Abstract
Autophagy is the sequential process whereby cell components are degraded, which can occur due to nutrient deprivation. Its regulation has an essential role in many diseases, functioning in both cell survival and cell death. Autophagy starts when mTORC1 is inhibited, resulting in the activation of several complexes to form a cargo that fuses with a lysosome, where it undergoes degradation. In this review, we describe a plant extract that is well known in Korea, namely Korean ginseng extract; we studied how its derivatives and metabolites can regulate autophagy and thus mediate the pathogenesis of certain diseases.
Keywords: Autophagosome, Autophagy, Ginsenosides, Panax ginseng
1. Introduction
Autophagy is the sequential process of cytoplasm component degradation by lysosomes [1], [2]. It plays an essential role in countering certain diseases and conditions, such as cancer, infection, aging, heart diseases, and neurodegradation [3], [4]. There are different types of autophagy, classified by their mechanisms. Certain articles have classified autophagy into two types: nonselective (macroautophagy) and selective (classified by the cytosolic cargo sequestered) [3], [5]. Other articles have classified autophagy into three main types: macroautophagy, microautophagy, and chaperone-mediated autophagy [4], [6], [7]. Autophagy generally occurs due to nutrient deprivation and/or starvation, functioning in both cell survival and cell death [8].
Macroautophagy, which is commonly referred as autophagy, is involved in several pathways to form double membrane vesicles (autophagosomes) and to sequester cytoplasmic components through the inhibition of the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway [9], [10]. This inhibition activates the downstream complex Unc-51 like autophagy activating kinase 1/2 (ULK1/2), composed of ULK1/2, Atg13, and FIP200 [11]. This complex then triggers the Beclin-1/Phosphatidylinositol 3-kinase catalytic subunit type 3 (PI3KC3) complex to initiate phagopore formation. This in turn leads to autophagosome formation through the microtubule light-chain 3 protein or LC3 by activation through ubiquitin-like conjugation pathways [autophagy-related protein (ATG) conjugates] [12]. The autophagosome then fuses with a lysosome (autolysosome), and the materials inside are degraded. Furthermore, the proteins involved in autophagy would also be recycled at the end of the process.
While autophagy has a functional role in cell survival, it has several pathways in common with apoptosis, a pathway that causes cell death. Autophagy and apoptosis regulate each another: For example, autophagy mediates cell death by degrading the negative regulator of apoptosis signaling, particularly in the caspase signaling pathway [13], or by degrading catalase, increasing reactive oxygen species (ROS) accumulation, and leading to cell death [14]. On the other hand, apoptosis regulators such as Bcl-2 and Bcl-XL prevent autophagy by interacting with Beclin-1 on their BH3 domain. This can be disrupted by BH3 mimetics, for example, Bcl-2-associated death promoter (BAD), to initiate autophagy [15]. The implication of autophagy involvement in some diseases is attracting attention. A number of cancer treatments involve autophagy inhibition or activation. In this review, we focus on how natural sources take part in autophagy regulation, in particular a very famous plant in Korea, Korean ginseng, and its components.
2. Korean ginseng extract and its components
Korean ginseng (Panax ginseng Meyer) belongs to the Araliaceae family [16]. Korean ginseng has been found to have antidiabetic, antiinflammatory, antimicrobial, antifungal, neuroprotective, antioxidant, anticancer, and antitumor activity [17], [18], [19], [20], [21], [22]. The plant is especially rich in ginsenosides, a group of triterpene saponins, which comprise the active components [23]. Ginsenosides are classified into two categories based on their chemical structures, 20-S-protopanaxadiol and 20-S-protopanaxatriol [24]. Some ginsenoside components have been reported to play a role in certain diseases. Some ginsenoside derivatives such as Rb1, Rb2, Rg3, Rg1, and Rb1's metabolite compound K (Fig. 1) have been reported to have antidiabetic activity, involving the sites of activation or inhibition of proteins responsible for diabetes [25], [26], [27], [28], [29]. Some ginsenoside derivatives also have antiinflammatory activity; for example, ginsenoside Rg1 decreases nitric oxide (NO) and tumor necrosis factor (TNF)-α levels in macrophage cells [30], as well as interleukin (IL)-1β and IL-6 in vivo [31]. There is also a report that the nuclear factor (NF)-κB signaling pathway is downregulated in the presence of ginsenoside Rg3 in lung tissue of humans with asthma [32]. Ginsenoside Rp1 downregulates the insulin-like growth factor 1 receptor (IGF-1R)/protein kinase B (Akt) pathway in breast cancer cells, reducing their proliferation and colony formation [33]. In addition, ginsenoside Rk3 has been found to downregulate the expression of cyclin D1 and CDK4, followed by the upregulation of p21, in lung cancer in vitro and in vivo [34].
