Abstract
Panax ginseng, known as Koran ginseng, one of the most commonly used traditional plants, has been demonstrated to show a wide range of pharmacological applications. Ginsenosides are the major active ingredients found in ginseng and are responsible for the biological and pharmacological activities, such as antioxidation, antiinflammation, vasorelaxation, and anticancer actions. Existing studies have mostly focused on identifying and purifying single ginsenosides and investigating pharmacological activities and molecular mechanisms in cells and animal models. However, ginsenoside studies based on clinical trials have been very limited. Therefore, this review aimed to discuss the currently available clinical trials on ginsenosides and provide insights and future directions for developing ginsenosides as efficacious and safe drugs for human disease.
Keywords: Clinical trial, Ginseng, Ginsenoside, Protopanaxadiol, Protopanaxatriol
1. Introduction
Korean ginseng (Panax ginseng Meyer) has long been used in several eastern Asian countries, such as China, Japan, and Korea, as a traditional tonic for longevity. In traditional medicine, ginseng was simply prepared in water as a drink. A number of clinical studies on ginseng have been conducted to characterize its pharmacological properties, including improving physical performance and sexual function and treating diabetes, hypertension, inflammatory diseases, and cancer [1]. The mechanism of ginseng's functions had not been known until ginsenosides were purified as the major active components of ginseng. Since this discovery, many efforts have been made to focus on evaluating their pharmacological functions and demonstrating the molecular mechanisms of each ginsenoside. The therapeutic potential of ginseng has been studied extensively, and ginsenosides have been reported to modulate various physiological activities [2] and treat many chronic diseases. One of the major advantages of ginsenosides is their ability to interact with various target molecules in the cells, leading to combined beneficial effects [3]. Despite the remarkable achievements in diagnosis and treatment, current clinical therapies using conventional chemotherapeutic agents and pharmaceutical drugs are often toxic and have limited effectiveness, suggesting a high demand for novel, more effective, and less toxic therapeutic agents. Therefore, much attention has been paid to the exploration of novel drugs isolated from natural resources with low adverse effects and high efficacy for the treatment of human diseases. Complementary alternative medicine is a diverse type of medical systems, practices, and products [4]. Among the complementary alternative medicines, ginseng has been extensively used in Asian and, currently, in Western medicines, with a history stretching back more than 5,000 years [5]. Extensive studies have identified ginsenosides as the major pharmacological ingredient in ginseng and confirmed that ginsenosides play pivotal roles in preventing and treating various human diseases [6], [7], [8], [9], [10]. However, most of the promising studies reporting the pharmacological effects of ginsenosides on human diseases are in vitro cell-based and animal studies; therefore, this review discusses the recent studies on ginsenosides in clinical trials.
2. Ginseng and ginsenosides
Ginseng belongs to the genus Panax in Araliaceae family with nine species, including Korean ginseng (Panax ginseng), South China ginseng (Panax notoginseng), and American ginseng (Panax quinquefolius), etc. [11]. Panax means “all-healing”, and people believed that it is a panacea promoting longevity [12]; the name “ginseng” arises from the Chinese character “rénshen”, literally meaning “essence of men” [13]. Ginseng includes a variety of active ingredients, such as ginsenosides, polysaccharides, flavonoids, volatile oils, amino acids, and vitamins [14], [15]. Among these, ginsenosides are steroidal saponins with a triterpenoid dammarane structure, having a 4-ring, steroid-like configuration with sugar moieties conjugated, and more than 40 different types of ginsenosides have been identified and isolated from ginseng roots [15]. Ginseng root contains 2–3% ginsenosides, and among these, Rb1, Rb2, Rg1, Rc, Rd, Re, and Rb0 are quantitatively the most abundant and critical ones [16]. Chemical structures of ginsenosides are generally divided into two groups: protopanaxadiol and protopanaxatriol [6], [9]. Protopanaxadiols, including Rb1, Rb2, Rc, Rd, Rg3, and Rh3, have the sugar moieties attached to the 3-position of dammarane-type triterpine, whereas protopanaxatriols, including Re, Rf, Rg1, Rg2 and Rh1, have them attaching to the 6-position of dammarane-type triterpene [6], [9]. There have been a large number of studies investigating the pharmacological effect of ginseng and ginsenosides and the underlying molecular mechanisms. Ginseng and ginsenosides have been reported to play critical roles in preventing and treating many human diseases, including cancers and neuronal, cardiovascular, inflammatory, and metabolic diseases [6], [7], [8], [9], [10]; furthermore, the molecular mechanisms of their pharmacological actions are due to the diverse biological activities of the natural products, such as proapoptosis, antiangiogenesis, stimulation of the central nervous system, antiinflammatory responses, protective effects on DNA, and chemoprophylaxis [6], [9], [17], [18], [19], [20], [21].
