Abstract
The present study was conducted to evaluate the effects of zinc-enriched probiotics (ZnP) on growth performance, antioxidant status, immune function, related gene expression, and morphological characteristics of Wistar rats raised under high heat stress condition during summer. 36, 6-week-old male Wistar rats were randomly divided into three groups; fed with basal diet (control), basal diet with probiotics (P), and basal diet with zinc-enriched probiotics supplementation (ZnP, 100 mg/L), for 40 consecutive days. Blood samples were collected through intracardiac method on the last day of experiment and tissues were collected from liver, heart, and kidneys. The results revealed that both P and ZnP significantly (P < 0.05) enhanced growth performance. However, ZnP remarkably increased glutathione content, glutathione peroxidase, and superoxide dismutase activities but reduced malondialdehyde level in serum of the Wistar rats. The concentration of IL-2, IL-6, and IFN-γ was significantly (P < 0.05) increased with treatments of P and ZnP compared to control group while IL-10 was significantly (P < 0.05) decreased. Additionally, the expression of SOD1, SOD2, MT1, and MT2 genes was significantly (P < 0.05) up-regulated with the treatment of ZnP, but Hsp90 and Hsp70 heat shock genes were significantly (P < 0.05) down-regulated with the treatment of P and ZnP, respectively. Hematoxylin and Eosin staining showed that both P and ZnP supplementation treatments induced changes in villus height and intestinal wall thickness. In conclusion, zinc-enriched probiotics supplementation can improve the growth performance of Wistar rats under high ambient temperature through enhancing antioxidant status, immune function, related genes expression, and intestinal morphological characteristics. This product may serves as a potential nutritive supplement for Wistar rats under high heat stress conditions.
Keywords: Zinc-enriched probiotics, Heat stress, Antioxidant status, Immune function, Heat shock protein, Wistar rats
Introduction
Heat stress is one of the major constraint having harmful effects on animal health and performance, particularly in hot regions of the world, and is a broad term that is often used to produce negative connotations. This stress results in animals when there is an imbalance between heat generated and dissipation, and it often leads to oxidative stress in animals. Reactive oxygen species (ROS) are the main culprits of oxidative stress and, as an integral part of metabolism, are constantly produced in the body and may cause oxidative stress when their levels exceed the threshold (Kumar et al. 2011; Pham-Huy et al. 2008). In mammals, heat stress can increase the production of ROS that induces oxidative stress in cells (Flanagan et al. 1998; Lord-Fontaine and Averill-Bates 2002) and hyperthermic rats (Hall et al. 1994). Adaptation to heat stress requires the physiological integration of many organs and systems, namely endocrine and immune systems (Altan et al. 2003). In addition, temperature stress has been reported to cause considerable losses to the livestock farmers as well as has a negative impact on both production and reproduction. It increases embryonic loss, reduces milk yield, negatively affect immunity, affect embryo quality and size, endocrine hormones, sperm quality, and finally heat stress negatively effects the reproduction (Krishnan et al. 2017; Jordan 2003). To avoid heat stress, previous studies recommended basic strategies to mitigate thermal stress for improving health status of the animals including changing the environment of cows, selection of heat stress-resistant heifers, nutritional management such as using trace minerals like zinc, manganese, copper, selenium, chromium, antioxidant treatment, and considering pharmaceuticals and nutraceuticals principles (De Rensis and Scaramuzzi 2003; Wolfenson et al. 2000; Kumar et al. 2011). Furthermore, antioxidants including both enzymatic and non-enzymatic provide necessary defense against oxidative stress generated due to high ambient temperature (Fridovich 1978).
Zinc (Zn) is an essential microelement for the normal growth and health of humans and other animals. Previous studies suggested that Zn might enhance immunity, growth, reproductive performance, and the ability to resist disease (Poulsen 1995; Hahn and Baker 1993). In addition, zinc protects the cells from the harmful effects of free oxygen radicals produced during immune activation through its antioxidant effect (King et al. 1995b). In addition, Zn is also a component of the cytosolic superoxide dismutase (SOD) enzyme, which protects the cell from oxidative stress by catalyzing the dismutation of the superoxide anion and is known to be activated following zinc treatment (Buzadžić et al. 2002). Zn is also reported to stimulate immune defenses while minimizing the adverse effect of immune cell activation by bacterial translocation on the epithelial layer (King et al. 1995a). Zinc is part of an essential component of Cu/Zn superoxide dismutase (SOD) which regulates expression of many genes involved in antioxidant processes, such as metallothioneins and glutathione peroxidase (Wood et al. 2011). The physiological and cellular Zn2+ concentrations are immensely regulated by metallothioneins (MTs), Zn2+ importers (ZIPs proteins), and Zn2+ transporters (ZnTs), which play an important role in the absorption, excretion, transportation, and intracellular storage of zinc (Dong et al. 2014; Kambe et al. 2015). Moreover, inorganic Zn is also toxic in excess amounts. In the recent years, due to some beneficial characteristic of organic Zn processing some good characteristics viz., lower toxicity, better palatability, higher absorption and bioavailability, and less environmental pollution, have been paid much more attention (Carlson et al. 2004b).
Probiotics (P) are non-pathogenic microorganisms that can resist small intestinal digestion within its host and reach the colon alive, where they perform beneficial effects for the health of the host animals (Musa et al. 2009). Previous studies of probiotics such as Lactobacillus bacteria and yeasts have demonstrated that either Lactobacillus acidophilus or Saccharomyces cerevisiae has strong effects on animal antioxidant status and immunity, and can inhibit lipid peroxidation in pigs under normal conditions (Zhang et al. 2008; Lessard et al. 2009). Similarly, studies have revealed that probiotics can reduce the adverse effects of heat stress in poultry (Zulkifli et al. 2000). Apparently, under appropriate conditions, probiotics are capable of accumulating large amounts of trace elements such as Se and Zn and incorporating them into organic compounds (Ren et al. 2011c), since Zn and probiotics work via different mechanisms and have been suggested to cooperate through a synergistic effect (Ren et al. 2011b).
