Abstract
Background
Several publications have described differences in cross-sectional comparisons of gut microbiota between patients with Parkinson's disease and control subjects, with considerable variability of the reported differentially abundant taxa. The temporal stability of such microbiota alterations and their relationship to disease progression have not been previously studied with a high-throughput sequencing based approach.
Methods
We collected clinical data and stool samples from 64 Parkinson's patients and 64 control subjects twice, on average 2·25 years apart. Disease progression was evaluated based on changes in Unified Parkinson's Disease Rating Scale and Levodopa Equivalent Dose, and microbiota were characterized with 16S rRNA gene amplicon sequencing.
Findings
We compared patients to controls, and patients with stable disease to those with faster progression. There were significant differences between microbial communities of patients and controls when corrected for confounders, but not between timepoints. Specific bacterial taxa that differed between patients and controls at both timepoints included several previously reported ones, such as Roseburia, Prevotella and Bifidobacterium. In progression comparisons, differentially abundant taxa were inconsistent across methods and timepoints, but there was some support for a different distribution of enterotypes and a decreased abundance of Prevotella in faster-progressing patients.
Interpretation
The previously detected gut microbiota differences between Parkinson's patients and controls persisted after 2 years. While we found some evidence for a connection between microbiota and disease progression, a longer follow-up period is required to confirm these findings.
Keywords: Parkinson's disease, Gut microbiota, Gut-brain-axis, Disease progression
Research in context.
Evidence before this study
The cause of idiopathic Parkinson's disease has been hypothesized to involve an external agent, for example a pathogen, and one potential route of entry for such an agent could be via the gastrointestinal system. The interactions of gut microbes and the central nervous system, also known as the microbiota–gut–brain axis, have recently become a topic of intense research. Within the past 5 years, a total of twelve studies from three continents have reported differences in the composition of gut microbiota of patients with Parkinson's disease when compared to non-parkinsonian control subjects, showing promise for this novel field of research.
Added value of this study
Our study is the first to use a high-throughput sequencing based approach to explore gut microbiota of Parkinson's patients at two different timepoints. We show that the differences detected at baseline can be replicated at a follow-up timepoint 2 years later, and that there might be changes in gut microbiota composition in patients with faster disease progression.
Implications of all the available evidence
The consistent differences in gut microbiota between Parkinson's patients and control subjects could lead to new diagnostic or therapeutic modalities.
Alt-text: Unlabelled Box
1. Introduction
The early non-motor symptoms of Parkinson's disease (PD), such as hyposmia and gastrointestinal (GI) disorders [1,2], have led to the hypothesis that the disease could originate outside of the central nervous system, for example in the olfactory bulb or the enteric nervous system [3]. Research comparing nasal microbiota of PD patients and control subjects has not revealed notable differences [4,5]. In contrast, several studies have suggested that patients' gut microbiota differ from controls' [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]], although the differentially abundant taxa reported in them vary considerably. This could be due to differences in subject populations or methodology, such as PCR primers, sequencing platforms, and statistical tools. Nevertheless, some microbial community alterations, including a decreased abundance of the family Prevotellaceae, the genus Prevotella, and the species Prevotella copri [6,9,12,13], and an increase in Akkermansia and Verrucomicrobiaceae [[5], [6], [7],9,11,12,15], Bifidobacterium and Bifidobacteriaceae [9,11,13], as well as Lactobacillus and Lactobacillaceae [6,11,13], have been detected multiple times.
Aside from a recent disease progression study using a qRT-PCR-based assay [17], all PD gut microbiota publications have been case-control studies with one timepoint. They have used varying approaches to control for the effects of potential confounders, such as diet and medications. Diet influences the gut microbiome [18]. Since it has been hypothesized to be an important determinant of the abundance of Prevotellaceae [18], it could be associated to the decrease of that family seen in PD [6,7]. Additionally, PD medications can affect gut microbiota [6,11]. They are a particularly important confounder when studying disease progression, since progression is measured based on symptoms, which respond to medications.
In the present study, we explore the gut microbiota of a previously recruited group of PD patients and control subjects at baseline and 2 years later, while also considering their diet, medications, and other clinical variables.
2. Materials and methods
2.1. Study subjects and clinical data
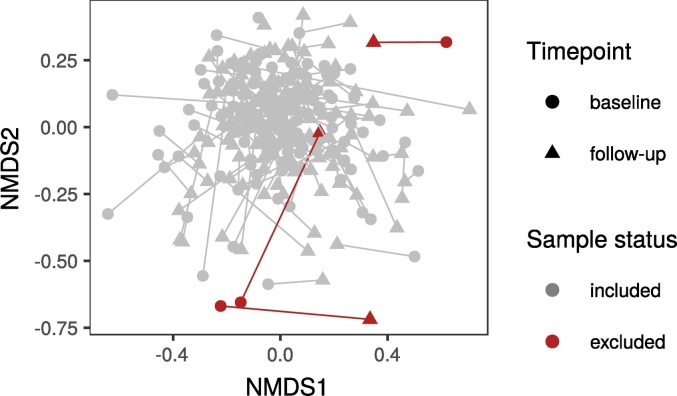
152 age and sex matched subjects (76 PD patients, 76 control subjects) originally recruited for a pilot study in Parkinson's disease and gut microbiota [6] were invited to a follow-up appointment on average 2·25 (SD: ± 0·20) years later. Out of the original subjects who returned, nine were excluded, while five subjects whose samples were not used in the pilot study for various reasons were included at follow-up, bringing the total number of subjects to 128 (64 PD patients, 64 control subjects; Table 1, Fig. 1). The study was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa. All participants gave informed consent.
Table 1.
Changes in subject inclusion/exclusion after pilot study.
| n (recruited for pilot study) | 152 |
| Excluded from pilot study, not included in current study: | |
| Excluded from pilot due to insufficient read count, drop-out from follow-up | 2 |
| Excluded from pilot due to lack of matching subject | 1 |
| Excluded from pilot study, included in current study: | |
| Not in pilot study due to insufficient read count | 2 |
| Not in pilot study because sample received too late | 2 |
| Not in pilot study: nasal polyps; deemed eligible for follow-up study | 1 |
| n (pilot study) | 144 |
| Excluded after pilot study: | |
| Exitus after pilot study | 1 |
| Drop-outs | 11 |
| Clinical exclusion: diagnosis changed to Lewy body dementia | 1 |
| Clinical exclusion: recent surgery | 3 |
| Technical exclusion: insufficient reads from baseline sample | 1 |
| Technical exclusion: missing sample | 1 |
| Outlier exclusion: microbial community outlier | 3 |
| n (follow-up study) | 128 |
Fig. 1.
NMDS ordination of samples excluded as microbiome outliers.
The subjects filled several questionnaires concerning non-motor symptoms, including the Wexner constipation score [19], Rome III IBS questionnaire [20], Non-Motor Symptoms Scale (NMSS) [21], Swallowing Disturbance Questionnaire (SDQ) [22], Sialorrhea Clinical Scale for PD (SCS-PD) [23], 15-item Geriatric Depression Scale (GDS-15) [24], REM sleep behavior disorder screening questionnaire (RBDSQ) [25] and Sniffin’ Sticks 16-item smell identification score [26]. The subjects' dietary habits were evaluated at follow-up with a 163 item Food Frequency Questionnaire (FFQ) with 9 frequency response options (based on [27]). The severity of the patients' parkinsonian symptoms was assessed with the Unified Parkinson's Disease Rating Scale (UPDRS) [28], and their total medication load was calculated using the Levodopa Equivalent Dose (LED) [29]. We also determined tremor and postural instability and gait difficulty (PIGD) symptom scores and derived motor phenotypes (postural instability and gait difficulty (PIGD), tremor dominant (TD), or mixed (MX)) [30]. At follow-up, we also collected stool consistency information using the Victoria Bowel Performance Scale (BPS) [31]; the patients kept a diary of their scores for one week leading up to sampling, and we used the weekly average stool consistency values for comparisons.
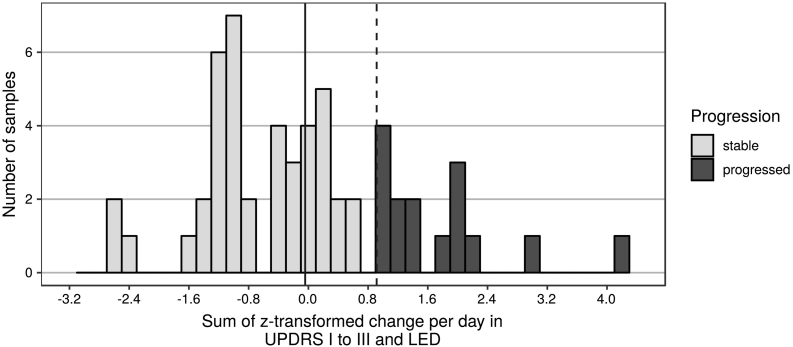
For comparisons of microbiota and disease progression, we excluded patients who were on Deep Brain Stimulation at either timepoint, those with missing UPDRS or LED values, and one patient whose LED score had decreased considerably between the two timepoints due to adjustment of overmedication symptoms. This left a subset of 56 PD patients for progression analyses. To classify patients into stable or progressed, we calculated changes in UPDRS I-III score (in the ON state) and LED between baseline and follow-up, divided each value by the number of days between appointments, z-transformed these two variables, and added them up. Based on the distribution of samples on this progression scale, we chose the 3rd quartile as a cut-off, resulting in 41 stable and 15 progressed patients (Fig. 2). We also used this subset of patients for additional analyses contrasting PD phenotypes, additionally excluding patients with a mixed (MX) phenotype, which resulted in a data set with 21 TD patients and 28 PIGD patients at baseline, and 20 TD patients and 35 PIGD patients at follow-up.
Fig. 2.
Histogram of the progression variable based on change in UPDRS I-III and LED.
Legend: Solid vertical line represents the median, dashed vertical line represents the 3rd quartile, which was used to categorize subjects into “stable” and “progressed”.
2.2. Sequencing and sequence analysis
Stool samples for both timepoints were collected at home by the study subjects into collection tubes with pre-filled DNA stabilizer (PSP Spin Stool DNA Plus Kit, STRATEC Molecular), and stored in the refrigerator until transport (for up to 3 days). Once received at the clinic, they were transferred to −80 °C. To minimize potential technical differences between baseline and follow-up samples, we reanalysed the baseline samples together with the follow-up samples, including new DNA extractions, PCR, and sequencing. Thus, the baseline samples, which had been frozen after collection, then thawed for sequencing at the time of the pilot study, re-frozen, and stored at −80 °C since, were thawed for a second time for this follow-up study.
We extracted bulk DNA from stool samples with the PSP Spin Stool DNA Plus Kit (STRATEC Molecular). Each extraction batch included one blank sample to assess potential contamination. The V3-V4 regions of the 16S rRNA gene were amplified following a previously published protocol [4], with the following changes: we used two technical replicates (25 μL reactions) per patient sample, and a mixture of the universal bacterial primers 341F1–4 (5′ CCTACGGGNGGCWGCAG 3′) and 785R1–4 (5′ GACTACHVGGGTATCTAATCC 3′) [4] with partial Illumina TruSeq adapter sequences added to the 5′ ends (F1; ATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT, F2; ATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTgt, F3; ATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTagag, F4; ATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTtagtgt and R1; GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT, R2; GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTa, R3; GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTtct, R4; GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTctgagtg). The additional nucleotides (small letters) are introduced for mixing in sequencing. The two-step PCR and subsequent quantification, pooling, and purification were done as described previously [4]. The obtained PCR amplicon pool was checked using Fragment Analyzer (Advanced Analytical Technologies Inc., Ankeny, IA, USA). Every PCR batch included a blank sample (no added DNA template) to assess potential contamination. Finally, the PCR products were sequenced with Illumina MiSeq (v3 600 cycle kit), with 325 bases for the forward and 285 bases for the reverse read.
The raw sequence data contained a total of 34 701 899 sequence reads. These sequences are available at the European Nucleotide Archive with the accession number PRJEB27564. Primers were removed from the reads using cutadapt (version 1.8.3) [32]. The reads were paired, quality trimmed, taxonomically classified, and clustered into OTUs following mothur's Standard Operating Procedure (SOP) for MiSeq (make.contigs: mothur 1.38.1, the rest of the workflow: mothur 1.39.5; SOP version last updated on 4 April 2018) [33,34]. The following changes were made to the SOP parameters: insert = 40 and deltaq = 10 in make.contigs; maxlength = 450 in the first screen.seqs step; start = 6428 and end = 23440 in the second screen.seqs step; diffs = 4 in pre.cluster. Additionally, singleton sequences were removed with split.abunds (cutoff = 1) before running classify.seqs. The references used were the full-length SILVA alignment release 128 for align.seqs and the RDP 16S rRNA reference (PDS) version 16 for classify.seqs. We inspected the extraction and PCR blank samples, which overall had low amounts of sequence reads (Supplementary material: R Markdown), and since these did not suggest any overall problems with contamination, we deleted these samples before downstream analyses. The final sequence data set (without the blanks) consisted of 18 867 278 good quality reads (median [IQR]: 73 078 [50 032–98 685]).
2.3. Statistics
All statistical comparisons and data visualization were performed with R (v. 3.5.1) [35]. The full analysis workflow is available in the supplementary materials (Supplementary material: R Markdown). In all comparisons, p-values <0·05, or adjusted p-values <0·05 in the case of multiple comparison corrected tests, were considered significant. False discovery rate [36] was used for multiple comparison correction. For basic comparisons of potentially confounding clinical variables, we used either Student's t-test, Wilcoxon signed rank test or Fisher's exact test depending on the type and distribution of each variable, and these comparisons were not corrected for multiple comparisons. Figures were plotted with ggplot2 (v. 3.1.0), except for the diet principal component analysis (PCA) biplot (which additionally utilized ggfortify v. 0.4.5) and Euler plots (drawn with eulerr v. 5.0.0).
Food and nutrient variables from the FFQ were adjusted for energy intake (divided by total energy intake in kilocalories and multiplied by 1000). For variable-specific comparisons, these continuous variables were split into categories by quintiles. To look for dietary patterns, we used PCA of a hand-picked set of 31 non-overlapping, continuous, energy-adjusted and z-transformed food items.
The R-package phyloseq (v. 1.26.0) [37] was used for microbiota data handling and calculating alpha diversity indices (observed richness, Shannon index and inverse Simpson index). Wilcoxon rank-sum test and linear regression were used for statistical testing of alpha diversity differences. Differences in Firmicutes/Bacteroidetes and Prevotella/Bacteroides ratios were also compared with the Wilcoxon rank-sum test. Enterotyping was run with the reference-based online tool [38], and distributions of enterotypes were compared with the chi-square test, for which simulate.p.value = TRUE was used when comparing PD patients subsetted according to progression, as the group sizes were small.
