Abstract
Background
Inflammation is a host-defensive innate immune response to protect the body from pathogenic agents and danger signals induced by cellular changes. Although inflammation is a host-defense mechanism, chronic inflammation is considered a major risk factor for the development of a variety of inflammatory autoimmune diseases, such as rheumatic diseases. Rheumatic diseases are systemic inflammatory and degenerative diseases that primarily affect connective tissues and are characterized by severe chronic inflammation and degeneration of connective tissues. Ginseng and its bioactive ingredients, genocides, have been demonstrated to have antiinflammatory activity and pharmacological effects on various rheumatic diseases by inhibiting the expression and production of inflammatory mediators.
Methods
Literature in this review was searched in a PubMed site of National Center for Biotechnology Information.
Results
The studies reporting the preventive and therapeutic effects of ginseng and ginsenosides on the pathogenesis of rheumatic diseases were discussed and summarized.
Conclusion
Ginseng and ginsenosides play an ameliorative role on rheumatic diseases, and this review provides new insights into ginseng and ginsenosides as promising agents to prevent and treat rheumatic diseases.
Keywords: Ginseng, Ginsenosides, Inflammation, Macrophages, Rheumatic diseases
Abbreviations: ACAN, Aggrecan; ACLT, Anterior cruciate ligament transection; BMP, Bone morphogenetic protein; CIA, Collagen-induced arthritic; CK, Compound K; COL, Collagen; DAMP, Danger-associated molecular pattern; LTMMR, Ligament transection and medial meniscus resection; OA, Osteoarthritis; PAMP, Pathogen-associated molecular pattern; PPD, Protopanaxadiol; PPT, Protopanaxatriol; PRR, Pattern-recognition receptor; RA, Rheumatoid arthritis; RNAKL, Receptor activator of NF-κB ligand; SLE, Systemic lupus erythematosus
1. Introduction
Inflammation is an innate immune mechanism to protect the body from invading pathogens and cellular danger signals [1], [2], [3]. Although an inflammatory response is a host-defense mechanism, chronic inflammation, defined as repeated and prolonged inflammation, is considered a major risk factor for the development of inflammatory and autoimmune diseases. Rheumatic diseases are chronic inflammatory and degenerative autoimmune diseases that primarily affect connective tissues such as cartilage and bones, ligaments, tendons, and muscles but can also affect nonconnective tissues and internal organs, leading to substantial morbidity [4]. More than 100 rheumatic diseases have been identified, and there are numerous patients who suffer from these diseases worldwide. This has resulted in extensive investigation of the mechanisms of disease pathogenesis and development of effective therapeutics. Representative rheumatic diseases include rheumatoid arthritis (RA), osteoarthritis (OA), systemic lupus erythematosus (SLE), ankylosing spondylitis, and Sjögren's syndrome. Despite uncertainty regarding the etiology of rheumatic diseases, chronic inflammation and autoimmunity are known to play critical roles in both the onset and progression of rheumatic diseases. Ginsengs and their active components, ginsenosides, have strong antiinflammatory properties; they may therefore have an ameliorative effect on the pathogenesis of rheumatic diseases. Indeed, many studies have investigated the therapeutic effects of ginsengs and ginsenosides on rheumatic diseases in vitro and in vivo.
Ginseng refers to species of slow-growing plants in the genus Panax and has been used to ameliorate and cure various human diseases for thousands of years. Many efforts have been made to purify and identify the bioactive components of ginseng, and ginsenosides have been identified as the main bioactive compounds. A large number of studies have investigated the pharmacological roles of various ginsenosides on the pathogenesis of human diseases, and many studies have reported that various ginsenosides exert an antiinflammatory effect during the inflammatory response and in inflammatory autoimmune diseases [5].
This review provides a brief summary of ginseng and ginsenosides and discusses recent studies that have investigated their pharmacological roles in the pathogenesis of rheumatic diseases. The aim of this review is not only to enhance understanding of the effects of ginseng and ginsenosides in rheumatic diseases but also to provide new insights that may spur the development of ginseng-containing remedies to prevent and treat rheumatic diseases.