3. Clinical trials on ginsenosides
3.1. Ginsenoside Rb1 and compound K
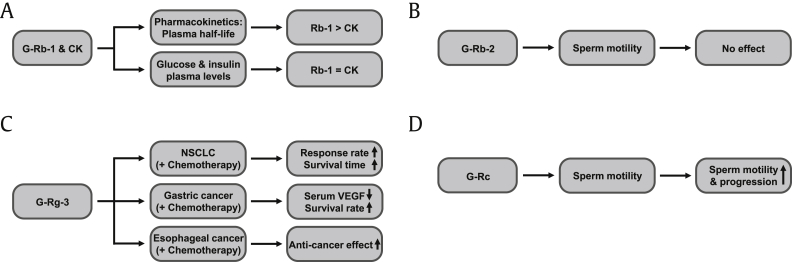
Ginsenoside Rb1 (G-Rb1) is abundant in roots and is also present in the stem and leaves [22]. Many investigations have suggested that Rb1 and its major metabolite, compound K (CK), have diverse in vitro and in vivo effects, including neuroprotective, antiinflammatory, and antiobesity actions [9]. However, only a few clinical trials of Rb1 have been reported. First, Kim orally administered healthy human individuals with red ginseng extract, followed by collection of blood samples for the pharmacokinetic analysis of both G-Rb1 and CK, and reported that maximum plasma levels of CK were significantly higher than those of G-Rb1, suggesting the delayed absorbance of CK compared with G-Rb1, which provides evidence that intestinal microflora plays a pivotal role in transforming G-Rb1 to CK [23]. Chang et al investigated the effect of G-Rb1 on insulin secretion and plasma glucose levels in healthy human individuals. Interestingly, G-Rb1 exerted no effect on plasma glucose levels and insulin secretion [24] although G-Rb1 was reported to decrease blood glucose levels in obese mice [25] and to increase insulin secretion in cultured insulinoma cells [26]. Despite these studies, further study of G-Rb1 in human patients to examine the pharmacological effects on human diseases is required.
3.2. Ginsenoside Rb2
Various pharmacological properties of ginsenoside Rb2 (G-Rb2) have been reported, including anticancer, antiadipocytic, antidiabetic, and antioxidative functions in cells and animal disease models [27], [28], [29], [30]. The anticancer activity of G-Rb2 was further reported; in uterine endometrial cancer cells, G-Rb2 suppressed matrix metalloproteinase 2 expression and cellular invasion and also inhibited neovascularization and tumor growth in a melanoma animal model [31]. Antiadipocytic activity of G-Rb2 was also reported; G-Rb2 decreased cholesterol and triglyceride levels and increased mRNA expression of the sterol-regulated element–binding protein, which is a lipid metabolism regulator in 3T3-L1 adipocytes [28]. Moreover, G-Rb2 was reported to show antiosteoporosis activity and to improve bone microarchitecture and bone mineral density by decreasing and increasing malondialdehyde and glutathione levels, respectively, in ovariectomized mice [32]. Despite these many pharmacological and mechanism studies of G-Rb2 in cells and animal models, only one clinical study has demonstrated no effect of G-Rb2 on the motility of human sperm in semen samples collected from human patients [33].
3.3. Ginsenoside Rg3
Ginsenoside Rg3 (G-Rg3) is highly enriched in Korean Red Ginseng [34] and exhibits various effects, including neuromodulatory, cognition-enhancing, antiinflammatory, antioxidative, antiangiogenic, and anticancer chemotherapeutic activities [7], [35], [36], [37], [38], [39]. Rg3 has enhanced anticancer activity compared with other ginsenosides and has the best proapoptosis effect [40]. Despite the useful antitumor effect of Rg3, only a few clinical studies have been demonstrated on ginsenoside Rg3. Many researchers have reported that combined chemotherapy with Rg3 improves the survival rate in cancer patients. For example, Sun et al and Lu et al reported that combined chemotherapy with Rg3 can improve the survival of cancer patients with advanced non–small cell lung cancer after surgery [41], [42], suggesting that Rg3 in combination with current anticancer drugs could improve a short-term therapeutic efficacy of non–small cell lung cancer. Shenyi capsule is a G-Rg3 monomer preparation, and using combined use of Shenyi capsules with current chemotherapy, it improved the postoperative survival of patients, mainly by improving immune functions and inhibiting angiogenesis in gastric cancer patients [43]. Huang et al also evaluated the therapeutic effect of Shenyi (95% G-Rg3) in combination with gemcitabine and cisplatin in advanced esophageal cancer patients and compared this patient group with the control patient group with chemotherapy alone. Results showed no significant difference in the total response rate between the Shenyi-administered and placebo-administered groups; however, the vascular endothelial growth factor in the Shenyi-administered group was found to be lower than that in the placebo-administered groups [44], suggesting the inhibitory effect of G-Rg3 on angiogenic activity. Moreover, the one-year survival rate in the Shenyi-administered group was higher than that in the placebo-administered groups [44]. Another clinical study also demonstrated that G-Rg3 is an effective antitumor agent [45], but more qualified clinical studies are needed to confirm the pharmacological effect of G-Rg3.
3.4. Ginsenoside Rh2
Ginsenoside Rh2 (G-Rh2) originally isolated from ginseng root has been suggested to have anticancer properties [46] and is considered one of the critical ginsenosides for antidiabetic, antiallergic, and antiinflammatory activities [47], [48], [49]. Recently, Qiu et al showed that G-Rh2 ameliorated Alzheimer's disease symptoms in mice by improving the learning and memory function in the diseased mice [50]. However, the poor oral bioavailability due to its low solubility in water greatly limits the use of G-Rh2 in clinical studies [51]. Therefore, additional studies are required to increase the solubility of G-Rh2 for better efficacy in clinical applications for long-term use.
