ABSTRACT
Gametocytes are the only form of the malaria parasite that is transmissible to the mosquito vector. They are present at low levels in blood circulation and significant knowledge gaps exist in their biology. Recent reductions in the global malaria burden have brought the possibility of elimination and eradication, with renewed focus on malaria transmission biology as a basis for interventions. This review discusses recent insights into gametocyte biology in the major human malaria parasite, Plasmodium falciparum and related species.
Keywords: Plasmodium falciparum, gametocyte, malaria, transmission
This review provides an update on our current understanding of the only parasite stage that can transmit malaria from man to mosquito, the gametocyte.
INTRODUCTION
Over the past decade, mass roll-outs of effective control tools for malaria have resulted in a significant reduction of malaria disease and death, as well as transmission rates in most endemic countries. Recently, this decline has stalled in many of these countries, and in some, a reversal of these gains is observed (WHO 2018). Importantly, the frontline antimalarials do not effectively clear gametocytes from infected people emphasising their unique biology compared to the asexual blood stage parasites (Delves et al. 2016; Bradley et al. 2019). Resurgence of human malaria and emergence of zoonotic infections emphasize the critical role of transmission for the persistence and spread of malaria in humans. Our knowledge about parasite transmission biology is mostly based on Plasmodium falciparum and the rodent malaria model Plasmodium berghei. By comparison, less research has been done on other parasites including Plasmodium vivax and Plasmodium knowlesi. However, major advances have been made in the past few years in our understanding of parasite transmission biology. Here, we discuss these recent findings with a focus on the only stage capable of transmission to mosquitoes, the gametocyte.
Plasmodium species relevant for human disease or research
Malaria parasites infect a variety of vertebrate hosts from mammals to birds and reptiles. The 6 human infective Plasmodium species of public health importance are P. falciparum, P. vivax, P. malariae, P. ovale curtisi, P. ovale wallikeri and P. knowlesi. Although P. falciparum malaria is the most lethal form that causes the bulk of morbidity and mortality, P. vivax malaria is the most prevalent form of disease outside of Africa and will persist once P. falciparum has been eliminated. In the past decade, P. knowlesi has emerged as a significant source of zoonotic infections in Southeast Asia (Singh and Daneshvar 2013), probably through increased contact of humans with the natural host and vector system. In addition to the canonical human malaria species, Plasmodium cynomolgi has been reported to infect humans asymptomatically in western Cambodia (Imwong et al. 2019) and P. brasilianum in the Venezuelan Amazon (Lalremruata et al. 2015). Plasmodium cynomolgi has been used as an in vivo model for P. vivax in non-human primates. Similarly, P. knowlesi has been established as an in vitro model for P. vivax. The most popular in vivo malaria models are, however, rodent malaria parasites used for different aspects of disease and immunological studies: P. berghei is the most studied of these parasites, as it can also be used to induce experimental cerebral malaria, thus modelling an important clinical complication of P. falciparum infection. The P. berghei model is also characterised by the ability to very efficiently generate all parasite life stages under laboratory conditions and its ease of genetic modification. It has therefore been very useful to elucidate many aspects of parasite infection in vivo including mosquito transmission. Recently P. falciparum gametocyte development has been established in the humanised mouse model as a system to study some features of gametocytogenesis in vivo (Duffier et al. 2016).
Phylogeny of the Plasmodium genus
Over the past decade, major genome sequencing projects have been completed providing a large library of reference genomes from different Plasmodium species across the major lineages (e.g. Gardner et al. 2002; Carlton et al. 2008; Otto et al. 2014; Rutledge et al. 2017). Subsequent comparative genomic analyses have started to clarify the evolutionary relationships between Plasmodium species. The first such analysis was done between the P. falciparum whole genome and P. yoelii yoelii partial genome, revealing a high level of synteny across the two species especially in the central chromosome regions (Carlton et al. 2002). The most recent phylogenomic analysis across Plasmodium confirms the ancient branching of bird and reptile parasites compared to all parasites infecting mammals (Bohme et al. 2018). In this analysis, rodent malaria parasites form a sister clade with the primate parasite lineage (represented by P. knowlesi and P. vivax) and the P. ovale lineage, whilst Laverania (the group of primate parasites including P. falciparum) and P. malariae are placed closer to the base (Fig. 1). Interestingly, a recent genetic analysis (analyzing 21 genetic loci) of the sister lineages Plasmodium and Haemoproteus including a large number of parasites infecting bats suggests that Plasmodium is polyphyletic and forms several independent clades (Laverania, rodent parasites, bird and reptile parasites, primate parasites and P. malariae/P. ovale) within the Haemoproteus lineage (Galen et al. 2018). These studies highlight the complicated evolutionary past of malaria parasites and suggest switches between and adaptations to host and vector. This is of particular relevance for parasite transmission, as some of the key aspects of this process appear to differ between Plasmodium lineages, although the overall structure of the life cycle remains the same.
Figure 1.
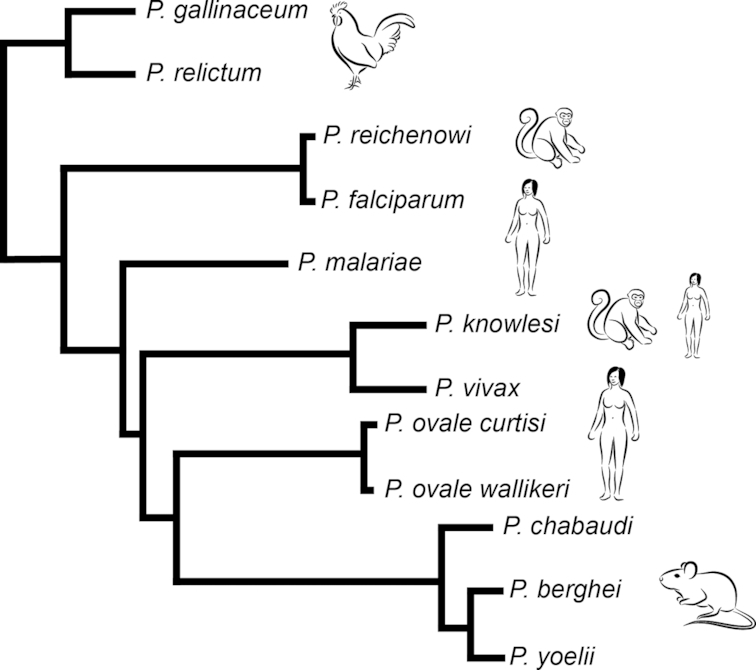
Phylogenetic representation of Plasmodium lineages. Schematic of phylogenetic tree based on most recent genome information across Plasmodium lineage. The tree is adapted from the recent publication by Boehme et al. (2018). The data show the major Plasmodium lineages: bird (P. gallinaceum, P. relictum) parasites are at the base of the tree whilst Laverania (including P. falciparum and P. reichenowi) are a sister group to other Plasmodium within the clade of mammalian parasites. The latter include rodent malaria parasites (P. berghei, P. chabaudi, P. yoelii) as well as primate (P. knowlesi) and human parasites (P. ovale curtisi and P. ovale wallikeri, P. vivax andP. malariae).
The Plasmodium life cycle
All malaria parasites leave the insect host through an infective bite by which they enter the vertebrate host and initiate the pre-erythrocytic developmental stage. In all mammalian species, this pre-erythrocytic stage occurs within the liver, where parasites invade hepatocytes, develop within the parasitophorous vacuole (PV) and produce tens of thousands of red blood cell (RBC) infective merozoites in a process called schizogony. Some species, such as P. vivax, P. ovale and P. cynomolgi, produce a latent phase (hypnozoite) during the pre-erythrocytic stage that remains in hepatocytes for prolonged durations (up to years). Notably, avian malaria parasites make hypnozoites during both the pre-erythrocytic cycle and in the erythrocytic cycle (Valkiunas 2005). Latent stages can be activated and initiate an infection, complicating P. vivax control strategies. From the pre-erythrocytic cycle, RBC-infective merozoites emerge and initiate the blood stage (erythrocytic) cycle of the parasite. After invasion of RBCs, these merozoites develop within a PV and undergo schizogony to produce daughter merozoites, which burst out of the host cell and reinvade new RBCs to perpetuate this asexual replication cycle. This asexual cycle causes all the symptoms associated with malaria infection and it can result in RBC infection rates >10% in malaria patients. The length of the intraerythrocytic cycle and the number of merozoites generated is species-specific. In some species, including P. berghei and P. vivax, merozoites preferentially invade young erythrocytes, increasing the likelihood of anemia (Cromer et al. 2006; Malleret et al. 2015).
A subset of asexually replicating parasites will produce gametocyte progeny and initiate the sexual cycle. Gametocytes develop in the intermediate vertebrate host until maturation before they are taken up by an arthropod. A series of recent studies suggest that in some species only mature gametocyte stages are present in the blood circulation, whilst immature gametocytes sequester in host tissues, particularly bone marrow and spleen (Joice et al. 2014; De Niz et al. 2018; Lee, Waters and Brewer 2018; Obaldia et al. 2018). Once ingested by the vector, the sexual reproduction phase of the life cycle occurs within the insect's midgut, allowing for recombination and generation of variation within the parasite population. During gametogenesis, the male gametocyte divides into up to eight flagellated microgametes, whereas the female gametocyte develops into a single macrogamete. Fertilization of a macrogamete by a microgamete results in the formation of a zygote that undergoes meiosis and develops into an ookinete, a motile form with apical organelles. The ookinete penetrates the mosquito gut wall and comes to rest near the basal lamina of the midgut. Here the ookinete rounds up and transforms into an oocyst, within which the parasite asexually replicates, forming several thousand sporozoites (sporogony). Upon oocyst rupture, these sporozoites migrate to and invade the salivary glands, where they can be transmitted back to the vertebrate host during a blood meal.