2. Ginseng and ginsenosides
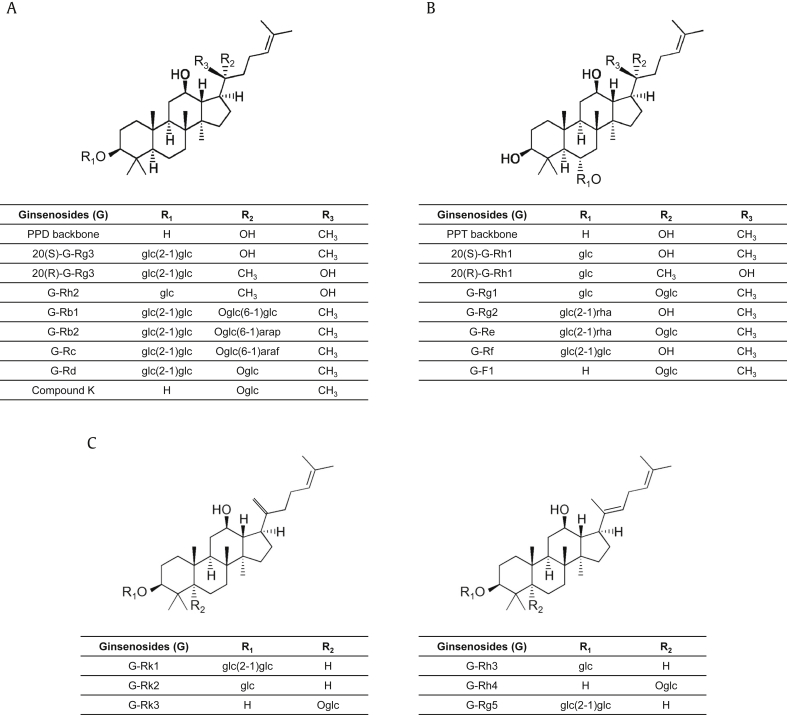
Ginseng refers to perennial plant species in the genus Panax in the family Araliaceae. Plants in this genus are found worldwide and are cultivated in Eastern Asia and North America. Ginseng has long been used as a traditional herbal medicine and as a health food to improve vitality and physical performance and to reduce or cure spiritlessness and fatigue [6]. There are 13 species in the genus Panax, of which the pharmacological properties of five have been investigated: Panax ginseng (Asian or Korean ginseng), American ginseng, Vietnamese ginseng, Japanese ginseng, and Pseudoginseng. Among these, Panax ginseng, also known as Korean ginseng, is the most popular and commonly used species for therapeutic purposes [7]. The meaning of “Panax” is “all healing,” which originates from traditional Oriental medicine beliefs that ginseng could treat all human diseases. A number of studies have demonstrated that ginseng can alleviate the symptoms of various diseases and conditions such as diabetes [8], [9], hypertension [10], [11], gastric ulcers [12], neuronal disease [13], [14], pain [15], [16], inflammatory diseases [5], [17], and cancers [18], [19]. Further studies have revealed the pharmacologically active compounds of ginseng: ginsenosides, ginsengosides, polysaccharides, peptides, phytosterols, polyacetylenes, polyacetylenic alcohols, and fatty acids [20], [21]. Among these, ginsenosides are the main bioactive compounds in ginseng, and most studies of the pharmacological and medicinal effects of ginseng have focused on ginsenosides. Ginsenosides are steroidal triterpenoid saponins [22]. More than 100 different types of ginsenosides have been identified, and ginsenosides are presented by G-Rx, where x is determined by the distance moved on thin layer chromatography from the polar segment marked “A” to the least polar segment marked “H” [23]. Ginsenosides are classified as protopanaxadiol, protopanaxatriol, or rare ginsenosides based on the backbone structure (Fig. 1), and various reactive groups and sugars are attached to the different areas of the backbone to generate distinctive ginsenoside molecules. A number of studies have successfully demonstrated that many types of ginsenosides have pharmacological effects in various human diseases, such as cardiovascular diseases [24], [25], neuronal diseases [14], diabetes [26], obesity [27], skin diseases [28], and cancers [29], [30]. Ginsenosides have also been reported to have antiinflammatory effects during inflammatory responses and in inflammatory diseases [5], and many studies have demonstrated that ginsenosides have a pharmacological effect on rheumatic diseases, which are the most common inflammatory autoimmune diseases.
Fig. 1.
Types of ginsenosides. (A) Backbone structure of PPD and the different types of ginsenosides with their side chains (R1, R2, and R3) in the PPD group. (B) Backbone structure of PPT and the different types of ginsenosides with their side chains (R1, R2, and R3) in the PPT group. (C) Backbone structures of rare ginsenosides and the different types of ginsenosides with their side chains (R1 and R2) in this group. PPD, protopanaxadiol; PPT, protopanaxatriol.
3. Effects of ginseng and ginsenosides in rheumatic diseases
3.1. Rheumatoid arthritis
RA is a long-term inflammatory autoimmune disease that causes chronic joint inflammation with the symptoms of pain, swelling, and stiffness in the hands, feet, knees, and wrists. RA is generally characterized by chronic synovial inflammation of the joints, resulting in the degradation and destruction of cartilages and bones, and has poor outcomes with limited treatment options [31], [32]. Despite the lack of robust epidemiological studies in some regions and the difference in RA prevalence between ethnicities, the prevalence of RA is the highest among the inflammatory autoimmune diseases, ranging from 0.5 to 1% of the population worldwide [33], [34]. The exact causes of RA pathogenesis remain unknown, but chemokines, pro-inflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6], and inflammatory mediators [e.g., reactive oxygen/nitrogen species, prostaglandins, cyclooxygenase-2, matrix metalloproteinases (MMPs)], and the influx of inflammatory immune cells (e.g., macrophages, neutrophils) are thought to play a critical role in RA pathogenesis [32], [35]. Therefore, many efforts have been made to develop RA therapies by suppressing these inflammatory products.