In this regard, the Institute of Nutritional and Metabolic disorders in Domestic Animal and Fowls laboratory has developed a zinc-enriched probiotic (ZnP), as a new feed additive product to promote the livestock industry. To produce this ZnP, two strains of microorganisms’ viz., Lactobacillus acidophilus and Saccharomyces cerevisiae (yeast) were cultured with zinc oxide (zinc, inorganic zinc) under appropriate micro-environment conditions and added to the medium. In the production of ZnP, inorganic zinc was converted into organic zinc and has proved to be very effective. Nevertheless, there is very limited knowledge about the effects of ZnP on Wistar rats exposed to high environmental heat stress. Therefore, no study has been conducted to investigate the effect of ZnP supplementation under heat stress on rats. The present study has been used to investigate the potential effects of zinc-enriched probiotics on growth performance, antioxidant status, immune functions, related genes expression, and morphological characteristics in Wistar rats growing under ambient heat stress condition in summer.
Materials and methods
Chemicals used in the present study
Antioxidant detection kits were bought from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The IL-2, IL-6, IL-10, and IFN-γ assay kits were obtained from Multi Sciences Biotechnology Co., Ltd. (Nanjing, China). Inorganic zinc oxide used in this study was purchased from Sigma (Shanghai, China). Reagents for real-time PCR were purchased from TakaRa (Dalian, China).
Probiotic strains and preparation of diets
The Institute of Nutritional and Metabolic Disorders in Domestic Animal and Fowls laboratory in Nanjing Agricultural University (Nanjing, China) provided two different probiotic strains including Lactobacillus acidophilus and S. cerevisiae strains. Preliminary results indicated that these two strains have a great tolerance to high concentrations of inorganic Zn, low pH conditions, and high concentrations of bile salts. Different type of diets viz., probiotics (P), zinc-enriched probiotics (ZnP) were prepared. The fermentative medium was composed of 20 g/L d-glucose, 10 g/L peptone, 5 g/L yeast extract, 10 g/L beef extract, 2 g/L triammonium citrate, 5 g/L sodium acetate, 1 ml/L Tween 80, 0.58 g/L MgSO4·7H2O, 0.05 g/L Mn SoO4·4H2O, 2 g/L K2HPO4. Additionally, the colony-forming units (CFU) of L. acidophilus and S. cerevisiae strains in all products were 1011 and 109 CFU mL−1, respectively. The total Zn2+ contents in ZnP were 100 mg/L. The cultures were grown in a rotating incubator at a temperature of 32 °C for 36 h at 100 rpm.
Experimental design and animals feeding
The experiment was carried out according to protocols approved by the Animal Care and Use Committee of Nanjing Agricultural University (Certification No.: SYXK (Su) 2011-0036). The formulation of the basal diets was performed according to nutrient requirements of the rat recommendations of National Research Council (Council 1995). Thirty-six male Wistar rats weighing 175 ± 5 g were bought from the Center of Laboratory Animals, Yangzhou University (Yangzhou, China). The rats were kept in a controlled condition at 25 ± 2 °C and a 12 h light/dark cycle for 5 days. The contents of zinc (inorganic zinc (zinc oxide)), were 100 mg/L, calculated according to the 1 kg basal diet. Probiotics (P) and zinc-enriched probiotics diets were fed 1 mL per day, via the stomach tube to each rat during the day time. 36 male Wistar rats were randomly divided into three equal groups (each group contained 12 Wistar rats). Each group was further divided into three replicates and each contained four rats. All rats were fed with the basal diet for 40 consecutive days (in the months of 01. July to 10. August 2018) as shown in Table 1.
Table 1.
Ingredient and nutrient composition of basal diet fed to Wistar rats
| Ingredient | Amount, g/kg diet |
|---|---|
| Casein (≥ 85% protein) | 200.0 |
| Corn starch | 150.0 |
| Sucrose | 500.0 |
| Maltose dextrin | 100.0 |
| Cellulose | 50.0 |
| Corn oil | 50.0 |
| Vitamin mix | 10.0 |
| Mineral mix | 35.0 |
| Choline bitartrate | 2.0 |
| DL-methionine | 3.0 |
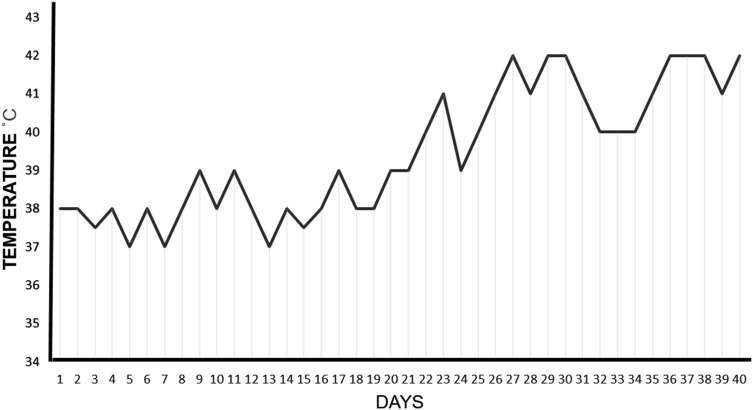
The treatment groups were fed with combination of basal and supplementation of P & ZnP products, respectively. During the initial 20 days, the daytime temperature in the rats’ room with natural ventilation ranged from 38 to 39 °C, and the terminal 10 days daytime room temperature ranged from 39 to 42 °C (Fig. 1), respectively. All of the basal diets for the Wistar rats were hanged daily, and fresh water was available at all times during the trial. Each rat was balanced at the beginning and the end of the feeding experiment. Feed intake was recorded daily; Average daily gain (ADG), average daily feed intake (ADFI), and feed/gain (F/G) ratio were calculated for each group.
Fig. 1.
Daily average temperature from 12:00 a.m. to 08:00 p.m. in entire 40-day experiment period
Sample collection and preparation
Blood samples were collected from each rat for all three treated groups on the last day of the experiment. Two milliliters (mL) of blood samples was collected directly from the heart of each rat via a syringe primed without EDTA and were gently ejected into Eppendorf tubes. Individually, blood samples were stored at 4 °C until analysis of GSH-Px activity, SOD activity, GSH content, MDA content, and IL-2, IL-6, IL-10, and IFN-γ concentration. We prepared 2 mL serum without EDTA and whole blood samples were kept in a sloping position at 37 °C for 2 h, and then at 4 °C overnight, followed by centrifugation at 3000 rpm for 10 min resulting supernatant; this supernatant was collected and stored at − 20 °C until analysis of antioxidant status.
At the end of the experiment, all rats were sacrificed. The liver, kidney, and heart tissues were quickly removed and dressed with ice-cold isotonic saline, then fasten frozen in liquid nitrogen and stored at − 80 °C until analysis of SOD1, SOD2, MT1, MT2, SHSP 70, and HSP 90 mRNA expression.
