Abstract
The importance of the commensal microbiota to human health and well-being has become increasingly evident over the past decades. From a therapeutic perspective, the popularity of fecal microbiota transplantation (FMT) to restore a disrupted microbiota and amend imbalances has increased. To date, most clinical experience with FMT originates from the treatment of recurrent or refractory Clostridioides difficile infections (rCDI), with resolution rates up to 90%. In addition to CDI, a role for the intestinal microbiome has been implicated in several disorders. FMT has been tested in several randomized controlled trials for the treatment of inflammatory bowel disease, irritable bowel disease and constipation with mixed results. FMT has also been explored for extra-gastrointestinal disorders such as metabolic syndrome, hepatic encephalopathy and graft-versus-host disease. With the exception of recurrent CDI, FMT is currently used in experimental settings only and should not yet be offered as standard care. In addition, it is critical to further standardize and optimize procedures for FMT preparation. This includes determination of active components of FMT to develop (personalized) approaches to treat disease.
Keywords: Fecal microbiota transplantation, Clostridioides difficile, Inflammatory bowel disease, Irritable bowel syndrome, Metabolic syndrome, Cancer
Abbreviations: ACG, American College of Gastroenterology; ARBs, Antibiotic resistant bacteria; ASD, Autism spectrum disorders; CD, Crohn's disease; CDI, Clostridioides difficile infection; CIPO, Chronic intestinal pseudo-obstruction; CRC, Colorectal cancer; CRE, Carbapenem-resistant Enterobacteriaceae; DJ, Duodenal/jejunal infusion; ESBL, Extended-spectrum beta-lactamase-producing; ESBL-E, Extended Spectrum Beta-Lactamase Enterobacteriaceae; ESCMID, European Society for Microbiology and Infectious disease; FMT, Fecal microbiota transplantation; GI, Gastrointestinal; GVHD, Graft-versus-host disease; HCT, Hematopoietic stem cell transplantation; HE, Hepatic encephalopathy; IBD, Inflammatory bowel disease; IBS, Irritable bowel syndrome; IBS-SSS, Irritable bowel syndrome severity scoring system; ICI, Immune checkpoint inhibitors; MS, Multiple sclerosis; NAFLD, Nonalcoholic fatty liver disease; NASH, Nonalcoholic steatohepatitis; PD, Parkinson's disease; rCDI, Recurrent Clostridioides difficile infection; RCT, Randomized controlled trial; SAE, Serious adverse event; SCFA, Short-chain fatty acid; STC, Slow transit constipation; TMAO, Trimethylamine-N-Oxide; UC, Ulcerative colitis; VRE, Vancomycin-resistant Enterococcus
1. Introduction
The human body harbors on average 10–100 trillion microbes, which is more than ten times the estimated number of human cells [1]. The majority of these microbial cells reside in the gastrointestinal (GI) tract and this complex community of microorganisms in the GI tract is termed the intestinal microbiota. The historical view that the gut microbiota is largely pathogenic has undergone a paradigm shift. Over the past few decades, the importance of the commensal microbiota to human health and well-being has become increasingly evident, as the impact of a healthy and diverse intestinal microbiota on metabolic activities, the immune system and homeostasis of the intestine has become more clear [2]. Furthermore, it has been shown that the gut microbiota influences the gut-brain axis, affecting brain function and development, and to confer colonization resistance against pathogenic bacteria [3,4].
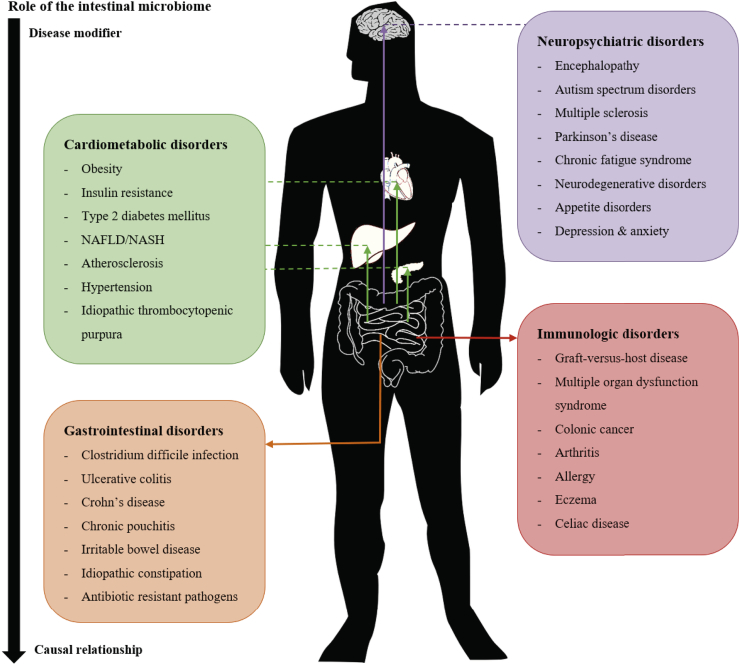
Dysbiosis of the intestinal microbiota is defined as decreased bacterial diversity or a shift in bacterial species compared to a healthy control, e.g. a decrease in butyrate producers [5]. Many studies have shown that this dysbiosis is implicated in the development of a wide range of diseases (Fig. 1) [5,6]. However, for most diseases it is currently unknown whether the changes in microbiota are causally related to the pathophysiology or merely a consequence of the disease. For Clostridioides difficile infection (CDI), there is a clear causal relationship with disease phenotype. For other diseases such as obesity and metabolic disease, a causal relationship still needs to be clarified [7]. In both scenarios, however, modulation of the intestinal microbiota to restore a balanced and diverse microbiota might hold merit to treat or prevent microbiome-related disease.
Fig. 1.
associations between the intestinal microbiome and disease. At this moment, for most diseases it is not known whether the microbiota is causally related or merely a result of the pathophysiology. Abbreviations: NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis [6,8,102].
Fecal microbiota transplantation (FMT), also called “feces transplantation”, “human intestinal microbiota transfer” and “fecal bacteriotherapy”, is the transfer of the fecal microbiota from a healthy, screened donor to a recipient [8]. FMT aims to restore a disrupted microbiota and amend imbalances through establishment of a stable, complex microbiota. The earliest documented administration of a fecal suspension was by the traditional Chinese doctor Ge Hong in the 4th century [9]. He used so-called ‘yellow soup’ as a treatment for food poisoning and severe diarrhea. However, it wasn't until the 16th century that another Chinese doctor named Li Shizhen recorded a range of fecal preparations for effective treatment of GI-diseases, such as constipation, fever, vomiting and pain. Subsequently during World War II, African Bedouins advised German soldiers stationed in Africa to consume fresh camel feces as a treatment for bacterial dysentery [10]. Although the potential health benefits of microbes were already mentioned by Metchnikoff in 1907, it wasn't until 1958 that fecal enemas were first described for the treatment of pseudomembranous enterocolitis by Dr. Ben Eiseman, an American surgeon [11]. Thereafter, a plethora of articles on the potential of FMT to treat recurrent CDI (rCDI) have been written. In this review, the potential of FMT beyond treatment of CDI and the current evidence in support of FMT as a therapeutic approach will be discussed.
2. Clostridioides difficile infection
Currently, most clinical experience with FMT is derived from the treatment of CDI, in particular recurrent or refractory infections [12]. Over the past decades, the incidence of CDI has risen, while the success rate of prolonged anti-microbial therapy is low (20–30% resolution rate) [13]. FMT has emerged as an important treatment option for rCDI with high resolution rates (up to 90%) [[13], [14], [15]]. Over 100 case reports and clinical trials on the treatment of rCDI with FMT have been published to date; most report high resolution rates of C. difficile associated diarrhea. The first randomized controlled clinical trial (RCT) for FMT in CDI was performed in the Netherlands by Van Nood et al. In this study, authors observed a primary and cumulative resolution of 81% and 94% after one and two FMTs, respectively, compared to 31% after a vancomycin regimen [16].
Subsequently, the number of RCTs addressing the use of healthy donor (allogenic) FMT to treat rCDI has increased. In several publications, FMT via colonoscopy has been shown to be superior to fidaxomicin, vancomycin and autologous FMT [[17], [18], [19]]. The cumulative resolution rate after FMT via colonoscopy was over 90% compared to 42% for fidaxomicin, <30% for vancomycin and 63% for autologous FMT [[17], [18], [19]]. Comparison of nasogastric and colonoscopic administration of a freeze-thawed fecal suspension could not demonstrate a significant difference in resolution rate (both 90%), although the patient groups in this RCT were fairly small [20]. One RCT showed that freeze-thawed feces was as effective as fresh feces, both administrated via enema, with resolution rates of 75% and 70% respectively [21]. In contrast, another RCT reported higher resolution rates with fresh FMT via colonoscopy compared to freeze-thawed and lyophilized FMT via colonoscopy, with resolution rates of 100%, 83% and 78%, respectively [22]. However, two RCTs found oral FMT capsules with either frozen or lyophilized fecal microbiota to be as effective as FMT via enema or colonoscopy, with resolution rates around 90% [23,24]. Recently, two small pilot RCTs evaluated the efficacy of FMT for primary CDI instead of rCDI with mixed results [25,26]. One study observed a higher primary resolution with FMT compared to metronidazole, while vancomycin performed better than FMT in the second study [25,26].
Several meta-analyses have confirmed the superiority of FMT over standard antibiotic treatment and indicated that FMT is a safe treatment for patients with rCDI [15,27,28]. In addition, colonoscopic delivery of FMT was associated with higher resolution rates, while duodenal infusion, enema and fecal amount < 50 g were associated with lower resolution rates [29]. Cost-effectiveness analyses have shown that FMT by colonoscopy (or enema, if colonoscopy is unavailable) is cost-effective, as the FMT procedure is relatively cheap and has a high efficacy [30,31]. The above findings have established FMT as an evidence-based treatment option for rCDI, which has been adopted by the European Society for Microbiology and Infectious disease (ESCMID) and the American College of Gastroenterology (ACG) (see Table 1) [32,33].
Table 1.
current and potential indications for fecal microbiota transplantation.
| Current indication | Studies | Quality of Evidence | Outcome |
|---|---|---|---|
| Recurrent Clostridioides difficile infection | >10 RCTs [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]] Meta-analyses [15,[27], [28], [29]] |
High | Highly effective, with resolution rates around 90% |
| Potential future indication | |||
| Gastrointestinal disorders | |||
| -Ulcerative colitis | 4 RCTs [34,[37], [38], [39]] Meta-analyses [36,40] |
Moderate | Clinical remission around 36–37% |
| -Crohn's disease | Cohort studies [[42], [43], [44]] Meta-analyses [36] |
Low | Clinical remission around 50–57%; decrease over time |
| -Irritable bowel syndrome | 2 RCTs [47,48] | Low | Mixed results |
| -Slow-transit constipation | 1 RCT [49] | Low | Clinical remission around 37%; decrease over time |
| -Antibiotic resistant bacteria | Cohort studies [[53], [54], [55], [56], [57]] open-label RCT [58] | Low | Promising results on decolonization of ESBL-producers, VREs and CREs |
| Metabolic disorders | |||
| -Metabolic syndrome | 2 RCTs [61,62] | Low | Increased insulin sensitivity, but no effect on clinical endpoints |
| -Cardiovascular disease | 1 RCT [68] | Low | Enrichment of SCFA-producers, but no clinical effect |
| Neuropsychiatric disorders | |||
| -Hepatic encephalopathy | 1 RCT [70] | Low | No new episodes of HE and fewer SAE's |
| -Autism spectrum disorder | Cohort study [74] | Low | Decrease in gastrointestinal and neurologic symptoms |
| Immunologic disorders | |||
| -Graft-versus-host disease | Cohort study [86] | Low | Overall survival and progression-free survival of 85% |
The above disorders were only listed if there was at least a cohort study published. Abbreviations: RCT = randomized controlled trial, ESBL = extended-spectrum beta-lactamase, VRE = vancomycin-resistant Enterococcus, CRE = carbapenem-resistant Enterobacteriaceae, SCFA = short-chain fatty acid, HE = hepatic encephalopathy, SAE = serious adverse event [103].