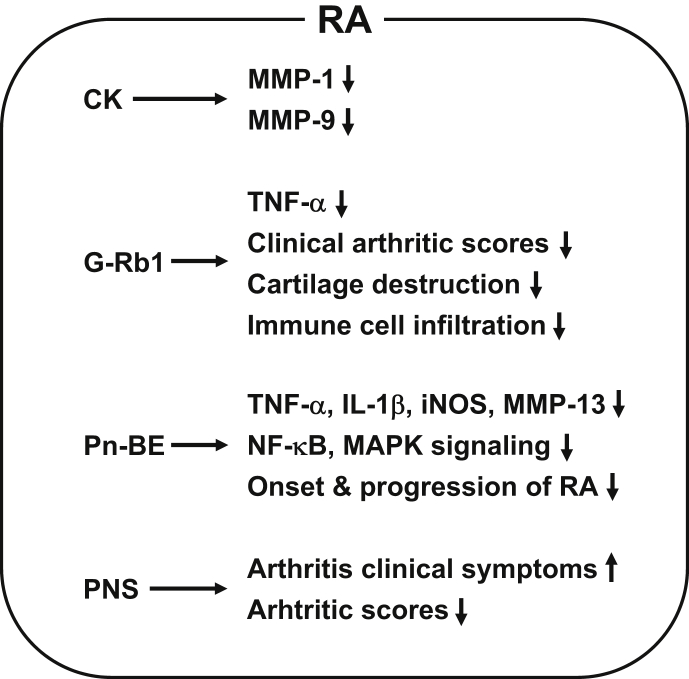
As discussed earlier, a number of studies have demonstrated the antiinflammatory properties of ginseng and ginsenosides, and their ameliorative effects on RA pathogenesis have also been studied. Joint-protective effects of compound K (CK) on RA pathogenesis were investigated in vitro. Choi et al demonstrated that CK significantly decreased the production of MMP-1 and MMP-3 from RA fibroblast-like synoviocytes by inhibiting janus kinase (JNK) and extracellular signal-regulated kinase (ERK) signaling pathways [36]. In vivo studies have also investigated the effects of ginseng and ginsenosides on RA pathogenesis using RA animal models. Kim et al demonstrated the antiarthritic effects of ginsenoside Rb1 (G-Rb1) in collagen-induced arthritic (CIA) mice and reported that oral administration of G-Rb1 markedly ameliorated clinical arthritis scores and also reduced immune cell infiltration and cartilage destruction by suppressing TNF-α expression, which is upregulated during inflammatory responses in CIA mice [37]. This study also demonstrated that G-Rb1 significantly inhibited TNF-α expression induced by interferon-γ in peripheral blood mononuclear cells, chondrocytes, and fibroblast-like synoviocytes isolated from human RA patients [37]. Chang et al investigated the antiinflammatory effects of BT-201, a Panax notoginseng butanol extract, in CIA mice and reported that BT-201 attenuated the onset and progression of arthritic symptoms and reduced arthritic histological scores [38]. The antiarthritic effects of BT-201 were achieved by inhibiting TNF-α and IL-1β secretion from THP-1 cells, inhibiting inducible nitric oxide synthase production in RAW264.7 cells, MMP-13 production in SW1353 cells by suppressing nuclear factor-kappa B, and mitogen-activated protein kinase signaling pathways [38]. Antiarthritic effects of ginseng were also investigated in 84 RA patients. Zhang et al demonstrated that Panax notoginseng saponin significantly improved the clinical symptoms and reduced arthritic indexes in RA patients [39]. Although protein-based antiarthritic blockbuster drugs, such as TNF-blockers, IL-1-blockers, and IL-6-blockers, are currently used to treat RA, alternative approaches are in great demand because of the instability of the protein drugs and existence of TNF-failure patient groups. Collectively, the studies described previously strongly suggest that ginseng and ginseng-derived components can suppress inflammatory responses and ameliorate RA pathogenesis (Fig. 2) and could therefore potentially be used as antiarthritic agents to prevent and treat RA.
Fig. 2.
Schematic summary of ameliorative effects of ginseng and ginsenosides on RA pathogenesis. CK, G-Rb1, Pn-BE, and PNS ameliorate RA by decreasing MMP-1, MMP-9, MMP-13, TNF-α, IL-1β, iNOS, and NF-kB/MAPK signaling, clinical arthritis scores, cartilage destruction, immune cell infiltration, and onset/progression of RA and by improving arthritis clinical symptoms in various cells, animal models, and human RA patients. CK, compound K; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; Pn-BE, Panax notoginseng butanol extract; PNS, Panax notoginseng saponin; RA, rheumatoid arthritis; TNF-α, tumor necrosis factor-α.
3.2. Osteoarthritis
OA is a complex and multifactorial joint degenerative disease resulting from the breakdown of cartilage and the underlying bones of the joint tissues and is characterized by the symptoms of pain, swelling, and stiffness in the hands, knees, and hips. Although the prevalence of OA varies based on the specific joints affected and characteristics of the study population, the prevalence of knee OA in the population aged above 60 years has been estimated to be 13% in women and 10% in men [40], and the proportion of the population with symptomatic knee OA is strongly associated with obesity and aging [41]. Prevalence of OA is expected to increase in the next few decades, with risk factors for OA pathogenesis thought to be damage from mechanical stress due to congenital causes, pathogen infection, excessive body weight, loss of strength in the muscles, and insufficient self-repair of damaged joint tissues [42]. Acetaminophen and nonsteroidal antiinflammatory drugs are prescribed to relieve pain, and several types of therapies, such as physical, occupational, and yoga therapies, have been applied to OA patients to improve their disease symptoms. Knee replacement surgery using titanium can relieve pain and disability. However, there are ongoing studies to develop disease-modifying agents for OA to target disease processes, not symptoms [43], [44], [45], [46], such as cell-mediated therapy with intraarticular injection [47], [48], [49], [50].