Beta-diversity comparisons were done with vegan (v. 2.5–3) [39] using Bray-Curtis dissimilarity of data subsampled to the lowest amount of sequences in a sample (2201), on three different taxonomic levels (OTU, genus and family). PERMANOVA was run with the command adonis2, with the parameters by = “margins” and perm = 9999 for all comparisons, except for the Timepoint + Parkinson + single confounder as well as the diet variable tests, which were run with 999 permutations. To narrow down the lists of potential confounders for testing, we focused on those clinical variables that differed significantly between groups (PD/control or progressed/stable), and a few common confounders that did not (age, sex, body mass index). Variables that were correlated with PD status (|Pearson's r| ≥ 0·5; Table 2) were only used for the within PD group comparisons; additionally, GDS 15 (Pearson's r = 0·491, close to the cutoff and with high values mainly present in PD patients at follow-up) and catechol-O-methyl transferase (COMT) inhibitor use (Pearson's r = 0·307, but with no controls using the medication) were also only used in the within PD group comparisons. To explore potential confounders for the PD/control comparisons, we first tested each variable for microbial community effects in a model that included timepoint (baseline or follow-up), PD status, and the variable in question. The variables that were significant (adonis2 p < 0·05 on at least one taxonomic level) in these single-confounder comparisons were then tested together in a combined adonis2 model. As an alternative test, we used the envfit function (run with permutations = 9999), fitting variables that were significant on at least one level with adonis2 onto a Nonmetric Multidimensional Scaling (NMDS) ordination performed with the metaMDS function (run with try = 500). Beta-diversity analysis for the progression comparisons was performed in a similar manner, with the categorical disease progression variable (progressed/stable) in place of the PD status variable.
Table 2.
Correlations with PD status for potential microbiome confounders.
| Variable | Pearson correlation coefficient with PD | Use for PD status comparisons | Use for progression comparisons |
|---|---|---|---|
| Rome III 9–15 sum score | 0.432 | Yes | No |
| RLS | 0.419 | Yes | No |
| Wexner total | 0.418 | Yes | No |
| Rome III IBS criteria fulfilled | 0.280 | Yes | No |
| Medication anticholinergic | 0.161 | Yes | No |
| Diet PC1 | 0.145 | Yes | No |
| BMI | 0.094 | Yes | No |
| Age at stool collection | 0.061 | Yes | No |
| Sex | 0.016 | Yes | No |
| MMSE total | −0.159 | Yes | No |
| Medication ACE-I/ARB | −0.160 | Yes | No |
| Medication Ca channel blockers | −0.165 | Yes | No |
| Medication warfarin | −0.205 | Yes | No |
| Medication statin | −0.332 | Yes | No |
| History TIA / ischemic stroke | −0.364 | Yes | No |
| Medication dopamine agonist | 0.807 | No | Yes |
| LED | 0.753 | No | Yes |
| Medication MAO inhibitor | 0.736 | No | Yes |
| NMSQuest total | 0.714 | No | Yes |
| Medication dopa | 0.662 | No | Yes |
| NMSS total | 0.607 | No | Yes |
| RBDSQ | 0.580 | No | Yes |
| SDQ total | 0.529 | No | Yes |
| SCS PD total | 0.520 | No | Yes |
| GDS 15 | 0.491 | No | Yes |
| Medication COMT inhibitora | 0.307 | No | Yes |
| Sniffin’ Sticks | −0.769 | No | Yes |
Table legend: RLS: Restless Legs Syndrome, IBS: Irritable Bowel Syndrome, PC: Principal Component, BMI: Body Mass Index, MMSE: Mini-Mental State Examination, ACE-I/ARB: angiotensin-converting-enzyme inhibitor / angiotensin II receptor blocker, TIA: Transient Ischemic Attack, LED: Levodopa Equivalent Dose, MAO: Monoamine Oxidase, NMSQuest: Non-Motor Symptoms Questionnaire, NMSS: Non-Motor Symptoms Scale, RBDSQ: REM Sleep Behavior Disorder Screening Questionnaire, SDQ: Swallowing Disturbance Questionnaire, SCS-PD: Sialorrhea Clinical Scale for PD, GDS 15: 15-item Geriatric Depression Scale, COMT: catechol-O-methyl transferase.
COMT inhibitor variable used only for progression although |Pearson's r| < 0.5, since only PD patients take this medication.
For differential abundance comparisons, we used ANCOM (v. 2.0; unpublished version shared online) [40,41], DESeq2 (v. 1.22.1) [42], and random forests ([43], packages randomForest (v. 4.6–14) [44] for the classification, rfUtilities (v. 2.1–3) [45] for estimating the significance of the classification, and rfPermute (v. 2.1.6) [46] for assessing the significances of specific taxa). All comparisons were performed on OTU, genus, and family levels, with data trimmed to include taxa that had more than one read in at least 1/10 of samples (26 samples for PD status comparisons, 11 for progression comparisons, and 10 for PD phenotype comparisons); additionally, OTUs were required to have at least 1000 sequence reads altogether. ANCOM and random forests were run separately for baseline and follow-up, classifying data by PD status or progression category. For ANCOM, we adjusted the PD/control comparisons for Rome III score and BMI, and the progression comparisons for COMT inhibitor use, and chose the less stringent multiple comparison correction option (multcorr = 2) and the 0.90 cutoff (prev.cut = 0.90) for proportion of zeroes. The results for the 0.6 detection level column were considered significant. DESeq2 comparisons were corrected for the same confounders and were run with the parameters fitType = “parametric” and sfType = “poscounts”. For the PD status comparisons, we used a model that was additionally corrected for subject (model: Rome III score + BMI + PD : subject + timepoint * PD) and extracted timepoint-specific contrasts from the results. Subjects that lacked baseline BMI information (2 PD, 3 control) were excluded from this analysis, which resulted in an unbalanced data set. Because of this and to assess the robustness of the results, the PD status DESeq2 comparison was performed with a leave-one-out approach, excluding each one of the 62 PD patients in turn, with the average FDR-adjusted p-value of the 62 rounds as the final result. DESeq2 comparisons for progression were run separately for each timepoint, correcting for COMT inhibitor medication (model: COMT + Progression). PD phenotype comparisons (TD vs PIGD, excluding patients with MX type) were run with ANCOM and DESeq2, separately for each timepoint, and not corrected for confounders due to the small number of subjects included in this subgroup analysis.
3. Results
3.1. Clinical and diet data
Control subjects and PD patients were matched for age and sex in the pilot study [6], and those who returned for the follow-up still had similar distributions for these variables, as well as body mass index (BMI) (Table 3). As expected, PD patients had higher scores on the non-motor symptoms scale for Parkinson's disease (NMSS, p < 0·001 at both timepoints), and the Rome III IBS questionnaire (p < 0·001 at both timepoints). Controls more commonly had a history of transient ischemic attack (TIA) or ischemic stroke (p < 0·001 at both timepoints) and were on several medications more often than patients: statins (p < 0·001 at both timepoints), warfarin at baseline (p (baseline) = 0·017, p (follow-up) = 0·076), and calcium channel blockers (CCB) at follow-up (p (baseline) = 0·271, p (follow-up) = 0·035) (Table 3).
Table 3.
Clinical variables in Parkinson's patients and control subjects at each timepoint.
| Variable | Timepoint | Control subjects (% (n) / mean ± SD / median [IQR]) | Parkinson's patients (% (n) / mean ± SD / median [IQR]) | p-value | Test |
|---|---|---|---|---|---|
| n | 64 | 64 | |||
| Age (at first stool collection) | 64.45 ± 6.9 | 65.2 ± 5.52 | 0.499 | t | |
| Sex (% males) | 50 (32) | 51.56 (33) | 1.000 | Fisher | |
| BMI | Baseline | 26.23 [24.1–28.05] | 26.51 [24.25–29.36] | 0.319 | Wilcox |
| Follow-up | 26.94 [24.32–28.64] | 27.24 [23.95–30.08] | 0.572 | Wilcox | |
| History of TIA or ischemic stroke | Baseline | 37.5 (24) | 6.25 (4) | <0.001 | Fisher |
| Follow-up | 37.5 (24) | 7.81 (5) | <0.001 | Fisher | |
| Medication: calcium channel blockers | Baseline | 15.62 (10) | 7.81 (5) | 0.271 | Fisher |
| Follow-up | 20.31 (13) | 6.25 (4) | 0.035 | Fisher | |
| Medication: statins | Baseline | 56.25 (36) | 21.88 (14) | <0.001 | Fisher |
| Follow-up | 50 (32) | 20.31 (13) | <0.001 | Fisher | |
| Medication: warfarin | Baseline | 14.06 (9) | 1.56 (1) | 0.017 | Fisher |
| Follow-up | 15.62 (10) | 4.69 (3) | 0.076 | Fisher | |
| NMSS total | Baseline | 8 [4–12] | 40 [23.75–55] | <0.001 | Wilcox |
| Follow-up | 6 [2–10.25] | 40 [19.75–58.75] | <0.001 | Wilcox | |
| Rome III constipation-defecation score (sum of items 9–15) | Baseline | 2 [1–4] | 6 [2.75–11] | <0.001 | Wilcox |
| Follow-up | 2 [0–3] | 8 [2.75–11] | <0.001 | Wilcox | |
| Rome III IBS criteria fulfilled | Baseline | 7.81 (5) | 23.44 (15) | 0.027 | Fisher |
| Follow-up | 7.81 (5) | 35.94 (23) | <0.001 | Fisher |
Table legend: Statistically significant p-values are marked in bold italic font. SD: Standard Deviation, IQR: Interquartile Range, BMI: Body Mass Index, TIA: Transient Ischemic Attack, NMSS: Non-Motor Symptoms Scale, IBS: Irritable Bowel Syndrome.
Contrasting stable and progressed patients (Table 4), defined based on between-timepoint change in Unified Parkinson's Disease Rating Scale (UPDRS) I-III sum and total medication load calculated using the Levodopa Equivalent Dose (LED), there were no differences in basic demographics. The UPDRS I-III sum was stable or even decreased slightly between timepoints in stable patients (p = 0·004) and increased in progressors (p = 0·004). While there was no statistically significant difference in UPDRS I-III sum between groups at baseline (p = 0·201), there was at follow-up (p = 0·020). LED was similar for both groups at both timepoints and increased in both between timepoints (p (stable) < 0·001; p (progressors) = 0·001). Medications that differed between progression groups at baseline were acetylsalicylic acid (p = 0·037), statins (p = 0·010) and ropinirole (p (dose (mg)) = 0·048). At follow-up, progressors used more COMT inhibitors (p (entacapone (mg)) = 0·027; p (yes/no) = 0·051) and less pramipexole (p (dose (mg)) = 0·001).
Table 4.
Clinical variables in stable and progressed Parkinson's patients at each timepoint.
| Variable | Timepoint | Stable patients (n = 41) |
Progressed patients (n = 15) |
||||
|---|---|---|---|---|---|---|---|
| % (n) / median [IQR] | p-value (baseline vs follow-up) | % (n) / median [IQR] | p-value (baseline vs follow-up) | p-value (progressed vs stable) | Test | ||
| Sex (% males) | 53.66 (22) | 46.67 (7) | 0.765 | Fisher | |||
| Age at stool collection | 65 [61–69] | 65 [62.5–66.5] | 0.802 | Wilcox | |||
| Age PD diagnosed | 60 [58–64] | 60 [57–65] | 0.963 | Wilcox | |||
| BMI | Baseline | 26.25 [24.15–29.8] | 0.225 | 26.53 [25.28–28.24] | 0.890 | 0.923 | Wilcox |
| Follow-up | 27.28 [24.27–30.21] | 26.9 [23.89–29.89] | 0.608 | ||||
| UPDRS I to III score total | Baseline | 45 [35–52] | 0.004 | 36 [33–46.5] | 0.004 | 0.201 | Wilcox |
| Follow-up | 38 [32–49] | 48 [41–49] | 0.020 | ||||
| UPDRS IV total | Baseline | 2 [0–3] | 0.062 | 1 [0.5–2] | 0.026 | 0.685 | Wilcox |
| Follow-up | 1 [0–5] | 1 [1–6] | 0.277 | ||||
| Hoehn & Yahr stage | Baseline | 2.5 [2–2.5] | 0.735 | 2.5 [2–2.5] | 0.021 | 0.453 | Wilcox |
| Follow-up | 2.5 [2–3] | 2.5 [2.5–3] | 0.038 | ||||
| Rome III IBS criteria fulfilled | Baseline | 24.39 (10) | 0.467 | 6.67 (1) | 0.330 | 0.255 | Fisher |
| Follow-up | 34.15 (14) | 26.67 (4) | 0.751 | ||||
| Jankovic subtypes | |||||||
| Baseline | MX: 9.76 (4), PIGD: 53.66 (22), TD: 36.59 (15) | 0.412 | MX: 20.00 (3), PIGD: 40.00 (6), TD: 40.00 (6) | 0.029 | 0.481 | Fisher | |
| Follow-up | MX: 2.44 (1), PIGD: 53.66 (22), TD: 43.9 (18) | MX: 0 (0), PIGD: 86.67 (13), TD: 13.33 (2) | 0.070 | ||||
| Medications | |||||||
| LED (mg) | Baseline | 420 [205–505] | <0.001 | 340 [210–585] | 0.001 | 0.774 | Wilcox |
| Follow-up | 505 [362–662] | 604 [440–811.75] | 0.177 | ||||
| Levodopa (%) | Baseline | 53.66 (22) | 0.171 | 46.67 (7) | 0.128 | 0.765 | Fisher |
| Follow-up | 70.73 (29) | 80 (12) | 0.735 | ||||
| Levodopa (mg) | Baseline | 100 [0–400] | <0.001 | 0 [0−300] | 0.034 | 0.715 | Wilcox |
| Follow-up | 300 [0–500] | 350 [100–475] | 0.708 | ||||
| COMT inhibitors (%) | Baseline | 12.2 (5) | 1.000 | 6.67 (1) | 0.080 | 1.000 | Fisher |
| Follow-up | 12.2 (5) | 40 (6) | 0.051 | ||||
| Entacapone (mg) | Baseline | 0 [0–0] | 0.850 | 0 [0–0] | 0.204 | 0.617 | Wilcox |
| Follow-up | 0 [0–0] | 0 [0–750] | 0.027 | ||||
| Acetylsalicylic acid (%) | Baseline | 17.07 (7) | 1.000 | 46.67 (7) | 0.450 | 0.037 | Fisher |
| Follow-up | 17.07 (7) | 26.67 (4) | 0.461 | ||||
| Statins (%) | Baseline | 12.2 (5) | 1.000 | 46.67 (7) | 0.710 | 0.010 | Fisher |
| Follow-up | 14.63 (6) | 33.33 (5) | 0.142 | ||||
| Pramipexole (mg) | Baseline | 0.26 [0–1.05] | 0.169 | 0 [0–0.52] | 0.098 | 0.160 | Wilcox |
| Follow-up | 0.26 [0–1.57] | 0 [0–0] | 0.001 | ||||
| Ropinirole (mg) | Baseline | 0 [0–0] | 0.887 | 8 [0–8] | 0.904 | 0.048 | Wilcox |
| Follow-up | 0 [0–0] | 0 [0–8] | 0.062 | ||||
Table legend: Statistically significant p-values are marked in bold italic font. IQR: Interquartile Range, BMI: Body Mass Index, UPDRS: Unified Parkinson's Disease Rating Scale, IBS: Irritable Bowel Syndrome, TD: tremor dominant, PIGD: postural instability and gait difficulty, MX: mixed disease phenotype, LED: Levodopa Equivalent Dose, COMT: catechol-O-methyl transferase.
