Abstract
Objective
To estimate the own‐price elasticity of demand for naloxone, a prescription medication that can counter the effects of an opioid overdose, and predict the change in pharmacy sales following a conversion to over‐the‐counter status.
Data Sources/Study Setting
The primary data source was a nationwide prescription claims dataset for 2010‐2017. The data cover 80 percent of US retail pharmacies and account for roughly 90 percent of prescriptions filled. Additional covariates were obtained from various secondary data sources.
Study Design
We estimated a longitudinal, simultaneous equation model of naloxone supply and demand. Our primary variables of interest were the quantity of naloxone sold, measured as total milligrams sold at pharmacies, and the out‐of‐pocket price paid per milligram, both measured per ZIP Code and quarter‐year.
Data Collection/Extraction Methods
Primary data came directly from payers and processors of prescription drug claims.
Principal Findings
We found that, on average, a 1 percent increase in the out‐of‐pocket price paid for naloxone would result in a 0.27 percent decrease in pharmacy sales. We predict that the total quantity of naloxone sold in pharmacies would increase 15 percent to 179 percent following conversion to over‐the‐counter status.
Conclusions
Naloxone is own‐price inelastic, and conversion to over‐the‐counter status is likely to lead to a substantial increase in total pharmacy sales.
Keywords: change in demand, naloxone, opioid overdose, over‐the‐counter conversion
1. INTRODUCTION
In 2016, approximately 2.1 million US persons, 12 years of age and older, had an opioid use disorder,1 and over 42 000 US persons died from a drug overdose attributed to opioids.2 More opioid overdose deaths could be prevented if bystanders had access to naloxone, a prescription opioid antagonist medication that can counter the effects of an opioid overdose.3 Naloxone has many appealing features from a public health standpoint. It has no effect on individuals who do not have opioids in their system, and the primary risk for those who do have opioids in their system is opioid withdrawal symptoms, which can be severe, but not when weighed against the risk of death for someone who is overdosing.4 Moreover, naloxone is non‐narcotic, has no abuse potential, and can be administered in some forms (eg, intranasal) by the general public with little‐to‐no training. Initiatives are underway across the country to increase layperson access to naloxone. All 50 states and the District of Columbia have modified laws related to naloxone access; for example, 23 states currently have statewide standing orders for naloxone, which allow pharmacists to dispense naloxone on request, and an additional 24 states allow jurisdictions to pass naloxone standing order laws.5 Major pharmacy corporations such as Walgreens and CVS have recently stated their commitment to stocking and selling naloxone; however, they must do so in accordance with the state's rules and regulations.6, 7 An alternative to standing orders that has received a great deal of support, including from the US Food and Drug Administration, is to change the prescribing status of naloxone from prescription‐only to over‐the‐counter (OTC) status.8, 9, 10, 11
Changing the prescribing status of naloxone to OTC is expected to further reduce access barriers, thereby resulting in an increase in demand and a concomitant increase in quantity demanded at any given price. However, if there is a loss of insurance coverage that accompanies the change to OTC status or manufacturers decide to increase prices for OTC products above prices for prescription products, out‐of‐pocket price increases would put downward pressure on the quantity of naloxone demanded. Which effect dominates (ie, the upward pressure on quantity demanded due to the increase in demand, or the downward pressure on quantity demanded due to the increase in out‐of‐pocket price) is an empirical question. Therefore, the initial objective of this study was to use nationwide, longitudinal, and comprehensive prescription claims data to estimate the demand and supply functions for naloxone purchased at US retail pharmacies. The price per unit for a given medication often varies by pharmacy, even within the same geographic location.12 Our ability to observe variation in the out‐of‐pocket price paid for naloxone by consumers at retail pharmacies across the United States, and the quantities purchased at those prices, while controlling for potentially confounding factors, allowed us to characterize the naloxone demand curve for this market. We used a simultaneous equation model to account for the endogeneity resulting from the simultaneous influence that demand and supply functions have on one another. We then estimated the own‐price elasticity of demand for naloxone purchased at pharmacies (ie, the percentage change in quantity demanded given a one percent increase in the out‐of‐pocket price), which allowed us to predict the change in total pharmacy naloxone sales following a conversion to OTC, based on different assumptions of changes in demand and price.
2. STUDY DATA AND METHODS
2.1. Analytic overview
The quantity exchanged of any good in a market is a function of both supply and demand; thus, econometric estimation of either the demand or supply function requires the consideration of the simultaneous influence that one has on the other.13 To account for this, we estimated a simultaneous equation model. The unit of observation was the pharmacy ZIP Code per quarter‐year. All values were adjusted to 2016 US dollars using the prescription drug consumer price index.14
There are currently three primary naloxone delivery systems (injection, auto‐injection, and intranasal). Even though the auto‐injection and intranasal systems are most likely to receive OTC status, we included all available formulations and delivery systems in our model. The reason for this is that the auto‐injection and intranasal delivery systems have only been on the market since 2014 and 2015, respectively, and therefore, by themselves, would provide very little information upon which to estimate our model.
