.82 | 11.52 | 9.49 | 4.18 |
| Carcass composition, % of EBW | |||||
| Total fat-free lean | 31.14 | 31.11 | 31.64 | 30.61 | 67.19 |
| Total bone | 66.62 | 66.49 | 66.05 | 67.06 | 4.73 |
| Total fat | 1.07 | 1.00 | 1.06 | 1.02 | 0.37 |
| Proximate composition of total lean, % | |||||
| Moisture | 80.78 | 79.76 | 80.43 | 80.12 | 3.28 |
| Crude protein | 12.50 | 12.60 | 12.68 | 12.42 | 2.91 |
| Crude fat | 2.15 | 2.22 | 2.08 | 2.30 | 0.68 |
| Ash | 0.88 | 0.93 | 0.90 | 0.90 | 0.16 |
| Glycogen (by difference) | 3.68 | 4.49 | 3.91 | 4.27 | 1.90 |
abcMeans in the same row with uncommon superscripts differ (P < 0.05).
defMeans in the same row with uncommon superscripts differ (P < 0.10).
1The interaction between fescue seed treatment and stage of gestation was nonsignificant (P > 0.15).
Muscle Fiber Histology
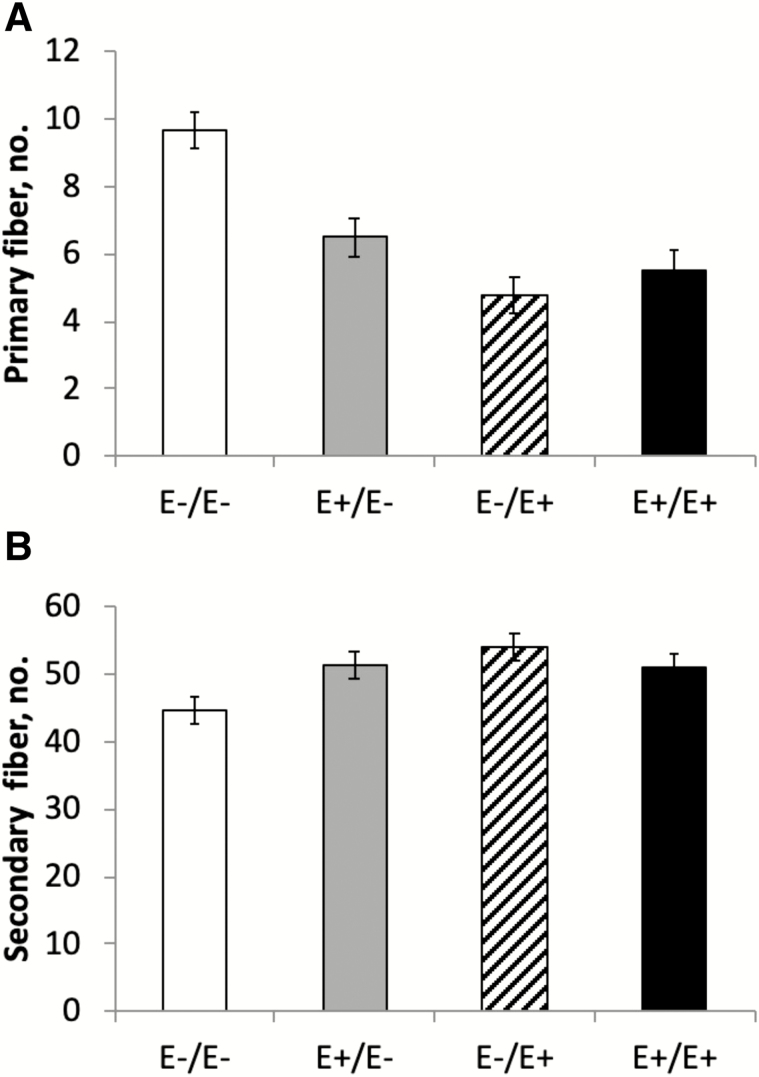
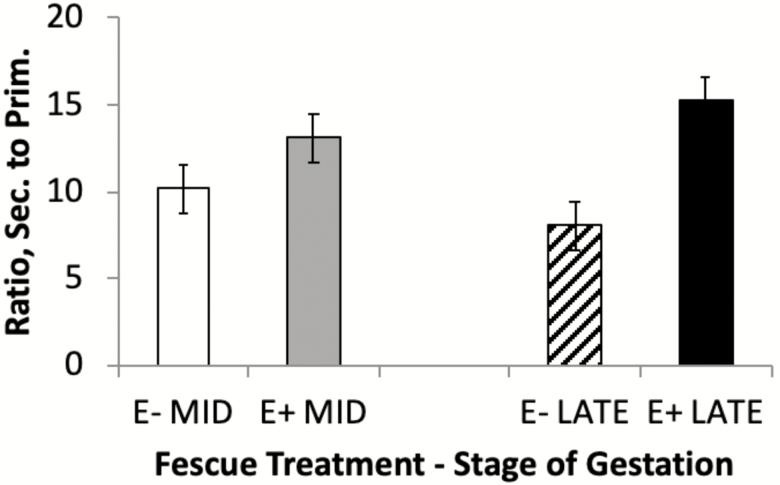
The interaction between fescue seed treatment and stage of gestation was significant for primary (P = 0.0005) and secondary (P = 0.027) fiber numbers. Semitendinosus muscles of fetuses exposed to E+ fescue seed during MID and/or LATE gestation had fewer (P = 0.0005) primary (Type I) muscle fiber numbers compared with E−/E− (Fig. 2A). Similarly, Yates et al. (2016) also observed a reduction in STN primary fiber number with hyperthermia-induced IUGR fetuses. In contrast, maternal undernutrition during early gestation (<90 d) increased primary fiber number in STN (Fahey et al., 2005a; Sen et al., 2016). Secondary (Type II) fiber number was greater (P = 0.027) in fetuses from ewes fed E+ fescue seed during LATE gestation only (E−/E+) compared with E−/E− (Fig. 2B). This translated to a higher ratio of secondary to primary muscle fibers in the STN muscle of fetuses from ewes fed E+ seed during MID (P = 0.040) and LATE (P < 0.0001) gestation compared with E− (Fig. 3). In contrast, Duckett et al. (2014a) reported a lower secondary fiber number and secondary to primary fiber ratio in STN muscle of Southdown lambs born to ewes fed E+ fescue seed from day 35 to parturition. These differences may be related to differences in muscle fiber formation between Suffolk and Southdown lambs. Rehfeldt et al. (2000) suggested that in pigs over 50% of phenotypic variation in muscle fiber number is due to genetic origin. Cross-sectional area of ST primary and secondary fibers did not differ (P > 0.20) among treatments. Our results show that E+ fescue exposure during both MID (days 35 to 85) and LATE (days 85 to 133) gestation reduced primary fiber number in STN. In the sheep, primary myogenesis is estimated to be complete by days 32 to 38 of gestation (Oksbjerg and Therkildsen, 2017), and secondary myogenesis is estimated to be complete by 85 to 90 d of gestation (Fahey et al., 2005b; Sen et al., 2016). Therefore, changes in primary fiber number with exposure to ergot alkaloids suggests heightened transition of primary to secondary fibers during both MID and LATE gestation in Suffolk lambs.
