Abstract
The objective of this study is to determine the incidence, socio-demographic and clinical risk factors for preeclampsia and associated maternal and perinatal adverse outcomes. This is a nested case-control derived from the multicentre cohort study Preterm SAMBA, in five different centres in Brazil, with nulliparous healthy pregnant women. Clinical data were prospectively collected, and risk factors were assessed comparatively between PE cases and controls using risk ratio (RR) (95% CI) plus multivariate analysis. Complete data were available for 1,165 participants. The incidence of preeclampsia was 7.5%. Body mass index determined at the first medical visit and diastolic blood pressure over 75 mmHg at 20 weeks of gestation were independently associated with the occurrence of preeclampsia. Women with preeclampsia sustained a higher incidence of adverse maternal outcomes, including C-section (3.5 fold), preterm birth below 34 weeks of gestation (3.9 fold) and hospital stay longer than 5 days (5.8 fold) than controls. They also had worse perinatal outcomes, including lower birthweight (a mean 379 g lower), small for gestational age babies (RR 2.45 [1.52–3.95]), 5-minute Apgar score less than 7 (RR 2.11 [1.03–4.29]), NICU admission (RR 3.34 [1.61–6.9]) and Neonatal Near Miss (3.65 [1.78–7.49]). Weight gain rate per week, obesity and diastolic blood pressure equal to or higher than 75 mmHg at 20 weeks of gestation were shown to be associated with preeclampsia. Preeclampsia also led to a higher number of C-sections and prolonged hospital admission, in addition to worse neonatal outcomes.
Subject terms: Medical research, Risk factors
Introduction
Preeclampsia is considered an important cause of maternal mortality and severe maternal morbidity1. For every woman who dies, it is estimated that around 20 other women suffer from severe morbidity and disability2,3. In view of the social and economic implications of this condition, great effort has been made to expeditiously prevent, diagnose and treat preeclampsia4–7.
The magnitude of the problem in some places across the world is still not fully known, especially in low and middle-income countries. In particular, the actual incidence of preeclampsia remains largely unknown8. There is usually suboptimal reporting of the disease, leading to constraints on public health applicability9. Another important aspect is the identification of pregnant women at risk of developing preeclampsia, especially in nulliparous women with no track record of any pregnancy outcomes3. From clinical risk factors to ‘omics’ technology, there is still currently no single good predictor of preeclampsia10–15.
Clinical factors remain an inexpensive and rapid way to predict the occurrence of preeclampsia. This current study intends to evaluate the incidence of preeclampsia and its sub-phenotypes (early-onset and late-onset), socio-demographic and clinical risk factors for preeclampsia, as well as assess the ability to predict this disorder in a cohort of healthy nulliparous Brazilian pregnant women.
Results
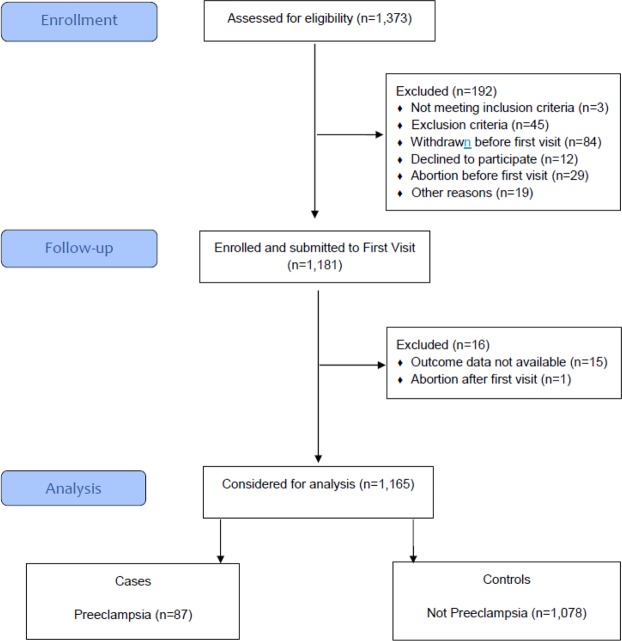
Among 1,373 participants screened for eligibility in the Preterm SAMBA study, complete pregnancy outcome data were available for 1,165 women (Fig. 1). Preeclampsia developed in 87 (7.5%) participants of whom 14 (16.1%) had early-onset preeclampsia while the remaining 73 were late-onset. The socio-demographic characteristics of women who developed preeclampsia and controls are shown in Table 1. Among patient characteristics, the rate of weight gain per week equal to or more than 0.75 kg, obesity (BMI > 30.9 Kg/m2) and diastolic blood pressure equal to or higher than 75 mmHg at 20 weeks of gestation were associated with more than twice the risk of preeclampsia (Table 1).
