Abstract
In recent years, researches focusing on PIWI-interacting RNAs (piRNAs) have increased rapidly. It has been revealed that piRNAs have strong association with a wide range of diseases; thus, it becomes very important to understand piRNAs’ role(s) in disease diagnosis, prognosis and assessment of treatment response. We searched more than 2500 articles using keywords, such as `PIWI-interacting RNAs’ and `piRNAs’, and further scrutinized the articles to collect piRNAs-disease association data. These data are highly complex and heterogeneous due to various types of piRNA idnetifiers (IDs) and different reference genome versions. We put considerable efforts into removing redundancy and anomalies and thus homogenized the data. Finally, we developed the piRDisease database, which incorporates experimentally supported data for piRNAs’ relationship with wide range of diseases. The piRDisease (piRDisease v1.0) is a novel, comprehensive and exclusive database resource, which provides 7939 manually curated associations of experimentally supported 4796 piRNAs involved in 28 diseases. piRDisease facilitates users by providing detailed information of the piRNA in respective disease, explored by experimental support, brief description, sequence and location information. Considering piRNAs’ role(s) in wide range of diseases, it is anticipated that huge amount of data would be produced in the near future. We thus offer a submitting page, on which users or researches can contribute in to update our piRDisease database.
Introduction
PIWI-interacting RNAs (piRNAs) are a type of small non-coding RNAs, first described in germ cells, represented as one of the major group of small non-coding RNAs such as miRNA and siRNA (1). piRNAs play a crucial role to safeguard genome, maintain the genome complexity and integrity, as they suppress the insertional mutations caused by transposable elements. Previously, the role of piRNAs was confined to gonad development (2–4), whereas existing studies have revealed that the expression profile of piRNAs vary from central nervous system (brain) to colon, heart, kidney, liver, lung, small intestine, spleen, stomach, ovary and testis (5–9). Evidently, piRNAs play critical roles in disease progression, diagnosis and assessment of treatment response (9–18). Genome-wide profiling studies have revealed that the expression of piRNAs was dysregulated in various diseases. However, target based mechanistic studies revealed the regulatory role of piRNAs in various diseases (26, 31). piRNAs regulate target genes through base paring mechanism (19). For instance, piR-823 binds to HSF1 to promote its phosphorylation, which contributes to colorectal tumorigenesis (19). Knocking down of piR-34736 results in high expression of Bax/Bcl2 and repression of EMT-mediator Vimentin in head and neck cancer (20, 21). Accumlating evidences sugest that the change in expression of piRNAs and abrerration in target genes regulation will be potential diagnostic marker (18, 22–25). In recent years, a few databases have been developed to provide basic information related to piRNAs, such as piRNABank and piRBase, which provide comprehensive piRNA sequence and location information for several species (26, 28). piRNAQuest is another database resource, which offers a diverse narrative focusing on pseudogenes and synteny information including sequence and location data (27).
Several databases are available, which document non-coding RNAs such as long non-coding RNAs' and small non-coding RNAs' association with disease. These databases include LncRNADisease, Lnc2cancer, miR2Disease, miRCancer, circRNAdisease and Circ2Disease (28–33). However, there is no online database resource offering data on piRNAs and disease relationship. Therefore, we developed manually curated piRNA and disease association database resource, which provides experimentally supported piRNAs with their disease associations from literature.
Construction and content
We searched PubMed for published research articles (34), using a list of keywords such as piRNAs, PIWI-interacting RNAs, PIWI-interacting RNAs involved in diseases and cancer, piRNAs and PIWI-interacting RNAs in diseases and cancers, respectively.
We retrieved 2572 articles, filtered these articles on the basis of piRNAs’ disease associations to acquire more than 50 articles (Figure 1) (15, 23). During data collection, we mainly focused on piRNAs’ association in respective diseases, illustrating their expression or mechanistic role in regulating target genes/proteins. Furthermore, we collected sequence and location information for those piRNAs, preceded by experimental methods, detail mechanism and description, in vivo or in vitro study, and the reference article’s PubMed identifier (ID) and title. After initial compilation of data, we observed that the data were in semantic form covering long textual strings including special characters, which usually creates problem during storage and retrieval of data from database. Therefore, before storing the data we applied several computational preprocessing methods, so that data can be curated smoothly (Figure 1).
Figure 1.
