Abstract
Metabolites have emerged as the quintessential effectors mediating the impact of the commensal microbiome on human physiology, both locally at the sites of microbial colonization and systemically. The endocrine activity of the microbiome and its involvement in a multitude of complex diseases has made microbiome-modulated metabolites an attractive target for the development of new therapies. Several properties make metabolites uniquely suited for interventional strategies: natural occurrence in a broad range of concentrations, functional pleiotropy, ease of administration, and tissue bioavailability. Here, we provide an overview of recently discovered physiological effects of microbiome-associated small molecules that may serve as the first examples of metabolite-based therapies. We also highlight challenges and obstacles that the field needs to overcome on the path toward successful clinical trials of microbial metabolites for human disease.
Keywords: Microbiome, Disease, Metabolites, Therapies, Post-biotics
1. Introduction
Large-scale genome-wide association studies (GWAS) have revealed numerous genetic contributions to modern human diseases, including metabolic, inflammatory, and neurodegenerative disorders. At the same time, the cases in which GWAS have come close to reaching saturation with respect to the number of genetic associations to a particular disease suggest that these diseases do not solely arise from human genetic determinants. Rather, environmental and lifestyle factors play a major role in disease pathogenesis. A significant component of our environmental exposure is represented by diet and by symbiotic microbes that reside in the gastrointestinal tract. The microbiota has been recognized to play a role in human disease, and the mechanisms by which these microorganisms contribute to host health have been extensively investigated over the past decade. Commensal bacteria can directly engage host responses through physical interactions or through secreted molecules that gain access to the systemic circulation and thereby affect human physiology and disease. Recent technical progress in the fields of untargeted metabolomics – applied to well-characterized clinical cohorts and coupled with mechanistic studies in animal models – have enabled the characterization of human disease-associated gut microorganisms and their metabolites. Given the pervasiveness and pleiotropy of metabolite effects on human physiology, the translational potential of these molecules for “post-biotic” therapies is enormous [1,2]. Herein, we broadly summarize recent advances in the identification and characterization of microbiome-associated metabolites that are of potential therapeutic value for clinical use (Fig. 1). These metabolites are either solely derived from bacterial metabolism or dietary nutrients that are modulated by intestinal bacteria.
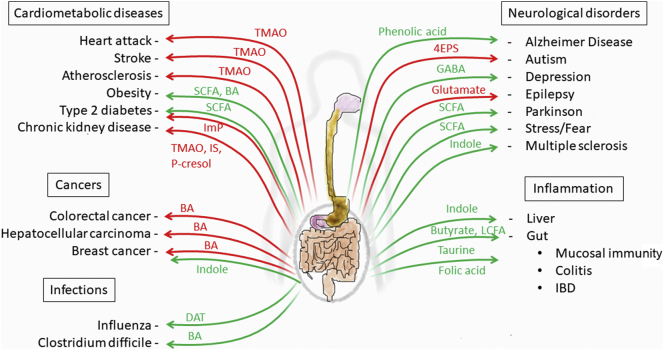
Fig. 1.
The impact of gut microbiome-derived metabolites in disease.
Arrows represent a beneficial (green) or detrimental (red) role for selected gut microbiome-associated metabolites in different diseases. 4EPS: 4-ethylphenylsulfate. BA: Bile acid. DAT: desaminotyrosine. GABA: gamma-aminobutyric acid. IBD: inflammatory bowel disease. ImP: Imidazole propionate. IS: Indoxyl sulfate. LCFA: Long-chain fatty acids. TMAO: Trimethylamine-N-oxide. SCFA: short-chain fatty acids.
1.1. Trimethylamine-N-oxide (TMAO)
TMAO may serve as a prototypical example for a microbiome-derived metabolite with strong translational potential. TMAO is linked to a broad range of diseases. In addition to cardiovascular and metabolic diseases, including atherosclerosis, type 2 diabetes, myocardial infarction, and stroke [[3], [4], [5], [6], [7]], studies identified TMAO as a potential predictive biomarker of chronic kidney disease and end-stage renal disease [8], although conflicting results were recently published [9]. Recent evidence also showed that TMAO is elevated in the cerebrospinal fluid of patients with mild cognitive impairment and Alzheimer's disease [10]. Initially, untargeted metabolomic analysis of human plasma samples from independent cohorts of patients who subsequently experienced myocardial infarction or stroke versus subjects who did not, led to the identification of TMAO as a metabolite strongly correlating with increased cardiovascular risk [11]. TMAO is formed by a diet-derived meta-organismal pathway. Eggs, milk, red meat and some seafood are rich in the lipid phosphatidylcholine, which is a source of the essential nutrient choline in omnivores. Choline and other trimethylamine (TMA)-containing species (l-carnitine; betaine) are catabolized by intestinal microbial TMA lyases and form the gas TMA [12]. TMA is rapidly absorbed by the host, enters the portal circulation, and is metabolized by the hepatic flavin monooxygenase FMO3 to TMAO [13]. An intact gut microbiome is required for the formation of TMAO from dietary choline, as germ-free mice failed to form TMAO after administration of deuterium-labelled PC, while the d9 isotopomer of TMAO was successfully produced in control mice [11]. TMAO supplementation in apolipoprotein E (ApoE)-deficient mice was observed to foster enhanced macrophage foam cell formation and to promote aortic root atherosclerotic plaque development [11]. TMAO induces alterations in cholesterol (reductions in reverse cholesterol transport), sterol metabolism, as well as in bile acid pool size and composition [14,15]. In experimental atherosclerosis, induced by 2% choline diet for 6 weeks in rodent, TMAO levels were reduced by oral supplementation with guggulsterone, a farnesoid X receptor antagonist, resulting in ameliorated experimental disease [16]. Although the receptor for TMAO is not yet known, this metabolite was shown to enhance platelet hyperreactivity and thrombosis risk [17].
Importantly, a natural inhibitor targeting the microbial TMA-generating enzyme gave promising results in a preclinal study of atherosclerosis [18] and protected against thrombosis risk. Indeed, a single oral dose of a structural analog to choline, 3,3-dimethyl-1-butanol (DMB), or iodomethylcholine (IMC), an inhibitor of the microbial CutC/D TMA-generating enzyme pair, significantly reduced plasma TMAO for 3 days in mice, rescuing the diet-induced hyperreactivity of platelets [19]. This effort represents a proof of concept that a microbial enzyme can be targeted to create new drugs for human diseases. Given that TMAO can be established as a cause rather than consequence of disease-promoting activity [20], the next step toward clinical application will be to establish the safety, efficacy and pharmacodynamics for this inhibitor in humans.
1.2. Short-chain fatty acids (SCFAs)
SCFAs are among the most concentrated metabolites in the gastrointestinal tract. Acetate, butyrate, and propionate are produced by members of the intestinal microbial community through fermentation of dietary fibers and starches, which are unable to be broken down by host-metabolism [21]. In turn, these metabolites are sensed by host cells through various G-protein coupled receptors (GPRs), known as free fatty acid receptors (FFARS), and the intracellular receptor PPARγ. Furthermore, SCFAs can also regulate cellular responses through inhibition of histone deacetylases (HDACs). Examples of SCFA effects on the host include regulatory T (Treg) cell and macrophage differentiation and downregulation of pro-inflammatory mediators. These effects underline the fine balance that SCFAs help maintain between intestinal immunity and inflammation [[21], [22], [23], [24]]. Furthermore, SCFAs have been seen to specifically alter hematopoiesis, promote IgA responses, alter T cell homeostasis, modulate fat accumulation and insulin sensitivity, and promote neurogenesis [[25], [26], [27], [28], [29], [30]]. Due to their clear role at the host-microbiota interface in shaping the immune system and metabolism, there has been increased interest to explore their potential roles in disease and uses as therapy. SCFAs are being used in clinical trials to treat a range of diseases affecting neurological functions, metabolism, and gastrointestinal health. Alterations in the microbiome have been correlated to the emergence of diabetes, obesity, and inflammatory diseases such as inflammatory bowel disease (IBD) [31]. Blood glucose regulation and lipid metabolism is highly implicated in metabolic diseases and some SCFAs, in particular butyrate, have been shown to affect these processes [32]. Due to butyrate's potential role, it is currently in Phase 2 and 3 trials to study its effect on insulin resistance and liver damage in children with obesity (NCT02721953). Furthermore, another clinical study administered butyrate enemas in patients in remission from ulcerative colitis or irritable bowel syndrome; however, relatively minor effects were observed [33]. Two studies aimed to look at the effect of either a SCFA mixture or acetate as interventional treatment in overweight male individuals with the potential of controlling obesity and type 2 diabetes mellitus. The results demonstrated whole body increase of fasting fat oxidation and plasma PYY, attenuation of fasting free glycerol concentrations, and decreased lipolysis, indicating the potential of acetate or SCFA colonic infusions as therapies for these metabolic conditions [[34], [35], [36]]. The potential therapeutic role of SCFAs is also being examined with respect to the gut-brain axis. Butyrate as an HDAC inhibitor, GPCR activator, and cellular energy source has been proposed to ameliorate symptoms in Alzheimer's, Parkinson's, autism, Huntington's, and schizophrenia [[37], [38], [39], [40], [41], [42]]. Trials are underway to assess butyrate's effect of cognitive function in patients with schizophrenia, in particular in regard to its epigenetic modulation of inflammation (NCT03010865). Currently, a SCFA mixture is being examined for its role in affective processing such as in stress, fear, and emotions (NCT03688854). Though these clinical studies are using SCFA directly as a therapy, there is also a growing interest in the use of pre-biotics such as resistant starches, which can be used by the microbiota to generate SCFA [40,43].