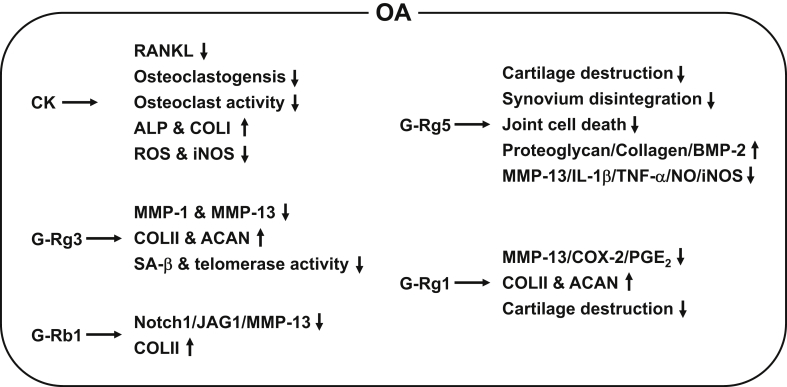
The in vitro and in vivo ameliorative effects of ginseng and ginsenosides on OA have been investigated using various cell types and OA animal models, respectively. Choi et al demonstrated that CK reduced the expression of reduced receptor activator of nuclear factor-kappa B ligand (RANKL) in fibroblast-like synoviocytes and significantly inhibited RANKL-induced osteoclastogenesis or osteoclast activity in RAW264.7 cells and human CD14+ monocytes by suppressing RANKL-mediated inhibitor of kappa B degradation and nuclear factor of activated T cells c1 expression [36]. Therapeutic potential of CK for treating OA was further investigated in silico and in vitro. Kang et al reported that CK had strong binding affinity for inhibitor of kappa B kinase based on in silico analysis and demonstrated that CK significantly increased the expression of the osteoblast differentiation markers alkaline phosphatase and type I collagen and reduced the production of reactive oxygen species and nitric oxide, which are critical molecules required for the development of OA, in H2O2-stimulated mouse osteoblast precursor cells (MC3T3-E1 cells) [51]. Another in vitro study investigated the protective effects of ginsenoside Rg3 (G-Rg3) on human osteoarthritic chondrocytes. So et al isolated chondrocytes from OA patients and demonstrated that G-Rg3 markedly reduced the expression of MMP-1 and MMP-13, which are chondrodegenerative proteins, and increased the expression of type 2 collagen (COLII) and aggrecan, which are chondroprotective proteins, in IL-1β–stimulated chondrocytes [52]. Moreover, G-Rg3 suppressed the expression of senescence-associated β-GAL, a biomarker of cellular senescence, and increased telomerase activity, which is known to be reduced during cellular aging, in chondrocytes [52].
In vivo studies have also investigated the therapeutic potential of ginseng and ginsenosides for treating OA in animal models. Wang et al generated an OA rat model by anterior cruciate ligament transection (ACLT) and demonstrated that intraarticular injection of G-Rb1 lowered levels of Notch1 and its ligand, jagged 1, which are overexpressed in OA [53], in the cartilage [54]. G-Rb1 treatment was strongly associated with decrease in levels of Notch1, jagged 1, and MMP-13 and increased level of COLII in IL-1β–stimulated human osteosarcoma cells (SW1353 cells) [53]. In addition, Zhang investigated the effect of ginsenoside Rg5 (G-Rg5) on the degradation of articular cartilage in an OA rat model prepared by ligament transection and medial meniscus resection. Histopathological analysis showed that G-Rg5 significantly alleviated OA symptoms by preventing cartilage degradation, synovial membrane disintegration, and apoptotic cell death in the knee joints in ligament transection and medial meniscus resection OA rats [55]. An in vitro study revealed that G-Rg5 increased proteoglycan, collagen, and bone morphogenetic protein 2 levels and decreased MMP-13, IL-1β, TNF-α, nitric oxide, and inducible nitric oxide synthase levels in the chondrocytes of OA rats [55]. Chang et al investigated the chondroprotective effects of ginsenoside Rg1 (G-Rg1) in human OA patients and an OA rat model by preparing articular chondrocytes from OA patients and an OA rat model that underwent ACLT. Histological analyses showed that G-Rg1 suppressed gene and protein expression of MMP-13, COX-2, and prostaglandin E2 and prevented the degradation of COLII and aggrecan in IL-1β–stimulated human OA articular chondrocytes [56]. G-Rg1 also ameliorated OA symptoms in ACLT OA rats by attenuating cartilage degradation and decreasing COLII loss and MMP-13 level [56]. Taken together, these results suggest that ginsenosides attenuate OA pathogenesis by decreasing the expression of cartilage-degrading factors and increasing the expression of cartilage-protecting factors (Fig. 3) and could therefore be useful as anti-OA remedies to prevent and treat OA.
Fig. 3.
Schematic summary of the ameliorative effects of ginsenosides on OA pathogenesis. CK, G-Rg1, G-Rg3, G-Rg5, and G-Rb1 ameliorate OA by decreasing RANKL, ROS, NO, iNOS, MMP-1, MMP-13, Notch1/JAG1, SA-β, IL-1β, TNF-α, COX-2, PGE2, osteoclastogenesis, osteoclast activity, telomerase activity, cartilage destruction, synovium disintegration, and joint cell death, by increasing expression of ALP, collagens, ACAN, proteoglycan, and BMP-2, and by improving OA clinical symptoms in cells and animal models. ALP, alkaline phosphatase; ACAN, aggrecan; BMP-2, bone morphogenetic protein 2; CK, compound K; COLI, type I collagen; COLII, type 2 collagen; COX-2, cyclooxygenase-2; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; JAG1, jagged 1; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; NO, nitric oxide; OA, osteoarthritis; PGE2, prostaglandin E2; RANKL, receptor activator of NF-κB ligand; ROS, reactive oxygen species; SA-β, senescence-associated β; TNF-α, tumor necrosis factor-α.