2.2. Demand function
The demand equation for naloxone was modeled as a function of the out‐of‐pocket price paid by consumers at the pharmacy, consumer income, other consumer demographic and socioeconomic characteristics that may be associated with the quantity of naloxone they are willing and able to purchase at any given price, the severity of the opioid use disorder epidemic in the area, local pharmacy naloxone access regulations, and other regional characteristics. Binary variables representing the presence of the auto‐injection (Evzio®, kaléo, Richmond, VA, USA) and intranasal (Narcan® , ADAPT Pharma, Radnor, PA, USA) delivery systems in the market were also included in the demand equation, since their introduction likely influenced the overall demand for naloxone.
2.3. Price function
The US pharmaceutical manufacturing industry for specific therapeutic markets is more accurately characterized as an oligopoly than a perfectly competitive market.15 As such, we estimated a price equation similar to that of Keeler et al,16 which better reflects the supply side of an oligopolistic market. The naloxone price equation was modeled as a function of the cost of production, distribution, and selling; variations in the price elasticity of demand over time; and market competition. The binary variables indicating the availability of the auto‐injection and intranasal naloxone delivery systems were also included to control for their potential effect on market competitiveness and the elasticity of demand.
2.4. Identification
To control for the endogeneity in a two‐equation system and ensure that the system is identified, each equation must contain at least one unique exogenous variable that is correlated with the endogenous right‐hand‐side variable, but not the residual, in the other equation. These “extra” exogenous variables serve as instruments for the endogenous right‐hand‐side variable. Consumer demographic and socioeconomic characteristics that are associated with their preferences for naloxone at any given price do not belong in the supply function from a theoretical standpoint, and therefore, those measures were excluded from the price equation. For the same reason, the production cost and market competition measures were excluded from the demand equation. Therefore, our simultaneous system of equations was likely overidentified, indicating that unique estimates for all parameters could be calculated. As an additional test of overidentification, we estimated a reduced‐form generalized method of moments equation and calculated Hansen's J statistic using Stata's overid command; the results indicated that the instruments are valid and the equation is overidentified.
2.5. Data and measures
Our primary data source was a nationwide prescription claims database from Symphony Health.17 The Symphony Health data contain information from over 80 percent of US retail pharmacies and account for roughly 90 percent of prescriptions filled in those locations. Our study focused solely on pharmacy sales to individual consumers, as opposed to facilities such as private and government hospitals, clinics, home health care providers, HMO captive pharmacies, mail‐order pharmacies, and prisons. Symphony data come directly from payers and processors of prescription drug claims, and contain information on consumer out‐of‐pocket costs, as well as consumer demographic and socioeconomic factors. All payers are captured by the data, including commercial insurers, Medicare fee‐for‐service, Medicare Advantage, Medicaid, and self‐pay. Additionally, pharmacies are required to resubmit files containing errors in more than 2 percent of required fields. Our study sample included all ZIP Code/quarter‐year observations for which naloxone pharmacy sales had been recorded, and we had information on the total milligrams of naloxone sold and the associated out‐of‐pocket prices for the time frame of 2010‐2017. In regions where ZIP Codes did not contain three or more unique pharmacies, pharmacy claims data were aggregated to the three‐digit ZIP Code level by the data distributor, for purposes of confidentiality.
Quantity of naloxone demanded was measured as total milligrams (mg) sold per ZIP Code, per quarter‐year. Out‐of‐pocket price paid for naloxone was measured as the out‐of‐pocket price paid per mg sold in a given ZIP Code, per quarter‐year. We included the following customer demographic and socioeconomic information to control for heterogeneity in their preferences for naloxone at any given price, all of which were operationalized as means or percentages per ZIP Code, per quarter‐year: age, race/ethnicity, gender, education, payer type, and annual income by quintile. The severity of the opioid use disorder epidemic in the area was measured using the reported opioid overdose death rate in the corresponding county during the prior year.18 Two binary variables were created to indicate whether the state in which the ZIP Code resides had a statewide standing order in place at the time or allowed standing orders to be implemented by jurisdiction; ZIP Codes in states that did not have either regulation in place at a given time served as the reference case. Other local characteristics, such as the political environment around naloxone distribution and influence of community members’ opinions, were controlled for using state fixed effects and Rural‐Urban Commuting Area (RUCA) codes to identify the degree of rurality associated with each ZIP Code.19 RUCA codes combine the Bureau of Census’ “urbanized area” and “urban cluster” definitions with information on work commutes to create a more refined measure of community isolation.
