Abstract
Background
Ginsenoside Rb1, a triterpene saponin, is derived from the Panax ginseng root and has potent antiinflammatory activity. In this study, we determined if Rb1 can increase macrophage phagocytosis and elucidated the underlying mechanisms.
Methods
To measure macrophage phagocytosis, mouse peritoneal macrophages or RAW 264.7 cells were cultured with fluorescein isothiocyanate–conjugated Escherichia coli, and the phagocytic index was determined by flow cytometry. Western blot analyses were performed.
Results
Ginsenoside Rb1 increased macrophage phagocytosis and phosphorylation of p38 mitogen-activated protein kinase (MAPK), but inhibition of p38 MAPK activity with SB203580 decreased the phagocytic ability of macrophages. Rb1 also increased Akt phosphorylation, which was suppressed by LY294002, a phosphoinositide 3-kinase inhibitor. Rb1-induced Akt phosphorylation was inhibited by SB203580, (5Z)-7-oxozeaenol, and small-interfering RNA (siRNA)–mediated knockdown of p38α MAPK in macrophages. However, Rb1-induced p38 MAPK phosphorylation was not blocked by LY294002 or siRNA-mediated knockdown of Akt. The inhibition of Akt activation with siRNA or LY294002 also inhibited the Rb1-induced increase in phagocytosis. Rb1 increased macrophage phagocytosis of IgG-opsonized beads but not unopsonized beads. The phosphorylation of p21 activated kinase 1/2 and actin polymerization induced by IgG-opsonized beads and Rb1 were inhibited by SB203580 and LY294002. Intraperitoneal injection of Rb1 increased phosphorylation of p38 MAPK and Akt and the phagocytosis of bacteria in bronchoalveolar cells.
Conclusion
These results suggest that ginsenoside Rb1 enhances the phagocytic capacity of macrophages for bacteria via activation of the p38/Akt pathway. Rb1 may be a useful pharmacological adjuvant for the treatment of bacterial infections in clinically relevant conditions.
Keywords: Akt, Ginsenoside Rb1, Macrophage, p38, Phagocytosis
1. Introduction
Phagocytosis by macrophages is the cornerstone of the innate immune response, which eliminates invading pathogens, cellular debris, and foreign bodies. Several phagocytic receptors including Fcγ receptors, the complement receptor, integrins such as α5β1 and αvβ3, the lipopolysaccharide (LPS) receptor CD14, and the mannose receptor are involved in the phagocytic uptake of particles [1], [2], [3], [4]. The binding of pathogens or specific molecules by these receptors results in the rapid responses of a series of intracellular signaling proteins, including the Rho family of G proteins, which play an important role in the reorganization of the actin cytoskeleton [1], [3]. Recent studies have reported that several kinases including p38 mitogen-activated protein kinase (MAPK) or adenosine monophosphate-activated protein kinase can activate Rac1, a subfamily of the Rho family, which plays a pivotal role in the phagocytic uptake of particles [5], [6], [7], [8].
Previous studies have revealed that the activation of p38 MAPK can regulate actin cytoskeletal reorganization, which plays an important role in phagocytosis, cell migration, and morphological changes in various cell populations [9], [10], [11]. Specifically, p38 MAPK activation by various stimuli enhances the ability of macrophages to phagocytose bacteria [7], [11], [12], [13], [14]. Stimulation of toll-like receptors (TLRs) with specific ligands enhances bacterial phagocytosis by macrophages and was blocked by p38 MAPK inhibitors or small-interfering RNA (siRNA)–mediated knockdown of p38 MAPK [11], [13]. Deprivation of nutrients, such as amino acids or glucose, has been shown to increase the phosphorylation of p38 MAPK, which is required for the starvation-induced increase in macrophage bacterial phagocytosis [12].
Ginsenosides are the major bioactive constituents of the Panax ginseng root, which contain many types of ginsenosides including Rb1, Rg1, Rc, and Rg3. The ginsenoside Rb1 appears to be the most abundant of the bioactive ginsenosides [15]. Rb1 exhibits antiinflammatory, anticarcinogenic, and antioxidant activities [16], [17], [18], [19], [20]. For example, Rb1 decreased TLR4 mRNA expression, production of tumor necrosis factor-α, and pathological changes in hepatic and lung tissues in septic rats [16], [17], [18], [19], [20]. Rb1 and its metabolite, compound K, significantly diminished the activation of interleukin-1 receptor-associated kinase-1, nuclear factor-κB, and MAPKs, and the production of inflammatory cytokines in macrophages stimulated with LPS [16], [17], [18], [19], [20]. These observations suggest that Rb1 may have beneficial effects in acute inflammatory conditions such as bacterial infection. In this study, we investigated whether Rb1 can increase macrophage phagocytosis of bacteria and the related intracellular signaling pathways.
2. Materials and methods
2.1. Mice
Male BALB/c mice (20–25 g, 8–10 weeks old) were purchased from Samtako Science (Daejeon, Korea). The mice were maintained in a 12 h light/dark cycle that allowed them to freely consume food and water. All experiments were conducted with the approval of the Animal Care and Ethics Committee of Chonnam National University Medical School (CNU IACUC-H-2015-28).
