Abstract
Background
A positive energy balance promotes white adipose tissue (WAT) expansion which is characterized by activation of a repertoire of events including hypoxia, inflammation and extracellular matrix remodelling. The transmembrane glycoprotein CD248 has been implicated in all these processes in different malignant and inflammatory diseases but its potential impact in WAT and metabolic disease has not been explored.
Methods
The role of CD248 in adipocyte function and glucose metabolism was evaluated by omics analyses in human WAT, gene knockdowns in human in vitro differentiated adipocytes and by adipocyte-specific and inducible Cd248 gene knockout studies in mice.
Findings
CD248 is upregulated in white but not brown adipose tissue of obese and insulin-resistant individuals. Gene ontology analyses showed that CD248 expression associated positively with pro-inflammatory/pro-fibrotic pathways. By combining data from several human cohorts with gene knockdown experiments in human adipocytes, our results indicate that CD248 acts as a microenvironmental sensor which mediates part of the adipose tissue response to hypoxia and is specifically perturbed in white adipocytes in the obese state. Adipocyte-specific and inducible Cd248 knockouts in mice, both before and after diet-induced obesity and insulin resistance/glucose intolerance, resulted in increased microvascular density as well as attenuated hypoxia, inflammation and fibrosis without affecting fat cell volume. This was accompanied by significant improvements in insulin sensitivity and glucose tolerance.
Interpretation
CD248 exerts detrimental effects on WAT phenotype and systemic glucose homeostasis which may be reversed by suppression of adipocyte CD248. Therefore, CD248 may constitute a target to treat obesity-associated co-morbidities.
Keywords: Adipocyte, Type 2 diabetes, Obesity, Mouse models, Endosialin, Fibrosis, Inflammation, Hypoxia, Angiogenesis, Insulin sensitivity, Glucose metabolism, Gene microarrays
Research in context.
Evidence before this study
White adipose tissue (WAT) is a highly dynamic organ that responds to changes in nutritional stresses and energy demands. Following a brief excess of food intake, WAT acutely expands to store energy in the form of lipids. In so doing, healthy WAT becomes mildly hypoxic, accommodating to the stress with a transient but effective inflammatory, angiogenic and extracellular matrix remodelling response, thereby returning it to a state of metabolic homeostasis. However, with chronic overfeeding, adipose tissue expansion exceeds the capacity of the angiogenic response. Hypoxia of the adipose tissue persists, with ensuing chronic inflammation, fibrosis, and generation of unhealthy adipose tissue. This results in ectopic lipid deposition, systemic insulin resistance and type 2 diabetes. Factors that determine the transition of adipose tissue from a healthy to an unhealthy phenotype remain unclear.
CD248 is a transmembrane glycoprotein that participates in hypoxic regulation, angiogenesis, inflammation and reorganization of the extracellular matrix. It is expressed postnatally at low levels in stromal and perivascular cells, but increased in the setting of tumor growth, inflammation and injuries associated with fibrosis. Lack of Cd248 in mice confers protection against the growth of some tumors and several inflammatory diseases, including arthritis, liver injury and atherosclerosis. Its potential role in the function of white adipose tissue and metabolic disease has not been explored.
Added value of this study
Our examinations of WAT from various human cohorts reveal that CD248 is expressed in mature white adipocytes, and that transcript levels correlate tightly and positively with obesity and insulin resistance. The findings suggest that CD248 may be a more sensitive marker of gluco-metabolic dysfunction than currently available. Gene ontology analyses, coupled with adipocyte and adipose tissue culture studies and gene knockdowns, support a role for CD248 in modulating the response of adipocytes to hypoxia and promoting inflammation, fibrosis and extracellular matrix remodelling. This was confirmed in a series of global and adipocyte-specific Cd248 gene knockouts in mice. Indeed, deletion of Cd248 in murine adipocytes reduces WAT hypoxia, inflammation and fibrosis, promotes WAT angiogenesis and improves glucose tolerance and insulin sensitivity. Strikingly, these improvements can be achieved in mice also after the onset of obesity and diabetes. Overall, we identify CD248 as an adipocyte sensor of the microenvironment that mediates the transition from healthy to unhealthy adipose.
Implications of all the available evidence
This study uncovers CD248 as a sensitive marker of adipocyte function, increased expression of which leads to activation of several pathways that result in disturbances in glucose metabolism and ectopic deposition of lipids. The tight correlation between adipocyte CD248, obesity and insulin resistance, together with our interventional studies in mice, suggest that it might not only be a valuable predictive marker of disordered glucose metabolism, but also a potential therapeutic target to reverse malfunctioning WAT and improve metabolic health.
Alt-text: Unlabelled Box
1. Introduction
Obesity results from an imbalance in energy homeostasis where surplus energy is stored in white adipose tissue (WAT) [1]. Increased WAT mass associates with the development of a number of cardiometabolic complications including insulin resistance/type 2 diabetes, dyslipidemia, hypertension and cardiovascular disease. While several different factors may contribute, it is clear that these conditions are closely linked to changes in WAT phenotype including adipocyte insulin resistance, local hypoxia, a chronic low-grade inflammation with leukocyte infiltration, predominantly comprising pro-inflammatory M1 versus alternatively activated M2 macrophages [2], and increased fibrosis with extracellular matrix (ECM) deposition [3,4]. These processes are believed to limit further WAT expansion, resulting in ectopic lipid deposition in peripheral organs which in turn lead to systemic insulin resistance and metabolic disturbances. Data suggest that the events needed for healthy WAT expansion are the same as those that become detrimental in response to a continuous positive energy balance [4]. Hence, short-term overfeeding leads to mild hypoxia and transient activation of inflammation. This allows the ECM to remodel and adipocyte precursors to differentiate into mature fat cells. In contrast, long-term over feeding leads to chronic hypoxia, low grade inflammation and fibrosis. Yet, little is known about the factors promoting the unresolved activation of these processes.
CD248 (endosialin/tumor endothelial marker 1) [5] is a type I transmembrane glycoprotein expressed at low levels postnatally in cells of mesenchymal origin (stromal fibroblasts, pericytes, vascular smooth muscle cells, osteoblasts) [6,7]. However, it is markedly upregulated in the setting of tumor growth, inflammation [8], and injury-induced fibrosis of several organs, including the liver [9] and kidney [10]. It is well-established that CD248 participates in vascular remodelling in part, by inducing an excessive hypoxic response characterized by activation of the transcription factors hypoxia inducible factor-1α (HIF-1α) and HIF-2α, as well as increased release/expression of pro-angiogenic factors [[11], [12], [13]]. This is in turn, accompanied by a pro-inflammatory state with increased recruitment of activated leukocytes, enhanced release of inflammatory cytokines, and upregulation of pro-fibrotic genes involved in remodelling of the ECM [14]. In line with these findings, global inactivation of Cd248 in mice attenuates disease severity in several models of human disease, including inflammatory arthritis [12] and atherosclerosis [8], with diminished tissue infiltration of pro-inflammatory leukocytes and reduced circulating levels of cyto−/chemokines such as IL1β, CCL2 and CCL5. Lack of Cd248 furthermore, ameliorates inflammation and fibrosis in models of acute and chronic liver injury [9] and renal fibrosis [15]. In concordance, humans with the corresponding diseases exhibit elevated expression levels of CD248 in their stromal fibroblasts which associates with disease severity [10].
The established role of CD248 in hypoxia, inflammation and ECM formation in other tissues led us to hypothesize that it could be involved in determining WAT function. Herein, based on analyses in several independent clinical cohorts, we demonstrate that CD248 expression in adipocytes associates positively with a pernicious WAT and metabolic phenotype. Furthermore, results in primary human adipocytes, corroborated by adipocyte-specific and inducible Cd248 gene knock-outs in mice, suggest that CD248 could be a potential target to improve WAT dysfunction and metabolic health in the obese and overweight state.
2. Materials and methods
2.1. Human cohorts
Five clinical cohorts were included all of which have been described in detail elsewhere [16]. Cohort 1 consisted of 26 non-obese (BMI: 24.1 ± 1.8, age: 43 ± 14) and 30 obese (BMI: 40.9 ± 6.9, age: 43 ± 10) women. Cohort 2 consisted of 15 obese women followed before and 2 years after bariatric surgery. At the follow-up, they were matched for BMI and age with 15 never-obese control women. Cohort 3 consisted of 40 obese insulin sensitive (BMI: 39.1 ± 3.0, age: 36 ± 6) and 40 obese insulin resistant (BMI: 42.7 ± 4.7, age: 36 ± 6) women subdivided according to homeostasis model of assessment insulin resistance (HOMA-IR) [17]. Cohort 4 included 27 non-obese men with (n = 14) (BMI: 25.9 ± 1.4, age: 57 ± 11) or without (n = 13) (BMI: 26.6 ± 1.6, age: 61 ± 9) type 2 diabetes [18]. Cohort 5 included 31 lean and obese women where gene expression analyses were performed on intact abdominal subcutaneous WAT and isolated adipocytes from the same samples [19]. Gene microarray data from cohorts 1 and 2 have been reported previously in the respective publications. Gene expression analyses in cohort 4 were performed by quantitative PCR as described [20]. Adipocytokine release from intact WAT in cohort 1 was determined by ELISA as detailed in the original publication [16].
2.2. Human white and brown adipose tissue
Excisional biopsies of adipose from the supraclavicular region of healthy human volunteers were performed at room temperature (20-22C°) under local anesthesia (lidocaine-epinephrine) by an experienced plastic surgeon. The site for the brown adipose tissue (BAT) biopsy was determined by PET/CT imaging based on accumulation of [18F]FDG. A subcutaneous white adipose tissue (WAT) sample was excised during the procedure via the same incision. Immediately after sampling, part of the tissue was fixed in formalin for immuno-histochemical analysis, and the rest of the sample was snap-frozen in liquid nitrogen.
2.3. Mice
Mice were housed in the Centre for Disease Modelling at the University of British Columbia, which is a 12:12 light:dark cycled, temperature and humidity-controlled, specific pathogen-free animal facility. Mice lacking Cd248 (Cd248−/−, KO) on a C57Bl6 background were previously generated [12]. Heterozygous (Cd248+/−) mice were interbred to generate Cd248+/+ (WT) and Cd248−/− (KO) littermates. For diet-induced obesity studies, mice were fed either a high-fat diet (HFD) (D12492, 60% fat caloric content, Research Diets, New Brunswick, NJ, USA) or low-fat diet (LFD) (D12450B, 10% fat caloric content, Research Diets). All animals were placed on the high/low fat diets at 7 weeks of age.