3.3. Systemic lupus erythematosus
SLE, the most common form of lupus, is a chronic autoimmune disease in which the immune system attacks healthy self-tissues in almost any part of the body. It is characterized by a wide range of clinical manifestations, including painful and swollen joints, fever, hair loss, chest pain, feeling tired, and malar “butterfly” rash. Despite the wide range of symptoms, one of the common clinical hallmarks of SLE is circulating autoantibodies and immune complexes that recognize host-derived substances, such as nucleic acids and insufficiently removed apoptotic bodies [57], [58]. The prevalence of SLE varies according to geographical region, study design, age, sex, and ethnicity, and SLE is much more common in women than in men (ratio of approximately 6:1) [59]. The highest prevalence of SLE was reported in North America (214 cases per 100,000 people), whereas the lowest prevalence was reported in Northern Australia (0 cases per 847 people) [60]. Current treatment options for SLE include inflammation-directed treatment, immune cell–targeted therapies, costimulatory signaling–targeted therapies, anticytokine therapies, anti-IgE therapy, and antikinase therapies [61]. Nevertheless, adverse effects related to treatment of SLE and active SLE refractory to traditional therapies remain major challenges, highlighting the need for complementary and alternative strategies to treat SLE.
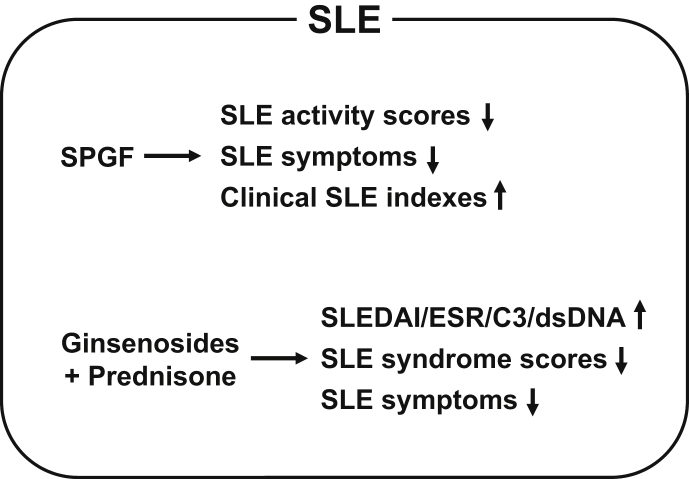
Several studies have reported that ginseng had an ameliorative effect on SLE pathogenesis. Yang and Zhang investigated the therapeutic effect of saponins of ginseng fruit on SLE pathogenesis in patients with active disease and demonstrated that saponins of ginseng fruit significantly decreased disease activity scores and ameliorated SLE symptoms by improving the clinical efficacy of current medications in SLE patients [62]. You et al evaluated the efficacy of ginsenosides combined with prednisone, a synthetic corticosteroid immunosuppressant drug used for SLE treatment, and reported that the clinical efficacy of the combined therapy was better than that of the corticosteroid alone, as evidenced by a significant improvement in SLE disease activity index, erythrocyte sedimentation rate, and complement 3 and anti-double-stranded DNA levels [63]. In another study, the same research group reported that combined prednisone and ginsenoside therapy significantly decreased disease syndrome scores and improved disease symptoms compared with the prednisone plus placebo group [64]. These studies suggest that ginseng itself as well as combined ginsenoside and current anti-SLE drug therapy could alleviate SLE symptoms and improve clinical outcomes of SLE patients (Fig. 4).
Fig. 4.
Schematic summary of ameliorative effects of ginseng and ginsenosides on SLE pathogenesis. SPGF and combination therapy with ginsenosides and prednisone ameliorate SLE by decreasing SLE activity scores; increasing SLEDAI, ESR, C3, and dsDNA expression; and improving SLE clinical indexes and SLE symptoms in human SLE patients. C3, complement 3; dsDNA, double-stranded DNA; ESR, erythrocyte sedimentation rate; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index; SPGF, saponins of ginseng fruit.
4. Conclusions and perspectives
Inflammation is an innate immune response to defend the host from infectious agents and alter cellular homeostasis. Although inflammation is a defensive response, chronic inflammation has been implicated in the development of several human inflammatory autoimmune diseases, among which rheumatic diseases are some of the most common. Ginseng and its main bioactive ingredients, ginsenosides, have well-documented pharmacological effects on the pathogenesis of inflammatory autoimmune diseases and rheumatic diseases such as RA, OA, and SLE, as described in Table 1. No studies to date have investigated the effects of ginseng and ginsenosides on the pathogenesis of other types of rheumatic diseases, such as ankylosing spondylitis, spondyloarthritis, and Sjögren's syndrome. Moreover, the effects of other types of ginsenosides and ginseng-derived ingredients on the pathogenesis of RA, OA, and SLE have not been explored. Future studies that address these knowledge gaps are required if ginseng and ginsenosides are to be used as novel therapeutics to treat rheumatic diseases.
Table 1.