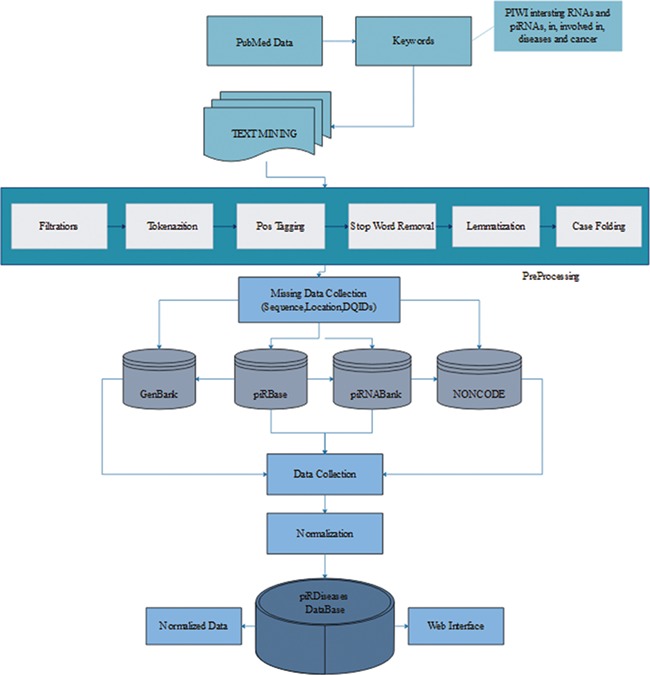
piRDisease database construction workflow. The piRDisease database was constructed from keywords search to data retrieval, preprocessing, normalization followed by adding missing data. Finally, all these data were stored in the piRDisease database.
We employed `Natural Language Toolkit’ and `TextBlob’ to apply natural language processing techniques on complex text data description (e.g. symbols, punctuation, double spaces, typo errors and long sentences etc.) extracted from literature particularly in two fields, such as `detailed mechanism’ and `description’ (39). Preprocessing involves several steps listed as follows.
Tokenization: The textual description of collected data from different research papers usually combines words and meaningless symbols e.g. special characters and punctuations. Such symbols create problems when we store the data in MYSQL. Tokenization filters out the meaningless symbols and divides the remaining text into tokens.
Spell correction: The unstructured attributes (e.g. detailed mechanism and description) of collected data may have spelling mistakes or typo errors. Therefore, we correct such mistakes in this step of preprocessing.
Stop-word removal: The text of a document often contains constructive terms (e.g. prepositions) and other language structures to connect sentences. Such terms are known as stop-words. We subtract stop-words from the preprocessed data.
Word inflection and lemmatization: Word inflection transforms words into their singular form and lemmatization shifts the comparative and superlative terms into their basic term. For example, inflection transforms the word `bugs’ into `bug’ and lemmatization shifts the word `computation’ into `compute’. We performed both word inflection and lemmatization to avoid the repetition of words that share the same basic term. Finally, we converted all the preprocessed words into lowercase (e.g. `Upregulated’ to `upregulated’).
After preprocessing, we categorized manually curated piRNAs’ disease association data in `annotation’ field based on experimental methods used in the reference studies. For example, piRNAs discovered from whole genome sequencing (WGS), RNA-Seq and microarray methods were denoted as `predicted’ (Table 1). However, if piRNAs expression was quantitatively measured by RT-qPCR following these WGS experiments they were categorized as `related’. Finally, when piRNAs’ mechanistic (regulatory) role was elaborated by a series of experiments (e.g. knock-down, northern blotting, MTT assay, cell cycle analysis etc.), they were called as `validated’. In order to validate these records, data extraction from relevant genome version and reference databases was considered. We obtained piRNAs' missing sequence and location information from piRNAs reference databases (e.g. piRNABank and piRBase), and from other non-coding RNA databases (e.g. NONCODE 3.0).
Table 1.
Annotation of piRNAs on the basis of experimental evidences
| Sr | Experiment methods in papers | Description | Annotation | |
|---|---|---|---|---|
| 1 | Microarray, RNA-Seq | WGS | Predicted | |
| 2 | Microarray, RNA-Seq, qPCR | WGS, RT-qPCR (expression validation) | Related | |
| 3 | Northern blot, MTT assay, knock down, Western Blot, xenograft model etc. | Multiple experiments (mechanistic role validation) | Validated |
After collection of the data, it was observed that data were highly diverse due to the complexity of nomenclature and various genome versions used by different non-coding RNAs databases in reference studies. piRNA-disease association studies incorporated data from various reference piRNA databases, and each of them has unique ID. For example, piRNABank and piRNAQuest use has_piR_000001 and piRBase follows piR_hsa_000001, which makes piRNA search quite challenging. However, DQ (accession ID) can be used to search exact piRNA in primary genome browsers such as GenBank as well as reference piRNA databases (26, 34, 35). Thus, we extracted DQ IDs for standardization, so that users can also use DQ ID data to search, explore and interpret results in piRDisease database (Figure 1). Before storing data into our database, the data were normalized by removing data redundancy and anomalies.
