In egg-laying chickens, the trend in the move away from the cage to alternative housing systems and restriction in antimicrobial use requires alternative approaches to maintain health and prevent diseases. There is increased research and commercial interest toward alternative gut health solutions while improving the performance and product safety in poultry production systems. One such approach, in recognition of the importance of the gut microbial community, is the use of microbes as feed supplements (such as probiotics). Unlike meat-type chickens, studies assessing the efficacy of such microbial supplements are limited for egg-laying chickens. Thus, by conducting a comprehensive assessment of the hen microbiota in response to various levels of B. subtilis DSM 29784 during the pullet phase (grower and developer) and the layer phase, the present study demonstrates the importance of direct-fed microbes in modulating gut microbiome, which may relate to improved performance efficiency in the pullet and layer phases.
KEYWORDS: Bacillus subtilis, pullets and laying hens, cecal microbiota, 16S rRNA gene, short-chain fatty acids, apparent retention of components
ABSTRACT
This study investigated the efficacy of a single strain of Bacillus subtilis (SSB) in modulating the composition of cecal microbiota and its link to the concentration of short-chain fatty acids (SCFA) and apparent retention (AR) of components. A total of 720, 4-week-old Shaver White chicks were allotted to control (CON), 1.1E+08 (low, LSSB), 2.2E+08 (medium, MSSB), or 1.1E+09 (high, HSSB) CFU/kg of diet groups. At grower (10-week), developer (16-week), and laying (28-week) phases, excreta and cecal digesta samples were taken for AR, microbial, and SCFA analyses. Microbial analysis involved high-throughput sequencing of the V3-V4 hypervariable regions of 16S rRNA gene. Bacterial diversity decreased (P < 0.05) at the developer phase as the SSB dose increased; however, a distinct clustering pattern (P < 0.05) of bacterial community was noted. Bacteroides and Faecalibacterium were differentially enriched in the developer for SSB-fed compared to CON-fed birds. Although no differences in microbial diversity were detected in grower and layer phases, different species of Clostridium (XVIII, XIVa, IV, and XIVb)—major butyrate producers—were identified in all phases, with stronger effect sizes for SSB-fed compared to CON-fed birds. Isobutyric acid was elevated in dose response (P = 0.034) in layer phase. In addition, the relative abundances of Alistipes, Lactobacillus, and Bifidobacterium were positively correlated (P < 0.05), with AR of most components for SSB-fed birds in the pullet phase. The results suggested that supplementing chickens’ diet with B. subtilis DSM 29784 may selectively enrich beneficial bacterial communities, which in turn are critical in promoting the growth and performance of hens.
IMPORTANCE In egg-laying chickens, the trend in the move away from the cage to alternative housing systems and restriction in antimicrobial use requires alternative approaches to maintain health and prevent diseases. There is increased research and commercial interest toward alternative gut health solutions while improving the performance and product safety in poultry production systems. One such approach, in recognition of the importance of the gut microbial community, is the use of microbes as feed supplements (such as probiotics). Unlike meat-type chickens, studies assessing the efficacy of such microbial supplements are limited for egg-laying chickens. Thus, by conducting a comprehensive assessment of the hen microbiota in response to various levels of B. subtilis DSM 29784 during the pullet phase (grower and developer) and the layer phase, the present study demonstrates the importance of direct-fed microbes in modulating gut microbiome, which may relate to improved performance efficiency in the pullet and layer phases.
INTRODUCTION
The development and maintenance of robust and healthy gastrointestinal tract are reflective of the composition of microbes in the gut. This is typically a result of the complex interactions that occur between gut microbes and the host (1–3). A number of distinct bacterial taxa (commensal and pathogenic) are involved, each of which inhabits a different region of the gastrointestinal tract (4). By and large, the balance of gut microbiome composition can determine the health (mutualists) and disease (pathological) state of the relationship between the gut microbes and the host (5).
In poultry, the disturbance of gut microbiota (dysbiosis) increases susceptibility to pathogen colonization and infectious diseases (6). In commercial poultry production, enteric diseases may lead to subsequent contamination of poultry products for human consumption (7). Interest in the creation of antibiotic-free gut health solutions for poultry has caused increased need for understanding the regulation of gut microbial community to benefit the health of the host (8). In this aspect, direct-fed microbes (probiotics) potentially developed to improve the balance of gut microbiota are regarded as an alternative to antibiotic growth promoters (7, 9).