3.5. Ginsenoside Rh3
Ginsenosides are generally metabolized by the bacteria in the human intestine to deglycosylated forms, which could be easily absorbed in the bloodstream and act as a bioactive ingredient [52]. Ginsenoside Rh3 (G-Rh3) is a bacterial metabolite of ginsenoside Rg5 (G-Rg5) [53], and treatment of human retinal cells with G-Rh3 induced the nuclear factor (erythroid-derived 2)-2 activation [54]. Moreover, G-Rh3 was reported to protect against memory deficits in mice by suppressing acetylcholinesterase activity as well as by inducing hippocampal brain-derived neurotrophic factor expression and cyclic adenosine monophosphate (cAMP) response element–binding protein activation [55]. However, no clinical study of G-Rh3 has been reported so far.
3.6. Ginsenoside Rc
Ginsenoside Rc (G-Rc) was found in tea [56] and has been reported to have various biological activities, such as inhibiting photoaging, oxidative stress, formalin-induced nociception, and inflammatory responses as well as increasing the life span of Caenorhabditis elegans [57], [58], [59], [60], [61]. Moreover, G-Rc was reported to have a potential antidiabetic activity by enhancing glucose uptake in C2C12 myotubes [62]. Despite these pharmacological and mechanism studies of G-Rc in cells and animal models, only one clinical study demonstrated that G-Rc enhanced the motility and progression of sperm in semen samples collected from human patients [33].
3.7. Ginsenoside Rg1
Ginsenoside Rg1 (G-Rg1) appears to be most abundant in Korean ginseng (Panax ginseng) [63] and has been demonstrated to have antiaging and antineurodegenerative activities [64], [65]. G-Rg1 was reported to improve memory impairment, to ameliorate behavioral abnormalities, and to modulate the hippocampal proteomic change in the mouse model of Alzheimer's disease [66], suggesting that Rg1 might be considered as a potential drug candidate to improve the behavioral deficits in Alzheimer's disease. However, the evidence that G-Rg1 plays a critical role in neurodegenerative diseases such as Alzheimer's disease is insufficient; in addition, there is a dearth of clinical trials investigating the effect of G-Rg1 in combination with other neuroprotective drugs, which needs to be explored in future clinical studies.
3.8. Ginsenoside Rg2
Ginsenoside Rg2 (G-Rg2) is found be more abundant in the stems and leaves of ginseng [67], and a number of studies have demonstrated that G-Rg2 has in vitro and in vivo pharmacological properties, such as neuroprotective and antidiabetic activities [68], [69]. The capacity of G-Rg2 regulating the expression of apoptosis-related proteins suggests that G-Rg2 might be a potential treatment for vascular dementia and other ischemic insults [70]. Despite these in vitro and animal studies regarding the pharmacological activity of G-Rg2, no clinical study of G-Rg2 has yet been reported, indicating the high demand for clinical studies of G-Rg2 to treat human diseases.
3.9. Ginsenoside Rh1
Ginsenoside Rh1 (G-Rh1) has been demonstrated to have antiinflammatory action in colitis in mice [71] and antioxidative activities in rat primary astrocytes [72]. Moreover, G-Rh1 was reported to exhibit neuroprotective effects on cognitive deficits in mice [73]. Despite these in vivo animal studies, no clinical study of G-Rh1 has been reported, and further clinical trials using G-Rh1 are highly recommended to provide more reliable evidence for its pharmacological activity.
The clinical studies on ginsenosides discussed in this chapter are summarized in Table 1 and conceptually described in Fig. 1.
Table 1.
Summary of the clinical trials using ginsenosides
| Ginsenosides | Participants | Study design | Clinical outcome | Ref. |
|---|---|---|---|---|
| G-Rb1 | 10 healthy volunteers | Pharmacokinetics of G-Rb1 and its major metabolite CK after oral administration | Seven times shorter CK half-life than G-Rb1 half-life in plasma | [23] |
| 12 healthy gymnasts | Improvement of glycemic status of patients during resistance training | No significant effect of G-Rb1 on the circulating glucose and insulin levels | [24] | |
| G-Rb2 | 20 patients with sperm motility between 20% and 40% of normal | G-Rb2 (0.1, 0.01, and 0.001 mg/mL) administration in patients | No effects of G-Rb2 on inferior human sperm motility in vitro | [33] |
| G-Rg3 | 115 patients with advanced NSCLC | Combined administration of G-Rg3 with vinorelbine plus cisplatin to stage III–IV NSCLC patients |
|
[41] |
| 133 patients with NSCLC | Combined administration of G-Rg3 with chemotherapy to NSCLC patients | Rg3 in combination with chemotherapy improved the life span of NSCLC patients after surgery | [42] | |
| 71 postoperative patients | Combined administration of G-Rg3 with mitomycin C plus tegafur to postoperative patients | The combined administration of G-Rg3 with chemotherapy decreased the serum VEGF level and improved the survival rate in advanced gastric cancer patients | [43] | |
| 60 patients with advanced esophageal cancer | Combined administration of G-Rg3 with gemcitabine plus cisplatin to advanced esophageal cancer patients | The anticancer effect of combined administration of G-Rg3 with chemotherapy was better than chemotherapy alone | [44] | |
| 124 patients with advanced NSCLC and EGFR-active mutation | Combined administration of G-Rg3 with EGFR-TKI to advanced NSCLC patients | Clinical benefit of combined administration of G-Rg3 with EGFR-TKI compared with EGFR-TKI alone in NSCLC patients harboring EGFR-active mutation | [45] | |
| G-Rc | 20 patients with sperm motility between 20% and 40% of normal | G-Rc (0.1, 0.01, and 0.001 mg/mL) administration in patients | G-Rc (0.01 and 0.001 mg/mL) enhanced the motility and progression of sperm | [33] |
CK, compound K; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Fig. 1.