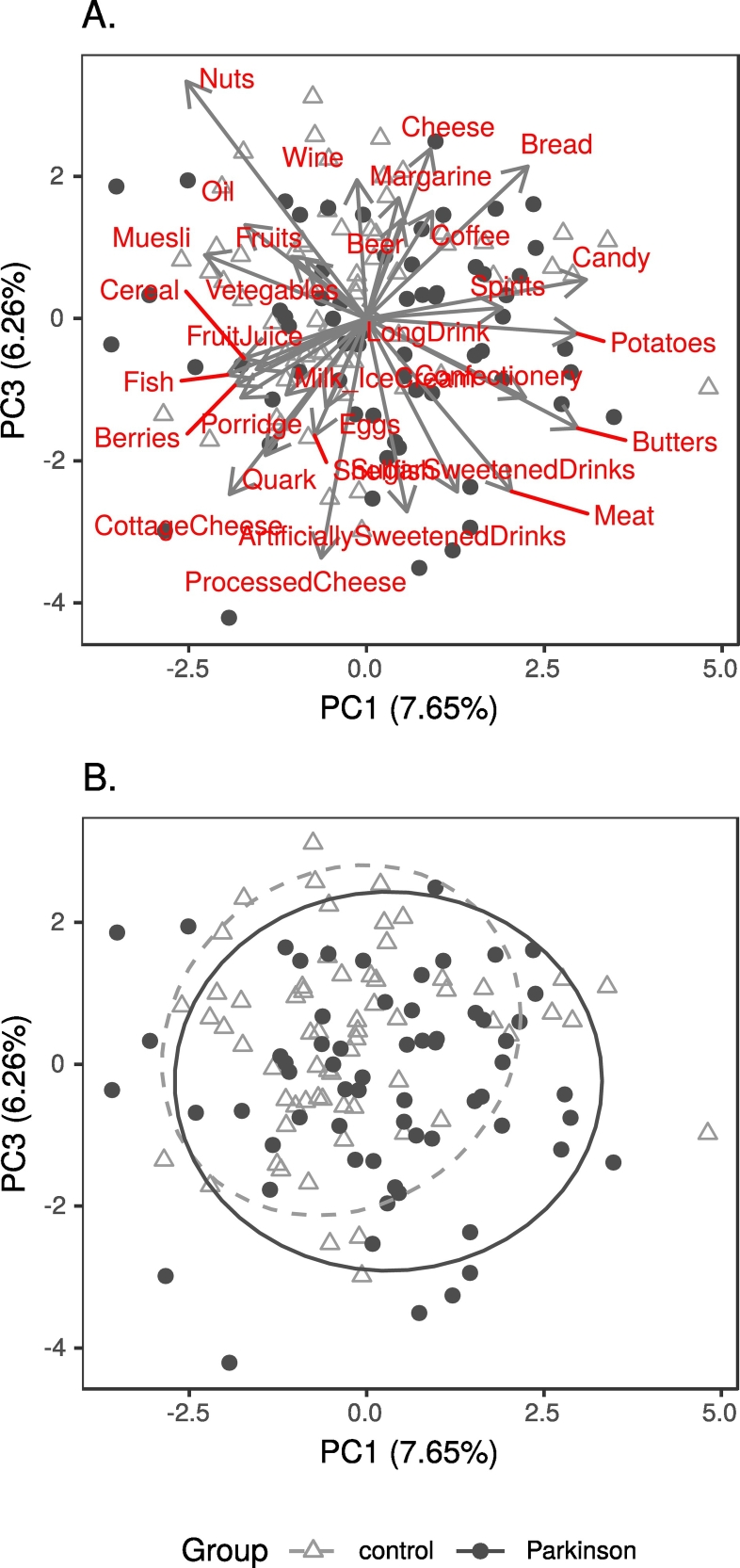
Regarding dietary data, there were no significant differences in intakes of any dietary items between patients and controls, or stable and progressed patients (Supplementary results). However, the first principal component (PC1) of a Principal Component Analysis (PCA) seemed to reflect diet healthiness (Table S1), with PD patients more often on the unhealthy side (Fig. 3). We kept PC1 as a potential confounder to be assessed in further analyses.
Fig. 3.
Biplot of principal component analysis of diet data, showing principal components 1 and 3.
Legend: A. Component loadings; B. PD patients and controls, with 90% confidence ellipses.
3.2. Microbiota data
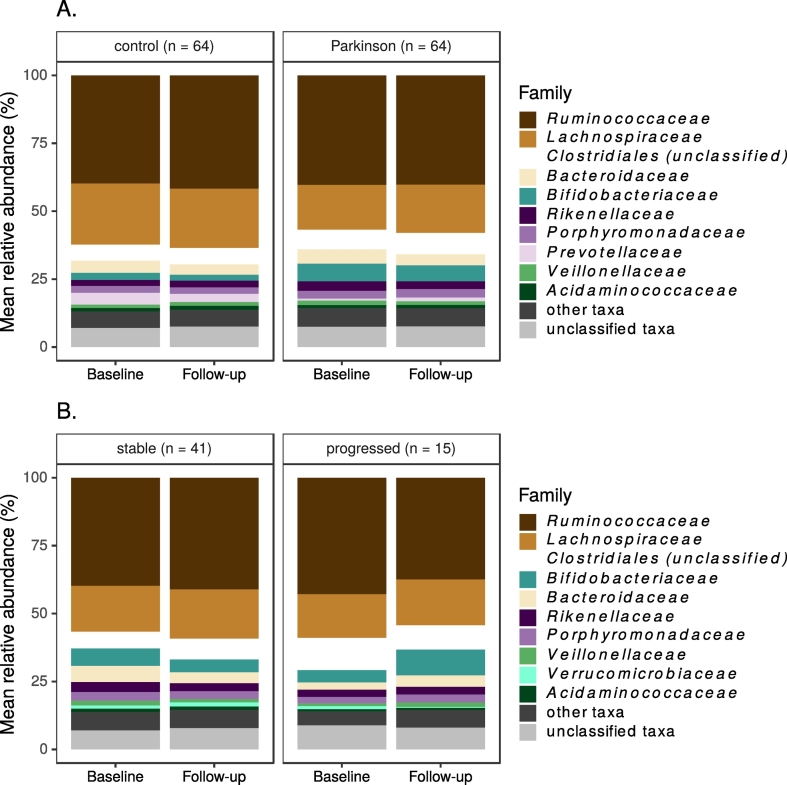
The 16S rRNA gene amplicon data contained 2836 OTUs, 198 genera and 77 families. The most common taxa were similar for the PD and control groups at both timepoints, with Ruminococcaceae and Lachnospiraceae dominating both groups' microbiota (Fig. 4A). Subsetting PD patients by progression status did not suggest major differences in microbial communities between these groups (Fig. 4B).
Fig. 4.
10 most common bacterial families at each timepoint.
Legend: A. All study subjects by PD status; B. PD patients by progression status.
Considering commonly used ratios of specific bacteria, the Firmicutes/Bacteroidetes ratio did not differ between patients and controls, but Prevotella/Bacteroides was higher in controls (p (baseline) = 0·052, p (follow-up) = 0·011). Comparing progressed and stable patients, Firmicutes/Bacteroidetes differed significantly at baseline (p = 0·012) but not at follow-up, while Prevotella/Bacteroides did not differ between groups.
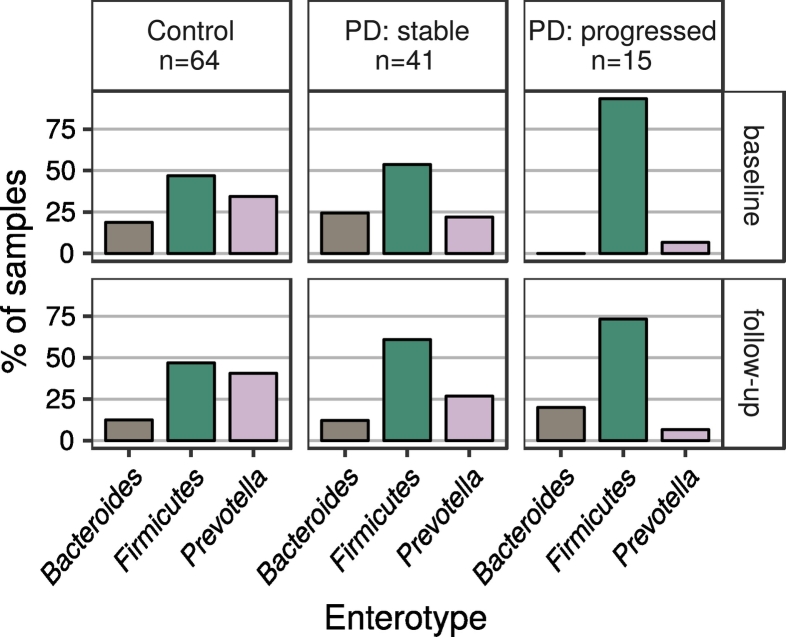
Enterotype analysis suggested that PD patients in general, and progressed patients in particular (Fig. 5), were overrepresented in the Firmicutes-dominated enterotype. This difference was statistically significant at both timepoints when contrasting controls and all patients (p (baseline) = 0·044; p (follow-up) = 0·025) or controls and progressed patients (p (baseline) < 0·001; p (follow-up) = 0·043), and at baseline when contrasting stable and progressed patients (p (baseline) = 0·016; p (follow-up) = 0·291). Enterotype distribution did not differ significantly between stable patients and controls (p (both timepoints) > 0·3).
Fig. 5.
Distributions of enterotypes in the subject groups.
Legend: Showing control subjects and PD patients classified by progression (excluding PD patients without a progression classification).
3.3. Alpha diversity
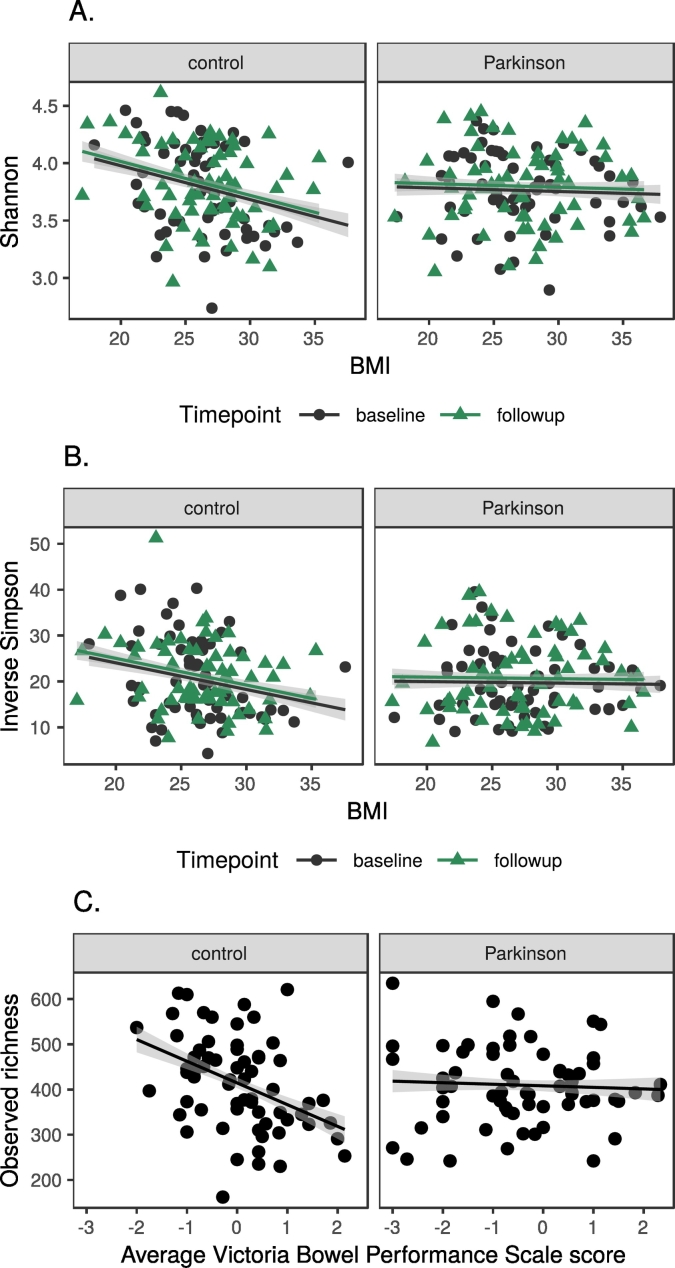
We explored alpha diversity (microbial community richness and evenness) using three different indices (observed richness, Shannon, inverse Simpson). There was no difference between timepoints (p > 0·1, all indices), controls and patients (p > 0·6, all indices, both timepoints), stable and progressed patients (p > 0·2, all indices, both timepoints), or PD phenotypes (postural instability and gait difficulty (PIGD) vs tremor dominant (TD); p > 0·3, all indices, both timepoints). To look for effects of potential confounders, we calculated their correlations with each index (Table 5). The only variable with significant adjusted p-values in these comparisons was CCB use (adjusted p (Shannon) = 0·042; adjusted p (inverse Simpson) = 0·039). BMI and history of ear, nose and throat (ENT) surgery had a significant unadjusted p for all indices. We also found interactions between PD status and BMI in linear regression for the Shannon and inverse Simpson indices, and between PD status and average Victoria Bowel Performance Scale (BPS) score (follow-up only) when modelling observed richness (Fig. 6, Supplementary results, Table S2). Dietary variables were not associated with differences in alpha diversity (Supplementary results).
Table 5.