Several studies (10, 11) have indicated that increased digestion and absorption of nutrients are among the major mechanisms responsible for the beneficial effect of probiotics in poultry acting through various modes of action. Probiotics act, in part, through the enhancement of digestive enzymes (12), improvement of intestinal integrity by stimulating the growth and proliferation of enterocytes (11, 13), and modulation of the intestinal immune response (14, 15). In addition, probiotics improve hindgut fermentation of dietary substrates (carbohydrates and proteins) that are undigested/unabsorbed by the host and convert them to monosaccharides and short‐chain fatty acids (SCFA), subsequently improving intestinal absorption of these nutrients (16–18). SCFA provide energy to the host (19, 20), with health‐promoting effects (21), and may act as metabolites in the regulation of the gut-brain axis (22). In general, supplementation of probiotics in poultry diets helps to maintain host-gut microbiota function and achieve intestinal homeostasis, which supports the metabolic activity of chickens (5, 23, 24).
The efficacy of probiotics may differ due to a number of factors, including diet, the dose of the probiotics, and the age of the animal (25–27). In addition, the gut microbiota is dynamic and is also influenced by numerous factors, including the age of animal, diet composition, the physiological status of the animal, the gut region, and the environment (25, 28–30). Because probiotics may act to maintain the dynamic equilibrium of the microbiota in the gut (8, 31), the complex interaction between host gastrointestinal tract and gut microbiota may change at different stages of life (32). Thus, understanding the effect of probiotics across the growth/production phases of egg-laying chickens is critical for developing an effective alternative to antibiotic growth promoters. Efficacy data for probiotics fed to egg-laying chickens cannot be extrapolated from findings in broiler studies due to various differences between the two species, including growth rate, diet composition, and intestinal physiology (33). Broiler chickens are selected for meat production and thus managed to gain a higher body weight compared to pullets (∼3 and 0.5 kg in 6 weeks, respectively). In this regard, compared to hens, broilers consume easily digestible high-energy feed and possess high rates of intestinal development (33, 34), providing an ideal niche for microbial colonization (35). Hence, in broilers, less nondigestible carbohydrate and more readily fermentable substrate may be available in the ceca for SCFA production (36–38), indicating that different microbes may exist in the ceca of broilers compared to the ceca of pullets and laying hens (30, 39).
The objective of this study was to evaluate the efficacy of supplementing various levels of Bacillus subtilis DSM 29784 (Alterion-NE50; Adisseo USA, Inc., Alpharetta, GA) in the diets of egg-laying chickens by investigating the composition of cecal microbiota in the grower (weeks 5 to 10), developer (weeks 11 to 16), and layer (weeks 19 to 28) phases. In addition, the correlation between relative abundance of bacterial taxa of the cecal content in birds and the concentration of SCFA, as well as the apparent retention (AR) of components, a measure of net disappearance from the digestive tract of ingested dietary components, including dry matter (DM), gross energy (GE), nitrogen (N), calcium (Ca), and total phosphorus (total P), as well as apparent metabolizable energy corrected for nitrogen (AMEn) (data extracted from Neijat et al. [40]), at different growth/production phases, was determined. Furthermore, this study also provides additional efficacy data for probiotic use in egg-laying chickens during the rearing stage, for which limited data exist.
RESULTS
Microbial community structure and diversity.
Regardless of treatments in each phase (grower, developer, and layer phases), Good’s coverage (>99%) indicated that the majority of the microbial phylotypes in the cecal samples were covered. This was consistent with the rarefaction curves (see Fig. S1 in the supplemental material), which showed reasonable sampling efforts to capture most of the species in the samples. Totals of 2,127, 2,107, and 2,250 operational taxonomic units (OTUs) were identified at a 0.03 cutoff for all treatment groups in the grower (n = 32), developer (n = 30), and layer (n = 31) phases, respectively (Fig. S2).