1.3. Long-chain fatty acids (LCFAs)
LCFAs have long been used in dietary supplementation, either as linseed or perilla oil containing linoleic acid and fish oils containing omega-3 and omega-6 fatty acids (FA). Considered to be essential, as they cannot be produced by mammals, LCFAs are obtained through diet and eventually metabolized to generate bioactive lipid mediators. These have been seen to reduce blood pressure (arachidonic acid) or modulate inflammation through attenuation of inflammatory cytokines [44,45]. Recently, LCFAs have been identified as microbial metabolites. Various conjugated linoleic acids (CLAs), oxy FA, and hydroxy FA are generated by commensal bacteria in the intestine: Lactobacillus plantarum, Enterococcus faecalis, and Bacteroides thetaiotaomicron [46]. Indeed, it has been shown that these microbial-derived linoleic acids can target PPARγ receptors in macrophages [47], while also fortifying epithelial barrier integrity through upregulation of tight junctions. Specifically, 10-hydroxy-cis-12 octadecenoic acid is one CLA which has been explored as a potential therapeutic for its role in epithelial barrier integrity [48,49]. Omega acids have long been shown to be beneficiary to the heart and brain, and now with accumulating evidence of microbially generated LCFAs, recent studies explore using CLAs in therapy as post-biotics. Indeed, studies have shown the potential of CLA to limit body fat percentage in healthy, overweight and obese adults possibly through modulation of fatty acid metabolism [50,51]. Additionally, one Phase 3 clinical study was performed to look for a role in attenuating atherosclerosis with a CLA, yet no effect was seen [52]. Another study posited that CLAs used as a supplement with vitamin and fiber could be effective as an adjuvant to ulcerative colitis therapy [53].
1.4. 4-ethylphenylsulfate (4-EPS)
4-EPS is a dietary fermentation product, suspected to be a uremic toxin. Specific pathogen-free and germ-free mice naturally have very low concentrations of 4-EPS. Interest in this metabolite stems from a report which showed an increase in 4-EPS in the maternal immune activation (MIA) mouse model of autism spectrum disorder (ASD). This elevated 4-EPS level correlated with behavioral abnormalities. These effects were then ameliorated through treatment with B. fragilis, indicating the possible use of pro-biotic and post-biotic therapies for ASD [54].
1.5. Indole
Amino-acid metabolism from the intestinal microbiota represents a major source of bioactive metabolites to the host [55]. The essential amino acid tryptophan is catalyzed into indole by tryptophanase, which is solely encoded in microbial genomes [56]. Tryptophan derivatives such as 3-indolepropionic acid (IPA) converted by Clostridium sporogenes or indole-3-aldehyde by Lactobacillus-encoded tryptophanase are recognized by the aryl hydrocarbon receptor (AhR). AhR is a ligand-activated transcription factor that integrates environmental, dietary, microbial and metabolic cues to control complex transcriptional programs in a ligand-specific, cell-type-specific, and context-specific manner [57]. Intestinal epithelial cell-specific deletion of Ahr results in failure to control C. rodentium infection [58]. AhR also mediates the effect of the microbiome on intra-epithelial lymphocytes [59]. Indole-3-aldehyde signaling through AhR mediates IL-22 production in type 3 innate lymphoid cells under pathogenic infection by Candida albicans [60]. Additional commensal microbiota metabolites are also endowed with AhR agonistic activity, such as indole-3-acetic acid, indole-3-acetylaldehyde, indole-3-aldehyde, 3-methylindole, and 2-(1ʹH-indole-3ʹ-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE). These metabolites are thought to limit intestinal inflammation via AhR-dependent mechanisms and may be dysfunctional in IBD [61]. Indole metabolites can also counteract detrimental inflammation in LPS-mediated liver inflammation through the modulation of the NLRP3 pathway [62]. In experimental autoimmune encephalomyelitis, a preclinal model of multiple sclerosis, indole metabolites modulated central nervous system inflammation through the reduction of pathogenic activity of astrocytes [[63], [64], [65]]. In neurodegenerative disorders, cerebellar syndrome is thought to be related to a neurochemical deficit of 5-hydroxytryptamine (5-HT). Current clinical trials will evaluate indole-3-propionic acid supplementation, a 5-HT precursor, as a therapeutic strategy for Friedreich's ataxia and multiple sclerosis. Indole can be further converted into indoxyl and indoxyl sulfate by host hepatic oxidases (CYP2E1 and SULT1A1). Indoxyl sulfate is suspected to be a central “uremic toxin”, whose removal from the host via renal excretion is impaired in kidney disease [66]. Indoxyl sulfate is also a potential vascular toxin that can induce oxidative stress in endothelial cells, increase vascular smooth muscle cell proliferation, and potentially contribute to the pathophysiology of atherosclerosis and sarcopenia in subjects with renal disease [66,67]. Over 90% of dietary tryptophan, however, is metabolized by the kynurenine pathway, which generates AhR ligands identified as trace-extended aromatic condensation products. Administration of a pharmacologically optimized kynureninase that degrades kynurenine into an immunologically inert, nontoxic metabolite inhibits tumor growth in a mouse model [68].
Several attempts to modulate indole metabolites for therapeutic purposes are under investigation. Arabinoxylan oligosaccharides (AXOS) are pre-biotic carbohydrates with promising health-promoting properties that stimulate the activity of specific colon bacteria, in particular Bifidobacteria. AXOS are tested in chronic kidney disease for lowering indole derivatives (indoxyl sulfate). An alternative to AXOS is AST-120 (also called Kremezin), which binds to indole and is currently tested in clinical trials as a therapeutic for chronic kidney disease (reduction of uremic toxin). An alternative therapeutic strategy would be to directly inhibit AhR by delivering antagonists alone or in nanoparticles or design enzyme optimized to metabolize AHR agonists [57].
p-cresol (or 4-methylphenol), and its derivative p-cresyl sulfate, is another bacterial metabolite from protein fermentation, not produced by human enzymes [69]. It is suspected to alter endothelial function [70], to promote vascular calcification [71] and has been associated with increased mortality in hemodialysis patients [72], pathogenesis of ulcerative colitis, and Clostridium difficile infection [73]. While increased dietary fat consumption in healthy subject results in an increase of p-cresol and indole [74], a dietary strategy with oligofructose-enriched inulin contributes to a lower generation of protein fermentation metabolites and constitutes a significant improvement for chronic kidney disease patients [75,76]. AXOS intake increases fecal bifidobacteria and reduces urinary p-cresol excretion [77].
1.6. Other protein-derived metabolites
Taurine is an amino sulfonic acid whose levels are modulated by commensal bacteria deconjugation of primary bile acids [78,79], causing an increase in luminal taurine levels. Taurine was observed to induce NLRP6 inflammasome signaling and was thus proposed to modulate inflammatory bowel diseases [80]. Taurine is also being studied as a protective metabolite in colorectal cancer and diabetes. One study looked at the abundance of sulfidogenic bacteria in African Americans with diets rich or low in taurine to observe for risk elements for colorectal cancer, such as oxidative stress, primary and secondary bile acid pools, and inflammation markers [81]. Meanwhile, two clinical studies administer taurine to treat vascular complications in diabetes that may arise through oxidative stress (NCT03410537, NCT01226537).
The protective effect of ketogenic diet (reduced carbohydrates and high-fat diet) has been shown to be mediated by the gut microbiome through the reduction of amino acid y-glutamylation, ultimately leading to increased GABA/glutamate content in the brain [82].
Desaminotyrosine (DAT) is a degradation product of tyrosine and naturally present in mammalian urine. A recent study showed that an intact gut microbiome, and in particular the presence of Clostridium orbiscendens, is required for detectable DAT levels in the feces and serum [83]. Reduction of DAT production was associated with increased susceptibility to viral infection, due to suboptimal amplification of the interferon type I signaling loop. Yet, no registered clinical study is assessing the translation of this finding to the clinic.
Imidazole propionate is a recently identified histidine-derived metabolite that plays an important role in the pathophysiology of type 2 diabetes. Higher concentrations of this metabolite tend to be found in type 2 diabetes subjects versus healthy subjects, and result in the impairment of the insulin signaling by activating p38γ, p62, and mTORC1 [84]. Therefore, clinical trials will evaluate diets (low-protein versus high-protein) that can inhibit imidazole propionate production (NCT03732690).
1.7. Polyamines
Polyamines have been implicated in the regulation of inflammation pathways and induction of inflammatory bowel diseases, such as colitis [80]. Metabolites such as spermidine are either generated by the microbiota, by the host, or consumed through the diet, and have pleiotropic effects on multiple different tissues [85]. Spermine can act on the NLRP6 inflammasome pathway, which subsequently impacts microbial composition. Indeed, several members of the Lactobacillus genus mediate polyamine synthesis through biosynthetic and transport pathways of spermine [80]. Spermine, which is produced through decarboxylation of amino acids, was observed to reduce colonic IL-18 levels and suppress NLRP6 inflammasome assembly. In patients with inflammatory bowel disease and in mice with chronic DSS-induced colitis, increased levels of the polyamine precursor spermidine were observed [86]. Insights into a possible role for polyamine inhibition as a viable strategy against inflammatory bowel disease in humans await clinical studies [87].