Figure 1.
Flowchart of women participating in the study.
Table 1.
Estimated risk of selected socio-demographic and some medical history and personal characteristics in preeclampsia.
| Characteristics | Preeclampsia | Controls | RR (95% CI) |
|---|---|---|---|
| Maternal age (years) | n (%) | n (%) | |
| <20 | 20 (23) | 271 (25.1) | 0.91 [0.48–1.74] |
| 20–34 | 60 (69) | 736 (68.2) | Ref. |
| >34 | 7 (8) | 71 (6.7) | 1.19 [0.50–2.85] |
| Ethnicity | |||
| White | 27 (31) | 435 (40.3) | Ref. |
| Others | 60 (69) | 643 (59.7) | 1.46 [0.84–2.55] |
| Marital status a | |||
| With partner | 63 (72.4) | 777 (72.0) | Ref. |
| Without partner | 24 (27.6) | 296 (27.4) | 1.00 [0.47–2.12] |
| Schooling (years) | |||
| <12 | 58 (66.6) | 733 (68.0) | Ref. |
| ≥12 | 29 (33.4) | 345 (32.0) | 1.06 [0.45–2.51] |
| Annual Family Income (US$) | |||
| Up to 3000 | 24 (27.6) | 280 (25.9) | 1.18 [0.63–2.23] |
| 3000 to 6000 | 31 (35.6) | 350 (32.4) | 1.22 [0.64–2.34] |
| Above 6000 | 32 (36.8) | 448 (41.7) | Ref. |
| Source of prenatal care | |||
| Entirely public | 81 (93.1) | 927 (85.9) | 2.10 [0.50–8.93] |
| Private/insurance/mixed | 6 (6.9) | 151 (14.1) | Ref. |
| Family history of hypertensive disease | |||
| Any hypertensive disorderb | 16 (18.4) | 141 (13.0) | 1.47 [0.83–2.60] |
| Pregnancy of participant’s mother | 4 (4.6) | 43 (3.9) | 1.15 [0.12–10.62] |
| Smoking | |||
| No smoking | 81 (93.1) | 997 (92.4) | Ref. |
| Stopped during pregnancy/current smoker | 6 (6.9) | 81 (7.5) | 0.92 [0.18–4.58] |
| Use of illicit drugs c | |||
| Non-user | 68 (78.2) | 873 (80.9) | Ref. |
| Ceased during pregnancy/current user | 2 (2.3) | 52 (4.8) | 0.51 [0.22–1.22] |
| Weight gain rate per week (kg) d | |||
| <0.25 | 14 (16.1) | 127 (11.7) | 1.67 [0.42–6.59] |
| 0.25–0.49 | 23 (26.4) | 364 (33.7) | Ref. |
| 0.50–0.74 | 26 (29.9) | 367 (34.0) | 1.11 [0.70–1.77] |
| ≥0.75 | 15 (17.2) | 109 (10.1) | 2.04 [1.12–3.69] |
| Body Mass Index at enrolment e,& | |||
| Underweight (<21.5 kg/m2) | 9 (10.3) | 190 (17.6) | 0.74 [0.38–1.45] |
| Normal weight (21.5–26.2) | 28 (32.2) | 433 (40.1) | Ref. |
| Overweight (26.3–30.9) | 22 (25.3) | 280 (25.9) | 1.20 [0.75–1.92] |
| Obesity (>30.9) | 28 (32.2) | 174 (16.1) | 2.28 [1.39–3.74] |
| Any previous maternal conditions (anaemia, thyroid, asthma, previous hypertensive disorder*, depression, POS) | |||
| No | 53 (60.9) | 730 (67.7) | Ref. |
| Yes | 34 (39.1) | 348 (32.3) | 1.31 [0.66–2.60] |
| Diastolic pressure at 20 weeks’ gestation e | |||
| <75 mmHg | 64 (73.6) | 937 (86.9) | Ref. |
| ≥75 mmHg | 23 (26.4) | 140 (12.9) | 2.21 [1.30–3.74] |
| Total | 87 | 1078 | |
Missing information for a5; b100; c170; d120; e1case; #RR and 95% CI not presented due to small numbers; values in bold mean they are significant.
*Without using medication.
& ref.41.