2.2. Reagents and antibodies
Ginsenoside Rb1 from P. ginseng (Korean ginseng) root, LY294002, and (5z)-7-oxozeaenol were purchased from Sigma-Aldrich (St Louis, MO, USA). Bovine serum albumin, Roswell Park Memorial Institute 1640 with L-glutamine, penicillin-streptomycin, and fetal bovine serum (FBS) were obtained from GIBCO (Gaithersburg, MD, USA). Antibodies specific for phosphor-Akt or total-Akt, p38, MAPK kinase 3/6 (MKK3/6), p21-activated kinase (PAK)1/2 and heat shock protein (HSP) 27 were obtained from Cell Signaling Technology (Beverly, MA, USA). SB203580 was purchased from Calbiochem (La Jolla, CA, USA). Fluorescein isothiocyanate (FITC)–conjugated Escherichia coli (K-12 strain), 1-μm fluorescent FluoSpheres beads, and opsonizing reagent were obtained from Invitrogen (Eugene, OR, USA).
2.3. Cell isolation and culture
Murine peritoneal macrophages were isolated as described previously [14], [21]. Briefly, 4% Brewer thioglycollate was intraperitoneally injected, and then peritoneal macrophages were isolated after 4 days and cultured at 37°C in Roswell Park Memorial Institute 1640 media containing 5% FBS in a 12-well plate. After 1 h, nonadherent cells were discarded by vigorous washing with culture medium. Murine macrophage RAW 264.7 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in Dulbecco's Modified Eagle's media supplemented with 5% FBS at 37°C.
2.4. Western blotting
The protein concentrations of whole cell lysates were measured by BCA protein assay kit to the manufacturer's manual (Thermo Fisher Scientific, Pierce, WI, USA). Equal amounts of protein were subjected to electrophoresis in 10–12% sodium dodecyl sulphate polyacrylamide gel, and then transferred to polyvinylidene difluoride membranes. After blocking with 5% nonfat dry milk, the membranes were maintained with primary antibodies diluted 1:1000 in tris-buffered salin-Tween with 5% bovine serum albumin for overnight at 4°C and then incubated with horseradish peroxidase-coupled secondary Ab in 5% nonfat dry milk. The signals were detected using enhanced chemiluminescence (Millipore, Billerica, MA, USA).
2.5. siRNA knockdown of p38α or Akt
RAW 264.7 cells (2 × 105 cells/well) in 12-well plates were incubated in Lipofectamine (Thermo Fisher Scientific, Pierce, WI, USA) with siRNA against mouse p38α MAPK, Akt, and control siRNA (50 nM) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 48 h. The transfected cells were assayed for phagocytosis or analyzed by Western blot as described in the figure legends.
2.6. In vitro phagocytosis assay
The phagocytic index was measured as described previously [7], [14]. FITC-conjugated E. coli and fluorescent beads were added to peritoneal macrophages or RAW 264.7 cells at a ratio of 2:1 for 20 min and 30 min, respectively, at 37°C. Fluorescent beads were opsonized with IgG opsonizing reagent for l h at 37°C. Nonphagocytized E. coli or fluorescent beads were removed by washing three times with cold phosphate buffered saline (PBS), and 0.2% trypan blue solution was added to the macrophages to quench fluorescence of the E. coli attached to the cells. The cells were isolated from the wells using 0.25% trypsin/EDTA in PBS. The phagocytic index was calculated as percentage of macrophages positive for fluorescent E. coli using flow cytometry.
2.7. In vivo phagocytosis assay
The phagocytic index was quantified by flow cytometry [7], [14]. Rb1 (0 or 5 mg/kg) was injected intraperitoneally into mice. Two hours after Rb1 injection, 50 μL PBS with FITC-labeled E. coli (10 × 106/mouse) was injected into the mouse lung via tracheotomy under anesthesia with sevoflurane. The cells in the lungs were obtained through three times of bronchoalveolar lavage (BAL) with 1 mL of ice-cold PBS with 5 mM EDTA 2 h after the administration of FITC-labeled E. coli. The cells in BAL fluid were washed with cold PBS and treated with 0.2% trypan blue solution. The number of E. coli positive cells was measured by flow cytometry.
2.8. Actin polymerzation
Actin polymerization was performed as described previously [6]. Briefly, macrophages were plated in 96-well plates. The cells were fixed with 4% paraformaldehyde for 20 min, washed, and permeabilized with 0.1% Triton X-100. The cells were washed and stained with Alexa Fluor 594-conjugated phalloidin (Invitrogen) and then stained with 4′,6-diamidino-2-phenylindole (DAPI). Actin polymerization was measured as a relative fluorescent unit of phalloidin to DAPI.
2.9. Statistical analysis
Data are expressed as means ± standard deviation. Statistical significance was determined using the Student t test for comparisons between two groups. Multigroup comparisons were performed using one-way analysis of variance with Tukey's post hoc test (SPSS, version 21.0). A value of p < 0.05 was considered significant.