3. Gastrointestinal disorders
3.1. Inflammatory bowel disease
Inflammatory bowel disease (IBD) comprises ulcerative colitis (UC) and Crohn's disease (CD). Both are characterized by recurring inflammation of the intestine. While UC is restricted to the large intestine, CD can affect the entire gastrointestinal tract. Both pathologies have been linked to dysbiosis of the gut microbiota, with a decreased diversity and decreases in Bacteroidetes and Firmicutes [34]. However, it remains unclear whether these shifts are a cause or a consequence of IBD. Some animal studies support a role for the gut microbiota in the pathogenesis of IBD, demonstrating that intestinal exposure to colitogenic microbiota induced spontaneous colitis [35]. Based on these findings, restoration of the gut microbiota through FMT has been explored as a treatment for IBD in several clinical trials.
3.2. Ulcerative colitis
Promising case reports and uncontrolled observational cohort studies have been published on the treatment of IBD with FMT, although the response rate to FMT is lower compared to rCDI. Of these studies, 53 were summarized in a systematic review, demonstrating clinical remission in 201/555 (36%) of UC, 42/83 (51%) of CD and 5/23 (22%) of pouchitis patients after FMT [36]. To date, four RCTs on the treatment of UC using FMT have been published, all showing promising results [34,[37], [38], [39]]. These results were pooled in a recent Cochrane meta-analysis, which demonstrated a significantly higher clinical remission at eight weeks in the FMT arm compared to the control arm, with 52/140 (37%) and 24/137 (18%) patients achieving remission, respectively [40]. Serious adverse events and adverse events did not significantly differ between groups. Importantly, methodology, FMT strategies and primary endpoints varied considerably among the RCTs. This exemplifies the necessity of additional dedicated studies.
Some studies indicated that the efficacy of a fecal transplant differed between transplant donors. These observations gave rise to the concept of super donors and highlight the importance of careful donor selection [37,38]. Furthermore, an enrichment in Eubacterium hallii and Roseburia inulivorans with increased levels of short-chain fatty acids and secondary bile acids was consistently found in patients in remission after FMT [41]. In addition, in patients that did not achieve remission, an enrichment in Fusobacterium gonidiaformans, Sutterella wadsworthensis, and Escherichia species was observed, characterized by increased levels of heme and lipopolysaccharide biosynthesis.
3.3. Crohn's disease
Evidence for FMT to treat CD is sparse and to date no RCTs have been published. In a meta-analysis of 11 uncontrolled observational cohort studies and case series in CD, 42/83 (51%) patients achieved clinical remission [36]. A prospective study observed clinical remission in 13/25 (52%) CD patients three months after FMT, which decreased to 5/22 (23%) 18 months after FMT [42]. Another study demonstrated that a second FMT, administered within 4 months after the first FMT, maintained the clinical benefits of the first FMT [43]. The largest prospective cohort study published to this day found a clinical remission in 79/139 (57%) patients one month after FMT and observed mild adverse events in 14% of patients one month after FMT [44]. However, the potential of FMT to treat CD is still uncertain and well-designed controlled studies addressing this question are needed. Compared to UC, CD is a more heterogeneous disease and it might be necessary to focus on specific disease phenotypes, instead of a general CD population.
3.4. Functional bowel disease
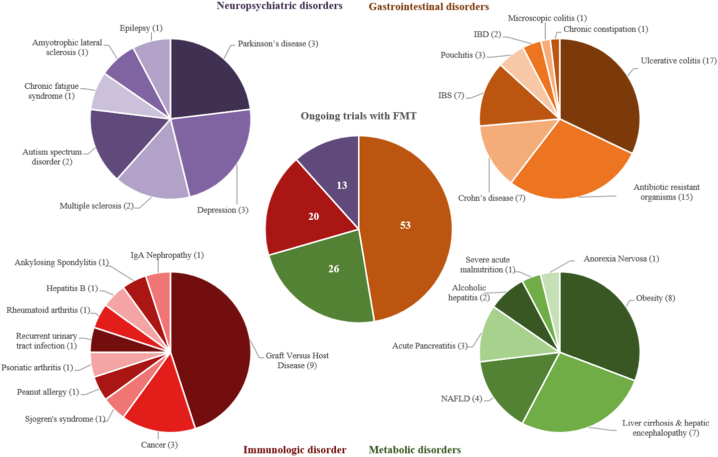
The efficacy of FMT is actively being explored in functional GI disorders, in particular irritable bowel syndrome (IBS) and chronic constipation. IBS is a chronic noninflammatory GI disorder, characterized by abdominal pain with diarrhea and/or constipation. Although the pathophysiology of IBS is not completely understood, several associations with the intestinal microbiota have been found. In line, this suggests a potential role for FMT to treat IBS [45,46]. Two RCTs were recently published showing conflicting results. In the first study, 36/55 (65%) patients in the FMT group had relief of IBS symptoms (>75 points reduction on IBS severity scoring system (IBS-SSS)) three months after a single FMT via colonoscopy compared to 12/28 (43%) patients in the placebo group [47]. The second study reported a larger reduction in IBS-SSS (−125·71) in the placebo group (n = 23) after three months compared to the FMT group (n = 22) that received FMT-capsules for 12 days (−52·45) [48]. Although diversity of the microbiota increased in patients receiving FMT capsules, clinical improvement of IBS symptoms was not achieved. Discrepancies in study outcomes might originate from the different FMT administration strategies or the included IBS-subtypes. Results of several ongoing RCTs (see Fig. 2) will further disentangle the therapeutic potential of FMT to treat IBS.
Fig. 2.
overview of ongoing trials in which the efficacy of FMT is tested for a range of diseases. Only intervention studies were included that were registered as ‘active’, ‘recruiting’, ‘enrolling via invitation’ or ‘not yet recruiting’. In addition, studies examining the effect of FMT in treating CDI were excluded, since this is out of the scope of this review. Table 2 gives a more detailed overview of the ongoing studies. Abbreviations: FMT = fecal microbiota transplantation, IBD = Inflammatory Bowel Disease, IBS = Irritable Bowel Syndrome, NAFLD = Non-Alcoholic Fatty Liver Disease.
The gut microbiota has also been implicated in the etiology of constipation. In one RCT, 60 adults with slow transit constipation (STC) received standard of care treatment (education, behavioral strategies, and oral laxatives) either with or without an additional 6 days FMT by nasoduodenal infusion [49]. After 12 weeks, clinical improvement was observed in 16/30 (53%) versus 6/30 (20%) patients and clinical cure (≥ three complete spontaneous bowel movements per week) in 11/30 (37%) versus 4/30 (13%) patients in the FMT and control group, respectively. Two prospective studies from the same group showed a decrease in clinical cure rate over time and observed higher efficacy of FMT in combination with the polysaccharide pectin in patients with STC [50,51]. A third prospective trial reported alleviation of bloating and pain symptoms in patients with chronic intestinal pseudo-obstruction (CIPO), a serious life-threatening motility disorder [52]. Although these results are promising, the FMT treatments were quite intense and invasive, with patients receiving up to 18 nasoduodenal FMTs over three months [51]. These limitations of dosing frequency and nasal tube placement could be addressed by using FMT capsules. Furthermore, the efficacy of the fecal microbiota in the FMT can be debated as the glycerol, used to protect the microbiota from freezing, could have a laxative effect.
3.5. Antibiotic-resistant bacteria
An increasing healthcare threat is intestinal colonization with multidrug-resistant organisms, which may cause life-threatening infections. Through direct ecological competition, FMT may potentially stimulate decolonization of antibiotic-resistant bacteria (ARBs) and increase resistance to colonization by these pathogens. This was first described in a case report where FMT was used for the successful eradication of an extended-spectrum beta-lactamase-producing (ESBL) Escherichia coli [53]. Thereafter, many case reports and small prospective cohort studies have been published, showing efficacy of FMT in decolonization of ESBL-producers, vancomycin-resistant Enterococcus (VRE), carbapenem-resistant Enterobacteriaceae (CRE) and other ARBs [[54], [55], [56], [57]]. One open-label RCT has been published, which demonstrated decolonization of CRE or ESBL producers in 9/22 (41%) patients who received a five-day course of oral antibiotics followed by FMT compared to 5/17 (29%) patients who didn't receive an intervention [58]. Interestingly, two prospective studies found a higher decolonization effect of FMT in the absence of periprocedural use of antibiotics, reporting decolonization in 6/7 (79%) and 7/8 (88%) patients after FMT [56,57]. Although the number of treated patients is small, these studies show that FMT might be an effective therapy for decolonization of antibiotic-resistant organisms from the GI-tract and more trials are currently under way to assess its safety and efficacy (see Fig. 2).
4. Metabolic disorders
4.1. Metabolic syndrome
There has been an increasing interest in the role of the gut microbiota in metabolic diseases, as microbes play a crucial role in digestion and absorption of nutrients from the diet. Furthermore, gut bacteria produce metabolites with critical properties for host metabolism including -but not limited to- short-chain fatty acids (SCFA) and bile acids. Dysbiosis of the intestinal microbiota has been linked to an impaired mucosal barrier function, also known as a “leaky gut”, a proinflammatory state and a disturbed production of signaling molecules, such as SCFAs and bile acids [59]. Animal studies suggest a causal link between the intestinal microbiota and obesity. For example, mice colonized with obesogenic microbiota were shown to have increased body fat and insulin resistance compared to mice colonized with lean donor microbiota [60]. Currently, two placebo-controlled RCTs have been published, which determined the effect of nasoduodenal FMT in obese Caucasian males with metabolic syndrome [61,62]. Six weeks after nasoduodenal infusion of lean donor feces, insulin sensitivity was significantly increased. This coincided with an increase in butyrate-producing intestinal microbiota. Importantly, the effect on insulin sensitivity disappeared after 18 weeks and no long-term clinical effects were found. In addition, metabolic response to FMT was found to be associated with a low microbial diversity at baseline [62]. Although the mechanisms underlying the favorable effects on insulin sensitivity are yet to be determined, these studies highlight a role for the intestinal microbiota in metabolic diseases.
4.2. NAFLD and NASH
Nonalcoholic fatty liver disease (NAFLD) is characterized by accumulation of fat in the liver, which may lead to inflammation and liver damage, commonly known as nonalcoholic steatohepatitis (NASH). NASH is a major cause of liver cirrhosis and liver cancer; both primary indications for liver transplantation. Differences in microbiota composition have been observed in patients with NAFLD or NASH compared to subjects with healthy liver [59]. In addition, increased intestinal permeability and a proinflammatory environment in the gut are frequently observed in NAFLD/NASH patients [63]. Although human studies are yet to be performed, studies in high-fat diet-fed mice found that FMT reduced weight gain and nonalcoholic fatty liver score [64]. To further investigate the potential of FMT in NASH there are several RCTs underway (summarized in Fig. 2), determining the efficacy of FMT compared to standard therapy in NASH related cirrhosis.
4.3. Cardiovascular disease
Accumulating evidence has implicated a role of the intestinal microbiota and microbial metabolites in the development of cardiovascular disease such as atherosclerosis and hypertension [65]. In an animal model, the introduction of a proinflammatory microbiota low in SCFA-producers enhanced systemic inflammation and accelerated atherogenesis [66]. In another study, mice with myocarditis were subjected to FMT. This resulted in reshaping of the microbiota composition and restoration of the Bacteroidetes population, which was accompanied by attenuation of myocarditis through reduced inflammatory infiltration of immune cells [67]. Currently, one small RCT in humans has addressed the effect of a single lean vegan-donor FMT on vascular inflammation and trimethylamine-N-Oxide (TMAO) production. TMAO is a microbial metabolite which increases atherosclerotic burden and stimulates a prothrombotic phenotype [68]. Although SCFA-producers were significantly enriched in the allogenic FMT group, no differences were detected in TMAO production or vascular inflammation at two weeks.
5. Neuropsychiatric disorders
5.1. Hepatic encephalopathy
Hepatic encephalopathy (HE) comprises a spectrum of neuropsychiatric abnormalities as a result of end-stage liver cirrhosis. HE has been associated with differences in the microbiota and an increased relative abundance of ammonia-producing bacteria [69]. Subsequent hyperammonemia is associated with impaired neuronal function [69]. Current treatment strategies for HE consist of lactulose supplementation and treatment with the nonabsorbable antibiotic rifaximin, which both influence the intestinal microbiota. Currently, one RCT has been published in which patients with HE were treated with a single FMT via enema in addition to standard of care treatment [70]. Fewer serious adverse events (2 versus 8) and new episodes of HE (0 versus 6) were observed in the FMT arm (n = 10) compared to the control group (n = 10) receiving solely standard of care. Furthermore, cognition and dysbiosis improved after FMT. In a small pilot study, eight patients with steroid-ineligible severe alcoholic hepatitis were treated with a nasoduodenal FMT for seven days [71]. Liver disease severity reduced and coincided with resolution of ascites and HE. Moreover, survival rate improved compared to historically matched controls. These studies show promising results and multiple clinical studies addressing the use of FMT in HE are underway (see Fig. 2).