Maternal and neonatal outcomes in preeclampsia were worse for both the mothers and their neonates (Table 2). Women with preeclampsia presented a relative risk of 3.58 for caesarean section, while hospital admission for 5 days or more was almost 6-fold higher. Women with preeclampsia had more preterm births at less than 34 weeks of gestation (3.97 fold) than controls. Neonates of women with preeclampsia had a significantly lower birthweight (a mean of 379 g lower), and there was a twofold to threefold higher occurrence of small for gestational age (SGA) babies, 5-minute Apgar scores less than 7, NICU admission and Neonatal Near Miss events. There was only one case of fetal death, occurring in a 26-year old woman, at 26 weeks of gestation. She was admitted to hospital, complaining of a headache. Arterial blood pressure was 170 × 110 mmHg, protein in dipstick urinalysis was +3 and no fetal heart beat was identified. The induction of labour lasted 24 hours, resulting in vaginal delivery of a baby weighing 620 g.
Table 2.
Maternal and neonatal outcomes associated with preeclampsia.
| Characteristics | Preeclampsia n (%) | Controls n (%) | RR (95% CI) |
|---|---|---|---|
| Mode of delivery | |||
| Vaginal | 21 (24.1) | 599 (55.5) | Ref. |
| C-section | 66 (75.9) | 479 (44.5) | 3.58 [1.57–8.12] |
| Onset of labour | |||
| Spontaneous | 18 (20.7) | 664 (61.5) | Ref. |
| Induced | 36 (41.4) | 211 (19.5) | 5.52 [2.21–13.83] |
| Elective C-section | 33 (37.9) | 203 (19.0) | 5.30 [1.25–22.38] |
| Gestational age at birth (weeks) | |||
| <34 | 11 (12.6) | 32 (3.0) | 3.97 [1.55–10.20] |
| 34–36 | 9 (10.3) | 73 (6.7) | 1.70 [0.57–5.06] |
| ≥37 | 67 (77.1) | 973 (90.3) | Ref. |
| Length of postpartum hospitalization | |||
| 1–4 days | 67 (77.0) | 1041 (96.5) | Ref. |
| ≥5 days | 20 (23.0) | 37 (3.5) | 5.80 [2.12–15.91] |
| Thromboembolic event before or after delivery | |||
| No | 87 (100.0) | 1072 (99.4) | Ref. |
| Yes | 0 | 4 (0.4) | # |
| Mean (SD) birthweight (g) | 2779.4 (±843.1) | 3158.8 (±558.9) | *WMD = −379.4 (−644.5 to −114.4) |
| Adequacy of birthweight to GA a | |||
| SGA (p < 10) | 22 (25.3) | 124 (11.5) | 2.45 [1.52–3.95] |
| AGA (10 < p < 90) | 54 (62.0) | 824 (76.4) | Ref. |
| LGA (p > 90) | 10 (11.5) | 118 (10.9) | 1.27 [0.76–2.13] |
| Fetal death | 1 (1.1) | 6 (0.5) | 1.92 [0.09–39.42] |
| Apgar score – at 5 minutes <7b | 3 (3.4) | 16 (1.5) | 2.11 [1.03–4.29] |
| Intubation required after birth | 7 (8.0) | 19 (1.7) | 3.89 [0.41–36.95] |
| NICU admission | 32 (36.7) | 141 (13.1) | 3.34 [1.61–6.90] |
| Neonatal Near Miss (Apgar 5 < 7 OR Birthweight <1750 g OR GA <33) c | 13 (14.9) | 39 (3.6) | 3.65 [1.78–7.49] |
| Total | 87 | 1078 | |
Missing information for a13 cases; b65 cases; c62 cases. Values in bold mean they are significant.
#RR and 95% CI not presented due to small numbers.
*WMD: weighted mean difference.
On multivariate analysis, diastolic blood pressure at 20 weeks of gestation and BMI at enrolment were independently associated with the occurrence of preeclampsia, with an adjusted risk ratio of 1.04 (Table 3).
Table 3.
Factors independently associated with preeclampsia on multivariate analysis [n = 1164].
| Characteristics | RRadj (95% CI) |
|---|---|
| Diastolic blood pressure at 20 weeks’ gestation (mmHg) | 1.04 [<1.01–1.06] |
| Body Mass Index at enrolment (kg/m2) | 1.04 [1.01–1.09] |
Variables included in the model (14): Maternal age (years); Ethnicity (White: 0/other: 1); Marital status (with partner: 0/without partner: 1); Schooling (<12 years: 0/≥12 years: 1); Annual Family Income (Up to US$6000: 1/>US$6000: 0); Source of prenatal care (entirely public: 1/other: 0); Family history of hypertensive disease: Any hypertensive disorder (yes: 1/no: 0); Pregnancy of participant´s mother (yes: 1/no: 0); Smoking (yes: 1/no smoking: 0); Use of illicit drugs (yes: 1/non-user:0); Weight gain rate per week (kg); Body Mass Index at enrolment (kg/m2); Any previous maternal conditions (yes: 1/no: 0); Diastolic blood pressure at 20 weeks’ gestation (mmHg).