3. Results
3.1. Ginsenoside Rb1 increases macrophage phagocytosis toward E.coli through activation of the MKK3/6–p38 MAPK pathway
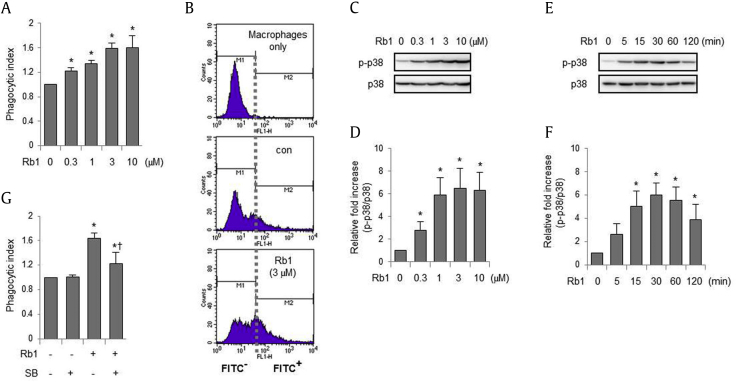
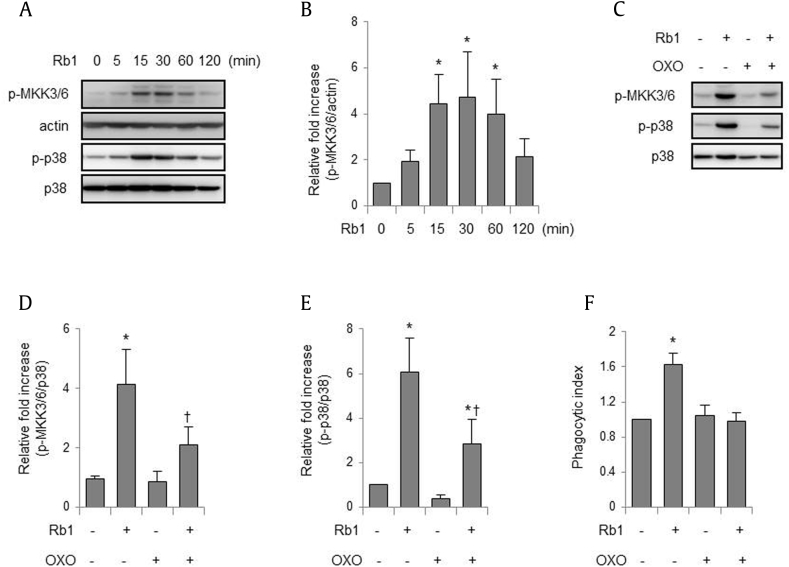
In the first set of experiments, we determined whether Rb1 could increase the phagocytic ability of macrophages toward E. coli. As shown in Figs. 1A, 1B, addition of Rb1 to peritoneal macrophage cultures enhanced phagocytosis of fluorescent E. coli in a dose-dependent manner, with a peak effect at a concentration of 3 μM. Because previous studies have shown that p38 MAPK may be involved in macrophage phagocytosis of bacteria [7], [14], we examined whether Rb1 can increase the activity of p38 MAPK in macrophages. Rb1 dose-dependently increased the phosphorylation of p38 MAPK in peritoneal macrophages, and maximal phosphorylation of p38 MAPK was attained at a concentration of 3 μM (Figs. 1C, 1D), in parallel with the increased phagocytic ability of macrophages. As shown in Figs. 1E, 1F, treatment of macrophages with Rb1 increased p38 MAPK phosphorylation, which began to rapidly increase within 15 min, peaked at 30 min, and lasted more than 2 h. Moreover, the addition of SB203580, a p38 MAPK inhibitor, before the addition of Rb1 to macrophage cultures, decreased Rb1-induced macrophage phagocytosis of E. coli (Fig. 1G). The activation of p38 MAPK can be regulated by upstream kinases including transforming growth factor-β-activated kinase 1 (TAK1), which phosphorylates MAPKs such as MKK3/6 [22]. As shown in Figs. 2A, 2B, treatment of macrophages with Rb1 induced the phosphorylation of MKK3/6, an upstream kinase of p38 MAPK, in a time-dependent manner, reaching maximum levels after 30 min. Addition of (5Z)-7-oxozeaenol, a TAK1 inhibitor, to macrophage cultures suppressed Rb1-induced phosphorylation of MKK3/6 and p38 MAPK (Figs. 2C–2E) and inhibited the Rb1-induced increase in macrophage phagocytosis (Fig. 2F).
Fig. 1.
Ginsenoside Rb1 enhances macrophage phagocytosis through p38 mitogen-activated protein kinase (MAPK) activation. (A) Peritoneal macrophages were cultured with Rb1 (0, 0.3, 1, 3, and 10 μM) for 2 h, and fluorescein isothiocyanate (FITC)-conjugated Escherichia coli was added to culture media for 20 min, followed by flow cytometry. (B) Representative flow cytometry histograms are shown. (C–F) Peritoneal macrophages were cultured with Rb1 (0, 0.3, 1, 3, and 10 μM) for 30 min (C, D) or with Rb1 (3 μM) for the indicated time periods (E, F). The whole cell lysates were subjected to electrophoresis in polyacrylamide gels. (G) Peritoneal macrophages were incubated with SB203580 (SB; 0 or 10 μM) for 30 min before culture with Rb1 (0 or 3 μM) for 2 h and then subjected to FITC-conjugated E. coli for 20 min. Each bar represents the mean ± standard deviation (n = 4). *p < 0.05 compared to the control.
Fig. 2.