Phases of the gametocyte developmental cycle
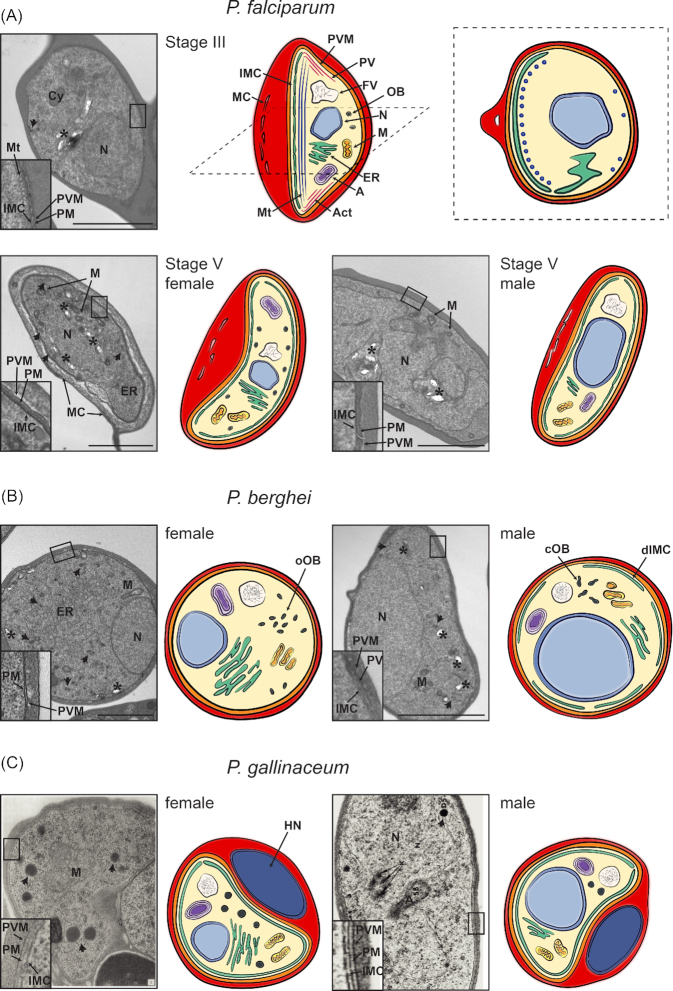
Most parts of the Plasmodium transmission cycle are conserved across the genus (Fig. 2); however, there are notable differences in cycle length and morphology between lineages. The conserved features enable utilization of animal models for in vivo studies of transmission biology. The first developmental stage that is functionally different from an asexual parasite is the sexually committed schizont. Commitment to gametocytogenesis is initiated by the activation of the transcription factor AP2-G, both in rodent parasites and P. falciparum (Kafsack et al. 2014; Sinha et al. 2014). Consistent with a previous report (Bruce et al. 1990), a recent study has demonstrated that P. falciparum schizonts produce either asexually or sexually committed progeny (Brancucci et al. 2018). Interestingly, conditional activation of AP2-G can produce mixed progeny, both in P. falciparum and P. berghei (Bancells et al. 2019; Kent et al. 2018). Upon invasion, the earliest phases of gametocyte development are morphologically indistinguishable from asexual development. Subsequently, the gametocytes of P. falciparum undergo five morphologically discernible stages of development in the course of 9–12 days (Hawking, Wilson and Gammage 1971). Stage I gametocytes have a very similar appearance as asexual trophozoites and cannot be distinguished morphologically; however, they can be identified using genetic reporters, as their transcriptome begins to differ from the one of asexual parasites. In stage II, slight changes in appearance become apparent, as the parasite takes the shape of a lemon or oat grain with one pointed end. The parasite then elongates whilst one side flattens and the opposite membrane curves, so that in stage III the shape of the parasite resembles the letter ‘D’ (Fig. 2A). The length of the parasite exceeds the diameter of the RBC, which exhibits signs of deformation. Developing into stage IV, the parasite elongates even more, now resembling the shape of a banana. The RBC cytoplasm is almost completely occupied with the parasite, except for a small membrane fold known as the Laveran's bib. Stage V gametocytes exhibit the characteristic crescent shape with rounded ends, as opposed to the pointed ends of stage IV gametocytes (Sinden 1982)(Fig. 2A). Apart from the likely conservation in Laverania, the morphological features of P. falciparum gametocytes are not conserved across the Plasmodium lineage: most gametocytes from rodent, bird and primate parasites are round and resemble the trophozoite stage (Sinden et al. 1978; Walzer et al. 2018)(Fig. 2B and C). The 9–12 day maturation time for P. falciparum gametocytes is exceptionally long, whereas the development in other primate, avian and rodent Plasmodium species ranges from 24 to 60 hours (Gautret and Motard 1999). In addition following development, and in contrast to species with shorter developmental periods that survive for less than 12 hours in circulation, mature P. falciparum gametocytes can survive for several days before being taken up by a mosquito (Smalley and Sinden 1977; Gautret and Motard 1999; Eichner et al. 2001).
Figure 2.
Morphological features of gametocytes across Plasmodium lineages. Shown are representative electron microscopy images and drawings of gametocytes of (A) human (P. falciparum), (B) rodent (P. berghei) and (C) avian (P. gallinaceum) malaria parasites. Mitochondria (M), apicoplast (A), nucleus (N), endoplasmatic reticulum (ER) and the food vacuole (FV) containing hemozoin crystals (marked with an asterisk) are found in all Plasmodium species in both male and female gametocytes. The gametocyte plasma membrane (PM) is in close association with the parasitophorous vacuolar membrane (PVM), and in most cases with the IMC (depicted in the closeup insets of the electron micrographs). Whilst the nucleus is smaller and more compact in female compared to male gametocytes, the ER is more developed in female gametocytes, in line with increased translation. Osmiophilic bodies (OB, marked with an arrow) are found in all species, with a higher number in female than in male gametocytes. (A) Ultrastructure of immature stage III and mature stage V P. falciparum gametocytes. Upper panel: Electron micrograph (left) and drawing (middle: longitudinal section, right: cross-section) of a stage III gametocyte. The IMC develops along one side of the gametocyte, supported by underlying microtubules (Mt)(depicted in the inset). Some microtubules are also found on the opposing side (see cross-section). Additionally, actin filaments (Act) are found, in particular at the tips of the developing gametocyte. MC are found within the erythrocyte cytoplasm. Lower panel: Electron microscope image and drawing of a female (left) and male (right) stage V gametocyte. The IMC plates now completely surround the gametocytes whilst actin and microtubules are absent. In the female gametocyte osmiophilic bodies are located along the periphery of the cell. In contrast they have not been described in male P. falciparum gametocytes. (B) Electron micrograph and drawing of mature female (left) and male (right) P. berghei gametocytes. Osmiophilic bodies are oval (oOB) and more abundant in female gametocytes, whilst male gametocytes have fewer and club-shaped osmiophilic bodies (cOB). The IMC is difficult to observe in female gametocytes and possibly absent, whilst a discontinuous IMC (dIMC) is clearly detectable in male P. berghei gametocytes (see insets) (Mons 1986). (C) Electron micrograph and scheme of mature female (left) and male (right) P. gallinaceum gametocytes. Electron micrographs are taken from (Aikawa et al. 1969)(female) and (Sterling and Aikawa 1973)(male) [PERMISSION PENDING]. In contrast to mammalian mature RBCs, avian mature RBCs are nucleated (depicted as host nucleus (HN)).
Regulation of gametocytogenesis
Sexual commitment is a facultative step in the malaria parasite cycle, and the rate of commitment is responsive to environmental cues. Similarly, gametocyte activation and subsequent gametogenesis depend on environmental cues presented upon parasite ingestion by the mosquito. Therefore, various steps in the transmission cycle are highly regulated and finely tuned at multiple levels. Gametocytes represent only a small fraction of the parasite population both in vitro and during infection, and rates of commitment vary (see above), representing a major barrier for their systematic investigation. Several environmental factors, including drugs, host cells and extracellular vesicles have been proposed to lead to sexual commitment of asexual blood stage parasites (Josling, Williamson and Llinas 2018). Whilst stage-specific effects of certain drugs may result in an increased sexual conversion rate, recent data do not support a physiological role for host cell age or extracellular vesicles in commitment (Brancucci et al. 2017). In contrast, a class of phospholipids abundant in serum, lysophosphatidylcholines (LysoPC), has been identified as sufficient to repress the gametocyte commitment at physiological levels in P. falciparum (Brancucci et al. 2017). LysoPC breakdown products are used for phospholipid biosynthesis via the Kennedy pathway in the parasite and blocking this pathway can induce sexual commitment (Brancucci et al. 2018). Altogether these data strongly suggest that the abundance of environmental sensors regulates both parasite metabolism and sexual commitment.
Parasites adapt to new host environments in mammalian host and arthropod vector in order to optimise the balance between persistence and transmission. Recent studies have shown that different Plasmodium species are able to detect fluctuations in nutrient availability and modify gene expression accordingly. In P. falciparum, limited LysoPC availability is translated into changes in rates of growth and sexual reproduction, whilst glucose sensing only affects growth. In rodent malaria parasites, both LysoPC and glucose sensing are only linked to growth but not transmission (Brancucci et al. 2017; Mancio-Silva et al. 2017), suggesting that there are significant differences in how these two parasite lineages respond to environmental changes. Notably, a recent study has also shown that P. falciparum parasites produce a higher proportion of gametocytes in low transmission settings and more asexual progeny in high transmission settings (Rono et al. 2018). These studies demonstrate that there are epigenetic and genetic differences between and within parasite species that result in distinct responses to environmental cues, as has been demonstrated for the variant expression of components of the Plasmodium surface anion channel (PSAC) on the iRBC surface (Nguitragool et al. 2011). It is likely that genetic differences also account for the adaptation of gametocytes in different host environments; however, systematic investigation of such phenotypes is currently lacking.