C57BL/6-Tg(Adipoq-cre/ERT2)1Soff/J (cat #025124) (adiponectin-cre-ERT2) mice were purchased from Jackson Labs (Bar Harbor, Maine, USA). Mice that lack Cd248 in mature adipocytes were generated by first cross-breeding adiponectin-cre-ERT2 mice [21,22] with Cd248lox/lox mice [12] to yield adiponectin-Cre-ERT2:Cd248lox/lox mice. At the indicated ages, in vivo excision of Cd248 from mature adipocytes was achieved by 5 daily ip injections of tamoxifen (Sigma Aldrich, cat #T5648) 75 mg/kg body weight, diluted at a concentration of 10% m/v in a solution of corn oil and ethanol, 9:1 v/v. Control mice were siblings with the same gene modifications, but were injected with the vehicle alone (Oil), without tamoxifen. Alternatively, Cd248lox/lox mice were generated with or without the adiponectin-cre-ERT2 transgene; these were placed on a HFD from the age of 6 wks, followed by a 5-day course of tamoxifen starting at 11 wks of age, as described in the Results.
2.4. Human adipocyte cultures
In vitro differentiated human adipocytes were established, cultured and differentiated as described [23] In brief, adipocyte precursors were obtained from the stromal vascular fraction (SVF) of abdominal subcutaneous WAT from subjects undergoing cosmetic surgery. In these subjects only BMI, age and gender was known.
2.5. Oil Red-O staining
Differentiated human adipocytes were washed three times with PBS (phosphate-buffered saline) and then fixed with 4% formaldehyde for 1 h. Oil Red-O (0.3% in isopropanol) was diluted with water (3:2), filtered through a 0.22 μm filter, and incubated with the fixed cells for 30 min at room temperature. Cells were then washed with water, whereupon stained neutral fat droplets were visualized by light microscopy and photographed. Oil Red-O was thereafter extracted using isopropanol and absorbance was measured at 570 nm for quantification.
2.6. RNA interference and hypoxia-reporter assays
CD248 expression was knocked down by RNAi in human in vitro differentiated adipocytes at day 10 of differentiation using 40 nM siRNA oligonucleotides (M-010720-00-0005, Dharmacon) and compared with non-silencing control RNAi (D-001206-13-05). The electroporation of siRNA was combined with 1 μg of plasmids containing the hypoxia response element (HRE) (previously described in [24]) coupled to a luciferase reporter and 0.1 μg Renilla. Cells were electroporated at day 10 of differentiation as previously described [19] and were then incubated in a hypoxic chamber at day 12 and harvested for subsequent analysis at day 13. Luciferase activity was measured in cell lysates using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions.
2.7. Gene-ontology analysis
Expression of CD248 in cohort 1 was correlated with the expression of all other coding genes measured by the microarray. Genes with a Pearson's r > 0.5 were included in the GO-analysis, which was performed using a web tool (https://toppgene.cchmc.org) with the gene limit in the included pathways set at >10 and <1000.
2.8. Gene microarray
Micro array was performed using Clariom™ S Assay, human (Catalog number: 902926) and analyzed as previously described [19]. Principal component analyses were performed using a previously described web tool (Metsalu, Tauno and Vilo, Jaak. Clustvis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Research, 43(W1):W566–W570, 2015. doi: https://doi.org/10.1093/nar/gkv468).
2.9. Metabolic studies in mice
Glucose tolerance tests (GTT) were performed by fasting mice for 4 h prior to intraperitoneal (ip) injection of glucose at a dose of 1 mg/kg body weight, followed by periodic saphenous vein bleeds over 90–120 min, for immediate blood glucose measurements with a glucometer (OneTouch UltraMini, Life Scan, Milpitas, CA). Insulin tolerance tests (ITT) were performed by ip injection of 0.75 units insulin/kg body weight (Novolin, Novo Nordisk, Denmark) followed by quantification of blood glucose levels at time intervals as noted. Area under the curves (AUCs) were calculated using the manufacturer's algorithm in the PRISM software package (GraphPad, San Diego, CA). Body composition was determined by dual-energy x-ray absorptiometry (DEXA) using a PIXImus Mouse Densitometer (Lunar Corporation, Madison, WI). Food and water intake were measured for 72 h with PhenoMaster metabolic cages (TSE Systems, Bad Homburg, Germany), after a 72 h acclimatization period [25].
2.10. Quantitative real-time PCR (qRT-PCR)
RNA was extracted from tissue lysates using the RNeasy Mini kit, as per the manufacturer's protocol (Qiagen, Mississauga, ON, Canada) or Nucleospin RNA II kit (Machery-Nagel, Düren, Germany). Isolated RNA was quantified and tested for the presence of contaminants using a NanoDrop 1000 or 2000 spectrophotometer (ThermoFisher). The qScript cDNA synthesis kit (Quanta Biosciences, Gaithersburg, MD, USA) or iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) was used to generate a cDNA library from isolated RNA samples. Relative transcript levels were analyzed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) or Thermal cycler CFX96 (Bio-Rad). Custom pre-designed primers (Taqman® Gene Expression Assays, ThermoFisher Scientific) for genes of interest were used. Gene expression was normalized to the Hprt housekeeping genes, with relative expression levels analyzed using the 2-∆∆Ct method and expression levels relative to Hprt were determined using the Expression Suite Software (Version 1.0.3, ThermoFisher Scientific).
2.11. Quantification of plasma levels of growth factors and adipokines
Plasma levels of Ccl2 and interleukin 6 (IL6) were quantified with specific ELISAs from Quantikine, R&D Systems (Minnesota, MN). ELISA kits from ALPCO (Salem, NH, USA) were used to measure plasma levels of adiponectin. Assays were performed in triplicate.
2.12. Histology and immunohistochemistry
Adipose depots were harvested and fixed overnight in 10% neutral buffered formalin. Sections obtained from paraffin-embedded tissues were stained with hematoxylin & eosin (H&E) using standard protocols. Formalin-fixed paraffin-embedded sections of murine adipose tissue were also used for immunodetection of specific antigens by first subjecting them to citrate buffer antigen retrieval prior to incubation with primary antibodies (rabbit anti-CD248 primary antibody (Proteintech, Chicago, IL, USA), rat monoclonal anti-F4/80 (Abcam #ab6640, 1:200), rat anti-mouse CD31 (BD Pharmingen, cat#550274, 1:200), or an isotype-matched control antibody. Immunostaining was detected using ultra-sensitive ABC peroxidase staining and metal enhanced DAB substrate kits (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's recommendations, or by immunofluorescence by methods as described [26], detecting with AF 488 goat anti-rat antibodies (green fluorescence, Invitrogen #A11006, 1:200) or AF 568 goat anti-rabbit antibodies (red fluorescence, Invitrogen #A11011, 1:200). Quantification of the area of adipocytes in H&E stained histologic sections was accomplished from 10 random high-resolution (20×) acquisitions per section using the automated software Adiposoft, as reported [27]. Alternatively, 6–10 random microscopic fields per section were imaged and fluorescence intensity was quantified with ImageJ software.
Average adipocyte size (Feret's diameter) in each animal was assessed using the ImageJ 1.45 software (National Institutes of Health, Bethesda, MD, USA) and the macro MRI's adipocyte tool. Adipocyte volume was calculated from the diameter using the following formula:V = 4/3 πr3. Adipocyte weight was obtained by multiplying the volume with the density of triglycerides which is 0.915 g/ml.
Adipose tissue hypoxia was measured by injecting mice with pimonidazole 60 mg/kg body weight intravenously ~10 min prior to sacrifice. Tissues were prepared as above and the Hydroxyprobe green kit (Hydroxyprobe Inc., Burlington, MA, #HP6) was used at a dilution of 1:50, according to the manufacturer's instructions. Detection was achieved with the AF 568 goat anti-rabbit antibody (Invitrogen #A11011, 1:200), and quantification was performed as above, using ImageJ software.
2.13. Ethics protocols
Experimental animal procedures were approved by the Institutional Animal Care Committee of the University of British Columbia. The described human studies were approved by the respective Institutional Review Boards and all subjects gave informed written consent to donate the tissue. Studies on BAT/WAT were performed on healthy volunteers at Turku University Hospital, Finland. Cohorts 1–4 were recruited and human adipocyte cultures were set up at the Karolinska Institute, Stockholm, Sweden. All described clinical studies were performed according to the statutes of the declaration of Helsinki.
2.14. Statistical methods
All data are expressed as means ± standard error of the mean (SEM) or standard deviation (SD) as noted. Significance was tested using 2-way analysis of variance (ANOVA) (P < .05), Kruskal-Wallis with uncorrected Dunn's post hoc test, or Student's t-test using P < .05.
2.15. Data deposition
Array data were uploaded to the GEO repository and assigned GEO accession number GSE131667. Supplementary files for “CD248 knockdown in in vitro differentiated adipocytes exposed to hypoxia” were assigned accession numbers GSM3801054-65.
3. Results
3.1. WAT CD248 expression is associated with metabolic disease
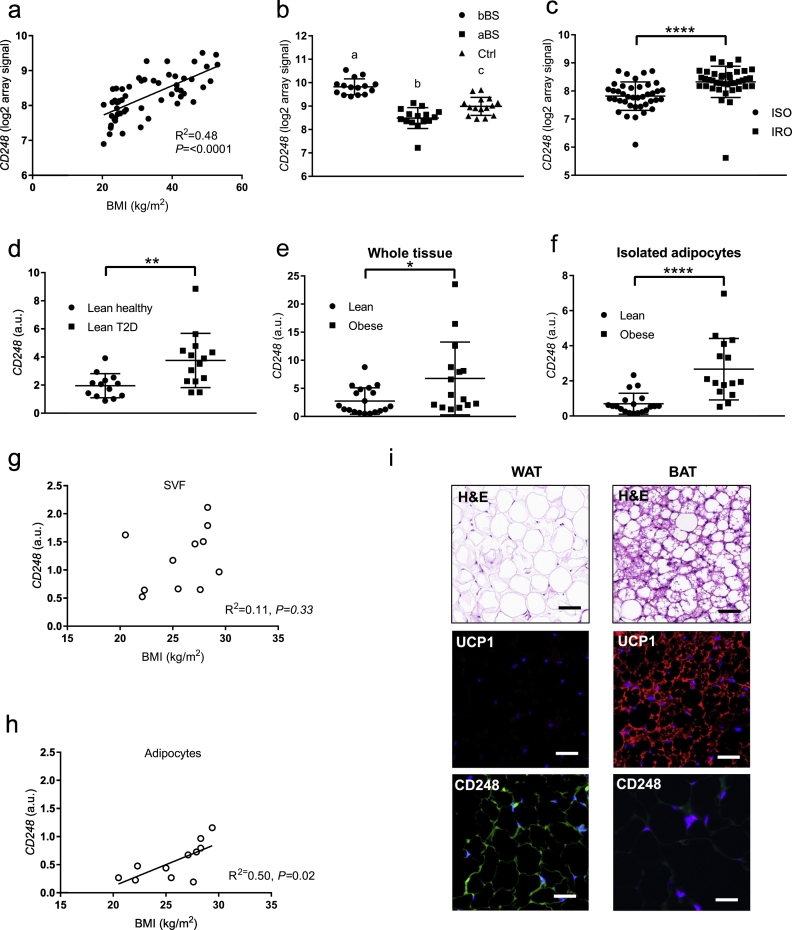
A query to the GTex Portal (Release V6p) of 53 human tissues showed that CD248 transcripts are most abundant in WAT. This, together with its demonstrated role in different processes of possible relevance for WAT, prompted us to map the expression of CD248 in human WAT. The associations between CD248 mRNA levels and clinical phenotypes were investigated in subcutaneous WAT samples from several independent cohorts. In 56 non-obese and obese women (cohort 1), CD248 expression correlated significantly with body mass index (BMI) (Fig. 1a). Hence, to evaluate additional clinical associations, BMI was included as an independent regressor in multiple regression analyses. This showed that CD248 was positively and BMI-independently associated with plasma glucose, insulin, insulin sensitivity (HOMA-IR), triglyceride levels, metabolic syndrome risk score (ATPIII) as well as fat cell volume (an adipocyte phenotype linked to metabolic complications) [28] (Table 1). The observed associations were strong, and the expression of CD248 associated much better with measures of metabolic health than BMI. In a second group of women (cohort 2), the expression of CD248 in WAT was significantly lower two years after bariatric surgery, as compared to before surgery (Fig. 1b, n = 15). Furthermore, the mRNA levels were reduced below the levels observed in age- and BMI-matched never-obese controls (Fig. 1b, n = 15). A BMI-independent association between CD248 expression and metabolic health was further supported by comparing the expression levels in samples of insulin sensitive obese (ISO, n = 40) and insulin resistant obese (IRO, n = 40) women (Fig. 1c, cohort 3) as well as non-obese men (cohort 4) subdivided into healthy (i.e. normal glucose tolerance, n = 13) and type 2 diabetes categories (Fig. 1d, n = 14).