The cost of naloxone production, distribution, and selling was controlled for using the average producer price index value for pharmaceutical preparation manufacturing overall, by quarter‐year.20 The number of naloxone manufacturers in the quarter‐year was included in the price equation as a measure of market competitiveness. An exponential time trend was included in the price equation to reflect changes in the price elasticity of demand over time, as well as technological improvements that could have increased the efficiency with which manufacturers were able to produce naloxone. Finally, binary variables indicating the availability of the auto‐injection and intranasal naloxone delivery systems were included in both the demand and price equations due to their potential effects on demand and supply.
2.6. Analysis
A multivariable generalized structural equation model (GSEM) with clustered standard errors was used to estimate the demand and price functions for naloxone. The GSEM combines the capabilities of structural equation models (SEMs) and generalized linear models (GLMs). SEMs are flexible multiple equation regression models that allow for complex relationships between factors of interest, and as such, SEMs encompass simultaneous equation models. The GLM, and thus the GSEM, allows the most appropriate mean and variance functions to be chosen for each equation in the system, according to the fit of the data.21 The modified Parks test was used as a guide to choosing the appropriate variance structure (ie, family), and the Pearson Correlation, Pregibon Link, and Modified Hosmer and Lemeshow tests were used to inform the decision of which mean (ie, link) function was most appropriate.21
The opioid overdose death rate was suppressed for regions with fewer than 10 reported overdose deaths.18 Given that we know the reason for the missingness of observations in this variable and had observed variables that were strong predictors of the probability of the missingness (ie, the RUCA code and state fixed effects), we used inverse probability weighting within a GLM framework to predict and replace these missing values prior to the inclusion of the opioid overdose death rate in the GSEM.22 The average missingness per quarter‐year ranged from 19 percent to 41 percent, with a mean of 26 percent across all time periods.
2.6.1. Prediction of change in quantity of naloxone sales
Our prediction of the change in the quantity of naloxone sold in pharmacies following a conversion to OTC was based on our estimated own‐price elasticity of demand for naloxone, and estimates obtained from the literature regarding (a) the difference between the out‐of‐pocket price for medications covered by insurance and the price paid for those medications after a change in prescribing status to OTC, (b) the own‐price elasticity of demand for nicotine replacement therapies following their conversion to OTC, and (c) the estimated effect that the conversion to OTC had on the quantity of nicotine replacement therapies demanded. Nicotine replacement therapies were chosen because they are the only other substance use disorder pharmacotherapy to have experienced a change from prescription‐only to OTC status in the United States, and the change to OTC status created an entirely new OTC market with potential public health benefits,23 as would naloxone.
We defined eight scenarios that describe a range of predicted changes in naloxone sales following OTC conversion. We considered two levels of demand increase: 78 percent (based on the low estimate for nicotine patches) and 180 percent (based on the estimate for nicotine gum).16 We also considered four out‐of‐pocket price increases based on ranges observed in Maryland for indemnity/managed care plan enrollees (2 percent and 113 percent) and Kaiser Permanente enrollees (54 percent and 233 percent), for four other OTC conversion products (cromolyn sodium—Nasalcrom®, tioconazole—Vagistat®, ketoconazole—Nizoral®, and terbinafine—Lamisil®).24
2.6.2. Sensitivity analyses
We conducted one‐way sensitivity analyses to find the threshold values for the percentage increase in price, percentage increase in quantity demanded, and own‐price elasticity of demand that would result in no change in total naloxone sales following OTC. We also varied the “extra” exogenous variables that serve as instruments in the demand and price functions. Finally, we calculated the most likely average out‐of‐pocket price increase that occurred in the market for nicotine patches and gum. The estimated price increases of 26 percent (patches) and 33 percent (gum) were calculated based on the own‐price elasticities of demand for these products following OTC conversion (−2.33 and −2.46, respectively),25 and the observed unadjusted increase in total sales that occurred after OTC conversion (18 percent and 100 percent, respectively).16
2.6.3. Strengths and limitations
A major strength of this paper is the longitudinal, nationwide, comprehensive pharmaceutical claims data, which included all payer types, out‐of‐pocket prices, and demographic and socioeconomic variables. Our generalized structural equation model, which allowed us to choose the most appropriate mean and variance functions according to our data and estimate a simultaneous system of supply and demand equations, is also a major strength.
The fact that our data failed to capture 20 percent of pharmacies is a limitation; however, according to the data distributor, pharmacies that are not included in the data are typically independent or associated with relatively small chains, and these pharmacies may be less likely to dispense naloxone.26, 27, 28, 29 The pharmacy ZIP Code serving as a proxy for a customer's geographic location is a limitation; however, since naloxone must be purchased in‐person, even under a standing order, we believe this to be a reasonable assumption. The CDC's compressed mortality file was only available at the county level, and data were suppressed for regions with fewer than 10 overdose deaths; however, these observations were accounted for using a proven technique to address missing‐data bias.22 We were unable to determine whether ZIP Codes in states that allowed local standing orders actually had one in place. Similarly, for ZIP Codes in states with statewide standing orders we were unable to determine whether pharmacies were actually adhering to the standing order. We were unable to measure the production, distribution, and selling costs for naloxone specifically, but were able to include the producer price index for pharmaceutical preparation manufacturing as a proxy. We do not have sufficient data to forecast the impact that a conversion of naloxone to OTC would have on opioid overdose education and naloxone distribution by harm reduction agencies, for example, information on the likelihood that persons who are unable or unwilling to purchase naloxone at a higher out‐of‐pocket price would obtain it by other means.