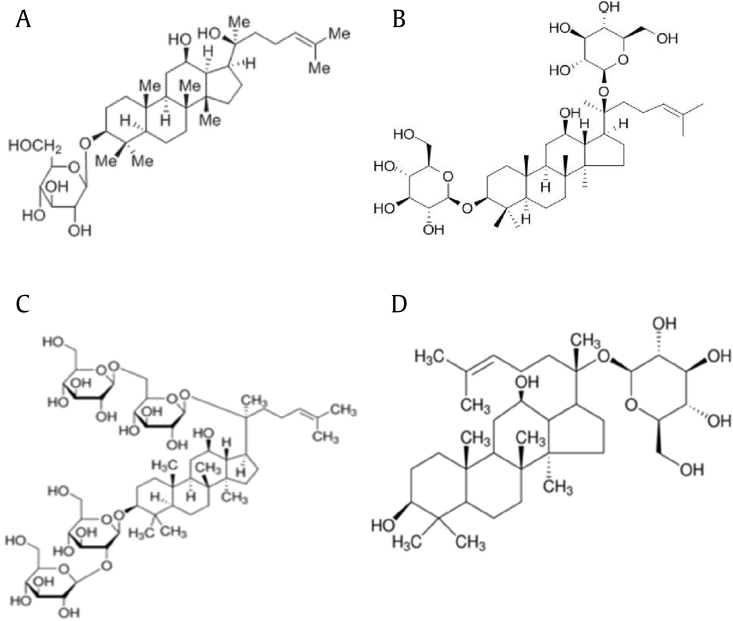
Fig. 1.
Chemical structure of ginseng components. (A) Ginsenoside Rh2. (B) Ginsenoside F2. (C) Ginsenoside Rb1. (D) Compound K.
3. Regulation of autophagy by Korean ginseng extract
The components and derivatives of Korean ginseng extract (KGE) play important roles in treating certain serious diseases, and the effects of Korean ginseng on autophagy may be one of the mechanisms (Table 1 and Fig. 2). Several studies have investigated KGE-mediated autophagy. Cha et al [35] reported that the extract had the potential to attenuate testicular dysfunction as treatment decreased molecules responsible for spermatogenesis and downregulated the mRNA levels of sex hormone receptors including the androgen, luteinizing hormone, and follicle-stimulating hormone receptors. Concomitantly, Korean Red Ginseng extract (RGE) also modulated autophagy responses by decreasing the mTORC1 mRNA and protein levels after they had been elevated by doxycycline (DOX)-induced testicular damage [35]. Nonetheless, further study is needed to determine the stage at which autophagy regulates testicular dysfunction and vice versa.
Table 1.
Target diseases and autophagy-mediated molecular targets responding to ginseng components
| Component | Target diseases | Autophagy-mediated molecular target |
|---|---|---|
| Korean Red Ginseng extract | Testicular dysfunction [35] | Inhibits mTORC1 and mTOR phosphorylation. |
| Diabetes mellitus [40] | Reduces LC3B puncta accumulation; increases LC3B-LAMP-2A colocalization. | |
| Hepatocellular carcinoma [36] | Increases LC3 puncta as indication of reducing the late stage of autophagy. | |
| Ginsenoside Rb1 | Melanoma [41] | Attenuates mTOR phosphorylation by induction of AMPK. |
| Compound K | Colon cancer [45] | Causes accumulation of LC3 after the upregulation of Atg5 and Atg7. Disrupts Atg6 (Beclin-1) and Bcl-2 interaction (also induces apoptosis). |
| Ginsenoside Rh2 | Acute and chronic myeloid leukemia [58] | Increases LC3B activation after downregulation of p62. |
| Ginsenoside F2 | Breast cancer [53] | Increases the accumulation of LC3, Beclin-1, and Atg-7. |
mTORC1, mammalian target of rapamycin complex 1.