Correlations for alpha diversity measures with variables of interest and confounders.
| Observed richness |
Shannon |
Inverse Simpson |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation | p-value | Adjusted p-value | Pearson correlation | p-value | Adjusted p-value | Pearson correlation | p-value | Adjusted p-value | |
| Timepoint | 0.104 | 0.098 | 0.496 | 0.042 | 0.506 | 0.832 | 0.055 | 0.382 | 0.873 |
| PD status | 0.005 | 0.942 | 0.990 | −0.023 | 0.718 | 0.908 | −0.026 | 0.678 | 0.915 |
| Meds CCB | 0.136 | 0.030 | 0.266 | 0.216 | 0.001 | 0.042 | 0.217 | <0.001 | 0.039 |
| History ENT surgery | 0.169 | 0.007 | 0.184 | 0.189 | 0.002 | 0.096 | 0.163 | 0.009 | 0.181 |
| BMI | −0.200 | 0.001 | 0.057 | −0.159 | 0.012 | 0.190 | −0.129 | 0.041 | 0.417 |
| History CAD | 0.105 | 0.096 | 0.496 | 0.168 | 0.007 | 0.153 | 0.169 | 0.007 | 0.181 |
| Tobacco 100 in life | 0.092 | 0.140 | 0.630 | 0.167 | 0.008 | 0.153 | 0.140 | 0.025 | 0.403 |
| Selegeline mg | −0.043 | 0.494 | 0.970 | −0.124 | 0.048 | 0.487 | −0.163 | 0.009 | 0.181 |
| History IBS | 0.149 | 0.017 | 0.232 | 0.116 | 0.064 | 0.517 | 0.131 | 0.036 | 0.417 |
| History hyper-thyreosis | −0.034 | 0.591 | 0.970 | −0.143 | 0.022 | 0.299 | −0.110 | 0.078 | 0.576 |
| History appendectomy | −0.054 | 0.392 | 0.970 | −0.133 | 0.034 | 0.388 | −0.110 | 0.078 | 0.576 |
| History lactose intolerance | 0.129 | 0.039 | 0.280 | 0.116 | 0.063 | 0.517 | 0.108 | 0.086 | 0.579 |
| History hernia repair | 0.013 | 0.835 | 0.990 | 0.101 | 0.108 | 0.729 | 0.135 | 0.031 | 0.417 |
| History hypothyreosis | 0.161 | 0.010 | 0.199 | 0.078 | 0.212 | 0.832 | 0.031 | 0.618 | 0.894 |
| Wexner total | 0.151 | 0.015 | 0.232 | 0.036 | 0.569 | 0.832 | 0.020 | 0.744 | 0.972 |
| Rome III 9–15 sum score | 0.212 | 0.001 | 0.052 | 0.031 | 0.616 | 0.832 | 0.010 | 0.872 | 0.985 |
| Rome III IBS criteria fulfilled | 0.128 | 0.040 | 0.280 | 0.033 | 0.597 | 0.832 | −0.007 | 0.913 | 0.985 |
| Meds thyroxine | 0.143 | 0.022 | 0.258 | 0.051 | 0.412 | 0.832 | 0.010 | 0.874 | 0.985 |
| Food PC1 | −0.127 | 0.042 | 0.280 | −0.087 | 0.164 | 0.832 | −0.055 | 0.379 | 0.873 |
| Pramipexole mg | −0.139 | 0.026 | 0.265 | −0.028 | 0.657 | 0.845 | −0.001 | 0.985 | 0.985 |
Table legend: Showing only variables with a significant p-value in at least one comparison, and the PD status and Timepoint variables. Statistically significant p-values are marked in bold italic font. CCB: Calcium Channel Blockers, ENT: Ear, Nose and Throat, BMI: Body Mass Index, CAD: Coronary Artery Disease, IBS: Irritable Bowel Syndrome, PC: Principal Component.
Fig. 6.
Interactions in linear regression of alpha diversity, PD status and confounders.
Legend: A. Shannon index and BMI; B. Inverse Simpson index and BMI, C. Observed richness and Victoria Bowel Performance Scale.
3.4. Beta diversity
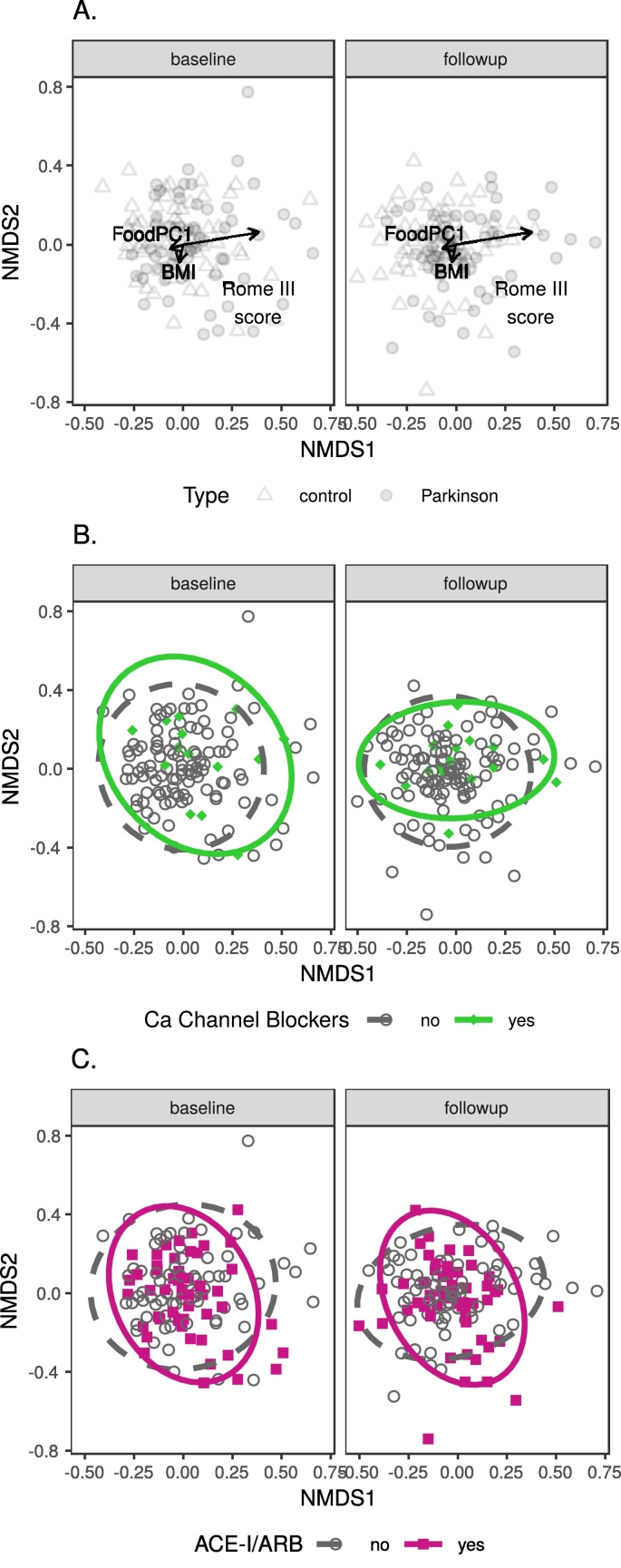
Regarding beta diversity (between-sample community dissimilarity), subjects' microbial communities did not differ between timepoints in any comparison, but they did between patients and controls (p (PD status) < 0·017, all comparisons; Table 6A–C, Fig. 7). The confounding variables with the most consistent effects were constipation scores (Rome III: p ≤ 0·001, all comparisons; Wexner: p ≤ 0·004 in all single-confounder comparisons, dropped from further comparisons due to collinearity) and BMI (p < 0·025 in all comparisons except envfit test at genus and family level); two medication variables, CCB and ACE-I/ARB (angiotensin-converting-enzyme inhibitor / angiotensin II receptor blocker), and PC1 from the diet analysis were also significant in more than one comparison (Table 6B-C, Fig. 8). Separate beta diversity comparisons for dietary variables revealed no strong dietary confounders (Table S3, Supplementary results).
Table 6.
Beta diversity comparisons of PD patients, control subjects and confounding variables.
|
A. Adonis: Timepoint + PD status (no confounders) | ||
|---|---|---|
| Level | p-value (Timepoint) | p-value (PD status) |
| OTU | 0.898 | <0.001 |
| Genus | 0.544 | <0.001 |
| Family | 0.647 | <0.001 |
|
B. Adonis: Timepoint + PD + single confounder | |||
|---|---|---|---|
| Variable | p-value (OTUs) | p-value (genera) | p-value (families) |
| BMI | 0.001 | 0.002 | 0.003 |
| ACEI / ARB | 0.032 | 0.046 | 0.018 |
| Rome III 9–15 sum score | 0.001 | 0.001 | 0.001 |
| Wexner total | 0.001 | 0.002 | 0.004 |
| Diet PC1 | 0.003 | 0.009 | 0.064 |
| CCB | 0.017 | 0.031 | 0.183 |
| RLS | 0.034 | 0.085 | 0.043 |
| TIA / ischemic stroke | 0.007 | 0.103 | 0.186 |
| Age at stool collection | 0.153 | 0.170 | 0.266 |
| Sex | 0.053 | 0.106 | 0.065 |
| Anticholinergics | 0.287 | 0.828 | 0.882 |
| Statins | 0.068 | 0.370 | 0.453 |
| Warfarin | 0.564 | 0.610 | 0.339 |
| MMSE total | 0.730 | 0.561 | 0.310 |
| Rome III IBS criteria fulfilled | 0.229 | 0.191 | 0.276 |
| C. Timepoint + PD status + multiple confounders | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
p-value |
||||||||||
| Test | Level | Timepoint | Parkinson | BMI | TIA / ischemic stroke | ACEI / ARB | CCB | Rome III 9–15 sum score | Diet PC1 | RLS |
| Adonis2 | OTU | 0.888 | 0.006 | 0.002 | 0.009 | 0.020 | 0.003 | <0.001 | 0.013 | 0.086 |
| Genus | 0.510 | 0.016 | 0.002 | 0.085 | 0.168 | 0.007 | 0.001 | 0.096 | 0.110 | |
| Family | 0.625 | 0.017 | 0.025 | 0.349 | 0.044 | 0.121 | 0.001 | 0.227 | 0.133 | |
| Envfit | OTU | 0.995 | 0.001 | 0.010 | 0.948 | 0.188 | 0.073 | <0.001 | 0.063 | NA |
| Genus | 0.083 | 0.001 | 0.372 | 0.660 | 0.097 | 0.162 | <0.001 | 0.560 | NA | |
| Family | 0.129 | 0.001 | 0.607 | 0.981 | 0.953 | 0.044 | <0.001 | 0.303 | NA | |
Table legend: Statistically significant p-values are marked in bold italic font. OTU: Operational Taxonomic Unit, BMI: Body Mass Index, ACE-I/ARB: angiotensin-converting-enzyme inhibitor / angiotensin II receptor blocker, PC: Principal Component, CCB: Calcium Channel Blocker, RLS: Restless Legs Syndrome, TIA: Transient Ischemic Attack, MMSE: Mini-Mental State Examination, IBS: Irritable Bowel Syndrome.
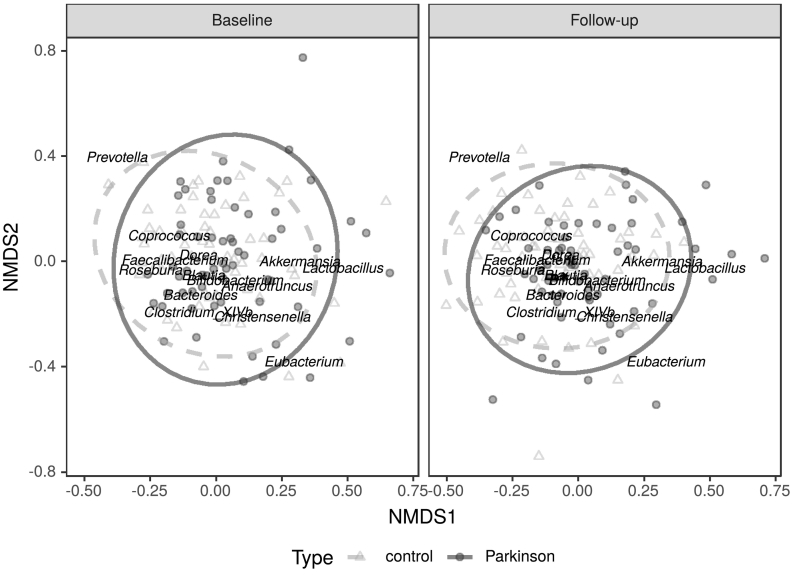
Fig. 7.
NMDS ordination illustrating microbial community differences between PD patients and control subjects.
Legend: Also showing centroid locations for microbial genera reported as differentially abundant between the two groups in previous studies.
Fig. 8.
Genus-level NMDS ordination plots of the main confounding variables.
Legend: A. BMI, diet and Rome III score; B. Calcium channel blockers (CCB) medication; C. ACE-I/ARB medication.
Comparing progressed and stable patients, there were no beta diversity differences between timepoints nor for progression (Table 7A–C), while COMT inhibitor use had a very significant community effect (p ≤ 0·001, all comparisons except the envfit test on genus and family levels; Table 7B–C). Other confounders which were significant in more than one comparison were statins, SCS-PD total, and NMSQuest. There were no differences for beta diversity between PD phenotypes (Supplementary results).
Table 7.