Rb1 increases activation of the p38 MAPK pathway. (A–B) Peritoneal macrophages were cultured with Rb1 for the indicated times. (C–E) Peritoneal macrophages were cultured with (5Z)-7-oxozeaenol (OXO; 0 or 1 μM) for 1 h before exposure to Rb1 (0 or 3 μM) for 30 min. The whole cell lysates were subjected to electrophoresis in polyacrylamide gels. (F) Peritoneal macrophages were cultured with OXO (0 or 1 μM) for 1 h prior to incubation with Rb1 (0 or 3 μM) for 2 h, and FITC-conjugated E. coli was added to the culture media for 20 min, followed by flow cytometry. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control. †p < 0.05 compared to Rb1 only. FITC, fluorescein isothiocyanate; MAPK, mitogen-activated protein kinase; SD, standard deviation.
3.2. p38 MAPK–Akt activation is responsible for the Rb1-dependent increase in macrophage phagocytosis
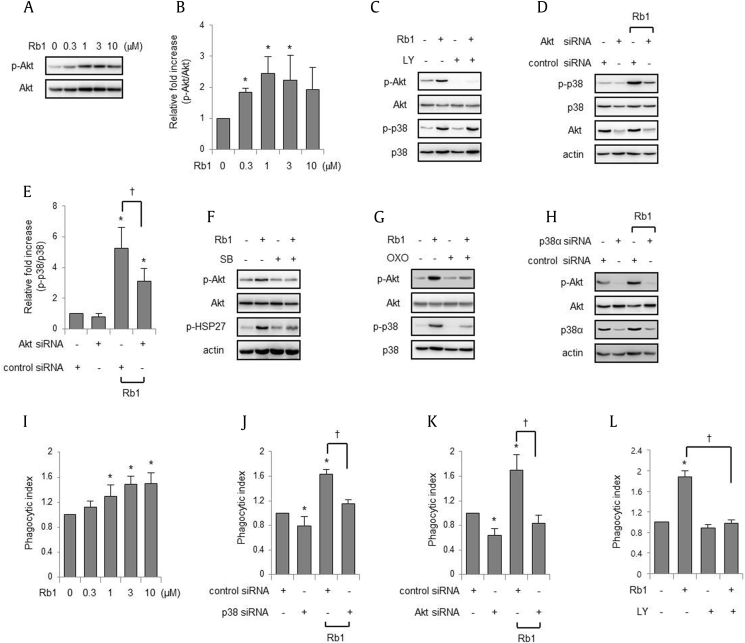
Although our initial experiments showed that p38 MAPK activation was involved in the Rb1-induced increase in macrophage phagocytosis, the mechanism through which Rb1 enhances macrophage phagocytosis is yet to be determined. Because previous studies have demonstrated that phosphoinositide 3-kinase (PI3K)/Akt plays an important role in macrophage phagocytosis [23], we evaluated if Rb1 could increase the activation of Akt, a well-recognized downstream target of PI3K in multiple cell types. As shown in Figs. 3A, 3B, the addition of Rb1 to macrophage cultures increased Akt phosphorylation. To determine whether Akt is an upstream regulator of p38 MAPK, macrophages were cultured with LY294002, a PI3K inhibitor, before the addition of Rb1 to cultures. Rb1 increased the phosphorylation of p38 MAPK in the presence of LY294002 or under the Akt knockdown condition (Figs. 3C–3E). However, addition of SB203580 or (5Z)-7-oxozeaenol to macrophage cultures before Rb1 exposure diminished Akt phosphorylation, p38 MAPK, and heat shock protein 27, a downstream target of p38 MAPK (Figs. 3F, 3G). In addition, knockdown of p38α in RAW 264.7 cells also inhibited the Rb1-induced increase in Akt phosphorylation (Fig. 3H). Addition of Rb1 to RAW 264.7 cells also enhanced the phagocytosis of fluorescent E. coli in a dose-dependent manner (Fig. 3I), consistent with the above results, which demonstrated that Rb1 increased peritoneal macrophage phagocytosis. siRNA-mediated knockdown of p38α inhibited the Rb1-induced increase in macrophage phagocytosis, and knockdown of Akt also blocked the Rb1-induced increase in macrophage phagocytosis (Figs. 3J, 3K). Moreover, the addition of LY294002 also inhibited the Rb1-induced increase in phagocytosis (Fig. 3L).
Fig. 3.
Rb1 increases the phosphorylation of Akt through the p38 MAPK pathway. (A–B) Peritoneal macrophages were cultured with Rb1 for 1 h at the indicated doses. The cell lysates were subjected to western blotting. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control. (C, F, and G) Macrophages were cultured with LY294002 (LY; 0 or 10 μM) for 30 min, SB203580 (SB; 0 or 10 μM) for 30 min or (5Z)-7-oxozeaenol (OXO; 0 or 1 μM) for 1 h before exposure to Rb1 (0 or 3 μM) for 1 h. (D–E) RAW 264.7 cells were treated with control small-interfering RNA (siRNA) or siRNA targeting mouse Akt and cultured with Rb1 (0 or 3 μM) for 1 h. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control siRNA; †p < 0.05. (H) RAW 264.7 cells were treated with control or p38α siRNA and cultured with Rb1 (0 or 3 μM) for 1 h. The whole cell lysates were subjected to electrophoresis in polyacrylamide gels. Representative gels are shown, and the other two independent experiments provided similar results. (I) RAW 264.7 cells were cultured with Rb1 (0, 0.3, 1, 3, and 10 μM) for 2 h and subjected to FITC-conjugated E. coli for 30 min, followed by flow cytometry. (J–K) RAW 264.7 cells were treated with control siRNA or siRNA targeting mouse p38α or Akt. The cells were cultured with Rb1 (0 or 3 μM) for 2 h and subjected to FITC-conjugated E. coli for 30 min, followed by flow cytometry. (L) LY294002 (LY; 0 or 10 μM) was added to peritoneal macrophage culture for 30 min before exposure to Rb1 (0 or 3 μM) for 2 h, and FITC-conjugated E. coli was added to the culture media for 20 min, followed by flow cytometry. Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control; †p < 0.05. FITC, fluorescein isothiocyanate; MAPK, mitogen-activated protein kinase; SD, standard deviation.