3. RESULTS
Descriptive statistics by ZIP Code/quarter‐year over the observation period are presented in Table 1. The mean number of milligrams of naloxone sold for a ZIP Code/quarter‐year where naloxone pharmacy sales had been recorded was 165, at an average out‐of‐pocket price of $28.11/mg (SE = $2.20/mg). On average, 34 percent of ZIP Code/quarter‐year observations were in a state with a statewide standing order and 48 percent were in a state that allowed local standing orders. The average age of naloxone consumers was 51 years, and the plurality of those for whom we had complete demographic and socioeconomic information was white, female, had a high school diploma or GED, and was making under $30 000 per year. Over 40 percent of naloxone prescriptions were purchased using Medicare or Medicare Advantage. Almost 79 percent of ZIP Code/quarter‐year observations that contained a recorded naloxone pharmacy sale were associated with a metropolitan area, and the mean opioid overdose death rate in the associated counties in the prior year was 9.71/100 000 persons. The national opioid overdose death rate over this period ranged from just under 7/100 000 to over 13/100 000 persons.
Table 1.
Descriptive statistics for naloxone pharmacy claims by ZIP Code/Quarter‐Year, 2010‐2017
| n = 10 468 | Mean | SE |
|---|---|---|
| Naloxone characteristics | ||
| Average total milligrams of naloxone | 165.00 | 5.78 |
| Average price per milligram of naloxone | $28.11 | $2.20 |
| Demographic characteristics of naloxone consumers | ||
| Average age | 50.76 | 0.12 |
| Race/ethnicity | ||
| % White | 49.64 | 0.36 |
| % Black | 8.13 | 0.20 |
| % Hispanic | 4.02 | 0.14 |
| % Other ethnicity | 1.11 | 0.07 |
| % Unknown ethnicity | 37.11 | 0.35 |
| Sex | ||
| % Male | 46.28 | 0.35 |
| % Female | 53.72 | 0.35 |
| Socioeconomic characteristics of naloxone consumers | ||
| Education | ||
| % College degree | 27.01 | 0.25 |
| % Associate's degree | 13.31 | 0.31 |
| % High school diploma or GED | 23.65 | 0.31 |
| % Unknown education | 36.03 | 0.34 |
| Income | ||
| % Under 30k | 18.31 | 0.27 |
| % 30k‐49k | 10.82 | 0.21 |
| % 49k‐79k | 10.89 | 0.21 |
| % 79k‐99k | 8.58 | 0.19 |
| % 100 + k | 13.76 | 0.25 |
| % Unknown income | 37.63 | 0.35 |
| Payer | ||
| % Commercial | 37.35 | 0.35 |
| % Assistance programs | 3.80 | 0.14 |
| % Cash | 8.80 | 0.22 |
| % Managed Medicare | 9.63 | 0.23 |
| % Medicaid | 7.61 | 0.19 |
| % Medicare | 32.80 | 0.34 |
| ZIP Code characteristics | ||
| % ZIP Code/quarter‐year with statewide standing order | 33.91 | 0.46 |
| % ZIP Code/quarter‐year with nonstatewide standing order law | 47.66 | 0.49 |
| ZIP Code size | ||
| % Microa | 9.13 | 0.28 |
| % Smallb | 12.20 | 0.32 |
| % Metroc | 78.67 | 0.40 |
| County opioid overdose death rate (per 100k persons) in preceding year | 9.71 | 0.06 |
RUCA19 codes 4 (primary flow within a “large urban cluster” = 10 000‐49 999 persons) through 6 (primary flow 10%‐30% to a “large urban cluster”).
RUCA codes 7 (primary flow within a “small urban cluster” = 2500‐9999 persons) through 10 (rural = primary flow outside an urban cluster or area).
RUCA codes 1 (primary flow within an “urbanized area” >9999) through 3 (primary flow 10%‐30% to an “urbanized area”).