Effects of ginseng and ginsenosides on rheumatic diseases
| Disease | Compound | Effects | Experimental models | Ref. |
|---|---|---|---|---|
| RA | CK | Decreases MMP-1 and MMP-9 production | Human RA fibroblast-like synoviocytes | [36] |
| G-Rb1 | Decreases TNF-α expression Ameliorates clinical arthritic scores Reduces immune cell infiltration and cartilage destruction |
Human RA fibroblast-like synoviocytes Human PBMCs Human chondrocytes Collagen-induced arthritic mice |
[37] | |
| Pn-BE | Inhibits TNF-α, IL-1β, iNOS, and MMP-13 expression Suppresses NF-κB and MAPK signaling pathways Attenuates onset and progression of CIA |
Human THP-1 cells Mouse RAW264.7 cells Human SW1353 cells Collagen-induced arthritic mice |
[38] | |
| PNS | Improves clinical symptoms Reduces arthritic indexes |
Human RA patients | [39] | |
| OA | CK | Reduces RANKL expression Inhibits RANKL-induced osteoclastogenesis/osteoclast activity Increases ALP and COLI levels Reduces ROS and NO levels |
Human RA fibroblast-like synoviocytes Mouse RAW264.7 cells Human CD14+ monocytes Mouse MC3T3-E1 cells |
[36] |
| [51] | ||||
| G-Rg3 | Decreases MMP-1 and MMP-13 expression Increases COLII and ACAN expression Suppresses SA-β-GAL level and telomerase activity |
Human chondrocytes | [52] | |
| G-Rb1 | Decreases Notch1, JAG1, and MMP-13 expression Increases COLII expression |
Human SW1353 cells ACLT OA rats |
[53] | |
| G-Rg5 | Prevents cartilage destruction, synovial membrane disintegration, and apoptotic cell death in knee joints Increases proteoglycan, collagen, and BMP-2 expression Decreases MMP-13, IL-1β, TNF-α, NO, and iNOS expression |
LTMMR OA rat chondrocytes LTMMR OA rats |
[55] | |
| G-Rg1 | Decreases MMP-13, COX-2, and PGE2 expression Increases COLII and ACAN expression Attenuates cartilage destruction |
Human OA chondrocytes ACLT OA rats |
[56] | |
| SLE | SPGF | Decreases disease activity scores Ameliorates SLE symptoms Improves clinical SLE indexes |
Human SLE patients | [62] |
| Ginsenosides + prednisone | Improves SLEDAI, ESR, C3, and anti-dsDNA levels Decreases disease syndrome scores Improves disease symptoms |
Human SLE patients Human SLE patients |
[63] | |
| [64] |
ACAN, aggrecan; ACLT, anterior cruciate ligament transection; ALP, alkaline phosphatase; BMP-2, bone morphogenetic protein 2; C3, complement 3; CIA, collagen-induced arthritic; CK, compound K; COLI, type I collagen; COLII, type 2 collagen; COX-2, cyclooxygenase-2; dsDNA, double-stranded DNA; ESR, erythrocyte sedimentation rate; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; JAG1, jagged 1; LTMMR, ligament transection and medial meniscus resection; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; NO, nitric oxide; OA, osteoarthritis; PBMCs, peripheral blood mononuclear cells; PGE2, prostaglandin E2; RA, rheumatoid arthritis; RANKL, receptor activator of NF-κB ligand; ROS, reactive oxygen species; SA-β-GAL, senescence-associated β-GAL; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index; SPGF, saponins of ginseng fruit; TNF-α, tumor necrosis factor-α.
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Yi Y.S. Folate receptor-targeted diagnostics and therapeutics for inflammatory diseases. Immune Netw. 2016;16:337–343. doi: 10.4110/in.2016.16.6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi Y.S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152:207–217. doi: 10.1111/imm.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi Y.S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J Physiol Pharmacol. 2018;22:1–15. doi: 10.4196/kjpp.2018.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong S.K., Oberholzer V.G. Ginseng–is there a use in clinical medicine? Postgrad Med J. 1988;64:841–846. doi: 10.1136/pgmj.64.757.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun T.K. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl):S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng J., Liu Y., Duan Z., Zhu C., Hui J., Mi Y., Ma P., Ma X., Fan D., Yang H. Protopanaxadiol and protopanaxatriol-type saponins ameliorate glucose and lipid metabolism in type 2 diabetes mellitus in high-fat diet/streptozocin-induced mice. Front Pharmacol. 2017;8:506. doi: 10.3389/fphar.2017.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang S., Ren D., Li J., Yuan G., Li H., Xu G., Han X., Du P., An L. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia. 2014;95:58–64. doi: 10.1016/j.fitote.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Zhao S., Zheng M.X., Chen H.E., Wu C.Y., Wang W.T. Effect of panax notoginseng saponins injection on the p38MAPK pathway in lung tissue in a rat model of hypoxic pulmonary hypertension. Chin J Integr Med. 2015;21:147–151. doi: 10.1007/s11655-014-1790-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.W., Lim H.J., Jun J.H., Choi J., Lee M.S. Ginseng for treating hypertension: a systematic review and meta-analysis of double blind, randomized, placebo-controlled trials. Curr Vasc Pharmacol. 2017;15:549–556. doi: 10.2174/1570161115666170713092701. [DOI] [PubMed] [Google Scholar]