Finally, all the mined data were stored in the form of database using MySQL (version 5.7.25). The web interface was built in HTML and CSS to make the web portal attractive. The data processing programs were written in PHP (5.7), ajax, JavaScript and the web services were built using Xamp server. The piRDisease database is freely available at http://piwirna2disease.org/.
In summary, piRDisease is a distinct database resource providing 7939 manually curated associations of experimentally supported 4796 piRNA involved in 28 different disease types.
User interface
piRDisease provides `search’, `browse’ and `submit’ options on the home page. Users can search the database, entering piRNA ID or DQ-ID and select the specific disease or any disease to explore the piRNAs’ association (expression) in relevant disease, and this will display result page for searched piRNA’s (or disease associated piRNAs’) expression or interaction type in relevant disease (Figure 3). Currently users can browse piRNA-disease association data for three organisms (human, mouse and rat). The `submit’ button allows researchers to add in new data, which will be significant for updating information in piRDisease database. Further, users can click on `detailed page’ and it will reveal piRNA target genes, and detailed mechanism of piRNA expression or regulation of target genes. piRNAs are categorized as predicted, related and validated in annotation field in the database on the bases of experimental methods. Description provides the overall functional relationship followed by tissues or cells used in reference study (Figure 3). `Detailed page’ also provides piRNA sequence, location, species, PubMed IDs and title of the study. piRDisease uses `non fuzzy’ search so that exact match will be found. piRDisease also contains novel piRNAs as well as piRNA-like RNAs (piRNA-like) implicated in some diseases. piRDisease provides its own search ID for the piRNAs that do not have DQ IDs, piRNA-like and novel piRNAs.
Figure 3.

A schematic workflow for piRDisease. (A) Users can search individual piRNA or disease that is associated with piRNAs. (B) Searching results were shown. (C, D) piRNA-disease association data for three species were shown. (E)piRDisease provided detailed information for relevant piRNA that was associated with specific disease.
Utility and discussion
Evidently, piRNAs’ spatial and temporal expression is critical for normal cellular development and differentiation, ranging from embryonic stage to gonad development (7, 36–38). Hence, piRNAs dysregulated expression and peculiarly their target genes’ regulation can be a potentially diagnostic marker in wide range of diseases (7, 37, 38). Recent progression of studies enforced the role of piRNAs in various type of diseases, specifically different cancer types (Figures S1 and S2). Enormous amount of piRNA-disease association data are expected to be produced in the near future. Hence, we developed piRDisease database by collecting piRNA-disease association data scattered in the literature. piRDisease is the first and novel piRNA database resource that contains 7939 piRNA-disease-associated entries, which comprises of 4796 unique piRNAs and 28 types of associated diseases in three species (human, mouse and rat; Figure 3, Table S1). However, piRNAs involved in deep regulatory mechanism is still to be explored. For instance, when we search piRDisease with the search term `piR-651’, which is one of the highly explored piRNAs in various diseases in literature, we will retrieve eight results. We found that `piR-651’ is mostly upregulated in various cancer types such as breast cancer, gastric cancer, colon cancer, mesothelium, liver cancer and cervical cancer. However, only a few studies revealed detailed mechanistic roles of piR-651 in some diseases. For example, estrogen and androgen hormones treatment resulted in higher expression of piR-651 in prostate cancer. In addition, this piRNA overexpression was highly correlated with tumor propagation, which was mediated by cyclin D1 and CDK4 pathway in `non-small cell lung carcinoma’. These results suggested that `piR-651’ aberrant expression is significant to many cancer types, but only in a few cancer types its detailed mechanism was revealed. Currently, piRNA-disease association data are available for 28 diseases, of which 54% are various types of cancers; 40% are cardiovascular diseases; 4% are neurodegenerative diseases; and 1% are spermatogenesis-related and other diseases (Figure 2B).
Figure 2.