Conceptual summary of clinical studies using ginsenosides. (A) The effects of G-Rb1 and CK on their plasma half-life and the plasma levels of glucose and insulin in healthy humans. (B) The effect of G-Rb2 on the sperm motility in the patients with reduced sperm motility. (C) The anticancer effect of G-Rg3 and chemotherapy on NSCLC, gastric cancer, and esophageal cancer patients. (D) The effect of G-Rc on the sperm motility in the patients with reduced sperm motility. CK, compound K; NSCLC, non–small cell lung cancer; VEGF, vascular endothelial growth factor.
4. Clinical trials on ginseng
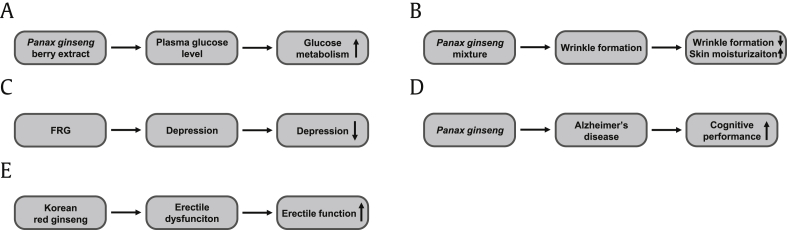
Although a large number of studies have successfully demonstrated both in vitro and in vivo pharmacological effects of ginseng in a variety of diseases, including cancers, inflammatory diseases, cardiovascular diseases, metabolic diseases, and hepatotoxicity, the efficacy of ginseng in human patients has not been clearly established, and there are insufficient reliable clinical data, meaning that the quality of most clinical studies using ginseng is relatively poor. However, several studies have demonstrated the effects of ginseng in humans. Choi et al investigated the antihyperglycemic effect of the Panax ginseng berry in healthy human individuals and reported that ginseng berries did not have an antihyperglycemic effect but showed potential to improve glucose metabolism in humans [74]. Hwang et al investigated the antiaging effect of a mixture of Panax ginseng and Crataegus pinnatifida in healthy human skin and reported that this mixture exerted an antiaging effect by suppressing wrinkle formation and increasing moisture in human skin [75]. Lee and Ji investigated the effect of fermented red ginseng on the depression in postmenopausal women and reported that fermented red ginseng ameliorated cognitive depression [76]. Lee et al also examined the effect of Panax ginseng on the cognitive performance of patients with Alzheimer's disease. The study reported that Panax ginseng improved the cognitive performance of patients with Alzheimer's disease and that discontinuing ginseng consumption exacerbated the cognitive performance of the patients [77]. Moreover, Choi et al demonstrated the effect of Korean Red Ginseng on the erectile dysfunction in patients and showed that Korean Red Ginseng improved disease symptoms [78]. Despite the small number of clinical studies, these studies demonstrated the clinical efficacy of ginseng for human diseases. The clinical studies on ginseng discussed in this chapter are summarized in Table 2 and conceptually described in Fig. 2.
Table 2.
Summary of the clinical trials using ginseng
| Ginseng | Participants | Study design | Clinical outcome | Ref. |
|---|---|---|---|---|
| Panax ginseng berry extract | 72 healthy volunteers | Administration of ginseng berry extract in participants with a fasting glucose level between 100 mg/dL and 140 mg/dL | Ginseng berry extract improved glucose metabolism | [74] |
| Panax ginseng mixture | 20 healthy female volunteers (aged 30–65 yr) with crow's feet wrinkles | Administration of a mixture of Panax ginseng and Crataegus pinnatifida | Panax ginseng mixture decreased wrinkle formation and increased moisture in the human skin | [75] |
| FRG | 93 postmenopausal female volunteers (aged 50–73 yr) | Administration of FRG | FRG ameliorated cognitive depression that occurred via the energy factor | [76] |
| Panax ginseng | 97 Alzheimer's disease patients | Administration of Panax ginseng | Panax ginseng enhanced cognitive performance of Alzheimer's disease patients | [77] |
| Korean Red Ginseng | 90 patients with erectile dysfunction | Administration of Korean Red Ginseng | Clinical efficacy on Korean Red Ginseng on erectile dysfunction | [78] |
FRG, fermented red ginseng.