5.2. Autism spectrum disorder
Autism spectrum disorders (ASD) are not only characterized by impairments in social interaction and communication, but often coincide with GI symptoms such as constipation or diarrhea [72]. Experiments in ASD mouse models have mechanistically linked the gut microbiota to abnormal metabolites and behavior [73]. To this day, one open-label study has explored the effect of FMT on GI and ASD symptoms in children aged 7–16 years [74]. 18 children were placed on a two-week antibiotic regimen, a bowel lavage and either an initial rectal or oral high FMT dose, followed by a daily lower oral maintenance dose for 7–8 weeks. After FMT, both GI and ASD symptoms significantly improved, which persisted for 8 weeks after treatment. These are promising results, although the potential causal contributions of the gut microbiota to ASD remain speculative.
5.3. Multiple sclerosis
A number of studies have shown intestinal microbiota dysbiosis in patients with multiple sclerosis (MS), a chronic inflammatory disease of the central nervous system [75]. Additionally, animal models have shown that feces from patients with MS could precipitate an MS-like autoimmune disease in mice, which suggests microbiota involvement in the pathogenesis of MS. [76] Some case reports describe improvement of neurological symptoms and disease stability after FMT, although more research is needed to determine the benefit and safety of FMT in MS. [77,78]
6. Parkinson's disease
Also for Parkinson's disease (PD) intestinal dysbiosis has been reported, with decreases in Prevotella and butyrate-producing bacteria [79]. An observational study found a decrease in total count of gut microbiota during PD progression and changes in gut microbiota could be correlated with a rapid or slow disease progression [79]. In a mouse model, gut microbiota transplantation from donor mice with PD reduced striatal neurotransmitter release with subsequent motor impairment in healthy recipient mice [80]. Furthermore, healthy mouse donor FMT had neuroprotective effects in PD mice through suppression of neuroinflammation and reduction of TLR4/TNF-α signaling [80]. FMT could have a potential benefit in PD, but studies in humans have not been performed yet.
7. Immunologic disorders
7.1. Graft-versus-host disease
Acute intestinal graft-versus-host disease (GVHD) is a major cause of mortality in patients that receive an allogenic hematopoietic stem cell transplantation (HCT). Standard treatment consists of immunosuppressive steroids, although some patients develop steroid-refractory GVHD for which no well-established treatment is available. Growing evidence suggests that the risk of GVHD is influenced by host-microbiota interactions and one study observed an increased mortality in recipients with a lower phylogenetic diversity [81]. In a mouse model, alterations in intestinal microbiota following HCT resulted in a decreased butyrate production, potentially contributing to a proinflammatory state of the intestine [82]. Results from several case series on FMT to treat acute steroid-refractory GVHD have shown some promise, demonstrating resolution of clinical symptoms, restoration of microbiota composition and a higher progression-free survival [[83], [84], [85]]. In a recent prospective open-label study, 13 patients were treated with FMT capsules to restore their intestinal microbiome diversity at a median of 27 days after HCT [86]. During a median follow-up period of 15 months after FMT, two patients developed acute GI GVHD which resulted in one death. The 12-month overall survival and progression-free survival were both 85%. Although the results of FMT in GVHD are promising, larger controlled studies are needed.
7.2. Cancer
Microbial dysbiosis has been extensively observed in human malignancies [87]. Several bacterial species are linked to colorectal cancer (CRC), including Enterococcus faecalis, Escherichia coli and Bacteroides fragilis [88]. This finding was supported by animal studies, in which the infusion of feces from patients with CRC could promote tumorigenesis in germ-free mice [89]. Although FMT has not been tested as a treatment for CRC, it has been used to increase the efficacy of immune checkpoint inhibitors (ICI). Only a minority of patients with CRC responds to ICIs, which has been linked to an abnormal gut microbiome composition [90]. Moreover, it has been shown that antibiotics inhibit the clinical benefit of ICIs [90]. It has been hypothesized that restoration of the microbiome reinforces the intestinal barrier integrity and reduces systemic inflammation [90]. When the feces from cancer patients who responded to ICIs was transplanted into germ-free mice, the antitumor effects of ICIs were ameliorated, whereas feces from non-responders failed to do so [90]. In one case-series, FMT was used to successfully treat refractory ICI-associated colitis, reconstitute the GI microbiome and increase the proportion of regulatory T-cells in the colonic mucosa [91]. These results indicate the important role of the microbiota in ICI-related toxicity and efficacy and point to a potential role for microbiota-modifying therapies, such as FMT. A recently published review by Wardill et al. extensively describes the use of FMT in supportive oncology more into depth [92].
8. Discussion & conclusion
Interest in FMT to treat disease has risen over the last few years and its therapeutic benefit is currently being explored for a variety of diseases. Table 1 provides an overview of the current and potential indications for FMT. Besides the above described disorders, the use of FMT has been described in case reports as treatment for multiple organ dysfunction syndrome, chronic pouchitis and celiac disease [[93], [94], [95]]. However, for most diseases it is not fully known whether the changes in microbiota are causally related to the pathophysiology, or merely a result of the disorder. If the intestinal microbiota plays a causal role in disease pathophysiology, altering the microbiota may influence its course. In most cases, however, a single microorganism is not likely to be a causal pathogen or missing beneficial microbe. Therefore, an advantage of FMT over prebiotics and probiotics is the introduction of a complete healthy gut microbiota. FMT can be used as a tool to dissect association from causality in human intervention studies by assessing the effect of the microbiota on a disease. Fig. 2 and Table 2 give an overview of currently ongoing clinical trials that study the potential of FMT as a treatment for a variety of disorders.
Table 2.
Ongoing trials with fecal microbiota transplantation for indications other than CDI.
| Indication | NCT number | Intervention | Study design | Primary outcome | Patients (n) |
|---|---|---|---|---|---|
| IBD | NCT03399188 | I: FMT via colonoscopy | Single group, open label trial | - Clinical remission (PUCAI / Mayo score / PCDAI / CDAI) [6 months] | 100 |
| NCT02575040 | I: FMT | Single group, open label trial | - number of patients with worsened disease [1 year] - Safety: (serious) adverse events [1 year] |
60 | |
| Ulcerative colitis | NCT01790061 | I: FMT via DJ C: standard of care treatment |
Controlled, open label trial | - Clinical remission (Montreal score S0) [1 year] | 500 |
| NCT03843385 | I: fecal microbiota filtrate capsules II: FMT capsules C: placebo capsules |
3- arm, randomized, placebo-controlled, quadruple blinded trial | - Clinical remission (Mayo score) [12 weeks] | 174 | |
| NCT03483246 | I: FMT via colonoscopy C: sham via colonoscopy |
Randomized, placebo-controlled, single blinded trial | - Steroid-free clinical and endoscopic remission [12 weeks] | 150 | |
| NCT03804931 | I: FMT C: Saline |
Randomized, placebo-controlled, single blinded trial | - Clinical remission (Mayo score) [12 weeks] - Clinical improvement (Mayo score) [12 weeks] |
120 | |
| NCT03110289 | I: super donor FMT C: autologous FMT |
Randomized, controlled, quadruple blinded trial | - Clinical remission (Mayo score) [8 weeks] - Endoscopic remission [8 weeks] |
108 | |
| NCT02291523 | I: allogenic FMT via colonoscopy C: autologous FMT via colonoscopy |
Randomized, controlled, quadruple blinded trial | - Clinical remission (PUCAI) [12 months] | 101 | |
| NCT03582969a | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, triple blinded trial | - Clinical remission (SCCAI) [12 weeks] | 100 | |
| NCT03561532 | I: allogenic FMT via colonoscopy C: autologous FMT via colonoscopy |
Randomized, controlled, quadruple blinded trial | - Clinical and endoscopic remission [52 weeks] | 80 | |
| NCT02606032 | I: FMT + metronidazole + doxycycline + terbinafine C: FMT |
Randomized, controlled, quadruple blinded trial | - Clinical and endoscopic remission [9 weeks] | 80 | |
| NCT03016780 | I: FMT C: Saline |
Randomized, placebo-controlled, open label trial | - Safety: (serious) adverse events [3 months] | 60 | |
| NCT03104036 | I: FMT via enema C: mesalazine enema |
Randomized, controlled, open label trial | - Clinical remission (Mayo score) [12 weeks] | 60 | |
| NCT03006809 | I: antibiotics + FMT via colonoscopy + FMT capsules II: FMT via colonoscopy + FMT capsules III: antibiotics + FMT via colonoscopy + FMT via enema IV: FMT via colonoscopy + FMT via enema |
4-arm, randomized, controlled, open label trial | - Safety: (serious) adverse events [1 year] - Clinical and endoscopic remission [8 weeks] |
40 | |
| NCT02734589 | I: FMT via colonoscopy II: diet for UC + FMT III: diet for UC |
3-arm, randomized, controlled, single blinded trial | - Clinical remission (SCCAI) [56 days] | 34 | |
| NCT03948919 | I: low sulfur FMT capsules C: placebo capsules |
Randomized, placebo-controlled, quadruple blinded trial | - Engraftment of sulfate reducing microbiota [12 weeks] | 20 | |
| NCT02390726 | I: FMT C: placebo FMT |
Randomized, placebo-controlled, double blinded trial | - Endoscopic stage [2 years] - Biologic inflammatory markers [2 years] - Symptomatology and quality of life [2 years] |
20 | |
| NCT03949257 | I: FMT via DJ | Single group, open label trial | - Gut microbiome [7 days] | 20 | |
| NCT03716388 | I: FMT + mesalazine granules II: FMT + placebo granules III: mesalazine granules + placebo FMT |
3-arm randomized, controlled, double blinded trial | - Clinical remission (Mayo score) [14 weeks] | 15 | |
| Crohn's disease | NCT01793831 | I: FMT via DJ | Single group, open label trial | - Clinical remission (HBI score) [1 year] | 200 |
| NCT03078803 | I: FMT via colonoscopy and capsules C: water via colonoscopy and placebo capsules |
Randomized, placebo-controlled, quadruple blinded trial | - Clinical and endoscopic remission [8 weeks] | 126 | |
| NCT03378167 | I: FMT via colonoscopy + FMT capsules C: Placebo FMT |
Randomized, placebo-controlled, triple blinded, crossover trial | - Monthly Recruitment Rate [30 weeks] - Dropout Rate Post Enrolment [30 weeks] - Rate of Patient Protocol Adherence [30 weeks] - Safety: (serious) adverse events [30 weeks] |
45 | |
| NCT02417974 | I: FMT via colonoscopy C: no intervention |
Randomized, controlled, single blinded trial | - Endoscopic recurrence after ileo-cecal resection (Rutgeert's score) [6 months] | 44 | |
| NCT03267238 | I: FMT | Single group, open label trial | - Treatment-related adverse events (CTCAE v4.0) [5 years] | 40 | |
| NCT03747718 | I: FMT via enema C: placebo via enema |
Randomized, placebo-controlled, triple blinded trial | - Safety: (serious) adverse events [6 months] | 30 | |
| NCT03194529 | I: FMT via DJ | Single group, open label trial | - Safety: (serious) adverse events [24 weeks] | 10 | |
| Pouchitis | NCT03524352 | I: FMT C: sterile saline |
Randomized, placebo-controlled, double blinded trial | - Clinical and endoscopic relapse [PDAI) [106 weeks] | 42 |
| NCT03545386 | I: FMT via enema C: saline via enema |
Randomized, placebo-controlled, triple blinded trial | - Clinical remission (PDAI) [7 weeks] | 34 | |
| NCT03378921 | I: allogenic FMT via colonoscopy C: autologous FMT via colonoscopy |
Randomized, controlled, triple blinded trial | - Clinical remission (PDAI) [52 weeks] | 26 | |
| Microscopic colitis | NCT03275467 | I: FMT via colonoscopy + FMT via enema | Single group, open label trial | - Remission (<3 stools/day) [6 weeks] | 10 |
| IBS | NCT03613545 | I: FMT via colonoscopy C: sham via colonoscopy |
Randomized, placebo-controlled, single blinded trial | - IBS severity (IBS-SSS) [6 months] | 120 |
| NCT03125564 | I: FMT C: placebo FMT |
Randomized, placebo-controlled, double blinded trial | - Proportion of responder [12 weeks] | 90 | |
| NCT02847481 | I: FMT C: placebo FMT |
Randomized, placebo-controlled, double blinded trial | - Engraftment of donor microbiota [10 weeks] | 80 | |