- 12.Yu T., Rhee M.H., Lee J., Kim S.H., Yang Y., Kim H.G., Kim Y., Kim C., Kwak Y.S., Kim J.H. Ginsenoside Rc from Korean red ginseng (panax ginseng C.A. Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am J Chin Med. 2016;44:595–615. doi: 10.1142/S0192415X16500336. [DOI] [PubMed] [Google Scholar]
- 13.Lee S., Youn K., Jeong W.S., Ho C.T., Jun M. Protective effects of red ginseng oil against abeta25-35-induced neuronal apoptosis and inflammation in PC12 cells. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong W.Y., Farooqui T., Koh H.L., Farooqui A.A., Ling E.A. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129. doi: 10.3389/fnagi.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn E.J., Choi G.J., Kang H., Baek C.W., Jung Y.H., Woo Y.C., Bang S.R. Antinociceptive effects of ginsenoside Rg3 in a rat model of incisional pain. Eur Surg Res. 2016;57:211–223. doi: 10.1159/000448001. [DOI] [PubMed] [Google Scholar]
- 16.Kim W.J., Kang H., Choi G.J., Shin H.Y., Baek C.W., Jung Y.H., Woo Y.C., Kim J.Y., Yon J.H. Antihyperalgesic effects of ginseng total saponins in a rat model of incisional pain. J Surg Res. 2014;187:169–175. doi: 10.1016/j.jss.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 17.Osada-Oka M., Hirai S., Izumi Y., Misumi K., Samukawa K., Tomita S., Miura K., Minamiyama Y., Iwao H. Red ginseng extracts attenuate skin inflammation in atopic dermatitis through p70 ribosomal protein S6 kinase activation. J Pharmacol Sci. 2018;136:9–15. doi: 10.1016/j.jphs.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.Z., Anderson S., Du W., He T.C., Yuan C.S. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14:7–16. doi: 10.3724/SP.J.1009.2016.00007. [DOI] [PubMed] [Google Scholar]
- 19.Dai D., Zhang C.F., Williams S., Yuan C.S., Wang C.Z. Ginseng on cancer: potential role in modulating inflammation-mediated angiogenesis. Am J Chin Med. 2017;45:13–22. doi: 10.1142/S0192415X17500021. [DOI] [PubMed] [Google Scholar]
- 20.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 21.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 23.Liu C.X., Xiao P.G. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Liu Y., Chen K. Roles and mechanisms of ginsenoside in cardiovascular diseases: progress and perspectives. Sci China Life Sci. 2016;59:292–298. doi: 10.1007/s11427-016-5007-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan H.D., Kim J.T., Kim S.H., Chung S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Virgous C., Si H. Ginseng and obesity: observations and understanding in cultured cells, animals and humans. J Nutr Biochem. 2017;44:1–10. doi: 10.1016/j.jnutbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Sabouri-Rad S., Sahebkar A., Tayarani-Najaran Z. Ginseng in dermatology: a review. Curr Pharm Des. 2017;23:1649–1666. doi: 10.2174/1381612822666161021152322. [DOI] [PubMed] [Google Scholar]
- 29.Sodrul I.M.D., Wang C., Chen X., Du J., Sun H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget. 2018;9:2931–2950. doi: 10.18632/oncotarget.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol- and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016:5738694. doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smolen J.S., Aletaha D., Barton A., Burmester G.R., Emery P., Firestein G.S., Kavanaugh A., McInnes I.B., Solomon D.H., Strand V. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 32.Yi Y.S., Ayala-Lopez W., Kularatne S.A., Low P.S. Folate-targeted hapten immunotherapy of adjuvant-induced arthritis: comparison of hapten potencies. Mol Pharm. 2009;6:1228–1236. doi: 10.1021/mp900070b. [DOI] [PubMed] [Google Scholar]
- 33.Myasoedova E., Crowson C.S., Kremers H.M., Therneau T.M., Gabriel S.E. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobon G.J., Youinou P., Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35:10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Szekanecz Z., Koch A.E. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y.S., Kang E.H., Lee E.Y., Gong H.S., Kang H.S., Shin K., Lee E.B., Song Y.W., Lee Y.J. Joint-protective effects of compound K, a major ginsenoside metabolite, in rheumatoid arthritis: in vitro evidence. Rheumatol Int. 2013;33:1981–1990. doi: 10.1007/s00296-013-2664-9. [DOI] [PubMed] [Google Scholar]
- 37.Kim H.A., Kim S., Chang S.H., Hwang H.J., Choi Y.N. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7:1286–1291. doi: 10.1016/j.intimp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Chang S.H., Choi Y., Park J.A., Jung D.S., Shin J., Yang J.H., Ko S.Y., Kim S.W., Kim J.K. Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model. Clin Nutr. 2007;26:785–791. doi: 10.1016/j.clnu.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J.H., Wang J.P., Wang H.J. Clinical study on effect of total panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:589–592. [PubMed] [Google Scholar]
- 40.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med. 2011;2:205–212. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandt K.D., Dieppe P., Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93:1–24. doi: 10.1016/j.mcna.2008.08.009. xv. [DOI] [PubMed] [Google Scholar]
- 43.Evans C.H., Ghivizzani S.C., Robbins P.D. Gene delivery to joints by intra-articular injection. Hum Gene Ther. 2018;29:2–14. doi: 10.1089/hum.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madry H., Cucchiarini M. Gene therapy for human osteoarthritis: principles and clinical translation. Expert Opin Biol Ther. 2016;16:331–346. doi: 10.1517/14712598.2016.1124084. [DOI] [PubMed] [Google Scholar]