3.1. Demand function
The results from the estimated demand function are presented in Table 2. The coefficient estimate for the log of the out‐of‐pocket price per mg of naloxone is −0.27 (95% CI: −0.32, −0.22; P < 0.01), indicating that a 1 percent increase in the out‐of‐pocket price would result in a 0.27 percent decrease in the quantity of naloxone demanded from pharmacies. Having a statewide standing order in place or having a law allowing standing orders were both associated with a significant increase in naloxone demanded from pharmacies (1.36 percent and 0.80 percent, respectively; P < 0.01), relative to ZIP Codes in states without such regulations. ZIP Codes located in counties with relatively high opioid overdose death rates in the preceding year sold more naloxone at pharmacies; specifically, every one point increase in the opioid overdose death rate was associated with a 0.04 percent increase in pharmacy naloxone sales in the subsequent year (P < 0.01). The introduction of the naloxone nasal spray (Narcan®) was associated with a 2 percent increase in naloxone pharmacy sales, while the effect of the introduction of the naloxone auto‐injector (Evzio®) was nonsignificant. Demographic and socioeconomic factors associated with higher pharmacy sales of naloxone included increased age; residing in a metropolitan area; having a 4‐year college degree, vs a high school diploma or GED; identifying as white/Caucasian, vs Hispanic; and having insurance coverage provided by Medicare, Medicare Advantage, or Medicaid, relative to commercial insurance.
Table 2.
GSEM demand and supply function results
| Coef. | Std. Err. | P > |z| | |
|---|---|---|---|
| Demand function | |||
| Naloxone characteristics | |||
| Log out‐of‐pocket price per mg | −0.27 | 0.02 | <0.01 |
| Auto‐injector on market | 0.19 | 0.13 | 0.15 |
| Nasal spray on market | 1.99 | 0.10 | <0.01 |
| Demographic characteristics | |||
| Average age | 0.01 | 0.00 | <0.01 |
| Race/ethnicity | |||
| White | Reference | ||
| Black | 0.10 | 0.21 | 0.63 |
| Hispanic | −0.59 | 0.22 | 0.01 |
| Other ethnicity | 0.36 | 0.49 | 0.47 |
| Unknown ethnicity | 0.06 | 0.41 | 0.88 |
| Sex | |||
| Male | Reference | ||
| Female | −0.06 | 0.09 | 0.49 |
| Opioid overdose death rate in preceding year | 0.04 | 0.01 | <0.01 |
| Socioeconomic characteristics | |||
| Education | |||
| College degree | Reference | ||
| Associate's degree | −0.26 | 0.20 | 0.21 |
| High school diploma or GED | −0.47 | 0.14 | <0.01 |
| Unknown education | −0.30 | 0.54 | 0.58 |
| Income | |||
| Under 30k | 0.33 | 0.17 | 0.06 |
| 30k‐49k | 0.16 | 0.17 | 0.36 |
| 49k‐79k | Reference | ||
| 79k‐99k | 0.24 | 0.19 | 0.20 |
| 100 + k | 0.13 | 0.18 | 0.46 |
| Unknown income | 0.24 | 0.36 | 0.50 |
| Payer | |||
| Commercial | Reference | ||
| Assistance programs | 0.44 | 0.29 | 0.12 |
| Cash | 0.10 | 0.12 | 0.43 |
| Medicaid | 0.54 | 0.22 | 0.01 |
| Medicare | 0.24 | 0.12 | 0.05 |
| Managed Medicare | 1.00 | 0.30 | <0.01 |
| ZIP Code characteristics | |||
| Statewide standing order present | 1.36 | 0.12 | <0.01 |
| Jurisdictional standing order law present | 0.80 | 0.10 | <0.01 |
| ZIP Code size | |||
| Micro | −0.96 | 0.12 | <0.01 |
| Small | −0.81 | 0.12 | <0.01 |
| Metro | Reference | ||
| Constant | 0.60 | 0.31 | 0.06 |
| Price function | |||
| Producer price index | −0.03 | 0.01 | 0.01 |
| Manufacturers on market | −0.69 | 0.15 | <0.01 |
| Time trend | 0.00 | 0.00 | 0.41 |
| Auto‐injector on market | 2.46 | 0.36 | <0.01 |
| Nasal spray on market | 1.69 | 0.42 | <0.01 |
| Constant | 5.71 | 13.02 | 0.66 |
Notes: Dependent variables are log of total milligrams of naloxone sold (demand function) and log of out‐of‐pocket price per mg of naloxone sold (supply function). State fixed effects (not shown) were included in the demand function equation.
3.2. Price function
The results from the price function are also displayed in Table 2. The number of naloxone manufacturers was negatively associated with the out‐of‐pocket pharmacy price; specifically, each additional naloxone manufacturer resulted in a 0.69 percent (95% CI: −0.98, −0.39; P < 0.01) decrease in the out‐of‐pocket price. The producer price index for pharmaceutical preparation manufacturing was also negatively associated with the out‐of‐pocket price, although the effect was small (B = −0.03, P = 0.01). According to Rosenberg et al,30 the raw material costs for naloxone were fairly stable over this time period, but unfortunately, we do not know how other costs of naloxone manufacturing, distribution, and selling were changing. The introductions of the naloxone nasal spray (Narcan®) and auto‐injector (Evzio®) were associated with significant increases in the out‐of‐pocket pharmacy price for naloxone (2.46 percent and 1.69 percent, respectively; P < 0.01).