Alpha-diversity indices (Chao1, Shannon, and Invsimpson) calculated in each phase are shown in Fig. 1. Although no significant differences were observed in Chao1 richness estimates in the grower and developer phases, in the layer phase the richness in birds fed medium concentrations of the Bacillus subtilis strain SSB (MSSB) was lower (P < 0.01) than for the other SSB-fed groups or for the control (CON) birds (Fig. 1a, Table 1). Shannon and Invsimpson diversity indices indicated a tendency (P ≤ 0.080) for increased richness and evenness in birds treated with low concentrations of SSB (LSSB) or with MSSB compared to CON birds or birds treated with high concentrations of SSB (HSSB) in the grower phase (Fig. 1b and c, Table 1). In the developer phase, Shannon and Invsimpson diversity indices indicated significant (P < 0.05, Table 1) reductions in bacterial diversity as the SSB dose increased, whereas these indices did not vary between treatments in the layer phase (Fig. 1b and c; Table 1).
FIG 1.
Alpha-diversity (a, Chao1 index; b, Shannon index; c, Invsimpson index) comparisons in cecal microbiomes of control and B. subtilis DSM 29784-fed chickens in the grower, developer, and layer phases. Doses (CFU/kg of feed): CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
TABLE 1.
Alpha-diversity comparisons (ANOVA P values) of control and B. subtilis DSM 29784-fed chickens
| Comparison | Chao1 |
Shannon index |
Invsimpson index |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Grower | Developer | Layer | Grower | Developer | Layer | Grower | Developer | Layer | |
| CON vs LSSB | 0.835 | 0.999 | 0.995 | 0.113 | 0.161 | 0.655 | 0.114 | 0.094 | 0.390 |
| CON vs MSSB | 0.990 | 1.000 | 0.009 | 0.080 | 0.013 | 0.546 | 0.073 | 0.003 | 0.960 |
| CON vs HSSB | 0.469 | 0.953 | 0.949 | 0.997 | 0.046 | 0.780 | 0.997 | 0.013 | 0.810 |
| LSSB vs MSSB | 0.663 | 0.999 | 0.004 | 0.999 | 0.758 | 0.069 | 0.997 | 0.591 | 0.167 |
| LSSB vs HSSB | 0.107 | 0.969 | 0.867 | 0.071 | 0.967 | 0.173 | 0.072 | 0.916 | 0.080 |
| MSSB vs HSSB | 0.659 | 0.977 | 0.053 | 0.048 | 0.945 | 0.986 | 0.045 | 0.915 | 0.976 |
Nonmetric multidimensional scaling (NMDS) analysis indicated that bacterial communities from cecal samples clustered not only on a treatment basis but also on a phase basis. The NMDS plots indicated significant differences between grower and developer, grower and layer, and developer and layer (analysis of molecular variance [AMOVA]; P = 0.014, P < 0.001, and P < 0.001, respectively) (Fig. S3). Compared to the grower or layer phases, cecal communities distinctively clustered in samples from the developer phase. In the grower phase, differences in the diversity of bacteria in SSB-supplemented chickens were small (Fig. 1, Table 1), and separations were not significant (Fig. 2a, Table 2). However, during the developer phase, there was distinct clustering (AMOVA, P < 0.05; Fig. 2b), which was consistent with the differences in the diversity of bacteria among treatments (Fig. 1b and c). In the layer phase, separations were poor (AMOVA, P > 0.05) (Fig. 2c), which is also consistent with the relatively low differences observed in the diversity of bacteria between treatments (Fig. 1).
FIG 2.
Nonmetric multidimensional scaling for control and single strain B. subtilis (SSB)-supplemented chickens during the grower (week 10) (a), developer (week 16) (b), and layer (week 28) (c) phases. Treatments represent B. subtilis DSM 29784 doses (CFU/kg of feed): CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09). Stress = 0.19, r2 = 0.87.
TABLE 2.
Beta-diversity comparisons of control and B. subtilis DSM 29784-fed chickens
| Treatment effect | AMOVA P value (iteration = 1,000) |
||
|---|---|---|---|
| Grower | Developer | Layer | |
| CON vs LSSB | 0.002 | 0.039 | 0.065 |
| CON vs MSSB | 0.063 | 0.006 | 0.225 |
| CON vs HSSB | 0.607 | 0.009 | 0.278 |
| LSSB vs MSSB | 0.547 | 0.016 | 0.089 |
| LSSB vs HSSB | 0.011 | 0.389 | 0.11 |
| MSSB vs HSSB | 0.061 | 0.067 | 0.821 |
Microbial community composition.