Figure 2.
Simple effects of fescue seed treatment [endophyte-infected (E+) or endophyte-free (E−)] by stage of gestation (MID, days 35 to 85 or LATE, days 86 to 133) on fetal semitendinosus primary (Type I) and secondary (Type II) muscle fiber number on day 133 of gestation. The interaction between fescue seed treatment and stage of gestation was significant for primary (P = 0.0005) and secondary (P = 0.027) fiber types. abcMeans with uncommon superscripts differ (P < 0.05).
Figure 3.
Main effects of fescue seed treatment [endophyte-infected (E+) or endophyte-free (E−)] and stage of gestation (MID, days 35 to 85 or LATE, days 86 to 133) on fetal semitendinosus ratio of secondary (sec.) to primary (Prim.) muscle fibers at day 133 of gestation. The interaction between fescue seed treatment and stage of gestation was nonsignificant (P = 0.27). abMeans with uncommon superscripts differ (P < 0.05).
miRNA Sequencing
The total number of mappable reads for the ST was 92,177,228 or 81.2% of total raw reads. Of the mappable reads, 27.5% of reads were mapped to miRNA that have been previously identified in Ovis aries (Gp1a; Fig. 4). In addition, 18.1% of reads were mapped to miRNA previously identified in other mammalian species, but are novel to O. aries (Gp1b). As such, these miRNAs are prefaced by a species identifier associated with the species genome to which they have previously been aligned. The most abundant length was 22 nt (23%), followed by 23 nt (14%) and 21 nt (12%; Fig. 5). This is consistent with the known 21 to 23 nt range for mature miRNA. Several myo-miRNAs have been identified (miR-1, −133, −206) and are known to be directly involved in skeletal muscle differentiation (Luo et al., 2013; Horak et al., 2016; Ma et al., 2017). miR-1 and miR-206 were present in high abundance in this study but were not differentially expressed due to fescue treatment. Similarly, others have reported the lack of differential regulation of myo-miRNAs in skeletal muscle of market pigs (Daza et al., 2017) and ovine fetuses (Lie et al., 2014).
Figure 4.
Effect of feeding endophyte-infected (E+) or endophyte-free (E−) tall fescue seed to ewes during MID (D 35–85) and/or LATE (d 86–133) gestation on fetal semitendinosus muscle miRNA transcriptome and percentage of reads (92,177,228) mapped to selected miRNA in miRbase.