3.3. Prediction of change in quantity demanded
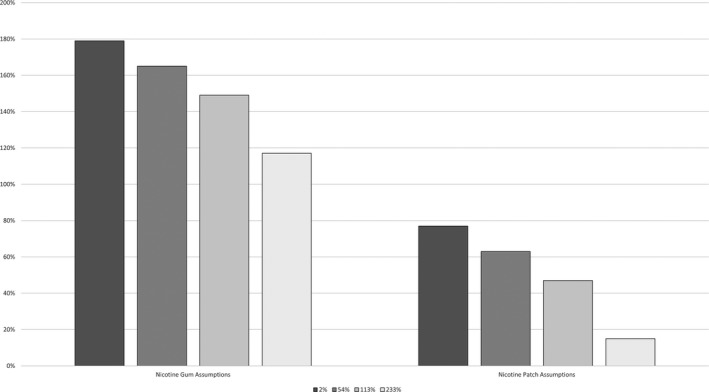
Applying the own‐price elasticity of demand for naloxone (−0.27) to the observed out‐of‐pocket price increases associated with OTC product conversions in Maryland (2 percent to 233 percent),24 and the estimated demand increases for nicotine patches and gum (78 percent and 180 percent, respectively),16 we predict an increase in naloxone sales of 15 percent to 77 percent using the estimated demand increase for nicotine patches, and 117 to 179 percent using the estimate for nicotine gum (Table 3, Figure 1). Predicted sales increases are substantially lower for nicotine patches and gum, due to their substantially higher estimated own‐price elasticities of demand. Based on the scenario that produced the lowest predicted naloxone sales increase (15 percent), we find that a 288 percent price increase (vs 233 percent), a 63 percent demand increase (vs 78 percent), or an own‐price elasticity of −0.34 (vs −0.27 percent) would result in no change in total naloxone pharmacy sales. Applying the estimated out‐of‐pocket price increases following OTC conversion for nicotine patches (26 percent) and nicotine gum (33 percent), our estimated own‐price elasticity of demand for naloxone, and the relevant estimated effects of OTC conversions on demand for nicotine patches and gum, we predict a 71 to 171 percent increase in total naloxone pharmacy sales.
Table 3.
Predicted changes in naloxone sales following conversion to OTC
| Price increasea | 2% | 54% | 113% | 233% |
|---|---|---|---|---|
| Nicotine gum assumptions | ||||
| Demand increaseb | 180% | 180% | 180% | 180% |
| OOP elasticity of demand | −0.27 | −0.27 | −0.27 | −0.27 |
| Change in quantity due solely to price increase | −0.54% | −14.58% | −30.51% | −62.91% |
| Change in total sales after accounting for all supply and demand side effects | 179% | 165% | 149% | 117% |
| Nicotine patch assumptions | ||||
| Demand increaseb | 78% | 78% | 78% | 78% |
| OOP elasticity of demand | −0.27 | −0.27 | −0.27 | −0.27 |
| Change in quantity due solely to price increase | −0.54% | −14.58% | −30.51% | −62.91% |
| Change in total sales after accounting for all supply and demand side effects | 77% | 63% | 47% | 15% |
Figure 1.
- Source: Authors’ analysis of the 2010‐2017 Symphony Health pharmacy claims database.
4. DISCUSSION
Our primary outcome of interest was the own‐price elasticity of demand for naloxone pharmacy sales. We found that the demand for naloxone was inelastic with regard to changes in its out‐of‐pocket price during our observed time period, with an elasticity of −0.27, which indicates that a 1 percent increase in the out‐of‐pocket price for naloxone would only result in a 0.27 percent decrease in the quantity of naloxone sold in pharmacies. This figure is much smaller in magnitude than the estimated own‐price elasticity of the nicotine gum and patch replacement therapies immediately following their conversion to OTC, −2.46 and −2.33, respectively,25 but slightly larger than recent own‐price elasticity of demand estimates of prescription medications for the period 2005‐2009, which ranged from −0.02 for NSAIDs/opioids to −0.16 for smoking cessation medications.31 The relatively large own‐price elasticities of demand for the OTC nicotine replacement therapies may be the result of the marginal consumer being less committed to smoking cessation, and thus more responsive to price changes, than those who seek the prescription products. Similarly, the marginal OTC consumer may feel there are more substitutes for the OTC products, including those that are prescription‐only, whereas prescription‐only consumers may have already tried the OTC products, with limited success.
Applying the estimated own‐price elasticity of demand for naloxone to estimates from the literature regarding changes in price and demand around OTC conversion, we predict that naloxone pharmacy sales would increase between 15 percent and 179 percent if naloxone was moved to OTC. After estimating the mean percentage price increase in nicotine replacement therapies that occurred at the point of purchase following OTC, we narrowed our prediction of the change in naloxone sales following OTC to a 71 percent to 171 percent increase.