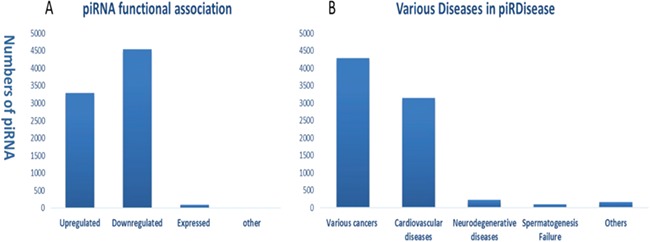
Statistics and distribution (A) of dysfunction types of piRNAs (B) in various disease types in piRDisease database.
Conclusions
In order to provide biological community central resource to search, explore and investigate the piRNA-disease relationships, we developed piRDisease database, which is a convenient, comprehensive web-based database resource, providing detailed information about piRNAs’ role in various diseases.
piRDisease provides scientific community inclusive insights into piRNAs functional relationship in wide range of diseases. This novel and unique database resource will lead toward further research ideas.
Future extension
Since piRNAs involved in diseases were explored vastly in the past few years, a huge amount of data is expected to be produced in the near future. We thus plan to update this data on yearly bases. In addition, we intend to build and incorporate some piRNA target prediction software based on some innovative algorithms.
Authors Contribution
Mr Muhammad Azhar conceptualized idea, collected, stored and managed the data. Mr Muhammad Azhar and Mr Waheed Ramay contributed in building the database. Mr Nauman khan and Miss Hong Jiang cross checked the database. Dr Xiaoyuan Song supervised this work and manuscript.
Supplementary Material
Acknowledgements
The authors thank Dr Zhang Yuanwei and Mr Ranjha Khan at University of Science and Technology China for their constructive suggestions and guidance. We also thank Mr Badar Iqbal for proofreading and revising language for the manuscript.
Database URL: http://www.piwirna2disease.org/index.php
Funding
National Natural Science Foundation of China (91540107). Chinese Academy of Sciences (CAS)-The World Academy of Sciences (TWAS) President’s Fellowship (to A.M.). Young Thousand Talents Program (KJ 2070000026 to X.S.).
Conflict of interest. None declared.
References
- 1. Iwasaki Y.W., Siomi M.C. and Siomi H.. PIWI-interacting RNA: its biogenesis and functions. Annu. Rev. Biochem., 84, 405–433. [DOI] [PubMed] [Google Scholar]
- 2. Ng K.W., Anderson C., Marshall E.A. et al. (2016) Piwi-interacting RNAs in cancer: emerging functions and clinical utility. Mol. Cancer, 15, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luteijn M.J. and Ketting R.F. (2013) PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nat. Rev. Genet., 14, 523–534. [DOI] [PubMed] [Google Scholar]
- 4. Kawaoka S., Izumi N., Katsuma S. et al. (2011) 3′ End formation of PIWI-interacting RNAs in vitro. Mol. Cell, 43, 1015–1022. [DOI] [PubMed] [Google Scholar]
- 5. Fu A., Jacobs D.I., Hoffman A.E. et al. (2015) PIWI-interacting RNA 021285 is involved in breast tumorigenesis possibly by remodeling the cancer epigenome. Carcinogenesis, 36, 1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ortogero N., Schuster A.S., Oliver D.K. et al. (2014) A novel class of somatic small RNAs similar to germ cell pachytene PIWI-interacting small RNAs. J. Biol. Chem., 289, 32824–32834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams Z., Morozov P., Mihailovic A. et al. (2015) Discovery and characterization of piRNAs in the human fetal ovary. Cell Rep., 13, 854–863. [DOI] [PubMed] [Google Scholar]
- 8. Huang X., Yuan T., Tschannen M. et al. (2013) Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics, 14, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quek C., Bellingham S.A., Jung C.-H. et al. (2016) Defining the purity of exosomes required for diagnostic profiling of small RNA suitable for biomarker discovery. RNA Biol., 14, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bahn J.H., Zhang Q., Li F. et al. (2015) The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem, 61, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bachmayr-Heyda A., Auer K., Sukhbaatar N. et al. (2016) Small RNAs and the competing endogenous RNA network in high grade serous ovarian cancer tumor spread. Oncotarget, 7, 39640–39653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy J., Sarkar A., Parida S. et al. (2017) Small RNA sequencing revealed dysregulated piRNAs in Alzheimer’ s disease and their probable role in pathogenesis. Mol. Biosyst., 13, 565–576. [DOI] [PubMed] [Google Scholar]
- 13. Daugaard I., Venø M.T., Yan Y. et al. (2017) Small RNA sequencing reveals metastasis-related microRNAs in lung adenocarcinoma. Oncotarget, 8, 27047–27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reeves M.E., Firek M., Jliedi A. et al. (2017) Identification and characterization of RASSF1C piRNA target genes in lung cancer cells. Oncotarget, 8, 34268–34282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y., Wu X., Gao H. et al. (2015) Piwi-interacting RNAs (piRNAs) are dysregulated in renal cell carcinoma and associated with tumor metastasis and cancer-specific survival. Mol. Med., 21, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faunes F., Sanchez N., Moreno M. et al. (2011) Expression of transposable elements in neural tissues during Xenopus development. PLoS One, 6, e22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cui L., Lou Y., Zhang X. et al. (2011) Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin. Biochem., 44, 1050–1057. [DOI] [PubMed] [Google Scholar]