From the cecal samples of all treatments and phases, 14 phyla, 26 classes, 41 orders, 70 families, and 130 genera were identified. Firmicutes and Bacteroidetes were the predominant phyla, accounting for ∼85% of the total sequence reads (Fig. 3). Proteobacteria represented a significant proportion of the sequences (up to ∼10%). However, no significant differences in the relative abundances of the dominant phyla (Firmicutes and Bacteroidetes) or the low-abundance phyla (Proteobacteria, Elusimicrobia, Fusobacteria, Actinobacteria, and Verrucomicrobia) were noted among treatment groups. At the class level, the relative abundances of Bacilli (% total sequences; Fig. 4S) did not differ between treatments in the grower phase but resulted in a significant decline in the developer, particularly with the LSSB (P < 0.01) and the HSSB (P < 0.007) doses and in the layer phase (P < 0.034) at higher doses of the probiotic supplementation compared to the control and the LSSB doses, respectively.
FIG 3.
Relative abundances (%) of bacterial communities at the phylum level in the ceca of birds fed B. subtilis DSM 29784 in the grower (week 10), developer (week 16), and layer (week 28) phases (n = 96). Treatments represent B. subtilis DSM 29784 doses (CFU/kg of feed): CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
The most dominant bacterial communities within the phylum Firmicutes were unclassified members (9.87% ± 2.28%), and members of Clostridiales represented about 16.7% ± 2.73% of Firmicutes (Fig. 4a). The phylum Bacteroidetes was predominantly Alistipes (15.4% ± 3.60%) and Bacteroides (15.1 ± 5.03%) (Fig. 4a). At the genus level, no significant difference was observed in the relative abundance of these taxa among treatments. Despite the lower relative abundance, an overall comparison of the CON- versus SSB-fed groups (based on statistical analysis of metagenomic profiles [STAMP]) indicated that the relative proportions of some genera such as Butyricimonas in the grower phase increased significantly (P = 0.037) for the SSB-fed group compared to the control group (Fig. 4b). In the developer phase, Bacteroides and Faecalibacterium were more abundant in the ceca of SSB-fed than in CON-fed birds (2-fold greater for each taxon, Fig. 4c). In contrast, Helicobacter and Lactobacillus were abundant in the ceca of the CON-fed hens compared to the SSB-fed group in the developer phase (Fig. 4c). In the layer phase, Butyricicoccus was the only differentially abundant genus for the SSB-fed group compared to the control group (P = 0.021; Fig. 4d).
FIG 4.
Relative abundances (%) of bacterial community at the genus level in the ceca of chickens fed B. subtilis DSM 29784 during the grower (week 10), developer (week 16), and layer (week 28) phases, overall (stacked graph) (a) and using STAMP to identify and compare differentially abundant communities between CON versus all other samples (B. subtilis treatment groups) in the grower (b), developer (c), and layer (d) phases. Doses (CFU/kg of feed) for B. subtilis DSM 29784: CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
Linear discriminant analysis (LDA), using the LDA effect size (LEfSe) algorithm (P = 0.05, LDA score > 2), identified 19, 17, and 24 phylotypes that discriminate and reveal the differences among treatments (levels of the SSB) within the grower, developer, and layer phases, respectively (Fig. 5). In the grower phase (Fig. 5a), differentially enriched bacterial communities, particularly for the HSSB- and MSSB-supplemented groups, were generally characterized by phylotypes at the genus level that were primarily members of the phylum Firmicutes, although some members of the phylum Proteobacteria were also present. The LSSB-fed group was enriched with the genus Rikenella belonging to the phylum Bacteroidetes. In this phase, the cecal contents of CON-fed birds were enriched with genera Bilophila and Akkermansia belonging to phyla Proteobacteria and Verrucomicrobia, respectively (Fig. 5a).
FIG 5.