Discussion
Our study revealed that the incidence of preeclampsia was 7.5% in a nulliparous group of healthy pregnant women from three different Brazilian regions, which is higher than values obtained from other cohorts of nulliparous pregnant women16–18. Current analysis was able to identify only three factors significantly associated with the development of preeclampsia: weight gain rate per week, obesity and value of diastolic blood pressure measured at 20 weeks of gestation equal to or higher than 75 mmHg. The low number of preeclampsia cases in this sample probably prevented us from identifying additional factors, limiting the capacity to predict preeclampsia by using a composition of factors. Not surprisingly, our findings on maternal and perinatal outcomes added support to other studies, showing an increased frequency of Caesarean sections, preterm births, neonatal near misses, 5-minute Apgar scores less than 7 and low birth weight in pregnancies complicated by preeclampsia19,20. This higher proportion of adverse perinatal outcomes, including the lower birthweight, are also related to the increased occurrence of preterm births among preeclamptic women.
To the best of our knowledge, this was the first time that a Brazilian cohort of healthy nulliparous pregnant women received follow-up with data acquisition on the incidence of preeclampsia. The manner of calculating the rate of weight gain was a limitation of our analysis. Patients were recruited from 19 to 21 weeks of gestation to the last measurement at the end of prenatal care. Owing that the last time measure was done between 37 and 39 weeks of gestation, a quarter of preeclampsia cases (22.9%) were not included. Potential bias – reverse causality – may occur, since after preeclampsia is diagnosed, weight is influenced by oedema, a potential feature of this disease. Another limiting characteristic is that the database does not have information on the precise time when antihypertensive drugs (if used) were initiated.
In our cohort, data on the actual incidence of preeclampsia was totally distinct from findings of a systematic review published in 2008 showing a prevalence of 1.5% for preeclampsia and 0.6% for eclampsia. According to those authors, their numbers were underestimated in some regions due to lack of information8. Almost 10 years later, a study implemented in Brazil showed that the prevalence of preeclampsia was 8.1% in specific regions21. Our study currently revealed that the incidence of preeclampsia is 7.5% in a nulliparous group of healthy pregnant women, which is higher than values obtained from other cohorts16–18. The high prevalence of obesity in our population may explain the incidence of preeclampsia. Despite the lack of data available on this topic in our country, a recent cross-sectional study involving 1,279 pregnant women showed that the prevalence of overweight or obesity during the first prenatal visit was almost 40%22.
Owing to the high incidence and the potential impact of preeclampsia2,23,24, it is essential to find an effective tool that provides early identification of pregnant women at high risk for this disease, in order to implement prophylactic measures and avoid harmful consequences. A search for a predictive model with widespread global applicability has begun, encouraged by the results achieved by studies using low-dose aspirin as a prophylactic measure25,26. However, the prediction of preeclampsia is challenging, in view of the complexity of its aetiology27. It is unlikely that a single risk factor will be able to predict the occurrence of this condition. In addition, our results come from women that were initially screened at around 20 weeks, after the period when some known prophylactic measures are recommended to be started.
Maternal clinical factors have emerged as an interesting screening alternative. In 2010, a guideline of the National Collaborating Centre for Women’s and Children’s Health recommended the use of maternal clinical factors as screening tests. According to the guideline, a previous history of gestational hypertensive disorder, autoimmune disease (systemic erythematous lupus or antiphospholipid syndrome), chronic renal disease, diabetes and chronic hypertension are considered high-risk factors. Once any of these factors are present, prophylactic measures must be initiated28. The NICE screening proposal was assessed in a prospective study involving a heterogeneous population composed of nulliparous and multiparous pregnant women. Detection rates of 37% and 28.9% were obtained for early-onset (before 34 weeks of gestation) and late-onset (at or after 34 weeks of gestation) preeclampsia cases, respectively29. These numbers were confirmed in another study applying the NICE criteria with a third of preeclampsia cases identified30.
Our cohort identified only three factors related to increased risk of preeclampsia: weight gain rate per week, obesity and value of diastolic blood pressure measured at 20 weeks of gestation equal to or higher than 75 mmHg. A cohort of more than 62,000 nulliparous pregnant women generated the same finding concerning the influence of weight gain per week on preeclampsia risk18. We also reinforced previous evidence that obesity predisposes to the occurrence of preeclampsia, especially in late-onset cases. This is probably associated with the inflammatory property of adipose tissue and its effects on endothelial function31. Considering that both BMI and weight gain rate are modifiable risk factors, consolidated knowledge of their predictive function emphasises the importance of antenatal counselling and prenatal care follow-up32,33.