Beta diversity comparisons of stable and progressed PD patients and confounding variables.
|
A. Adonis: Timepoint + progression category (no confounders) | ||
|---|---|---|
| Level | p-value (Timepoint) | p-value (progression) |
| OTU | 1.000 | 0.245 |
| Genus | 0.945 | 0.344 |
| Family | 0.850 | 0.142 |
|
B. Adonis: Timepoint + progression category single confounder | |||
|---|---|---|---|
| Variable | p-value (OTUs) | p-value (genera) | p-value (families) |
| SDQ | 0.012 | 0.007 | 0.010 |
| COMT inhibitors | 0.001 | 0.001 | 0.001 |
| Levodopa entacapone (mg) | 0.001 | 0.001 | 0.001 |
| Entacapone (mg) | 0.001 | 0.001 | 0.001 |
| NMSQuest | 0.003 | 0.018 | 0.137 |
| SCS-PD | 0.001 | 0.005 | 0.166 |
| Statins | 0.010 | 0.038 | 0.065 |
| GDS 15 | 0.016 | 0.395 | 0.308 |
| L-dopa | 0.025 | 0.061 | 0.088 |
| NMSS | 0.018 | 0.070 | 0.306 |
| RBDSQ | 0.007 | 0.131 | 0.079 |
| Ropinirole (mg) | 0.365 | 0.045 | 0.223 |
| Dopamine agonists | 0.050 | 0.365 | 0.074 |
| MAO inhibitors | 0.545 | 0.826 | 0.442 |
| Sniffin’ Sticks | 0.422 | 0.342 | 0.093 |
| Acetylsalicylic acid | 0.430 | 0.824 | 0.943 |
| PIGD score Jankovic | 0.057 | 0.176 | 0.484 |
| Pramipexole (mg) | 0.075 | 0.060 | 0.059 |
| C. Timepoint + progression category + multiple confounders | ||||||
|---|---|---|---|---|---|---|
| adonis2 |
envfit |
|||||
| Variable | p-value (OTUs) | p-value (genera) | p-value (families) | p-value (OTUs) | p-value (genera) | p-value (families) |
| Timepoint | 0.865 | 0.515 | 0.582 | 0.797 | 0.372 | 0.074 |
| Progression | 0.396 | 0.334 | 0.204 | 0.330 | 0.592 | 0.889 |
| COMT inhibitors | 0.001 | 0.001 | 0.001 | <0.001 | 0.295 | 0.888 |
| NMSQuest | 0.036 | 0.027 | 0.321 | 0.227 | 0.024 | 0.102 |
| SCS-PD | 0.002 | 0.035 | 0.271 | 0.031 | 0.427 | 0.479 |
| Statins | 0.042 | 0.214 | 0.442 | 0.475 | <0.001 | 0.005 |
| SDQ | 0.114 | 0.116 | 0.067 | NA | NA | NA |
| GDS 15 | 0.127 | 0.804 | 0.660 | NA | NA | NA |
| L-dopa | 0.421 | 0.344 | 0.208 | NA | NA | NA |
| NMSS | 0.447 | 0.216 | 0.498 | NA | NA | NA |
| RBDSQ | 0.062 | 0.513 | 0.312 | NA | NA | NA |
| Ropinirole (mg) | 0.411 | 0.098 | 0.440 | NA | NA | NA |
Table legend: Statistically significant p-values are marked in bold italic font. OTU: Operational Taxonomic Unit, SDQ: Swallowing Disturbance Questionnaire, COMT: catechol-O-methyl transferase, NMSQuest: Non-Motor Symptoms Questionnaire, SCS-PD: Sialorrhea Clinical Scale for PD, GDS 15: 15-item Geriatric Depression Scale, NMSS: Non-Motor Symptoms Scale, RBDSQ: REM Sleep Behavior Disorder Screening Questionnaire, MAO: Monoamine Oxidase, PIGD: postural instability and gait difficulty.
3.5. Differential abundance of microbial taxa
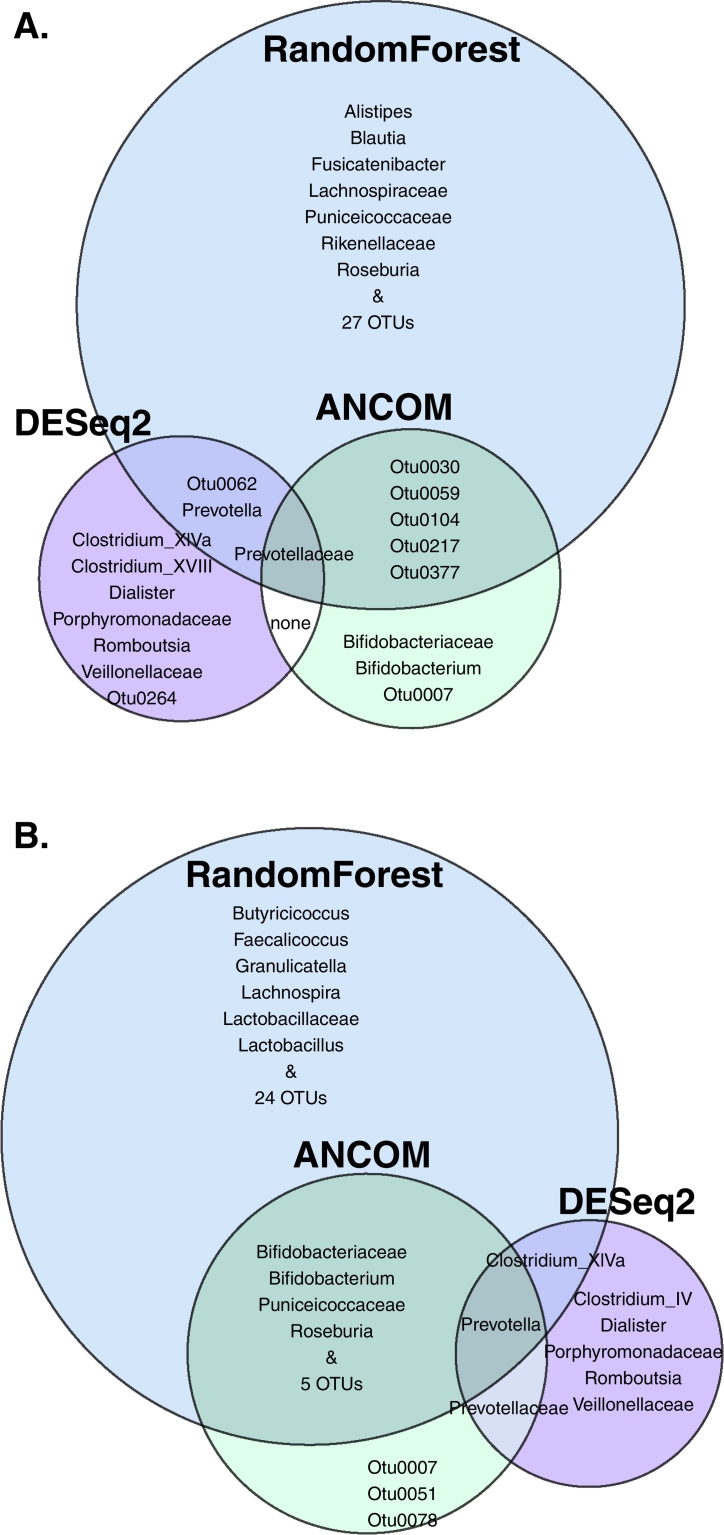
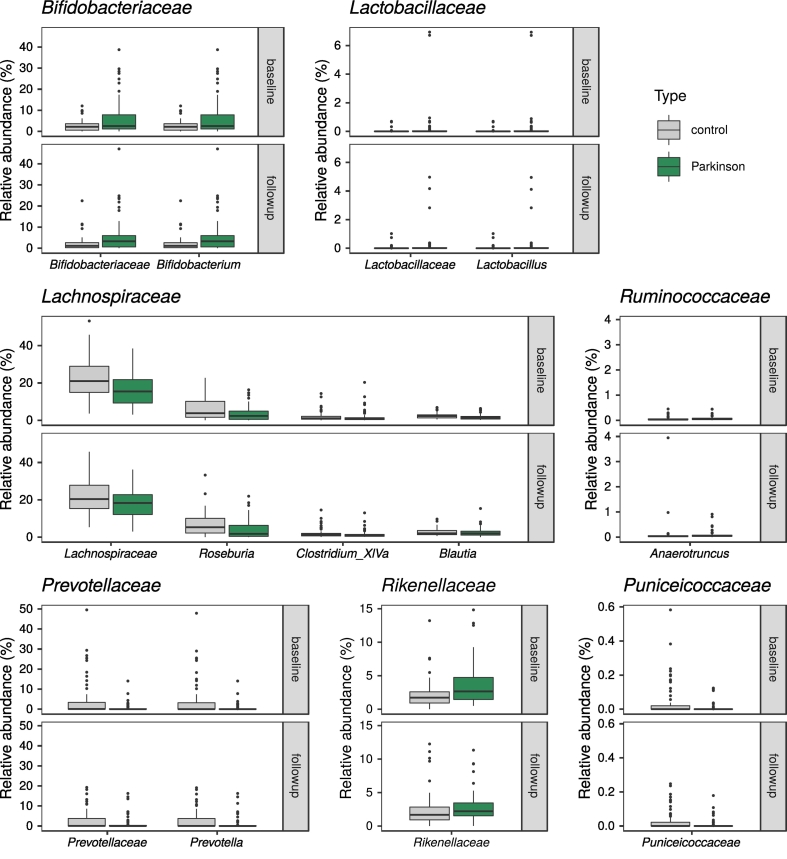
We used three differential abundance comparison methods, all of which suggested several taxa as differing significantly between PD patients and controls (ANCOM: 2 families, 1 genus and 6 OTUs for baseline and 3 families, 3 genera and 8 OTU for follow-up; random forests: 4 families, 5 genera and 33 OTUs for baseline, 3 families, 9 genera and 29 OTUs for follow-up; DESeq2: 3 families, 5 genera and 2 OTUs at baseline, 3 families and 5 genera at follow-up; Fig. 9, Supplementary material: Table S4). All random forest classifiers were significantly better than chance (p ≤ 0·046 for all comparisons; Table S5). Combining the results of the three methods, a handful of taxa overlapped at one or both timepoints, mainly the families Bifidobacteriaceae, Prevotellaceae, and Puniceicoccaceae, and the genera Bifidobacterium, Roseburia, Prevotella, and Clostridium XIVa (Table 8, Fig. 9, Fig. 10). A few other taxa reported as differentially abundant in previous studies were significant only according to random forests: the family Lachnospiraceae and the genus Blautia at baseline, and Lactobacillaceae and Lactobacillus at follow-up. Considering the confounders included in DESeq2, 2 OTUs, the genera Clostridium and Coprococcus, and the family Clostridiaceae 1 were associated with BMI, and Bifidobacterium and Bifidobacteriaceae with the Rome III score (Supplementary material: Table S4C). Bifidobacteriaceae were not associated with PD status according to DESeq2 at either timepoint (Table 8). Finally, the DESeq2 model enabled the detection of microbes with a difference in between-timepoint trends when comparing controls and PD patients, but we found no such taxa.
Fig. 9.
Differentially abundant taxa between control subjects and PD patients according to three different tools.
Legend: A. Baseline; B. Follow-up.
Table 8.
Summary of differential abundance results contrasting PD patients and control subjects.
| Baseline |
Follow-up |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | Previously published | Relative abundance (%, mean ± SD) |
p-value |
Relative abundance (%, mean ± SD) |
p-value |
|||||||
| Control | PD | ANCOM | Random Forests | DESeq2 | Control | PD | AN-COM | Random Forests | DESeq2 | |||
| Bifidobacteriaceae | Family | Yes | 2.629 ± 2.719 | 6.528 ± 8.573 | s.s. | 0.129 | 0.151 | 2.189 ± 3.531 | 5.919 ± 8.256 | s.s. | 0.040 | 0.150 |
| Prevotellaceae | Family | Yes | 4.345 ± 9.22 | 0.725 ± 2.12 | s.s. | 0.010 | 0.002 | 2.972 ± 4.882 | 1.395 ± 3.474 | s.s. | 0.079 | 0.002 |
| Rikenellaceae | Family | Yes | 2.201 ± 2.035 | 3.494 ± 2.899 | n.s. | 0.030 | 0.075 | 2.479 ± 2.559 | 2.836 ± 2.131 | n.s. | 0.386 | 0.172 |
| Lachnospiraceae | Family | Yes | 22.478 ± 10.241 | 16.48 ± 9.121 | n.s. | 0.020 | 0.990 | 21.787 ± 8.964 | 17.753 ± 8.198 | n.s. | 0.416 | 0.995 |
| Pasteurellaceae | Family | Yes | 0.036 ± 0.097 | 0.009 ± 0.027 | n.s. | 0.089 | 0.625 | 0.059 ± 0.28 | 0.014 ± 0.052 | n.s. | 0.158 | 0.585 |
| Lactobacillaceae | Family | Yes | 0.032 ± 0.124 | 0.28 ± 1.199 | n.s. | 0.129 | 0.993 | 0.04 ± 0.159 | 0.226 ± 0.867 | n.s. | 0.040 | 0.995 |
| Puniceicoccaceae | Family | No | 0.045 ± 0.103 | 0.01 ± 0.029 | n.s. | 0.010 | 0.993 | 0.032 ± 0.061 | 0.01 ± 0.03 | s.s. | 0.010 | 0.995 |
| Bifidobacterium | Genus | Yes | 2.628 ± 2.717 | 6.524 ± 8.57 | s.s. | 0.089 | 0.244 | 2.188 ± 3.53 | 5.917 ± 8.256 | s.s. | 0.040 | 0.410 |
| Roseburia | Genus | Yes | 7.014 ± 6.944 | 3.588 ± 4.096 | n.s. | 0.020 | 0.147 | 6.683 ± 6.162 | 4.395 ± 5.298 | s.s. | 0.010 | 0.121 |
| Prevotella | Genus | Yes | 4.187 ± 9.003 | 0.659 ± 2.102 | n.s. | 0.050 | 0.042 | 2.85 ± 4.854 | 1.306 ± 3.355 | s.s. | 0.030 | 0.043 |
| Anaerotruncus | Genus | Yes | 0.056 ± 0.079 | 0.071 ± 0.084 | n.s. | 0.079 | 0.986 | 0.119 ± 0.501 | 0.098 ± 0.163 | n.s. | 0.693 | 0.990 |
| Blautia | Genus | Yes | 2.419 ± 1.516 | 1.829 ± 1.598 | n.s. | 0.040 | 0.742 | 2.63 ± 1.847 | 2.429 ± 2.334 | n.s. | 0.644 | 0.927 |
| Lactobacillus | Genus | Yes | 0.031 ± 0.125 | 0.278 ± 1.197 | n.s. | 0.356 | 0.989 | 0.04 ± 0.159 | 0.221 ± 0.862 | n.s. | 0.030 | 0.996 |
| Clostridium XlVa | Genus | No | 1.971 ± 2.631 | 1.83 ± 3.265 | n.s. | 0.168 | <0.001 | 2.136 ± 2.589 | 1.501 ± 2.076 | n.s. | 0.020 | <0.001 |
| Otu0003 Roseburia | OTU | Yes | 6.506 ± 6.71 | 3.115 ± 3.742 | n.s. | 0.020 | 0.342 | 6.144 ± 5.933 | 4.109 ± 5.027 | n.s. | 0.020 | 0.275 |
| Otu0007 Bifidobacterium | OTU | Yes | 1.563 ± 1.808 | 4.579 ± 6.456 | s.s. | 0.079 | 0.613 | 1.518 ± 3.155 | 4.029 ± 6.243 | s.s. | 0.089 | 0.692 |
| Otu0024 Blautia | OTU | Yes | 0.904 ± 0.875 | 0.649 ± 0.903 | n.s. | 0.020 | 1.000 | 1.057 ± 1.14 | 0.712 ± 0.884 | n.s. | 0.020 | 1.000 |
| Otu0027 Ruminococcus | OTU | Yes | 0.679 ± 0.959 | 0.437 ± 0.895 | n.s. | 0.089 | 1.000 | 0.832 ± 1.515 | 0.604 ± 1.23 | n.s. | 0.960 | 1.000 |
| Otu0030 Alistipes | OTU | Yes | 0.404 ± 0.861 | 0.955 ± 1.392 | s.s. | 0.010 | 1.000 | 0.711 ± 1.627 | 0.623 ± 1.134 | n.s. | 0.535 | 1.000 |
| Otu0036 Roseburia | OTU | Yes | 0.483 ± 0.757 | 0.468 ± 1.279 | n.s. | 0.089 | 1.000 | 0.534 ± 0.771 | 0.282 ± 0.689 | n.s. | 0.030 | 1.000 |
| Otu0041 Alistipes | OTU | Yes | 0.278 ± 0.247 | 0.448 ± 0.499 | n.s. | 0.099 | 1.000 | 0.33 ± 0.342 | 0.412 ± 0.37 | n.s. | 0.713 | 1.000 |
| Otu0055 Prevotella | OTU | Yes | 0.344 ± 1.243 | 0.197 ± 1.048 | n.s. | 0.129 | 1.000 | 0.493 ± 1.758 | 0.342 ± 1.413 | n.s. | 0.079 | 1.000 |
| Otu0062 Blautia | OTU | Yes | 0.446 ± 0.576 | 0.266 ± 0.358 | n.s. | 0.030 | 0.049 | 0.328 ± 0.46 | 0.217 ± 0.299 | n.s. | 0.198 | 0.111 |
| Otu0098 Bacteroides | OTU | Yes | 0.105 ± 0.239 | 0.29 ± 0.599 | n.s. | 0.030 | 0.499 | 0.111 ± 0.22 | 0.231 ± 0.407 | n.s. | 0.079 | 0.371 |
| Otu0109 Ruminococcus | OTU | Yes | 0.343 ± 1.142 | 0.122 ± 0.844 | n.s. | 0.465 | 1.000 | 0.369 ± 1.175 | 0.001 ± 0.002 | s.s. | 0.010 | 1.000 |
| Otu0110 Ruminococcus | OTU | Yes | 0.189 ± 0.332 | 0.136 ± 0.279 | n.s. | 0.020 | 1.000 | 0.205 ± 0.519 | 0.14 ± 0.322 | n.s. | 0.139 | 1.000 |
| Otu0131 Bacteroides | OTU | Yes | 0.183 ± 0.654 | 0.079 ± 0.256 | n.s. | 0.406 | 0.998 | 0.209 ± 0.613 | 0.049 ± 0.205 | s.s. | 0.020 | 0.995 |
| Otu0363 Lactobacillus | OTU | Yes | 0.019 ± 0.107 | 0.056 ± 0.225 | n.s. | 0.792 | 1.000 | 0.016 ± 0.122 | 0.006 ± 0.035 | n.s. | 0.050 | 1.000 |
| Otu0379 Alistipes | OTU | Yes | 0.001 ± 0.003 | 0.041 ± 0.2 | n.s. | 0.317 | 1.000 | 0.004 ± 0.033 | 0.047 ± 0.236 | n.s. | 0.020 | 1.000 |
| Otu0464 Lactobacillus | OTU | Yes | 0.002 ± 0.009 | 0.004 ± 0.016 | n.s. | 0.446 | 1.000 | 0.001 ± 0.006 | 0.044 ± 0.236 | n.s. | 0.010 | 1.000 |
| Otu0468 Faecalibacterium | OTU | Yes | 0.016 ± 0.026 | 0.009 ± 0.019 | n.s. | 0.723 | 1.000 | 0.021 ± 0.033 | 0.007 ± 0.016 | n.s. | 0.050 | 1.000 |
| Otu0513 Anaerotruncus | OTU | Yes | 0.006 ± 0.006 | 0.011 ± 0.011 | n.s. | 0.030 | 0.998 | 0.006 ± 0.005 | 0.012 ± 0.019 | n.s. | 0.208 | 0.995 |
Table legend: This table includes (1) taxa that are differentially abundant according to more than one method at either timepoint, and (2) taxa that have been reported as differentially abundant in previous studies and have p < 0.1 with at least one method at either timepoint in this study. Statistically significant p-values are marked in bold italic font. OTU: Operational Taxonomic Unit, SD: Standard Deviation, s.s.: statistically significant, n.s.: not significant.