Fig. 2.
Conceptual summary of clinical studies using ginseng. (A) The effect of Panax ginseng berry extract on the plasma glucose level in healthy humans. (B) The effect of Panax ginseng on wrinkle formation and skin moisturization. (C) The effect of FRG on the depression in postmenopausal patients. (D) The effect of Panax ginseng on the cognitive performance in patients with Alzheimer's disease. (E) The effect of Korean Red Ginseng on the erectile function in the patients with erectile dysfunction. FRG, fermented red ginseng.
5. Why are clinical studies of ginseng and ginsenosides so few?
The importance of ginseng and ginsenosides as natural remedies has long been recognized based on their versatile efficacies that have been proven in in vitro cells and in vivo animal models. Although many ginsenosides have showed clear anticancer activities in cancer cells and animal tumor models, it is required to examine their comparable effects in human patients. However, the use of ginsenosides has been limited to cells and animal models due to their low bioavailability, such as poor water solubility and biomembrane permeability, instability in gastrointestinal tract, and high metabolic rate in the body. Therefore, a number of efforts have been made on solving these problems by developing effective delivery systems and a variety of administration routes to improve the bioavailability of ginsenosides, resulting in remarkable improvements in their bioavailability. Moreover, ginsenosides exhibit antioxidative, antiinflammatory, apoptotic, and immunostimulatory properties with diverse molecular mechanisms of action; with this said, it is often difficult to standardize the studies due to the diversity of ginsenosides in both chemical structure and biological activity. Therefore, further systemic studies using ginsenosides with improved bioavailability and a series of related compounds are required.
6. Conclusion and future perspectives
Panax ginseng is an herbal medicine traditionally used for a long time worldwide for a wide range of indications. Clinical trials using ginseng and ginsenosides and systematic reviews are now available, and although their conclusions vary, they are promising for some indications. The active ingredients have been mainly isolated and quantified in roots, extracts, and finished products of ginseng, and many studies have shown significant variations between finished preparations. Moreover, many European and American recommendations for using ginseng and its products involve dosages about 10 times less than those generally used in Asian countries. Recent emphasis on the standardized products, which may provide an impression that it is stronger than root powders or other ginseng extracts, might mask the fact that the ginseng products are often standardized to quite low dosages of active ingredients. Recent studies provide the support for using ginseng to treat hyperlipidemia, diabetes, heart failure, and hypertension with the administration doses used in Asian countries, but the evidence supporting performance-enhancing and energy-boosting effects is weak. In addition, there exists considerable confusion for the potential side effects of ginseng, but most of the warnings may not be justified.
Poor bioavailability of ginsenosides and their metabolites indicates that these compounds could not reach the intended biological system when orally administered. Ginsenoside studies could become physiologically relevant only when (a) a large amount of pure ginsenosides are available, (b) ginsenosides are stabilized to increase their stability and absorption in the gastrointestinal tract, and/or (c) special delivery strategies for ginsenosides to reach the treatment areas are established.
Most studies investigating the efficacy of ginseng and ginsenosides have been conducted in cell lines and animal models; relatively few have been undertaken in humans despite the fact that ginseng products have been widely believed and accepted to have therapeutic effects when used alone or in combination with other therapeutic agents in the management of a wide range of chronic diseases. Therefore, active studies demonstrating the therapeutic effects of ginseng and ginsenosides are in high demand. Moreover, further studies using a single ginsenoside need to include the molecular and cellular mechanisms of action, specificity, the structure–function relationship, pharmacokinetics profile, and toxicity in animal models and humans. These studies could maximize the potential of ginsenosides as promising herbal medicines, thereby further contributing to the promotion of global health.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the grant from the National Cancer Center (1810960-1), Republic of Korea.
Contributor Information
Young-Su Yi, Email: ysyi@cju.ac.kr.
Byong Chul Yoo, Email: yoo_akh@ncc.re.kr.