Fig. 1.
Associations between CD248 and clinical parameters. a. The association between CD248 mRNA expression (array signal) and BMI in cohort 1. b. The expression levels of CD248 (array signal) in cohort 2 in obese women before (bBS) and after (aBS) bariatric surgery compared with that in never-obese controls. Significant differences at p < .05 between groups by ANOVA are indicated by connecting letters. c. The expression of CD248 (array) in cohort 3, obese women subdivided according to insulin sensitive (ISO) and insulin resistant (IRO). d. The expression of CD248 (by quantitative RT-PCR, qPCR) in cohort 4 consisting of non-obese men subdivided into healthy and T2D groups. e-f. Expression of CD248 (by qPCR) in paired samples from intact WAT (e) and isolated adipocytes (f) from cohort 5. g, h. Simple regression analyses of the association between BMI and CD248 (by qPCR) in stromal vascular cells of WAT (g) and isolated adipocytes (h). i. Representative microphotographs of hematoxylin & eosin (H&E) (upper panels) stainings of paired samples of WAT and brown adipose tissue (BAT) from adult human subjects. Immunohistochemistry of UCP1 and CD248 are shown. Blue represents DAPI staining of nuclei. Scale bar = 25 μm. In panels a, g and h, R2- and p-values are shown. In panels c-f, significant differences were determined by Student's unpaired t-test, * = p < .05, ** = p < .01 and **** = p < .0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Associations between clinical and WAT secretion parameters and CD248 gene expression by gene microarray and BMI. Results are derived from cohort 1. Analyses were performed by multiple regression using CD248 and BMI as independent regressors and each clinical/WAT variable as dependent regressor. Results are presented as standardized beta-coefficients and p-values for CD248 and BMI, respectively. Note that CD248 displays significantly stronger associations with every parameter compared with BMI.
| Independents |
||||
|---|---|---|---|---|
| CD248 |
BMI |
|||
| Std. β | P | Std. β | P | |
| P-glucose | 0.70 | 0.00003 | −0.18 | 0.250 |
| P-insulin | 0.52 | 0.0002 | 0.27 | 0.046 |
| Log HOMA-IR | 0.56 | 0.00003 | 0.27 | 0.032 |
| Log P-triglycerides | 0.49 | 0.004 | 0.11 | 0.511 |
| HDL | −0.25 | 0.117 | −0.41 | 0.014 |
| ATPIII risk score | 0.50 | 0.0007 | 0.24 | 0.091 |
| Adipocyte size | 0.31 | 0.009 | 0.55 | 0.00001 |
3.2. CD248 is associated with body weight only in the mature adipocyte fraction and is not expressed in brown adipocytes
WAT is heterogeneous, consisting of various cell types where mature adipocytes only constitute 20–40% of the cell population [3]. To map CD248 expression, we compared CD248 levels determined by gene microarray in different cell fractions of human WAT using recently published data [29]. This indicated that CD248 is expressed in all cell types including adipocytes, adipocyte progenitors and leukocytes/macrophages (Fig. S1a). Using qPCR, CD248 was confirmed to be expressed in whole WAT (Fig. 1e) and in the isolated mature adipocyte fraction of the same individuals (Fig. 1f, n = 34, cohort 5). In both cases, expression was significantly higher in the obese subjects (n = 15). In additional available samples (n = 11), CD248 was expressed in both the stromal vascular fraction (SVF, consisting of all cell types in WAT except mature adipocytes) and adipocytes (Fig. S1b, left panel). However, there was no significant association between CD248 expression and BMI in the SVF (Fig. 1g) while there was a significant positive correlation in isolated mature adipocytes (Fig. 1h). Results on gene expression were confirmed at the protein level by immunohistochemistry (Fig. 1i, left panels). In contrast to WAT, CD248 was not detectable in human brown adipose tissue (UCP1 staining used as a positive control, Fig. 1i, right panels).
3.3. CD248 expression is associated with expression of genes involved in extracellular matrix remodelling and inflammation
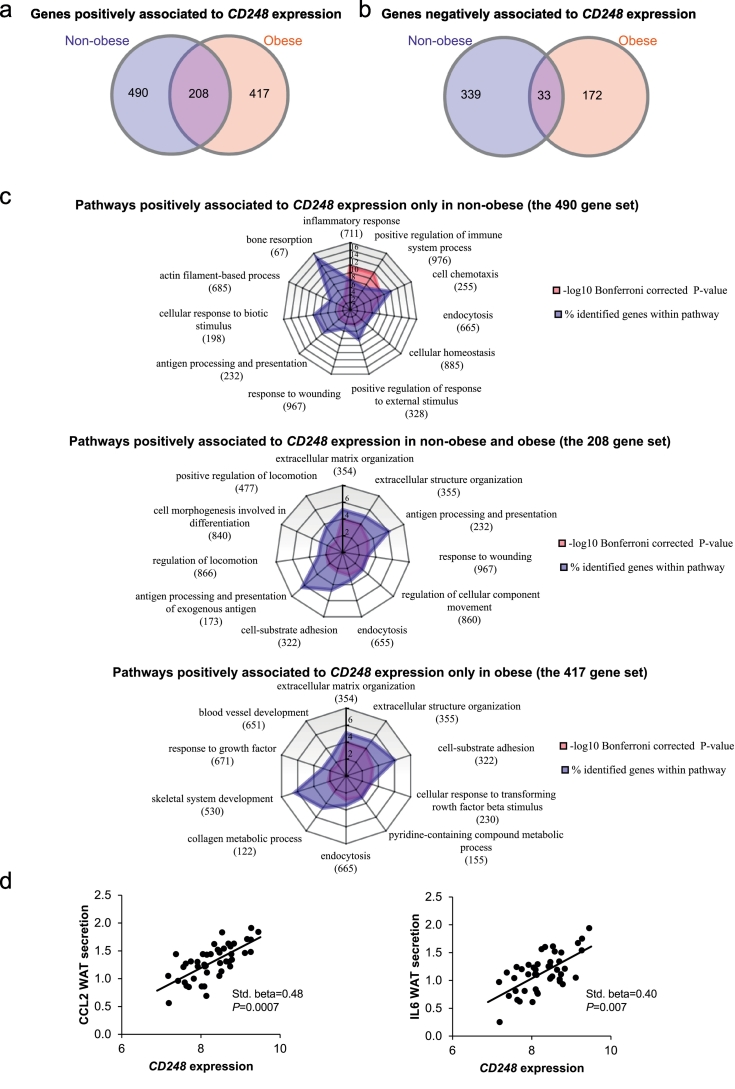
The strong association between CD248 expression and metabolic health prompted us to perform further bioinformatic studies in order to identify potential underlying mechanisms. Correlation analyses in gene microarrays were compared in non-obese and obese individuals (cohort 1). This demonstrated that 208 genes were positively associated (Fig. 2a) and only 33 were negatively associated (Fig. 2b) with CD248 expression, independently of body weight status. The latter set of genes was not enriched for any specific pathway. In contrast, in both non-obese and obese individuals, the genes positively correlated with CD248 were enriched in pathways involved in ECM remodelling and inflammation (Fig. 2c). A link to inflammation was supported by an analysis of ex vivo secretion from human WAT of CCL2 and IL6 (two pro-inflammatory adipokines), demonstrating their BMI-independent association with CD248 expression (cohort 1, Fig. 2d).
Fig. 2.