3.3. Rb1-induced actin polymerization is mediated by p38 MAPK and Akt activation
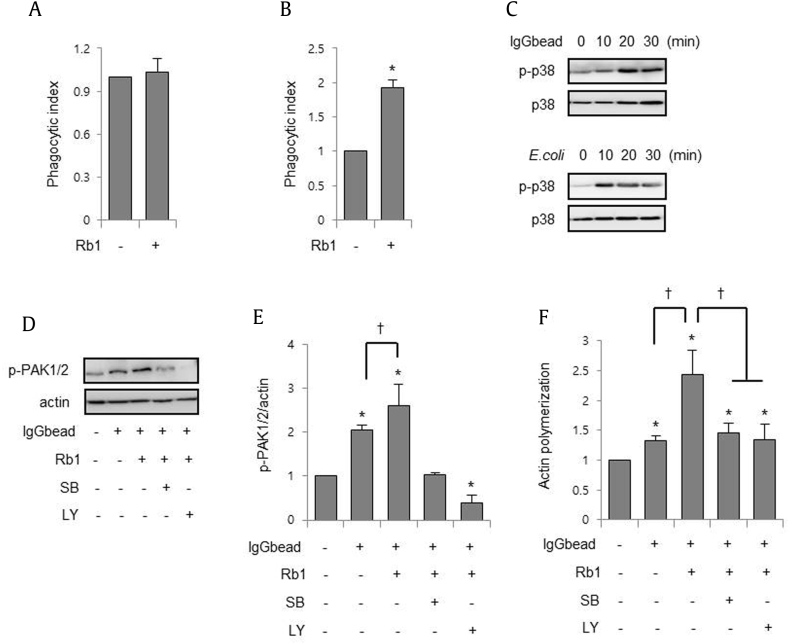
Because Fcγ receptor (FcgR) plays a pivotal role in the removal of extracellular pathogens, we investigated whether Rb1 is involved in FcgR-mediated phagocytosis using IgG-opsonized beads (IgGbead). Rb1 did not affect macrophage phagocytosis of unopsonized beads, but increased phagocytosis of IgGbead (Fig. 4A, 4B). In addition, culture of macrophages with E. coli or IgGbead increased p38 MAPK phosphorylation (Fig. 4C). Reorganization of actin cytoskeleton is required for FcgR-mediated phagocytosis and PAKs; downstream effectors of small GTPases of the Rho family such as Rac1/Cdc42 are mainly involved in actin polymerization [5], [24]. In our studies, FcgR engagement with IgGbead increased phosphorylation of PAK1/2 and co-culture of macrophages with IgGbead and Rb1 further increased the phosphorylation of PAK1/2 (Figs. 4D, 4E). A previous study suggested that Akt was involved in PAK activation [25]. Therefore, we examined whether Rb1-induced p38/Akt activation could modulate PAK activation. Addition of SB203580 or LY294002 to macrophage cultures inhibited the phosphorylation of PAK1/2 (Fig. 4D, 4E) and actin polymerization induced by IgGbead or/and Rb1 (Fig. 4F). These results suggest that Rb1-induced p38 MAPK activation can modulate FcgR-mediated phagocytic pathways.
Fig. 4.
Rb1 increased actin polymerization through p38 MAPK and Akt activation. (A–B) Peritoneal macrophages were cultured with (A) unopsonized beads or (B) IgG-opsonized beads (IgGbead) for 20 min and followed by flow cytometry. (C) Macrophages were cultured with E.coli or IgGbead for the indicated times, and the whole cell lysates were subjected to electrophoresis in polyacrylamide gels. (D–E) Macrophages were cultured with LY294002 (LY; 10 μM) or SB203580 (SB; 10 μM) for 30 min, before exposure to Rb1 (3 μM) for 1 h and then subjected to IgGbead for 20 min. (D) The whole cell lysates were subjected to western blotting. (E) Relative fluorescent units of phalloidine to DAPI were measured by fluorescence plate reader. Each bar represents the mean ± SD (n = 5). *p < 0.05 compared to the control. †p < 0.05. MAPK, mitogen-activated protein kinase; PAK, p21-activated kinase; SD, standard deviation.