Our cohort of nulliparous healthy pregnant women also indicated that a diastolic blood pressure higher than 75 mmHg was correlated with the occurrence of preeclampsia. This finding conflicts with another study34, which showed that mean arterial blood pressure was a better predictor of preeclampsia in a healthy group of pregnant women.
The modest predictive power achieved through models with only maternal clinical factors have prompted prospective studies among heterogeneous populations. These studies used multivariate analysis combining maternal clinical factors to other elements such as uterine artery Doppler and biomarkers, in order to develop algorithms of preeclampsia prediction. Although the detection rates of the resulting algorithms were high, these studies were implemented among a heterogeneous population of pregnant women at high risk of developing preeclampsia29,30. Furthermore, these studies did not segregate nulliparous women, which is a limitation. It is well-known that the most consistent predictive clinical factor for preeclampsia, which is a previous history of preeclampsia, cannot be applied to first-time mothers3.
Biochemical factors have been studied, with modest results in terms of prediction potential35,36. Furthermore, the potential costs incurred and technologies available for biomarker processing represent a limiting factor for use on a large scale, especially in low and middle-income countries. Thus, clinical risk factors continue to play a crucial role as a cheap and tangible screening instrument for preeclampsia.
To date, no single screening test has shown sufficiently accurate specificity and sensitivity to predict preeclampsia cases37. The value of clinical risk factors, biochemical markers, uterine Doppler as predictive markers addressed separately remains modest at best for all women destined to develop preeclampsia38. This is probably due to the multifactorial aetiology of the condition. Genetic, immunological, environmental and maternal factors have all made their contributions and remain to be fully elucidated. Therefore, preeclampsia is a heterogeneous disease concerning clinical presentation, pathology and outcomes. Notwithstanding decades of research, an enigma still persists surrounding a useful and accurate screening test model to identify early on pregnant women, primarily in the nulliparous group, at high risk for preeclampsia. Research into this field is of considerable importance.
Methods
This is a nested case-control study derived from a secondary analysis of the Preterm-SAMBA study (Preterm Screening and Metabolomics in Brazil and Auckland), a prospective multicentre cohort study conducted in five Brazilian centres between July 2015 and March 2018. The research protocol was previously published elsewhere39. Briefly, the original study design was based on the primary goal of developing a predictive model for preterm birth. The study was developed in two phases: a discovery phase and a validation phase. The first phase was a case-control study, involving participants from the previously described SCOPE study3. In the validation phase, a prediction model was validated in the Preterm SAMBA Brazilian cohort. Other maternal and perinatal outcomes of interest were considered as secondary objectives included preeclampsia (currently addressed), gestational diabetes mellitus and fetal growth restriction. For this nested case-control approach, cases were women who developed preeclampsia and controls were all the remaining women free from the disorder. Preterm-SAMBA study was conducted according to Declaration of Helsinki guidelines. Appropriate approval was obtained from the five centres involved in the study. All recruited participants gave their written informed consent.
Participants
The study enrolled healthy nulliparous pregnant women between 19 and 20 + 6 weeks of gestation, with a singleton pregnancy, from five different centres in Brazil (from Campinas, Botucatu, Recife, Fortaleza and Porto Alegre). Exclusion criteria were: 3 or more previous abortions; cervical suture; fetal malformation; chronic hypertension requiring antihypertensive drugs and/or diabetes and/or renal disease; arterial blood pressure higher than 160 × 100 mmHg at the time of enrolment; Systemic Lupus Erythematosus and/or antiphospholipid syndrome; sickle cell disease; HIV infection; congenital uterine anomalies (bicornuate uterus, septate uterus); previous cervical knife cone biopsy; chronic exposure to corticosteroids or calcium at a dosage above 1 g or fish oil at a dosage above 2.7 g per day or vitamin C above 1000 mg per day or vitamin E above 400 UI per day; heparin or aspirin use (any dosage or presentation form). Our inclusion and exclusion criteria were decided to be aligned with another study, previously published called SCOPE3 that used exactly the same criteria. This is the reason why obesity was not considered exclusion criteria, although for some authors it is considered a major risk factor for preeclampsia27.