Genetics of gametocyte development
Initial studies of the genetics of gametocytogenesis relied on parasites which have lost the ability to produce gametocytes when maintained in in vitro culture. Indeed, gene loss or amplification can occur during culture adaptation, in particular if resulting in a fitness advantage such as loss of gametocyte production. Several P. berghei (Sinha et al. 2014) and P. falciparum (Alano et al. 1995; Eksi et al. 2012) parasite lines have lost the ability to form gametocytes during continuous blood passage in the mouse (P. berghei) and in vitro (P. falciparum) due to loss of specific chromosomal loci. Importantly, whole genome sequencing identified mutations in the ap2-g locus in most instances (Kafsack et al. 2014; Sinha et al. 2014; Claessens et al. 2017), demonstrating that loss of AP2-G function results in a fitness advantage under optimised in vitro conditions and during propagation in the mouse. Further functional studies identified AP2-G as the key switch initiating the sexual commitment process in both P. falciparum and P. berghei (Kafsack et al. 2014; Sinha et al. 2014). Intriguingly, two recent studies using inducible expression systems have demonstrated that conditional ap2-g activation in ring stage parasites alone results in reprogramming of these cells into gametocytes (Bancells et al. 2019; Kent et al. 2018), without passing through the committed schizont stage. The physiological relevance of this alternative sexual commitment process is unclear. Another gene, gdv-1 has been identified via genome sequencing of a gametocyte-deficient P. falciparum line (Eksi et al. 2012). Recent data suggest that GDV-1, possibly regulated by its own anti-sense transcript is essential for activating AP2-G (Filarsky et al. 2018), moving the unknown trigger further upstream. Interestingly, the gdv-1 locus has been lost in the rodent malaria lineage (Brancucci et al. 2017)(see also Fig. 3), suggesting that alternative pathways of the AP2-G activation may be available in this lineage. It is currently unknown how LysoPC levels translate into gdv-1 and ap2-g activation and, hence, what the upstream trigger regulating commitment is.
Figure 3.
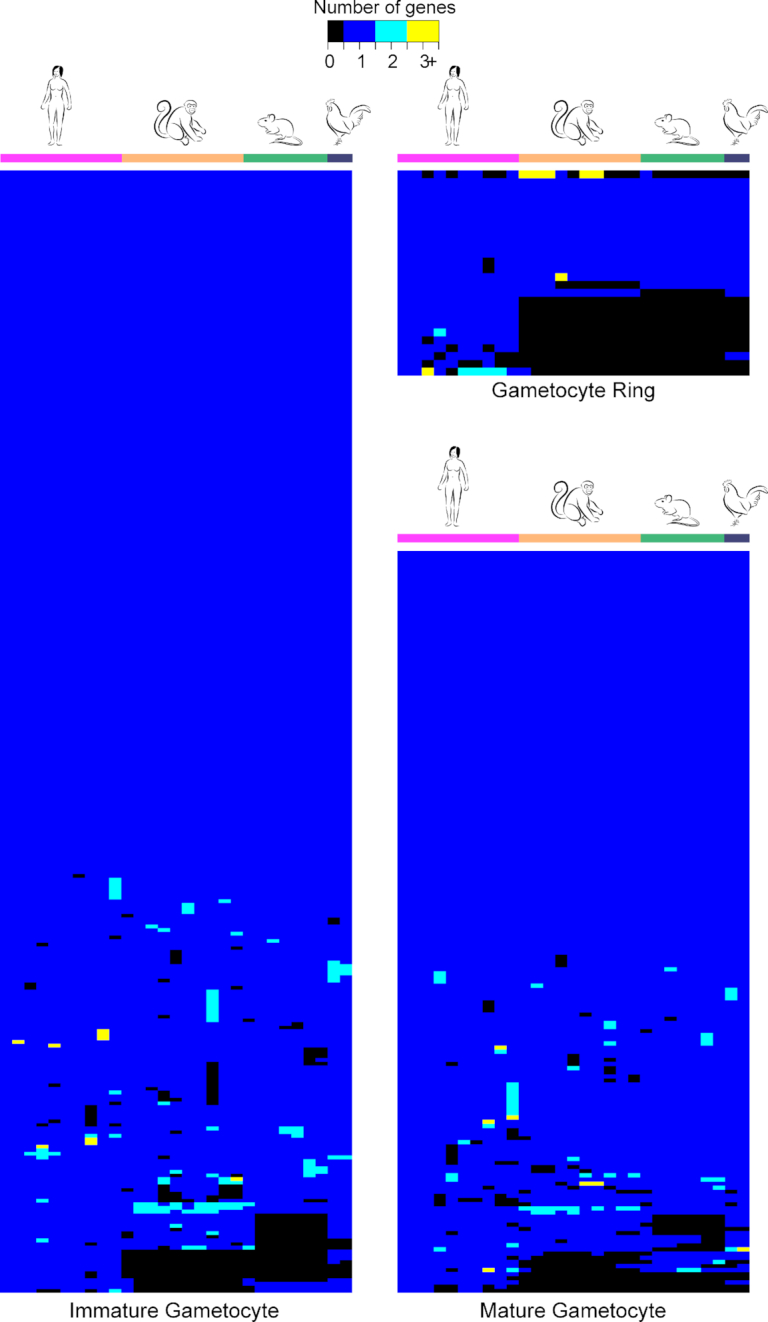
Comparative genomic view of gametocytogenesis across Plasmodium lineages. Heatmap showing the number of orthologous genes annotated as expressed specifically in P. falciparum gametocytes as defined by Pelle et al. (2015)(in rows) in major Plasmodium lineages. Apart from P. falciparum we have included P. vivax, P. berghei and P. gallinaceum. The orthologues of the P. falciparum gametocyte genes were retrieved from PlasmoDB (Aurrecoechea et al. 2009). The genes (rows) were reordered by hierarchical clustering using the R function hclust (www.R-project.org). Using P. falciparum as a template (as there are no other species-specific sets of gametocyte genes) the majority of gametocyte-specific genes is conserved across the 4 species (see also Supplementary Table S1 (Supporting Information)). Most P. falciparum-specific genes in the gametocyte ring encode exported proteins. Interestingly, a putative zinc finger protein (PF3D7_1 134 600) has been lost in the rodent lineage only. Only a few immature and mature gametocyte genes are unique to P. falciparum, and most of them encode hypotheticals. Amongst the few exceptions are two putative protein kinases that have been selectively lost in primate (PF3D7_031 1400) and rodent malaria parasites (PF3D7_020 3100), respectively.
Additional loci have been identified that are commonly lost in long term culturing experiments, but their putative role in gametocytogenesis remains to be investigated (Claessens et al. 2017). An initial study exploiting the piggybac transposon system in P. falciparum enabled identification of additional genes important for formation and development of gametocytes (Ikadai et al. 2013). Amongst parasite mutants with defective gametocyte production, 16 unique loci were identified–9 genes interfered with formation of gametocytes and 7 with gametocyte development. More recently, a genome-wide knock-out study in P. berghei (Bushell et al. 2017) and saturation mutagenesis using piggybac in P. falciparum (Zhang et al. 2018) have provided a first map of essential genes in Plasmodium. As expected, the proportion of essential genes is significantly lower amongst gametocyte genes compared to the rest of the genome (our own analysis; Pvalue < 1.5e-15, one-tailed Pearson's χ2). In contrast to the recent genetic dissection of sexual commitment, very little progress has been made to systematically investigate genetic determinants of gametocyte development. Most of the genes identified as selectively expressed during gametocyte development in P. falciparum are conserved between Plasmodium species (Fig. 3). A small subset of these genes appears to be lineage specific: genes specific to the Laverania subgenus expressed in the gametocyte ring stage are mainly annotated as exported proteins (see Supplementary Table S1, Supporting Information), whilst the function of most of the other lineage-specific genes is still unknown.
Gene regulation during gametocytogenesis
Early studies have suggested that in Plasmodium gene expression is a tightly regulated cascade such that a gene is expressed just in time when the protein is required (Bozdech et al. 2003). This paradigm has meanwhilst been corroborated through identification and functional investigation of a family of transcription factors of the ApiAP-2 family (Coulson, Hall and Ouzounis 2004), including AP2-G. Moreover, a series of studies has demonstrated that many processes in response to environmental changes, such as antigenic variation, invasion switching and sexual commitment are also under strong epigenetic control (e.g. Stubbs et al. 2005; Voss et al. 2006; Brancucci et al. 2014; Coleman et al. 2014; Fraschka et al. 2018). For example, in most Plasmodium species studied so far, virulence genes are localised in the subtelomeric regions and in a few chromosome internal clusters, tracking with the genomic distribution of heterochromatin patterns (Fraschka et al. 2018). A few additional loci are similarly heterochromatic in asexual parasites and the only one conserved across Plasmodium encodes ap2-g (Fraschka et al. 2018), supporting its essential role in sexual commitment across species and suggesting that epigenetic regulation of this master switch is highly conserved. A series of single cell transcriptomics studies provided a first glimpse into the transcriptome of asexually and sexually committed schizonts (Poran et al. 2017; Brancucci et al. 2018). Apart from ap2-g, these studies identified a number of merozoite antigens with unique expression in sexually committed schizonts, including MSRP1 and DBLMSP2. These data suggest that the sexual merozoites membrane may be functionally distinct from their asexual counterparts. It is tempting to speculate that such differences relate to altered tissue or host cell tropism between asexual and gametocyte stages. Indeed, both P. berghei and P. falciparum gametocytes preferentially develop in the extravascular space of the spleen and bone marrow, whilst asexual stages do not show such a preference (Joice et al. 2014; De Niz et al. 2018; Lee, Waters and Brewer 2018).