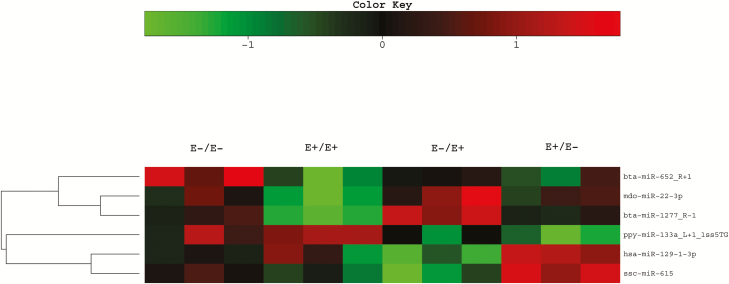
Six miRNA were differentially expressed (P < 0.010) in STN muscle of fetuses exposed to E+ or E− fescue seed during MID and LATE gestation (Fig. 6). The differentially regulated miRNA include bta-miR-652_R+1, mdo-miR-22-3p, bta-miR-1277_R-1, ppy-miR-133a_L+1_1ss5TG, hsa-miR-129-1-3p, and ssc-miR-615. These miRNAs were previously mapped in other species but are new to the ovine genome. miRNA are highly conserved across species (Meunier et al., 2013) and function as post-transcriptional regulators of gene expression to promote mRNA degradation (Guo et al., 2010). Others have shown that maternal over and under nutrition alters mRNA expression of key myogenic factors (Reed et al., 2014) and differentially regulate mRNA involved in protein synthesis and muscle metabolism using RNA sequencing (Hoffman et al., 2016b). However, the effects of E+ fescue exposure on fetal muscle miRNA expression have not been explored previously and may be involved in the regulation of myogenesis.
Figure 6.
Effect of feeding endophyte-infected (E+) or endophyte-free (E−) tall fescue seed to ewes during MID (d 35–85) and/or LATE (d 86–133) gestation on fetal semitendinosus muscle miRNA that were differentially regulated (P < 0.01) by maternal dietary treatment.
Feeding E+ fescue seed during MID gestation only (E+/E−) downregulated (P = 0.0057) ppy-miR-133a_L+1_1ss5TG expression in fetal STN when compared with E−/E− and E+/E+ treatments. The miR-133a sequence is highly conserved across multiple species. miR-133a is considered a muscle tissue-specific miRNA (McCarthy et al., 2009) and has been shown to indirectly target solute carrier family 2 member 4 (SLC2A4 or GLUT4) and serum response factor (Esteves et al., 2017). GLUT4 is the primary glucose transporter in both brown and white adipose tissue as well as skeletal and cardiac muscle tissue (Uldry and Thorens, 2004). miR-133a and miR-133b have been shown to target Klf15 (Krüeppel-like factor 15), which is the transcription factor for GLUT4, leading to a reduction in GLUT4 and insulin-stimulated glucose uptake in rat cardiomyocytes (Horie et al., 2009). Chen et al. (2018) showed that a reduction in miR-133a can cause an increase in the amount of cell proliferation and differentiation in developing chicken skeletal muscle.
Feeding ewes E+/E+ seed reduced (P = 0.0057) abundance of mdo-miR-22-3p in fetal ST tissue when compared with all other treatments. Krauss et al. (2018) found downregulation of miR-22-3p in heart muscle of near-term (140 to 142 d) or pre-term (127 to 129 d) fetuses compared with lambs at birth. Lie et al. (2014) reported upregulation of miR-22-3p in quadriceps muscle at days 136 to 138 of gestation in twin fetuses from ewes underfed (70% of control) during periconceptional (−60 to 0 d) and preimplantation (0 to 6 d) periods compared with controls. Lie et al. (2014) found 22 miRs in fetal skeletal muscle that were differentially regulated and target specific proteins in the insulin-signaling pathway. Switching from E+ to E− fescue seed upregulated (P = 0.009) abundance of has-miR-129-1-3p in fetal STN tissue; however, switching from E− to E+ downregulated (P = 0.0010) abundance of has-miR-129-1-3p. miR-129-3p is associated with angiotensin II type 1 receptor in cardiac myocytes, which regulates blood pressure (Jeppesen et al., 2011).
ssc-miR-615 was upregulated (P = 0.00076) in STN muscle of fetuses from E+/E− treatment compared with all other treatments. Based on in vitro results from C2C12 cells, miR-615 levels, along with 13 other miRNAs, are negatively correlated with intracellular ATP levels (Siengdee et al., 2015). The study also found that there are 6 possible targets for miR-615 (Cox4i2, Cox6a2, Ndufb7, Ndufs4, Ndufs5, and Ndufv1). The COX genes function in the electron transport chain in oxidative phosphorylation (Quintens et al., 2013). Electron transport chain complex 1 is made of the NADH dehydrogenase family of proteins that encompasses Ndufb7, Ndufs4, Ndufs5, and Ndufv1 (Triepels et al., 2001).
bta-652_R+1 was downregulated (P = 0.0055) by maternal E+ treatment in MID gestation (E+/E− and E+/E+) when compared with E−/E− treatment. Muroya et al. (2016) also identified differential expression of bta-miR-652 levels in the LM of grass-fed cattle vs. grain-fed cattle. A partial gene target for miR-652 is phosphatase and tension homolog, which is responsible for inhibiting phosphatidylinositol-3-kinase (PI3K) signaling. The PI3K signaling pathway is a key element in insulin-dependent glucose uptake in muscle and adipose tissue, and insulin resistance can prevent the maturation of myofibers (Hu et al., 2010). bta-miR-1277_R-1 was upregulated (P = 0.000017) in fetal ST muscle from ewes fed E+ fescue seed during LATE gestation only (E−/E+) compared with all other treatments. The hsa-miR-1277-3p and bta-miR-1277-R-1 sequences are identical. In humans, has-miR-1277-3p downregulates lipoprotein lipase, which hydrolyzes lipoprotein triacylglycerides (Caussy et al., 2016). Placental insufficiency-induced IUGR has been linked to the accumulation of adipose tissue and insulin resistance in postnatal life (Garofano et al., 1997; Béringue et al., 2002). In the present study, E+ fescue seed exposure during MID and LATE gestation altered the miRNA profile of fetal STN compared to E−/E−. Six miRNA were identified and are associated with glucose transport, insulin signaling, intracellular ATP, hypertension, or adipogenesis.
Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
The gene targets of differentially expressed miRNAs were annotated using the Kyoto Encyclopedia of Genes and Genomes pathway. One hundred and sixty-five differentially expressed genes were utilized, and 13 pathways were identified as significantly enriched (false discovery rate < 0.05; Table 7). These pathways included cytokine–cytokine receptor interaction, Jak-STAT signaling pathway, hematopoietic cell lineage, osteoclast differentiation, and several disease/immune response factors. Skeletal muscle development is regulated by a variety of cytokines and growth factors, many of which overlap with those involved in osteogenesis and bone formation (DiGirolamo et al., 2013). McKinney-Freeman et al. (2002) have confirmed that there are hematopoietic stem and progenitor cell populations, which are distinct from satellite cell populations, located in muscle tissue. In myoblasts, the Jak-STAT pathway has an important role in promoting proliferation and differentiation (Sun et al., 2007; Wang et al., 2008). Collectively, cytokine–cytokine receptor interaction and the pathways of Jak-STAT and the toll-like receptor indicate that exposure to E+ fescue seed in utero may influence both intracellular and extracellular communication in the STN muscle of fetal lambs.
Table 7.
mirRanda results (P < 0.01) based on semitendinosus miRNA sequencing
| KEGG1-pathway term | Count | P-value | Fold enrichment | FDR2 |
|---|---|---|---|---|
| oas040603: Cytokine–cytokine receptor interaction | 24 | 1.63E-09 | 4.57 | 2.08E-06 |
| oas04630: Jak-STAT signaling pathway | 20 | 1.72E-09 | 5.63 | 2.19E-06 |
| oas04620: Toll-like receptor signaling pathway | 14 | 2.69E-07 | 6.27 | 3.43E-04 |
| oas04640: Hematopoietic cell lineage | 13 | 2.71E-07 | 6.95 | 3.45E-04 |
| oas05162: Measles | 15 | 8.45E-07 | 5.23 | 1.08E-03 |
| oas05133: Pertussis | 12 | 1.16E-06 | 6.80 | 1.48E-03 |
| oas05140: Leishmaniasis | 12 | 1.31E-06 | 6.72 | 1.67E-03 |
| oas05152: Tuberculosis | 17 | 3.65E-06 | 4.06 | 4.66E-03 |
| oas05142: Chagas disease (American trypanosomiasis) | 13 | 4.53E-06 | 5.36 | 5.77E-03 |
| oas04380: Osteoclast differentiation | 13 | 9.20E-06 | 5.01 | 1.17E-02 |
| oas05144: Malaria | 9 | 1.52E-05 | 7.84 | 1.94E-02 |
| oas05205: Proteoglycans in cancer | 16 | 2.93E-05 | 3.64 | 3.74E-02 |
| oas05164: Influenza A | 15 | 3.59E-05 | 3.79 | 4.58E-02 |
1KEGG = Kyoto Encyclopedia of Genes and Genomes.
2FDR = false discovery rate.
3oas = Ovis aries.
Conclusion
Fetuses from ewes fed E+ seed during LATE gestation (days 85 to 133) were smaller with a higher brain to EBW ratio, which demonstrates IUGR. Muscle mass was reduced in fetuses from ewes fed E+ seed during LATE gestation. Changes in primary muscle fiber number and miRNA of STN indicate that E+ fescue fed during MID and LATE gestation altered fetal muscle development. Our results show that ergot alkaloid exposure during gestation altered expression of 6 miRNA that are involved in glucose transport, insulin signaling, intracellular ATP, hypertension, or adipogenesis. Future research will continue to investigate mechanisms by which ergot alkaloids disrupt normal developmental processes and strategies that can be employed to mitigate these disruptions.
Footnotes
Technical Contribution No. 6695 of the Clemson University Experiment Station. This research was supported by United States Department of Agriculture National Institute of Food and Agriculture Grant No. 2015-67015-23218. The research reported in this publication was conducted using a Leica SPE Confocal, housed in the Clemson Light Imaging Facility (CLIF). CLIF is supported, in part, by the Clemson University Division of Research, NIH EPIC COBRE Award No. P20GM109094 and NIH SCBiocraft COBRE Award No. 5P20RR021949-03. The content of this material and any opinions, findings, conclusions, or recommendations expressed in this material are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Appreciation is expressed to M. C. Miller, R. L. Smith, and AVS 4220 Fetal Fescue Research class for animal management and laboratory assistance; J. G. Andrae, G. Aiken, and J. R. Strickland for experimental design assistance; and H. Holliday, C. M. Nigro, S. L. Pratt, and N. M. Long for surgical assistance.