Sample size estimation
Sample size was calculated according to the primary outcome - preterm birth. Assuming a type I error of 5% and accuracy of the test of at least 0.68 according to the area under the ROC curve, and to test the hypotheses with adequate power (80% of power, β = 0.2), the sample size would need to approach 80 cases of preterm delivery. The minimum expected prevalence of this outcome was presumed to be 7% in Brazil, therefore the sample size was calculated at 1150 women. In addition, considering that the mean prevalence of preeclampsia observed in larger studies of around 5–6%8,17 among nulliparous women, it was anticipate that this cohort would incorporate around 58 to 69 cases of preeclampsia.
Procedures
All steps of the main study have been previously described39. Data were collected at three different set points (visits) during follow-up. On the first visit, between 19 and 21 weeks of gestation, a full assessment was performed to gather information on sociodemographic characteristics, reproductive family history, current or previous diseases, personal habits, with a complete follow-up until delivery and immediate postpartum period. During the interview, data were entered into a central database with internet access and complete audit trail (MedSciNet). Anthropometric measurements plus nutritional assessment were also performed. The same evaluation was conducted on both subsequent visits, at 27–29 weeks of gestation and at 37–39 weeks of gestation.
Outcome
The outcome of interest for the current analyses is preeclampsia. In this study, preeclampsia was defined as the occurrence of hypertension (SBP ≥ 140 and/or DBP > 90 mmHg) in at least two different time periods, combined with proteinuria (300 mg/24 hour or at least 1 g/L [2+] on dipstick testing or spot urine protein/creatinine >30 mg/mmol [0.3 mg/mg]). Preeclampsia was also classified as early-onset when diagnosed before 34 weeks of gestation and as late-onset otherwise. In the absence of proteinuria, the disorder was also defined as the occurrence of any systemic complications/organ dysfunction40 including:
Haematological complications (thrombocytopenia - platelet count below 100,000/dL, DIC, haemolysis);
Hepatic dysfunction (elevated liver enzymes – at least twice the upper limit of normal + right upper quadrant or epigastric abdominal pain);
Neurological dysfunction (eclampsia, altered mental status, blindness, stroke, hyperreflexia with clonus, severe headaches, visual scotomata when persistent);
Renal dysfunction (creatinine ≥1.2 mg/dL).
Statistical analysis
We determined the general incidence of preeclampsia and early-onset and late-onset preeclampsia. Several socio-demographic, clinical factors and lifestyle habits were regarded as potential risk factors. Furthermore, maternal and neonatal outcomes associated with preeclampsia were addressed. Bivariate analysis was performed, estimating the Risk Ratios (RR) and their respective 95% Confidence Intervals, using Student’s t, chi-square or Fisher’s exact tests accordingly. Finally, a multivariate analysis with a Poisson regression model was performed to identify which factors were independently associated with preeclampsia in this sample, estimating the adjusted RR for those identified. Each centre/hospital was considered as a Primary Sampling Unit (PSU) in every analysis. SPSS software version 20.0 and Stata software version 7.0 were used for analysis.
Ethical considerations
The current study is an ancillary analysis (preeclampsia) of the outcome from a Brazilian cohort of low-risk nulliparous women entitled “Preterm SAMBA” which was financially supported by the Bill and Melinda Gates Foundation and the Brazilian CNPq. The Preterm SAMBA study has been reviewed and approved by the Brazilian National Committee for Ethics in Research (CONEP) and by the Institutional Review Board (IRB) of the coordinating centre (Letter of approval 1.048.565 issued on 28th April 2015) and of all other Brazilian participating centres. Before enrolment, each woman was full explained about the study and signed an informed consent form.
Acknowledgements
We thanks The Preterm SAMBA study group that also included: Mary A. Parpinelli, Karayna G Fernandes, José P Guida, Danielly S Santana, Ricardo M Barbosa and Rafael B F Galvao, from the School of Medical Sciences, University of Campinas, Brazil; Bianca F. Cassettari, School of Medicine of Botucatu, UNESP, Brazil; Lucia Pfitscher, School of Medicine, Federal University of Rio Grande do Sul, Porto Alegre, Brazil; Daisy Lucena de Feitosa, School of Medicine, Federal University of Ceará, Fortaleza, Brazil; Elias de Melo Ferreira Júnior, Danilo Anacleto, School of Medicine, Federal University of Pernambuco, Recife, Brazil; Vilma Zotareli and Marcia Alice Silva, from the University of Campinas. In addition, we acknowledge the contribution of all institutions involved in the entire study, including the funders and also the participants, who kindly agreed to take part in the full study. This study was jointly funded by the Bill and Melinda Gates Foundation (grant OPP1107597) and CNPq (grant 401636/2013-5). The funders played no role whatsoever in study development, data collection, data analysis or data interpretation.