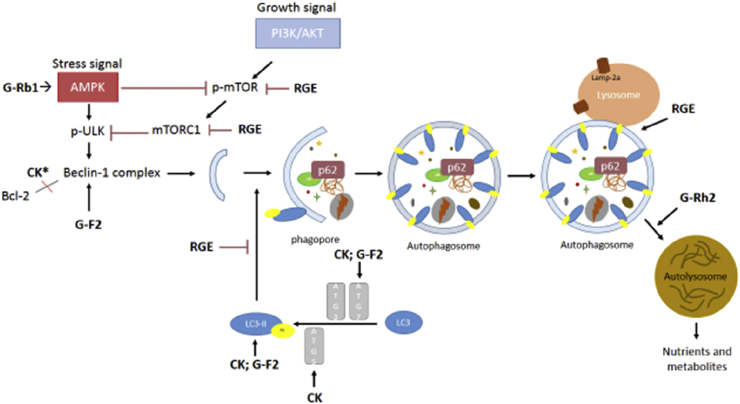
Fig. 2.
Schematic flow chart of autophagy-mediated molecular targets regarding ginseng components.
AMPK, AMP-activated protein kinase; CK, compound K; mTORC1, mammalian target of rapamycin complex 1; PI3KC3, Phosphatidylinositol 3-kinase catalytic subunit type 3; RGE, Red Ginseng extract; ULK1/2, Unc-51 like autophagy activating kinase 1/2.
On the other hand, RGE inhibited autophagic flux synergistically with doxorubicin, leading to cell death in hepatocarcinoma cell lines [36]. RGE-mediated prevention of autophagic flux increased the sensitivity of the cancer cells to the doxorubicin. Autophagic flux inhibition increases cell starvation by preventing autophagosome–lysosome fusion, which is marked by a decrease in the protein level of p62 due to autophagy cargo degradation [37], [38]. When chloroquine treatment was used to block lysosome function, in turn inhibiting autophagy, RGE did not upregulate the expression levels of both LC3 and p62, which indicates that RGE upregulates LC3 in the last stage of autophagy [38].
Through regulation of autophagy, KGE may reduce immunosuppressant-associated adverse effects, such as diabetes mellitus associated with tacrolimus [39], [40]. Tacrolimus-induced diabetes mellitus is characterized by the accumulation of autophagic vacuoles, whose conversion to autophagosomes results from the activation of LC3 [39]. These autophagosomes mediate beta cell injury, leading to diabetes mellitus. RGE can modulate this activity to prevent the sustainable formation of autophagosomes, markedly by the inhibition of LC3B as an autophagosome marker after enhancement of the colocalization of LC3B and lysosome-associated membrane protein-2A during autophagosome–lysosome fusion, to enhance the autophagy clearance [40].
4. Regulation of autophagy by ginsenoside derivatives
KGE contains many derivatives and metabolites, which have divergent roles in regulating autophagy (Table 1 and Fig. 2). One ginsenoside Rb1 metabolite, 20-O-β-D-glucopyranosil-20(S)-protopanaxadiol (compound K), has been shown to play a role in malignancy by inducing autophagy and apoptosis via activation of AMP-activated protein kinase (AMPK)/c-Jun N-terminal kinase (JNK) [41]. The parallel activity in the mitogen activated protein kinase (MAPK) and AMPK signaling pathways initiates autophagy as follows: JNK phosphorylates Bcl-2 to dissociate with Beclin-1, inhibiting mTOR [42], whereas mTOR generally is inhibited by AMPK activation to initiate autophagy processes and negatively regulate the transformation and proliferation of melanoma cells [43], [44]. Compound K also has been reported to induce autophagy in human colon cancer by regulating JNK and ROS generation [45] because in cancer cells, intracellular ROS concentration is found to be increased, which could mediate autophagy [46]. Activation of autophagy is also part of atherosclerosis: Macrophages form cholesterol-laden foam cells after engulfing oxygenized low-density lipoproteins, resulting in the formation of atherosclerotic plaques [47]. In brief, autophagy regulates lipid metabolism in macrophages through the hydrolysis of intracellular lipids and cholesterol efflux promotion. Moreover, autophagy dysfunction may lead to the abnormalities of atherosclerotic plaque formation and progression of atherosclerosis [48], [49], [50].