3.4. Rb1 increases phagocytosis of bacteria in mouse lungs
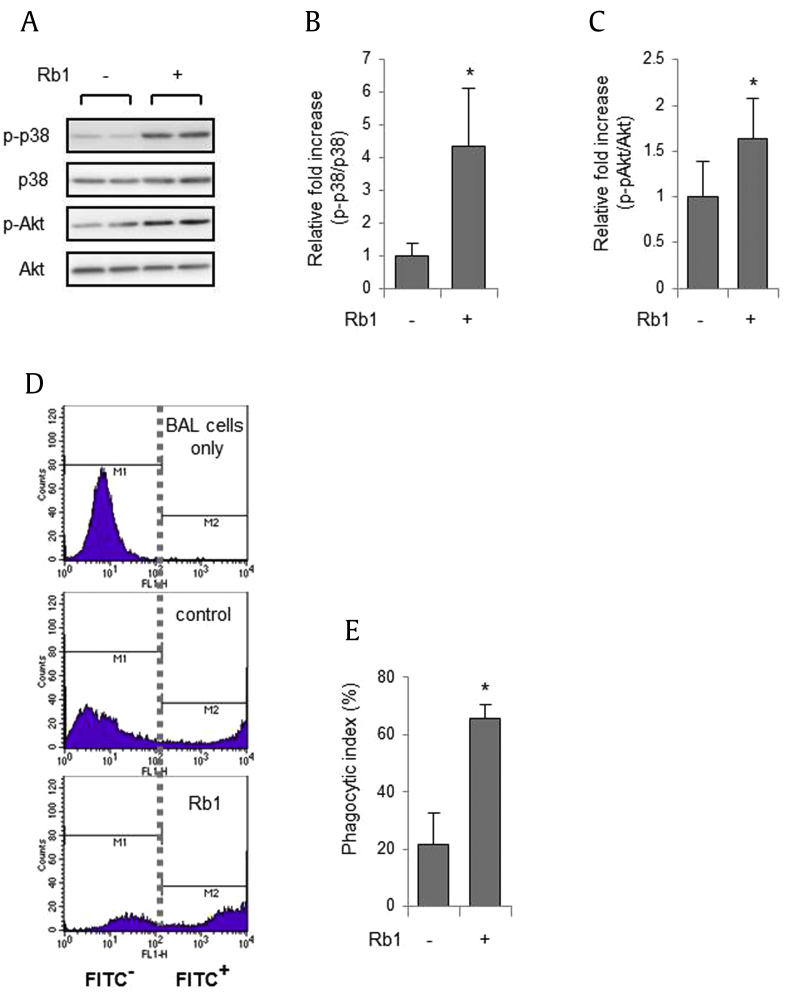
Although our experiments showed that Rb1 could increase macrophage phagocytosis in vitro, we investigated whether such results could also be seen in vivo. Mice were subjected to intraperitoneal administration of Rb1 for 2 h. As shown in Figs. 5A–5C, Rb1 increased the phosphorylation of p38 MAPK and Akt in the lungs of mice, consistent with the in vitro results. Mice were injected intraperitoneally with Rb1 for 2 h before intratracheal administration of FITC-conjugated E. coli. The number of E. coli-positive cells in the BAL fluid was measured using flow cytometry, and a significant increase in the number of cells was found in mice treated with Rb1 compared to control mice (Figs. 5D, 5E).
Fig. 5.
Rb1 increases macrophage phagocytosis in the mouse lung. (A–C). Mice were injected intraperitoneally with Rb1 (0 or 5 mg/kg) for 2 h and bronchoalveolar lavage (BAL) cells were obtained from mouse lungs. The cell lysates were subjected to electrophoresis in polyacrylamide gels. (A) Representative western blots and (B and C) quantitative density ratio of phospho-p38 or total-p38 and Akt from five mice in each group. *p < 0.05 compared to the control. (D–E) Mice were injected intraperitoneally with Rb1 (0 or 5 mg/kg) for 2 h and FITC-conjugated E. coli was added to mouse lungs for 2 h. BAL cells isolated from the lungs were assayed by flow cytometry. (D) Representative flow cytometry histograms are shown. (E) Each bar represents the mean ± SD (n = 4). *p < 0.05 compared to the control. FITC, fluorescein isothiocyanate; SD, standard deviation.
4. Discussion
Phagocytic processes require finely controlled actin cytoskeletal remodeling for the internalization of foreign particles and phagosome maturation [1], [3]. p38 MAPK is involved in the reorganization of the actin cytoskeleton, which is essential for cell migration and internalization of bacteria [6], [10]. In this study, ginsenoside Rb1 increased phagocytosis of bacteria in cultured macrophages and in the mouse lung. Rb1 dose-dependently increased p38 MAPK in cultured macrophages, in parallel with enhanced engulfment of bacteria. However, inhibition of Rb1-induced activation of p38 MAPK by pharmacologic inhibitors or siRNA targeting p38α MAPK suppressed the increase in macrophage phagocytosis. These results showed that the Rb1-induced increase in p38 MAPK activity was involved in the enhanced phagocytic uptake of bacteria by macrophages. This is consistent with previous observations that activation of p38 MAPK by various agents increases the phagocytic ability of macrophages, but inhibition of p38 MAPK activation suppresses macrophage bacterial phagocytosis [6], [7], [14].
TAK1 is a member of the serine/threonine protein kinase family and is activated in response to a diverse range of intracellular or extracellular stimuli. It is a major upstream signaling molecule of MKK3/6–p38 MAPK [26]. In this study, Rb1 increased the activation of MKK3/6, but addition of the TAK1 inhibitor (5Z)-7-oxozeaenol to macrophage cultures inhibited Rb1-mediated activation of MKK3/6 and p38 MAPK and internalization of bacteria. Stimulation of TLRs activates the MyD88-dependent pathway, which results in activation of TAK1 [27], [28]. A previous study demonstrated that activation of the bacterial-mediated TLR-p38 MAPK pathway is important for bacterial phagocytosis [29]. Recognition of E. coli by TLR4 promotes activation of the MyD88-p38 MAPK pathway in macrophages, which leads to the internalization of bacteria and phagosome maturation. Macrophages from TLR or MyD88 knockout mice were impaired in engulfment of bacteria compared to wild-type macrophages, similar to the addition of p38 inhibitors to macrophage cultures [29]. These results suggest that Rb1-induced activation of the TAK1–p38 MAPK pathway may potentiate the phagocytic ability of macrophages induced by the bacterial-mediated TLR4–p38 MAPK pathway, although it is presently unclear whether Rb1 directly affects the activation of TLR or MyD88.