Pathway analyses of the CD248-associated transcriptome in human WAT. Investigations were performed using transcriptomic data from cohort 1. a, b. Venn diagrams detailing the number of genes positively (a) and negatively (b) associated with CD248 expression in non-obese and obese subjects. c. Pathway analyses of the genes positively associated with CD248 in non-obese (upper panel), non-obese and obese combined (middle panel) and obese (lower panel) summarized in radar charts. Numbers of genes within each pathway are indicated in parentheses. The percentage of identified genes within each pathway are shown in blue while the p-value (after Bonferroni correction) is indicated in light red. d. Multiple regression analyses of the correlation between CD248 (by array) and secreted levels of CCL2 and IL6 from intact WAT pieces. Standardized beta-coefficients and p-values after correction for BMI are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. CD248 is involved in mediating the transcriptional response to hypoxia
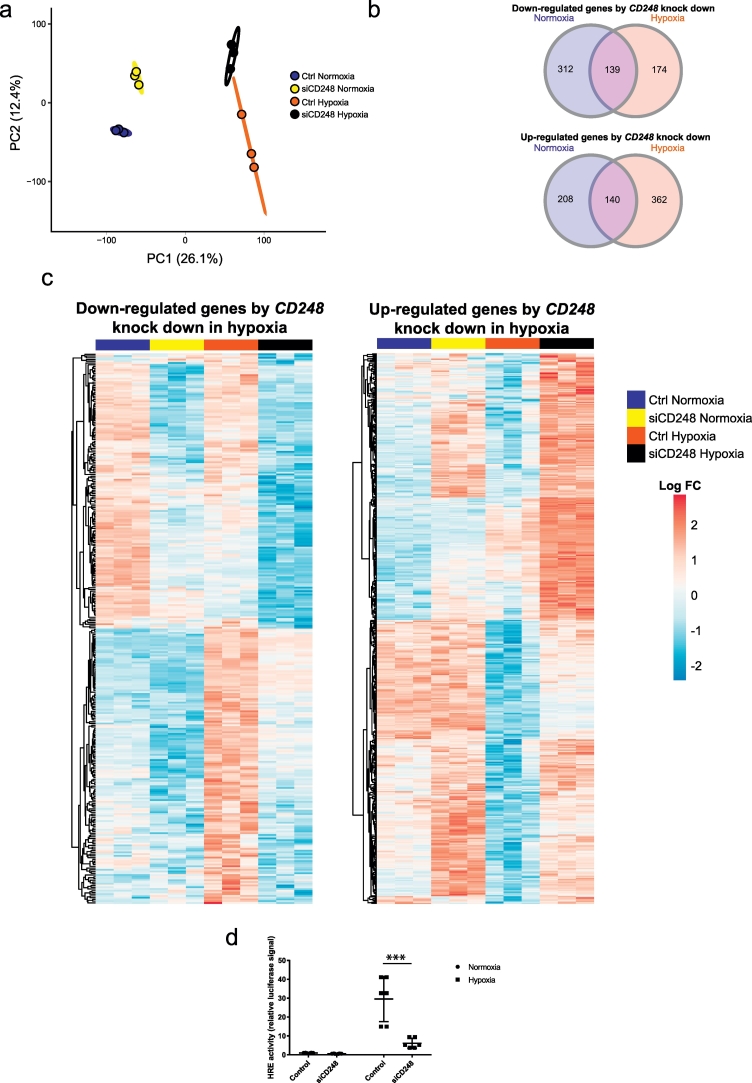
Both ECM remodelling and inflammation are induced by hypoxia. This, together with evidence in non-adipose cells that CD248 participates in driving these pathways [12,30], led us to hypothesize that CD248 may be involved in regulating the transcriptional response to hypoxia in adipocytes. To test this, human in vitro differentiated adipocytes (day 10 post-induction) were transfected with non-silencing control or CD248-targeting siRNAs. RNAi (up to at least 72 h post-transfection) did not affect adipocyte differentiation as determined by Oil Red-O staining (Fig. S1c). After 48 h, cells were incubated in normoxic (21% O2) or hypoxic (1% O2) conditions for an additional 24 h before harvesting the RNA. CD248 protein and mRNA were suppressed to almost undetectable levels by the specific siRNAs (Fig. S1d). Principal component analyses of gene micoarrays from the RNA demonstrated a clear separation between the four conditions wherein the first principal component (PC1) separated normoxic versus hypoxic conditions and PC2 separated the gene knockdowns (Fig. 3a). CD248 knockdown resulted in both attenuated and increased gene expression in response to normoxia or hypoxia (Fig. 3b). However, there was a proportional shift in the directionality depending on the oxygen level, as CD248 knockdown resulted predominantly in reduced gene expression in normoxia but increased gene expression in hypoxia (Fig. 3b). Based on our hypothesis that CD248 impacted on the hypoxic response, we analyzed genes regulated by CD248 knockdown in hypoxia (Fig. 3c). Hierarchical clustering of these genes identified two major clusters; the first one consisted mainly of genes that were regulated by CD248 independently of oxygen levels (upper half of the heatmaps in Fig. 3c, consisting of 139 and 140 genes, respectively from the Venn diagrams in panel 3b). However, in the second one (lower half of both heat maps in Fig. 3c, consisting of 174 and 362 genes, respectively from panel 3c), transcripts were significantly regulated by hypoxia in control cells but the response was markedly attenuated upon CD248 knockdown. This suggests that CD248 modulates the response to hypoxia in human adipocytes. This was confirmed in additional experiments demonstrating that the promoter activity (Fig. 3d) of HIF-1α, the central transcriptional regulator of hypoxia [31], was attenuated following CD248 knockdown in cells incubated under low oxygen tension.
Fig. 3.
Transcriptomic analyses of human adipocytes in relation to CD248 knockdown and hypoxia. Human adipocytes were differentiated in vitro and transfected at day 10 post-induction with siRNA oligonucleotides directed against CD248 (siCD248) or non-silencing control (Ctrl). After 48 h, cells were exposed to hypoxia (1% O2) or continued normoxia (21% O2) for 24 h. After this, cells were lysed and RNA subjected to gene microarray analyses. a. Principal component analysis demonstrating a clear separation between samples based on both CD248 knockdown and hypoxia. b. Venn diagram of the number of genes significantly (FDR 5%) down- (upper panel) or up-regulated (lower panel) by CD248 knockdown and hypoxia. c. Heat-map summarizing the log2-fold changes of the genes significantly (down- or up-) regulated by CD248-knockdown under hypoxic conditions. d. HIF-activity was determined by a luciferase-based hypoxia-response element (HRE) assay in human adipocytes treated as described above. *** = p < .001.
3.5. Cd248 deficiency protects against high fat diet-induced insulin resistance and WAT dysfunction
To obtain further insights into the in vivo relevance of CD248 in WAT, we developed a set of gene knockout (KO) models. Similar to the findings in human tissues, Cd248 was clearly expressed in WAT but not in BAT (Fig. S2a, b). Cd248 in cells from the SVF and mature adipocytes of epi-gonadal and inguinal WAT from mice, was distributed similar to that from humans (Fig. S1b, right panel). We confirmed that the global knockout of Cd248 in mice [12] resulted in lack of Cd248 in WAT at the protein level (Fig. S2b, c) and all other surveyed tissues at the gene expression level (Fig. S2d). As compared to wild-type (WT) littermates, whole body Cd248−/− animals displayed attenuated body weight gain upon high fat diet (HFD), in the absence of changes in food intake (Fig. S3a, b). At 17 weeks of age, HFD fed Cd248−/− mice were significantly leaner (Fig. S3c), and plasma levels of adipocytokines Ccl2 and IL-6 were lower (Fig. S3d). Cd248−/− mice had improved glucose tolerance and a trend towards improved insulin sensitivity compared with WT littermates (Fig. S3e, f). At sacrifice, the livers of the WT mice on the HFD were approximately twice the weight of those from the KO mice, with gross and histologic evidence of increased fat deposition (Fig. S3g). iWAT fat pads from the HFD-fed Cd248−/− mice were significantly reduced in weight (Fig. S3h). Similarly, epi-gonadal (gWAT), mesenteric (mWAT) and subcutaneous (sWAT) fat pads from the HFD-fed Cd248−/− mice weighed slightly less as compared to those from WT mice.
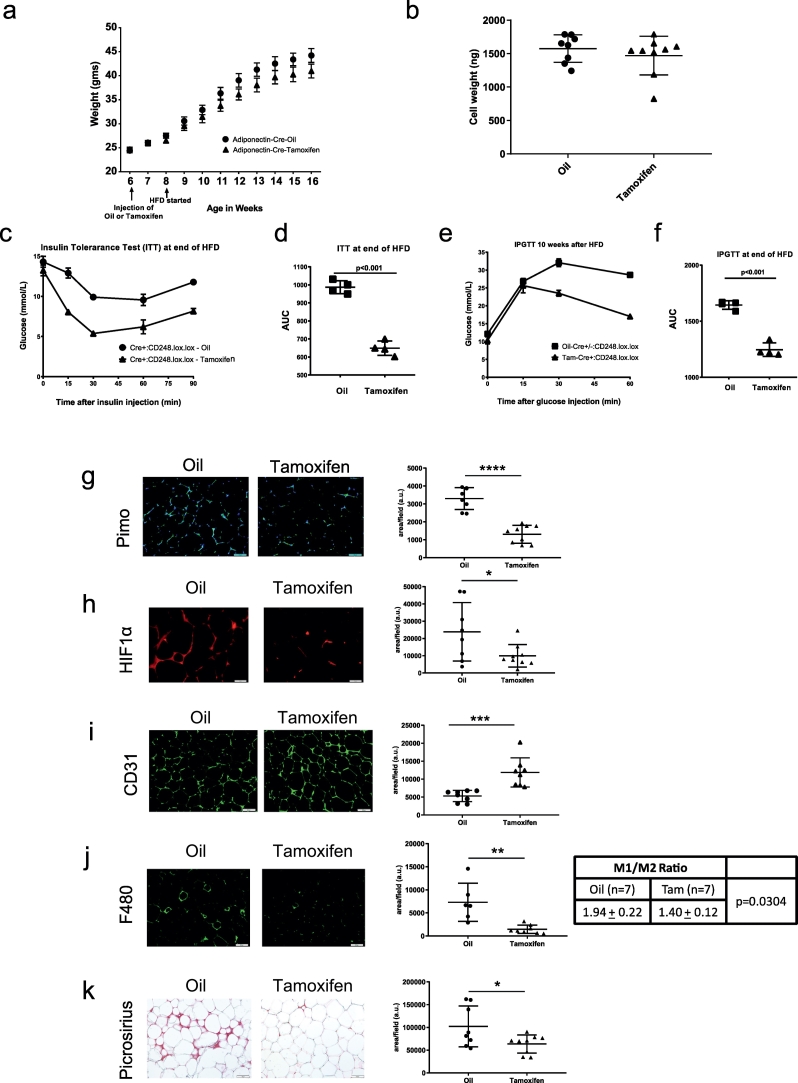
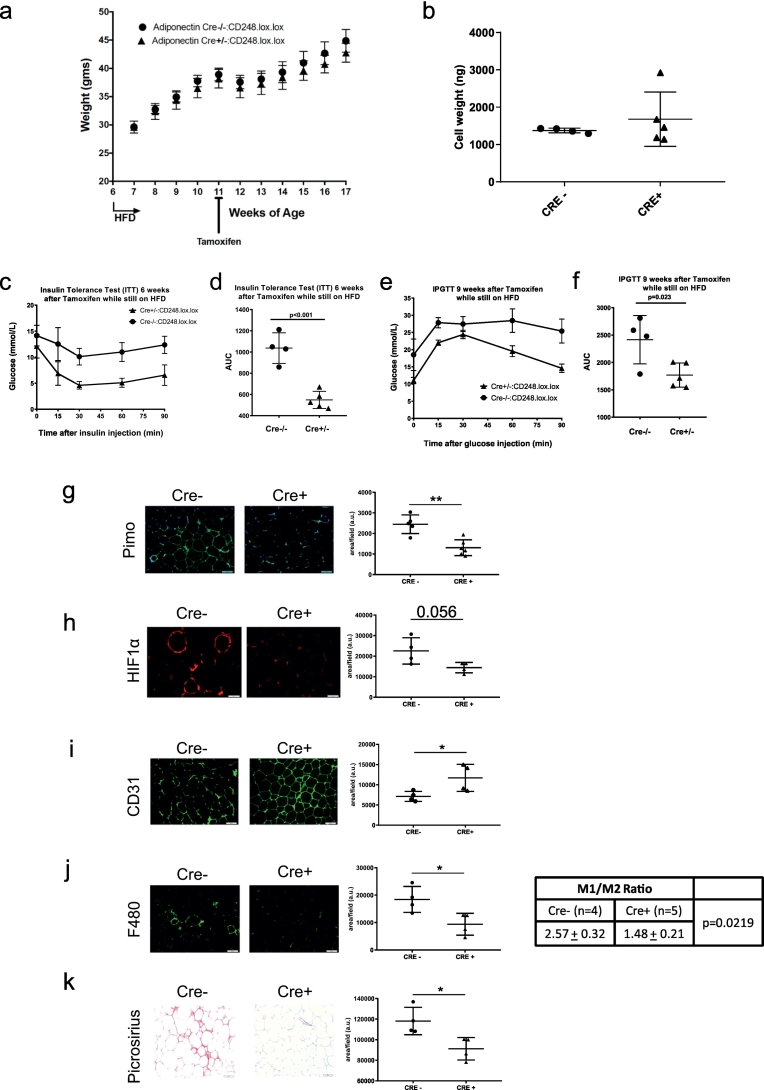
Since Cd248 is expressed in several different tissues, the global Cd248 KO model was limited in terms of identifying underlying mechanisms. To specifically assess the role of Cd248 in mature adipocytes, a tamoxifen-inducible adipocyte-specific knockout model was developed. In these animals, tamoxifen injection resulted in a virtually complete KO of Cd248 specifically in adipocytes, as confirmed 2 weeks after injection (not shown) and after 8-weeks of HFD (Figs. S4a, b, S2d). In contrast to the genotype effect of the HFD in the Cd248−/− mice, there was no significant difference in total body weight between animals injected with tamoxifen or oil (Fig. 4a). There were also no differences in average fat cell size in the epi-gonadal WAT depot (Fig. 4b). Even more prominent than with the whole-body KOs, there was a significant improvement in both glucose (Fig. 4c, d) and insulin tolerance (Fig. 4e, f) in the tamoxifen-treated animals. Immunohistochemistry of WAT showed that tamoxifen-treated animals displayed an attenuated hypoxic response, evidenced by a significant reduction in pimonidazole staining (Fig. 4g). There was also a reduction in overall HIF1α protein levels although there was no obvious difference in nuclear staining (Fig. 4h). Interestingly, microvessel density as measured by CD31 staining, was significantly increased in WAT of the tamoxifen-treated animals (Fig. 4i). Macrophage infiltration, quantified by F4/80 staining (Fig. 4j), was also lower in adipocytes lacking Cd248, with a significant reduction in the M1/M2 ratio, measured by CD68/CD163 staining, respectively. In addition, fibrosis/ECM deposition, as shown by picrosirius staining of collagens (Fig. 4k), was lower. An improvement in WAT phenotype was further corroborated by higher plasma levels of circulating adiponectin (Fig. S4c). There was also histologic evidence of reduced lipid accumulation in the liver (Fig. S4d), but this was not corroborated by a reduction in levels of hepatic triglycerides. Moreover, the pattern of hepatic gene expression was not influenced to any significant degree in the tamoxifen-treated animals (Fig. S4e).