In breast cancer, metastasis can lead to drug resistance [51], [52]. Mai et al [53] found an association between apoptosis and autophagy with ginsenoside F2 in breast cancer stem cells. They concluded that ginsenoside F2 blocks proliferative activity via activation of the intrinsic apoptotic pathway and triggers mitochondrial dysfunction. Moreover, it also induced the recruitment of LC3, Beclin-1, and Atg-7 in the context of autophagy, controlled by the apoptosis–autophagy conjunction controller protein p53. The upregulation of p53 phosphorylation resulted in the activation of AMPK, which inhibits mTOR as an initiator of autophagy via AMPK-TSC/1/TSC2-mTOR [54], [55]. In short, ginsenoside F2 in this case has a dual role including activation of apoptosis after protective autophagy against breast cancer stem cells.
Another ginsenoside derivative, Rh2 (G-Rh2), was shown to induce apoptosis in leukemia cells by upregulating transforming growth factor (TGF)-β expression and regulating the mitochondria signaling pathway [56], [57]. Furthermore, autophagy may play another role in leukemia by upregulating the activation of LC3-II from LC3-I, together with downregulating p62, which indicates the autophagy flux in a dose-dependent manner [58]. This regulation protects the cells against apoptosis. Conclusively, to increase the efficiency of the therapeutic effect of G-Rh2 in both acute and chronic myeloid leukemia, it is necessary to inhibit autophagy to increase cancer cell death.
5. Conclusion
Studies of KGE and its components are helping to provide an understanding of the role of autophagy in the pathogenesis of certain diseases (Fig. 2). More studies are needed to determine the role of each stage of autophagy in disease. These studies would provide a better understanding of how this extract and its components could be used to treat diseases such as cancer. Conflicts of interest All authors declare no conflicts of interest.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea (2016R1D1A1B03932512).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2018.12.011.
Contributor Information
Mi-Yeon Kim, Email: kimmy@ssu.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Tooze S.A., Abada A., Elazar Z. Endocytosis and autophagy: exploitation or cooperation? Cold Spring Harbor Perspect Biol. 2014;6:a018358. doi: 10.1101/cshperspect.a018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maria Cuervo A. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Lamb C.A., Dooley H.C., Tooze S.A. Endocytosis and autophagy: shared machinery for degradation. BioEssays. 2013;35:34–45. doi: 10.1002/bies.201200130. [DOI] [PubMed] [Google Scholar]
- 4.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z., Klionsky D.J. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch-Day M.A., Klionsky D.J. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baehrecke E.H. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 9.Paquette M., El-Houjeiri L., Pause A. mTOR pathways in cancer and autophagy. Cancers. 2018;10:18. doi: 10.3390/cancers10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi A.M.K., Ryter S.W., Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 11.Randall-Demllo S., Chieppa M., ERI R. Intestinal epithelium and autophagy: partners in gut homeostasis. Front Immunol. 2013;4 doi: 10.3389/fimmu.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das G., Shravage B.V., Baehrecke E.H. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harbor Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L., Wan F., Dutta S., Welsh S., Liu Z., Freundt E., Baehrecke E.H., Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiuri M.C., Le Toumelin G., Criollo A., Rain J.-C., Gautier F., Juin P., Tasdemir E., Pierron G., Troulinaki K., Tavernarakis N. Functional and physical interaction between bcl-x(l) and a bh3-like domain in beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho C.-W., Kim Y.-C., Rhee Y.K., Lee Y.-C., Kim K.-T., Hong H.-D. Chemical composition characteristics of Korean straight ginseng products. J Ethn Foods. 2014;1:24–28. [Google Scholar]