Microbiota-associated polyamines have also been implicated in the regulation of epithelial circadian rhythms [88]. The microbiome undergoes diurnal oscillations, and so do intestinal polyamine levels [[88], [89], [90], [91]]. Alteration of polyamine rhythms is associated with clock dysfunction [92], and thus control of intestinal polyamine levels might present a rational approach to controlling transcriptional oscillations in intestinal epithelial cells.
1.8. Retinoic acid
Retinoic acid, derived from dietary vitamin A, has been implicated largely in the development and regulation of the immune system. Retinoic acid concentration in the gut is in turn maintained by Clostridia commensal bacteria by suppression of retinol dehydrogenase 7 in intestinal epithelial cells [93]. This lipid metabolite has been shown to contribute to the modulation of immune responses through IgA production [27]. Moreover, retinoic acid maintains T-cell immunity through regulatory T (Treg) cell differentiation and T helper 17 cell control [94]. Indeed, deficiencies in retinoic acid or its receptors have been associated with disease states such as colitis-associated colon cancer and IBD [95]. Due to several studies implicating its role in T cell development, all trans-retinoic acid (ATRA) is being explored as a potential therapy in diseases of deregulated immune states such as thrombocytopenia, rheumatoid arthritis, cancer, and auto-immune disorders [[96], [97], [98], [99]]. To date there has been one completed Phase 2 study demonstrating that patients with primary immune thrombocytopenia treated with ATRA showed enhanced response of sustained platelet levels (NCT01667263). ATRA is also currently used to treat leukemia and acne under the name tretinoin, though its mechanism of action is still currently unknown. Further studies are required to explore the use of retinoic acid in ATRA form or 13-cis retinoic form in modulation of inflammation in the gut.
1.9. Bile acids (BAs)
Primary BAs, chenodeoxycholic acid (CDCA) and cholic acid (CA), are synthesized in the liver from cholesterol by hepatocytes and represent more than 75% of the body's total BA content. In the small and large intestine, the bacterial deconjugation, dehydrogenation, 7α-dehydroxylation, and epimerization of the primary BAs produces the “secondary” BAs. CA is converted to dihydroxy deoxycholic acid (DCA) and CDCA to the monohydroxy lithocholic acid (LCA). Colonic bacteria can further metabolize 7a-oxo-LCA to a “tertiary” BA, dihydroxy ursodeoxycholic acid (UDCA) [100]. BAs play a key role in maintaining cholesterol homeostasis through the absorption and emulsification of lipids. Bacteria with bile salt hydrolase activity, such as Lactobacillus and Clostridium, facilitate various aspects of BA metabolism including deconjugation, dehydrogenation, and dihydroxylation [101,102]. Germ-free mice present higher overall BA levels, mostly ursodeoxycholic acid (UDCA), as well as bigger gallbladders due to activation of the transmembrane G protein-coupled receptor 5 (TGR5) pathway [103]. Exposure to cold temperatures in mice triggers the transformation of cholesterol to BAs in the liver, alters gut microbiome composition, stimulates lipoprotein processing in brown adipose tissue, and generates heat [104].
Recent studies have indicated that UDCA improves insulin sensitivity and promotes weight loss in patients with type 2 diabetes (NCT02033876). It also plays a key role in fighting infection. UDCA inhibited the growth of Clostridium difficile in fecal samples from patients with recurring C. difficile infections and was effective in treating a patient with C. difficile-associated pouchitis [105]. A clinical trial will aim to validate this result by administering ursidol/UDCA to patients with recurring C. difficile infections (NCT02748616). Additionally, higher concentrations of deoxycholic acid (DCA) are associated with colonic crypt regeneration after wounding [106]. DCA utilized FXR to hinder prostaglandin E2 synthesis (a substance that inhibits crypt repair), thereby promoting wound repair [106].
Moreover, studies have focused on inhibiting secondary BAs as a form of treatment. Many studies have linked high concentrations of secondary BAs to increased risk for various types of cancer (colorectal, pancreatic, and liver cancer) and recognized their ability to promote DNA mutations and cell death. For example, one paper reported that in a murine model of liver cancer, the administration of secondary BAs (e.g. ω-muricholic acid) reduced natural killer T (NKT) cell accumulation and decreased CXCL16 expression, thereby reversing the antitumor effect of primary BAs [107]. Other studies have reported significantly higher DCA concentrations in colorectal cancer patients, and increased hepatocellular carcinoma (HCC) risk with rising DCA levels [108,109]. However, lowering DCA levels (using either difructose anhydride III or ursodeoxycholic acid) decreased HCC development in obese mice [109].
1.10. Flavonoids
Flavonoids are polyphenolic secondary plant metabolites that are known to possess a vast range of biological abilities, including antioxidant function, protective effects on vascular endothelial cells, and anti-carcinogenic activity. These metabolites are a specific subgroup within the larger category of polyphenols, and the microbiome plays a significant role in flavonoid metabolism [110]. Members of the colonic microbiota are responsible for mediating both the hydrolysis and fermentation of ingested flavonoids [111], and germ-free or antibiotics-treated mice consequently have high levels of flavonoids in their intestines [112]. For example, Bacteroides distasonis and Bacteroides uniformis are two of several microbial species that are known to be involved in the hydrolysis of polyphenols. Therefore, the gut microbiota is key to facilitating these processes, but the polyphenols and flavonoids themselves may also alter gut microbiota composition. For example, a study revealed that the flavonoids rutin and quercetin can inhibit E. coli and Serratia marscens growth [113].
Furthermore, flavonoids seem to have a disease-ameliorating effect in obese mice. A study reported a decrease in hyperlipidemia, hepatic steatosis, and insulin resistance in high-fat diet mice after oral gavage with a flavonoid-rich Paulownia fortunei extract [114]. Clinical trials aim at evaluating the post-biotic effect of polyphenols derived from dietary plant Extra Virgin Olive Oil and/or Red Wine (NCT03101436) in obese and diabetic patients. In addition, the flavonoid-metabolizing activity of the gut microbiome becomes physiologically relevant in the post-obesity situation [115]. In a mouse model of post-dieting weight regain, persistent post-obesity microbiome alterations maintained a paucity of the two flavonoids apigenin and naringenin in the intestine [112]. Post-biotic supplementation of these metabolites increased host energy expenditure and ameliorated relapsing obesity.
Moreover, studies have reported that flavonoids like luteolin are powerful antioxidants that can inhibit mast cells, which are known to be involved in inflammatory responses. A study reported that tetramethoxyluteolin, a luteolin analog, inhibited the release of inflammatory mediators such as beta-hexosaminidase, histamine secretion, and tumor necrosis factor (TNF) from mast cells [116]. Additionally, luteolin was also shown to inhibit the release of IL-6 [106]. Treatment with luteolin and diosmin, another luteolin analog, inhibits the IL-6 induced JAK2/STAT3 pathway in MIA mice and improves social skills [117]. This result has also been replicated in human studies. A study reported decreased levels of IL-6 and TNF, as well as improvements in social skills, in ASD children after luteolin formulation treatment [118]. Indeed, flavonoids seem to have beneficial affects across various types of disorders and diseases of the brain. For example, flavonoids have shown promising results in Alzheimer's disease (AD). Grape seed polyphenolic extract (GSPE), a potent antioxidant, has been reported to inhibit amyloid-β (Aβ) oligomerization in Tg2576 mice and tau peptide aggregations in vitro [119].
Together, these studies indicate that modulating the microbiome to increase intestinal flavonoid levels might be beneficial for a wide range of conditions.
1.11. N-acyl amides
N-acyl amides are bioactive lipids that play multiple roles in mammalian physiology. Commendamide (or N-acyl-3-hydroxypalmitoyl-glycine) is a metabolite from the human gut microbiome that was identified by functional metagenomic screening for NF-kB activators. It resembles long chain N-acyl-amides and activates a G-protein coupled receptor G2A/GPR132, implicated in autoimmunity and atherosclerosis [120]. Genes predicted to encode similar lipids were further identified, synthesized, and inserted into E. coli. These lipids were then tested against G-protein coupled receptors (GPCR). The microbial lipid N-acyl serinol is similar to a human ligand for GPR119, which once activated stimulates glucagon-like peptide-1 (GLP-1) release from the intestinal L-cells and regulated metabolic hormone activity and glucose homeostasis. Similar results were found when N-acyl serinol is fed to mice [121]. No registered clinical trial is yet evaluating its potential translation to human pathologies.
2. Conclusion
The examples discussed here illustrate the functional versatility of metabolites that are produced or modulated by the intestinal microbiome. While the concept of adjusting the levels of these small molecules for therapeutic purposes is intuitive and congruent with decades of experience in the field of pharmacology, we believe that numerous challenges need to be overcome on the path toward using microbial metabolites for therapies.