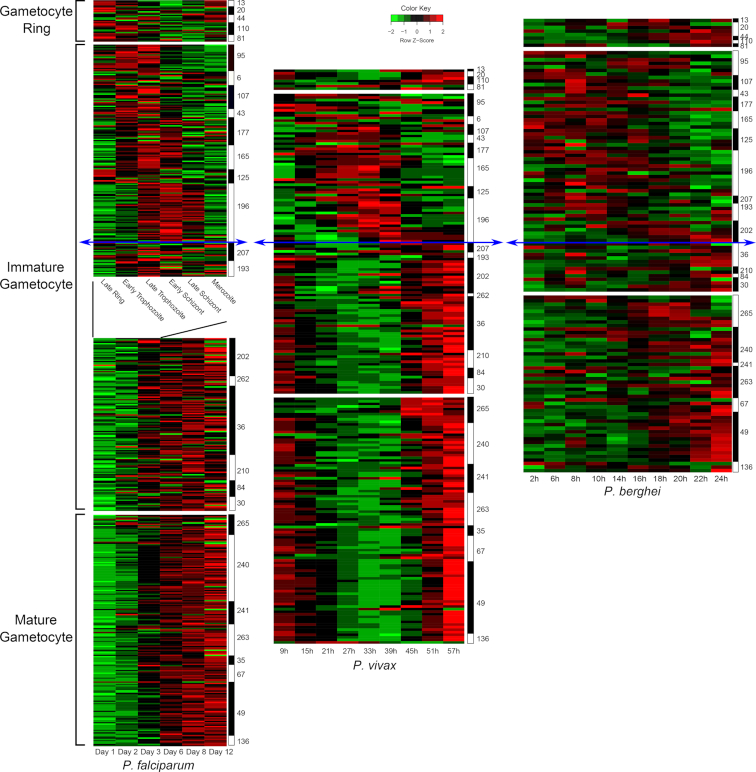
Activation of ap2-g expression in a subset of schizonts triggers expression of a series of early gametocyte genes including Pfs16, Pfg27/25, Pfg14.744, Pfg14.745 and Pfg14.748 (Kafsack et al. 2014) that are needed in the gametocyte progeny. Many of the genes activated in response to AP2-G are required for early gametocyte development, including several members of the PHIST family (Eksi et al. 2005). In contrast, genes involved in asexual host cell remodeling, including components of the knob complex required for antigen display on the iRBC surface are epigenetically silenced during sexual stages (Fraschka et al. 2018). As in the asexual cycle, it is anticipated that gene expression during gametocyte development is tightly synchronised and coordinated. Notably, many factors required for parasite development in the mosquito stage are transcribed in gametocytes and the mRNAs are stored via translational repression (Mair et al. 2006; Guerreiro et al. 2014), providing an additional level of expression regulation. Unfortunately, the only P. falciparum time course data available are more than a decade old and the samples used contain mixtures of asexual and gametocyte stages (Eksi et al. 2005; Young et al. 2005). Nevertheless, one of the time courses was used, in conjunction with a large array of expression data across the entire parasite cycle, to deconvolve these mixtures by generating a transcriptional network of P. falciparum gene expression (Pelle et al. 2015)(Fig. 4). The network predicts 29 expression clusters peaking at different phases during the gametocyte cycle and probably provides the most accurate analysis of transcriptional dynamics in gametocytes to date. Most recently, both transcriptional and proteomic data were combined to generate a short list of high confidence gametocyte markers in P. falciparum (Meerstein-Kessel et al. 2018). Also, artificial ap2-g activation and reprogramming within the same cycle was used for transcriptional profiling of gametocyte development in P. berghei (Kent et al. 2018). Altogether these data demonstrate that virulence and transmission traits are tightly controlled and interlinked in Plasmodium.
Figure 4.
Transcriptional profiles across gametocyte development and Plasmodium lineages. Time-course data (Log2 Fold change) of gametocyte-specific genes were extracted from previously published datasets. For P. falciparum data are used from an asexual time course (Le Roch et al. 2003)(top part, in hours) and a gametocyte time course (Young et al.2005)(bottom part, in days). For P. vivax, data are used from an ex vivo time course (Bozdech et al. 2008)(in hours), and for P. berghei data are from an in vivo time course (Hoo et al. 2016)(in hours). Comparing the temporal transcription of the gametocyte-specific genes as defined in Pelle et al. and their orthologues in P. berghei and P. vivax shows that their profile is qualitatively conserved. The initial phase of development from the gametocyte ring stage to early immature gametocyte development shows a similar cascade of gene expression and takes a similar amount of time across the three species (20–39 hours). In contrast further gametocyte maturation lasts significantly longer in P. falciparum (9–10 days) than in P. vivax (about 17–20 hours) and P. berghei (about 4 hours). These differences in maturation time are likely related to the physiological differences in their respective hosts. The numbers on the right side indicate the cluster number as defined previously (Pelle et al. 2015). The blue arrows separate the time courses between the initial stages of development (top) with conserved duration and the later stages with variable in time scale.
Metabolic adaptations during gametocytogenesis
Most metabolic pathways are similar between parasite and its host, but there are key differences in the enzymes involved. Plasmodium possesses most pathways for de novo biosynthesis of important metabolites (curiously, several have been lost in the rodent malaria lineage (Frech and Chen 2013)) but heavily relies on salvaging nutrients from host cells. As the parasite encounters different environments within and between hosts, it must cope with the different physiological environments in the various tissues and hosts. Nutrient sensing thereby links parasite metabolism (e.g. salvage vsde novo biosynthesis) to cell fate (e.g. replication vs differentiation). For example, replicating parasites have different requirements for energy metabolism than gametocytes, and the two stages may develop in different environmental niches and host cells.
Proliferative parasites rely on glycolysis to sustain high energy requirements for generating large numbers of progeny. However, Plasmodium parasites are unable to generate glucose or catabolise glycogen and, therefore, they rely on glucose from the host cells. Within the vertebrate host blood glucose levels are maintained at constant rate and provide the parasite a stable source for glycolysis. In turn parasites produce lactic acid as a byproduct, which can cause lactic acidosis during malaria infection. Plasmodium also lack the gene encoding the mitochondrial pyruvate dehydrogenase; however, both asexual stages and gametocytes catabolise both glucose and glutamine in a canonical TCA cycle for energy production (MacRae et al. 2013). Whilst asexual parasites rely mostly on glucose uptake and aerobic glycolysis for energy, gametocytes mostly use the mitochondrial TCA cycle for energy production (MacRae et al. 2013), probably due to their sequestration outside of the blood stream. It is confirmed by the fact that the deletion of the enzymes of this cycle allows for asexual replication but tends to be deleterious for gametocyte formation (Ke et al. 2015). Interestingly, African Trypanosomes show a similar pattern of energy usage: proliferating slender forms perform glycolysis whilst non-proliferating stumpy forms required for transmission use the TCA (Giffin and McCann 1989). Gametocytes possess larger mitochondria with tubular cristae (Okamoto et al. 2009), and a majority of mitochondrial TCA cycle enzymes are upregulated in gametocytes (Khan et al. 2005; Young et al. 2005). Male gametes have no mitochondria (Okamoto et al. 2009) and utilize glycolysis for motility (Talman et al. 2014), whilst female gametes as well as ookinetes maintain TCA use. Of note, some Plasmodium parasites including P. vivax and to a lesser extent P. berghei are restricted to reticulocytes that are metabolically active and have a functional TCA cycle maintained by glutamine as the main carbon source (Srivastava et al. 2017). Because of their enrichment in hematopoietic niches, where erythroid cells are predominant, gametocytes can potentially exploit carbon reserves in these cells in addition to utilisation of TCA for energy metabolism.
Lipids are essential in Plasmodium for signaling, protein trafficking, hemoglobin degradation and membrane biosynthesis. Lipid metabolism is an essential process of the parasite, which involves uptake from host cells, transport and synthesis from fatty acids (FA). Plasmodium parasites are capable of obtaining FA from host plasma for lipid synthesis (Grellier et al. 1991; Ofulla et al. 1993). Alternatively they can synthesise them de novo using a (type II) fatty acid biosynthesis pathway (Tarun, Vaughan and Kappe 2009). This process is dispensable in asexual blood stages but crucial in mosquito and liver stages (Yu et al. 2008; Vaughan et al. 2009; van Schaijk et al. 2014), whilst there are currently no data for gametocytes. However, there are significant differences in the lipid composition of asexual stages and gametocytes (Gulati et al. 2015). Overall phospholipid and glycerolipid levels are decreased in gametocyte stages, probably as a result of altered substrate utilization following the switch to gametocytogenesis (Brancucci et al. 2017). Gametocytes may require less lipids for membrane biosynthesis compared to the proliferative asexual stages. Interestingly, sphingolipids, and in particular ceramide levels, are increased in gametocytes and essential for their maturation (Gulati et al. 2015; Tran et al. 2016).