| NCT02857257 | I: Anaerobic cultured human intestinal microbiota | Single group, open label trial | - Symptom relief (IBS-SSS) [4 weeks] | 50 | |
| NCT03074227 | I: allogenic FMT via DJ C: autologous FMT via DJ |
Randomized, controlled, quadruple blinded trial | - >50% reduction in severity and frequency of abdominal pain [12 weeks] | 30 | |
| NCT02092402 | I: allogenic FMT C: autologous FMT |
Randomized, controlled, quadruple blinded trial | - Symptoms of IBS patients (GSRS-IBS) [6 months] | 17 | |
| NCT02651740 | I: Rifaximin 400 mg + FMT via DJ | Single group, open label trial | - Relief of IBS condition [6 months] | 10 | |
| Chronic constipation | NCT03018613 | I: FMT C: Saline |
Randomized, placebo-controlled, open label trial | - Safety: (serious) adverse events [3 months] | 60 |
| Antibiotic resistant organisms | NCT03061097 | I: autologous FMT via enema C: placebo via enema |
Randomized, placebo-controlled, triple blinded trial | - Safety: (serious) adverse events [7 days] | 180 |
| NCT03643887 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, triple blinded trial | - VRE/CRE decolonization [6 months] | 90 | |
| NCT03391674 | I: FMT capsules + omeprazole C: no intervention |
Randomized, controlled, open label trial | - CRE Decolonization (3 consecutive negative stool samples for CRE) [3 months] | 60 | |
| NCT03167398 | I: FMT capsules + omeprazole | Single group, open label trial | - CRE Decolonization (3 consecutive negative stool samples for CRE) [1 month] | 60 | |
| NCT03834051 | I: FMT via enema | Single group, open label trial | - Clearance of antimicrobial resistant organism [2 years] | 50 | |
| NCT03029078 | I: FMT via DJ | Single group, open label trial | - Intestinal colonization with CRE/VRE [6 months] | 50 | |
| NCT03802461 | I: FMT via enema C: standard of care treatment |
Randomized, controlled, open label trial | - Intestinal colonization with CRE [3 months] - Feasibility: randomization rate [12 months] - Feasibility: retention of 90% of patients [6 months] |
40 | |
| NCT03479710 | I: FMT via DJ C: no intervention |
Controlled, open label trial | - Intestinal colonization with CRE/VRE [12 months] | 40 | |
| NCT02472600 | I: colistin + neomycin, followed by FMT capsules or ND infusion C: no intervention |
Randomized, controlled, open label trial | - Intestinal colonization with ESBL-E / CRE [48 days] | 39 | |
| NCT02922816 | I: FMT via enema C: no intervention |
Randomized, controlled, open label trial | - Safety and feasibility (CTCAE v4.0) [30 weeks] | 20 | |
| NCT03527056 | I: FMT capsules C: no intervention |
Controlled, open label trial | - CRE decolonization [10 days] - Safety: (serious) adverse events [10 days] |
20 | |
| NCT02543866 | I: FMT via DJ | Single group, open label trial | - Safety and Tolerability of FMT [1–5 years] | 20 | |
| NCT02312986 | I: FMT via enema | Single group, open label trial | - Safety: (serious) adverse events [12 months] | 20 | |
| NCT02816437 | I: FMT via enema | Single group, open label trial | - Safety: (serious) adverse events [10 months] | 20 | |
| NCT03063437 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, quadruple blinded trial | - VRE decolonization [10 days] - Safety: (serious) adverse events [10 days] |
9 | |
| Obesity | NCT03727321a | I: FMT + fiber supplement II: FMT + placebo supplement III: fiber supplement C: placebo supplement |
4-arm, randomized, controlled, quadruple blinded trial | - Insulin resistance (HOMA-IR) [12 weeks] | 68 |
| NCT03127696 | I: FMT + lifestyle modification program II: FMT III: lifestyle modification program |
3 –arm randomized, placebo-controlled, double blinded trial | - 5% reduction in weight [24 weeks] | 60 | |
| NCT03273855 | I: allogenic FMT via enema C: autologous FMT via enema |
Randomized, controlled, quadruple blinded trial | - Changes in body weight [12 months] | 60 | |
| NCT02970877 | I: allogenic FMT C: autologous FMT |
Randomized, controlled, quadruple blinded trial | - Insulin Resistance (HOMA-IR) [3 months] | 48 | |
| NCT03391817 | I: allogenic FMT C: autologous FMT |
Randomized, controlled, triple blinded trial | - Reduction of weight [1,5 years] | 40 | |
| NCT02530385 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, double blinded trial | - Insulin resistance (HOMA-IR) [6 weeks] | 24 | |
| NCT02741518 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, triple blinded trial | - Safety: (serious) adverse events [52 weeks] | 22 | |
| NCT03789461 | I: FMT | Single group, open label trial | - 10% reduction in weight [6 weeks] | 20 | |
| NAFLD | NCT02868164 | I: FMT via DJ C: standard of care treatment |
Randomized, controlled, open label trial | - Adverse events complication rate in NASH [1 year] | 120 |
| NCT02721264 | I: FMT via DJ C: standard of care treatment |
Randomized, controlled, open label trial | - Hepatic Venous Pressure Gradient [1 year] | 112 | |
| NCT03803540 | I: FMT via DJ | Single group, open label trial | - Histological resolution of NASH [72 weeks] | 15 | |
| NCT02469272 | I: FMT via DJ | Single group, open label trial | - Hepatic steatosis (by MRI) [12 weeks] | 5 | |
| Alcoholic hepatitis | NCT03091010 | I: FMT via DJ C: prednisolone 40 mg |
Randomized, controlled, open label trial | - Overall Survival [3 months] | 130 |
| NCT03827772 | I: FMT via DJ C: standard of care treatment |
Controlled, open label trial | - Overall Survival [3 months] | 40 | |
| Liver cirrhosis & hepatic encephalopathy | NCT03796598 | I: FMT capsules + FMT enema II: FMT capsules + placebo enema III: placebo capsules + FMT enema C: placebo capsules + placebo enema |
4-arm, randomized, placebo-controlled, triple blinded trial | - Serious adverse events related to FMT [6 months] | 100 |
| NCT03363022 | I: FMT via enema C: placebo via enema |
Randomized, placebo-controlled, double blinded trial | - Survival [21 days] | 40 | |
| NCT02862249 | I: FMT via DJ C: placebo via DJ |
Randomized, placebo-controlled, single blinded trial | - Feasibility of FMT [18 months] - Safety: (serious) adverse events [18 months] |
32 | |
| NCT03439982 | I: FMT via colonoscopy + FMT via enema | Single group, open label trial | - Time to hepatic encephalopathy breakthrough [9 weeks] | 30 | |
| NCT03420482 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, quadruple blinded trial | - Psychometric Hepatic Encephalopathy Score [28 days] | 30 | |
| NCT03416751 | I: FMT via enema C: saline via enema |
Randomized, placebo-controlled, double blinded trial | - Safety: (serious) adverse events [15 days] - Related transmissible infectious disease [15 days] |
20 | |
| NCT02255617 | I: FMT via colonoscopy and enema | Single group, open label trial | - Time to hepatic encephalopathy breakthrough [6 months] | 10 | |
| Acute Pancreatitis | NCT03015467 | I: FMT C: Saline |
Randomized, placebo-controlled, open label trial | - Mortality [3 months] | 80 |
| NCT02318134 | I: FMT via enema + traditional treatment C: traditional treatment |
Randomized, controlled, single blinded trial | - Gastrointestinal Failure score [7–14 days] | 60 | |
| NCT02318147 | I: FMT via enema + traditional treatment C: traditional treatment |
Randomized, controlled, single blinded trial | - Control of infectious complications [from admission to discharge] | 60 | |
| Severe acute malnutrition | NCT03087097 | I: FMT via enema C: placebo via enema |
Randomized, placebo-controlled, quadruple blinded trial | - Safety: serious adverse events [56 days] | 20 |
| Anorexia Nervosa | NCT03928808 | I: FMT via DJ | Single group, open label trial | - Safety: (serious) adverse events [30 days] - Feasibility: participants recruited [3 years] - Tolerability: participants able to complete 4 FMT administrations [3 years] - Tolerability: GI distress Post FMT [3 years] |
10 |
| Graft Versus Host Disease | NCT03862079 | I: total gut decontamination + FMT via enema II: FMT via enema C: standard of care treatment |
3-arm, randomized, controlled, open label trial | - Development of acute GVHD [100 days] - Relapse-free survival [6 months] |
120 |
| NCT03678493 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, open label trial | - Incidence of infections [4 months] | 120 | |
| NCT03492502 | I: autologous FMT via DJ | Single group, open label trial | - Safety: (serious) adverse events [7 days] | 70 | |
| NCT03720392 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, double blinded trial | - Gut microbiome diversity [1 month] | 48 | |
| NCT03359980 | I: FMT | Single group, open label trial | - GI and overall GVHD response [28 days] | 32 | |
| NCT03812705 | I: FMT via colonoscopy of ND tube | Single group, open label trial | - Response rate [12 weeks] | 30 | |
| NCT03549676 | I: FMT via DJ | Single group, open label trial | - Efficacy in the treatment of refractory GVHD on day 7 and 28. | 15 | |
| NCT03819803 | I: FMT via colonoscopy | Single group, open label trial | - GI-GVHD remission [90 days] | 15 | |
| NCT03214289 | I: FMT capsules | Single group, open label trial | - Safety: (serious) adverse events [28 days] | 4 | |
| Cancer | NCT03819296 | I: FMT | Single group, open label trial | - Safety: (serious) adverse events [4 months] - Incidence of toxicities [1 year] |
100 |
| NCT03353402 | I: FMT via colonoscopy + FMT capsules with feces of responders to immunotherapy | Single group, open label trial | - Safety: (serious) adverse events [4 years] - Engraftment of fecal microbiota [4 years] |
40 | |
| NCT03341143 | I: FMT via colonoscopy + pembrolizumab | Single group, open label trial | - Objective Response Rate [3 years] | 20 | |
| Psoriatic arthritis | NCT03058900 | I: FMT via DJ + methotrexate C: saline via DJ + methotrexate |
Randomized, placebo-controlled, triple blinded trial | - Treatment failure [6 months] | 80 |
| Rheumatoid arthritis | NCT03944096 | I: allogenic FMT via DJ + methotrexate C: autologous FMT + methotrexate |
Randomized, controlled, triple blinded trial | - Clinical response (ACR20) [16 weeks] | 30 |
| Ankylosing Spondylitis | NCT03726645 | I: allogenic FMT C: autologous FMT |
Randomized, controlled, triple blinded trial | - Clinical activity of ankylosing spondylitis [12 months] | 20 |
| Hepatitis B | NCT03429439 | I: FMT + antiviral therapy C: antiviral therapy |
Randomized, controlled, open label trial | - Serum hepatitis B virus e antigen [6 months] | 60 |
| IgA Nephropathy | NCT03633864 | I: FMT via enema | Single group, open label trial | - Change of Urinary protein [8 weeks] | 30 |
| Recurrent urinary tract infection | NCT03050515 | I: FMT via enema | Single group, open label trial | - Frequency of culture proven urinary tract infections [6 months] | 12 |
| Peanut allergy | NCT02960074 | I: FMT capsules | Single group, open label trial | - Safety: (serious) adverse events [12 months] | 10 |
| Sjogren's syndrome | NCT03926286 | I: FMT via enema | Single group, open label trial | - Safety: (serious) adverse events [7 months] - Stable microbiome engraftment [3 months] |
10 |
| Parkinson's disease | NCT03876327 | I: FMT C: no intervention |
Controlled, open label trial | - Motor symptoms (UPDRS III) [6 months] - Constipation level [6 months] |
100 |
| NCT03808389 | I: allogenic FMT via DJ C: autologous FMT via DJ |
Randomized, controlled, quadruple blinded trial | - Changes in clinical symptoms (MDS-UPDRS) [12 months] | 40 | |
| NCT03671785 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, single blinded trial | - Microbiome diversity in fecal Samples [9 months] - Microbiome Richness in Fecal Samples [9 months] - Safety: (serious) adverse events [9 months] |
12 | |
| Multiple sclerosis | NCT03183869 | I: FMT C: no intervention |
Randomized, controlled, open label, crossover trial | - Cytokines levels in peripheral blood [6 months] | 40 |
| NCT03594487 | I: FMT via colonoscopy C: no intervention |
Controlled, open label trial | - Subjects who complete the study protocol [1 year] - Change in fecal microbiota [12 weeks] - Safety: (serious) adverse events [12 weeks] |
30 | |
| Amyotrophic lateral sclerosis | NCT03766321 | I: FMT via DJ C: sham FMT via DJ |
Randomized, placebo-controlled, quadruple blinded trial | - Tregs number [6 months] | 42 |
| Depression | NCT03279224 | I: allogenic FMT via colonoscopy C: autologous FMT via colonoscopy |
Randomized, controlled, quadruple blinded trial | - Depressive symptoms (MADRS) [24 weeks] | 60 |
| NCT03281044 | I: FMT capsules C: placebo capsules |
Randomized, placebo-controlled, triple blinded trial | - Depressive symptoms (HRSD) [8 months] | 40 | |
| NCT03233100 | I: FMT | Single group, open label trial | - Complete spontaneous bowl movements [12 weeks] - Anxiety symptoms (HAMA) [12 weeks] - Depressive symptoms (HAMD) [12 weeks] |
40 | |
| Autism spectrum disorder | NCT03408886 | I: FMT capsules C: placebo capsules |
Randomized, controlled, quadruple blinded trial | - Autism symptoms CARS) [10 weeks] | 84 |