- 45.Zhang D.W., Yang Q.S., Zhu J.Y., Cao X.R., Li L.W., Zhu Q.S. Amelioration of osteoarthritis by intra-articular hyaluronan synthase 2 gene therapy. Med Hypotheses. 2007;69:1111–1113. doi: 10.1016/j.mehy.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 46.Mason J.B., Gurda B.L., Hankenson K.D., Harper L.R., Carlson C.S., Wilson J.M., Richardson D.W. Wnt10b and Dkk-1 gene therapy differentially influenced trabecular bone architecture, soft tissue integrity, and osteophytosis in a skeletally mature rat model of osteoarthritis. Connect Tissue Res. 2017;58:542–552. doi: 10.1080/03008207.2016.1267153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noh M.J., Lee K.H. Orthopedic cellular therapy: an overview with focus on clinical trials. World J Orthop. 2015;6:754–761. doi: 10.5312/wjo.v6.i10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasadam I., Akuien A., Friis T.E., Fang W., Mao X., Crawford R.W., Xiao Y. Mixed cell therapy of bone marrow-derived mesenchymal stem cells and articular cartilage chondrocytes ameliorates osteoarthritis development. Lab Invest. 2018;98:106–116. doi: 10.1038/labinvest.2017.117. [DOI] [PubMed] [Google Scholar]
- 49.McIntyre J.A., Jones I.A., Han B., Vangsness C.T., Jr. Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. Am J Sports Med. 2017 doi: 10.1177/0363546517735844. 363546517735844. [DOI] [PubMed] [Google Scholar]
- 50.Deng M.W., Wei S.J., Yew T.L., Lee P.H., Yang T.Y., Chu H.Y., Hung S.C. Cell therapy with g-CSF-mobilized stem cells in a rat osteoarthritis model. Cell Transplant. 2015;24:1085–1096. doi: 10.3727/096368914X680091. [DOI] [PubMed] [Google Scholar]
- 51.Kang S., Siddiqi M.H., Yoon S.J., Ahn S., Noh H.Y., Kumar N.S., Kim Y.J., Yang D.C. Therapeutic potential of compound K as an IKK inhibitor with implications for osteoarthritis prevention: an in silico and in vitro study. In Vitro Cell Dev Biol Anim. 2016;52:895–905. doi: 10.1007/s11626-016-0062-9. [DOI] [PubMed] [Google Scholar]
- 52.So M.W., Lee E.J., Lee H.S., Koo B.S., Kim Y.G., Lee C.K., Yoo B. Protective effects of ginsenoside Rg3 on human osteoarthritic chondrocytes. Mod Rheumatol. 2013;23:104–111. doi: 10.1007/s10165-012-0635-8. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson C., Brantsing C., Egell S., Lindahl A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs. 2008;188:287–298. doi: 10.1159/000121610. [DOI] [PubMed] [Google Scholar]
- 54.Wang W., Zeng L., Wang Z.M., Zhang S., Rong X.F., Li R.H. Ginsenoside Rb1 inhibits matrix metalloproteinase 13 through down-regulating Notch signaling pathway in osteoarthritis. Exp Biol Med (Maywood) 2015;240:1614–1621. doi: 10.1177/1535370215587918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang P. GinsenosideRg5 treatment inhibits apoptosis of chondrocytes and degradation of cartilage matrix in a rat model of osteoarthritis. Oncol Rep. 2017;37:1497–1502. doi: 10.3892/or.2017.5392. [DOI] [PubMed] [Google Scholar]
- 56.Cheng W., Jing J., Wang Z., Wu D., Huang Y. Chondroprotective effects of ginsenoside Rg1 in human osteoarthritis chondrocytes and a rat model of anterior cruciate ligament transection. Nutrients. 2017;9 [Google Scholar]
- 57.Arbuckle M.R., McClain M.T., Rubertone M.V., Scofield R.H., Dennis G.J., James J.A., Harley J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 58.Yi Y.S. Functional role of milk fat globule-epidermal growth factor VIII in macrophage-mediated inflammatory responses and inflammatory/autoimmune diseases. Mediators Inflamm. 2016;2016:5628486. doi: 10.1155/2016/5628486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rees F., Doherty M., Grainge M., Davenport G., Lanyon P., Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Ann Rheum Dis. 2016;75:136–141. doi: 10.1136/annrheumdis-2014-206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rees F., Doherty M., Grainge M.J., Lanyon P., Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford) 2017;56:1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 61.Yildirim-Toruner C., Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. J Allergy Clin Immunol. 2011;127:303–312. doi: 10.1016/j.jaci.2010.12.1087. quiz 313–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H.T., Zhang J.R. Treatment of systemic lupus erythematosus with saponin of ginseng fruit (SPGF): an immunological study. Zhong Xi Yi Jie He Za Zhi. 1986;6 157-159, 131-2. [PubMed] [Google Scholar]
- 63.You Y.L., Ling C.Q., Feng Y.L. Efficacy of combined therapy with ginsenosides and prednisone in treating systemic lupus erythematosus–a randomized, controlled and double-blinded trial. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:776–779. [PubMed] [Google Scholar]
- 64.You Y.L., Feng Y.L., Cai Q., Guan J.L., Zhang L.L., Xu M.J., Xu X., Ling C.Q. Efficacy of ginsenosides combined with prednisone in patients with systemic lupus erythematosus: a prospective, randomized, double-blind, placebo-controlled trial. Zhong Xi Yi Jie He Xue Bao. 2010;8:762–766. doi: 10.3736/jcim20100806. [DOI] [PubMed] [Google Scholar]