Emerging roles for IMC and cytoskeleton during gametocyte development
A common feature of the Alveolates—which include the Dinoflagellates, Ciliates and Apicomplexa—are the alveoli, flattened membrane sacs beneath the plasma membrane (Gould et al. 2008). In Apicomplexa, the alveoli are usually referred to as inner membrane complex (IMC). Together with the plasma membrane, alveoli form the so-called pellicle, a structure that is closely associated with cytoskeletal elements. The most conspicuous function of the pellicle is to generate and maintain cell shape, such as funnel shaped Tintinnids in Ciliates, crescent shaped sporozoites in Plasmodium, and the horned appearance of the Ceratium species (Dinoflagellates). Furthermore, the pellicle serves as an anchor and scaffold for various parasite structures. For example, gliding motility—a hallmark of apicomplexan locomotion required for various migration processes—is facilitated by the glideosome, consisting of an actin-myosin motor complex anchored to the IMC and the plasma membrane (Baum et al. 2006). The pellicle also harbours the apical complex that Apicomplexan parasites require for host cell invasion and in some cases egress. In P. falciparum parasites, the IMC is only present in merozoites, sporozoites, ookinetes and gametocytes. Whilst merozoites, sporozoites and ookinetes actively invade or traverse host cells, and sporozoites and ookinetes exhibit gliding motility, active motility or invasion has not been conclusively shown in gametocytes. Thus, the IMC in this stage seems to predominantly play a structural role; however, recent studies imply that motility might be a feature of gametocytes as well (De Niz et al. 2018).
IMC and microtubule dynamics during gametocyte development
In contrast to gametocytes, merozoites, sporozoites and ookinetes possess a polar ring at the apical end that functions as a microtubule organising centre (MTOC). In these stages, microtubules originate at the polar ring and grow towards the basal end of the cell, whilst at the same time IMC membranes spread over the cell body, finally engulfing it. In P. falciparum gametocytes IMC assembly is initiated by unknown factors and starts in stage I/II gametocytes with the deposition of a single patch of vacuolar membrane and microtubules underneath the parasite membrane (Sinden 1982; Dearnley et al. 2012; Dearnley et al. 2016)(Fig. 2A). Additional membrane sacs, originated from the endoplasmic reticulum (ER), are deposited at the periphery along one side of the developing gametocyte in a string of beads pattern. This side will form the ‘foot’, the flattened membrane of the stage III gametocyte. Even though gametocytes, as opposed to the motile stages of P. falciparum, do not possess an apical-basal polarity (Parkyn Schneider et al. 2017), they are clearly laterally polarised during their development. Whether this polarisation has any functional consequences, however, remains unknown.
Microtubules are present underneath the membrane sacs, indicating that they nucleate and polymerise at the developing IMC membranes. However, it is also possible that microtubule polymerisation is mediated by unknown factors in these regions and triggers or attracts the deposition of IMC membranes (Parkyn Schneider et al. 2017). Further experiments are needed to test this hypothesis, especially live cell imaging that facilitates a better temporal resolution of cellular processes. Whilst microtubule bundles are concentrated along the nascent IMC plates, they also start extending through the cytoplasm in early stage II gametocytes. Further microtubule polymerisation accompanied by the extension of the IMC plates then leads to the elongation of the gametocyte ‘foot’, giving it the characteristic D-shape of stage III gametocytes. In stage III gametocytes, the IMC plates start to extend laterally to finally engulf the gametocyte almost entirely in stage IV. During the lateral extension of the IMC, the division of the IMC into individual plates becomes very apparent. Proteinaceous sutures connect the plates that are approximately 800 nm wide. The sutures start forming as soon as IMC plates are deposited in stage I-II gametocytes (Kono et al. 2012). Apart from connecting the IMC plates, the sutures are believed to play a role in the parallel alignment of microtubules along the longitudinal axis of the gametocyte, as is apparent in stage IV gametocytes (Meszoely et al. 1987; Kaidoh et al. 1993). In stage V gametocytes, the microtubule network is disassembled and only patches of short microtubule bundles remain that are associated with the IMC (Parkyn Schneider et al. 2017). This leads to rounding up of the gametocyte tips and a relaxation of the IMC membranes, where the sutures are now less pronounced. The disassembly of the microtubule network coincides with a steep increase in cellular deformability compared to stage IV and younger gametocytes (Aingaran et al. 2012; Dearnley et al. 2012; Tiburcio et al.2012a). However, it has recently been shown that microtubules are not the only, or not even the major structural determinant to gametocyte cellular rigidity. Instead, rearrangements of the RBC cytoskeleton play a dominant role in the deformability switch (Dearnley et al. 2016). Functionally, this high deformability allows mature gametocytes to leave their site of sequestration in the bone marrow or spleen and to enter the circulation to be taken up by a mosquito, as shown in the case of P. berghei (De Niz et al. 2018).
Whilst IMC development in gametocytes has been extensively studied in P. falciparum, less is known about it in other Plasmodium species. Based on early electron microscopy studies, it appears that the gametocytes of most Plasmodium species, including avian and reptilian malaria parasites, possess an IMC (Aikawa, Huff and Sprinz 1969). However, some species, e.g. P. berghei (Olivieri et al. 2015) and P. knowlesi (Aikawa, Huff and Sprinz 1969) exhibit discontinuities within the IMC, i.e. the IMC does not completely engulf the gametocyte (see also Fig. 2B). Interestingly, in P. berghei the discontinuous IMC is easily detected in male gametocytes, whilst in female gametocytes it may be absent (Mons 1986). As the main function of the IMC in gametocytes seems to be forming and stabilising the shape of the cell, Plasmodium species with round gametocytes might have less requirement for a stable IMC. Further systematic ultrastructural and light microscopy studies of different Plasmodium species will be required to learn more about the IMC and its function(s) in gametocytes.
Different roles for actin in gametocytes?
Plasmodium expresses two distinct actin isoforms, Actin-I and Actin-II. Actin-I is expressed throughout the whole life cycle whilst Actin-II is only expressed in the mosquito stages, gametocytes (mainly males) and gametes (Wesseling et al. 1989). A recent study has investigated Actin-I localisation through gametocyte development in P. falciparum. It was shown that actin forms filaments that are present underneath the microtubules and can be closely associated with them. These filaments appear very stable as they cannot be depolymerised using the actin polymerisation inhibitor Cytochalasin D. Throughout gametocyte development, actin accumulated at the opposing ends of the cell (Fig. 2A) and co-localised with the actin nucleation factor formin-1, indicating that these are the sites of actin polymerisation. In contrast to the actin filaments associated with microtubules, these filaments are sensitive to treatment with Cytochalasin D. The function of the actin network in gametocytes is not fully understood, but it appears that it is required for maintaining a normal mitochondrial architecture (Hliscs et al. 2015) and for successful release of male gametes (Deligianni et al. 2011). Another potential function could be the stabilisation of the cell, thus contributing to the high rigidity of developing gametocytes: actin filaments, similar to microtubules are disassembled in stage V gametocytes. Interestingly, treatment of P. berghei gametocytes with Cytochalasin D affects homing to bone marrow and spleen upon infection, suggesting that actin function is required for parasite circulation and tissue homing (De Niz et al. 2018).
Extreme makeover: host cell remodelling during gametocyte development
Protein export and the modification of the host RBC are hallmarks of Plasmodium blood stage infection and conserved across the genus, but they have hitherto been most extensively studied in P. falciparum. The discovery of an N-terminal motif termed PEXEL or HT motif (Hiller et al. 2004; Marti et al. 2004) required for protein export has facilitated the prediction of the P. falciparum exportome. Including exported proteins without a PEXEL motif (termed PEXEL-negative exported proteins, PNEPs), 5–10% of the P. falciparum proteome is estimated to be exported (Spielmann and Gilberger 2015). Such a high fraction of exported proteins seems to be an exception amongst Plasmodium species. Plasmodium falciparum was found to have largely expanded its repertoire of exported PEXEL proteins compared to other human and rodent malaria species. Remarkably, the P. falciparum genome both encodes more exported protein families than other species and the families that are shared between species contain more members in P. falciparum (Sargeant et al. 2006). Whilst the number of PEXEL proteins indicates that P. falciparum indeed has a much larger repertoire of exported proteins than most other Plasmodium species, it is also possible that a majority of the exportome of those other species consists of PNEPs. However, the lack of a known export motif within PNEPs and their small number has made the prediction of complete exportomes so far impossible (Gruring et al. 2012; Heiber et al. 2013; Spielmann and Gilberger 2015).
Defining the Plasmodium Gexportome
Whilst protein export and host cell modifications are essential for the asexual parasite's survival both in vivo and in vitro, less is known in the sexual stages. A comparative proteome analysis of P. falciparum gametocytes vs asexual trophozoites revealed an upregulation of a number of exported proteins during the early phases of gametocytogenesis (Stage I/II) (Silvestrini et al. 2010). These proteins, termed GEXPs (P. falciparum gametocyte-exported proteins) represent one-tenth of the entire proteome detected in this study, indicating that protein export plays an important role during early gametocytogenesis. Interestingly, only four of the 26 GEXPs have orthologues in P. berghei, indicating that the cellular processes GEXPs are involved in during early P. falciparum gametocytogenesis differ significantly from P. berghei (Silvestrini et al. 2010). Remarkably, one-third of the GEXPs belong to the PHIST (Plasmodium helical interspersed subtelomeric) protein family (Sargeant et al. 2006), a Plasmodium-specific protein family with important roles in host cell remodelling and protein trafficking (reviewed in Warncke, Vakonakis and Beck (2016)). In contrast, components of the asexual knob complex and its major surface antigen PfEMP1 are absent in P. falciparum gametocytes (Tiburcio et al. 2012b; Fraschka et al. 2018), suggesting that there are significant differences in terms of host cell remodelling between asexual and gametocyte stages.