Fig. 4.
Metabolic role of adipocyte Cd248 in response to high fat diet. a. Adiponectin-cre-ERT2:Cd248lox/lox mice were treated with Oil or tamoxifen x 5 d at the age of 6 wks, followed 2 wks later by introduction of a HFD. Weights were monitored weekly. b. Analysis of histologic sections of epi-gonadal adipocytes revealed no differences in adipocyte weight at the end of the HFD. c, d. Insulin tolerance tests (ITT) and e, g glucose tolerance tests (IPGTT) performed at the end of the HFD, showed that deletion of adipocyte Cd248 confers significant protection against insulin resistance and glucose intolerance. g-k. Representative histologic stains of epi-gonadal WAT are shown for pimonidazole (hypoxia), HIF-1α, CD31, F4/80 (macrophages), and picrosirius red (collagens) (scale bars = 50 μm), with corresponding quantifications, including the M1/M2 ratio based on CD68/CD163 staining. Each point represents data from a single mouse. * = p < .05, ** = p < .01 and **** = p < .0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Attenuated Cd248 expression in the obese state reverses impaired glucose metabolism
Finally, in order to determine whether suppression of Cd248 in the obese state would confer improvements in glucometabolic disease, we induced an adipocyte-specific KO five weeks after initiating a HFD, by administering tamoxifen to Cd248lox/lox mice carrying (Cre+/−) or not carrying (Cre−/−) the adiponectin cre-ERT2 transgene. Analyses over the subsequent 6 weeks showed that there was a transient but similar dip in body weight in both groups immediately after the first tamoxifen injection. This was followed by a comparable increase with no significant differences in body weight at the time of sacrifice (Fig. 5a) when adipocyte-specific Cd248 excision was confirmed (Figs. S4f, g, S2d). Similar to the findings in Fig. 4, adipocyte-specific excision of Cd248 from WAT after obesity was established, did not affect fat cell size (Fig. 5b). Notably, there was again a significant improvement in glucose (Fig. 5c, d) and insulin (Fig. 5e, f) tolerance. This was also associated with a reduction in pimonidazole (Fig. 5g) and HIF-1α staining (Fig. 5h) as well as increased microvessel density detected by CD31 staining (Fig. 5i). Again, there was less macrophage infiltration, a reduction in the M1/M2 ratio, and diminished fibrosis (Fig. 5j, k). Also in this model, lack of Cd248 resulted in increased circulating plasma and WAT mRNA levels of adiponectin (Fig. S4 h). There was again reduced hepatic fat accumulation by histology (Fig. S4i), but without an effect on hepatic levels of triglycerides, and without significant changes in hepatic gene expression (Fig. S4j).
Fig. 5.
Reversal of HFD-induced glucose intolerance and insulin resistance by deletion of adipocyte Cd248. a. Adiponectin-cre-ERT2:Cd248lox/lox mice and Cd248lox/lox mice were started on a HFD from the age of 6 weeks. At 11 weeks of age, when the IPGTT and ITTs were similar (not shown), all mice were treated with tamoxifen x 5 days. Weights were monitored weekly. No weight differences were observed. b. Analysis of histologic sections of gonadal adipocytes revealed no differences in adipocyte weight at the end of the HFD. c, d. Insulin tolerance tests (ITT) and e, f glucose tolerance tests (IPGTT) performed at the end of the HFD, showed that deletion of adipocyte Cd248 reversed the HFD-induced insulin resistance and glucose intolerance. g-k. Representative histologic stains of epi-gonadal WAT are shown for pimonidazole (hypoxia), HIF-1α, CD31, F4/80 (macrophages), and picrosirius red (collagens) (scale bars = 50 μm), with corresponding quantifications, including the M1/M2 ratio based on CD68/CD163 staining. Each point represents data from a single mouse. * = p < .05, ** = p < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The mechanisms underlying the close link between metabolic disease and the pernicious WAT triad (hypoxia, inflammation and fibrosis) have been unclear. The present study suggests that CD248 acts as a common mediator in this process by affecting several pathways involved in tissue remodelling, possibly by affecting the transcriptional response to hypoxia in adipocytes and modulating the vascularity of WAT. The observation that metabolic health, glucose homeostasis and WAT phenotype can be improved by selectively down-regulating adipocyte CD248, makes it an attractive target for future drug development.
In the GTex data base, which includes >50 tissues, CD248 displays the highest expression levels in WAT. Despite this, the role of CD248 in WAT has not been studied. In our transcriptomic analyses of WAT, obesity and associated metabolic complications correlated strongly with increased CD248 expression, primarily in white adipocytes. Pathway analyses of transcriptomic data from human WAT showed that CD248 expression was positively associated with inflammation, ECM remodelling and angiogenesis. Increased adipose CD248 expression was reversible, as demonstrated by the significant reduction following weight loss induced by bariatric surgery. A causal link between CD248 and adipocyte function was confirmed in in vitro differentiated human adipocytes following RNAi. In concordance with findings in non-adipose cells and tissues (see Introduction), this suggested that CD248 augments the hypoxic response. This notion was supported by a significantly altered transcriptional response to hypoxia and blunted HIF-1α activation following CD248 knockdown. It is worth noting that CD248 is particularly highly expressed in tumor stroma and WAT, tissue types with marked expansion and remodelling potential. The observation that CD248+ cells from the adipose stroma promote wound healing more efficiently than non-sorted or CD248− cells, support a direct role of CD248 in tissue remodelling [13].
The pathophysiological role of CD248 was evaluated in vivo using various transgenic and inducible models. Collectively, the results suggest that Cd248 impacts on insulin sensitivity and glucose tolerance via effects on WAT phenotype. Given that attenuated Cd248 expression did not affect the increase in either body weight or fat cell size upon HFD, the positive effects are not likely secondary to changes in adipogenesis but rather to other mechanisms that improve WAT function, including hypoxic response, inflammation (reduced M1/M2 ratio) and remodelling of the extracellular matrix. It is noteworthy, that microvessel density in the WAT of Cd248 knockdowns was increased, with a corresponding reduction in hypoxia as determined by pimonidazole staining. While we also observed a reduction in HIF-1α by immunohistochemistry, we did not observe a clear nuclear localization in the WT animals and the gene expression of classical HIF-1α target genes (Glut1, Vegfa, Plgf etc) was not different between genotypes. Complex angiogenic responses to reduced Cd248 in mice have been observed in several previous studies, i.e., in models of postnatal hypoxia of the retina [32] and with several tumors, including glioblastoma [33], colon cancer [34] and melanoma [32]. The differences in vascularity/pimonidazole staining cannot depend on fat cell hypertrophy as adipocyte size was not affected by loss of Cd248. Altogether, this suggests that reduced adipocyte Cd248 expression impacts on several aspects of adipose vascularity and hypoxic response, the mechanisms of which remain elusive. Indeed, the role of Cd248 in physiologic and pathologic angiogenesis is intricate, with context-dependent evidence of pro- and anti-angiogenic properties [14]. It is therefore difficult to directly compare the effects of CD248 knockdown in vitro and in vivo. Nonetheless, our findings indicate that suppression of adipocyte Cd248 confers beneficial effects on glucometabolic function by mechanisms that involve, in the least, regulation of the hypoxic response and vascular remodelling. These changes may in turn attenuate pathways involved in inflammation and ECM production.
Although reduction of adipocyte Cd248 expression did not affect hepatic triglyceride levels, there was histologic evidence of attenuated hepatic lipid deposition. This effect was probably secondary to the improvement in WAT function, as plasma and WAT mRNA adiponectin levels were increased and hepatic gene expression was not altered. While the impact on hepatic fat was mild, it is possible that longer HFD interventions (i.e. >8 weeks) could result in more pronounced genotype-dependent differences. Importantly, the observed effects were not secondary to tamoxifen per se as similar changes in WAT/metabolic phenotypes were observed in the Cre+/− model where WT littermates received the same treatment. The adipocyte-specific KO/knockdowns displayed larger improvements in glucose metabolism compared with the whole body KO. Given that CD248 is expressed in several different tissues and has been implicated in pathological processes in e.g. the liver and kidney (see Introduction), the lung [35,36], skin [37], vasculature [8], bone [6], and several tumors [34,38], it is possible that CD248 in non-adipose tissues and cells may modulate its overall metabolic effects. Together with the human expression data, our results from the inducible KO mice demonstrate that CD248 expression, specifically in white adipocytes, plays an important role in whole body metabolism. Nevertheless, the beneficial metabolic effects observed in whole body KO animals underlines the potential value of testing CD248 antagonists/blocking antibodies to treat obesity-related metabolic disease.