In sensitivity analyses we estimated that even at a price increase of 233 percent for naloxone, the increase in demand associated with OTC would have to fall below 68 percent, or the absolute value of the own‐price elasticity of demand would have to increase to −0.34, before total pharmacy sales would decline. The own‐price elasticity of demand was robust to varying the instruments in the demand and price functions. Moreover, theoretically, we would not expect the elasticity of demand for naloxone to change much following a move to OTC given that it is a life‐saving drug for which there are no substitutes, and, at a current retail price of $150 ($18.75/mg) for two intranasal devices,32 we do not expect it to become a significantly larger proportion of the average consumer's budget, even if most insurers choose not to cover the drug. Of course, the larger the increase in demand, the more robust the findings are to increases in price and elasticity of demand. We posit that a relatively large increase in naloxone demand is reasonable given the significant increase associated with state laws that allow local naloxone standing orders, the even larger effect associated with statewide standing orders, and the continued calls to increase naloxone among the general public, including from the US Surgeon General33; especially when considered in the context of the recent New York Times investigation, which found that of the 720 pharmacies in New York City that were on the city's list of pharmacies who sell naloxone under the state's standing order, only 38 percent had it in stock and were willing to dispense it without a prescription.34 Additionally, moving naloxone to OTC would help normalize the purchasing process, and likely reduce concerns of stigma by customers, which would also serve to increase demand.35 Finally, the potential to obtain FDA approval to sell naloxone OTC would likely draw additional manufacturers into the marketplace, similar to the nicotine replacement therapy market following OTC conversion,23 which in turn would put downward pressure on price.
The degree to which a conversion of naloxone to OTC would affect public health will depend on how the change affects those persons who would be most likely to observe an overdose and use the product. That is, will the number of opioid overdose reversals among new purchasers of naloxone be greater than the number forgone by current purchasers who would be unwilling or unable to purchase naloxone at a higher out‐of‐pocket price? An additional consideration is the manner in which these two groups of potential consumers differ. For example, if insurers for low‐income individuals, who may be more sensitive to price increases, turn out to be less likely to continue coverage of naloxone after OTC conversion, this could lead to large out‐of‐pocket price increases for this population,24 in which case public health could actually be adversely affected and economic disparities could increase.
5. CONCLUSION
Converting naloxone from prescription‐only to over‐the‐counter status is likely to lead to a substantial increase in total pharmacy sales. All else constant, as the prevalence of naloxone increases among the general public, so too should the opportunities to reverse opioid overdoses, thereby giving overdose survivors another chance to initiate treatment. However, the public health impact will depend on how likely the new population of OTC naloxone consumers are to encounter an overdose and use the product relative to the population of existing naloxone consumers.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: All authors are funded by the National Institute on Drug Abuse (Grant No. P30DA040500). Dr. Murphy has consulted for Sandoz Inc. on an unrelated project. The authors wish to thank David Powell, PhD, for assistance with the study design. The authors report no other disclosures.
Murphy SM, Morgan JR, Jeng PJ, Schackman BR. Will converting naloxone to over‐the‐counter status increase pharmacy sales? Health Serv Res. 2019;54:764–772. 10.1111/1475-6773.13125
[The copyright line in this article was changed on 8 March 2019 after online publication.]
REFERENCES
- 1. Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park‐Lee E. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- 2. Centers for Disease Control and Prevention . Provisional Drug Overdose Death Counts. Atlanta, GA: Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 3. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. Morb Mortal Wkly Rep. 2015;64(23):631‐635. [PMC free article] [PubMed] [Google Scholar]
- 4. Wermeling DP. Review of naloxone safety for opioid overdose: practical considerations for new technology and expanded public access. Therap Adv Drug Saf. 2015;6(1):20‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis C, Chang S, Hernandez‐Delgado H. Legal Interventions to Reduce Overdose Mortality: Naloxone Access and Overdose Good Samaritan Laws. Saint Paul, MN: The Network for Public Health Law; 2017. [Google Scholar]
- 6. CVS . Naloxone. 2018. https://www.cvs.com/content/prescription-drug-abuse/save-a-life. Accessed December 10, 2018.
- 7. Walgreens . About Naloxone. 2018. https://www.walgreens.com/topic/pharmacy/naloxone.jsp. Accessed December 10, 2018.
- 8. Mahoney K. FDA supports greater access to naloxone to help reduce opioid overdose deaths. 2016. https://blogs.fda.gov/fdavoice/index.php/2016/08/fda-supports-greater-access-to-naloxone-to-help-reduce-opioid-overdose-deaths/. Accessed December 18, 2018.