The GSH-Px and SOD activity and GSH and MDA content in serum were, respectively, determined using the commercial kits in accordance with the manufacture’s instructions. GSH-Px activity, SOD activity, and GSH and MDA content were expressed as U/L, U/mL, µmol/g protein, and nmol/mL, respectively.
Jejunum morphological examination
After fixation overnight, 0.5-cm cubes of the middle part of jejunum were embedded in paraffin and 5 µm sections were cut. The sections were stained using hematoxylin and eosin (H&E) as previously described (Hamid et al. 2017). The villus height and muscle thickness of the jejunum were photographed using a light microscope according to the method described previously (Wang et al. 2016). Morphometric measurements of jejunum villus and intestinal wall thickness were performed at a 40× magnification. All measurements were analyzed using Image J software. Morphometric measurements, including villus height were recorded according to the method described previously (Ma et al. 2018). Also, the muscle thickness of the jejunum was measured according to the method described by Wang et al. (2015).
Determination of IL-2, IL-6, IL-10, and IFN-γ concentrations in serum
The IL-2, IL-6, IL-10, and IFN-γ concentrations in the serum samples were measured using enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s protocol (Multi Sciences Biotechnology Co., Ltd.). The concentration units were expressed as pg/mL.
SYBR green real-time PCR analysis
Real-time quantitative PCR method was employed for mRNA detection. The PCR primers (Table 2) for six genes, SOD1, SOD2, MT1, MT2, HSP70, and HSP90 and one reference gene (control) (β-actin) were designed using primer software on the basis of the known Rattus norvegicus sequences reported in the NCBI database.
Table 2.
Primers used for real-time quantitative PCR
| Gene | Accession No. | Forward primer (5′–3′) | Reverse primer (5′–3′) | Product length (bp) |
|---|---|---|---|---|
| β-Actin | NM_031144.3 | CACGGCATTGTCACCAACTG | AACACAGCCTGGATGGCTAC | 203 |
| SOD1 | NM_017050.1 | GAAAGGACGGTGTGGCCAAT | TCGTGGACCACCATAGTACG | 94 |
| SOD2 | NM_017051.2 | CGGGGGCCATATCAATCACA | GCCTCCAGCAACTCTCCTTT | 84 |
| MT1 | NM_138826.4 | TCCTGCAAGAAGAGCTGCTG | CACTTGTCCGAGGCACCTTT | 89 |
| MT2 | NM_001137564.1 | ACAGATGGATCCTGCTCCTG | CCGAAGCCTCTTTGCAGATG | 139 |
| HSP70 | NM_001329896.1 | CAAGAATGCGCTCGAGTCCT | CGCTGATCTTGCCCTTGAGA | 77 |
| HSP90 | NM_001004082.3 | GTACAGCAGCTCAAGGAGTTC | GGGGGAAGACACAAGCCTATT | 201 |
Real-time quantitative PCR (qPCR)
The mRNA expression of genes (SOD1, SOD2, MT1, and MT2) and heat shock protein genes (Hsp70 and Hsp90) were quantitatively analyzed by real-time PCR (qRT-PCR). Primers of the β-actin (reference gene) and target genes are shown in Table 1. The total RNA was extracted from the frozen heart, liver, and kidney samples using the RNAiso Plus (TaKaRa) reagent according to the manufacturer’s protocol. The isolated RNA pellets were resuspended in 50 µL of diethyl pyrocarbonate (DEPC) water, quantified by measurement of the absorbance ratio at 260/280 nm, and then stored at − 80 °C prior to cDNA synthesis. First-strand cDNA was synthesized from 2 µL of total RNA using 4 µL 5xprimerScript RT Master Mix and 14 µL RNase free water (TaKaRa) according to the manufacturer’s instructions. The real-time PCR was performed as described previously (Gan et al. 2013). With some changes, reactions were carried out in a 20 µL reaction combination holding 10 µL of 2 × SYBR Green I PCR Mater Mix (TaKaRa BIO INC), 0.4 µL Rox Reference Dye 1, 0, 4 µL of each primer, 2 µL cDNA, and 6.8 µL of PCR grade water. The whole processes were conducted in a Step OnePlus Real-Time PCR system (Applied Biosystems, USA). Relative mRNA expression levels of the above genes were detected using the ΔCt (Δ cycle threshold) procedure (Gan et al. 2014). The result was applied to each gene by calculating the expression 2−ΔΔ Ct.
Statistical analysis
The results are expressed as the mean values with their standard errors. SPSS Statistics version 19 was used for statistical analysis. Significant differences were analyzed by one-way analysis of variance (ANOVA) followed by the Duncan post hoc test. A P < 0.05 was considered as statistically significant.
Results
Growth performance and feed intake
After continuous feeding for 40 days, the zinc-enriched probiotic group showed significantly (P < 0.05) increased body weight of Wistar rats compared to control and other treated groups under heat stress condition. There was significant (P < 0.05) enhancement in the growth performance of Wistar rats under both the treatments viz., probiotics (P) and Zn-enriched probiotics (ZnP) groups relative to control under heat stress conditions. However, ZnP-supplemented rats group showed better growth performance compared to other treated groups (Table 3). There were no significant (P < 0.05) differences between the treated and control groups in ADFI, but significant (P < 0.05) difference was exhibited among the treated groups in F/G ratio, which can be elucidated due to the effects of ZnP supplementation (Table 3).
Table 3.
Growth performance and feed intake of rats
| Growth performance | Groups | ||
|---|---|---|---|
| Control | P | ZnP | |
| Initial BW(g) | 177.16 ± 1.77a | 179.0 ± 1.53a | 176.37 ± 1.86a |
| Final BW(g) | 204.04 ± 2.56c | 232.48 ± 5.34b | 259.57 ± 3.24a |
| ADG (g/day) | 0.672 ± 0.13c | 1.34 ± 0.32b | 2.08 ± 0.60a |
| ADFI (g/day) | 22.63 ± 2.39a | 25.84 ± 1.45a | 24.79 ± 2.96a |
| F/G | 33.67 ± 7.52c | 19.29 ± 4.26b | 11.91 ± 2.23a |
GSH-Px activity, GSH content, SOD activity, and MDA content in blood serum
The non-significant difference was found in GSH-Px activity among the control and P groups under heat stress. However, GSH-Px activity increased significantly (P < 0.05) in ZnP-supplemented group compared to P treated and control groups under heat stress condition (Table 4). The serum GSH content was significantly (P < 0.05) higher in the ZnP-supplemented group compared to control and other treated groups under heat stress conditions. Similarly, P-supplemented group also showed significant (P < 0.05) difference compared to control (Table 4). Moreover, the control showed significantly (P < 0.05) decreased level of SOD activity, but ZnP-treated group revealed significantly (P < 0.05) increased level of SOD compared to control and other treated groups under heat stress (Table 4). In addition, the control group showed significantly (P < 0.05) higher level of MDA content compared to P- and ZnP-supplemented groups under thermal stress. Finally, ZnP supplementation significantly (P < 0.05) decreased the level of MDA content compared to control and P-treated groups, respectively (Table 4).