References
- 1.Choi J., Kim T.H., Choi T.Y., Lee M.S. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42:239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jong M.C., van de Vijver L., Busch M., Fritsma J., Seldenrijk R. Integration of complementary and alternative medicine in primary care: what do patients want? Patient Educ Couns. 2012;89:417–422. doi: 10.1016/j.pec.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.J., Zhang D., Yang D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33:717–735. doi: 10.1016/j.biotechadv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja A., Kim J.H., Yi Y.S., Cho J.Y. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 2018;42:248–254. doi: 10.1016/j.jgr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nah S.Y. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014;5:98. doi: 10.3389/fphys.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J., Gan X.T., Haist J.V., Rajapurohitam V., Zeidan A., Faruq N.S., Karmazyn M. Ginseng inhibits cardiomyocyte hypertrophy and heart failure via NHE-1 inhibition and attenuation of calcineurin activation. Circ Heart Fail. 2011;4:79–88. doi: 10.1161/CIRCHEARTFAILURE.110.957969. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J.B., Zhang R., Han X., Piao C.L. Ginsenoside Rg1 inhibits dietary-induced obesity and improves obesity-related glucose metabolic disorders. Braz J Med Biol Res. 2018;51:e7139. doi: 10.1590/1414-431X20177139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelova N., Kong H.W., van der Heijden R., Yang S.Y., Choi Y.H., Kim H.K., Wang M., Hankemeier T., van der Greef J., Xu G. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19:2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 12.Sodrul I.M.D., Wang C., Chen X., Du J., Sun H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget. 2018;9:2931–2950. doi: 10.18632/oncotarget.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong A.S., Che C.M., Leung K.W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–272. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]
- 14.Wu W., Jiao C., Li H., Ma Y., Jiao L., Liu S. LC-MS based metabolic and metabonomic studies of Panax ginseng. Phytochem Anal. 2018;29:331–340. doi: 10.1002/pca.2752. [DOI] [PubMed] [Google Scholar]
- 15.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang O.J., Kim J.S. Comparison of ginsenoside contents in different parts of Korean ginseng (Panax ginseng C.A. Meyer) Prev Nutr Food Sci. 2016;21:389–392. doi: 10.3746/pnf.2016.21.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Zhang S., Sun Q., Jiao W., Yan Y. Compound K induces endoplasmic reticulum stress and apoptosis in human liver cancer cells by regulating STAT3. Molecules. 2018;23 doi: 10.3390/molecules23061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek S.H., Shin B.K., Kim N.J., Chang S.Y., Park J.H. Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo. J Ginseng Res. 2017;41:233–239. doi: 10.1016/j.jgr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong A., Lee H.J., Jeong S.J., Lee E.O., Bae H., Kim S.H. Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biol Pharm Bull. 2010;33:945–950. doi: 10.1248/bpb.33.945. [DOI] [PubMed] [Google Scholar]
- 21.Lu H., Zhou X., Kwok H.H., Dong M., Liu Z., Poon P.Y., Luan X., Ngok-Shun Wong R. Ginsenoside-Rb1-mediated anti-angiogenesis via regulating PEDF and miR-33a through the activation of PPAR-gamma pathway. Front Pharmacol. 2017;8:783. doi: 10.3389/fphar.2017.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan T.H., Xu Z.W., Wang Z., Wu Y.L., Wu W.K., Tan H.M. Ginsenoside Rb1 prevents homocysteine-induced endothelial dysfunction via PI3K/Akt activation and PKC inhibition. Biochem Pharmacol. 2011;82:148–155. doi: 10.1016/j.bcp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res. 2013;37:451–456. doi: 10.5142/jgr.2013.37.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang W.H., Tsai Y.L., Huang C.Y., Hsieh C.C., Chaunchaiyakul R., Fang Y., Lee S.D., Kuo C.H. Null effect of ginsenoside Rb1 on improving glycemic status in men during a resistance training recovery. J Int Soc Sports Nutr. 2015;12:34. doi: 10.1186/s12970-015-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang W.B., Yu X.Z., Wang G.Q., Zhao J. Effect of ginsenoside Rb1 in ameliorating insulin resistance and ectopic fat deposition in obese mice induced by high fat diet. Zhongguo Zhong Yao Za Zhi. 2013;38:4119–4123. [PubMed] [Google Scholar]
- 26.Park S., Ahn I.S., Kwon D.Y., Ko B.S., Jun W.K. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3-L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci Biotechnol Biochem. 2008;72:2815–2823. doi: 10.1271/bbb.80205. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto J., Sakaguchi H., Aoki I., Toyoki H., Khatun S., Tamaya T. Inhibitory effect of ginsenoside-Rb2 on invasiveness of uterine endometrial cancer cells to the basement membrane. Eur J Gynaecol Oncol. 2001;22:339–341. [PubMed] [Google Scholar]
- 28.Kim E.J., Lee H.I., Chung K.J., Noh Y.H., Ro Y., Koo J.H. The ginsenoside-Rb2 lowers cholesterol and triacylglycerol levels in 3T3-L1 adipocytes cultured under high cholesterol or fatty acids conditions. BMB Rep. 2009;42:194–199. doi: 10.5483/bmbrep.2009.42.4.194. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.T., Jung T.W., Lee H.J., Kim S.G., Shin Y.S., Whang W.K. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res. 2011;34:1201–1208. doi: 10.1007/s12272-011-0719-6. [DOI] [PubMed] [Google Scholar]