| NCT03426826 | I: FMT via DJ C: placebo via DJ |
Randomized, placebo-controlled, quadruple blinded trial | - Safety and tolerability [24 weeks] - Symptom changes (RBS-R) [24 weeks] |
10 | |
| Chronic fatigue syndrome | NCT03691987 | I: allogenic FMT via enema C: autologous FMT via enema |
Randomized, controlled, quadruple blinded trial | - Clinical response (FSS) [3 months] | 80 |
| Epilepsy | NCT02889627 | I: FMT via DJ | Single group, open label trial | - Frequency of the seizures [3 months] | 50 |
The above table summarizes ongoing trials studying the effect of FMT for a variety of diseases. Studies are grouped in ‘gastrointestinal’, ‘metabolic’, immunologic’ and ‘neuropsychiatric’ disorders, sorted by disease and number of included patients. These studies were derived from Clinicaltrial.gov in May 2019. Ongoing trials were included when they were registered as ‘Active’, ‘recruiting’, ‘enrolling via invitation’ or ‘not yet recruiting’. Only intervention studies were included. In addition, studies examining the effect of FMT in treating CDI were excluded, since this is out of the scope of this review. There were several methods for upper administration of FMT (i.e. gastroscopy, nasoduodenal tube, transendoscopic enteral tubing, etc.), which were pooled for simplicity as duodenal/jejunal infusion (DJ). Within brackets, the time frame of a primary outcome can be found. Abbreviations: I = intervention, C = control, FMT = fecal microbiota transplantation, DJ = duodenal/jejunal infusion, IBD = Inflammatory Bowel Disease, UC = Ulcerative Colitis, IBS = Irritable Bowel Syndrome, CRE = Carbapenem-Resistant Enterobacteriaceae, VRE = Vancomycin-Resistant Enterococci, ESBL-E = Extended Spectrum Beta-Lactamase Enterobacteriaceae, NAFLD = Non-Alcoholic Fatty Liver Disease, NASH = Non-Alcoholic Steatohepatitis, GI = Gastrointestinal, GVHD = Graft Versus Host Disease.
Entry NCT03582969 was the same as NCT03273465, which were therefore merged. The same goes for entry NCT03477916, which was the same as NCT03727321
Currently, FMT is a non-standardized treatment which should be optimized and standardized for specific indications. This is supported by the finding of super donors, which suggests a specific bacterial composition can be more effective to treat a certain disease [37,38]. In addition, treatment strategy and route can impact the microbiota composition and colonization, which can influence the therapeutic effect. With the development of FMT capsules, the therapy became less invasive, more standardized, and less expensive [96]. However, some microbes (or metabolites) critical for the efficacy of an FMT might not survive the processing required for capsulation. Therefore, it is important to determine the differences between fresh and processed fecal microbiota and the efficacy in particular diseases. Furthermore, optimal location of delivery and ‘dose’ of FMT to treat microbiota-mediated diseases are largely unknown. A small cohort study showed that capsules releasing fecal microbiota in the colon achieved a slightly higher cure rate (81%) compared to gastric release (75%) in treating patients with rCDI [97]. Delivery of microbiota to a specific area of the intestine via targeted opening of a capsule might be an interesting future approach to further investigate.
Given the variable composition of feces, FMT will probably be replaced by other microbiome-targeting therapeutics. While the knowledge of the microbiota and host-commensal interactions in dysbiotic environments increases, it is to be expected that dietary manipulation and specific alteration of key microbes will be emerging in the future. Furthermore, it appears FMT is not a one-size-fits-all therapy and needs a more personalized approach for several disorders. For instance, donors with a specific microbiota profile are more likely to provide a beneficial effect for patients with IBD [37,38]. Other studies have shown that the microbiota profile of the recipient is predictive for the outcome of the FMT [62]. Future studies should therefore focus more on donor-recipient compatibility and suitability prior to FMT.
With the rapid increase in novel and more affordable techniques to analyze the gut microbiota, implications for a role of this ‘endocrine organ’ in disease development has risen exponentially. It is important to emphasize however, that besides bacteria, the microbiota consists of archaea, viruses (especially bacteriophages) and fungi. Bacteriophages, viruses that specifically infect and eliminate bacteria, were found to be 20 times more abundant than bacteria in mucosal samples [98]. Given the high number of bacteriophages in an FMT (1–10 times the number of bacteria), these viruses might be important drivers of FMT efficacy. In a small prospective study, the effect of a sterile (bacteria-free) FMT was tested in rCDI patients [99]. Although only five patients were included, all patients had resolution of their CDI-associated diarrhea. Interestingly, shifts in viral and bacterial composition towards the donor's microbiota profile were observed. Another prospective study observed highly individualized virus colonization patterns depending on specific donor-recipient pairings [100]. The intestinal microbiome is a complex ecosystem with many yet to be identified components likely to affect human metabolism.
In addition, there is a significant knowledge gap in the link between the (small) intestinal microbiota and disease development and progression in humans. This is in large part because the accessible, fecal microbiome is usually used to analyze the microbiome composition and associate with the disease of interest (fecal bias). The small intestinal microbiome differs significantly form the fecal microbiome [101]. Together with the fact that the small intestine plays a major role in human metabolism and disease development, it is critical to develop strategies to sample small intestinal microbiome. In line, nasoduodenal administration of FMT exposes the upper GI to a lower GI/fecal microbiome. This might be a potential drawback for diseases where the upper GI microbiota or the mucosal microbiota is the main culprit. Future research needs to investigate whether and to what extent FMT is capable of modifying the upper and mucosal microbiota.
In conclusion, FMT is a promising treatment strategy for many microbiota-related indications. However, with exception of rCDI, FMT is still experimental and should not be offered as treatment option outside of a research setting. More controlled trials are needed to assess the potential benefit of FMT compared to or in addition to standard therapy.
9. Outstanding questions
FMT remains an unstandardized procedure and the optimal location of delivery and ‘dose’ of FMT to treat specific microbiota-mediated diseases remain largely unknown. Some studies show a specific microbiota profile is more effective in treating disease, while others show the microbiota profile of the recipient is predictive for the outcome of the FMT. For treating rCDI, the above appears less relevant, as high effectivity rates are observed regardless of the route, dose, processing or donor. However, FMT as a treatment for other disease comes with a smaller effect size along with a larger group of nonresponders. Further optimization and personalization of the FMT strategy might improve the outcome in diseases beyond CDI.
The gut microbiota remains a complex ecosystem with lot of unknowns and therefore, future research should focus more on other key players beside bacteria. In addition, there is a significant difference in microbiota composition throughout the GI tract and the role of each section in human metabolism and disease development is hardly understood. Therefore, it is critical to develop strategies to sample the microbiome throughout the GI tract. In line, future research needs to investigate whether and to what extent FMT is capable of modifying the upper and mucosal microbiota.
Funding sources
K. Wortelboer is supported by a Novo Nordisk Foundation, Denmark CAMIT grant 2018. M. Nieuwdorp is supported by a ZONMW-VIDI, Netherlands grant 2013 [016.146.327] and a grant from the Dutch Heart Foundation, Netherlands, CVON IN CONTROL. H. Herrema is supported by a Le Ducq consortium, France grant 17CVD01. None of the funding bodies had any role in writing of the manuscript of selection of the literature.
Declaration of interests
M. Nieuwdorp is on the scientific advisory board of Caelus Health, the Netherlands. The remaining authors have nothing to disclose.
Author contributions
K. Wortelboer performed the literature search, wrote and corrected the article and made the figs. H. Herrema and M. Nieuwdorp critically reviewed and corrected the manuscript.
Search strategy and selection criteria
Embase, Web of Science and Pubmed were searched in December 2018 for articles using the search term “Fecal Microbiota Transplantation”, which resulted in 2027, 1581 and 1414 articles respectively. Duplicates were merged. Articles on clinical trials, systematic reviews and meta-analyses, written in English and published before 1 January 2019 were included. To identify additional relevant studies, reference lists were manually searched. In addition, we searched ClinicalTrials.gov for new and ongoing trials with FMT for indications other than CDI using the search term “[disease]” in combination with “FMT” OR “fecal microbiota transplantation”.
Acknowledgements
None.
References
- 1.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007 Oct 18;449(7164) doi: 10.1038/nature06244. (804–10) [DOI] [PMC free article] [PubMed] [Google Scholar]; Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007 Oct 18;449(7164):804–10. [DOI] [PMC free article] [PubMed]
- 2.Jung Lee W., Lattimer L.D.N., Stephen S., Borum M.L., Doman D.B. Fecal microbiota transplantation: a review of emerging indications beyond relapsing clostridium difficile toxin colitis. Gastroenterol Hepatol (N Y) 2015 Jan;11(1):24–32. [PMC free article] [PubMed] [Google Scholar]; Jung Lee W, Lattimer LDN, Stephen S, Borum ML, Doman DB. Fecal Microbiota Transplantation: A Review of Emerging Indications Beyond Relapsing Clostridium difficile Toxin Colitis. Gastroenterol Hepatol (N Y). 2015 Jan;11(1):24–32. [PMC free article] [PubMed]
- 3.Dinan T.G., Cryan J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]; Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017;46(1):77–89. [DOI] [PubMed]
- 4.Kim S., Covington A., Pamer E.G. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017 Sep;279(1):90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim S, Covington A, Pamer EG. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017 Sep;279(1):90–105. [DOI] [PMC free article] [PubMed]
- 5.Machiels K., Joossens M., Sabino J., De Preter V., Arijs I., Eeckhaut V. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014 Aug;63(8) doi: 10.1136/gutjnl-2013-304833. (1275–83) [DOI] [PubMed] [Google Scholar]; Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014 Aug;63(8):1275–83. [DOI] [PubMed]
- 6.Cani P.D. Gut microbiota-at the intersection of everything? Nat Rev Gastroenterol Hepatol. 2017;14(6) doi: 10.1038/nrgastro.2017.54. (321–2) [DOI] [PubMed] [Google Scholar]; Cani PD. Gut microbiota-at the intersection of everything? Nat Rev Gastroenterol Hepatol. 2017;14(6):321–2. [DOI] [PubMed]
- 7.Scheithauer T.P.M., Dallinga-Thie G.M., de Vos W.M., Nieuwdorp M., van Raalte D.H. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5(9) doi: 10.1016/j.molmet.2016.06.002. (759–70) [DOI] [PMC free article] [PubMed] [Google Scholar]; Scheithauer TPM, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5(9):759–70. [DOI] [PMC free article] [PubMed]
- 8.Bakker G.J., Nieuwdorp M. Fecal microbiota transplantation: therapeutic potential for a multitude of diseases beyond Clostridium difficile. Microbiol Spectr. 2017 Aug;5(4) doi: 10.1128/microbiolspec.bad-0008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bakker GJ, Nieuwdorp M. Fecal Microbiota Transplantation: Therapeutic Potential for a Multitude of Diseases beyond Clostridium difficile. Microbiol Spectr. 2017 Aug;5(4). [DOI] [PMC free article] [PubMed]
- 9.Zhang F., Luo W., Shi Y., Fan Z., Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012 Nov;107(11) doi: 10.1038/ajg.2012.251. 1755;1755–6. [DOI] [PubMed] [Google Scholar]; Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012 Nov;107(11):1755;1755-6. [DOI] [PubMed]
- 10.Lewin R.A. 1st ed. Random House; New York: 1999. Merde: Excursions in scientific, cultural, and socio-historical coprology; p. 208. [Google Scholar]; Lewin RA. Merde: Excursions in Scientific, Cultural, and Socio-Historical Coprology. 1st ed. New York: Random House; 1999. 208 p.