LITERATURE CITED
- Aberle E. D. 1984. Myofiber differentiation in skeletal muscles of newborn runt and normal weight pigs. J. Anim. Sci. 59:1651–1656. doi:10.2527/jas1984.5961651x [DOI] [PubMed] [Google Scholar]
- Alexander G., and Williams D.. 1971. Heat stress and development of the conceptus in domestic sheep. J. Agric. Sci. 76:53–72. [Google Scholar]
- Béringue F., Blondeau B., Castellotti M. C., Bréant B., Czernichow P., and Polak M.. 2002. Endocrine pancreas development in growth-retarded human fetuses. Diabetes 51:385–391. [DOI] [PubMed] [Google Scholar]
- Britt J. L., Greene M. A., Bridges W. C., Klotz J. L., Aiken G. E., Andrae J. G., Pratt S. L., Long N. M., Schrick F. N., Strickland J. R., et al. 2019. Ergot alkaloid exposure during gestation alters. I. Maternal characteristics and placental development of pregnant ewes. J. Anim. Sci. 97:1874–1890. doi: 10.1093/jas/skz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. D., Coffey K. P., Jennings J. A., Philipp D., Young A. N., Tucker J. D., Hubbell D. S. 3rd, Hess T., Looper M. L., West C. P., et al. 2013. Performance by spring and fall-calving cows grazing with full, limited, or no access to toxic Neotyphodium coenophialum-infected tall fescue. J. Anim. Sci. 91:465–476. doi: 10.2527/jas.2011-4603 [DOI] [PubMed] [Google Scholar]
- Camacho L. E., Chen X., Hay W. W. Jr, and Limesand S. W.. 2017. Enhanced insulin secretion and insulin sensitivity in young lambs with placental insufficiency-induced intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 313:R101–R109. doi: 10.1152/ajpregu.00068.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussy C., Charrière S., Meirhaeghe A., Dallongeville J., Lefai E., Rome S., Cuerq C., Euthine V., Delay M., Marmontel O., et al. 2016. Multiple microRNA regulation of lipoprotein lipase gene abolished by 3’UTR polymorphisms in a triglyceride-lowering haplotype harboring p.ser474ter. Atherosclerosis 246:280–286. doi: 10.1016/j.atherosclerosis.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Chen X., Ouyang H., Wang Z., Chen B., and Nie Q.. 2018. A novel circular RNA generated by FGFR2 gene promotes myoblast proliferation and differentiation by sponging miR-133a-5p and miR-29b-1-5p. Cells 7:11. doi: 10.3390/cells7110199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Bird J. A., Lomax M. A., and Symonds M. E.. 1996. Effect of beta 3-adrenergic agonist (Zeneca D7114) on thermoregulation in near-term lambs delivered by cesarean section. Pediatr. Res. 40:330–336. doi: 10.1203/00006450-199608000-00023 [DOI] [PubMed] [Google Scholar]
- Daza K. R., Steibel J. P., Velez-Irizarry D., Raney N. E., Bates R. O., and Ernst C. W.. 2017. Profiling and characterization of a longissimus dorsi muscle microRNA dataset from an F2 Duroc × Pietrain pig resource population. Genom. Data 13:50–53. doi: 10.1016/j.gdata.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo D. J., Kiel D. P., and Esser K. A.. 2013. Bone and skeletal muscle: Neighbors with close ties. J. Bone Miner. Res. 28:1509–1518. doi: 10.1002/jbmr.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett S. K., Andrae J. G., and Pratt S. L.. 2014a. Exposure to ergot alkaloids during gestation reduces fetal growth in sheep. Front. Chem. 2:68. doi: 10.3389/fchem.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett S. K., Pratt S. L., and Pavan E.. 2009. Corn oil or corn grain supplementation to steers grazing endophyte-free tall fescue. II. Effects on subcutaneous fatty acid content and lipogenic gene expression. J. Anim. Sci. 873:1120–1128. doi: 10.2527/jas.2008-1420 [DOI] [PubMed] [Google Scholar]
- Duckett S. K., Volpi-Lagreca G., Alende M., and Long N. M.. 2014b. Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep. Diabetes Metab. Syndr. Obes. 7:553–563. doi: 10.2147/DMSO.S72695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:PL223–PL228. [DOI] [PubMed] [Google Scholar]
- Esteves J. V., Enguita F. J., and Machado U. F.. 2017. MicroRNAs-mediated regulation of skeletal muscle GLUT4 expression and translocation in insulin resistance. J. Diabetes Res. 2017:7267910. doi: 10.1155/2017/7267910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey A. J., Brameld J. M., Parr T., and Buttery P. J.. 2005a. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J. Anim. Sci. 83:2564–2571. doi: 10.2527/2005.83112564x [DOI] [PubMed] [Google Scholar]
- Fahey A. J., Brameld J. M., Parr T., and Buttery P. J.. 2005b. Ontogeny of factors associated with proliferation and differentiation of muscle in the ovine fetus. J. Anim. Sci. 83:2330–2338. doi: 10.2527/2005.83102330x [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Bhattacharyya S. N., and Sonenberg N.. 2008. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Foote A. P., Harmon D. L., Brown K. R., Strickland J. R., McLeod K. R., Bush L. P., and Klotz J. L.. 2012. Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. J. Anim. Sci. 90:1603–1609. doi: 10.2527/jas.2011-4513. [DOI] [PubMed] [Google Scholar]