First, as discussed here, the function of these metabolites is pleiotropic, but highly context-dependent. SCFAs can serve as energetic fuel, modulate gene expression via modification of histone residues, and function as signaling molecules by binding to receptors and triggering a downstream signaling cascade. The precise therapeutic effect is thus highly dependent on cell type, concentration, tissue context, and receptor expression patterns.
Second, depending on the effect of a particular metabolite on the molecular etiology of a disease, different strategies can be envisioned for pharmacological intervention (Table 1). In case the metabolite levels sink below physiological levels, supplementation strategies need to consider the route and frequency of administration, side effects of exceeding physiological concentrations, and inter-individual differences in pharmacokinetics. In case the metabolite contributes to disease pathophysiology, the inhibition of enzymatic steps in the production of the metabolite is promising; however, tools to study pharmacological inhibition of prokaryotic proteins are currently limited.
Table 1.
Gut-derived metabolites, from bench to bedside.
| Metabolite | Major physiological effect | Reference for basic science study | Clinical trial (condition, intervention, stage) | Reference for clinical trial |
|---|---|---|---|---|
| TMAO | Alterations of cholesterol, sterol metabolism and bile acid pool size and composition | [11,14] | Chronic kidney disease, Rifaximin antibiotic, phase 4 | NCT03718988 |
| Platelet hyperreactivity and thrombosis risk | [13,18] | Heart failure, Rifaximin vs S. boulardii, phase 2 | NCT02637167 | |
| Chronic kidney disease, Sevelamer Carbonate, phase 3 | NCT03596749 | |||
| Chronic Kidney Disease, AXOS vs Maltodextrine, phase 2 | NCT02141815 | |||
| SCFAs | Hematopoietic alterations through HDACs, gut-brain axis | [24,30,41,42] | Stress and attention, SCFA oral supplementation, N/A | NCT03688854 |
| Butyrate | Epigenetic modulation of inflammation | Schizophrenia | NCT03010865 | |
| Increased fatty acid oxidation | [124] | Childhood Obesity: Liver damage and insulin resistance, dietary supplement, Phase 2 and 3 | NCT02721953 | |
| Inflammation and oxidative stress | [124,125] | Minor effects on oxidative stress, enemas sodium butyrate, N/A | NCT00696098 | |
| Conjugated linoleic acid (CLA) | Fortification of epithelial barrier integrity | [48,49] | obesity, oral supplementation, N/A | PMID: 11592727 |
| N-3 long chain fatty acid | Attenuation of inflammation | [126] | Ulcerative Colitis, oral supplement, Phase 3 | PMID: 15822041 |
| Indole-3-carbinol | Ahr mediated control of intestinal epithelial cells proliferation and differentiation | [58] | Squamous Cell Head and Neck Cancer, SCB01A, phase 2 | NCT03020823 |
| Prostate cancer, phase 2/3 | NCT00579332 | |||
| Breast Cancer, DIM supplementation, phase 3 | NCT02525159 | |||
| Indole | Ahr mediated mucosal immunity | [59,61] | Healthy, L. reuteri recolonization, N/A | NCT03501082 |
| Indole-3-carbinol | Ahr mediated immunoregulatory effects | [127] | Systemic Lupus Erythematosus, DIM supplementation, phase 1 | NCT02483624 |
| Obesity, Indole 3 carbinol supplementation, phase 2 | NCT00988845 | |||
| Indole-3-propionic acid | Friedreich's Ataxia, VP 20629, phase1 | NCT01898884 | ||
| Indoxyl sulfate | Uremic and vascular toxin | [67] | Chronic kidney disease, AST-120 (Kremezin®), phase 4 | NCT01157260 |
| p-cresol | Uremic toxin altering of endothelial cells function and promoting vascular calcification | [70,71] | Chronic Kidney Disease, BENEO synergy1 (inulin/oligofructose), Phase1/2 | NCT00695513 |
| Chronic Renal Failure, Synbiotic Probinul-Neutro®, phase 4 | NCT02008331 | |||
| Taurine | Modulation of gut bile acid metabolism, anti-inflammatory, oxidative stress | [79,80] | Diabetes, oral supplement, N/A | NCT03410537NCT01226537 |
| Imidazole propionate | Impairment of the insulin signaling | [84] | type 2 diabetes, high vs low protein diets, N/A | NCT03732690 |
| Retinoic Acid | T-Cell development | [93] | Primary immune thrombocytopenia, ATRA supplement, phase 2 | NCT01667263 |
| UDCA | Ameliorate insulin insensitivity | Type 2 diabetes, Ursodiol, phase 2 | NCT02033876 | |
| Inhibition of Clostridium difficile spore germination and vegetative growth | [105] | Diarrhea, Ursodiol, phase 4 | NCT02748616 | |
| DCA | Colonic crypt regeneration | [106] | Esophageal Carcinoma, Ursodiol, phase 2 | NCT01097304 |
| Flavonoid | Antioxidant and anti-inflammatory properties | [116,117] | Autism Spectrum Disorders, Luteolin, Quercetin and Rutin dietary supplement, phase 2 | NCT01847521 |
| Metabolic Syndrome, chlorogenic acid and luteolin, N/A | NCT03444558 | |||
| Alzheimer's diseases or T2DM, polyphenolic extract, phase 1 | NCT02502253 | |||
| N-acyl amides | Ligand of G-protein coupled receptor | [120,121] | Type 2 Diabetes Mellitus, dietary supplementation, phase 1 | NCT01453842 |
Finally, given the dynamic nature of the microbiome and its disease relevance [122,123], optimal concentrations of intestinal metabolites might be highly context-dependent. As such, time-resolved studies are necessary to determine the optimal timing for metabolite interventions of the course of disease development.
These difficulties notwithstanding, targeting the meta-organismal pathways involved in the generation and signaling of microbiome-associated metabolites, presents an enormous and mostly untapped opportunity to modulate disease susceptibility.
2.1. Outstanding Questions
-
-
How can we interfere with disease-associated microbiome-derived metabolites and enhance the effect of beneficial microbial metabolites in a durable and context-dependent manner?
-
-
How can we identify and validate microbial metabolite synthesis pathways using metagenomic sequencing information, and how can the microbial versus dietary or host-derived sources for the metabolites be distinguished?
-
-
Can we identify host sensors for theses metabolites and generate drugs targeting either microbial enzymes for metabolite production or their receptors on host tissues?
-
-
How many clinically relevant metabolites are microbiome derived and how many of them can be therapeutically targeted?
2.2. Search strategy and selection criteria
Data for this Review were identified by searches of PubMed, Google Scholar, and clinical trial database (clinicaltrials.gov). References were used from relevant articles using the search terms “metabolite”, and “gut microbiota”/”microbiome”. Only articles published in English between 1980 and February 2019 were included. Particular emphasis was given on literature published in peer-reviewed journals in the past 5 years, reporting a mechanism of action or clinical data.
Acknowledgments
Acknowledgements
We thank the members of the Thaiss lab for valuable input and apologize to those colleagues whose relevant work could not be cited owing to space constraints. C.A.T. is supported by the Edward Mallinckrodt, Jr. Foundation, the Agilent Early Career Professor Award, the Global Probiotics Council, and grants by the PennCHOP Microbiome Program, the Penn Institute for Immunology, the Penn Center for Molecular Studies in Digestive and Liver Diseases (P30-DK-050306), the Penn Skin Biology and Diseases Resource-based Center (P30-AR-069589), and the Penn Diabetes Research Center (P30-DK-019525).
Conflict of interest
Authors declare that they have no competing interests.