- 11.Eiseman B., Silen W., Bascom G.S., Kauvar A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958 Nov;44(5) (854–9) [PubMed] [Google Scholar]; Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958 Nov;44(5):854–9. [PubMed]
- 12.Wang J.-W., Kuo C.-H., Kuo F.-C., Wang Y.-K., Hsu W.-H., Yu F.-J. Fecal microbiota transplantation: review and update. J Formos Med Assoc. 2019 Mar;118(Suppl. 1):S23–S31. doi: 10.1016/j.jfma.2018.08.011. [DOI] [PubMed] [Google Scholar]; Wang J-W, Kuo C-H, Kuo F-C, Wang Y-K, Hsu W-H, Yu F-J, et al. Fecal microbiota transplantation: Review and update. J Formos Med Assoc. 2019 Mar;118 Suppl 1:S23-S31 [DOI] [PubMed]
- 13.Quraishi M.N., Widlak M., Bhala N., Moore D., Price M., Sharma N. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017 Sep;46(5) doi: 10.1111/apt.14201. (479–93) [DOI] [PubMed] [Google Scholar]; Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017 Sep;46(5):479–93. [DOI] [PubMed]
- 14.Sha S., Liang J., Chen M., Xu B., Liang C., Wei N. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10) doi: 10.1111/apt.12699. (1003–32) [DOI] [PubMed] [Google Scholar]; Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, et al. Systematic review: Faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–32. [DOI] [PubMed]
- 15.Li Y.-T., Cai H.-F., Wang Z.-H., Xu J., Fang J.-Y. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for clostridium difficile infection. Aliment Pharmacol Ther. 2016 Feb;43(4) doi: 10.1111/apt.13492. (445–57) [DOI] [PubMed] [Google Scholar]; Li Y-T, Cai H-F, Wang Z-H, Xu J, Fang J-Y. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016 Feb;43(4):445–57. [DOI] [PubMed]
- 16.Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, De Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013/01/18. 2013;368(5):(407–15). [DOI] [PubMed]
- 17.Cammarota G., Masucci L., Ianiro G., Bibbò S., Dinoi G., Costamagna G. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9) doi: 10.1111/apt.13144. (835–43) [DOI] [PubMed] [Google Scholar]; Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–43. [DOI] [PubMed]
- 18.Kelly C.R., Khoruts A., Staley C., Sadowsky M.J., Abd M., Alani M. Effect of Fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016 Nov;165(9) doi: 10.7326/M16-0271. (609–16) [DOI] [PMC free article] [PubMed] [Google Scholar]; Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med. 2016 Nov;165(9):609–16. [DOI] [PMC free article] [PubMed]
- 19.Hvas C.L., Jørgensen S.M.D., Jørgensen S.P., Storgaard M., Lemming L., Hansen M.M. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology. 2019 Apr;156(5) doi: 10.1053/j.gastro.2018.12.019. (1324–1332.e3) [DOI] [PubMed] [Google Scholar]; Hvas CL, Jørgensen SMD, Jørgensen SP, Storgaard M, Lemming L, Hansen MM, et al. Fecal Microbiota Transplantation is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology. 2019 Apr;156(5):1324-1332.e3 [DOI] [PubMed]
- 20.Youngster I., Sauk J., Pindar C., Wilson R.G., Kaplan J.L., Smith M.B. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014 Jun;58(11) doi: 10.1093/cid/ciu135. (1515–22) [DOI] [PMC free article] [PubMed] [Google Scholar]; Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014 Jun;58(11):1515–22. [DOI] [PMC free article] [PubMed]
- 21.Lee C.H., Steiner T., Petrof E.O., Smieja M., Roscoe D., Nematallah A. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2016 Jan;315(2):142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]; Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2016 Jan;315(2):142–9. [DOI] [PubMed]
- 22.Jiang Z.D., Ajami N.J., Petrosino J.F., Jun G., Hanis C.L., Shah M. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection - fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther. 2017 Apr;45(7):899–908. doi: 10.1111/apt.13969. [DOI] [PubMed] [Google Scholar]; Jiang ZD, Ajami NJ, Petrosino JF, Jun G, Hanis CL, Shah M, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection - fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther. 2017 Apr;45(7):899–908. [DOI] [PubMed]
- 23.Jiang Z.-D., Jenq R.R., Ajami N.J., Petrosino J.F., Alexander A.A., Ke S. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang Z-D, Jenq RR, Ajami NJ, Petrosino JF, Alexander AA, Ke S, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: A randomized clinical trial. PLoS One. 2018;13(11):e0205064. [DOI] [PMC free article] [PubMed]
- 24.Kao D., Roach B., Silva M., Beck P., Rioux K., Kaplan G.G. Effect of oral capsule– vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA J Am Med Assoc. 2017;318(20) doi: 10.1001/jama.2017.17077. (1985–93) [DOI] [PMC free article] [PubMed] [Google Scholar]; Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule– vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: A randomized clinical trial. JAMA - J Am Med Assoc. 2017;318(20):1985–93. [DOI] [PMC free article] [PubMed]
- 25.Juul F.E., Garborg K., Bretthauer M., Skudal H., Oines M.N., Wiig H. Fecal microbiota transplantation for primary Clostridium difficile infection. N Engl J Med. 2018 Jun;378(26) doi: 10.1056/NEJMc1803103. (2535–6) [DOI] [PubMed] [Google Scholar]; Juul FE, Garborg K, Bretthauer M, Skudal H, Oines MN, Wiig H, et al. Fecal Microbiota Transplantation for Primary Clostridium difficile Infection. N Engl J Med. 2018 Jun;378(26):2535–6. [DOI] [PubMed]
- 26.Camacho-Ortiz A., Gutierrez-Delgado E.M., Garcia-Mazcorro J.F., Mendoza-Olazaran S., Martinez-Melendez A., Palau-Davila L. Randomized clinical trial to evaluate the effect of fecal microbiota transplant for initial Clostridium difficile infection in intestinal microbiome. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189768. [DOI] [PMC free article] [PubMed] [Google Scholar]; Camacho-Ortiz A, Gutierrez-Delgado EM, Garcia-Mazcorro JF, Mendoza-Olazaran S, Martinez-Melendez A, Palau-Davila L, et al. Randomized clinical trial to evaluate the effect of fecal microbiota transplant for initial Clostridium difficile infection in intestinal microbiome. PLoS One. 2017;12(12):e0189768. [DOI] [PMC free article] [PubMed]
- 27.Khan M.Y., Dirweesh A., Khurshid T., Siddiqui W.J. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018 Nov;30(11) doi: 10.1097/MEG.0000000000001243. (1309–17) [DOI] [PubMed] [Google Scholar]; Khan MY, Dirweesh A, Khurshid T, Siddiqui WJ. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018 Nov;30(11):1309–17. [DOI] [PubMed]
- 28.Moayyedi P., Yuan Y., Baharith H., Ford A.C. Faecal microbiota transplantation for <em>Clostridium difficile</em>-associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust. 2017 Aug;207(4) doi: 10.5694/mja17.00295. (166–72) [DOI] [PubMed] [Google Scholar]; Moayyedi P, Yuan Y, Baharith H, Ford AC. Faecal microbiota transplantation for <em>Clostridium difficile</em>-associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust. 2017 Aug;207(4):166–72. [DOI] [PubMed]
- 29.Ianiro G., Maida M., Burisch J., Simonelli C., Hold G., Ventimiglia M. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta-analysis. United Eur Gastroenterol J. 2018 Oct;6(8) doi: 10.1177/2050640618780762. (1232–44) [DOI] [PMC free article] [PubMed] [Google Scholar]; Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, et al. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur Gastroenterol J. 2018 Oct;6(8):1232–44. [DOI] [PMC free article] [PubMed]
- 30.Lapointe-Shaw L., Tran K.L., Coyte P.C., Hancock-Howard R.L., Powis J., Poutanen S.M. Cost-effectiveness analysis of six strategies to treat recurrent Clostridium difficile infection. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149521. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lapointe-Shaw L, Tran KL, Coyte PC, Hancock-Howard RL, Powis J, Poutanen SM, et al. Cost-Effectiveness Analysis of Six Strategies to Treat Recurrent Clostridium difficile Infection. PLoS One. 2016;11(2):e0149521. [DOI] [PMC free article] [PubMed]
- 31.Varier R.U., Biltaji E., Smith K.J., Roberts M.S., Kyle Jensen M., LaFleur J. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015 Apr;36(4) doi: 10.1017/ice.2014.80. (438–44) [DOI] [PubMed] [Google Scholar]; Varier RU, Biltaji E, Smith KJ, Roberts MS, Kyle Jensen M, LaFleur J, et al. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015 Apr;36(4):438–44. [DOI] [PubMed]
- 32.Surawicz C.M., Brandt L.J., Binion D.G., Ananthakrishnan A.N., Curry S.R., Gilligan P.H. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013 Apr;108(4) doi: 10.1038/ajg.2013.4. (478–98; [quiz 499]) [DOI] [PubMed] [Google Scholar]; Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013 Apr;108(4):478–98; quiz 499. [DOI] [PubMed]
- 33.Tacconelli E., Cataldo M.A., Dancer S.J., De Angelis G., Falcone M., Frank U. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014 Jan;20(Suppl. 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]; Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014 Jan;20 Suppl 1:1–55. [DOI] [PubMed]
- 34.Rossen N.G., Fuentes S., van der Spek M.J., Tijssen J.G., Hartman J.H.A., Duflou A. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015 Jul;149(1):110–118. doi: 10.1053/j.gastro.2015.03.045. (e4) [DOI] [PubMed] [Google Scholar]; Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JHA, Duflou A, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015 Jul;149(1):110–118.e4. [DOI] [PubMed]
- 35.Garrett W.S., Gallini C.A., Yatsunenko T., Michaud M., DuBois A., Delaney M.L. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010 Sep 16;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010 Sep 16;8(3):292–300. [DOI] [PMC free article] [PubMed]
- 36.Paramsothy S., Paramsothy R., Rubin D.T., Kamm M.A., Kaakoush N.O., Mitchell H.M. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017 Oct;11(10) doi: 10.1093/ecco-jcc/jjx063. (1180–99) [DOI] [PubMed] [Google Scholar]; Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017 Oct;11(10):1180–99. [DOI] [PubMed]
- 37.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized, controlled trial. Gastroenterology. 2015 Jul;149(1):102–109. doi: 10.1053/j.gastro.2015.04.001. (e6) [DOI] [PubMed] [Google Scholar]; Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized, Controlled Trial. Gastroenterology. 2015 Jul;149(1):102-109.e6. [DOI] [PubMed]
- 38.Paramsothy S., Kamm M.A., Kaakoush N.O., Walsh A.J., van den Bogaerde J., Samuel D. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017 Mar;389(10075):1218–1228. doi: 10.1016/S0140-6736(17)30182-4. (London, England) [DOI] [PubMed] [Google Scholar]; Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet (London, England). 2017 Mar;389(10075):1218–28. [DOI] [PubMed]
- 39.Costello S.P., Waters O., Bryant R.V., Katsikeros R., Makanyanga J., Schoeman M. Short duration, low intensity, pooled fecal microbiota transplantation induces remission in patients with mild-moderately active ulcerative colitis: a randomised controlled trial. Gastroenterology. 2017;152(5) (S198–9) [Google Scholar]; Costello SP, Waters O, Bryant R V., Katsikeros R, Makanyanga J, Schoeman M, et al. Short Duration, Low Intensity, Pooled Fecal Microbiota Transplantation Induces Remission in Patients with Mild-Moderately Active Ulcerative Colitis: A Randomised Controlled Trial. Gastroenterology. 2017;152(5):S198–9.