Table 4.
The GSH-Px activity, GSH content, SOD activity, and MDA Content in blood serum
| Antioxidant status | Groups | ||
|---|---|---|---|
| Con | P | ZnP | |
| GSH-Px Activity (U/L) | 22.49 ± 4.35b | 23.68 ± 4.6b | 53.91 ± 5.99a |
| GSH content (µmol/g protein) | 26.23 ± 1.76c | 46.34 ± 1.82b | 59.63 ± 2.63a |
| SOD activity (U/mL) | 51.46 ± 4.99c | 82.05 ± 3.79b | 122.26 ± 5.34a |
| MDA content (nmol/ML) | 54.17 ± 2.99c | 32.81 ± 2.54b | 19.27 ± 3.23a |
IL-2, IL-6, IL-10, and IFN-γ concentration in serum
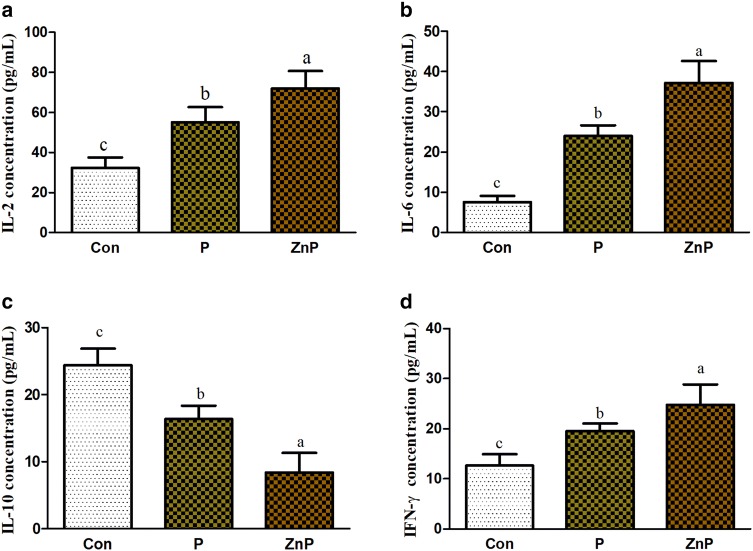
The IL-2, IL-6, IL-10, and IFN-γ concentrations in serum from three treatment groups are shown in Fig. 2a–d. However, on the last day of the experiment, there were significant (P < 0.05) differences between all three groups for IL-2, IL-6, IL-10, and IFN-γ, when compared to each other. The heat-stressed control group showed significantly (P < 0.05) lower level of IL-2, IL-6, and IFN-γ while the IL-10 showed significantly (P < 0.05) higher level in serum. Although the P group showed significantly higher (P < 0.05) value relative to control group, but the ZnP group was even more highly significant (P < 0.05) than the P and control groups, respectively.
Fig. 2.
Effect of P and ZnP on IL-2, IL-6, IL-10, and IFN-γ concentrations in whole blood serum pg/mL, respectively, of rats under heat stress. a IL-2 concentration (pg/mL), b IL-6 concentration (pg/mL), c IL-10 concentration (pg/mL), d IFN-γ (pg/mL). The values with different superscript letters are significantly different from each other at (P < 0.05)
Effect of ZnP on the mRNA expression of SOD1 and SOD2 genes
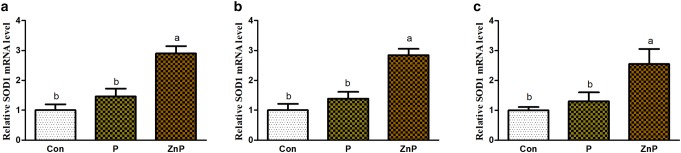
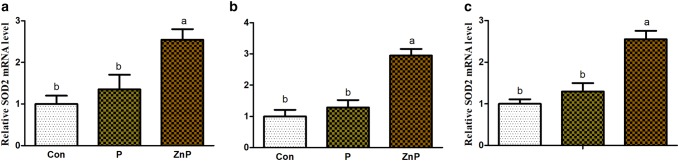
The expressions of SOD1 and SOD2 genes were up-regulated (P < 0.05) in the ZnP group as compared to control and P groups. In addition, the mRNA expressions of the antioxidant-related gene showed a better effect (P < 0.05) in the ZnP group relative to the P group. Similarly, the expression of SOD1 and SOD2 genes was increased significantly (P < 0.05) in ZnP group compared to group. However, no significant differences were found in expressions of SOD1 and SOD2 genes between control and P groups (Figs. 3, 4).
Fig. 3.
Effect of P and ZnP on mRNA levels of SOD1 in the liver (a), heart (b), and kidney (c) of heat-stressed rats. The means with different superscript letters are significantly different from each other at (P < 0.05)
Fig. 4.
Effect of P and ZnP on mRNA levels of SOD2 in the liver (a), heart (b), and kidney (c) of heat-stressed rats. The means with different superscript letters are significantly different from each other at (P < 0.05)
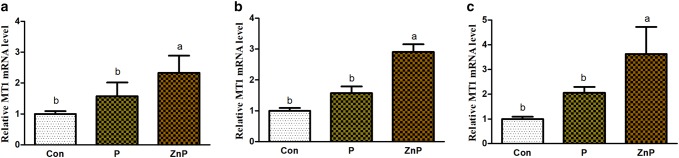
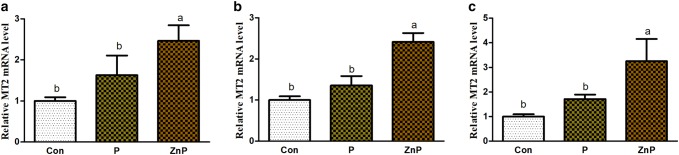
Effect of ZnP on MT1 and MT2 mRNA genes expressions
Expression levels of MT1 and MT2 genes in liver, heart, and kidney tissues are shown in Figs. 5 and 6. The MT1 and MT2 genes expressions in liver, heart, and kidney of rats were up-regulated under both P and ZnP treatment compared to the control group, respectively. Additionally, there were no significant (P < 0.05) differences between control and P-supplemented groups in the up-regulation level of MT1 and MT2 genes expression. However, the expression level of ZnP-supplemented group was significantly (P < 0.05) up-regulated relative to control and treated groups.