Despite the fact that CD248 was identified almost three decades ago and has been extensively studied both in vitro and in vivo, the molecular mechanisms by which CD248 exerts its effects are not yet fully delineated. As noted above, this is further exemplified by its complex role in angiogenesis [14]. However, there are strong data showing that components of the ECM, including fibronectin, collagen type 1, collagen type IV, and multimerin-2 play an important role by binding to CD248, altering growth factor-triggered cellular responses to hypoxia, inflammation and injury [[39], [40], [41], [42]]. In line with this, isolated stromal fibroblasts and perivascular cells lacking CD248 are resistant to the pro-migratory properties of platelet derived growth factor (PDGF)-BB [40,41], release lower amounts of active matrix metalloproteinase-9 [39] and angiogenic and chemotactic factors [8], deposit less collagen, and exhibit a blunted response to the pro-fibrotic effects of TGFβ [15]. While the signalling pathways of CD248 remain largely unknown, they may involve activation of ERK1/2, dampening of tumor suppressor genes [41], and upregulation of Src/PI-3 kinase and c-fos via co-operation with PDGF-BB [40]. CD248 signalling may therefore be induced together with other cell membrane bound receptors through ECM binding where the number of interactions are increased in the growing tissue. The cytoplasmic tail of CD248 participates in its biological effects, as mice lacking this domain phenocopy the full-gene knockout [12,41]. An improved understanding of the signalling mechanisms induced by CD248 is essential to define its role in WAT and whether adipocyte-specific targeting approaches are feasible.
The mechanisms that regulate CD248 expression and function remain unclear at the moment. The BMI-independent association between CD248 and fat cell volume in human WAT suggests that increased fat cell size may trigger its gene expression. Although it is well established that increased fat cell size associates with a number of processes, including insulin resistance, hypoxia, inflammation and fibrosis, very little is known about how increased adipocyte volume induces these changes. Moreover, the observation that Cd248 knockdown did not impact on cell size in murine WAT, indicates that if there is a causal link between adipocyte size and CD248 expression, it is uni-directional (i.e. cell size may affect CD248 expression but not vice versa). Additional factors that could affect CD248 expression may include epigenetic mechanisms. An unresolved question is whether transient vs chronic overexpression of CD248 in white adipocytes plays different roles. It is possible that transient CD248 activation enables physiological WAT remodelling but that chronic overexpression leads to a pernicious WAT phenotype. Although we acknowledge that these are all important points to address, it was beyond the scope of the present study to identify the factors involved in the transcriptional regulation of CD248.
Taken together, we identify adipocyte CD248 as a potent modulator of insulin sensitivity. Enlarged adipocytes express higher levels of CD248, possibly to transduce signals needed for WAT remodelling. In obesity, this may lead to an unresolved hypoxic response, inflammation and fibrosis which is abrogated by Cd248 knockdown/KO. CD248 may therefore constitute a potential target to rescue malfunctioning WAT and improve metabolic health.
The following are the supplementary data related to this article.
(related to Fig. 1, Fig. 3, Fig. 4, Fig. 5): Patterns of expression of CD248. a. CD248 was detected by gene microarray in multiple cells from human adipose tissue, including mature adipocytes, pre-adipocytes, M1 and M2 macrophages, and T-cells. b. Relative expression of CD248 determined by qPCR in mature adipocytes relative to the stromal vascular fraction (SVF) in adipose tissue from humans (left) and mice (right). c. CD248 expression was knocked down by RNAi (siCD248) in human in vitro differentiated adipocytes at day 10 of differentiation and compared with non-silencing control (siCtrl). Cells were stained with Oil Red-O. d. siRNA knockdown of CD248 in human differentiated adipocytes was confirmed by Western blot (left) and qRT-PCR (right).
(related to Fig. 4, Fig. 5): Expression of Cd248 in global and adipocyte-specific KO mice. a. Representative histologic sections of epi-gonadal white adipose tissue (WAT) and brown adipose tissue (BAT) that were stained for H&E or with specific antibodies for expression of UCP1 (red) or CD248 (green). Blue represents DAPI staining of nuclei. (Scale bar = 100 μm). b. Lysates of inguinal WAT (iWAT) and BAT from adult wild-type (WT) and CD248 knockout (KO) male mice were separated by SDS-PAGE for Western blotting to detect CD248. c. Histologic sections of iWAT from adult WT and KO mice were immunostained with specific anti-CD248 antibodies or isotype control non-specific antibodies (NSIg). Detection of CD248 with secondary antibodies followed by peroxidase staining is only evident in the WAT of WT mice. d. qRT-PCR was used to detect Cd248 in livers, cultured aortic vascular smooth muscle cells and kidneys from mice at the end of the HFDs from the global KO model, and the adipocyte-specific KO models (WT/KO re: Fig. S3, Oil/Tam re: Fig. 4, Cre-/Cre + re: Fig. 5). Cd248 is absent in all cells/organs from the global KO mice. Oil alone has no effect on Cd248 expression. Adipocyte-specific KO of Cd248 has no effect on Cd248 expression in non-adipose organs/cells.
Effects of global KO of Cd248 in mice a, b. WT and KO mice were fed a high fat diet (HFD) or low fat diet (LFD) from the age of 7 weeks. On the HFD, KO mice gained significantly less weight (a), during which time there was no difference in food intake (b). c-h. At 17 weeks, HFD-fed KO mice had reduced fat tissue and more lean tissue (c), with lower plasma levels of adipocytokines (d), and improved glucose tolerance tests (GTT) (e) and insulin tolerance tests (ITT) (f). Liver weight and sizes were markely reduced in the HFD-fed KO mice, with gross (scale bar 1 cm) and histologic (scale bar 50 um) evidence of less lipid accumulation as compared to the HFD-fed WT mice (g). The weights of HFD-fed KO WAT fat pads from different depots (inguinal (iWAT), epigonadal (gWAT), mesenteric (mWAT), perirenal (pWAT)) were reduced. All results are representative of independent experiments performed 3 times with n = 5–10 mice per group. * = p < .05, ** = p < .01 and *** = p < .001.
(related to Fig. 4, Fig. 5): Adipocyte-specific excision of Cd248. a, b. At the end of the HFD following treatment with Oil (vehicle) or tamoxifen (see Fig. 4a), WAT was isolated from the adiponectin-cre:Cd248flox mice. Immunodetection of Cd248 in gWAT (a) and Cd248 qRT-PCR in different fat depots (b) confirmed efficient excision in tamoxifen-treated mice. c. Circulating adiponectin levels were measured by ELISA in Oil and tamoxifen treated animals, respectively. c-e. At the end of the HFD, livers were surgically removed and stained for H&E, the fat areas were quantified, and mRNA expression levels in the livers were measured by qRT-PCR. f, g. WAT was isolated from Cre−/− and Cre+/− mice treated with tamoxifen (see Fig. 5). Immunodetection of Cd248 in gWAT (f) and Cd248 qRT-PCR in different fat depots (g) confirmed efficient excision in the Cre+/− mice. h. Adiponectin levels in plasma and gWAT were measured by ELISA and qRT-PCR, respectively. i, j. At the end of the HFD, livers were surgically removed and stained for H&E, the fat areas were quantified, and mRNA expression levels in the livers were measured by qRT-PCR. Excision of adipocyte Cd248 reduced the amount of fat, but had no effect on liver mRNAs. * = p < .05, ** = p < .01, *** = p < .001.
Acknowledgments
Acknowledgements
We thank the following for valuable technical support and advice: Jacqueline Rollins (UBC), Alice M. O'Byrne (UBC), Piyushkumar Kapopara (UBC), Houra Loghmani Khouzani (UBC), Denis Richard (Laval University), Kanta Chechi (Laval University), Tarja Niemi (University of Turku), Markku Taittonen (University of Turku), Pirjo Nuutila (University of Turku), Martin Klingenspor (Technische Universitat Munchen), Stefanie Maurer (Technische Universitat Munchen), Tobias Fromme (Technische Universitat Munchen), Gareth Lim (UBC, current address Université de Montreal).
Funding sources
EMC was supported by operating grants from the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Canada Foundations for Innovation (CFI). He holds a Tier 1 Canada Research Chair in Endothelial Cell Biology and is an adjunct Scientist with the Canadian Blood Services. TJK and JDJ were supported by grants from the CIHR (Foundation Grant for TJK). MMK received support from NSERC and a Killam Scholarship. SBC received funding from the Swedish Research Council, Stockholm County and Strategic Research Programe Diabetes. MR received funding from the Swedish Research Council, the Novo Nordisk Foundation including the Tripartite Immuno-metabolism Consortium (TrIC) Grant Number NNF15CC0018486 and the MSAM consortium NNF15SA0018346, the Swedish Diabetes Foundation, EFSD, Stockholm County Council and the Diabetes Research Program at the Karolinska Institutet. EA and DJO are employees of Morphotek Inc.
Role of the funding sources
No funding sources were involved in the writing of the manuscript or the decision to submit it for publication. No authors were paid to write this article from any source or agency. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of interest
JDJ was an employee of Novo Nordisk Ltd., although the company had no involvement in this study; all collaborative work described here was conducted in his academic laboratory at the University of British Columbia (UBC). EMC received partial funding from Morphotek Inc. for these studies. EA and DJO were employed by Morphotek Inc.
Author contributions
PP and TLF designed and performed experiments, interpreted data, and helped to write the manuscript. MMK, DJO, EA, KV, SMC, TJK, ACC, JDJ provided critical guidance in designing studies and reviewing the manuscript. The following provided technical support and helped interpret data: MMK, JH, VL, NSS, SK, XZ, SBC, JL, CN. EMC and MR supervised the design and performance of the studies, helped write the manuscript and bear responsibility for the content.
Contributor Information
Mikael Rydén, Email: mikael.ryden@ki.se.
Edward M. Conway, Email: ed.conway@ubc.ca.