- 40.Imdad A., Nicholson M.R., Tanner-Smith E.E., Zackular J.P., Gomez-Duarte O.G., Beaulieu D.B. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018 Nov;11 doi: 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane database Syst Rev. 2018 Nov;11:CD012774. [DOI] [PMC free article] [PubMed]
- 41.Paramsothy S., Nielsen S., Kamm M.A., Deshpande N.P., Faith J.J., Clemente J.C. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019 Apr;156(5):1440–1454. doi: 10.1053/j.gastro.2018.12.001. (.e2) [DOI] [PubMed] [Google Scholar]; Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology. 2019 Apr;156(5):1440-1454.e2. [DOI] [PubMed]
- 42.He Z., Li P., Zhu J., Cui B., Xu L., Xiang J. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn's disease complicated with inflammatory mass. Sci Rep. 2017 Jul;7(1):4753. doi: 10.1038/s41598-017-04984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; He Z, Li P, Zhu J, Cui B, Xu L, Xiang J, et al. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn's disease complicated with inflammatory mass. Sci Rep. 2017 Jul;7(1):4753. [DOI] [PMC free article] [PubMed]
- 43.Li P., Zhang T., Xiao Y., Tian L., Cui B., Ji G. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn's disease. Appl Microbiol Biotechnol. 2019 Jan;103(1):349–360. doi: 10.1007/s00253-018-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li P, Zhang T, Xiao Y, Tian L, Cui B, Ji G, et al. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn's disease. Appl Microbiol Biotechnol. 2019 Jan;103(1):349-360. [DOI] [PMC free article] [PubMed]
- 44.Wang H., Cui B., Li Q., Ding X., Li P., Zhang T. The safety of fecal microbiota transplantation for Crohn's disease: findings from a long-term study. Adv Ther. 2018 Nov;35(11):1935–1944. doi: 10.1007/s12325-018-0800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang H, Cui B, Li Q, Ding X, Li P, Zhang T, et al. The Safety of Fecal Microbiota Transplantation for Crohn's Disease: Findings from A Long-Term Study. Adv Ther. 2018 Nov;35(11):1935–44. [DOI] [PMC free article] [PubMed]
- 45.Mazzawi T., Lied G.A., Sangnes D.A., El-Salhy M., Hov J.R., Gilja O.H. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0194904. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mazzawi T, Lied GA, Sangnes DA, El-Salhy M, Hov JR, Gilja OH, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One. 2018;13(11):e0194904. [DOI] [PMC free article] [PubMed]
- 46.Rodino-Janeiro B.K., Vicario M., Alonso-Cotoner C., Pascua-Garcia R., Santos J. A review of microbiota and irritable bowel syndrome: future in therapies. Adv Ther. 2018 Mar;35(3):289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rodino-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-Garcia R, Santos J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther. 2018 Mar;35(3):289–310. [DOI] [PMC free article] [PubMed]
- 47.Johnsen P.H., Hilpusch F., Cavanagh J.P., Leikanger I.S., Kolstad C., Valle P.C. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018 Jan;3(1):17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]; Johnsen PH, Hilpusch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. lancet Gastroenterol Hepatol. 2018 Jan;3(1):17–24. [DOI] [PubMed]
- 48.Halkjaer S.I., Christensen A.H., Lo B.Z.S., Browne P.D., Gunther S., Hansen L.H. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018 Dec;67(12):2107–2115. doi: 10.1136/gutjnl-2018-316434. [DOI] [PubMed] [Google Scholar]; Halkjaer SI, Christensen AH, Lo BZS, Browne PD, Gunther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018 Dec;67(12):2107–15. [DOI] [PubMed]
- 49.Tian H., Ge X., Nie Y., Yang L., Ding C., McFarland L.V. Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171308. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tian H, Ge X, Nie Y, Yang L, Ding C, McFarland L V, et al. Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial. PLoS One. 2017;12(2):e0171308. [DOI] [PMC free article] [PubMed]
- 50.Ding C., Fan W., Gu L., Tian H., Ge X., Gong J. Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: results from a prospective study with long-term follow-up. Gastroenterol Rep. 2018 May;6(2):101–107. doi: 10.1093/gastro/gox036. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ding C, Fan W, Gu L, Tian H, Ge X, Gong J, et al. Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: results from a prospective study with long-term follow-up. Gastroenterol Rep. 2018 May;6(2):101–7. [DOI] [PMC free article] [PubMed]
- 51.Zhang X., Tian H., Gu L., Nie Y., Ding C., Ge X. Long-term follow-up of the effects of fecal microbiota transplantation in combination with soluble dietary fiber as a therapeutic regimen in slow transit constipation. Sci China Life Sci. 2018 Jul;61(7):779–786. doi: 10.1007/s11427-017-9229-1. [DOI] [PubMed] [Google Scholar]; Zhang X, Tian H, Gu L, Nie Y, Ding C, Ge X, et al. Long-term follow-up of the effects of fecal microbiota transplantation in combination with soluble dietary fiber as a therapeutic regimen in slow transit constipation. Sci China Life Sci. 2018 Jul;61(7):779–86. [DOI] [PubMed]
- 52.Gu L., Ding C., Tian H., Yang B., Zhang X., Hua Y. Serial frozen fecal microbiota transplantation in the treatment of chronic intestinal pseudo-obstruction: a preliminary study. J Neurogastroenterol Motil. 2017 Apr;23(2):289–297. doi: 10.5056/jnm16074. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gu L, Ding C, Tian H, Yang B, Zhang X, Hua Y, et al. Serial Frozen Fecal Microbiota Transplantation in the Treatment of Chronic Intestinal Pseudo-obstruction: A Preliminary Study. J Neurogastroenterol Motil. 2017 Apr;23(2):289–97. [DOI] [PMC free article] [PubMed]
- 53.Singh R., van Nood E., Nieuwdorp M., van Dam B., Ten Berge I.J.M., Geerlings S.E. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin Microbiol Infect. 2014 Nov;20(11) doi: 10.1111/1469-0691.12683. (O977–8) [DOI] [PubMed] [Google Scholar]; Singh R, van Nood E, Nieuwdorp M, van Dam B, ten Berge IJM, Geerlings SE, et al. Donor feces infusion for eradication of Extended Spectrum beta-Lactamase producing Escherichia coli in a patient with end stage renal disease. Clin Microbiol Infect. 2014 Nov;20(11):O977-8. [DOI] [PubMed]
- 54.Singh R., de Groot P.F., Geerlings S.E., Hodiamont C.J., Belzer C., Ten Berge I.J.M. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: a proof of principle study. BMC Res Notes. 2018 Mar;11(1):190. doi: 10.1186/s13104-018-3293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Singh R, de Groot PF, Geerlings SE, Hodiamont CJ, Belzer C, Berge IJM Ten, et al. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: a proof of principle study. BMC Res Notes. 2018 Mar;11(1):190. [DOI] [PMC free article] [PubMed]
- 55.Dinh A., Fessi H., Duran C., Batista R., Michelon H., Bouchand F. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 2018 Aug;99(4):481–486. doi: 10.1016/j.jhin.2018.02.018. [DOI] [PubMed] [Google Scholar]; Dinh A, Fessi H, Duran C, Batista R, Michelon H, Bouchand F, et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 2018 Aug;99(4):481–6. [DOI] [PubMed]
- 56.Davido B., Batista R., Fessi H., Michelon H., Escaut L., Lawrence C. Fecal microbiota transplantation to eradicate vancomycin-resistant enterococci colonization in case of an outbreak. Med Mal Infect. 2019 May;49(3):214–218. doi: 10.1016/j.medmal.2018.11.002. [DOI] [PubMed] [Google Scholar]; Davido B, Batista R, Fessi H, Michelon H, Escaut L, Lawrence C, et al. Fecal microbiota transplantation to eradicate vancomycin-resistant enterococci colonization in case of an outbreak. Med Mal Infect. 2019 May;49(3):214-218. [DOI] [PubMed]
- 57.Bilinski J., Grzesiowski P., Sorensen N., Madry K., Muszynski J., Robak K. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective. Single-Center Study Clin Infect Dis. 2017 Aug;65(3):364–370. doi: 10.1093/cid/cix252. [DOI] [PubMed] [Google Scholar]; Bilinski J, Grzesiowski P, Sorensen N, Madry K, Muszynski J, Robak K, et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin Infect Dis. 2017 Aug;65(3):364–70. [DOI] [PubMed]
- 58.Huttner B.D., de Lastours V., Wassenberg M., Maharshak N., Mauris A., Galperine T. A five-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant enterobacteriaceae: a randomized clinical trial. Clin Microbiol Infect. 2019 Jan 25 doi: 10.1016/j.cmi.2018.12.009. https://www.ncbi.nlm.nih.gov/pubmed/30616014 (pii: S1198-743X(19)30030–8) [DOI] [PubMed] [Google Scholar]; Huttner BD, de Lastours V, Wassenberg M, Maharshak N, Mauris A, Galperine T, et al. A five-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A Randomized Clinical Trial. Clin Microbiol Infect. 2019 Jan 25. pii: S1198-743X(19)30030-8. [DOI] [PubMed]
- 59.de Groot P.F., Frissen M.N., de Clercq N.C., Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes. 2017 May;8(3):253–267. doi: 10.1080/19490976.2017.1293224. [DOI] [PMC free article] [PubMed] [Google Scholar]; de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes. 2017 May;8(3):253–67. [DOI] [PMC free article] [PubMed]
- 60.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]; Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec;444(7122):1027–31. [DOI] [PubMed]
- 61.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F.W.M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916. doi: 10.1053/j.gastro.2012.06.031. (e7) [DOI] [PubMed] [Google Scholar]; Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916.e7. [DOI] [PubMed]
- 62.Kootte R.S., Levin E., Salojärvi J., Smits L.P., Hartstra A.V., Udayappan S.D. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017 Oct;26(4):611–619. doi: 10.1016/j.cmet.2017.09.008. (e6) [DOI] [PubMed] [Google Scholar]; Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra A V., Udayappan SD, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017 Oct;26(4):611–619.e6. [DOI] [PubMed]
- 63.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009 Jun;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]; Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009 Jun;49(6):1877–87. [DOI] [PubMed]
- 64.Zhou D., Pan Q., Shen F., Cao H.-X., Ding W.-J., Chen Y.-W. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017 May;7(1):1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou D, Pan Q, Shen F, Cao H-X, Ding W-J, Chen Y-W, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017 May;7(1):1529. [DOI] [PMC free article] [PubMed]
- 65.Peng J., Xiao X., Hu M., Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018 Dec;214:153–157. doi: 10.1016/j.lfs.2018.10.063. [DOI] [PubMed] [Google Scholar]; Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018 Dec;214:153–7. [DOI] [PubMed]
- 66.Brandsma E., Kloosterhuis N.J., Koster M.H., Dekker D., Gijbels M., van der Velden S. A pro-inflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res. 2019 Jan 4;124(1):94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brandsma E, Kloosterhuis NJ, Koster MH, Dekker D, Gijbels M, van der Velden S, et al. A Pro-Inflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ Res. 2019 Jan 4;124(1):94-100. [DOI] [PMC free article] [PubMed]
- 67.Hu X.-F., Zhang W.-Y., Wen Q., Chen W.-J., Wang Z.-M., Chen J. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. 2018 Nov;139:412–421. doi: 10.1016/j.phrs.2018.11.042. [DOI] [PubMed] [Google Scholar]; Hu X-F, Zhang W-Y, Wen Q, Chen W-J, Wang Z-M, Chen J, et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. 2018 Nov;139:412–21. [DOI] [PubMed]
- 68.Smits L.P., Kootte R.S., Levin E., Prodan A., Fuentes S., Zoetendal E.G. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018 Mar;7(7) doi: 10.1161/JAHA.117.008342. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018 Mar;7(7). [DOI] [PMC free article] [PubMed]
- 69.Zhang Z., Zhai H., Geng J., Yu R., Ren H., Fan H. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013 Oct;108(10):1601–1611. doi: 10.1038/ajg.2013.221. [DOI] [PubMed] [Google Scholar]; Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, et al. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013 Oct;108(10):1601–11. [DOI] [PubMed]
- 70.Bajaj J.S., Kassam Z., Fagan A., Gavis E.A., Liu E., Cox I.J. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017 Dec;66(6):1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017 Dec;66(6):1727–38. [DOI] [PMC free article] [PubMed]
- 71.Philips C.A., Pande A., Shasthry S.M., Jamwal K.D., Khillan V., Chandel S.S. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017 Apr;15(4):600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]; Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017 Apr;15(4):600–2. [DOI] [PubMed]
- 72.Slykerman R.F., Thompson J., Waldie K.E., Murphy R., Wall C., Mitchell E.A. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2017 Jan;106(1):87–94. doi: 10.1111/apa.13613. [DOI] [PubMed] [Google Scholar]; Slykerman RF, Thompson J, Waldie KE, Murphy R, Wall C, Mitchell EA. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2017 Jan;106(1):87–94. [DOI] [PubMed]
- 73.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013 Dec 19;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013 Dec 19;155(7):1451–63. [DOI] [PMC free article] [PubMed]
- 74.Kang D.-W., Adams J.B., Gregory A.C., Borody T., Chittick L., Fasano A. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017 Jan;5(1):10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017 Jan;5(1):10. [DOI] [PMC free article] [PubMed]
- 75.Freedman S.N., Shahi S.K., Mangalam A.K. The “gut feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics. 2018 Jan;15(1):109–125. doi: 10.1007/s13311-017-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Freedman SN, Shahi SK, Mangalam AK. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics. 2018 Jan;15(1):109–25. [DOI] [PMC free article] [PubMed]
- 76.Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114(40):10719–24. [DOI] [PMC free article] [PubMed]
- 77.Makkawi S., Camara-Lemarroy C., Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol neuroinflammation. 2018 Jul;5(4) doi: 10.1212/NXI.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]; Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol neuroinflammation. 2018 Jul;5(4):e459. [DOI] [PMC free article] [PubMed]
- 78.Borody T, SM L, J C, M T, A N. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am J Gastroenterol 2011;106:S352.