Cecal microbiota of chickens fed B. subtilis DSM 29784 during the grower (week 10) (a), developer (week 16) (b), and layer (week 28) (c) phases. LEfSe analysis generated a taxonomic cladogram of the 16S sequences indicating taxa with a significant LDA, a threshold value of >2, and a P value of <0.05. Doses (CFU/kg of feed) for B. subtilis DSM 29784: CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
In the developer phase, cecal contents of the HSSB-supplemented birds were mainly enriched with communities at a higher taxonomic level, i.e., unclassified communities of Bacteria and Firmicutes (Fig. 5b). In this phase, despite the low diversity of microbial species in the probiotic-supplemented group compared to the control group, the enriched microbes showing the greatest effect with LSSB and MSSB supplementation were Bacteroides, Barnesiella, Bifidobacterium, Ruminococcus, and Clostridium cluster IV (Fig. 5b). On the other hand, in the ceca of CON-fed pullets, the genus Parabacteroides was the most enriched (Fig. 5b).
In the layer phase, enriched microbial communities were primarily from low-abundance phyla, including Proteobacteria, Verrucomicrobia, Fusobacteria, Lentisphaerae, and Elusimicrobia. No differential enrichment of microbial communities was noted with HSSB supplementation in the layer phase (Fig. 5c). At a genus level, the cecal contents of the MSSB-fed birds were characterized by an abundance of Fusobacterium, Escherichia/Shigella, Victivallis, and Clostridium cluster XIVb, members of phyla Fusobacteria, Proteobacteria, Lentisphaerae, and Firmicutes, respectively. In contrast, LSSB-fed chickens were differentially enriched with Helicobacter and Sutterella (phylum Proteobacteria), Elusimicrobium (phylum Elusimicrobia), Lactobacillus (phylum Firmicutes), and Coprobacter and Odoribacter (phylum Bacteroidetes). The CON-fed chickens only enriched the genus Romboutsia, a member of phylum Firmicutes (Fig. 5c).
Metabolites of cecal fermentation and apparent retention of nutrients.
Dietary supplementation of B. subtilis had no significant effect on the concentration of simple sugars (lactose, citric, glucose, xylose, or arabinose) in the cecal digesta of the chickens (Table 3). In the grower phase, a linear increase (P = 0.005) in the concentration of propionate was observed for birds fed probiotic diets compared to the control group. Isobutyric acid (a branched-chain fatty acid) accumulated in linear (P = 0.076) and quadratic (P = 0.066) trends with probiotic inclusion and was highest with LSSB-fed birds compared to the other treatments. Although the concentrations of lactate and acetate did not differ between treatments in the grower phase, the levels of these acids linearly decreased (P = 0.006 and P < 0.001, respectively; Table 3) in the developer phase. The lactate concentration was lower (P = 0.032) for the HSSB-fed than for the CON-fed birds, while acetate was lower (P = 0.001) for all levels of probiotic-fed birds compared to the control group (Table 3). In the layer phase, lactic acid increased in a quadratic manner (P = 0.005), being highest for LSSB- and MSSB-fed birds compared to CON- or HSSB-fed groups. However, across phases, the concentrations of lactic acid increased in the layer compared to the pullet phase (9.47 ± 0.95 [standard error], 6.82 ± 0.95, and 17.45 ± 0.95 μmol/g of wet cecal digesta for the grower, developer, and layer phases, respectively).
TABLE 3.