- 30.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 31.Sato K., Mochizuki M., Saiki I., Yoo Y.C., Samukawa K., Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 32.Huang Q., Gao B., Jie Q., Wei B.Y., Fan J., Zhang H.Y., Zhang J.K., Li X.J., Shi J., Luo Z.J. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone. 2014;66:306–314. doi: 10.1016/j.bone.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Chen J.C., Chen L.D., Tsauer W., Tsai C.C., Chen B.C., Chen Y.J. Effects of ginsenoside Rb2 and Rc on inferior human sperm motility in vitro. Am J Chin Med. 2001;29:155–160. doi: 10.1142/S0192415X01000174. [DOI] [PubMed] [Google Scholar]
- 34.Jovanovski E., Bateman E.A., Bhardwaj J., Fairgrieve C., Mucalo I., Jenkins A.L., Vuksan V. Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: a randomized controlled trial. J Am Soc Hypertens. 2014;8:537–541. doi: 10.1016/j.jash.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee B., Sur B., Park J., Kim S.H., Kwon S., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther (Seoul) 2013;21:381–390. doi: 10.4062/biomolther.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F., Eckman E.A., Eckman C.B. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20:1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- 37.Kang A., Xie T., Zhu D., Shan J., Di L., Zheng X. Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J Agric Food Chem. 2017;65:6861–6869. doi: 10.1021/acs.jafc.7b02386. [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Yu X.F., Zhao J.J., Shi S.M., Fu L., Sui D.Y. Ginsenoside Rg3 attenuated omethoate-induced lung injury in rats. Hum Exp Toxicol. 2016;35:677–684. doi: 10.1177/0960327115597984. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J., Yuan Z., Sun Y., Bu Y., Li W., Fei Z. Ginsenoside Rg3 enhances the anti-proliferative activity of erlotinib in pancreatic cancer cell lines by downregulation of EGFR/PI3K/Akt signaling pathway. Biomed Pharmacother. 2017;96:619–625. doi: 10.1016/j.biopha.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 40.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol- and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016:5738694. doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y., Lin H., Zhu Y., Feng J., Chen Z., Li G., Zhang X., Zhang Z., Tang J., Shi M. A randomized, prospective, multi-centre clinical trial of NP regimen (vinorelbine+cisplatin) plus Gensing Rg3 in the treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2006;9:254–258. doi: 10.3779/j.issn.1009-3419.2006.03.09. [DOI] [PubMed] [Google Scholar]
- 42.Lu P., Su W., Miao Z.H., Niu H.R., Liu J., Hua Q.L. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z.J., Cheng J., Huang Y.P., Han S.L., Liu N.X., Zhu G.B., Yao J.G. Effect of adjuvant chemotherapy of ginsenoside Rg3 combined with mitomycin C and tegafur in advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:64–66. [PubMed] [Google Scholar]
- 44.Huang J.Y., Sun Y., Fan Q.X., Zhang Y.Q. Efficacy of Shenyi Capsule combined with gemcitabine plus cisplatin in treatment of advanced esophageal cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2009;7:1047–1051. doi: 10.3736/jcim20091105. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Wang Y., Niu K., Chen X., Xia L., Lu D., Kong R., Chen Z., Duan Y., Sun J. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget. 2016;7:70535–70545. doi: 10.18632/oncotarget.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M.J., Yun H., Kim D.H., Kang I., Choe W., Kim S.S., Ha J. AMP-activated protein kinase determines apoptotic sensitivity of cancer cells to ginsenoside-Rh2. J Ginseng Res. 2014;38:16–21. doi: 10.1016/j.jgr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park E.K., Choo M.K., Kim E.J., Han M.J., Kim D.H. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 48.Gupta R.C., Chang D., Nammi S., Bensoussan A., Bilinski K., Roufogalis B.D. Interactions between antidiabetic drugs and herbs: an overview of mechanisms of action and clinical implications. Diabetol Metab Syndr. 2017;9:59. doi: 10.1186/s13098-017-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinoth Kumar R., Oh T.W., Park Y.K. Anti-inflammatory effects of ginsenoside-Rh2 inhibits LPS-induced activation of microglia and overproduction of inflammatory mediators via modulation of TGF-beta1/smad pathway. Neurochem Res. 2016;41:951–957. doi: 10.1007/s11064-015-1804-x. [DOI] [PubMed] [Google Scholar]
- 50.Qiu J., Li W., Feng S.H., Wang M., He Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet Mol Res. 2014;13:3586–3598. doi: 10.4238/2014.May.9.2. [DOI] [PubMed] [Google Scholar]
- 51.Fu B.D., Bi W.Y., He C.L., Zhu W., Shen H.Q., Yi P.F., Wang L., Wang D.C., Wei X.B. Sulfated derivatives of 20(S)-ginsenoside Rh2 and their inhibitory effects on LPS-induced inflammatory cytokines and mediators. Fitoterapia. 2013;84:303–307. doi: 10.1016/j.fitote.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Bae E.A., Park S.Y., Kim D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 53.Zheng M.M., Xu F.X., Li Y.J., Xi X.Z., Cui X.W., Han C.C., Zhang X.L. Study on transformation of ginsenosides in different methods. Biomed Res Int. 2017;2017:8601027. doi: 10.1155/2017/8601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang C.Z., Li K.R., Yu Q., Jiang Q., Yao J., Cao C. Activation of Nrf2 by Ginsenoside Rh3 protects retinal pigment epithelium cells and retinal ganglion cells from UV. Free Radic Biol Med. 2018;117:238–246. doi: 10.1016/j.freeradbiomed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Kim E.J., Jung I.H., Van Le T.K., Jeong J.J., Kim N.J., Kim D.H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol. 2013;146:294–299. doi: 10.1016/j.jep.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 56.Luchtefeld R., Kostoryz E., Smith R.E. Determination of ginsenosides Rb1, Rc, and Re in different dosage forms of ginseng by negative ion electrospray liquid chromatography-mass spectrometry. J Agric Food Chem. 2004;52:4953–4956. doi: 10.1021/jf040104j. [DOI] [PubMed] [Google Scholar]