Fig. 10.
Genera and families that differ significantly between control subjects and PD patients.
Legend: Showing taxa that differ at either timepoint according to more than one method, or have been reported as differing in a previous publication and have p < 0.1 at one timepoint according to at least one method.
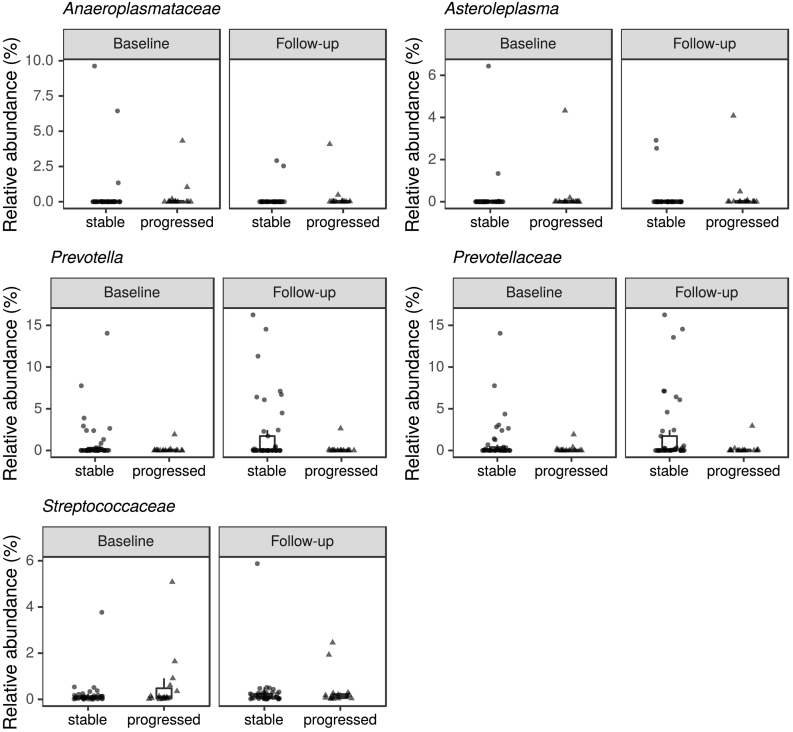
Analyses contrasting progressed patients to stable ones also resulted in lists of several differentially abundant taxa (Supplementary material: Table S6A—C). However, random forest models failed to differentiate progressors and stable patients better than chance; the only comparison with a significant p-value was OTU level at follow-up (p = 0·044), and even there, the difference between actual and randomly permuted error values was small (Table S7). The differential abundance results of the three methods did not overlap substantially. One low-abundance, unclassified Lachnospiraceae OTU was significant according to all methods at follow-up; DESeq2 and random forests agreed on one Streptococcus OTU and the family Streptococcaceae at baseline, and three OTUs, the genus Asteroleplasma, and the family Anaeroplasmataceae at follow-up; and a single Bifidobacterium OTU, which was more common in progressors at follow-up, was supported by both ANCOM and random forests (Table 9). The genus Prevotella was more abundant in stable patients at both timepoints according to DESeq2, while the family Prevotellaceae only differed at follow-up (Fig. 11; Table 9; Supplementary results: Table S6C). There were as many or more taxa associated with COMT inhibitors in the DESeq2 results as there were with progression, although these were also inconsistent across timepoints (Supplementary results: Table S6C). Among them, Bifidobacterium was more common in COMT users than non-users at follow-up.
Table 9.
Summary of differential abundance results contrasting progressed and stable PD patients.
| Baseline |
Follow-up |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative abundance (%, mean ± SD) |
p-value |
Relative abundance (%, mean ± SD) |
p-value |
||||||||
| Level | Stable | Progressed | AN-COM | Random Forests | DESeq2 | Stable | Progressed | AN-COM | Random Forests | DESeq2 | |
| Streptococcaceae | Family | 0.207 ± 0.584 | 0.614 ± 1.316 | n.s. | 0.010 | 0.001 | 0.299 ± 0.904 | 0.398 ± 0.74 | n.s. | 0.663 | 0.176 |
| Anaeroplasmataceae | Family | 0.426 ± 1.792 | 0.371 ± 1.125 | n.s. | 0.198 | 0.966 | 0.133 ± 0.596 | 0.311 ± 1.05 | n.s. | 0.010 | <0.001 |
| Prevotella | Genus | 0.964 ± 2.571 | 0.147 ± 0.493 | n.s. | 0.554 | <0.001 | 1.966 ± 4.04 | 0.187 ± 0.678 | n.s. | 0.386 | <0.001 |
| Asteroleplasma | Genus | 0.191 ± 1.022 | 0.302 ± 1.113 | n.s. | 0.455 | 0.767 | 0.133 ± 0.596 | 0.31 ± 1.051 | n.s. | 0.050 | <0.001 |
| Otu0148 Bifidobacterium | OTU | 0.06 ± 0.252 | 0.143 ± 0.498 | n.s. | 0.465 | 0.862 | 0.051 ± 0.125 | 0.744 ± 1.324 | s.s. | 0.010 | 0.330 |
| Otu0327 Lachnospiraceae unclassified | OTU | 0.013 ± 0.032 | 0.104 ± 0.282 | n.s. | 0.050 | 0.965 | 0.021 ± 0.091 | 0.178 ± 0.357 | s.s. | 0.010 | 0.016 |
| Otu0118 Ruminococcaceae unclassified | OTU | 0.084 ± 0.102 | 0.246 ± 0.221 | n.s. | 0.010 | 0.094 | 0.135 ± 0.261 | 0.165 ± 0.14 | n.s. | 0.040 | 0.838 |
| Otu0166 Ruminococcaceae unclassified | OTU | 0.069 ± 0.102 | 0.166 ± 0.203 | n.s. | 0.020 | 0.531 | 0.082 ± 0.097 | 0.194 ± 0.227 | n.s. | 0.040 | 0.603 |
| Otu0222 Phascolarctobacterium | OTU | 0.091 ± 0.281 | 0 ± 0 | n.s. | 0.436 | 0.002 | 0.043 ± 0.137 | 0 ± 0 | n.s. | 0.911 | 0.000 |
| Otu0111 Streptococcus | OTU | 0.127 ± 0.369 | 0.46 ± 1.187 | n.s. | 0.050 | 0.007 | 0.217 ± 0.643 | 0.254 ± 0.501 | n.s. | 0.743 | 0.438 |
| Otu0042 Coprococcus | OTU | 0.344 ± 1.26 | 0.002 ± 0.002 | n.s. | 0.733 | <0.001 | 0.511 ± 1.44 | 0.004 ± 0.011 | n.s. | 0.525 | 0.000 |
| Otu0268 Desulfovibrio | OTU | 0.059 ± 0.168 | 0.034 ± 0.131 | n.s. | 0.475 | <0.001 | 0.042 ± 0.102 | 0.001 ± 0.002 | n.s. | 0.792 | 0.009 |
| Otu0115 Lachnospiraceae unclassified | OTU | 0.167 ± 0.404 | 0.607 ± 2.296 | n.s. | 0.921 | 0.018 | 0.04 ± 0.086 | 0.776 ± 2.121 | n.s. | 0.010 | 0.014 |
| Otu0049 Ruminococcaceae unclassified | OTU | 0.26 ± 0.707 | 0.595 ± 0.919 | n.s. | 0.040 | 0.864 | 0.286 ± 0.663 | 0.56 ± 0.896 | n.s. | 0.030 | 0.297 |
| Otu0241 Clostridiales unclassified | OTU | 0.029 ± 0.054 | 0.044 ± 0.064 | n.s. | 0.446 | 0.698 | 0.015 ± 0.022 | 0.084 ± 0.159 | n.s. | 0.040 | 0.020 |
| Otu0084 Clostridium IV | OTU | 0.313 ± 0.978 | 0.335 ± 0.726 | n.s. | 0.485 | 0.864 | 0.071 ± 0.091 | 0.275 ± 0.345 | n.s. | 0.010 | 0.001 |
Table legend: This table includes (1) bacterial taxa that are differentially abundant at one timepoint according to more than one method, or (2) at both timepoints according to one method. Statistically significant p-values are marked in bold italic font. OTU: Operational Taxonomic Unit, SD: Standard Deviation, s.s.: statistically significant, n.s.: not significant.
Fig. 11.
Genera and families that differ significantly between stable and progressed PD patients.
Legend: Showing taxa that differ at one timepoint according to more than one method, or at both timepoints according to a single method.
When contrasting PD phenotypes (TD and PIGD), ANCOM detected no differentially abundant families or genera, and only 2 OTUs at baseline, while DESeq2 detected longer lists of taxa (Supplementary results; Table S8).
4. Discussion
Several studies have demonstrated that in healthy subjects, intersubject variability is greater than temporal variability in individuals [47,48]. Based on our results, this seems to also hold true for PD patients. In contrast to the lack of differences between timepoints, the microbial communities clearly differed between PD patients and controls, which is in line with previous studies of PD and gut microbiota: they have consistently reported differences in beta diversity between PD patients and controls [ [5], [6], [7],[10], [11], [12], [13], [14], [15], [16]].
The results of differential abundance comparison methods based on different statistical approaches can vary widely [49], offering one explanation for the varying results of the specific taxa reported in previous publications on PD and gut microbiota [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]]. Other potential sources of variation include differences in the subjects' demographics and clinical details, technical differences in the sampling, storage and sequencing protocols, and different sample sizes. Our study ranks among the larger publications when it comes to the number of subjects, but it is still possible that we have missed some subtle effects due to not having enough samples.
In the present study, we used three different tools to look for taxa that differ between PD patients and controls, and our results underlined the variation between methods: the lists of detected taxa overlapping between methods were short. The only taxa significant at both timepoints according to multiple methods were Prevotella and Prevotellaceae [6,9,12,13]. Some taxa reported to differ between PD patients and control subjects by earlier studies, such as Akkermansia [5,7,11,12] and Verrucomicrobiaceae [[5], [6], [7],11,13,15], did not differ significantly in our comparisons, while many others, including Bifidobacterium and Bifidobacteriaceae [9,11,13], Lactobacillus and Lactobacillaceae [6,11,13], Lachnospiraceae [7,11,15] and Roseburia [7,11], were inconsistent across tools and timepoints. An additional, new taxon that was detected as significant at both timepoints in our study was Puniceicoccaeae, but as this family mainly contains environmental species [50] and was present at a very low abundance (mean ± SD (%): 0·025 ± 0·065), it seems unlikely to be of interest.