Already during RBC invasion, the parasite modifies its host cell by secreting proteins and lipids into the RBC, including the proposed PSAC component CLAG3, that is essential for nutrient uptake (Nguitragool et al. 2011). When invasion is complete, the parasite is engulfed by a parasitophorous vacuole membrane, creating a barrier between the parasite and its host cell (Dvorak et al. 1975). For proteins to reach the host cell and subsequent instalment of host cell modifications, these proteins have to cross the PVM. This is achieved by inserting a protein translocon, the Plasmodium translocon of exported proteins (PTEX) into the PVM membrane (de Koning-Ward et al. 2009). It has been shown that both PEXEL proteins and PNEPs are translocated through PTEX (Gehde et al. 2009; Gruring et al. 2012). Furthermore, the PTEX core components are conserved throughout the Plasmodium genus indicating a similar export mechanism for all exported proteins and in all Plasmodium species. PTEX, and thus protein export, has been shown to be essential for asexual blood stage development in both P. falciparum and P. berghei (Matthews et al. 2013; Matz, Matuschewski and Kooij 2013). However, in gametocytes the function of PTEX is only essential during the first 24–48 hours (stage I/II) of gametocytogenesis. This coincides with the upregulation of GEXPs during early gametocytogenesis and highlights the importance of protein export in this stage.
During further progression of P. falciparum gametocytogenesis, the expression levels of PTEX components decrease and their localisation in the PV and PVM becomes less pronounced. In contrast, PTEX components could either not be detected or were not localised to the PV in mature P. berghei gametocytes, raising the question whether protein export even occurs at this stage (Matthews et al. 2013). In P. falciparum, it has not been conclusively shown if protein export still occurs in stage III or later. In fact, the IMC engulfs the entire parasite in stage IV and V gametocytes and could represent a major obstacle for proteins to be trafficked to the parasite plasma membrane (PPM) and beyond.
Remodelling of host membranes in gametocytes
There are several major changes to the host cell that occur in stage III-V gametocytes, which could be caused by the activation of previously exported effector proteins or by de novo export. One of these changes is the deformability switch that occurs during the transition from stage IV to stage V gametocytes. The STEVOR family of exported proteins has been shown to play an important role in mediating host cell rigidity. Whilst still present at the RBC membrane in stage IV, STEVOR is internalised in stage V, potentially contributing to the increase in deformability at this stage (Tiburcio et al.2012a; Ramdani et al. 2015; Naissant et al. 2016). Likewise, changes in the host cytoskeleton network, including spectrin and band 3 (Dearnley et al. 2016) may be required for the observed deformability switch during gametocyte maturation.
In asexual P. falciparum parasites, Maurer's clefts play an important role in the trafficking of exported proteins within the host cell. Especially, proteins that contain one or more transmembrane domains are temporarily localised to the Maurer's clefts before being further trafficked to the RBC surface (e.g. McMillan et al. 2013; Oberli et al. 2014). A similar mechanism could exist in gametocytes with effectors being stored at or in the Maurer's clefts and then further trafficked to the site of action when required. Maurer's clefts, originally believed to be injuries to the host cell caused by the attachment of parasites (Mundwiler-Pachlatko and Beck 2013), are generated within hours after invasion of a RBC by P. falciparum (Gruring et al. 2011). Initially mobile, they are tethered to the RBC membrane and PVM by proteinaceous tethers containing the protein MAHRP2 during the transition from a ring stage parasite to a trophozoite (Hanssen et al. 2008; Pachlatko et al. 2010; Gruring et al. 2011). These tethers have so far only been described in P. falciparum, and MAHRP2 is specific to the subgenus Laverania, indicating that this mechanism is lineage specific. In P. falciparum Maurer's clefts can be detected throughout gametocytogenesis (Dearnley et al. 2016). In contrast to trophozoites, Maurer's clefts seem to be mobile in later gametocytes (stage III); however, it is unclear whether they are mobile throughout gametocytogenesis or initially tethered (Dearnley et al. 2016). The function of Maurer's clefts tethering is unknown, but it could potentially contribute to its function as a trafficking hub. In P. falciparum, a major function of Maurer's clefts is the trafficking of PfEMP1. Disruption of Maurer's clefts architecture by knock-out or truncation of the resident Maurer's cleft protein REX1 prevents the display of PfEMP1 on the RBC surface (Spycher et al. 2008; Dixon et al. 2011). Whilst REX1 is present at the Maurer's clefts throughout gametocyte development (Dearnley et al. 2016), both PfEMP1 and the knob structures that anchor the protein at the RBC membrane are absent in gametocytes (Tiburcio et al. 2012b). However, the presence of REX1 indicates that the maintenance of Maurer's clefts architecture throughout gametocytogenesis might be important for transmission. Maurer's clefts or similar structures seem to exist throughout the Plasmodium genus, suggesting a conserved function (Aikawa, Miller and Rabbege 1975). There is evidence that they serve similar functions in the trafficking of virulence proteins across species, as the knock-out of two Maurer's cleft resident proteins (MAHRP1, SBP1) results in reduced iRBC receptor binding in both P. falciparum and P. berghei (De Niz et al. 2016). In line with the observed absence of host cell modifications on the iRBC surface of immature gametocytes, binding studies have shown no significant interactions with endothelial cells (Silvestrini et al. 2012) or erythroid cells (Neveu et al. 2018); however, recent work suggests some binding to mesenchymal stem cells (Messina et al. 2018).
Most research into protein export and host cell modifications has been focused on asexual P. falciparum stages. The knowledge about iRBC surface antigens in gametocytes will be of great interest for the development of transmission blocking vaccines. Furthermore, understanding the mechanisms of protein export and antigen display during gametocyte development will expand our knowledge about parasite biology and could explain some of the differences between Plasmodium species.
Concluding remarks
Mature gametocytes were first discovered by Alphonse Laveran in the blood of an Algerian soldier more than 130 years ago (Laveran 1881), yet their biology remains elusive to this day. Recent efforts in malaria elimination and eradication have resulted in renewed interest in basic and translational aspects of malaria transmission.
In the past 5 years a series of technical breakthroughs in the malaria field have made the study of parasites and in particular of gametocytes more tractable—(i) forward genetic screens in P. berghei and P. falciparum (Bushell et al. 2017; Zhang et al. 2018) allow genome-wide investigation of gene function, which is particularly interesting for facultative processes such as gametocytogenesis; (ii) reproducible protocols for the efficient production of gametocytes (Brancucci et al. 2015; Brancucci et al. 2017; Filarsky et al. 2018) allow for systematic and synchronous investigation of gametocyte development; (iii) successful isolation of subpopulations of cells for transcriptomics (Pelle et al. 2015) and proteomics (Khan et al. 2005; Silvestrini et al. 2010); (iv) development of various inducible expression systems (de Koning-Ward, Gilson and Crabb 2015) and (v) high throughput single cell approaches (Poran et al. 2017; Brancucci et al. 2018; Reid et al. 2018) provide a template for the analysis of rare populations in vitro and in vivo at scale. These and other emerging tools will enable comparative transcriptomic, proteomic and metabolomic analyses of various parasite species and stages in vitro and in vivo. At the same time the continuous development in imaging technologies, including super resolution and high content imaging approaches, provide the corresponding phenotypic data at increasing resolution in time and space.
We anticipate that the systematic application of these tools to the study of gametocytogenesis will unravel some of the major questions in the field as follows: (i) how are external signals such as LysoPC translated in the cell to regulate sexual commitment? (ii) how and when is gender determined? (iii) what is the function of IMC, cytoskeleton and parasite surface antigens during gametocyte development in vivo? (iv) what is the regulatory program governing gametocyte maturation and mosquito infectivity? (v) what are the functional differences between gametocytes of different lineages? Closing these knowledge gaps may translate into novel opportunities to block the transmission cycle of this deadly human parasite.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Drs Katarzyna Modrzynska, Christopher Moxon and Leandro Lemgruber for critical reading of this manuscript. Research in the Marti lab is supported by a Wellcome Senior Investigator award (MM), European Research Council Consolidator award BoneMalar (MM) and a WT Centre award to WCMP. Additional funding comes from a Royal Society Wolfson Merit award (MM) and a DFG postdoctoral fellowship (FH).
Contributor Information
Priscilla Ngotho, Wellcome Centre for Integrative Parasitology, Institute of Infection, Immunity and Inflammation, University of Glasgow, 120 University Road, Glasgow G12 8TA, UK.
Alexandra Blancke Soares, Wellcome Centre for Integrative Parasitology, Institute of Infection, Immunity and Inflammation, University of Glasgow, 120 University Road, Glasgow G12 8TA, UK.
Franziska Hentzschel, Wellcome Centre for Integrative Parasitology, Institute of Infection, Immunity and Inflammation, University of Glasgow, 120 University Road, Glasgow G12 8TA, UK.
Fiona Achcar, Wellcome Centre for Integrative Parasitology, Institute of Infection, Immunity and Inflammation, University of Glasgow, 120 University Road, Glasgow G12 8TA, UK.
Lucia Bertuccini, Core Facilities, Microscopy Area, Instituto Superiore di Sanita, Via Regina Elena 299, 00161 Rome, Italy.
Matthias Marti, Wellcome Centre for Integrative Parasitology, Institute of Infection, Immunity and Inflammation, University of Glasgow, 120 University Road, Glasgow G12 8TA, UK; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston 02115, MA, USA.
Conflict of interest. None declared.