- Forbes W. B., Tracy C., Resnick O., and Morgane P. J.. 1977. Effects of maternal dietary protein restriction on growth of the brain and body in the rat. Brain Res. Bull. 2:131–135. [DOI] [PubMed] [Google Scholar]
- Galan H. L., Anthony R. V., Rigano S., Parker T. A., de Vrijer B., Ferrazzi E., Wilkening R. B., and Regnault T. R.. 2005. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am. J. Obstet. Gynecol. 192:272–279. doi: 10.1016/j.ajog.2004.05.088 [DOI] [PubMed] [Google Scholar]
- Gale C. R., Martyn C. N., Kellingray S., Eastell R., and Cooper C.. 2001. Intrauterine programming of adult body composition. J. Clin. Endocrinol. Metab. 86:267–272. doi: 10.1210/jcem.86.1.7155 [DOI] [PubMed] [Google Scholar]
- Garofano A., Czernichow P., and Bréant B.. 1997. In utero undernutrition impairs rat beta-cell development. Diabetologia 40:1231–1234. doi: 10.1007/s001250050812 [DOI] [PubMed] [Google Scholar]
- Guo H., Ingolia N. T., Weissman J. S., and Bartel D. P.. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840. doi: 10.1038/nature09267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimonet V., Bussieres L., A. A. Medjebeur, B. Gasser, B. Lelongt, and K. Laborde. 1998. Nephrogenesis and angiotendin II receptors subtypes gene expression in the fetal lamb. Am. J. Physiol. 2746(6):F1062–F1069. doi:10.1152/ajprenal.1998.274.6.F1062 [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Peck K. N., Forella M. E., Fox A. R., Govoni K. E., and Zinn S. A.. 2016a. The effects of poor maternal nutrition during gestation on postnatal growth and development of lambs. J. Anim. Sci. 94:789–99. doi: 10.2527/jas2015-9933 [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Peck K. N., Wegrzyn J. L., Reed S. A., Zinn S. A., and Govoni K. E.. 2016b. Poor maternal nutrition during gestation alters the expression of genes involved in muscle development and metabolism in lambs. J. Anim. Sci. 94:3093–3099. doi: 10.2527/jas.2016-0570 [DOI] [PubMed] [Google Scholar]
- Horak M., Novak J., and Bienertova-Vasku J.. 2016. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 410:1–13. doi: 10.1016/j.ydbio.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Horie T., Ono K., Nishi H., Iwanaga Y., Nagao K., Kinoshita M., Kuwabara Y., Takanabe R., Hasegawa K., Kita T., et al. 2009. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 389:315–320. doi: 10.1016/j.bbrc.2009.08.136 [DOI] [PubMed] [Google Scholar]
- Hu Z., Wang H., Lee I. H., Modi S., Wang X., Du J., and Mitch W. E.. 2010. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 59:1312–1320. doi: 10.2337/db09-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. L., Christensen G. L., Schneider M., Nossent A. Y., Jensen H. B., Andersen D. C., Eskildsen T., Gammeltoft S., Hansen J. L., and Sheikh S. P.. 2011. Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br. J. Pharmacol. 164:394–404. doi: 10.1111/j.1476-5381.2011.01375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Britt J. L., Miller M. F., Snider M. A., Aiken G. E., Long N. M., Pratt S. L., Andrae J. G., and Duckett S. K.. 2019. Ergot alkaloid exposure during gestation alters: II. Uterine and umbilical artery vasoactivity1. J. Anim. Sci. 97:1891–1902. doi: 10.1093/jas/skz069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrominova T. Y. 2011. Application of WGA lectin staining for visualization of the connective tissue in skeletal muscle, bone, and ligament/tendon studies. Microsc. Res. Tech. 74:18–22. doi: 10.1002/jemt.20865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. H., Phipson B., Oshlack A., Prasad-Gupta N., Cheung M. M., Smolich J. J., and Pepe S.. 2018. Shifts in ovine cardiopulmonary microRNA expression in late gestation and the perinatal period. PLoS One 13:e0204038. doi: 10.1371/journal.pone.0204038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang U., Baker R. S., Khoury J., and Clark K. E.. 2000. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R53–R59. doi: 10.1152/ajpregu.2000.279.1.R53 [DOI] [PubMed] [Google Scholar]
- Lie S., Morrison J. L., William-Wyss O., Suter C. M., Humphreys D. T., Ozanne S. E., Zhang S., MacLaughlin S. M., Kleemann D. O., Walker S. K., Roberts C. T., and McMillen I. C.. 2014. Periconceptional undernutrition programs changes in insulin-signaling molecules and microRNAs in skeletal muscle in singleton and twin fetal sheep. Biol. Reprod. 901:1–10. doi: 10.1095/biolreprod.113.109751 [DOI] [PubMed] [Google Scholar]