References
- 1.Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. Apr 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]; Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nature reviews Immunology 2017. [DOI] [PubMed]
- 2.Thaiss C.A., Elinav E. The remedy within: will the microbiome fulfill its therapeutic promise? J Mol Med (Berl) 2017;95(10):1021–1027. doi: 10.1007/s00109-017-1563-z. [DOI] [PubMed] [Google Scholar]; Thaiss CA, Elinav E. The remedy within: will the microbiome fulfill its therapeutic promise? J Mol Med (Berl) 2017; 95(10): 1021-7. [DOI] [PubMed]
- 3.Bennett B.J., de Aguiar Vallim T.Q., Wang Z. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell metabolism 2013; 17(1): 49-60. [DOI] [PMC free article] [PubMed]
- 4.Tan Y., Sheng Z., Zhou P. Plasma Trimethylamine N-oxide as a novel biomarker for plaque rupture in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2019;12(1) doi: 10.1161/CIRCINTERVENTIONS.118.007281. [DOI] [PubMed] [Google Scholar]; Tan Y, Sheng Z, Zhou P, et al. Plasma Trimethylamine N-Oxide as a Novel Biomarker for Plaque Rupture in Patients With ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Interv 2019; 12(1): e007281. [DOI] [PubMed]
- 5.Zhuang R., Ge X., Han L. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes Rev. Jun 2019;20(6):883–894. doi: 10.1111/obr.12843. [DOI] [PubMed] [Google Scholar]; Zhuang R, Ge X, Han L, et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes Rev 2019. [DOI] [PubMed]
- 6.Haghikia A., Li X.S., Liman T.G. Gut microbiota-dependent Trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to Proinflammatory monocytes. Arterioscler Thromb Vasc Biol. 2018;38(9):2225–2235. doi: 10.1161/ATVBAHA.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]; Haghikia A, Li XS, Liman TG, et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol 2018; 38(9): 2225-35. [DOI] [PMC free article] [PubMed]
- 7.Li X.S., Wang Z., Cajka T. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight. 2018;3(6) doi: 10.1172/jci.insight.99096. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li XS, Wang Z, Cajka T, et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018; 3(6). [DOI] [PMC free article] [PubMed]
- 8.Missailidis C., Hallqvist J., Qureshi A.R. Serum Trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]; Missailidis C, Hallqvist J, Qureshi AR, et al. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PloS one 2016; 11(1): e0141738. [DOI] [PMC free article] [PubMed]
- 9.Stubbs J.R., Stedman M.R., Liu S. Trimethylamine N-oxide and cardiovascular outcomes in patients with end-stage kidney disease receiving maintenance Hemodialysis. Clin J Am Soc Nephrol. Jan 21 2019 doi: 10.2215/CJN.06190518. (pii: CJN.06190518) [DOI] [PMC free article] [PubMed] [Google Scholar]; Stubbs JR, Stedman MR, Liu S, et al. Trimethylamine N-Oxide and Cardiovascular Outcomes in Patients with End-stage Kidney Disease Receiving Maintenance Hemodialysis. Clin J Am Soc Nephrol 2019. [DOI] [PMC free article] [PubMed]
- 10.Vogt N.M., Romano K.A., Darst B.F. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):124. doi: 10.1186/s13195-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vogt NM, Romano KA, Darst BF, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimers Res Ther 2018; 10(1): 124. [DOI] [PMC free article] [PubMed]
- 11.Wang Z., Klipfell E., Bennett B.J. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472(7341): 57-63. [DOI] [PMC free article] [PubMed]
- 12.Koeth R.A., Wang Z., Levison B.S. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]; Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine 2013; 19(5): 576-85. [DOI] [PMC free article] [PubMed]
- 13.Zhu W., Buffa J.A., Wang Z. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost. 2018;16(9):1857–1872. doi: 10.1111/jth.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhu W, Buffa JA, Wang Z, et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost 2018; 16(9): 1857-72. [DOI] [PMC free article] [PubMed]
- 14.Warrier M., Shih D.M., Burrows A.C. The TMAO-generating enzyme Flavin Monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. Jan 20 2015;10(3):326–338. doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]; Warrier M, Shih DM, Burrows AC, et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell reports 2015. [DOI] [PMC free article] [PubMed]
- 15.Ding L., Chang M., Guo Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018;17(1):286. doi: 10.1186/s12944-018-0939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ding L, Chang M, Guo Y, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis 2018; 17(1): 286. [DOI] [PMC free article] [PubMed]
- 16.Gautam A., Paudel Y.N., Abidin S., Bhandari U. Guggulsterone, a farnesoid X receptor antagonist lowers plasma trimethylamine- N-oxide levels: an evidence from in vitro and in vivo studies. Hum Exp Toxicol. 2018;960327118817862 doi: 10.1177/0960327118817862. [DOI] [PubMed] [Google Scholar]; Gautam A, Paudel YN, Abidin S, Bhandari U. Guggulsterone, a farnesoid X receptor antagonist lowers plasma trimethylamine- N-oxide levels: An evidence from in vitro and in vivo studies. Hum Exp Toxicol 2018: 960327118817862. [DOI] [PubMed]
- 17.Zhu W., Gregory J.C., Org E. Gut microbial metabolite TMAO enhances platelet Hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016; 165(1): 111-24. [DOI] [PMC free article] [PubMed]
- 18.Wang Z., Roberts A.B., Buffa J.A. Non-lethal inhibition of gut microbial Trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang Z, Roberts AB, Buffa JA, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015; 163(7): 1585-95. [DOI] [PMC free article] [PubMed]
- 19.Roberts A.B., Gu X., Buffa J.A. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407–1417. doi: 10.1038/s41591-018-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nature medicine 2018; 24(9): 1407-17. [DOI] [PMC free article] [PubMed]
- 20.Velasquez M.T., Ramezani A., Manal A., Raj D.S. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel) 2016;8(11) doi: 10.3390/toxins8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]; Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins (Basel) 2016; 8(11). [DOI] [PMC free article] [PubMed]
- 21.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary Fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]; Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016; 165(6): 1332-45. [DOI] [PubMed]
- 22.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America 2014; 111(6): 2247-52. [DOI] [PMC free article] [PubMed]
- 23.Schulthess J., Pandey S., Capitani M. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432–45 e7. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schulthess J, Pandey S, Capitani M, et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019; 50(2): 432-45 e7. [DOI] [PMC free article] [PubMed]
- 24.Sun M., Wu W., Chen L. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9(1):3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun 2018; 9(1): 3555. [DOI] [PMC free article] [PubMed]
- 25.Smith P.M., Howitt M.R., Panikov N. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341(6145): 569-73. [DOI] [PMC free article] [PubMed]
- 26.Arpaia N., Campbell C., Fan X. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504(7480): 451-5. [DOI] [PMC free article] [PubMed]
- 27.Wu W., Sun M., Chen F. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10(4):946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu W, Sun M, Chen F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal immunology 2017; 10(4): 946-56. [DOI] [PMC free article] [PubMed]
- 28.Kim M., Friesen L., Park J., Kim H.M., Kim C.H. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur J Immunol. 2018;48(7):1235–1247. doi: 10.1002/eji.201747122. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim M, Friesen L, Park J, Kim HM, Kim CH. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. European journal of immunology 2018; 48(7): 1235-47. [DOI] [PMC free article] [PubMed]
- 29.Kimura I., Ozawa K., Inoue D. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013; 4: 1829. [DOI] [PMC free article] [PubMed]
- 30.Yoo D.Y., Kim W., Nam S.M. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res. 2011;36(10):1850–1857. doi: 10.1007/s11064-011-0503-5. [DOI] [PubMed] [Google Scholar]; Yoo DY, Kim W, Nam SM, et al. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res 2011; 36(10): 1850-7. [DOI] [PubMed]
- 31.Slingerland A.E., Schwabkey Z., Wiesnoski D.H., Jenq R.R. Clinical evidence for the microbiome in inflammatory diseases. Front Immunol. 2017;8:400. doi: 10.3389/fimmu.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]; Slingerland AE, Schwabkey Z, Wiesnoski DH, Jenq RR. Clinical Evidence for the Microbiome in Inflammatory Diseases. Front Immunol 2017; 8: 400. [DOI] [PMC free article] [PubMed]
- 32.Puddu A., Sanguineti R., Montecucco F., Viviani G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm 2014; 2014: 162021. [DOI] [PMC free article] [PubMed]
- 33.Hamer H.M., Jonkers D.M., Bast A. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28(1):88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]; Hamer HM, Jonkers DM, Bast A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 2009; 28(1): 88-93. [DOI] [PubMed]
- 34.van der Beek C.M., Canfora E.E., Lenaerts K. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) 2016;130(22):2073–2082. doi: 10.1042/CS20160263. [DOI] [PubMed] [Google Scholar]; van der Beek CM, Canfora EE, Lenaerts K, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) 2016; 130(22): 2073-82. [DOI] [PubMed]
- 35.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]; Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015; 11(10): 577-91. [DOI] [PubMed]
- 36.Canfora E.E., van der Beek C.M., Jocken J.W.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):2360. doi: 10.1038/s41598-017-02546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Canfora EE, van der Beek CM, Jocken JWE, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep 2017; 7(1): 2360. [DOI] [PMC free article] [PubMed]