Fig. 5.
Effect of P and ZnP on mRNA levels of MT1 gene in the liver (a), heart (b), and kidney (c) of heat-stressed rats. The means with different superscript letters are significantly different from each other at (P < 0.05)
Fig. 6.
Effect of P and ZnP on mRNA levels of MT2 gene in the liver (a), heart (b), and kidney (c) of heat-stressed rats. The means with different superscript letters are significantly different from each other at (P < 0.05)
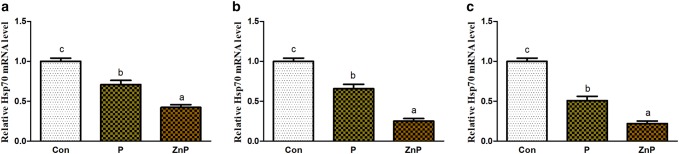
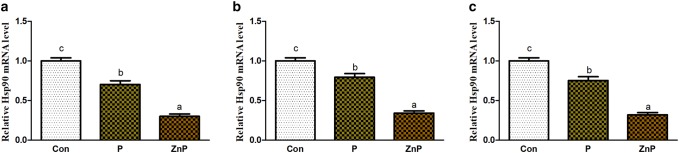
Effect of ZnP on Hsp70 and Hsp90 mRNA genes expressions
Expression levels of Hsp70 and Hsp90 genes in liver, heart, and kidney tissues are shown in Figs. 7 and 8. The expression of Hsp70 and Hsp90 genes in liver, heart, and kidney of rats was significantly down-regulated (P < 0.05) in diet supplied with P or ZnP relative to the control group. However, the expression level of ZnP-supplemented group is significantly (P < 0.05) down-regulated compared to control and treated groups.
Fig. 7.
Effect of P and ZnP on mRNA levels of Hsp70 gene in the liver (a), heart (b), and kidney (c) of heat-stressed rats. The means with different superscript letters are significantly different from each other at (P < 0.05)
Fig. 8.
Effect of P and ZnP on mRNA levels of Hsp90 gene in the liver (a), heart (b), and kidney (c) of heat-stressed rats. The means with different superscript letters are significantly different from each other at (P < 0.05)
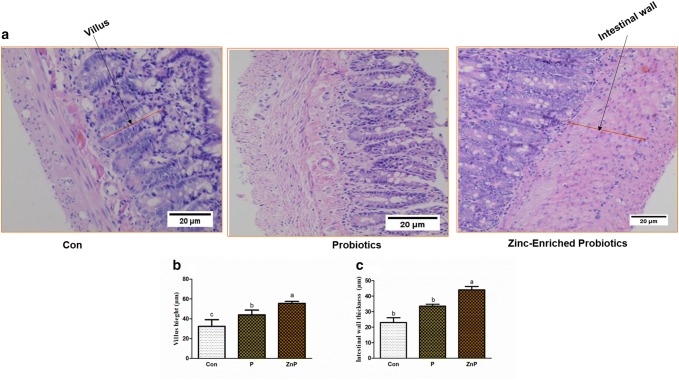
Dietary of probiotics and zinc-enriched probiotics affected the development of villus height and muscle thickness of jejunum
Hematoxylin and eosin staining showed that P and ZnP diet-induced changes in the middle part of jejunum morphology are shown in Fig. 9. Compared to the control, the probiotics and zinc-enriched probiotics fed rats had a significantly increased villus height but it is non-significantly increased among the P and control groups while compared to the intestinal wall thickness. Moreover, the ZnP diet fed rats were showing a significant increase in villus height and intestinal wall thickness compared to P and control groups.
Fig. 9.
Effect of dietary P and ZnP on the middle part of jejunum histology. a Hematoxylin and eosin (H & E) staining images (magnification, × 40). b Villus height. c. Intestinal wall thickness. a, b Means with different letters differed significantly (P < 0.05)
Discussion
The present study demonstrated that zinc-enriched probiotics (ZnP) supplementation has improved both final BW and ADG of Wistar rats, while as it decreased F/G ratio under heat stress condition. Hence, it proved the hypothesis suggesting that ZnP supplementation could improve growth performance under heat stress. It has been reported that heat stress enhances metabolic activity (Bernabucci et al. 2010), as well as leads to oxidative stress due to increased accumulation of ROS content along with increased lipid peroxidation products (Zhang et al. 2003). Previous studies have revealed that pigs fed with diets enriched of zinc oxide reveal significant differences in ADG, final BW, and F/G compared to control group, while as ZnP diet decreased the F/G ratio (Cho et al. 2018; Stehlik-Tomas et al. 2004; Li et al. 2018), and also pigs fed with Zn in the form of ZnO supplements had showed significant increase in ADG and F/G compared to pigs fed with basal diet (Li et al. 2018).
Piglets raised under high ambient temperature showed that dietary P supplementation decreased MDA content as well as increased the GPX activity (Carlson et al. 2004a). Although the mechanism of probiotics on increasing GSH content and SOD activity is still unclear, the reduced MDA content might be associated with the increased GSH content and SOD activity. Many earlier studies have revealed the role of probiotics (L. acidophilus) in improving the function of swine antioxidant defense system (Wang et al. 2009, 2012). Furthermore, Zn supplementation and dietary zinc oxide nanoparticle of Japanese quails and broiler chickens improved the total antioxidant capacity and reduced the MDA concentrations compared to controls (Zhao et al. 2014; Atakisi et al. 2009), and supplementation with zinc improved antioxidant status of birds, and the effects of ZnPic were relatively greater than those of ZnSo2H2O in heat-stressed quail (Sahin et al. 2005). Similar to above mentioned previous studies, the present study reported that combined effect of ZnP supplementation significantly increased GPX and SOD activities as well as GSH content, and reduced MDA content under heat stress conditions in Wistar rats. Hence, our results strongly suggest that the ZnP supplementation has more beneficial effects in terms of improving animal antioxidant status than P alone under heat stress in Wistar rats. Similarly, Ren et al. also demonstrated that selenium/zinc-enriched probiotics significantly increased the activities of glutathione peroxidase, SOD, and total antioxidant capacity, and decreased MDA content in the blood of canine (Ren et al. 2011a).