The baseline samples compared in this analysis were a subset of our earlier study [6], but the new results differed from our previous ones. For example, the new analyses did not support a difference in Lactobacillaceae at baseline. As the earlier study used different PCR primers, sequencing platform, and statistical tools, it is not unexpected that the results also differ. Additionally, the baseline stool samples were stored longer and thawed twice, compared to the follow-up samples which were stored for a briefer period and only thawed once. Although we could detect no overall differences between timepoints in alpha or beta diversity comparisons, some of the variation in lists of differentially abundant taxa could relate to this.
Regarding disease progression, the differentially abundant taxa supported by more than one method did not overlap between timepoints aside from two unclassified Lachnospiraceae OTUs. According to DESeq2, Prevotella was more abundant in stable subjects at both timepoints, and Prevotellaceae at follow-up. We also contrasted patients with different disease phenotypes (TD and PIGD). Although the difference in Enterobacteriaceae between phenotypes detected in our pilot study with the metastats method [6] was significant in the resequenced baseline data when tested with DESeq2, there was no significant difference for this family at follow-up. The family Anaeroplasmataceae and the genus Asteroleplasma were significant in both progression and PD phenotype comparisons, but both taxa had values close to zero in nearly all samples, so these are likely to be false positives caused by the outlier subjects.
One previous publication on gut microbiota and PD progression, also with a 2-year follow-up period, found that stable and deteriorated patients had similar microbiota at each timepoint, although Bifidobacterium was less abundant in deteriorated patients at baseline [17]. Several taxa, including Prevotella, decreased between timepoints, and the abundances of Bifidobacterium and B. fragilis were negatively correlated to UPDRS I [17]. In our study, we saw no overall decreasing trend for Prevotella, although the genus was more abundant in stable subjects. Bifidobacterium was more abundant in COMT inhibitor users at follow-up. The previous study assessed only selected microbial taxa and did not consider the changes in medication over time in their progression classification [17]. To get a more universal measure of PD symptom severity than pure motor function, and to reduce dependency on the rater, we chose to use the sum of UPDRS I-III as a symptom severity measure for progression calculations [51]. For practical reasons, UPDRS-III assessments in medication OFF-state were only available for the follow-up timepoint of our cohort. Therefore, we chose to use UPDRS-III in medication ON-state for our calculations [51]. Thus, UPDRS scores are to some degree influenced by PD medication load. A dichotomous classification into progressed and stable, only based on this score, could falsely classify patients with progressed disease as stable e.g. if they had been undertreated at baseline, and this was compensated during the follow-up interval by overproportional medication increase. This may have influenced the results of the previous longitudinal study on microbiota in PD [17]. If disease severity is assessed in ON-state, the progression classifier must at the same time account for the PD medication load that may make motor symptom severity appear less than it actually is. The severity measure that we used to classify our patients by progression is driven by symptom severity and medication load and thus increase in either one will increase the probability of a patient being classified as a progressor. Even though not commonly used previously, our measure is based on a widely used indicator of disease severity (UPDRS I-III in ON-state) and is adjusted for medication load, which we deem essential in this context.
Bearing in mind the methodological differences, the discordant results of the two follow-up studies are unsurprising. Taken together, they seem to imply that there is no strong microbial signal associated with PD progression. However, measuring progression is challenging. A 2-year follow-up period is short, and the number of patients classified as progressed in our comparisons was low (15 patients). A longer follow-up period and a larger patient cohort might be required to observe progression-related changes in microbiota.
The lowered abundance of the family Prevotellaceae in PD was a key finding of our pilot study [6]. Considering the results of our follow-up analysis, it remains a taxon of interest. Based on our FFQ comparisons, the abundance of this taxon was not strongly affected by dietary factors. The difference in Prevotella abundance also remained significant when correcting for Rome III constipation score, although this does not entirely exclude the possibility that the results are confounded by the constipation commonly seen in PD [52]. Prevotella is a common colonizer of the human gut, and the dominant taxon in one of the three suggested enterotypes [38]. It has been linked to numerous medical conditions, its role varying from beneficial to detrimental, possibly depending on the species or strain [53]. Two PD studies have reported a lowered abundance particularly for the species P. copri [[10], [12]]. Additionally, one study found the genus less abundant in PD patients with IBS-like symptoms [1]. We have no strain level information in the present study, as 16S rRNA gene amplicon sequencing lacks the resolution for reliably defining species or strains. Hopefully, future research into the features of different Prevotella taxa will shed more light on their potential relationship to PD.
Numerous factors, such as BMI, sex, diet, and medications, can influence gut microbiota [11,18,54]. Our subjects represent a small and fairly uniform population, and patients and controls were matched for age and sex, but some differences between groups are inevitable: for example, PD patients have more GI symptoms than the general population [1,2,52], and take PD-specific medications. Additionally, stroke or TIA were more common among our controls, who also used CCBs, statins and warfarin more often than patients. Diversity comparisons suggested that CCBs might influence gut microbiota diversity, but with so few subjects using them (baseline: 15, follow-up: 17), evaluating the significance of this effect is difficult. The same was true for COMT inhibitors: they were associated with differences in beta diversity and many differentially abundant taxa but were only used by a small subset of patients (baseline: 6, follow-up: 11). Nevertheless, as they have been previously linked to microbiota changes [6,11], future studies of PD and microbiota should consider COMT inhibitors as an important confounder.
Since diet could underlie some microbiota differences detected in earlier studies, we also collected FFQ data. PC1 from a dietary PCA implied a less healthy diet for patients and was associated with beta diversity differences in single-confounder comparisons, but in multiple-confounder models it was only significant on OTU level with one test, while other variables (PD status, Rome III score, BMI) remained significant on multiple taxonomic levels with two different tests. Overall, diet did not appear to be a major confounder. Dietary fibre, which is thought to influence gut microbiota (and specifically the abundance of Prevotella), was included in all diet comparisons, with separate variables for total fibre, insoluble fibre, and soluble fibre. There were no differences in fibre consumption between PD patients and controls, and fibre was not associated with significant differences in alpha or beta diversity. Another potentially important confounder, the use of probiotics, was equally insignificant in all comparisons. This is likely to reflect the fact that our data set is small, particularly considering the semiquantitative nature of FFQs, and may not have enough statistical power to catch the microbial influences of these diet variables.
Intriguing incidental findings were the interactions between PD and BMI in relation to alpha diversity indices that include richness and evenness, and PD and stool consistency for observed richness. An inverse correlation between BMI and alpha diversity has been reported in several previous publications [[55], [56], [57]]; in our analyses, this effect was noticeably weaker in PD patients. Additionally, the inverse correlation between the BPS stool consistency scale and richness, analogous to a previously reported inverse correlation between richness and the Bristol Stool Scale [58], was also stronger in control subjects than PD patients. These results offer support for PD patients having abnormal gut microbial communities that do not follow trends seen in analyses with healthy subjects. Statistical modelling that goes beyond linear regression between alpha diversity and a few key confounders could offer further information regarding the complex relationships between PD and the other variables associated with changes in alpha diversity.
5. Conclusions
In this study, we have shown that the differences in gut microbiota of PD patients and controls persist at follow-up sampling 2 years later. As all previous studies have only included data from a single timepoint, this is an important first confirmation of replicability of such findings. Our lists of differentially abundant taxa between patients and controls included many previously reported bacteria (such as Bifidobacterium, Prevotella, Lactobacillus, and Roseburia), although the results varied considerably between statistical tools. Progressed PD patients had a Firmicutes-dominated enterotype more often than stable patients or control subjects. Additionally, Prevotella, a genus already shown to be less abundant in PD patients compared to controls, also appears less abundant in patients with faster disease progression. This further underlines the potential importance of this genus in PD. A longer follow-up period might be warranted for capturing the trends in microbial community changes during disease progression.
Acknowledgments
Acknowledgments
We would like to thank Reeta Levo for her invaluable contribution as the research nurse. We also thank the staff of the DNA sequencing and genomics laboratory, particularly Ursula Lönnqvist and Simo Soila, for performing the sequencing assays for this study, and Olli-Pekka Smolander for discussions on statistical methods.
Funding sources
This study was funded by the Michael J. Fox Foundation for Parkinson's Research, the Academy of Finland (295724, 310835), the Finnish Medical Foundation, the Finnish Parkinson Foundation, Helsinki University Hospital (T1010NL101), and Hyvinkää Hospital (M6095PEV12). None of the funding bodies had any role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Declaration of interests
VTEA has a patent FI127671B issued, a patent US10139408B2 issued, a patent US16/186,663 pending, and a patent EP3149205 pending.
PABP has a patent FI127671B issued, a patent US10139408B2 issued, a patent US16/186,663 pending, and a patent EP3149205 pending.
SV has nothing to disclose.
LP has a patent FI127671B issued, a patent US10139408B2 issued, a patent US16/186,663 pending, and a patent EP3149205 pending.
EP reports personal fees from Finnish Patient Insurance Centre, personal fees and non-financial support from Abbott, personal fees, non-financial support and other from Abbvie, non-financial support from Boston Scientific, personal fees from NordicInfu care, personal fees from Orion, personal fees from Zambon, and is the person responsible for a multicenter study organized by Abbvie, outside the submitted work.
PA reports grants from Michael J. Fox Foundation for Parkinson's Research during the conduct of the study; In addition, Dr. Auvinen has a patent FI127671B issued, a patent US10139408B2 issued, a patent US16/186,663 pending, and a patent EP3149205 pending.
FS reports grants from Finnish Parkinson Foundation, grants from Finnish Medical Foundation, grants from Academy of Finland, grants from Michael J. Fox Foundation for Parkinson's Research, grants from Helsinki University Hospital, grants from Hyvinkää Hospital during the conduct of the study; personal fees and non-financial support from Abbvie, personal fees and non-financial support from UCB, non-financial support from NordicInfu Care, non-financial support from Medtronic, personal fees and other from NeuroInnovation Oy, personal fees and non-financial support from Herantis Pharma, non-financial support from Global Kinetics Corporation, personal fees and non-financial support from Zambon, personal fees from Orion, non-financial support from Abbott, other from LivaNova, other from Axial Biotherapeutics, grants from Renishaw, outside the submitted work; In addition, Dr. Scheperjans has a patent FI127671B issued, a patent US10139408B2 issued, a patent US16/186,663 pending, and a patent EP3149205 pending.
Authors' contributions
VTEA performed all bioinformatic and statistical analyses and wrote the first draft of the manuscript. PABP assisted in designing the statistical analyses and contributed to interpreting the results. SV designed the dietary data collection and performed the initial dietary data analysis. LP was responsible for the design and execution of the amplicon sequencing. EP contributed to the study design and the clinical part of the investigation. PA participated in the conception, study design and organization of the project. FS participated in the conception and the study design, was responsible for the overall execution of the project, performed the clinical evaluations of subjects, and contributed to the statistical analyses. All authors read and approved the final manuscript.
Availability of data and material
The raw sequence data generated and analysed during the current study are available in the European Nucleotide Archive with the accession number PRJEB27564. Due to participant privacy, full clinical metadata is only available from the corresponding author upon request. The final OTU and taxonomy tables and all additional files and scripts used to run the analyses in R are also available on request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.05.064.
Appendix A. Supplementary data
Supplementary results, including dietary data analyses, additional diversity comparisons, Parkinson's disease phenotype comparisons, and supplementary tables S1-S8; Supplementary R Markdown, showing all the R code used to run the analyses and to produce the figures and tables in this manuscript.