- 18. Krishnan P., Ghosh S., Wang B. et al. (2016) Profiling of small nucleolar RNAs by next generation sequencing: potential new players for breast cancer prognosis. PLoS One, 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yin J., Jiang X.-Y., Qi W. et al. (2017) piR-823 contributes to colorectal tumorigenesis by enhancing the transcriptional activity of HSF1. Cancer Sci., 108, 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou A.E., Zheng H., Saad M.A. et al. (2016) The non-coding landscape of head and neck squamous cell carcinoma. Oncotarget, 7, 51211–51222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brock M. and Mei Y. (2017) Protein functional effector sncRNAs (pfeRNAs) in lung cancer. Cancer Lett., 403, 138–143. [DOI] [PubMed] [Google Scholar]
- 22. Lim R.S.M. and Kai T. (2015) A piece of the pi(e): the diverse roles of animal piRNAs and their PIWI partners. Semin. Cell Dev. Biol., 47–48, 17–31. [DOI] [PubMed] [Google Scholar]
- 23. Martinez V.D., Vucic E.A., Thu K.L. et al. (2015) Unique somatic and malignant expression patterns implicate PIWI-interacting RNAs in cancer-type specific biology. Sci. Rep., 5, 10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krishnan P., Ghosh S., Graham K. et al. (2016) Piwi-interacting RNAs and PIWI genes as novel prognostic markers for breast cancer. Oncotarget, 7, 37944–37956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krishnan A.R., Korrapati A., Zou A.E. et al. (2017) Smoking status regulates a novel panel of PIWI-interacting RNAs in head and neck squamous cell carcinoma. Oral Oncol., 65, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sai Lakshmi S. and Agrawal S. (2008) piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. Nucleic Acids Res., 36, 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarkar A., Maji R.K., Saha S. et al. (2014) piRNAQuest: searching the piRNAome for silencers. BMC Genomics, 15, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen G., Wang Z., Wang D. et al. (2013) LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res., 41, 983–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ning S., Zhang J., Wang P. et al. (2016) Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res., 44, D980–D985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang Q., Wang Y., Hao Y. et al. (2009) miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res., 37, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie B., Ding Q., Han H. et al. (2013) MiRCancer: a microRNA-cancer association database constructed by text mining on literature. Bioinformatics, 29, 638–644. [DOI] [PubMed] [Google Scholar]
- 32. Zhao Z., Wang K., Wu F. et al. (2018) circRNA disease: a manually curated database of experimentally supported circRNA-disease associations. Cell Death Dis., 9, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yao D., Zhang L., Zheng M. et al. (2018) Circ2Disease: a manually curated database of experimentally validated circRNAs in human disease. Sci. Rep., 2018, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwala R., Barrett T., Beck J. et al. (2016) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res., 44, D7–D19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang P., Si X., Skogerbø G. et al. (2014) PiRBase: a web resource assisting piRNA functional study. Database, 2014, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang H., Wang B., Liu X. et al. (2017) Identification and expression of piwil2 in turbot Scophthalmus maximus, with implications of the involvement in embryonic and gonadal development. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol., 208–209, 84–93. [DOI] [PubMed] [Google Scholar]
- 37. Hong Y., Wang C., Fu Z. et al. (2016) Systematic characterization of seminal plasma piRNAs as molecular biomarkers for male infertility. Sci. Rep., 6, 24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiong C. (2014) Identification of microRNAs predominately derived from testis and epididymis in human seminal plasma. Clin. Biochem., 47, 967–972. Bird S, Klein E, Loper E. Natural Language Processing in Python. Forthcoming. [DOI] [PubMed]
- 39. Bird S, Klein E, Loper E. Natural Language Processing in Python. Forthcoming.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