- 17.Kim J.H., Yi Y.-S., Kim M.-Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attele A.S., Zhou Y.-P., Xie J.-T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 19.Xue P., Yao Y., Yang X.-S., Feng J., Ren G.-X. Improved antimicrobial effect of ginseng extract by heat transformation. J Ginseng Res. 2017;41:180–187. doi: 10.1016/j.jgr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng M., Xin Y., Li Y., Xu F., Xi X., Guo H., Cui X., Cao H., Zhang X., Han C. Ginsenosides: a potential neuroprotective agent. BioMed Res Int. 2018;2018:11. doi: 10.1155/2018/8174345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahuja A., Kim J.H., Kim J.-H., Yi Y.-S., Cho J.Y. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 2018;42:248–254. doi: 10.1016/j.jgr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J.-T., Shao Z.-H., Vanden Hoek T.L., Chang W.-T., Li J., Mehendale S., Wang C.-Z., Hsu C.-W., Becker L.B., Yin J.-J. Antioxidant effects of ginsenoside re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Cho C.-W., Kim Y.-C., Kang J.-H., Rhee Y.K., Choi S.Y., Kim K.-T., Lee Y.-C., Hong H.-D. Characteristic study on the chemical components of Korean curved ginseng products. J Ginseng Res. 2013;37:349–354. doi: 10.5142/jgr.2013.37.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung K.W., Wong A.S.-T. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5 doi: 10.1186/1749-8546-5-20. 20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K.-T., Jung T.W., Lee H.-J., Kim S.-G., Shin Y.-S., Whang W.-K. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharmacol Res. 2011;34:1201. doi: 10.1007/s12272-011-0719-6. [DOI] [PubMed] [Google Scholar]
- 26.Saba E., Kim S.-H., Kim S.-D., Park S.-J., Kwak D., Oh J.-H., Park C.-K., Rhee M.H. Alleviation of diabetic complications by ginsenoside Rg3-enriched red ginseng extract in western diet-fed ldl–/– mice. J Ginseng Res. 2018;42:352–355. doi: 10.1016/j.jgr.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanan P., Subramaniyam S., Mathiyalagan R., Yang D.-C. Molecular signaling of ginsenosides Rb1, Rg1, andRrg3 and their mode of actions. J Ginseng Res. 2018;42:123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen L., Haas M., Wang D.Q.H., May A., Lo C.C., Obici S., Tso P., Woods S.C., Liu M. Ginsenoside Rb1 increases insulin sensitivity by activating AMP-activated protein kinase in male rats. Physiol Rep. 2015;3:e12543. doi: 10.14814/phy2.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang S., Ren D., Li J., Yuan G., Li H., Xu G., Han X., Du P., An L. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia. 2014;95:58–64. doi: 10.1016/j.fitote.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Song Y., Zhao F., Zhang L., Du Y., Wang T., Fu F. Ginsenoside Rg1 exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vitro. Fitoterapia. 2013;91:173–179. doi: 10.1016/j.fitote.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Kim M.K., Kang H., Baek C.W., Jung Y.H., Woo Y.C., Choi G.J., Shin H.Y., Kim K.S. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. J Ginseng Res. 2018;42:183–191. doi: 10.1016/j.jgr.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee I.-S., Uh I., Kim K.-S., Kim K.-H., Park J., Kim Y., Jung J.-H., Jung H.-J., Jang H.-J. Anti-inflammatory effects of ginsenoside Rg3 via Nf-κb pathway in a549 cells and human asthmatic lung tissue. J Immunol Res. 2016;2016 doi: 10.1155/2016/7521601. 7521601–7521601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang J.-H., Song K.-H., Woo J.-K., Park M.H., Rhee M.H., Choi C., Oh S.H. Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer activity by down-regulation of the IGF-1R/Akt pathway in breast cancer cells. Plant Foods Human Nutr. 2011;66:298. doi: 10.1007/s11130-011-0242-4. [DOI] [PubMed] [Google Scholar]
- 34.Duan Z., Deng J., Dong Y., Zhu C., Li W., Fan D. Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: in vitro and in vivo. Food Funct. 2017;8:3723–3736. doi: 10.1039/c7fo00385d. [DOI] [PubMed] [Google Scholar]
- 35.Cha K.-M., Kopalli S.R., Han S.Y., Lee S.-H., Jeong M.-S., Cho J.Y., Han C.-G., Lee S.-H., Kim S.-N., Kim J.-C. Korean red ginseng attenuates doxorubicin-induced testicular dysfunction in rats by modulating inflammatory, oxidative, and autophagy responses. J Funct Foods. 2018;40:736–743. [Google Scholar]