References
- 1.Mertsalmi T.H., Aho V.T.E., Pereira P.A.B. More than constipation – bowel symptoms in Parkinson's disease and their connection to gut microbiota. Eur. J. Neurol. 2017;24(11):1375–1383. doi: 10.1111/ene.13398. [DOI] [PubMed] [Google Scholar]; Mertsalmi TH, Aho VTE, Pereira PAB, et al. More than constipation – bowel symptoms in Parkinson's disease and their connection to gut microbiota. Eur J Neurol 2017; 24(11): 1375–83. doi:10.1111/ene.13398. [DOI] [PubMed]
- 2.Fasano A., Visanji N.P., Liu L.W.C., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2015;14(6):625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]; Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol 2015; 14(6): 625–39. doi:10.1016/S1474-4422(15)00007-1. [DOI] [PubMed]
- 3.Hawkes C.H., Del Tredici K., Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33(6):599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 2007; 33(6): 599–614. doi:10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed]
- 4.Pereira P.A.B., Aho V.T.E., Paulin L., Pekkonen E., Auvinen P., Scheperjans F. Oral and nasal microbiota in Parkinson's disease. Parkinsonism Relat. Disord. 2017;38:61–67. doi: 10.1016/j.parkreldis.2017.02.026. [DOI] [PubMed] [Google Scholar]; Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Oral and nasal microbiota in Parkinson's disease. Parkinsonism Relat Disord 2017; 38: 61–7. [DOI] [PubMed]
- 5.Heintz-Buschart A., Pandey U., Wicke T. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018;33(1):88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heintz-Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 2018; 33(1): 88–98. doi:10.1002/mds.27105. [DOI] [PMC free article] [PubMed]
- 6.Scheperjans F., Aho V., Pereira P.A.B. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]; Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015; 30(3): 350–8. [DOI] [PubMed]
- 7.Keshavarzian A., Green S.J., Engen P.A. Colonic bacterial composition in Parkinson's disease. Mov. Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]; Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord 2015; 30(10): 1351–60. doi:10.1002/mds.26307. [DOI] [PubMed]
- 8.Hasegawa S., Goto S., Tsuji H. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hasegawa S, Goto S, Tsuji H, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS ONE 2015; 10(11): e0142164. [DOI] [PMC free article] [PubMed]
- 9.Unger M.M., Spiegel J., Dillmann K. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]; Unger MM, Spiegel J, Dillmann K, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord 2016; 32: 66–72. [DOI] [PubMed]
- 10.Petrov V.A., Saltykova I.V., Zhukova I.A. Analysis of gut microbiota in patients with Parkinson's disease. Bull. Exp. Biol. Med. 2017;162(6):734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]; Petrov VA, Saltykova IV, Zhukova IA, et al. Analysis of gut microbiota in patients with Parkinson's disease. Bull Exp Biol Med 2017; 162(6): 734–7. doi:10.1007/s10517-017-3700-7. [DOI] [PubMed]
- 11.Hill-Burns E.M., Debelius J.W., Morton J.T. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017;32(5):739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord 2017; 32(5): 739–49. doi:10.1002/mds.26942. [DOI] [PMC free article] [PubMed]
- 12.Bedarf J.R., Hildebrand F., Coelho L.P. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med. 2017;9:39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bedarf JR, Hildebrand F, Coelho LP, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med 2017; 9: 39. doi:10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed]
- 13.Hopfner F., Künstner A., Müller S.H. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–45. doi: 10.1016/j.brainres.2017.04.019. [DOI] [PubMed] [Google Scholar]; Hopfner F, Künstner A, Müller SH, et al. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 2017; 1667: 41–5. doi: 10.1016/j.brainres.2017.04.019. [DOI] [PubMed]
- 14.Li W., Wu X., Hu X. Structural changes of gut microbiota in Parkinson's disease and its correlation with clinical features. Sci. China Life Sci. 2017;60(11):1223–1233. doi: 10.1007/s11427-016-9001-4. [DOI] [PubMed] [Google Scholar]; Li W, Wu X, Hu X, et al. Structural changes of gut microbiota in Parkinson's disease and its correlation with clinical features. Science China Life Sciences 2017; 60(11): 1223–33. doi:10.1007/s11427-016-9001-4. [DOI] [PubMed]
- 15.Lin A., Zheng W., He Y. Gut microbiota in patients with Parkinson's disease in southern China. Parkinsonism Relat. Disord. 2018;53:82–88. doi: 10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed] [Google Scholar]; Lin A, Zheng W, He Y, et al. Gut microbiota in patients with Parkinson's disease in southern China. Parkinsonism Relat Disord 2018; 53: 82–8. doi:10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed]
- 16.Qian Y., Yang X., Xu S. Alteration of the fecal microbiota in Chinese patients with Parkinson's disease. Brain Behav. Immun. 2018;70:194–202. doi: 10.1016/j.bbi.2018.02.016. [doi:S0889-1591(18)30028-X [pii] [DOI] [PubMed] [Google Scholar]; Qian Y, Yang X, Xu S, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson's disease. Brain Behav Immun 2018; 70: 194–202. doi:S0889-1591(18)30028-X [pii]. [DOI] [PubMed]
- 17.Minato T., Maeda T., Fujisawa Y. Progression of Parkinson's disease is associated with gut dysbiosis: two-year follow-up study. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]; Minato T, Maeda T, Fujisawa Y, et al. Progression of Parkinson's disease is associated with gut dysbiosis: Two-year follow-up study. PLOS ONE 2017; 12(11): e0187307. [DOI] [PMC free article] [PubMed]
- 18.David L.A., Maurice C.F., Carmody R.N. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]; David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505(7484): 559–63. [DOI] [PMC free article] [PubMed]
- 19.Agachan F., Chen T., Pfeifer J., Reissman P., Wexner S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon Rectum. 1996;39(6):681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]; Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon rRectum 1996; 39(6): 681–5. [DOI] [PubMed]
- 20.Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]; Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130(5): 1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed]
- 21.Chaudhuri K.R., Pablo Martinez-Martin, Brown R.G. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov. Disord. 2007;22(13):1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]; Chaudhuri KR, Martinez-Martin Pablo, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Mov Disord 2007; 22(13): 1901–11. doi:10.1002/mds.21596. [DOI] [PubMed]
- 22.Lam K., Kwai Yi Lam F., Kwong Lau K. Simple clinical tests may predict severe oropharyngeal dysphagia in Parkinson's disease. Mov. Disord. 2007;22(5):640–644. doi: 10.1002/mds.21362. [DOI] [PubMed] [Google Scholar]; Lam K, Kwai Yi Lam F, Kwong Lau K, et al. Simple clinical tests may predict severe oropharyngeal dysphagia in Parkinson's disease. Mov Disord 2007; 22(5): 640–4. doi:10.1002/mds.21362. [DOI] [PubMed]
- 23.Perez Lloret S., Pirán Arce G., Rossi M., Caivano Nemet M.L., Salsamendi P., Merello M. Validation of a new scale for the evaluation of sialorrhea in patients with Parkinson's disease. Mov. Disord. 2007;22(1):107–111. doi: 10.1002/mds.21152. [DOI] [PubMed] [Google Scholar]; Perez Lloret S, Pirán Arce G, Rossi M, Caivano Nemet ML, Salsamendi P, Merello M. Validation of a new scale for the evaluation of sialorrhea in patients with Parkinson's disease. Mov Disord 2007; 22(1): 107–11. doi:10.1002/mds.21152. [DOI] [PubMed]
- 24.Sheikh J.I., Yesavage J.A. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin. Gerontol. 1986;5(1–2):165–173. [Google Scholar]; Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontologist 1986; 5(1-2): 165–73. doi:10.1300/J018v05n01_09.
- 25.Stiasny-Kolster K., Mayer G., Schäfer S., Möller J.C., Heinzel-Gutenbrunner M., Oertel W.H. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov. Disord. 2007;22(16):2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]; Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—A new diagnostic instrument. Mov Disord 2007; 22(16): 2386–93. doi:10.1002/mds.21740. [DOI] [PubMed]
- 26.Boesveldt S., de Muinck Keizer R.O., Knol D.L., Wolters E.C., Berendse H.W. Extended testing across, not within, tasks raises diagnostic accuracy of smell testing in Parkinson's disease. Mov. Disord. 2009;24(1):85–90. doi: 10.1002/mds.22325. [DOI] [PubMed] [Google Scholar]; Boesveldt S, de Muinck Keizer RO, Knol DL, Wolters EC, Berendse HW. Extended testing across, not within, tasks raises diagnostic accuracy of smell testing in Parkinson's disease. Mov Disord 2009; 24(1): 85–90. doi:10.1002/mds.22325. [DOI] [PubMed]
- 27.Pietinen P., Hartman A.M., Haapa E. Reproducibility and validity of dietary assessment instruments. II. A qualitative food frequency questionnaire. Am. J. Epidemiol. 1988;128(3):667–676. doi: 10.1093/oxfordjournals.aje.a115014. [DOI] [PubMed] [Google Scholar]; Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. II. A qualitative food frequency questionnaire. Am J Epidemiol 1988; 128(3): 667–76. [DOI] [PubMed]
- 28.Fahn S., Elton R.L. Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S., Marsden C.D., Goldstein M., Calne D.B., editors. Recent Developments in Parkinsons Disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163. Vol. 2 ed. [Google Scholar]; Fahn S, Elton RL, Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinsons Disease. Vol. 2 ed. Florham Park, NJ: Macmillan Healthcare Information; 1987. p.153–63.
- 29.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]; Tomlinson CL, Rebecca S, Smitaa P, Caroline R, Richard G, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010; 25(15): 2649–53. doi:10.1002/mds.23429. [DOI] [PubMed]
- 30.Jankovic J., McDermott M., Carter J. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]; Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990; 40(10): 1529–34. [DOI] [PubMed]
- 31.Hawley P., Barwich D., Kirk L. Implementation of the Victoria bowel performance scale. J. Pain Symptom Manag. 2011;42:946–953. doi: 10.1016/j.jpainsymman.2011.02.021. [DOI] [PubMed] [Google Scholar]; Hawley P, Barwich D, Kirk L. Implementation of the Victoria Bowel Performance Scale. Journal of Pain and Symptom Management 2011; 42: 946–53. [DOI] [PubMed]
- 32.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12. [Google Scholar]; Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011; 17(1): 10–2.
- 33.Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79(17):5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013; 79(17): 5112–20. doi:10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed]
- 34.Schloss P.D. mothur: Standard Operating Procedure for MiSeq sequenced 16S data. 2017. https://www.mothur.org/wiki/MiSeq_SOP Available at. Accessed April 12, 2017.; Schloss PD. mothur: Standard Operating Procedure for MiSeq sequenced 16S data. 2017; Available at: http://www.mothur.org/wiki/Schloss_SOP. Accessed April 12, 2017.
- 35.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and environment for statistical computing. [Google Scholar]; R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
- 36.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal. Stat. Soc. 1995;57(1):289–300. [Google Scholar]; Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc 1995; 57(1): 289–300.
- 37.McMurdie P.J., Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061217. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]; McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8(4): e61217. doi:10.1371/journal.pone.0061217 [doi]. [DOI] [PMC free article] [PubMed]
- 38.Costea P.I., Hildebrand F., Manimozhiyan A. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018;3(1):8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Costea PI, Hildebrand F, Manimozhiyan A, et al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 2018; 3(1): 8–16. doi:10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed]
- 39.Oksanen J., Blanchet F.G., Kindt R. 2015. Vegan: Community Ecology Package. 2.3–2. [Google Scholar]; Oksanen J, Blanchet FG, Kindt R, et al. vegan: Community Ecology Package. 2015; 2.3-2.
- 40.Mandal S., Van Treuren W., White R.A., Eggesbø M., Knight R., Peddada S.D. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015;26 doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 2015; 26. doi:10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed]
- 41.Mandal S. ANCOM 2: Updated R code. 2018. https://sites.google.com/site/siddharthamandal1985/research Available from. 2018.; Mandal S. ANCOM 2: updated R code. 2018; Available at: https://sites.google.com/site/siddharthamandal1985/research, 2018.
- 42.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15(12): 550. [DOI] [PMC free article] [PubMed]
- 43.Breiman L. Random forests. Mach. Learn. 2001;45(1):5–32. [Google Scholar]; Breiman L. Random Forests. Mach Learning 2001; 45(1): 5–32. doi:10.1023/A:1010933404324.
- 44.Liaw A., Wiener M. Classification and regression by randomForest. R News. 2002;2(3):18–22. [Google Scholar]; Liaw A, Wiener M. Classification and regression by randomForest. R News 2002; 2(3): 18–22.
- 45.Murphy M.A., Evans J.S., Storfer A. Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology. 2010;91(1):252–261. doi: 10.1890/08-0879.1. [DOI] [PubMed] [Google Scholar]; Murphy MA, Evans JS, Storfer A. Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 2010; 91(1): 252–61. [DOI] [PubMed]
- 46.rfPermute Archer E. Estimate Permutation p-values for Random Forest Importance Metrics. https://CRAN.R-project.org/package=rfPermute R package version 2.1.5. 2016; Available from.; Archer E. rfPermute: Estimate permutation p-values for random forest importance metrics. R package version 2.1.5. 2016; Available at: https://CRAN.R-project.org/package=rfPermute. Accessed 01/15, 2018.
- 47.Rajilić-Stojanović M., Heilig H.G.H.J., Tims S., Zoetendal E.G., de Vos W.M. Long-term monitoring of the human intestinal microbiota composition. Environ. Microbiol. 2013;15(4):1146–1159. doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]; Rajilić-Stojanović M, Heilig HGHJ, Tims S, Zoetendal EG, de Vos WM. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol 2013; 15(4): 1146–59. doi:10.1111/1462-2920.12023. [DOI] [PubMed]
- 48.Flores G.E., Caporaso J.G., Henley J.B. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 2014;15(12):531. doi: 10.1186/s13059-014-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Flores GE, Caporaso JG, Henley JB, et al. Temporal variability is a personalized feature of the human microbiome. Genome Biol 2014; 15(12): 531. doi:10.1186/s13059-014-0531-y. [DOI] [PMC free article] [PubMed]
- 49.Weiss S., Xu Z.Z., Peddada S. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome. 2017;5(1):27. doi: 10.1186/s40168-017-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Weiss S, Xu ZZ, Peddada S, et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017; 5(1): 27. doi:10.1186/s40168-017-0237-y. [DOI] [PMC free article] [PubMed]
- 50.Yoon J. The family Puniceicoccaceae. In: Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes: Other Major Lineages of Bacteria and the Archaea. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. pp. 847–848. [Google Scholar]; Yoon J. The Family Puniceicoccaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes: Other Major Lineages of Bacteria and The Archaea. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. p.847–8.
- 51.Maetzler W., Liepelt I., Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. Lancet Neurol. 2009;8(12):1158–1171. doi: 10.1016/S1474-4422(09)70291-1. [DOI] [PubMed] [Google Scholar]; Maetzler W, Liepelt I, Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. Lancet Neurol 2009; 8(12): 1158–71. doi:10.1016/S1474-4422(09)70291-1. [DOI] [PubMed]
- 52.Knudsen K., Fedorova T.D., Bekker A.C. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson's disease: a colon transit and volume study. J. Park. Dis. 2017;7(2):359–367. doi: 10.3233/JPD-161050. doi. [DOI] [PubMed] [Google Scholar]; Knudsen K, Fedorova TD, Bekker AC, et al. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson's disease: A colon transit and volume study. J Parkinsons Dis 2017; 7(2): 359–67. doi:10.3233/JPD-161050 [doi]. [DOI] [PubMed]
- 53.Ley R.E. Prevotella in the gut: choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016;13(2):69–70. doi: 10.1038/nrgastro.2016.4. [DOI] [PubMed] [Google Scholar]; Ley RE. Prevotella in the gut: choose carefully. Nature Reviews Gastroenterology & Hepatology 2016; 13(2): 69–70. [DOI] [PubMed]
- 54.Davis S.C., Yadav J.S., Barrow S.D., Robertson B.K. Gut microbiome diversity influenced more by the westernized dietary regime than the body mass index as assessed using effect size statistic. MicrobiologyOpen. 2017;6(4) doi: 10.1002/mbo3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis SC, Yadav JS, Barrow SD, Robertson BK. Gut microbiome diversity influenced more by the Westernized dietary regime than the body mass index as assessed using effect size statistic. MicrobiologyOpen 2017; 6(4): e00476. doi:10.1002/mbo3.476. [DOI] [PMC free article] [PubMed]
- 55.Chen J., Ryu E., Hathcock M. Impact of demographics on human gut microbial diversity in a US Midwest population. PeerJ. 2016;4 doi: 10.7717/peerj.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen J, Ryu E, Hathcock M, et al. Impact of demographics on human gut microbial diversity in a US Midwest population. PeerJ 2016; 4: e1514. doi:10.7717/peerj.1514. [DOI] [PMC free article] [PubMed]
- 56.Turnbaugh P.J., Hamady M., Yatsunenko T. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]; Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009; 457(7228): 480–4. [DOI] [PMC free article] [PubMed]
- 57.Verdam F.J., Fuentes S., de Jonge C. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21(12):E607–E615. doi: 10.1002/oby.20466. [DOI] [PubMed] [Google Scholar]; Verdam FJ, Fuentes S, de Jonge C, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013; 21(12): E607-15. doi:10.1002/oby.20466. [DOI] [PubMed]
- 58.Vandeputte D., Falony G., Vieira-Silva S., Tito R.Y., Joossens M., Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65(1):57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016; 65(1): 57–62. doi:10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary results, including dietary data analyses, additional diversity comparisons, Parkinson's disease phenotype comparisons, and supplementary tables S1-S8; Supplementary R Markdown, showing all the R code used to run the analyses and to produce the figures and tables in this manuscript.
Data Availability Statement
The raw sequence data generated and analysed during the current study are available in the European Nucleotide Archive with the accession number PRJEB27564. Due to participant privacy, full clinical metadata is only available from the corresponding author upon request. The final OTU and taxonomy tables and all additional files and scripts used to run the analyses in R are also available on request.