- 37.Cuomo A., Maina G., Rosso G. The microbiome: a new target for research and treatment of schizophrenia and its resistant presentations? A systematic literature search and review. Front Pharmacol. 2018;9:1040. doi: 10.3389/fphar.2018.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cuomo A, Maina G, Rosso G, et al. The Microbiome: A New Target for Research and Treatment of Schizophrenia and its Resistant Presentations? A Systematic Literature Search and Review. Front Pharmacol 2018; 9: 1040. [DOI] [PMC free article] [PubMed]
- 38.Erny D., Hrabe de Angelis A.L., Jaitin D. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015; 18(7): 965-77. [DOI] [PMC free article] [PubMed]
- 39.Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016; 165(7): 1762-75. [DOI] [PMC free article] [PubMed]
- 40.Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett 2016; 625: 56-63. [DOI] [PMC free article] [PubMed]
- 41.Govindarajan N., Agis-Balboa R.C., Walter J., Sananbenesi F., Fischer A. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26(1):187–197. doi: 10.3233/JAD-2011-110080. [DOI] [PubMed] [Google Scholar]; Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. Journal of Alzheimer's disease : JAD 2011; 26(1): 187-97. [DOI] [PubMed]
- 42.Lopes-Borges J., Valvassori S.S., Varela R.B. Histone deacetylase inhibitors reverse manic-like behaviors and protect the rat brain from energetic metabolic alterations induced by ouabain. Pharmacol Biochem Behav. 2015;128:89–95. doi: 10.1016/j.pbb.2014.11.014. [DOI] [PubMed] [Google Scholar]; Lopes-Borges J, Valvassori SS, Varela RB, et al. Histone deacetylase inhibitors reverse manic-like behaviors and protect the rat brain from energetic metabolic alterations induced by ouabain. Pharmacol Biochem Behav 2015; 128: 89-95. [DOI] [PubMed]
- 43.Burokas A., Arboleya S., Moloney R.D. Targeting the microbiota-gut-brain Axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]; Burokas A, Arboleya S, Moloney RD, et al. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry 2017; 82(7): 472-87. [DOI] [PubMed]
- 44.Shibata N., Kunisawa J., Kiyono H. Dietary and microbial metabolites in the regulation of host immunity. Front Microbiol. 2017;8:2171. doi: 10.3389/fmicb.2017.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shibata N, Kunisawa J, Kiyono H. Dietary and Microbial Metabolites in the Regulation of Host Immunity. Front Microbiol 2017; 8: 2171. [DOI] [PMC free article] [PubMed]
- 45.Hirata S.I., Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol Int. 2017;66(4):523–528. doi: 10.1016/j.alit.2017.06.008. [DOI] [PubMed] [Google Scholar]; Hirata SI, Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol Int 2017; 66(4): 523-8. [DOI] [PubMed]
- 46.Kishino S., Takeuchi M., Park S.B. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. 2013;110(44):17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kishino S, Takeuchi M, Park SB, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proceedings of the National Academy of Sciences of the United States of America 2013; 110(44): 17808-13. [DOI] [PMC free article] [PubMed]
- 47.Bassaganya-Riera J., Hontecillas R., Horne W.T. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn's disease. Clin Nutr. 2012;31(5):721–727. doi: 10.1016/j.clnu.2012.03.002. [DOI] [PubMed] [Google Scholar]; Bassaganya-Riera J, Hontecillas R, Horne WT, et al. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn's disease. Clin Nutr 2012; 31(5): 721-7. [DOI] [PubMed]
- 48.Kaikiri H., Miyamoto J., Kawakami T. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/nga mice. Int J Food Sci Nutr. 2017;68(8):941–951. doi: 10.1080/09637486.2017.1318116. [DOI] [PubMed] [Google Scholar]; Kaikiri H, Miyamoto J, Kawakami T, et al. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/nga mice. Int J Food Sci Nutr 2017; 68(8): 941-51. [DOI] [PubMed]
- 49.Miyamoto J., Mizukure T., Park S.B. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015;290(5):2902–2918. doi: 10.1074/jbc.M114.610733. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miyamoto J, Mizukure T, Park SB, et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. The Journal of biological chemistry 2015; 290(5): 2902-18. [DOI] [PMC free article] [PubMed]
- 50.Gaullier J.M., Halse J., Hoivik H.O. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr. 2007;97(3):550–560. doi: 10.1017/S0007114507381324. [DOI] [PubMed] [Google Scholar]; Gaullier JM, Halse J, Hoivik HO, et al. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr 2007; 97(3): 550-60. [DOI] [PubMed]
- 51.Smedman A., Vessby B. Conjugated linoleic acid supplementation in humans--metabolic effects. Lipids. 2001;36(8):773–781. doi: 10.1007/s11745-001-0784-7. [DOI] [PubMed] [Google Scholar]; Smedman A, Vessby B. Conjugated linoleic acid supplementation in humans--metabolic effects. Lipids 2001; 36(8): 773-81. [DOI] [PubMed]
- 52.Sluijs I., Plantinga Y., de Roos B., Mennen L.I., Bots M.L. Dietary supplementation with cis-9,trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr. 2010;91(1):175–183. doi: 10.3945/ajcn.2009.28192. [DOI] [PubMed] [Google Scholar]; Sluijs I, Plantinga Y, de Roos B, Mennen LI, Bots ML. Dietary supplementation with cis-9,trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr 2010; 91(1): 175-83. [DOI] [PubMed]
- 53.Seidner D.L., Lashner B.A., Brzezinski A. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: a randomized, controlled trial. Clin Gastroenterol Hepatol. 2005;3(4):358–369. doi: 10.1016/s1542-3565(04)00672-x. [DOI] [PubMed] [Google Scholar]; Seidner DL, Lashner BA, Brzezinski A, et al. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: a randomized, controlled trial. Clin Gastroenterol Hepatol 2005; 3(4): 358-69. [DOI] [PubMed]
- 54.Hsiao E.Y., McBride S.W., Hsien S. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155(7): 1451-63. [DOI] [PMC free article] [PubMed]
- 55.Sridharan G.V., Choi K., Klemashevich C. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun. 2014;5:5492. doi: 10.1038/ncomms6492. [DOI] [PubMed] [Google Scholar]; Sridharan GV, Choi K, Klemashevich C, et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun 2014; 5: 5492. [DOI] [PubMed]
- 56.Devlin A.S., Marcobal A., Dodd D. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe. 2016;20(6):709–715. doi: 10.1016/j.chom.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Devlin AS, Marcobal A, Dodd D, et al. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell host & microbe 2016; 20(6): 709-15. [DOI] [PMC free article] [PubMed]
- 57.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19(3):184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]; Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nature reviews Immunology 2019; 19(3): 184-97. [DOI] [PubMed]
- 58.Metidji A., Omenetti S., Crotta S. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2018;49(2):353–62 e5. doi: 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Metidji A, Omenetti S, Crotta S, et al. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 2018; 49(2): 353-62 e5. [DOI] [PMC free article] [PubMed]
- 59.Cervantes-Barragan L., Chai J.N., Tianero M.D. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science. 2017;357(6353):806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cervantes-Barragan L, Chai JN, Tianero MD, et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science 2017; 357(6353): 806-10. [DOI] [PMC free article] [PubMed]
- 60.Zelante T., Iannitti R.G., Cunha C. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]; Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013; 39(2): 372-85. [DOI] [PubMed]
- 61.Lamas B., Richard M.L., Leducq V. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nature medicine 2016; 22(6): 598-605. [DOI] [PMC free article] [PubMed]
- 62.Beaumont M., Neyrinck A.M., Olivares M. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018;32:6681–6693. doi: 10.1096/fj.201800544. (fj201800544) [DOI] [PMC free article] [PubMed] [Google Scholar]; Beaumont M, Neyrinck AM, Olivares M, et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J 2018: fj201800544. [DOI] [PMC free article] [PubMed]
- 63.Rothhammer V., Mascanfroni I.D., Bunse L. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rothhammer V, Mascanfroni ID, Bunse L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nature medicine 2016; 22(6): 586-97. [DOI] [PMC free article] [PubMed]
- 64.Rothhammer V., Borucki D.M., Tjon E.C. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rothhammer V, Borucki DM, Tjon EC, et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018; 557(7707): 724-8. [DOI] [PMC free article] [PubMed]
- 65.Apetoh L., Quintana F.J., Pot C. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]; Apetoh L, Quintana FJ, Pot C, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature immunology 2010; 11(9): 854-61. [DOI] [PMC free article] [PubMed]
- 66.Duranton F., Cohen G., De Smet R. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23(7):1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]; Duranton F, Cohen G, De Smet R, et al. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 2012; 23(7): 1258-70. [DOI] [PMC free article] [PubMed]
- 67.Nakano T., Katsuki S., Chen M. Uremic toxin Indoxyl Sulfate promotes Proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-notch Signaling. Circulation. 2019;139(1):78–96. doi: 10.1161/CIRCULATIONAHA.118.034588. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nakano T, Katsuki S, Chen M, et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019; 139(1): 78-96. [DOI] [PMC free article] [PubMed]
- 68.Triplett T.A., Garrison K.C., Marshall N. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36(8):758–764. doi: 10.1038/nbt.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]; Triplett TA, Garrison KC, Marshall N, et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol 2018; 36(8): 758-64. [DOI] [PMC free article] [PubMed]