- 79.Minato T., Maeda T., Fujisawa Y., Tsuji H., Nomoto K., Ohno K. Progression of Parkinson's disease is associated with gut dysbiosis: two-year follow-up study. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]; Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, et al. Progression of Parkinson'’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS One. 2017;12(11):e0187307. [DOI] [PMC free article] [PubMed]
- 80.Sun M.-F., Zhu Y.-L., Zhou Z.-L., Jia X.-B., Xu Y.-D., Yang Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. 2018 May;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]; Sun M-F, Zhu Y-L, Zhou Z-L, Jia X-B, Xu Y-D, Yang Q, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson'’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. 2018 May;70:48–60. [DOI] [PubMed]
- 81.Liu C., Frank D.N., Horch M., Chau S., Ir D., Horch E.A. Associations between acute gastrointestinal GvHD and the baseline gut microbiota of allogeneic hematopoietic stem cell transplant recipients and donors. Bone Marrow Transplant. 2017 Dec;52(12):1643–1650. doi: 10.1038/bmt.2017.200. [DOI] [PubMed] [Google Scholar]; Liu C, Frank DN, Horch M, Chau S, Ir D, Horch EA, et al. Associations between acute gastrointestinal GvHD and the baseline gut microbiota of allogeneic hematopoietic stem cell transplant recipients and donors. Bone Marrow Transplant. 2017 Dec;52(12):1643–50. [DOI] [PubMed]
- 82.Mathewson N.D., Jenq R., Mathew A.V., Koenigsknecht M., Hanash A., Toubai T. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mathewson ND, Jenq R, Mathew A V, Koenigsknecht M, Hanash A, Toubai T, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–13. [DOI] [PMC free article] [PubMed]
- 83.Kakihana K., Fujioka Y., Suda W., Najima Y., Kuwata G., Sasajima S. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016 Oct;128(16):2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016 Oct;128(16):2083–8. [DOI] [PMC free article] [PubMed]
- 84.Qi X., Li X., Zhao Y., Wu X., Chen F., Ma X. Treating steroid refractory intestinal acute graft-vs.-host disease with fecal microbiota transplantation: a pilot study. Front Immunol. 2018;9:2195. doi: 10.3389/fimmu.2018.02195. [DOI] [PMC free article] [PubMed] [Google Scholar]; Qi X, Li X, Zhao Y, Wu X, Chen F, Ma X, et al. Treating Steroid Refractory Intestinal Acute Graft-vs.-Host Disease With Fecal Microbiota Transplantation: A Pilot Study. Front Immunol. 2018;9:2195. [DOI] [PMC free article] [PubMed]
- 85.Spindelboeck W., Schulz E., Uhl B., Kashofer K., Aigelsreiter A., Zinke-Cerwenka W. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica. 2017 May;102(5) doi: 10.3324/haematol.2016.154351. (e210–3) [DOI] [PMC free article] [PubMed] [Google Scholar]; Spindelboeck W, Schulz E, Uhl B, Kashofer K, Aigelsreiter A, Zinke-Cerwenka W, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica. 2017 May;102(5):e210–3. [DOI] [PMC free article] [PubMed]
- 86.DeFilipp Z., Peled J.U., Li S., Mahabamunuge J., Dagher Z., Slingerland A.E. Third-party fecal microbiota transplantation following Allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018 Apr;2(7):745–753. doi: 10.1182/bloodadvances.2018017731. [DOI] [PMC free article] [PubMed] [Google Scholar]; DeFilipp Z, Peled JU, Li S, Mahabamunuge J, Dagher Z, Slingerland AE, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018 Apr;2(7):745–53. [DOI] [PMC free article] [PubMed]
- 87.Boursi B., Mamtani R., Haynes K., Yang Y.-X. Recurrent antibiotic exposure may promote cancer formation--another step in understanding the role of the human microbiota? Eur J Cancer. 2015 Nov;51(17):2655–2664. doi: 10.1016/j.ejca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Boursi B, Mamtani R, Haynes K, Yang Y-X. Recurrent antibiotic exposure may promote cancer formation--Another step in understanding the role of the human microbiota? Eur J Cancer. 2015 Nov;51(17):2655–64. [DOI] [PMC free article] [PubMed]
- 88.Dai Z., Zhang J., Wu Q., Chen J., Liu J., Wang L. The role of microbiota in the development of colorectal cancer. Int J Cancer. 2018 Nov 26 doi: 10.1002/ijc.32017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dai Z, Zhang J, Wu Q, Chen J, Liu J, Wang L, et al. The role of microbiota in the development of colorectal cancer. Int J cancer. 2018 Nov 26. doi: 10.1002/ijc.32017.
- 89.Wong S.H., Zhao L., Zhang X., Nakatsu G., Han J., Xu W. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017 Dec;153(6):1621–1633. doi: 10.1053/j.gastro.2017.08.022. (e6) [DOI] [PubMed] [Google Scholar]; Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of Fecal Samples From Patients With Colorectal Cancer Promotes Intestinal Carcinogenesis in Germ-Free and Conventional Mice. Gastroenterology. 2017 Dec;153(6):1621–1633.e6. [DOI] [PubMed]
- 90.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillere R. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018 Jan;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]; Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018 Jan;359(6371):91–7. [DOI] [PubMed]
- 91.Wang Y., Wiesnoski D.H., Helmink B.A., Gopalakrishnan V., Choi K., DuPont H.L. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018 Dec;24(12):1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018 Dec;24(12):1804–8. [DOI] [PMC free article] [PubMed]
- 92.Wardill H.R., Secombe K.R., Bryant R.V., Hazenberg M.D., Costello S.P. Adjunctive fecal microbiota transplantation in supportive oncology: emerging indications and considerations in immunocompromised patients. EBioMedicine. 2019 Mar 30 doi: 10.1016/j.ebiom.2019.03.070. https://www.ncbi.nlm.nih.gov/pubmed/30940601 (pii: S2352–3964(19)30215–4) [DOI] [PMC free article] [PubMed] [Google Scholar]; Wardill HR, Secombe KR, Bryant RV, Hazenberg MD, Costello SP. Adjunctive fecal microbiota transplantation in supportive oncology: Emerging indications and considerations in immunocompromised patients. EBioMedicine. 2019 Mar 30. pii: S2352-3964(19)30215-4. [DOI] [PMC free article] [PubMed]
- 93.Wei Y., Yang J., Wang J., Yang Y., Huang J., Gong H. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016 Oct;20(1):332. doi: 10.1186/s13054-016-1491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016 Oct;20(1):332. [DOI] [PMC free article] [PubMed]
- 94.Fang S., Kraft C.S., Dhere T., Srinivasan J., Begley B., Weinstein D. Successful treatment of chronic Pouchitis utilizing fecal microbiota transplantation (FMT): a case report. Int J Colorectal Dis. 2016 May;31(5):1093–1094. doi: 10.1007/s00384-015-2428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fang S, Kraft CS, Dhere T, Srinivasan J, Begley B, Weinstein D, et al. Successful treatment of chronic Pouchitis utilizing fecal microbiota transplantation (FMT): a case report. Int J Colorectal Dis. 2016 May;31(5):1093–4. [DOI] [PMC free article] [PubMed]
- 95.van Beurden Y.H., van Gils T., van Gils N.A., Kassam Z., Mulder C.J.J., Aparicio-Pages N. Serendipity in refractory celiac disease: full recovery of duodenal villi and clinical symptoms after fecal microbiota transfer. J Gastrointestin Liver Dis. 2016 Sep;25(3):385–388. doi: 10.15403/jgld.2014.1121.253.cel. [DOI] [PubMed] [Google Scholar]; van Beurden YH, van Gils T, van Gils NA, Kassam Z, Mulder CJJ, Aparicio-Pages N. Serendipity in Refractory Celiac Disease: Full Recovery of Duodenal Villi and Clinical Symptoms after Fecal Microbiota Transfer. J Gastrointestin Liver Dis. 2016 Sep;25(3):385–8. [DOI] [PubMed]
- 96.Hirsch B.E., Saraiya N., Poeth K., Schwartz R.M., Epstein M.E., Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15(1):1–9. doi: 10.1186/s12879-015-0930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15(1):1–9. [DOI] [PMC free article] [PubMed]
- 97.Allegretti J.R., Fischer M., Sagi S.V., Bohm M.E., Fadda H.M., Ranmal S.R. Fecal microbiota transplantation capsules with targeted colonic versus gastric delivery in recurrent Clostridium difficile infection: a comparative cohort analysis of high and lose dose. Dig Dis Sci. 2019 Jun;64(6):1672–1678. doi: 10.1007/s10620-018-5396-6. [DOI] [PubMed] [Google Scholar]; Allegretti JR, Fischer M, Sagi S V, Bohm ME, Fadda HM, Ranmal SR, et al. Fecal Microbiota Transplantation Capsules with Targeted Colonic Versus Gastric Delivery in Recurrent Clostridium difficile Infection: A Comparative Cohort Analysis of High and Lose Dose. Dig Dis Sci. 2019 Jun;64(6):1672-1678. [DOI] [PubMed]
- 98.Barr J.J., Auro R., Furlan M., Whiteson K.L., Erb M.L., Pogliano J. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10771–6. [DOI] [PMC free article] [PubMed]
- 99.Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2016/11/22. 2017;152(4):799–811.(e7). [DOI] [PubMed]
- 100.Draper L.A., Ryan F.J., Smith M.K., Jalanka J., Mattila E., Arkkila P.A. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6(1):220–229. doi: 10.1186/s40168-018-0598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Draper LA, Ryan FJ, Smith MK, Jalanka J, Mattila E, Arkkila PA, et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6(1):220–9. [DOI] [PMC free article] [PubMed]
- 101.Zmora N., Zilberman-Schapira G., Suez J., Mor U., Dori-Bachash M., Bashiardes S. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405. doi: 10.1016/j.cell.2018.08.041. (e21) [DOI] [PubMed] [Google Scholar]; Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. 2018;174(6):1388–1405.e21. [DOI] [PubMed]
- 102.Smits L.P., Bouter K.E.C., de Vos W.M., Borody T.J., Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013 Nov;145(5):946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]; Smits LP, Bouter KEC, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013 Nov;145(5):946–53. [DOI] [PubMed]
- 103.Cammarota G., Ianiro G., Tilg H., Rajilic-Stojanovic M., Kump P., Satokari R. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017 Apr;66(4):569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017 Apr;66(4):569–80. [DOI] [PMC free article] [PubMed]