REFERENCES
- Aikawa M, Huff CG, Sprinz H. Comparative fine structure study of the gametocytes of avian, reptilian, and mammalian malarial parasites. J Ultrastruct Res. 1969;26:316–31. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Miller LH, Rabbege J. Caveola–vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivaxand P. cynomolgi. Unique structures related to Schuffner's dots. Am J Pathol. 1975;79:285–300. [PMC free article] [PubMed] [Google Scholar]
- Aingaran M, Zhang R, Law SKet al.. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell Microbiol. 2012;14:983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano P, Roca L, Smith Det al.. Plasmodium falciparum: parasites defective in early stages of gametocytogenesis. Exp Parasitol. 1995;81:227–35. [DOI] [PubMed] [Google Scholar]
- Aurrecoechea C, Brestelli J, Brunk BPet al.. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;; 37:D539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancells C, Llora-Batlle O, Poran Aet al.. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat Microbiol. 2019;4:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Richard D, Healer Jet al.. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197–208. [DOI] [PubMed] [Google Scholar]
- Bohme U, Otto TD, Cotton JAet al.. Complete avian malaria parasite genomes reveal features associated with lineage-specific evolution in birds and mammals. Genome Res. 2018;28:547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Llinas M, Pulliam BLet al.. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Mok S, Hu Get al.. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA. 2008;; 105::16290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Soumare HM, Mahamar Aet al.. Transmission-blocking effects of primaquine and methylene blue suggest P. falciparumgametocyte sterilisation rather than effects on sex ratio. Clin Infect Dis. 2019, DOI: 10.1093/cid/ciz134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci N, De Niz M, Straub Tet al.. Probing Plasmodium falciparum sexual commitment at the single-cell level. Wellcome Open Res. 2018;; 3::70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci NM, Goldowitz I, Buchholz Ket al.. An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nat Protoc. 2015;10:1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci NM, Witmer K, Schmid Cet al.. A var gene upstream element controls protein synthesis at the level of translation initiation in Plasmodium falciparum. PLoS One. 2014;9:e100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci NMB, Gerdt JP, Wang Cet al.. Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell. 2017;171:1532–1544.e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MC, Alano P, Duthie Set al.. Commitment of the malaria parasite Plasmodium falciparumto sexual and asexual development. Parasitology. 1990;100(Pt 2):191–200. [DOI] [PubMed] [Google Scholar]
- Bushell E, Gomes AR, Sanderson Tet al.. Functional profiling of a plasmodium genome reveals an abundance of essential genes. Cell. 2017;170:260–272.e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JCet al.. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Angiuoli SV, Suh BBet al.. Genome sequence and comparative analysis of the model rodent malaria parasiteP lasmodium yoelii yoelii. Nature. 2002;419:512–9. [DOI] [PubMed] [Google Scholar]
- Claessens A, Affara M, Assefa SAet al.. Culture adaptation of malaria parasites selects for convergent loss-of-function mutants. Sci Rep. 2017;7:41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BI, Skillman KM, Jiang RHet al.. A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion. Cell Host Microbe. 2014;16:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson RM, Hall N, Ouzounis CA. Comparative genomics of transcriptional control in the human malaria parasite Plasmodium falciparum. Genome Res. 2004;14:1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D, Evans KJ, Schofield Let al.. Preferential invasion of reticulocytes during late-stage Plasmodium berghei infection accounts for reduced circulating reticulocyte levels. Int J Parasitol. 2006;36:1389–97. [DOI] [PubMed] [Google Scholar]
- Dearnley M, Chu T, Zhang Yet al.. Reversible host cell remodeling underpins deformability changes in malaria parasite sexual blood stages. Proc Natl Acad Sci USA. 2016;113:4800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnley MK, Yeoman JA, Hanssen Eet al.. Origin, composition, organization and function of the inner membrane complex of Plasmodium falciparum gametocytes. J Cell Sci. 2012;125:2053–63. [DOI] [PubMed] [Google Scholar]
- de Koning-Ward TF, Gilson PR, Boddey JAet al.. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning-Ward TF, Gilson PR, Crabb BS. Advances in molecular genetic systems in malaria. Nat Rev Microbiol. 2015;13:373–87. [DOI] [PubMed] [Google Scholar]
- Deligianni E, Morgan RN, Bertuccini Let al.. Critical role for a stage-specific actin in male exflagellation of the malaria parasite. Cell Microbiol. 2011;13:1714–30. [DOI] [PubMed] [Google Scholar]
- Delves MJ, Straschil U, Ruecker Aet al.. Routine in vitro culture of P. falciparum gametocytes to evaluate novel transmission-blocking interventions. Nat Protoc. 2016;11:1668–80. [DOI] [PubMed] [Google Scholar]
- De Niz M, Meibalan E, Mejia Pet al.. Plasmodium gametocytes display homing and vascular transmigration in the host bone marrow. Sci Adv. 2018;4:eaat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Niz M, Ullrich AK, Heiber Aet al.. The machinery underlying malaria parasite virulence is conserved between rodent and human malaria parasites. Nat Commun. 2016;7:11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MW, Kenny S, McMillan PJet al.. Genetic ablation of a Maurer's cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol. 2011;81:982–93. [DOI] [PubMed] [Google Scholar]
- Duffier Y, Lorthiois A, Cistero Pet al.. A humanized mouse model for sequestration of Plasmodium falciparum sexual stages and in vivo evaluation of gametocytidal drugs. Sci Rep. 2016;6:35025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak JA, Miller LH, Whitehouse WCet al.. Invasion of erythrocytes by malaria merozoites. Science. 1975;187:748–50. [DOI] [PubMed] [Google Scholar]
- Eichner M, Diebner HH, Molineaux Let al.. Genesis, sequestration and survival of Plasmodium falciparumgametocytes: parameter estimates from fitting a model to malariatherapy data. Trans R Soc Trop Med Hyg. 2001;95:497–501. [DOI] [PubMed] [Google Scholar]
- Eksi S, Haile Y, Furuya Tet al.. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:90–99. [DOI] [PubMed] [Google Scholar]
- Eksi S, Morahan BJ, Haile Yet al.. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog. 2012;8:e1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filarsky M, Fraschka SA, Niederwieser Iet al.. GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science. 2018;359:1259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschka SA, Filarsky M, Hoo Ret al.. Comparative heterochromatin profiling reveals conserved and unique epigenome signatures linked to adaptation and development of malaria parasites. Cell Host Microbe. 2018;23:407–420.e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech C, Chen N. Variant surface antigens of malaria parasites: functional and evolutionary insights from comparative gene family classification and analysis. BMC Genomics. 2013;14:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen SC, Borner J, Martinsen ESet al.. The polyphyly of Plasmodium: comprehensive phylogenetic analyses of the malaria parasites (order Haemosporida) reveal widespread taxonomic conflict. R Soc Open Sci. 2018;5:171780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung Eet al.. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Motard A. Periodic infectivity of Plasmodium gametocytes to the vector. A review. Parasite. 1999;6:103–11. [DOI] [PubMed] [Google Scholar]
- Gehde N, Hinrichs C, Montilla Iet al.. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol Microbiol. 2009;71:613–28. [DOI] [PubMed] [Google Scholar]
- Giffin BF, McCann PP. Physiological activation of the mitochondrion and the transformation capacity of DFMO induced intermediate and short stumpy bloodstream form trypanosomes. Am J Trop Med Hyg. 1989;40:487–93. [DOI] [PubMed] [Google Scholar]
- Gould SB, Tham WH, Cowman AFet al.. Alveolins, a new family of cortical proteins that define the protist infrakingdom Alveolata. Mol Biol Evol. 2008;25:1219–30. [DOI] [PubMed] [Google Scholar]
- Grellier P, Rigomier D, Clavey Vet al.. Lipid traffic between high density lipoproteins and Plasmodium falciparum-infected red blood cells. J Cell Biol. 1991;112:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruring C, Heiber A, Kruse Fet al.. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nature Commun. 2011;2:165. [DOI] [PubMed] [Google Scholar]
- Gruring C, Heiber A, Kruse Fet al.. Uncovering common principles in protein export of malaria parasites. Cell Host & Microbe. 2012;12:717–29. [DOI] [PubMed] [Google Scholar]
- Guerreiro A, Deligianni E, Santos JMet al.. Genome-wide RIP-Chip analysis of translational repressor-bound mRNAs in the Plasmodium gametocyte. Genome Biol. 2014;15:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati S, Ekland EH, Ruggles KVet al.. Profiling the essential nature of lipid metabolism in asexual blood and gametocyte stages of Plasmodium falciparum. Cell Host Microbe. 2015;18:371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen E, Hawthorne P, Dixon MWet al.. Targeted mutagenesis of the ring-exported protein-1 of Plasmodium falciparum disrupts the architecture of Maurer's cleft organelles. Mol Microbiol. 2008;69:938–53. [DOI] [PubMed] [Google Scholar]
- Hawking F, Wilson ME, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:549–59. [DOI] [PubMed] [Google Scholar]
- Heiber A, Kruse F, Pick Cet al.. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog. 2013;9:e1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller NL, Bhattacharjee S, van Ooij Cet al.. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–7. [DOI] [PubMed] [Google Scholar]
- Hliscs M, Millet C, Dixon MWet al.. Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell Microbiol. 2015;17:207–25. [DOI] [PubMed] [Google Scholar]
- Hoo R, Zhu L, Amaladoss Aet al.. Integrated analysis of the Plasmodium species transcriptome. EBioMedicine. 2016;7:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikadai H, Shaw Saliba K, Kanzok SMet al.. Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. PNAS. 2013;110:E1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M, Madmanee W, Suwannasin Ket al.. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgiand Plasmodium knowlesi. J Infect Dis. 2019;219:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joice R, Nilsson SK, Montgomery Jet al.. Plasmodium falciparumtransmission stages accumulate in the human bone marrow. Sci Transl Med. 2014;6:244re245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josling GA, Williamson KC, Llinas M. Regulation of sexual commitment and gametocytogenesis in malaria parasites. Annu Rev Microbiol. 2018;72:501–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack BF, Rovira-Graells N, Clark TGet al.. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidoh T, Nath J, Okoye Vet al.. Novel structure in the pellicular complex ofPlasmodium falciparum gametocytes. J Eukaryot Microbiol. 1993;40:269–71. [DOI] [PubMed] [Google Scholar]