References
- 1.Gesta S., Tseng Y.H., Kahn C.R. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]; Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242-56. [DOI] [PubMed]
- 2.Castoldi A., Naffah de Souza C., Camara N.O., Moraes-Vieira P.M. The macrophage switch in obesity development. Front Immunol. 2015;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]; Castoldi A, Naffah de Souza C, Camara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Frontiers in immunology. 2015;6:637. [DOI] [PMC free article] [PubMed]
- 3.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1-2):20-44. [DOI] [PMC free article] [PubMed]
- 4.Crewe C., An Y.A., Scherer P.E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]; Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. The Journal of clinical investigation. 2017;127(1):74-82. [DOI] [PMC free article] [PubMed]
- 5.Christian S., Ahorn H., Koehler A., Eisenhaber F., Rodi H.P., Garin-Chesa P. Molecular cloning and characterization of endosialin, a C-type lectin- like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276(10):7408–7414. doi: 10.1074/jbc.M009604200. [DOI] [PubMed] [Google Scholar]; Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi HP, Garin-Chesa P, et al. Molecular cloning and characterization of endosialin, a C-type lectin- like cell surface receptor of tumor endothelium. The Journal of biological chemistry. 2001;276(10):7408-14. [DOI] [PubMed]
- 6.Naylor A.J., Azzam E., Smith S., Croft A., Poyser C., Duffield J.S. The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice. Arthritis Rheum. 2012;64(10):3334–3343. doi: 10.1002/art.34556. [DOI] [PMC free article] [PubMed] [Google Scholar]; Naylor AJ, Azzam E, Smith S, Croft A, Poyser C, Duffield JS, et al. The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice. Arthritis and rheumatism. 2012;64(10):3334-43. [DOI] [PMC free article] [PubMed]
- 7.Dolznig H., Schweifer N., Puri C., Kraut N., Rettig W.J., Kerjaschki D. Characterization of cancer stroma markers: in silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer Immun. 2005;5:10. [PubMed] [Google Scholar]; Dolznig H, Schweifer N, Puri C, Kraut N, Rettig WJ, Kerjaschki D, et al. Characterization of cancer stroma markers: in silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer Immun. 2005;5:10. [PubMed]
- 8.Hasanov Z., Ruckdeschel T., Konig C., Mogler C., Kapel S.S., Korn C. Endosialin promotes atherosclerosis through phenotypic Remodeling of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2017;37(3):495–505. doi: 10.1161/ATVBAHA.116.308455. [DOI] [PubMed] [Google Scholar]; Hasanov Z, Ruckdeschel T, Konig C, Mogler C, Kapel SS, Korn C, et al. Endosialin Promotes Atherosclerosis Through Phenotypic Remodeling of Vascular Smooth Muscle Cells. Arteriosclerosis, thrombosis, and vascular biology. 2017;37(3):495-505. [DOI] [PubMed]
- 9.Mogler C., Wieland M., Konig C., Hu J., Runge A., Korn C. Hepatic stellate cell-expressed endosialin balances fibrogenesis and hepatocyte proliferation during liver damage. EMBO Mol Med. 2015;7(3):332–338. doi: 10.15252/emmm.201404246. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mogler C, Wieland M, Konig C, Hu J, Runge A, Korn C, et al. Hepatic stellate cell-expressed endosialin balances fibrogenesis and hepatocyte proliferation during liver damage. EMBO molecular medicine. 2015;7(3):332-8. [DOI] [PMC free article] [PubMed]
- 10.Smith S.W., Eardley K.S., Croft A.P., Nwosu J., Howie A.J., Cockwell P. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int. 2011;80(2):199–207. doi: 10.1038/ki.2011.103. [DOI] [PubMed] [Google Scholar]; Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, et al. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int. 2011;80(2):199-207. [DOI] [PubMed]
- 11.Ohradanova A., Gradin K., Barathova M., Zatovicova M., Holotnakova T., Kopacek J. Hypoxia upregulates expression of human endosialin gene via hypoxia-inducible factor 2. Br J Cancer. 2008;99(8):1348–1356. doi: 10.1038/sj.bjc.6604685. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ohradanova A, Gradin K, Barathova M, Zatovicova M, Holotnakova T, Kopacek J, et al. Hypoxia upregulates expression of human endosialin gene via hypoxia-inducible factor 2. British journal of cancer. 2008;99(8):1348-56. [DOI] [PMC free article] [PubMed]
- 12.Maia M., de Vriese A., Janssens T., Moons M., van Landuyt K., Tavernier J. CD248 and its cytoplasmic domain: a therapeutic target for arthritis. Arthritis Rheum. 2010;62(12):3595–3606. doi: 10.1002/art.27701. [DOI] [PubMed] [Google Scholar]; Maia M, de Vriese A, Janssens T, Moons M, van Landuyt K, Tavernier J, et al. CD248 and its cytoplasmic domain: a therapeutic target for arthritis. Arthritis and rheumatism. 2010;62(12):3595-606. [DOI] [PubMed]
- 13.Brett E., Zielins E.R., Chin M., Januszyk M., Blackshear C.P., Findlay M. Isolation of CD248-expressing stromal vascular fraction for targeted improvement of wound healing. Wound Repair Regen Off Publ Wound Healing Soc Eur Tissue Repair Soc. 2017;2017(3):414–422. doi: 10.1111/wrr.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brett E, Zielins ER, Chin M, Januszyk M, Blackshear CP, Findlay M, et al. Isolation of CD248-expressing stromal vascular fraction for targeted improvement of wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2017;2017(3):414-22. [DOI] [PMC free article] [PubMed]
- 14.Kontsekova S., Polcicova K., Takacova M., Pastorekova S. Endosialin: molecular and functional links to tumor angiogenesis. Neoplasma. 2016;63(2):183–192. doi: 10.4149/202_15090N474. [DOI] [PubMed] [Google Scholar]; Kontsekova S, Polcicova K, Takacova M, Pastorekova S. Endosialin: molecular and functional links to tumor angiogenesis. Neoplasma. 2016;63(2):183-92. [DOI] [PubMed]
- 15.Smith S.W., Croft A.P., Morris H.L., Naylor A.J., Huso D.L., Isacke C.M. Genetic deletion of the stromal cell marker CD248 (Endosialin) protects against the development of renal fibrosis. Nephron. 2015;131(4):265–277. doi: 10.1159/000438754. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith SW, Croft AP, Morris HL, Naylor AJ, Huso DL, Isacke CM, et al. Genetic Deletion of the Stromal Cell Marker CD248 (Endosialin) Protects against the Development of Renal Fibrosis. Nephron. 2015;131(4):265-77. [DOI] [PMC free article] [PubMed]
- 16.Arner E., Mejhert N., Kulyte A., Balwierz P.J., Pachkov M., Cormont M. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arner E, Mejhert N, Kulyte A, Balwierz PJ, Pachkov M, Cormont M, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986-93. [DOI] [PMC free article] [PubMed]
- 17.Arner P., Sahlqvist A.S., Sinha I., Xu H., Yao X., Waterworth D. The epigenetic signature of systemic insulin resistance in obese women. Diabetologia. 2016;59(11):2393–2405. doi: 10.1007/s00125-016-4074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arner P, Sahlqvist AS, Sinha I, Xu H, Yao X, Waterworth D, et al. The epigenetic signature of systemic insulin resistance in obese women. Diabetologia. 2016;59(11):2393-405. [DOI] [PMC free article] [PubMed]
- 18.Acosta J.R., Douagi I., Andersson D.P., Backdahl J., Rydén M., Arner P. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560–570. doi: 10.1007/s00125-015-3810-6. [DOI] [PubMed] [Google Scholar]; Acosta JR, Douagi I, Andersson DP, Backdahl J, Rydén M, Arner P, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560-70. [DOI] [PubMed]
- 19.Petrus P., Bialesova L., Checa A., Kerr A., Naz S., Backdahl J. Adipocyte expression of SLC19A1 links DNA Hypermethylation to adipose tissue inflammation and insulin resistance. J Clin Endocrinol Metab. 2018;103(2):710–721. doi: 10.1210/jc.2017-01382. [DOI] [PubMed] [Google Scholar]; Petrus P, Bialesova L, Checa A, Kerr A, Naz S, Backdahl J, et al. Adipocyte Expression of SLC19A1 Links DNA Hypermethylation to Adipose Tissue Inflammation and Insulin Resistance. The Journal of clinical endocrinology and metabolism. 2018;103(2):710-21. [DOI] [PubMed]
- 20.Eriksson Hogling D., Petrus P., Gao H., Backdahl J., Dahlman I., Laurencikiene J. Adipose and circulating CCL18 levels associate with metabolic risk factors in women. J Clin Endocrinol Metab. 2016;101(11):4021–4029. doi: 10.1210/jc.2016-2390. [DOI] [PubMed] [Google Scholar]; Eriksson Hogling D, Petrus P, Gao H, Backdahl J, Dahlman I, Laurencikiene J, et al. Adipose and Circulating CCL18 Levels Associate With Metabolic Risk Factors in Women. The Journal of clinical endocrinology and metabolism. 2016;101(11):4021-9. [DOI] [PubMed]
- 21.Sassmann A., Offermanns S., Wettschureck N. Tamoxifen-inducible Cre-mediated recombination in adipocytes. Genesis. 2010;48(10):618–625. doi: 10.1002/dvg.20665. [DOI] [PubMed] [Google Scholar]; Sassmann A, Offermanns S, Wettschureck N. Tamoxifen-inducible Cre-mediated recombination in adipocytes. Genesis. 2010;48(10):618-25. [DOI] [PubMed]
- 22.Kang S., Kong X., Rosen E.D. Adipocyte-specific transgenic and knockout models. Methods Enzymol. 2014;537:1–16. doi: 10.1016/B978-0-12-411619-1.00001-X. [DOI] [PubMed] [Google Scholar]; Kang S, Kong X, Rosen ED. Adipocyte-specific transgenic and knockout models. Methods in enzymology. 2014;537:1-16. [DOI] [PubMed]
- 23.Gao H., Mejhert N., Fretz J.A., Arner E., Lorente-Cebrian S., Ehrlund A. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014;19(6):981–992. doi: 10.1016/j.cmet.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gao H, Mejhert N, Fretz JA, Arner E, Lorente-Cebrian S, Ehrlund A, et al. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell metabolism. 2014;19(6):981-92. [DOI] [PMC free article] [PubMed]
- 24.Forsberg E.A., Botusan I.R., Wang J., Peters V., Ansurudeen I., Brismar K. Carnosine decreases IGFBP1 production in db/db mice through suppression of HIF-1. J Endocrinol. 2015;225(3):159–167. doi: 10.1530/JOE-14-0571. [DOI] [PubMed] [Google Scholar]; Forsberg EA, Botusan IR, Wang J, Peters V, Ansurudeen I, Brismar K, et al. Carnosine decreases IGFBP1 production in db/db mice through suppression of HIF-1. The Journal of endocrinology. 2015;225(3):159-67. [DOI] [PubMed]
- 25.Kim S.J., Nian C., Karunakaran S., Clee S.M., Isales C.M., McIntosh C.H. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CH. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PloS one. 2012;7(7):e40156. [DOI] [PMC free article] [PubMed]
- 26.Suresh Babu S., Valdez Y., Xu A., O'Byrne A., Calvo F., Lie V. TGFβ-mediated suppression of CD248 in non-cancer cells via canonical Smad-dependent signaling pathways is uncoupled in cancer cells. BMC Cancer. 2014;14:113. doi: 10.1186/1471-2407-14-113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Suresh Babu S, Valdez Y, Xu A, O'Byrne A, Calvo F, Lie V, et al. TGFβ-mediated suppression of CD248 in non-cancer cells via canonical Smad-dependent signaling pathways is uncoupled in cancer cells. BMC Cancer. 2014;14:113. [DOI] [PMC free article] [PubMed]
- 27.Galarraga M., Campion J., Munoz-Barrutia A., Boque N., Moreno H., Martinez J.A. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53(12):2791–2796. doi: 10.1194/jlr.D023788. [DOI] [PMC free article] [PubMed] [Google Scholar]; Galarraga M, Campion J, Munoz-Barrutia A, Boque N, Moreno H, Martinez JA, et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. Journal of lipid research. 2012;53(12):2791-6. [DOI] [PMC free article] [PubMed]