- 69.Hamer H.M., De Preter V., Windey K., Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G1–G9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? American journal of physiology Gastrointestinal and liver physiology 2012; 302(1): G1-9. [DOI] [PMC free article] [PubMed]
- 70.Meijers B.K., Van Kerckhoven S., Verbeke K. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis. 2009;54(5):891–901. doi: 10.1053/j.ajkd.2009.04.022. [DOI] [PubMed] [Google Scholar]; Meijers BK, Van Kerckhoven S, Verbeke K, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 2009; 54(5): 891-901. [DOI] [PubMed]
- 71.Opdebeeck B., Maudsley S., Azmi A. Indoxyl Sulfate and p-Cresyl Sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol. 2019;30(5):751–766. doi: 10.1681/ASN.2018060609. [DOI] [PMC free article] [PubMed] [Google Scholar]; Opdebeeck B, Maudsley S, Azmi A, et al. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J Am Soc Nephrol 2019; 30(5): 751-66. [DOI] [PMC free article] [PubMed]
- 72.Bammens B., Evenepoel P., Keuleers H., Verbeke K., Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69(6):1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]; Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 2006; 69(6): 1081-7. [DOI] [PubMed]
- 73.Patel M., Fowler D., Sizer J., Walton C. Faecal volatile biomarkers of Clostridium difficile infection. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0215256. [DOI] [PMC free article] [PubMed] [Google Scholar]; Patel M, Fowler D, Sizer J, Walton C. Faecal volatile biomarkers of Clostridium difficile infection. PloS one 2019; 14(4): e0215256. [DOI] [PMC free article] [PubMed]
- 74.Wan Y., Wang F., Yuan J. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut. 2019:1–31. doi: 10.1136/gutjnl-2018-317609. [DOI] [PubMed] [Google Scholar]; Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019. [DOI] [PubMed]
- 75.De Preter V., Falony G., Windey K., Hamer H.M., De Vuyst L., Verbeke K. The prebiotic, oligofructose-enriched inulin modulates the faecal metabolite profile: an in vitro analysis. Mol Nutr Food Res. 2010;54(12):1791–1801. doi: 10.1002/mnfr.201000136. [DOI] [PubMed] [Google Scholar]; De Preter V, Falony G, Windey K, Hamer HM, De Vuyst L, Verbeke K. The prebiotic, oligofructose-enriched inulin modulates the faecal metabolite profile: an in vitro analysis. Mol Nutr Food Res 2010; 54(12): 1791-801. [DOI] [PubMed]
- 76.Lecerf J.M., Depeint F., Clerc E. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr. 2012;108(10):1847–1858. doi: 10.1017/S0007114511007252. [DOI] [PubMed] [Google Scholar]; Lecerf JM, Depeint F, Clerc E, et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 2012; 108(10): 1847-58. [DOI] [PubMed]
- 77.Cloetens L., Broekaert W.F., Delaedt Y. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: a randomised, placebo-controlled cross-over study. Br J Nutr. 2010;103(5):703–713. doi: 10.1017/S0007114509992248. [DOI] [PubMed] [Google Scholar]; Cloetens L, Broekaert WF, Delaedt Y, et al. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: a randomised, placebo-controlled cross-over study. Br J Nutr 2010; 103(5): 703-13. [DOI] [PubMed]
- 78.Wahlstrom A., Sayin S.I., Marschall H.U., Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]; Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell metabolism 2016; 24(1): 41-50. [DOI] [PubMed]
- 79.Yao L., Seaton S.C., Ndousse-Fetter S. A selective gut bacterial bile salt hydrolase alters host metabolism. Elife. 2018;7 doi: 10.7554/eLife.37182. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yao L, Seaton SC, Ndousse-Fetter S, et al. A selective gut bacterial bile salt hydrolase alters host metabolism. Elife 2018; 7. [DOI] [PMC free article] [PubMed]
- 80.Levy M., Thaiss C.A., Zeevi D. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163(6):1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; Levy M, Thaiss CA, Zeevi D, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015; 163(6): 1428-43. [DOI] [PMC free article] [PubMed]
- 81.Yazici C., Wolf P.G., Kim H. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yazici C, Wolf PG, Kim H, et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut 2017; 66(11): 1983-94. [DOI] [PMC free article] [PubMed]
- 82.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the Ketogenic diet. Cell. 2018;174(2):497. doi: 10.1016/j.cell.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]; Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018; 174(2): 497. [DOI] [PMC free article] [PubMed]
- 83.Steed A.L., Christophi G.P., Kaiko G.E. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357(6350):498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]; Steed AL, Christophi GP, Kaiko GE, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017; 357(6350): 498-502. [DOI] [PMC free article] [PubMed]
- 84.Koh A., Molinaro A., Stahlman M. Microbially produced imidazole propionate impairs insulin Signaling through mTORC1. Cell. 2018;175(4):947–61 e17. doi: 10.1016/j.cell.2018.09.055. [DOI] [PubMed] [Google Scholar]; Koh A, Molinaro A, Stahlman M, et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018; 175(4): 947-61 e17. [DOI] [PubMed]
- 85.Madeo F., Eisenberg T., Pietrocola F., Kroemer G. Spermidine in health and disease. Science. 2018;359(6374) doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]; Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science 2018; 359(6374). [DOI] [PubMed]
- 86.Weiss T.S., Herfarth H., Obermeier F. Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(5):529–535. doi: 10.1097/00054725-200409000-00006. [DOI] [PubMed] [Google Scholar]; Weiss TS, Herfarth H, Obermeier F, et al. Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm Bowel Dis 2004; 10(5): 529-35. [DOI] [PubMed]
- 87.Levy M., Shapiro H., Thaiss C.A., Elinav E. NLRP6: a multifaceted innate immune sensor. Trends Immunol. 2017;38(4):248–260. doi: 10.1016/j.it.2017.01.001. [DOI] [PubMed] [Google Scholar]; Levy M, Shapiro H, Thaiss CA, Elinav E. NLRP6: A Multifaceted Innate Immune Sensor. Trends Immunol 2017; 38(4): 248-60. [DOI] [PubMed]
- 88.Thaiss C.A., Levy M., Korem T. Microbiota diurnal rhythmicity programs host Transcriptome oscillations. Cell. 2016;167(6):1495–510 e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]; Thaiss CA, Levy M, Korem T, et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016; 167(6): 1495-510 e12. [DOI] [PubMed]
- 89.Thaiss C.A., Zeevi D., Levy M., Segal E., Elinav E. A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes. 2015;6(2):137–142. doi: 10.1080/19490976.2015.1016690. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thaiss CA, Zeevi D, Levy M, Segal E, Elinav E. A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes 2015; 6(2): 137-42. [DOI] [PMC free article] [PubMed]
- 90.Thaiss C.A., Zeevi D., Levy M. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]; Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014; 159(3): 514-29. [DOI] [PubMed]
- 91.Thaiss C.A., Levy M., Elinav E. Chronobiomics: the biological clock as a new principle in host-microbial interactions. PLoS Pathog. 2015;11(10) doi: 10.1371/journal.ppat.1005113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thaiss CA, Levy M, Elinav E. Chronobiomics: The Biological Clock as a New Principle in Host-Microbial Interactions. PLoS pathogens 2015; 11(10): e1005113. [DOI] [PMC free article] [PubMed]
- 92.Zwighaft Z., Aviram R., Shalev M. Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab. 2015;22(5):874–885. doi: 10.1016/j.cmet.2015.09.011. [DOI] [PubMed] [Google Scholar]; Zwighaft Z, Aviram R, Shalev M, et al. Circadian Clock Control by Polyamine Levels through a Mechanism that Declines with Age. Cell metabolism 2015; 22(5): 874-85. [DOI] [PubMed]
- 93.Grizotte-Lake M., Zhong G., Duncan K. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity. 2018;49(6):1103–15 e6. doi: 10.1016/j.immuni.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grizotte-Lake M, Zhong G, Duncan K, et al. Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity 2018; 49(6): 1103-15 e6. [DOI] [PMC free article] [PubMed]
- 94.Clemente J.C., Manasson J., Scher J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360 doi: 10.1136/bmj.j5145. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018; 360: j5145. [DOI] [PMC free article] [PubMed]
- 95.Wada Y., Hisamatsu T., Kamada N., Okamoto S., Hibi T. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflamm Bowel Dis. 2009;15(10):1548–1556. doi: 10.1002/ibd.20934. [DOI] [PubMed] [Google Scholar]; Wada Y, Hisamatsu T, Kamada N, Okamoto S, Hibi T. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflamm Bowel Dis 2009; 15(10): 1548-56. [DOI] [PubMed]
- 96.Feng F.E., Feng R., Wang M. Oral all-trans retinoic acid plus danazol versus danazol as second-line treatment in adults with primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2017;4(10) doi: 10.1016/S2352-3026(17)30170-9. (e487-e96) [DOI] [PubMed] [Google Scholar]; Feng FE, Feng R, Wang M, et al. Oral all-trans retinoic acid plus danazol versus danazol as second-line treatment in adults with primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol 2017; 4(10): e487-e96. [DOI] [PubMed]
- 97.Nozaki Y., Tamaki C., Yamagata T. All-trans-retinoic acid suppresses interferon-gamma and tumor necrosis factor-alpha; a possible therapeutic agent for rheumatoid arthritis. Rheumatol Int. 2006;26(9):810–817. doi: 10.1007/s00296-005-0076-1. [DOI] [PubMed] [Google Scholar]; Nozaki Y, Tamaki C, Yamagata T, et al. All-trans-retinoic acid suppresses interferon-gamma and tumor necrosis factor-alpha; a possible therapeutic agent for rheumatoid arthritis. Rheumatol Int 2006; 26(9): 810-7. [DOI] [PubMed]