PI3K regulates the internalization of various types of particles including IgG-opsonized particles, apoptotic cells, zymosan, and bacteria [30], [31]. Akt is a serine-threonine kinase and downstream target of the PI3K pathway, which regulates macrophage survival, migration, polarization, and response to extracellular stimuli including inflammatory signals [23]. A recent study showed that ginsenoside Rb1 enhanced the activation of PI3K/Akt and protected against neuronal cell death induced by ischemic insults [32]. In fact, macrophages transfected with a constitutively active form of Akt showed enhanced Fc-receptor–mediated phagocytosis [33]. Macrophages from phosphatase and tensin homolog knockout mice displayed enhanced Akt activity and a subsequent increase in phagocytic ability [34]. In our experiments, Rb1 increased Akt phosphorylation in macrophages, but phagocytosis was suppressed by inhibiting PI3K/Akt activation with the PI3K inhibitor LY294002 or siRNA targeting Akt. These results indicate that the PI3K/Akt pathway is involved in the Rb1-induced increase in macrophage phagocytosis. Next, we investigated the interplay between the p38 MAPK and PI3K/Akt pathways in our experimental setting. A previous study demonstrated that p38 MAPK activation in MAPK phosphatase-1 deficient macrophages resulted in PI3K/Akt activation [35]. Deficiency of p38α MAPK inhibited Akt activation in macrophages, which resulted in endoplasmic reticulum stress–induced macrophage apoptosis [36]. In this study, Rb1-induced Akt activation was suppressed by inhibition of the p38 MAPK pathway. However, inhibition of PI3k/Akt activation by LY294002 or siRNA targeting Akt did not block Rb1-induced p38 MAPK activation. These results suggest that PI3K/Akt acts downstream of p38 MAPK in the Rb1-induced phagocytic pathway in macrophages.
In this study, Rb1 increased the phosphorylation of p38 MAPK and Akt and macrophage phagocytosis of bacteria in mouse lung cells, consistent with the in vitro results. A recent study showed that Rb1 diminished the severity of lung injury in rats exposed to LPS. Rb1 inhibited the LPS-induced increase in inflammatory mediators, including tumor necrosis factor-α, monocyte chemoattractant protein-1, and interleukin-8 [37]. In addition, Rb1 ameliorated pathologic changes in the liver and lung, decreased mortality, and stabilized arterial oxygenation and blood pressure in a polymicrobial septic shock model [16]. Therefore, Rb1 may be a good adjuvant for the treatment of bacterial infection and the resolution of acute inflammatory conditions, such as sepsis and acute lung injury.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by a grant (HCRI15015-22) from the Chonnam National University Hwasun Hospital Institute for Biomedical Science and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A3A01016932).
Contributor Information
Hong-Beom Bae, Email: nextphil2@jnu.ac.kr.
Jeong-Il Choi, Email: jichoi@jnu.ac.kr.
References
- 1.May R.C., Machesky L.M. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 2.Bae H.B., Tadie J.M., Jiang S., Park D.W., Bell C.P., Thompson L.C., Peterson C.B., Thannickal V.J., Abraham E., Zmijewski J.W. Vitronectin inhibits efferocytosis through interactions with apoptotic cells as well as with macrophages. J Immunol. 2013;190:2273–2281. doi: 10.4049/jimmunol.1200625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groves E., Dart A.E., Covarelli V., Caron E. Molecular mechanisms of phagocytic uptake in mammalian cells. Cell Mol Life Sci. 2008;65:1957–1976. doi: 10.1007/s00018-008-7578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuy A.G., Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci. 2008;121:1773–1783. doi: 10.1242/jcs.018036. [DOI] [PubMed] [Google Scholar]
- 5.Bae H.B., Zmijewski J.W., Deshane J.S., Tadie J.M., Chaplin D.D., Takashima S., Abraham E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011;25:4358–4368. doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ninković J., Roy S. Morphine decreases bacterial phagocytosis by inhibiting actin polymerization through cAMP-, Rac-1-, and p38 MAPK-dependent mechanisms. Am J Pathol. 2012;180:1068–1079. doi: 10.1016/j.ajpath.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan H., Hur Y.H., Xin C., Kim J.M., Choi J.I., Kim M.Y., Bae H.B. Stearoyl lysophosphatidylcholine enhances the phagocytic ability of macrophages through the AMP-activated protein kinase/p38 mitogen activated protein kinase pathway. Int Immunopharmacol. 2016;39:328–334. doi: 10.1016/j.intimp.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Quan H., Kim J.M., Lee H.J., Lee S.H., Choi J.I., Bae H.B. AICAR enhances the phagocytic ability of macrophages towards apoptotic cells through P38 mitogen activated protein kinase activation independent of AMP-activated protein kinase. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127885. e0127885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuenda A., Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer Z.A., Aga M., Prabhu U., Watters J.J., Hall D.J., Bertics P.J. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- 11.Doyle S.E., O'Connell R.M., Miranda G.A., Vaidya S.A., Chow E.K., Liu P.T., Suzuki S., Suzuki N., Modlin R.L., Yeh W.C. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinet W., Schrijvers D.M., Timmermans J.P., Herman A.G., De Meyer G.R. Phagocytosis of bacteria is enhanced in macrophages undergoing nutrient deprivation. FEBS J. 2009;276:2227–2240. doi: 10.1111/j.1742-4658.2009.06951.x. [DOI] [PubMed] [Google Scholar]