- 28.Lonn M., Mehlig K., Bengtsson C., Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24(1):326–331. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]; Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24(1):326-31. [DOI] [PubMed]
- 29.Acosta J.R., Joost S., Karlsson K., Ehrlund A., Li X., Aouadi M. Single cell transcriptomics suggest that human adipocyte progenitor cells constitute a homogeneous cell population. Stem Cell Res Ther. 2017;8(1):250. doi: 10.1186/s13287-017-0701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Acosta JR, Joost S, Karlsson K, Ehrlund A, Li X, Aouadi M, et al. Single cell transcriptomics suggest that human adipocyte progenitor cells constitute a homogeneous cell population. Stem Cell Res Ther. 2017;8(1):250. [DOI] [PMC free article] [PubMed]
- 30.Tian Y., Deng H., Han L., Hu S., Qi X. Hypoxia-inducible factor may induce the development of liver fibrosis in Budd-Chiari syndrome by regulating CD248/endosialin expression: a hypothesis. J Transl Int Med. 2018;6(2):66–69. doi: 10.2478/jtim-2018-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tian Y, Deng H, Han L, Hu S, Qi X. Hypoxia-inducible Factor may Induce the Development of Liver Fibrosis in Budd-Chiari Syndrome by Regulating CD248/endosialin Expression: A Hypothesis. J Transl Int Med. 2018;6(2):66-9. [DOI] [PMC free article] [PubMed]
- 31.Semenza G.L. A compendium of proteins that interact with HIF-1alpha. Exp Cell Res. 2017;356(2):128–135. doi: 10.1016/j.yexcr.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]; Semenza GL. A compendium of proteins that interact with HIF-1alpha. Experimental cell research. 2017;356(2):128-35. [DOI] [PMC free article] [PubMed]
- 32.Simonavicius N., Ashenden M., van Weverwijk A., Lax S., Huso D.L., Buckley C.D. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120(7):1516–1527. doi: 10.1182/blood-2011-01-332338. [DOI] [PubMed] [Google Scholar]; Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, et al. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120(7):1516-27. [DOI] [PubMed]
- 33.Carson-Walter E.B., Winans B.N., Whiteman M.C., Liu Y., Jarvela S., Haapasalo H. Characterization of TEM1/endosialin in human and murine brain tumors. BMC Cancer. 2009;9:417. doi: 10.1186/1471-2407-9-417. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carson-Walter EB, Winans BN, Whiteman MC, Liu Y, Jarvela S, Haapasalo H, et al. Characterization of TEM1/endosialin in human and murine brain tumors. BMC Cancer. 2009;9:417. [DOI] [PMC free article] [PubMed]
- 34.Nanda A., Karim B., Peng Z., Liu G., Qiu W., Gan C. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci U S A. 2006;103(9):3351–3356. doi: 10.1073/pnas.0511306103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, et al. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3351-6. [DOI] [PMC free article] [PubMed]
- 35.Bartis D., Crowley L.E., D'Souza V.K., Borthwick L., Fisher A.J., Croft A.P. Role of CD248 as a potential severity marker in idiopathic pulmonary fibrosis. BMC Pulm Med. 2016;16(1):51. doi: 10.1186/s12890-016-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bartis D, Crowley LE, D'Souza VK, Borthwick L, Fisher AJ, Croft AP, et al. Role of CD248 as a potential severity marker in idiopathic pulmonary fibrosis. BMC Pulm Med. 2016;16(1):51. [DOI] [PMC free article] [PubMed]
- 36.Barozzi C., Galletti M., Tomasi L., De Fanti S., Palazzini M., Manes A. A combined targeted and whole exome sequencing approach identified novel candidate genes involved in heritable pulmonary arterial hypertension. Sci Rep. 2019;9(1):753. doi: 10.1038/s41598-018-37277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Barozzi C, Galletti M, Tomasi L, De Fanti S, Palazzini M, Manes A, et al. A Combined Targeted and Whole Exome Sequencing Approach Identified Novel Candidate Genes Involved in Heritable Pulmonary Arterial Hypertension. Scientific reports. 2019;9(1):753. [DOI] [PMC free article] [PubMed]
- 37.Di Benedetto P., Liakouli V., Ruscitti P., Berardicurti O., Carubbi F., Panzera N. Blocking CD248 molecules in perivascular stromal cells of patients with systemic sclerosis strongly inhibits their differentiation toward myofibroblasts and proliferation: a new potential target for antifibrotic therapy. Arthritis Res Ther. 2018;20(1):223. doi: 10.1186/s13075-018-1719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Di Benedetto P, Liakouli V, Ruscitti P, Berardicurti O, Carubbi F, Panzera N, et al. Blocking CD248 molecules in perivascular stromal cells of patients with systemic sclerosis strongly inhibits their differentiation toward myofibroblasts and proliferation: a new potential target for antifibrotic therapy. Arthritis research & therapy. 2018;20(1):223. [DOI] [PMC free article] [PubMed]
- 38.Valdez Y., Maia M., Conway E.M. CD248: reviewing its role in health and disease. Curr Drug Targets. 2012;13(3):432–439. doi: 10.2174/138945012799424615. [DOI] [PubMed] [Google Scholar]; Valdez Y, Maia M, Conway EM. CD248: reviewing its role in health and disease. Curr Drug Targets. 2012;13(3):432-9. [DOI] [PubMed]
- 39.Tomkowicz B., Rybinski K., Foley B., Ebel W., Kline B., Routhier E. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci U S A. 2007;104(46):17965–17970. doi: 10.1073/pnas.0705647104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E, et al. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):17965-70. [DOI] [PMC free article] [PubMed]
- 40.Tomkowicz B., Rybinski K., Sebeck D., Sass P., Nicolaides N.C., Grasso L. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther. 2010;9(11) doi: 10.4161/cbt.9.11.11731. [DOI] [PubMed] [Google Scholar]; Tomkowicz B, Rybinski K, Sebeck D, Sass P, Nicolaides NC, Grasso L, et al. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer biology & therapy. 2010;9(11). [DOI] [PubMed]
- 41.Maia M., DeVriese A., Janssens T., Moons M., Lories R.J., Tavernier J. CD248 facilitates tumor growth via its cytoplasmic domain. BMC Cancer. 2011;11:162. doi: 10.1186/1471-2407-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]; Maia M, DeVriese A, Janssens T, Moons M, Lories RJ, Tavernier J, et al. CD248 facilitates tumor growth via its cytoplasmic domain. BMC Cancer. 2011;11:162. [DOI] [PMC free article] [PubMed]
- 42.Khan K.A., Naylor A.J., Khan A., Noy P.J., Mambretti M., Lodhia P. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene. 2017;36(44):6097–6108. doi: 10.1038/onc.2017.214. [DOI] [PMC free article] [PubMed] [Google Scholar]; Khan KA, Naylor AJ, Khan A, Noy PJ, Mambretti M, Lodhia P, et al. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene. 2017;36(44):6097-108. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(related to Fig. 1, Fig. 3, Fig. 4, Fig. 5): Patterns of expression of CD248. a. CD248 was detected by gene microarray in multiple cells from human adipose tissue, including mature adipocytes, pre-adipocytes, M1 and M2 macrophages, and T-cells. b. Relative expression of CD248 determined by qPCR in mature adipocytes relative to the stromal vascular fraction (SVF) in adipose tissue from humans (left) and mice (right). c. CD248 expression was knocked down by RNAi (siCD248) in human in vitro differentiated adipocytes at day 10 of differentiation and compared with non-silencing control (siCtrl). Cells were stained with Oil Red-O. d. siRNA knockdown of CD248 in human differentiated adipocytes was confirmed by Western blot (left) and qRT-PCR (right).
(related to Fig. 4, Fig. 5): Expression of Cd248 in global and adipocyte-specific KO mice. a. Representative histologic sections of epi-gonadal white adipose tissue (WAT) and brown adipose tissue (BAT) that were stained for H&E or with specific antibodies for expression of UCP1 (red) or CD248 (green). Blue represents DAPI staining of nuclei. (Scale bar = 100 μm). b. Lysates of inguinal WAT (iWAT) and BAT from adult wild-type (WT) and CD248 knockout (KO) male mice were separated by SDS-PAGE for Western blotting to detect CD248. c. Histologic sections of iWAT from adult WT and KO mice were immunostained with specific anti-CD248 antibodies or isotype control non-specific antibodies (NSIg). Detection of CD248 with secondary antibodies followed by peroxidase staining is only evident in the WAT of WT mice. d. qRT-PCR was used to detect Cd248 in livers, cultured aortic vascular smooth muscle cells and kidneys from mice at the end of the HFDs from the global KO model, and the adipocyte-specific KO models (WT/KO re: Fig. S3, Oil/Tam re: Fig. 4, Cre-/Cre + re: Fig. 5). Cd248 is absent in all cells/organs from the global KO mice. Oil alone has no effect on Cd248 expression. Adipocyte-specific KO of Cd248 has no effect on Cd248 expression in non-adipose organs/cells.
Effects of global KO of Cd248 in mice a, b. WT and KO mice were fed a high fat diet (HFD) or low fat diet (LFD) from the age of 7 weeks. On the HFD, KO mice gained significantly less weight (a), during which time there was no difference in food intake (b). c-h. At 17 weeks, HFD-fed KO mice had reduced fat tissue and more lean tissue (c), with lower plasma levels of adipocytokines (d), and improved glucose tolerance tests (GTT) (e) and insulin tolerance tests (ITT) (f). Liver weight and sizes were markely reduced in the HFD-fed KO mice, with gross (scale bar 1 cm) and histologic (scale bar 50 um) evidence of less lipid accumulation as compared to the HFD-fed WT mice (g). The weights of HFD-fed KO WAT fat pads from different depots (inguinal (iWAT), epigonadal (gWAT), mesenteric (mWAT), perirenal (pWAT)) were reduced. All results are representative of independent experiments performed 3 times with n = 5–10 mice per group. * = p < .05, ** = p < .01 and *** = p < .001.
(related to Fig. 4, Fig. 5): Adipocyte-specific excision of Cd248. a, b. At the end of the HFD following treatment with Oil (vehicle) or tamoxifen (see Fig. 4a), WAT was isolated from the adiponectin-cre:Cd248flox mice. Immunodetection of Cd248 in gWAT (a) and Cd248 qRT-PCR in different fat depots (b) confirmed efficient excision in tamoxifen-treated mice. c. Circulating adiponectin levels were measured by ELISA in Oil and tamoxifen treated animals, respectively. c-e. At the end of the HFD, livers were surgically removed and stained for H&E, the fat areas were quantified, and mRNA expression levels in the livers were measured by qRT-PCR. f, g. WAT was isolated from Cre−/− and Cre+/− mice treated with tamoxifen (see Fig. 5). Immunodetection of Cd248 in gWAT (f) and Cd248 qRT-PCR in different fat depots (g) confirmed efficient excision in the Cre+/− mice. h. Adiponectin levels in plasma and gWAT were measured by ELISA and qRT-PCR, respectively. i, j. At the end of the HFD, livers were surgically removed and stained for H&E, the fat areas were quantified, and mRNA expression levels in the livers were measured by qRT-PCR. Excision of adipocyte Cd248 reduced the amount of fat, but had no effect on liver mRNAs. * = p < .05, ** = p < .01, *** = p < .001.