Till date, the effects of ZnP supplementation on increasing IL-2, IL-6, and IFN-γ and effects on decreasing IL-10 concentrations in Wistar rats under high-temperature stress condition have not been reported. In this context, data obtained in the present study showed that dietary supplementation of P and ZnP increased serum IL-2, IL-6, and IFN-γ and decreased the IL-10 concentrations in Wistar rats under heat stress environment, respectively, which is consistent with some previous studies that report same findings under normal temperature conditions (Kirchner and Rink 2000). Furthermore, this study also showed that the effects of ZnP supplementation on increased IL-2, IL-6, and IFN-γ and decreased IL-10 secretions were more dramatic than that of P supplementation alone, suggesting that ZnP is more effective in boosting Wistar rat’s immune function than P used alone. The reason may be because of the synergistic effect between Zn and P on IL-2, IL-6, IL-10, and IFN-γ secretions. It was earlier reported that the probiotics of L. acidophilus increased the IL-2 secretion in pigs raised under normal temperature conditions (Tortuero et al. 1995; Babinska et al. 2005).
The present study also revealed that the combinational effect of ZnP showed significantly better results compared to using either of them (i.e., Zn or P) alone, that suggests that Zn-enriched probiotics promoted the expression of SOD1 and SOD2 genes in liver, heart, and kidney. This showed that zinc-enriched probiotics can significantly improve antioxidant genes in rats that were subjected to heat stress. Likewise, the previous study observed the effects of dietary zinc oxide (ZnO) on E. fetida, and results have revealed that expression of Cu/Zn-SOD and MT genes was significantly up-regulated to the highest levels in a dose-dependent manner at day 10, respectively (Xiong et al. 2012).
Metallothioneins are encoded by zinc-activated transcription genes group of proteins and are involved in protecting cells against ROS accumulation (Tapiero and Tew 2003). The MT-1 and MT-2 are the predominant MTs exerting as the potent ROS scavengers (Thornalley and Vašák 1985). In this study, we investigated the expression of MT-1 and MT-2 in liver, heart, and kidney using qRT-PCR. In the present study, zinc-enriched supplementation resulted in a significant increase in MT-1 and MT-2 mRNA levels, which is similar to the observation of OT-treated Coco-2/TC7 cells with zinc supplementation (Ranaldi et al. 2009).
Furthermore, in this study, the combined effect of zinc-enriched probiotics supplementation showed significant down-regulation of Hsp70 and Hsp90 mRNA expression in the liver, kidney, and heart of Wistar rats in each experimental group than their separate supplementation under heat stress. Previous studies have shown that thermal stress in mammals exposed over a period of time enhances Hsp70 and Hsp27 expression (Kadzere et al. 2002). Similarly, it has been revealed that the effect of organic supplementation of trace minerals selenium (Se), chromium (Cr), and zinc (Zn) reduced Hsp70 mRNA expression level significantly indicating the reduced heat stress in broilers during tropical summer (Rao et al. 2016). Therefore, based on the results of the present study, it can be hypothesized that effects of P and ZnP on down-regulating Hsp70 and Hsp90 mRNA levels of heat-stressed Wistar rats may be due to the increasing tissue P and Zn concentration as well as GSH-Px enzyme activity and mRNA levels. The ZnP supplementation in Wistar rats’ diet has maximum effects on decreasing Hsp70 and Hsp90 mRNA levels, suggesting that ZnP is more beneficial to Wistar rats under heat stress conditions than P or Zn alone. Clearly, the combination of P and Zn in ZnP has great effects on decreasing Hsp70 and Hsp90 mRNA.
In the present study, the villus height and intestinal wall thickness in the middle part of jejunum in P and ZnP groups were significantly (P < 0.05) higher and thicker compared to the control group. Previous studies have shown that zinc supplementation significantly (P < 0.05) increased the villus height in the small intestine in rats (Khan et al. 2013). On the other hand, Gunal et al. (2006) reported that villus height of jejunum and ileum increased in broiler chicks supplied with the multi-microbe probiotic product, and also it has been reported that villus height was increased in gnotobiotic piglets inoculated Lactobacillus fermentum (monoassociated with Lactobacillus fermentum) (Matur and Eraslan 2012).These results suggest that the effects of P and ZnP on Wistar rats changed the jejunum morphology.
Conclusion
The present study was the first report to study the effect of combined Zn and P as ZnP supplementation on the growth performance of Wistar rats under heat stress. It was reported that ZnP supplementation could alleviate the negative effects of heat stress, and improved growth performance, antioxidant capacity, immune functions, genes expression of Hsp90, Hsp70, SOD1, SOD2, MT1, and MT2, and change the morphological characteristics of villus height and intestinal wall thickness in the middle part of the jejunum. The probiotics and zinc may enhance the function due to their synergetic effects on growth performance of Wistar rats under heat stress. Our results suggest that ZnP (100 mg Zn/L) is a potential nutritive supplement for Wistar rats under heat stress conditions. It is concluded that dietary use of ZnP is a potential nutritive feed supplement in subtropical and tropical areas for reducing the harmful effects of persistent summer heat stress.
Authors’ contributions
RMM conceived and designed experiments and wrote the manuscript. GF and HLi designed the structure of this manuscript. JM, XXC, and KH gave some valuable advices about structure of manuscript. RMM, HE, DL, and JKB analyzed the data and revised the manuscript. RAF complied information; RMM and HL performed the experiments. All authors read and approved the final manuscript.