- 13.Kong L., Ge B.X. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008;18:745–755. doi: 10.1038/cr.2008.65. [DOI] [PubMed] [Google Scholar]
- 14.Jeong K.M., Choi J.I., Lee S.H., Lee H.J., Son J.K., Seo C.S., Song S.W., Kwak S.H., Bae H.B. Effect of sauchinone, a lignan from Saururus chinensis, on bacterial phagocytosis by macrophages. Eur J Pharmacol. 2014;728:176–182. doi: 10.1016/j.ejphar.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L.L., Jia B.H., Sun J., Chen J.X., Liu Z.Y., Liu Y. Protective effects of ginsenoside Rb1 on septic rats and its mechanism. Biomed Environ Sci. 2014;27:300–303. doi: 10.3967/bes2014.053. [DOI] [PubMed] [Google Scholar]
- 17.Chen W., Wang J., Luo Y., Wang T., Li X., Li A., Li J., Liu K., Liu B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res. 2016;40:351–358. doi: 10.1016/j.jgr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na J.Y., Kim S., Song K., Lim K.H., Shin G.W., Kim J.H., Kim B., Kwon Y.B., Kwon J. Anti- apoptotic activity of ginsenoside Rb1 in Hydrogen Peroxide-treated Chondrocytes: Stabilization of Mitochondria and the inhibition of Caspase-3. J Ginseng Res. 2012;36:242–247. doi: 10.5142/jgr.2012.36.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng S., Wong C.K.C., Lai H.C., Wong A.S.T. Ginsenoside-Rb1 targets chemotherapy- resistant ovarian cancer stem cells via simultaneous inhibition of Wnt/β-catenin signaling and epithelial-to-mesenchymal transition. Oncotarget. 2017;8:25897–25914. doi: 10.18632/oncotarget.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joh E.H., Lee I.A., Jung I.H., Kim D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation–the key step of inflammation. Biochem Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.M., Han H.J., Hur Y.H., Quan H., Kwak S.H., Choi J.I., Bae H.B. Stearoyl lysophosphatidylcholine prevents lipopolysaccharide-induced extracellular release of high mobility group box-1 through AMP-activated protein kinase activation. Int Immunopharmacol. 2015;28:540–545. doi: 10.1016/j.intimp.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Platanias L.C. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 23.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 24.Iden S., Collard J.G. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 25.Menard R.E., Mattingly R.R. Gbetagamma subunits stimulate p21-activated kinase 1 (PAK1) through activation of PI3-kinase and Akt but act independently of Rac1/Cdc42. FEBS Lett. 2004;556:187–192. doi: 10.1016/s0014-5793(03)01406-6. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson M.G., Millar J.B. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J. 2000;14:2147–2157. doi: 10.1096/fj.00-0102rev. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S., Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 29.Blander J.M., Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 30.Leverrier Y., Okkenhaug K., Sawyer C., Bilancio A., Vanhaesebroeck B., Ridley A.J. Class I phosphoinositide 3-kinase p110beta is required for apoptotic cell and Fcgamma receptor- mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–38442. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 31.Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 2003;187:S340–S345. doi: 10.1086/374747. [DOI] [PubMed] [Google Scholar]
- 32.Luo T., Liu G., Ma H., Lu B., Xu H., Wang Y., Wu J., Ge P., Liang J. Inhibition of autophagy via activation of PI3K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults. Int J Mol Sci. 2014;15:15426–15442. doi: 10.3390/ijms150915426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganesan L.P., Wei G., Pengal R.A., Moldovan L., Moldovan N., Ostrowski M.C., Tridandapani S. The serine/threonine kinase Akt Promotes Fc gamma receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J Biol Chem. 2004;279:54416–54425. doi: 10.1074/jbc.M408188200. [DOI] [PubMed] [Google Scholar]
- 34.Cao X., Wei G., Fang H., Guo J., Weinstein M., Marsh C.B., Ostrowski M.C., Tridandapani S. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol. 2004;172:4851–4857. doi: 10.4049/jimmunol.172.8.4851. [DOI] [PubMed] [Google Scholar]
- 35.Perdiguero E., Sousa-Victor P., Ruiz-Bonilla V., Jardí M., Caelles C., Serrano A.L. Muñoz- Cánoves P: p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seimon T.A., Wang Y., Han S., Senokuchi T., Schrijvers D.M., Kuriakose G., Tall A.R., Tabas I.A. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan Q., Jiang Y.W., Ma T.T., Fang Q.H., Pan L. Attenuating effect of Ginsenoside Rb1 on LPS-induced lung injury in rats. J Inflamm (Lond) 2014;11:40. doi: 10.1186/s12950-014-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]