- Ke H, Lewis IA, Morrisey JMet al.. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015; 11:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RS, Modrzynska KK, Cameron Ret al.. Inducible developmental reprogramming redefines commitment to sexual development in the malaria parasite Plasmodium berghei. Nat Microbiol. 2018;3:1206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Franke-Fayard B, Mair GRet al.. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–87. [DOI] [PubMed] [Google Scholar]
- Kono M, Herrmann S, Loughran NBet al.. Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol Biol Evol. 2012;29:2113–32. [DOI] [PubMed] [Google Scholar]
- Lalremruata A, Magris M, Vivas-Martinez Set al.. Natural infection ofPlasmodium brasilianumin humans: man and monkey share quartan malaria parasites in the Venezuelan Amazon. EBioMedicine. 2015;2:1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laveran A. Un nouveau parasite trouvé dans le sang de malades atteints de fièvre palustre. Origine parasitaire des accidents de l'impaludisme. Bull Mém Soc Méd Hôpitaux Paris. 1881;17:158–64. [Google Scholar]
- Lee RS, Waters AP, Brewer JM. A cryptic cycle in haematopoietic niches promotes initiation of malaria transmission and evasion of chemotherapy. Nat Commun. 2018;9:1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PLet al.. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;; 301:1503–8. [DOI] [PubMed] [Google Scholar]
- MacRae JI, Dixon MW, Dearnley MKet al.. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LSet al.. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B, Li A, Zhang Ret al.. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood. 2015;125:1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancio-Silva L, Slavic K, Grilo Ruivo MTet al.. Nutrient sensing modulates malaria parasite virulence. Nature. 2017;547:213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Good RT, Rug Met al.. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Kalanon M, Chisholm SAet al.. The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol Microbiol. 2013;89:1167–86. [DOI] [PubMed] [Google Scholar]
- Matz JM, Matuschewski K, Kooij TW. Two putative protein export regulators promote Plasmodium blood stage development in vivo. Mol Biochem Parasitol. 2013;191:44–52. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, Millet C, Batinovic Set al.. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 2013;15:1401–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerstein-Kessel L, van der Lee R, Stone Wet al.. Probabilistic data integration identifies reliable gametocyte-specific proteins and transcripts in malaria parasites. Sci Rep. 2018;8:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina V, Valtieri M, Rubio Met al.. Gametocytes of the malaria parasite Plasmodium falciparum interact with and stimulate bone marrow mesenchymal cells to secrete angiogenetic factors. Front Cell Infect Microbiol. 2018;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszoely CA, Erbe EF, Steere RLet al.. Plasmodium falciparum: freeze-fracture of the gametocyte pellicular complex. Exp Parasitol. 1987;64:300–9. [DOI] [PubMed] [Google Scholar]
- Mons B. Intra erythrocytic differentiation of Plasmodium berghei. Acta Leiden. 1986;54:1–124. [PubMed] [Google Scholar]
- Mundwiler-Pachlatko E, Beck HP. Maurer's clefts, the enigma of Plasmodium falciparum. Proc Natl Acad Sci USA. 2013;110:19987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naissant B, Dupuy F, Duffier Yet al.. Plasmodium falciparum STEVOR phosphorylation regulates host erythrocyte deformability enabling malaria parasite transmission. Blood. 2016;127:e42–53. [DOI] [PubMed] [Google Scholar]
- Neveu G, Dupuy F, Ladli Met al.. Plasmodium falciparum gametocyte-infected erythrocytes do not adhere to human primary erythroblasts. Sci Rep. 2018;8:17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguitragool W, Bokhari AA, Pillai ADet al.. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell. 2011;145:665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaldia N 3rd, Meibalan E, Sa JMet al.. Bone marrow is a major parasite reservoir in Plasmodium vivax infection. MBio. 2018;9:pii: e00625–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberli A, Slater LM, Cutts Eet al.. A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface. FASEB J. 2014;28:4420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofulla AV, Okoye VC, Khan Bet al.. Cultivation of Plasmodium falciparumparasites in a serum-free medium. Am J Trop Med Hyg. 1993;49:335–40. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Spurck TP, Goodman CDet al.. Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryotic Cell. 2009;8:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A, Bertuccini L, Deligianni Eet al.. Distinct properties of the egress-related osmiophilic bodies in male and female gametocytes of the rodent malaria parasite Plasmodium berghei. Cell Microbiol. 2015;17:355–68. [DOI] [PubMed] [Google Scholar]
- Otto TD, Rayner JC, Bohme Uet al.. Genome sequencing of chimpanzee malaria parasites reveals possible pathways of adaptation to human hosts. Nat Commun. 2014;5:4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachlatko E, Rusch S, Muller Aet al.. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer's cleft tethers. Mol Microbiol. 2010;77:1136–52. [DOI] [PubMed] [Google Scholar]
- Parkyn Schneider M, Liu B, Glock Pet al.. Disrupting assembly of the inner membrane complex blocks P lasmodium falciparum sexual stage development. PLoS Pathog. 2017;13:e1006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelle KG, Oh K, Buchholz Ket al.. Transcriptional profiling defines dynamics of parasite tissue sequestration during malaria infection. Genome Med. 2015;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poran A, Notzel C, Aly Oet al.. Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites. Nature. 2017;551:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdani G, Naissant B, Thompson Eet al.. cAMP-signalling regulates gametocyte-iunfected erythrocyte deformability required for Malaria parasite transmission. PLoS Pathog. 2015;11:e1004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AJ, Talman AM, Bennett HMet al.. Single-cell RNA-seq reveals hidden transcriptional variation in malaria parasites. Elife. 2018;7:pii: e33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rono MK, Nyonda MA, Simam JJet al.. Adaptation of Plasmodium falciparum to its transmission environment. Nat Ecol Evol. 2018;2:377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge GG, Bohme U, Sanders Met al.. Plasmodium malariae and P. ovale genomes provide insights into malaria parasite evolution. Nature. 2017;542:101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant TJ, Marti M, Caler Eet al.. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 2006;7:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini F, Lasonder E, Olivieri Aet al.. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9:1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini F, Tiburcio M, Bertuccini Let al.. Differential adhesive properties of sequestered asexual and sexual stages of Plasmodium falciparum on human endothelial cells are tissue independent. PLoS One. 2012;7:e31567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE, Canning EU, Bray RSet al.. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci. 1978;201:375–99. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Gametocytogenesis of Plasmodium falciparum in vitro: an electron microscopic study. Parasitology. 1982;84:1–11. [DOI] [PubMed] [Google Scholar]
- Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Hughes KR, Modrzynska KKet al.. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74:1–8. [DOI] [PubMed] [Google Scholar]
- Spielmann T, Gilberger TW. Critical steps in protein export ofPlasmodium falciparum blood stages. Trends Parasitol. 2015;31:514–25. [DOI] [PubMed] [Google Scholar]
- Spycher C, Rug M, Pachlatko Eet al.. The Maurer's cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Pl asmodium falciparum-infected erythrocytes. Mol Microbiol. 2008;68:1300–14. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Evans KJ, Sexton AEet al.. Metabolomics-based elucidation of active metabolic pathways in erythrocytes and HSC-derived reticulocytes. J Proteome Res. 2017;16:1492–505. [DOI] [PubMed] [Google Scholar]
- Sterling CR, Aikawa M. A comparative study of gametocyte ultrastructure in avian haemosporidia. J Protozool. 1973;20:81–92. [DOI] [PubMed] [Google Scholar]
- Stubbs J, Simpson KM, Triglia Tet al.. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science. 2005;309:1384–7. [DOI] [PubMed] [Google Scholar]
- Talman AM, Prieto JH, Marques Set al.. Proteomic analysis of the Plasmodiummale gamete reveals the key role for glycolysis in flagellar motility. Malar J. 2014;13:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarun AS, Vaughan AM, Kappe SH. Redefining the role of de novo fatty acid synthesis in Plasmodium parasites. Trends Parasitol. 2009;25:545–50. [DOI] [PubMed] [Google Scholar]
- Tiburcio M, Niang M, Deplaine Get al.. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood. 2012a;119:e172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio M, Silvestrini F, Bertuccini Let al.. Early gametocytes of the malaria parasite Plasmodium falciparumspecifically remodel the adhesive properties of infected erythrocyte surface. Cell Microbiol. 2012. b;15:647−59. [DOI] [PubMed] [Google Scholar]
- Tran PN, Brown SH, Rug Met al.. Changes in lipid composition during sexual development of the malaria parasitePlasmodium falciparum. Malar J. 2016;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiunas G. Avian Malaria Parasites and Other Haemosporidia. Boca Raton, FL: CRC Press. 2005. [Google Scholar]
- van Schaijk BC, Kumar TR, Vos MWet al.. Type II fatty acid biosynthesis is essential for Plasmodium falciparumsporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell. 2014;13:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, O'Neill MT, Tarun ASet al.. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss TS, Healer J, Marty AJet al.. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparummalaria. Nature. 2006;439:1004–8. [DOI] [PubMed] [Google Scholar]
- Walzer KA, Kubicki DM, Tang Xet al.. Single-cell analysis reveals distinct gene expression and heterogeneity in male and female Plasmodium falciparumgametocytes. mSphere. 2018;3, pii: e00130–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke JD, Vakonakis I, Beck HP. Plasmodium helical interspersed subtelomeric (PHIST) proteins, at the center of host cell remodeling. Microbiol Mol Biol Rev. 2016;80:905–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling JG, Snijders PJ, van Someren Pet al.. Stage-specific expression and genomic organization of the actin genes of the malaria parasitePlasmodium falciparum. Mol Biochem Parasitol. 1989;35:167–76. [DOI] [PubMed] [Google Scholar]
- WHO. WHO World Malaria Report 2018. Luxemburg: World Health Organization, 2018.
- Young JA, Fivelman QL, Blair PLet al.. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. [DOI] [PubMed] [Google Scholar]
- Yu M, Kumar TR, Nkrumah LJet al.. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe. 2008;4:567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang C, Otto TDet al.. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science. 2018;360:pii: eaap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.