Funding
This project is funded by the National Key R & D Program (2017YFD0501000), National Natural Science Foundation of China (31811530300), MOE Joint International Research Laboratory of Animal Health and Food Safety, College of Veterinary Medicine, Nanjing Agricultural University, Project of National Center for International Research on Animal Gut Nutrition and supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Jiangsu, China).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Altan Ö, Pabuçcuoğlu A, Altan A, Konyalioglu S, Bayraktar Ö. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br Poult Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Atakisi O, Atakisi E, Kart A. Effects of dietary zinc and l-arginine supplementation on total antioxidants capacity, lipid peroxidation, nitric oxide, egg weight, and blood biochemical values in Japanase quails. Biol Trace Elem Res. 2009;132(1–3):136–143. doi: 10.1007/s12011-009-8378-x. [DOI] [PubMed] [Google Scholar]
- Babinska I, Rotkiewicz T, Otrocka-Domagala I. The effect of Lactobacillus acidophilus and Bifidobacterium spp. administration on the morphology of the gastrointestinal tract, liver and pancreas in piglets. Polish J Vet Sci. 2005;8(1):29–35. [PubMed] [Google Scholar]
- Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal. 2010;4(7):1167–1183. doi: 10.1017/s175173111000090x. [DOI] [PubMed] [Google Scholar]
- Buzadžić B, Korać B, Lazić T, Obradović D. Effect of supplementation with Cu and Zn on antioxidant enzyme activity in the rat tissues. Food Res Int. 2002;35(2–3):217–220. doi: 10.1016/S0963-9969(01)00187-9. [DOI] [Google Scholar]
- Carlson M, Boren C, Wu C, Huntington C, Bollinger D, Veum T. Evaluation of various inclusion rates of organic zinc either as polysaccharide or proteinate complex on the growth performance, plasma, and excretion of nursery pigs. J Anim Sci. 2004;82(5):1359–1366. doi: 10.2527/2004.8251359x. [DOI] [PubMed] [Google Scholar]
- Carlson MS, Boren CA, Wu C, Huntington CE, Bollinger DW, Veum TL. Evaluation of various inclusion rates of organic zinc either as polysaccharide or proteinate complex on the growth performance, plasma, and excretion of nursery pigs. J Anim Sci. 2004;82(5):1359–1366. doi: 10.2527/2004.8251359x. [DOI] [PubMed] [Google Scholar]
- Cho JH, Liu SD, Yun W, Kim KS, Kim IH. Effect of supplemented microencapsulated zinc oxide and organic acids and pure botanicals on growth performance, nutrient digestibility, blood profiles, feces microflora, and zinc level of feces in weanling pigs. Can J Anim Sci. 2018;99(1):66–73. doi: 10.1139/cjas-2017-0114. [DOI] [Google Scholar]
- Wood CM, Farrell AP, Brauner CJ (2011) Contents of Homeostasis and Toxicology of Essential Metals, Volume 31A. Fish physiology, vol 31A. Academic Press, pp ix–xiii. 10.1016/S1546-5098(11)31037-0
- Council NR. Nutrient requirements of laboratory animals. 4. Washington, DC: The National Academies Press; 1995. [PubMed] [Google Scholar]
- De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology. 2003;60(6):1139–1151. doi: 10.1016/S0093-691X(03)00126-2. [DOI] [PubMed] [Google Scholar]
- Dong G, Chen H, Qi M, Dou Y, Wang Q. Balance between metallothionein and metal response element binding transcription factor 1 is mediated by zinc ions. Mol Med Rep. 2014;11:1582–1586. doi: 10.3892/mmr.2014.2969. [DOI] [PubMed] [Google Scholar]
- Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431(2):285–286. doi: 10.1016/S0014-5793(98)00779-0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gan F, Ren F, Chen X, Lv C, Pan C, Ye G, Shi J, Shi X, Zhou H, Shituleni SA. Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions. J Agric Food Chem. 2013;61(10):2385–2391. doi: 10.1021/jf300249j. [DOI] [PubMed] [Google Scholar]
- Gan F, Chen X, Liao SF, Lv C, Ren F, Ye G, Pan C, Huang D, Shi J, Shi XJ. Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agric Food Chem. 2014;62(20):4502–4508. doi: 10.1021/jf501065d. [DOI] [PubMed] [Google Scholar]
- Gunal M, Yayli G, Kaya O, Karahan N, Sulak O. The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int J Poult Sci. 2006;5:149–155. doi: 10.3923/ijps.2006.149.155. [DOI] [Google Scholar]
- Hahn JD, Baker DH. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci. 1993;71(11):3020–3024. doi: 10.2527/1993.71113020x. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Matthes RD, Gisolfi CV. Hyperthermia stimulates nitric oxide formation: electron paramagnetic resonance detection of NO-heme in blood. J Appl Physiol (Bethesda, Md: 1985) 1994;77(2):548–553. doi: 10.1152/jappl.1994.77.2.548. [DOI] [PubMed] [Google Scholar]
- Hamid M, Liu D, Abdulrahim Y, Liu Y, Qian G, Khan A, Gan F, Huang K. Amelioration of CCl4-induced liver injury in rats by selenizing Astragalus polysaccharides: role of proinflammatory cytokines, oxidative stress and hepatic stellate cells. Res Vet Sci. 2017;114:202–211. doi: 10.1016/j.rvsc.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Jordan ER. Effects of heat stress on reproduction. J Dairy Sci. 2003;86:E104–E114. doi: 10.3168/jds.S0022-0302(03)74043-0. [DOI] [Google Scholar]
- Kadzere C, Murphy M, Silanikove N, Maltz E. Heat stress in lactating dairy cows: a review. Livest Prod Sci. 2002;77(1):59–91. doi: 10.1016/S0301-6226(01)00330-X. [DOI] [Google Scholar]
- Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95(3):749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- Khan J, Wan Saudi WS, Islam M. Effect of zinc on chronic stress induced small intestinal changes in rats. Int Med J. 2013;20:29–33. [Google Scholar]
- King L, Osati-Ashtiani F, Fraker PJI. Depletion of cells of the B lineage in the bone marrow of zinc-deficient mice. Immunology. 1995;85(1):69. [PMC free article] [PubMed] [Google Scholar]
- King LE, Osati-Ashtiani F, Fraker PJ. Depletion of cells of the B lineage in the bone marrow of zinc-deficient mice. Immunology. 1995;85(1):69–73. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H, Rink L. Zinc-altered immune function and cytokine production. J Nutr. 2000;130(5):1407S–1411S. doi: 10.1093/jn/130.5.1407S. [DOI] [PubMed] [Google Scholar]
- Krishnan G, Bagath M, Pragna MKVP, Vidya MK, Aleena J, Archana PR, Sejian V, Bhatta R. Mitigation of the heat stress impact in livestock reproduction. Theriogenology. 2017;8:8–9. doi: 10.5772/intechopen.69091. [DOI] [Google Scholar]