Author Contributions
The idea for the study arose from J.G.C., P.N.B. and M.L.C. During development of the research proposal, important input was provided by F.E.F., E.A.R.F., J.V. and I.M.C., the PIs from the other participating centres. After approval, implementation was performed by R.T.S., J.M., D.F.L., J.G.C., F.E.F., E.A.R.F., J.V. and I.M.C. J.M., J.G.C. and M.L.C. planned the analysis which was performed by M.H.S. The first draft of the manuscript was prepared by J.M. All authors discussed the results, gave suggestions and agreed to the final version of the manuscript.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request. The participating women did not give their consent to make their own data publicly available.
Competing Interests
The authors declare no competing interests.
Footnotes
A comprehensive list of consortium members appears at the end of the paper
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jose G. Cecatti, Email: cecatti@unicamp.br
Preterm SAMBA study group:
Mary A. Parpinelli, Karayna G. Fernandes, José P. Guida, Danielly S. Santana, Ricardo M. Barbosa, Rafael B. F. Galvao, Bianca F. Cassettari, Lucia Pfitscher, Daisy Lucena de Feitosa, Elias Melo Ferreira Júnior, Danilo Anacleto, Vilma Zotareli, and Marcia Alice Silva
References
- 1.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e541–544.e512. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl 1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 3.Kenny LC, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014;64:644–652. doi: 10.1161/HYPERTENSIONAHA.114.03578. [DOI] [PubMed] [Google Scholar]
- 4.Jauniaux E, Steer P. Predicting pre-eclampsia: 100 years of trying and failing. BJOG. 2016;123:1066. doi: 10.1111/1471-0528.13858. [DOI] [PubMed] [Google Scholar]
- 5.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Xu TT, et al. Low-Dose Aspirin for Preventing Preeclampsia and Its Complications: A Meta-Analysis. J Clin Hypertens (Greenwich) 2015;17:567–573. doi: 10.1111/jch.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS One. 2014;9:e91198. doi: 10.1371/journal.pone.0091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208:476.e471–475. doi: 10.1016/j.ajog.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Villar J, Lindheimer M. World Health Organization systematic review of screening tests for preeclampsia. Obstet Gynecol. 2004;104:1367–1391. doi: 10.1097/01.AOG.0000147599.47713.5d. [DOI] [PubMed] [Google Scholar]
- 11.Gabbay-Benziv R, Oliveira N, Baschat AA. Optimal first trimester preeclampsia prediction: a comparison of multimarker algorithm, risk profiles and their sequential application. Prenat Diagn. 2016;36:34–39. doi: 10.1002/pd.4707. [DOI] [PubMed] [Google Scholar]
- 12.Gallo D, Poon LC, Fernandez M, Wright D, Nicolaides KH. Prediction of preeclampsia by mean arterial pressure at 11-13 and 20-24 weeks’ gestation. Fetal Diagn Ther. 2014;36:28–37. doi: 10.1159/000360287. [DOI] [PubMed] [Google Scholar]
- 13.Halscott TL, Ramsey PS, Reddy UM. First trimester screening cannot predict adverse outcomes yet. Prenat Diagn. 2014;34:668–676. doi: 10.1002/pd.4407. [DOI] [PubMed] [Google Scholar]
- 14.Anderson UD, Olsson MG, Kristensen KH, Åkerström B, Hansson SR. Review: Biochemical markers to predict preeclampsia. Placenta. 2012;33(Suppl):S42–47. doi: 10.1016/j.placenta.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JM, Himes KP. Pre-eclampsia: Screening and aspirin therapy for prevention of pre-eclampsia. Nat Rev Nephrol. 2017;13:602–604. doi: 10.1038/nrneph.2017.121. [DOI] [PubMed] [Google Scholar]
- 16.Kenny LC, Broadhurst DI, Myers JE, North RA, Baker PN. A high throughput and accurate early pregnancy-screening test for preeclampsia. Reproductive Sciences. 2012;19:323A. doi: 10.1177/1933719112442493. [DOI] [Google Scholar]
- 17.Boutin Amélie, Demers Suzanne, Gasse Cédric, Giguère Yves, Tétu Amélie, Laforest Geneviève, Bujold Emmanuel. First-Trimester Placental Growth Factor for the Prediction of Preeclampsia in Nulliparous Women: The Great Obstetrical Syndromes Cohort Study. Fetal Diagnosis and Therapy. 2018;45(2):69–75. doi: 10.1159/000487301. [DOI] [PubMed] [Google Scholar]
- 18.Hutcheon JA, et al. Pregnancy Weight Gain Before Diagnosis and Risk of Preeclampsia: A Population-Based Cohort Study in Nulliparous Women. Hypertension. 2018;72:433–441. doi: 10.1161/HYPERTENSIONAHA.118.10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kale PL, Mello-Jorge MHP, Silva KSD, Fonseca SC. Neonatal near miss and mortality: factors associated with life-threatening conditions in newborns at six public maternity hospitals in Southeast Brazil. Cad Saude Publica. 2017;33:e00179115. doi: 10.1590/0102-311X00179115. [DOI] [PubMed] [Google Scholar]