- 98.Smith M.A., Adamson P.C., Balis F.M. Phase I and pharmacokinetic evaluation of all-trans-retinoic acid in pediatric patients with cancer. J Clin Oncol. 1992;10(11):1666–1673. doi: 10.1200/JCO.1992.10.11.1666. [DOI] [PubMed] [Google Scholar]; Smith MA, Adamson PC, Balis FM, et al. Phase I and pharmacokinetic evaluation of all-trans-retinoic acid in pediatric patients with cancer. J Clin Oncol 1992; 10(11): 1666-73. [DOI] [PubMed]
- 99.Abdelhamid L., Luo X.M. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients. 2018;10(8) doi: 10.3390/nu10081016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abdelhamid L, Luo XM. Retinoic Acid, Leaky Gut, and Autoimmune Diseases. Nutrients 2018; 10(8). [DOI] [PMC free article] [PubMed]
- 100.Urdaneta V., Casadesus J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front Med (Lausanne) 2017;4:163. doi: 10.3389/fmed.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]; Urdaneta V, Casadesus J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front Med (Lausanne) 2017; 4: 163. [DOI] [PMC free article] [PubMed]
- 101.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]; Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006; 47(2): 241-59. [DOI] [PubMed]
- 102.Sayin S.I., Wahlstrom A., Felin J. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]; Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell metabolism 2013; 17(2): 225-35. [DOI] [PubMed]
- 103.Selwyn F.P., Csanaky I.L., Zhang Y., Klaassen C.D. Importance of large intestine in regulating bile acids and glucagon-like Peptide-1 in germ-free mice. Drug Metab Dispos. 2015;43(10):1544–1556. doi: 10.1124/dmd.115.065276. [DOI] [PMC free article] [PubMed] [Google Scholar]; Selwyn FP, Csanaky IL, Zhang Y, Klaassen CD. Importance of Large Intestine in Regulating Bile Acids and Glucagon-Like Peptide-1 in Germ-Free Mice. Drug Metab Dispos 2015; 43(10): 1544-56. [DOI] [PMC free article] [PubMed]
- 104.Worthmann A., John C., Ruhlemann M.C. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23(7):839–849. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]; Worthmann A, John C, Ruhlemann MC, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nature medicine 2017; 23(7): 839-49. [DOI] [PubMed]
- 105.Weingarden A.R., Chen C., Zhang N. Ursodeoxycholic acid inhibits Clostridium difficile spore germination and vegetative growth, and prevents the recurrence of Ileal Pouchitis associated with the infection. J Clin Gastroenterol. 2016;50(8):624–630. doi: 10.1097/MCG.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]; Weingarden AR, Chen C, Zhang N, et al. Ursodeoxycholic Acid Inhibits Clostridium difficile Spore Germination and Vegetative Growth, and Prevents the Recurrence of Ileal Pouchitis Associated With the Infection. Journal of clinical gastroenterology 2016; 50(8): 624-30. [DOI] [PMC free article] [PubMed]
- 106.Jain U., Lai C.W., Xiong S. Temporal regulation of the bacterial metabolite Deoxycholate during colonic repair is critical for crypt regeneration. Cell Host Microbe. 2018;24(3):353–63 e5. doi: 10.1016/j.chom.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jain U, Lai CW, Xiong S, et al. Temporal Regulation of the Bacterial Metabolite Deoxycholate during Colonic Repair Is Critical for Crypt Regeneration. Cell host & microbe 2018; 24(3): 353-63 e5. [DOI] [PMC free article] [PubMed]
- 107.Ma C., Han M., Heinrich B. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391) doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018; 360(6391). [DOI] [PMC free article] [PubMed]
- 108.Bayerdorffer E., Mannes G.A., Ochsenkuhn T., Dirschedl P., Wiebecke B., Paumgartner G. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut. 1995;36(2):268–273. doi: 10.1136/gut.36.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bayerdorffer E, Mannes GA, Ochsenkuhn T, Dirschedl P, Wiebecke B, Paumgartner G. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut 1995; 36(2): 268-73. [DOI] [PMC free article] [PubMed]
- 109.Yoshimoto S., Loo T.M., Atarashi K. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]; Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013; 499(7456): 97-101. [DOI] [PubMed]
- 110.Braune A., Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7(3):216–234. doi: 10.1080/19490976.2016.1158395. [DOI] [PMC free article] [PubMed] [Google Scholar]; Braune A, Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016; 7(3): 216-34. [DOI] [PMC free article] [PubMed]
- 111.Cassidy A., Minihane A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. 2017;105(1):10–22. doi: 10.3945/ajcn.116.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr 2017; 105(1): 10-22. [DOI] [PMC free article] [PubMed]
- 112.Thaiss C.A., Itav S., Rothschild D. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540(7634):544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]; Thaiss CA, Itav S, Rothschild D, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016. [DOI] [PubMed]
- 113.Rodriguez Vaquero M.J., Manca de Nadra M.C. Growth parameter and viability modifications of Escherichia coli by phenolic compounds and argentine wine extracts. Appl Biochem Biotechnol. 2008;151(2–3):342–352. doi: 10.1007/s12010-008-8197-0. [DOI] [PubMed] [Google Scholar]; Rodriguez Vaquero MJ, Manca de Nadra MC. Growth parameter and viability modifications of Escherichia coli by phenolic compounds and Argentine wine extracts. Appl Biochem Biotechnol 2008; 151(2-3): 342-52. [DOI] [PubMed]
- 114.Liu C., Ma J., Sun J. Flavonoid-rich extract of Paulownia fortunei flowers attenuates diet-induced Hyperlipidemia, hepatic Steatosis and insulin resistance in obesity mice by AMPK pathway. Nutrients. 2017;9(9) doi: 10.3390/nu9090959. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu C, Ma J, Sun J, et al. Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients 2017; 9(9). [DOI] [PMC free article] [PubMed]
- 115.Thaiss C.A., Shapiro H., Elinav E. Post-dieting weight gain: the role of persistent microbiome changes. Future Microbiol. 2017;12:555–559. doi: 10.2217/fmb-2017-0045. [DOI] [PubMed] [Google Scholar]; Thaiss CA, Shapiro H, Elinav E. Post-dieting weight gain: the role of persistent microbiome changes. Future Microbiol 2017; 12: 555-9. [DOI] [PubMed]
- 116.Weng Z., Patel A.B., Panagiotidou S., Theoharides T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. 2015;135(4):1044–52 e5. doi: 10.1016/j.jaci.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; Weng Z, Patel AB, Panagiotidou S, Theoharides TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. The Journal of allergy and clinical immunology 2015; 135(4): 1044-52 e5. [DOI] [PMC free article] [PubMed]
- 117.Parker-Athill E., Luo D., Bailey A. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol. 2009;217(1–2):20–27. doi: 10.1016/j.jneuroim.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Parker-Athill E, Luo D, Bailey A, et al. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. Journal of neuroimmunology 2009; 217(1-2): 20-7. [DOI] [PMC free article] [PubMed]
- 118.Tsilioni I., Taliou A., Francis K., Theoharides T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry. 2015;5:e647. doi: 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry 2015; 5: e647. [DOI] [PMC free article] [PubMed]
- 119.Wang J., Ho L., Zhao W. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang J, Ho L, Zhao W, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 2008; 28(25): 6388-92. [DOI] [PMC free article] [PubMed]
- 120.Cohen L.J., Kang H.S., Chu J. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc Natl Acad Sci U S A. 2015;112(35):E4825–E4834. doi: 10.1073/pnas.1508737112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cohen LJ, Kang HS, Chu J, et al. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proceedings of the National Academy of Sciences of the United States of America 2015; 112(35): E4825-34. [DOI] [PMC free article] [PubMed]
- 121.Cohen L.J., Esterhazy D., Kim S.H. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549(7670):48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cohen LJ, Esterhazy D, Kim SH, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017; 549(7670): 48-53. [DOI] [PMC free article] [PubMed]
- 122.Thaiss C.A. Microbiome dynamics in obesity. Science. 2018;362(6417):903–904. doi: 10.1126/science.aav6870. [DOI] [PubMed] [Google Scholar]; Thaiss CA. Microbiome dynamics in obesity. Science 2018; 362(6417): 903-4. [DOI] [PubMed]
- 123.Uhr G.T., Dohnalova L., Thaiss C.A. The dimension of time in host-microbiome interactions. mSystems. 2019;4(1) doi: 10.1128/mSystems.00216-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uhr GT, Dohnalova L, Thaiss CA. The Dimension of Time in Host-Microbiome Interactions. mSystems 2019; 4(1). [DOI] [PMC free article] [PubMed]
- 124.Hamer H.M., Jonkers D.M., Vanhoutvin S.A. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr. 2010;29(6):738–744. doi: 10.1016/j.clnu.2010.04.002. [DOI] [PubMed] [Google Scholar]; Hamer HM, Jonkers DM, Vanhoutvin SA, et al. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr 2010; 29(6): 738-44. [DOI] [PubMed]
- 125.Chen G., Ran X., Li B. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen G, Ran X, Li B, et al. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018; 30: 317-25. [DOI] [PMC free article] [PubMed]
- 126.Grisham M.B., Demichele S.J., Garleb K.A., Specian R.D. Sulfasalazine or enteral diets containing fish oil or oligosaccharides attenuate chronic colitis in rats. Inflamm Bowel Dis. 1996;2(3):178–188. [PubMed] [Google Scholar]; Grisham MB, Demichele SJ, Garleb KA, Specian RD. Sulfasalazine or enteral diets containing fish oil or oligosaccharides attenuate chronic colitis in rats. Inflamm Bowel Dis 1996; 2(3): 178-88. [PubMed]
- 127.Mohammadi S., Memarian A., Sedighi S., Behnampour N., Yazdani Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: a crucial role for aryl hydrocarbon receptor. Autoimmunity. 2018;51(5):199–209. doi: 10.1080/08916934.2018.1494161. [DOI] [PubMed] [Google Scholar]; Mohammadi S, Memarian A, Sedighi S, Behnampour N, Yazdani Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity 2018; 51(5): 199-209. [DOI] [PubMed]