- Kumar BVS, Kumar A, Kataria M. Effect of heat stress in tropical livestock and different strategies for its amelioration. J Stress Physiol Biochem. 2011;7(1):45–54. [Google Scholar]
- Lessard M, Dupuis M, Gagnon N, Nadeau E, Matte J, Goulet J, Fairbrother JM. Administration of Pediococcus acidilactici or Saccharomyces cerevisiae boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia coli challenge. J Anim Sci. 2009;87(3):922–934. doi: 10.2527/jas.2008-0919. [DOI] [PubMed] [Google Scholar]
- Li H-H, Jiang X-R, Wang W-J, Qiao J-Y. Effects of Lactobacillus acidophilus and zinc oxide on the growth performance, jejunal morphology and immune function of weaned piglet following an Escherichia coli K88 challenge. Ital J Anim Sci. 2018;17(1):114–120. doi: 10.1080/1828051X.2017.1344573. [DOI] [Google Scholar]
- Lord-Fontaine S, Averill-Bates DA. Heat shock inactivates cellular antioxidant defenses against hydrogen peroxide: protection by glucose. Free Radic Biol Med. 2002;32(8):752–765. doi: 10.1016/S0891-5849(02)00769-4. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhou G, Li Y, Zhu Y, Yu X, Zhao F, Li H, Xu X, Li C. Intake of fish oil specifically modulates colonic Muc2 expression in middle-aged rats by suppressing the glycosylation process. Mol Nutr Food Res. 2018;62(4):8–9. doi: 10.1002/mnfr.201700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matur Erdal, Erasl Evren. New Advances in the Basic and Clinical Gastroenterology. 2012. The Impact of Probiotics on the Gastrointestinal Physiology. [Google Scholar]
- Musa HH, Wu S, Zhu C, Seri H, Zhu GJ. The potential benefits of probiotics in animal production and health. J Anim Vet Adv. 2009;8(2):313–321. [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- Poulsen HD. Zinc oxide for weanling piglets. Acta Agric Scand Sect A Anim Sci. 1995;45(3):159–167. doi: 10.1080/09064709509415847. [DOI] [Google Scholar]
- Ranaldi G, Caprini V, Sambuy Y, Perozzi G, Murgia C. Intracellular zinc stores protect the intestinal epithelium from Ochratoxin A toxicity. Toxicol Vitro. 2009;23(8):1516–1521. doi: 10.1016/j.tiv.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Rao SVR, Prakash B, Raju MVLN, Panda AK, Kumari R, Reddy EPK. Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. Biol Trace Elem Res. 2016;172:511–520. doi: 10.1007/s12011-015-0587-x. [DOI] [PubMed] [Google Scholar]
- Ren Z, Zhao Z, Wang Y, Huang K. Preparation of selenium/zinc-enriched probiotics and their effect on blood selenium and zinc concentrations, antioxidant capacities, and intestinal microflora in canine. Biol Trace Elem Res. 2011;141(1–3):170–183. doi: 10.1007/s12011-010-8734-x. [DOI] [PubMed] [Google Scholar]
- Ren Z, Zhao Z, Wang Y, Huang K. Preparation of selenium/zinc-enriched probiotics and their effect on blood selenium and zinc concentrations, antioxidant capacities, and intestinal microflora in canine. Biol Trace Elem Res. 2011;141(1–3):170–183. doi: 10.1007/s12011-010-8734-x. [DOI] [PubMed] [Google Scholar]
- Sahin K, Smith M, Onderci M, Sahin N, Gursu M, Kucuk O. Supplementation of zinc from organic or inorganic source improves performance and antioxidant status of heat-distressed quail. Poult Sci. 2005;84(6):882–887. doi: 10.1093/ps/84.6.882. [DOI] [PubMed] [Google Scholar]
- Stehlik-Tomas V, Gulan Zetić V, Stanzer D, Grba S, Vahčić N. Zinc, copper and manganese enrichment in yeast saccharomyces cerevisiae. Food Technol Biotechnol. 2004;42:115–120. [Google Scholar]
- Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57(9):386–398. doi: 10.1016/S0753-3322(03)00012-X. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Vašák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochem Biophys Acta. 1985;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Tortuero F, Rioperez J, Fernandez E, Luisa Rodriguez M. Response of piglets to oral administration of lactic acid bacteria. J Food Protect. 1995;58:1369–1374. doi: 10.4315/0362-028x-58.12.1369. [DOI] [PubMed] [Google Scholar]
- Wang A, Yi X, Yu H, Dong B, Qiao S. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing–finishing pigs. J Appl Microbiol. 2009;107(4):1140–1148. doi: 10.1111/j.1365-2672.2009.04294.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ji H, Wang S, Zhang D, Liu H, Shan D, Wang Y. Lactobacillus plantarum ZLP001: in vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian Aust J Anim Sci. 2012;25(8):1153. doi: 10.5713/ajas.2012.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Peebles ED, Morgan TW, Harkess RL, Zhai W. Protein source and nutrient density in the diets of male broilers from 8 to 21 d of age: effects on small intestine morphology. Poult Sci. 2015;94(1):61–67. doi: 10.3382/ps/peu019. [DOI] [PubMed] [Google Scholar]
- Wang X, Farnell YZ, Peebles ED, Kiess AS, Wamsley KGS, Zhai W. Effects of prebiotics, probiotics, and their combination on growth performance, small intestine morphology, and resident Lactobacillus of male broilers. Poult Sci. 2016;95(6):1332–1340. doi: 10.3382/ps/pew030. [DOI] [PubMed] [Google Scholar]
- Wolfenson D, Roth Z, Meidan R. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim Reprod Sci. 2000;60:535–547. doi: 10.1016/S0378-4320(00)00102-0. [DOI] [PubMed] [Google Scholar]
- Xiong W, Bai L, Muhammad R-U-H, Zou M, Sun Y. Molecular cloning, characterization of copper/zinc superoxide dismutase and expression analysis of stress-responsive genes from Eisenia fetida against dietary zinc oxide. Comput Biochem Physiol C Toxicol Pharmacol. 2012;155(2):416–422. doi: 10.1016/j.cbpc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Xu L, Drake VJ, Xie L, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J Off Publ Fed Am Soc Exp Biol. 2003;17(15):2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan LJV. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26(29–30):3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C-Y, Tan S-X, Xiao X-Y, Qiu X-S, Pan J-Q, Tang Z-X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res. 2014;160(3):361–367. doi: 10.1007/s12011-014-0052-2. [DOI] [PubMed] [Google Scholar]
- Zulkifli I, Abdullah N, Azrin NM, Ho YW. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br Poult Sci. 2000;41(5):593–597. doi: 10.1080/713654979. [DOI] [PubMed] [Google Scholar]