- Lopez-Tello J., Arias-Alvarez M., Jimenez-Martinez M. A., Garcia-Garcia R. M., Rodriguez M., Lorenzo Gonzalez P. L., Bermejo-Poza R., Gonzalez-Bulnes A., and Garcia Rebollar P.. 2017. Competition for materno-fetal resource partitioning in a rabbit model of undernourished pregnancy. PLoS One 12:e0169194. doi: 10.1371/journal.pone.0169194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Nie Q., and Zhang X.. 2013. MicroRNAs involved in skeletal muscle differentiation. J. Genet. Genomics 40:107–116. doi: 10.1016/j.jgg.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Lyons P. C., Plattner R. D., and Bacon C. W.. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232:487–489. doi: 10.1126/science.3008328 [DOI] [PubMed] [Google Scholar]
- Ma Z., Li H., Zheng H., Jiang K., Yan F., Tian Y., Kang X., Wang Y., and Liu X.. 2017. Hepatic ELOVL6 mRNA is regulated by the gga-mir-22-3p in egg-laying hen. Gene 623:72–79. doi: 10.1016/j.gene.2017.04.040 [DOI] [PubMed] [Google Scholar]
- McCann M. A., Craddock B. F., Preston R. L., and Ramsey C. B.. 1990. Digestibility of cotton plant by-product diets for sheep at two levels of intake. J. Anim. Sci. 68:285–295. [DOI] [PubMed] [Google Scholar]
- McCarthy J. J., Esser K. A., Peterson C. A., and Dupont-Versteegden E. E.. 2009. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol. Genomics 39:219–226. doi: 10.1152/physiolgenomics.00042.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney-Freeman S. L., Jackson K. A., F. D. Camargo, G. Ferrari, F. Mavilio, and M. A. Goodell. 2002. Muscle-dervied hematopoietic stem cells are hematopoietic in orgin. Proc. Natl. Acad. Sci. USA 99(3):1341–1346. doi:10.1073/pnas.032438799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J., Lemoine F., Soumillon M., Liechti A., Weier M., Guschanski K., Hu H., Khaitovich P., and Kaessmann H.. 2013. Birth and expression evolution of mammalian microRNA genes. Genome Res. 23:34–45. doi: 10.1101/gr.140269.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroya S., Shibata M., Hayashi M., Oe M., and Ojima K.. 2016. Differences in circulating microRNAs between grazing and grain-fed wagyu cattle are associated with altered expression of intramuscular microRNA, the potential target PTEN, and lipogenic genes. PLoS One 11:e0162496. doi: 10.1371/journal.pone.0162496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter D. R. 2000. Effects of ewe age and season of lambing on prolificacy in US Targhee, Suffolk, and Polypay sheep. Small Rumin. Res. 38:1–7. [DOI] [PubMed] [Google Scholar]
- NRC 2007. Nutrient requirements of small ruminants: Sheep, goats, cervids, and New World Camelids. National Research Council, The National Academies Press, Washington, DC. [Google Scholar]
- Oksbjerg N., and Therkildsen M.. 2017. Myogenesis and muscle growth and meat quality. In: Purslow P. P., editor, New aspects of meat quality – From genes to ethics. Woodhead Publishing, Cambridge, MA; p. 33–62. [Google Scholar]
- Pardo C. E., Berard J., Kreuzer M., and Bee G.. 2013. Intrauterine crowding impairs formation and growth of secondary myofibers in pigs. Animal 73:430–438. doi: 10.1017/S1751731112001802 [DOI] [PubMed] [Google Scholar]
- Pope M., Budge H., and Symonds M. E.. 2012. The developmental transition of ovine adipose tissue through early life. Acta Physiol. 210:20–30. doi: 10.1111/apha.12053 [DOI] [PubMed] [Google Scholar]
- Quintens R., Singh S., Lemaire K., De Bock K., Granvik M., Schraenen A., Vroegrijk I. O., Costa V., Van Noten P., Lambrechts D., et al. 2013. Mice deficient in the respiratory chain gene cox6a2 are protected against high-fat diet-induced obesity and insulin resistance. PLoS One 8:e56719. doi: 10.1371/journal.pone.0056719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin O., Lefauconnier J. M., Chanez C., Bernard G., and Bourre J. M.. 1994. Developmental effects of intrauterine growth retardation on cerebral amino acid transport. Pediatr. Res. 35:640–648. doi: 10.1203/00006450-199406000-00005 [DOI] [PubMed] [Google Scholar]
- Rattray P. V., Garrett W. N., East N. E., and Hinman N.. 1974. Growth, development and composition of the ovine conceptus and mammary gland during pregnancy. J. Anim. Sci. 38:613–626. [DOI] [PubMed] [Google Scholar]
- Redmer D. A., Milne J. S., Aitken R. P., Johnson M. L., Borowicz P. P., Reynolds L. P., Caton J. S., and Wallace J. M.. 2012. Decreasing maternal nutrient intake during the final third of pregnancy in previously overnourished adolescent sheep: Effects on maternal nutrient partitioning and feto-placental development. Placenta 33:114–121. doi: 10.1016/j.placenta.2011.11.023 [DOI] [PubMed] [Google Scholar]
- Reed S. A., Raja J. S., Hoffman M. L., Zinn S. A., and Govoni K. E.. 2014. Poor maternal nutrition inhibits muscle development in ovine offspring. J. Anim. Sci. Biotech. 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeldt C., Fiedler I., Dietl G., and Ender K.. 2000. Myogenesis and postnatal skeletal muscle cell growth as influenced by selection. Livest. Prod. Sci. 66:177–188. doi: 10.1016/S0301-6226(00)00225-6 [DOI] [Google Scholar]