- 36.Park H.-H., Choi S.-W., Lee G.J., Kim Y.-D., Noh H.-J., Oh S.-J., Yoo I., Ha Y.-J., Koo G.-B., Hong S.-S. A formulated red ginseng extract inhibits autophagic flux and sensitizes to doxorubicin-induced cell death. J Ginseng Res. 2017 doi: 10.1016/j.jgr.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takanezawa Y., Nakamura R., Kojima Y., Sone Y., Uraguchi S., Kiyono M. Cytochalasin E increased the sensitivity of human lung cancer a549 cells to bortezomib via inhibition of autophagy. Biochem Biophys Res Commun. 2018;498:603–608. doi: 10.1016/j.bbrc.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Dupont N., Orhon I., Bauvy C., Codogno P. Chapter four – autophagy and autophagic flux in tumor cells. In: Galluzzi L., Kroemer G., editors. Methods in enzymology. Academic Press; 2014. pp. 73–88. [DOI] [PubMed] [Google Scholar]
- 39.Lim S.W., Jin L., Jin J., Yang C.W. Effect of exendin-4 on autophagy clearance in beta cell of rats with tacrolimus-induced diabetes mellitus. Sci Rep. 2016;6:29921. doi: 10.1038/srep29921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim S.W., Jin L., Luo K., Jin J., Yang C.W. Ginseng extract reduces tacrolimus-induced oxidative stress by modulating autophagy in pancreatic beta cells. Lab Invest. 2017;97:1271. doi: 10.1038/labinvest.2017.75. [DOI] [PubMed] [Google Scholar]
- 41.Kang S., Kim J.-E., Song N.R., Jung S.K., Lee M.H., Park J.S., Yeom M.-H., Bode A.M., Dong Z., Lee K.W. The ginsenoside 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol induces autophagy and apoptosis in human melanoma via AMPK/JNK phosphorylation. PLoS One. 2014;9:e104305. doi: 10.1371/journal.pone.0104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikoletopoulou V., Markaki M., Palikaras K., Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Vakana E., Altman J.K., Platanias L.C. Targeting AMPK in the treatment of malignancies. J Cell Biochem. 2012;113:404–409. doi: 10.1002/jcb.23369. [DOI] [PubMed] [Google Scholar]
- 45.Kim A.D., Kang K.A., Kim H.S., Kim D.H., Choi Y.H., Lee S.J., Kim H.S., Hyun J.W. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ling L.U., Tan K.B., Lin H., Chiu G.N.C. The role of reactive oxygen species and autophagy in safingol-induced cell death. Cell Death Dis. 2011;2:e129. doi: 10.1038/cddis.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao B-z, Han B-z, Zeng Y-x, Su D-f, Liu C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol Sin. 2016;37:150–156. doi: 10.1038/aps.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouimet M., Franklin V., Mak E., Liao X., Tabas I., Marcel Yves L. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W., Sultana N., Siraj N., Ward L.J., Pawlik M., Levy E., Jovinge S., Bengtsson E., Yuan X.-M. Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J Cell Mol Med. 2016;20:1664–1672. doi: 10.1111/jcmm.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razani B., Feng C., Coleman T., Emanuel R., Wen H., Hwang S., Ting Jenny P., Virgin Herbert W., Kastan Michael B., Semenkovich Clay F. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gottesman M.M. Mechanisms of cancer drug resistance. Ann Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 52.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 53.Mai T.T., Moon J., Song Y., Viet P.Q., Phuc P.V., Lee J.M., Yi T.-H., Cho M., Cho S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012;321:144–153. doi: 10.1016/j.canlet.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Balaburski G.M., Hontz R.D., Murphy M.E. P53 and ARF: unexpected players in autophagy. Trends Cell Biol. 2010;20:363–369. doi: 10.1016/j.tcb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Z., Chen C., Wu Z., Miao Y., Muhammad I., Ding L., Tian E., Hu W., Ni H., Li R. A dual role of p53 in regulating colistin-induced autophagy in PC-12 cells. Front Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00768. 768–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia T., Wang J.-C., Xu W., Xu L.-H., Lao C.-H., Ye Q.-X., Fang J.-P. 20(S)-ginsenoside Rh2 induces apoptosis in human leukaemia reh cells through mitochondrial signaling pathways. Biol Pharmaceut Bull. 2014;37:248–254. doi: 10.1248/bpb.b13-00667. [DOI] [PubMed] [Google Scholar]
- 57.Chung K.-S., Cho S.-H., Shin J.-S., Kim D.-H., Choi J.-H., Choi S.Y., Rhee Y.K., Hong H.-D., Lee K.-T. Ginsenoside Rh2 induces cell cycle arrest and differentiation in human leukemia cells by upregulating TGF-β expression. Carcinogenesis. 2013;34:331–340. doi: 10.1093/carcin/bgs341. [DOI] [PubMed] [Google Scholar]
- 58.Zhuang J., Yin J., Xu C., Mu Y., Lv S. 20(S)-ginsenoside Rh2 induce the apoptosis and autophagy in U937 and K562 cells. Nutrients. 2018:10. doi: 10.3390/nu10030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.