- 57.Oh Y., Lim H.W., Park K.H., Huang Y.H., Yoon J.Y., Kim K., Lim C.J. Ginsenoside Rc protects against UVBinduced photooxidative damage in epidermal keratinocytes. Mol Med Rep. 2017;16:2907–2914. doi: 10.3892/mmr.2017.6943. [DOI] [PubMed] [Google Scholar]
- 58.Kim D.H., Park C.H., Park D., Choi Y.J., Park M.H., Chung K.W., Kim S.R., Lee J.S., Chung H.Y. Ginsenoside Rc modulates Akt/FoxO1 pathways and suppresses oxidative stress. Arch Pharm Res. 2014;37:813–820. doi: 10.1007/s12272-013-0223-2. [DOI] [PubMed] [Google Scholar]
- 59.Shin Y.H., Jung O.M., Nah J.J., Nam K.Y., Kim C.Y., Nah S.Y. Ginsenosides that produce differential antinociception in mice. Gen Pharmacol. 1999;32:653–659. doi: 10.1016/s0306-3623(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 60.Yu T., Yang Y., Kwak Y.S., Song G.G., Kim M.Y., Rhee M.H., Cho J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res. 2017;41:127–133. doi: 10.1016/j.jgr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J.H., Choi S.H., Kwon O.S., Shin T.J., Lee B.H., Yoon I.S., Pyo M.K., Rhim H., Lim Y.H., Shim Y.H. Effects of ginsenosides, active ingredients of Panax ginseng, on development, growth, and life span of Caenorhabditis elegans. Biol Pharm Bull. 2007;30:2126–2134. doi: 10.1248/bpb.30.2126. [DOI] [PubMed] [Google Scholar]
- 62.Lee M.S., Hwang J.T., Kim S.H., Yoon S., Kim M.S., Yang H.J., Kwon D.Y. Ginsenoside Rc, an active component of Panax ginseng, stimulates glucose uptake in C2C12 myotubes through an AMPK-dependent mechanism. J Ethnopharmacol. 2010;127:771–776. doi: 10.1016/j.jep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 63.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Y., Shen L.H., Zhang J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 65.Huang L., Liu L.F., Liu J., Dou L., Wang G.Y., Liu X.Q., Yuan Q.L. Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res. 2016;11:319–325. doi: 10.4103/1673-5374.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nie L., Xia J., Li H., Zhang Z., Yang Y., Huang X., He Z., Liu J., Yang X. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of Alzheimer's disease. Oxid Med Cell Longev. 2017;2017:6473506. doi: 10.1155/2017/6473506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H., Peng D., Xie J. Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin Med. 2009;4:20. doi: 10.1186/1749-8546-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X.F., Lui C.N., Jiang Z.H., Ken Y.K. Neuroprotective effects of ginsenosides Rh1 and Rg2 on neuronal cells. Chin Med. 2011;6:19. doi: 10.1186/1749-8546-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan H.D., Kim D.Y., Quan H.Y., Kim S.J., Jung M.S., Chung S.H. Ginsenoside Rg2 induces orphan nuclear receptor SHP gene expression and inactivates GSK3beta via AMP-activated protein kinase to inhibit hepatic glucose production in HepG2 cells. Chem Biol Interact. 2012;195:35–42. doi: 10.1016/j.cbi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Zhang G., Liu A., Zhou Y., San X., Jin T., Jin Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol. 2008;115:441–448. doi: 10.1016/j.jep.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 71.Lee S.Y., Jeong J.J., Eun S.H., Kim D.H. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. 2015;762:333–343. doi: 10.1016/j.ejphar.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Jung J.S., Lee S.Y., Kim D.H., Kim H.S. Protopanaxatriol ginsenoside Rh1 upregulates phase II antioxidant enzyme gene expression in rat primary astrocytes: involvement of MAP kinases and Nrf2/ARE signaling. Biomol Ther (Seoul) 2016;24:33–39. doi: 10.4062/biomolther.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu C., Lv J., Dong L., Jiang N., Wang Y., Wang Q., Li Y., Chen S., Fan B., Wang F. Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phytother Res. 2018;32:1056–1063. doi: 10.1002/ptr.6044. [DOI] [PubMed] [Google Scholar]
- 74.Choi H.S., Kim S., Kim M.J., Kim M.S., Kim J., Park C.W., Seo D., Shin S.S., Oh S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: a 12-wk randomized, double-blind, and placebo-controlled clinical trial. J Ginseng Res. 2018;42:90–97. doi: 10.1016/j.jgr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang E., Park S.Y., Yin C.S., Kim H.T., Kim Y.M., Yi T.H. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J Ginseng Res. 2017;41:69–77. doi: 10.1016/j.jgr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee K.J., Ji G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr Neurosci. 2014;17:7–15. doi: 10.1179/1476830513Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 77.Lee S.T., Chu K., Sim J.Y., Heo J.H., Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 78.Choi H.K., Seong D.H., Rha K.H. Clinical efficacy of Korean red ginseng for erectile dysfunction. Int J Impot Res. 1995;7:181–186. [PubMed] [Google Scholar]