- 20.Nakimuli A, et al. Still births, neonatal deaths and neonatal near miss cases attributable to severe obstetric complications: a prospective cohort study in two referral hospitals in Uganda. BMC Pediatr. 2015;15:44. doi: 10.1186/s12887-015-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano JC, et al. The burden of eclampsia: results from a multicenter study on surveillance of severe maternal morbidity in Brazil. PLoS One. 2014;9:e97401. doi: 10.1371/journal.pone.0097401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morais SS, Nascimento SL, Godoy-Miranda AC, Kasawara KT, Surita FG. Body Mass Index Changes during Pregnancy and Perinatal Outcomes - A Cross-Sectional Study. Rev Bras Ginecol Obstet. 2018;40:11–19. doi: 10.1055/s-0037-1608885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacagnella RC, et al. Delays in receiving obstetric care and poor maternal outcomes: results from a national multicentre cross-sectional study. BMC Pregnancy Childbirth. 2014;14:159. doi: 10.1186/1471-2393-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silveira C, et al. A cohort study of functioning and disability among women after severe maternal morbidity. Int J Gynaecol Obstet. 2016;134:87–92. doi: 10.1016/j.ijgo.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 25.O’Gorman N, Wright D, Rolnik DL, Nicolaides KH, Poon LC. Study protocol for the randomised controlled trial: combined multimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE) BMJ Open. 2016;6:e011801. doi: 10.1136/bmjopen-2016-011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolnik DL, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 27.Brown Mark A., Magee Laura A., Kenny Louise C., Karumanchi S. Ananth, McCarthy Fergus P, Saito Shigeru, Hall David R., Warren Charlotte E., Adoyi Gloria, Ishaku Salisu. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertension. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Visintin C, et al. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207. doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 29.Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010;24:104–110. doi: 10.1038/jhh.2009.45. [DOI] [PubMed] [Google Scholar]
- 30.North RA, et al. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342:d1875. doi: 10.1136/bmj.d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eastabrook G, Aksoy T, Bedell S, Penava D, de Vrijer B. Preeclampsia biomarkers: An assessment of maternal cardiometabolic health. Pregnancy Hypertens. 2018;13:204–213. doi: 10.1016/j.preghy.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Phillippi JC, Roman MW. The Motivation-Facilitation Theory of Prenatal Care Access. J Midwifery Womens Health. 2013;58:509–515. doi: 10.1111/jmwh.12041. [DOI] [PubMed] [Google Scholar]
- 33.Villar J, et al. WHO antenatal care randomised trial for the evaluation of a new model of routine antenatal care. Lancet. 2001;357:1551–1564. doi: 10.1016/S0140-6736(00)04722-X. [DOI] [PubMed] [Google Scholar]
- 34.Cnossen JS, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. BMJ. 2008;336:1117–1120. doi: 10.1136/bmj.39540.522049.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myatt L, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119:1234–1242. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goetzinger KR, et al. Efficiency of first-trimester uterine artery Doppler, a-disintegrin and metalloprotease 12, pregnancy-associated plasma protein a, and maternal characteristics in the prediction of preeclampsia. J Ultrasound Med. 2013;32:1593–1600. doi: 10.7863/ultra.32.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane SC, Da Silva Costa F, Brennecke SP. New directions in the prediction of pre-eclampsia. Aust N Z J Obstet Gynaecol. 2014;54:101–107. doi: 10.1111/ajo.12151. [DOI] [PubMed] [Google Scholar]
- 38.Benton SJ, Ly C, Vukovic S, Bainbridge SA. Andrée Gruslin award lecture: Metabolomics as an important modality to better understand preeclampsia. Placenta. 2017;60:S32–S40. doi: 10.1016/j.placenta.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Cecatti JG, et al. Use of metabolomics for the identification and validation of clinical biomarkers for preterm birth: Preterm SAMBA. BMC Pregnancy Childbirth. 2016;16:212. doi: 10.1186/s12884-016-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tranquilli AL, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Morais SS, Ide M, Morgan AM, Surita FG. A novel body mass index reference range - an observational study. Clinics (Sao Paulo) 2017;72:698–707. doi: 10.6061/clinics/2017(11)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request. The participating women did not give their consent to make their own data publicly available.