- 9. Singer JA. Why Doesn't the Surgeon General Seek FDA Reclassification of Naloxone to OTC? 2018. https://www.cato.org/blog/why-doesnt-surgeon-general-seek-fda-reclassification-naloxone-otc. Accessed May 30, 2018.
- 10. McLemore M, Davis C. A simple move to save thousands of lives from overdose. The New York Times 2017; Opinion.
- 11. American Society of Addiction Medicine . Public Policy Statement on the Use of Naloxone for the Prevention of Opioid Overdose Deaths. 2016; https://www.asam.org/docs/default-source/public-policy-statements/use-of-naloxone-for-the-prevention-of-opioid-overdose-deaths-final.pdf. Accessed December 18, 2018.
- 12. Furberg CD, Furberg BD, Sasich LD. Knowing Your Medications. New York, NY: Potata Inc.; 2016. [Google Scholar]
- 13. Greene WH. Econometric Analysis, 8th ed New York, NY: Pearson Education, Inc.; 2018. [Google Scholar]
- 14. U.S. Department of Labor Bureau of Labor Statistics ‐ Consumer Price Index. http://www.bls.gov/cpi/. Accessed December 18, 2018.
- 15. Santerre RE, Neun SP. Health Economics: Theories, Insights, and Industry Studies, 5th ed Mason, OH: Cengage Learning//South‐Western; 2010. [Google Scholar]
- 16. Keeler TE, Hu TW, Keith A, et al. The benefits of switching smoking cessation drugs to over‐the‐counter status. Health Econ. 2002;11(5):389‐402. [DOI] [PubMed] [Google Scholar]
- 17. Symphony Health . n.d. https://symphonyhealth.com. Accessed December 04, 2018.
- 18. Centers for Disease Control and Prevention . CDC WONDER. 2017. https://wonder.cdc.gov. Accessed June 24, 2017.
- 19. United States Department of Agriculture Economic Research Service . Rural‐Urban Commuting Area Codes. n.d. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Accessed July 05, 2017.
- 20. Bureau of Labor Statistics . Producer Price Indexes. n.d. https://www.bls.gov/ppi/. Accessed December 18, 2018.
- 21. Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford, UK: Oxford University Press; 2014. [Google Scholar]
- 22. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278‐295. [DOI] [PubMed] [Google Scholar]
- 23. Mahecha LA. Rx‐to‐OTC switches: trends and factors underlying success. Nat Rev Drug Discovery. 2006;5(5):380. [DOI] [PubMed] [Google Scholar]
- 24. Gianfrancesco F, Manniing B, Wang R‐H. Effects of prescription‐to‐OTC switches on out‐of‐pocket health care costs and utilization. Drug Benefit Trends. 2002;14(3):13. [Google Scholar]
- 25. Tauras JA, Chaloupka FJ. The demand for nicotine replacement therapies. Nicotine Tob Res. 2003;5(2):237‐243. [DOI] [PubMed] [Google Scholar]
- 26. Bachyrycz A, Shrestha S, Bleske BE, Tinker D, Bakhireva LN. Opioid overdose prevention through pharmacy‐based naloxone prescription program: innovations in health care delivery. Subst Abus. 2017;38(1):55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green TC, Dauria EF, Bratberg J, Davis CS, Walley AY. Orienting patients to greater opioid safety: models of community pharmacy‐based naloxone. Harm Reduct J. 2015;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyerson B, Agley J, Davis A, et al. Predicting pharmacy naloxone stocking and dispensing following a statewide standing order, Indiana 2016. Drug Alcohol Depend. 2018;188:187‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casey MM, Klingner J, Moscovice I. Pharmacy services in rural areas: is the problem geographic access or financial access? J Rural Health. 2002;18(3):467‐477. [DOI] [PubMed] [Google Scholar]
- 30. Rosenberg M, Chai G, Mehta S, Schick A. Trends and economics drivers for United States naloxone pricing, January 2006 to February 2017. Addict Behav. 2018;86:86‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gatwood J, Gibson TB, Chernew ME, et al. Price elasticity and medication use: cost sharing across multiple clinical conditions. J Manag Care Pharmacy. 2014;20(11):1102‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta R, Shah ND, Ross JS. The rising price of naloxone—risks to efforts to stem overdose deaths. N Engl J Med. 2016;375(23):2213‐2215. [DOI] [PubMed] [Google Scholar]
- 33. Adams J. Surgeon General's Advisory on Naloxone and Opioid Overdose. 2018. https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed May 14, 2018.
- 34. Correal A. Overdose antidote is supposed to be easy to get. It's not. The New York Times 04/12/2018, 2018; New York.
- 35. Green TC, Case P, Fiske H, et al. Perpetuating stigma or reducing risk? Perspectives from naloxone consumers and pharmacists on pharmacy‐based naloxone in 2 states. J Am Pharm Assoc. 2017;57(2):S19‐S27.e14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