- Rozance P. J., Zastoupil L., Wesolowski S. R., Goldstrohm D. A., Strahan B., Cree-Green M., Sheffield-Moore M., Meschia G., Hay W. W. Jr, Wilkening R. B., et al. 2018. Skeletal muscle protein accretion rates and hindlimb growth are reduced in late gestation intrauterine growth-restricted fetal sheep. J. Physiol. 596:67–82. doi: 10.1113/JP275230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer A. A., Syddall H. E., Dennison E. M., Gilbody H. J., Duggleby S. L., Cooper C., Barker D. J., and Phillips D. I.. 2004. Birth weight, weight at 1 y of age, and body composition in older men: Findings from the Hertfordshire cohort study. Am. J. Clin. Nutr. 80:199–203. doi: 10.1093/ajcn/80.1.199 [DOI] [PubMed] [Google Scholar]
- Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., et al. 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967. doi: 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U., Sirin E., Yildiz S., Aksoy Y., Ulutas Z., and Kuran M.. 2016. The effect of maternal nutrition level during the periconception period on fetal muscle development and plasma hormone concentrations in sheep. Animal 10:1689–1696. doi: 10.1017/S1751731116000835 [DOI] [PubMed] [Google Scholar]
- Shoup L. M., Miller L. M., Srinivasan M., Ireland F. A., and Shike D. W.. 2016. Effects of cows grazing toxic endophyte-infected tall fescue or novel endophyte-infected tall fescue in late gestation on cow performance, reproduction, and progeny growth performance and carcass characteristics. J. Anim. Sci. 94:5105–5113. doi: 10.2527/jas.2016-0819 [DOI] [PubMed] [Google Scholar]
- Siengdee P., Trakooljul N., Murani E., Schwerin M., Wimmers K., and Ponsuksili S.. 2015. MicroRNAs regulate cellular ATP levels by targeting mitochondrial energy metabolism genes during C2C12 myoblast differentiation. PLoS One 10:e0127850. doi: 10.1371/journal.pone.0127850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., and Horwitz B. A.. 1969. Brown fat and thermogenesis. Physiol. Rev. 49:330–425. doi: 10.1152/physrev.1969.49.2.330 [DOI] [PubMed] [Google Scholar]
- Strickland J. R., Looper M. L., Matthews J. C., Rosenkrans C. F. Jr, Flythe M. D., and Brown K. R.. 2011. Board-invited review: St. Anthony’s fire in livestock: Causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi: 10.2527/jas.2010-3478 [DOI] [PubMed] [Google Scholar]
- Sun L., Ma K., Wang H., Xiao F., Gao Y., Zhang W., Wang K., Gao X., Ip N., and Wu Z.. 2007. JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J. Cell Biol. 179:129–138. doi: 10.1083/jcb.200703184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds M. E. 2013. Brown adipose tissue growth and development. Scientifica (Cairo) 2013:305763. doi: 10.1155/2013/305763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triepels R. H., Van Den Heuvel L. P., Trijbels J. M., and Smeitink J. A.. 2001. Respiratory chain complex I deficiency. Am. J. Med. Genet. 106:37–45. doi: 10.1002/ajmg.1397 [DOI] [PubMed] [Google Scholar]
- Uldry M., and Thorens B.. 2004. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 447:480–489. doi: 10.1007/s00424-003-1085-0 [DOI] [PubMed] [Google Scholar]
- Wallace L. R. 1948. The growth of lambs before and after birth in relation to the level of nutrition. J. Agric. Sci. 38:243–300. [Google Scholar]
- Wang K., Wang C., Xiao F., Wang H., and Wu Z.. 2008. JAK2/STAT2/STAT3 are required for myogenic differentiation. J. Biol. Chem. 283:34029–34036. doi: 10.1074/jbc.M803012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. H., McCann M. A., Parish J. A., Hoveland C. S., Thompson F. N., and Bouton J. H.. 2004. Productivity of cow-calf pairs grazing tall fescue pastures infected with either the wild-type endophyte or a nonergot alkaloid-producing endophyte strain, AR542. J. Anim. Sci. 82:3388–3393. doi: 10.2527/2004.82113388x [DOI] [PubMed] [Google Scholar]
- Xie F., Xiao P., Chen D., Xu L., and Zhang B.. 2012. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 80(1):75–84. doi: 10.1007/s11103-012-9885-2 [DOI] [PubMed] [Google Scholar]
- Yates D. T., Cadaret C. N., Beede K. A., Riley H. E., Macko A. R., Anderson M. J., Camacho L. E., and Limesand S. W.. 2016. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive type I fibers near term. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R1020–R1029. doi: 10.1152/ajpregu.00528.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. J., Ford S. P., Nathanielsz P. W., and Du M.. 2004. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol. Reprod. 71:1968–1973. doi: 10.1095/biolreprod.104.034561 [DOI] [PubMed] [Google Scholar]