Effect of B. subtilis DSM 29784 on the concentrations of cecal simple sugars and short-chain fatty acids in pullets during grower (week 10), developer (week 16), and layer (week 28) phasesa
| Parameter | Concn (μmol/g of wet cecal digesta)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simple sugars |
Short-chain fatty acids |
|||||||||||
| Lactose | Citric | Glucose | Xylose | Arabinose | Total sugarsc | Lactic | Acetic | Propionic | Isobutyric | n-Butyric | Total SCFAd | |
| Growers (wk 10) | ||||||||||||
| CON | 5.76 | 1.78 | 13.2 | 2.65 | 0.702 | 22.0 | 10.1 | 61.1 | 4.10B | 4.86 | 7.48 | 76.8 |
| LSSB | 8.06 | 2.02 | 7.60 | 2.17 | 0.668 | 18.4 | 8.42 | 54.9 | 5.17AB | 7.17 | 5.41 | 72.6 |
| MSSB | 6.11 | 1.82 | 11.4 | 2.75 | 0.765 | 19.0 | 8.08 | 70.1 | 7.05A | 6.97 | 9.17 | 93.3 |
| HSSB | 6.27 | 2.34 | 7.35 | 2.10 | 0.664 | 15.2 | 11.3 | 54.4 | 7.69A | 6.77 | 8.84 | 77.7 |
| SE | 1.03 | 0.43 | 3.41 | 0.43 | 0.082 | 3.24 | 1.84 | 4.99 | 0.89 | 0.64 | 1.92 | 7.11 |
| P | 0.38 | 0.78 | 0.56 | 0.63 | 0.78 | 0.53 | 0.59 | 0.12 | 0.036 | 0.089 | 0.51 | 0.21 |
| Response to SSB | ||||||||||||
| Linear | 0.92 | 0.46 | 0.39 | 0.59 | 0.96 | 0.18 | 0.70 | 0.83 | 0.005 | 0.076 | 0.37 | 0.47 |
| Quadratic | 0.32 | 0.75 | 0.83 | 0.85 | 0.69 | 0.98 | 0.20 | 0.36 | 0.81 | 0.066 | 0.66 | 0.43 |
| Developer (wk 16) | ||||||||||||
| CON | 5.58 | 1.74 | 12.2 | 2.56 | 0.659 | 21.0 | 9.01A | 96.9A | 6.91 | 5.00 | 13.9 | 124.5A |
| LSSB | 4.52 | 1.67 | 13.8 | 1.68 | 0.495 | 19.4 | 6.50AB | 75.7B | 5.67 | 4.26 | 12.6 | 99.3B |
| MSSB | 5.19 | 2.11 | 11.5 | 2.09 | 0.729 | 19.5 | 6.65AB | 68.5B | 5.84 | 5.34 | 14.0 | 93.7B |
| HSSB | 5.78 | 1.73 | 10.9 | 1.76 | 0.597 | 19.0 | 5.12B | 71.4B | 6.30 | 5.91 | 14.3 | 97.9B |
| SE | 0.54 | 0.32 | 1.58 | 0.27 | 0.079 | 1.42 | 0.87 | 4.53 | 0.70 | 0.46 | 1.63 | 5.96 |
| P | 0.40 | 0.74 | 0.60 | 0.11 | 0.25 | 0.78 | 0.032 | 0.001 | 0.601 | 0.10 | 0.88 | 0.005 |
| Response to SSB | ||||||||||||
| Linear | 0.59 | 0.79 | 0.39 | 0.096 | 0.89 | 0.37 | 0.006 | <0.001 | 0.60 | 0.073 | 0.73 | 0.004 |
| Quadratic | 0.14 | 0.63 | 0.48 | 0.313 | 0.84 | 0.70 | 0.58 | 0.013 | 0.23 | 0.16 | 0.62 | 0.021 |
| Layer (wk 28) | ||||||||||||
| CON | 6.65 | 1.21 | 15.5 | 3.36 | 0.502 | 25.2 | 11.8B | 79.0 | 12.23 | 5.19 | 14.4 | 110.8 |
| LSSB | 5.31 | 0.88 | 21.8 | 3.29 | 0.613 | 30.8 | 23.6A | 73.5 | 11.63 | 4.51 | 17.2 | 106.3 |
| MSSB | 5.81 | 1.24 | 25.2 | 4.39 | 0.580 | 34.6 | 19.0AB | 71.7 | 11.03 | 5.86 | 14.0 | 101.8 |
| HSSB | 5.42 | 1.01 | 23.2 | 3.92 | 0.552 | 33.0 | 15.4B | 72.3 | 10.48 | 6.21 | 15.3 | 104.4 |
| SE | 0.50 | 0.22 | 4.7 | 0.53 | 0.060 | 5.20 | 2.49 | 4.83 | 1.11 | 0.45 | 1.42 | 6.86 |
| P | 0.26 | 0.60 | 0.51 | 0.45 | 0.60 | 0.61 | 0.014 | 0.70 | 0.711 | 0.065 | 0.40 | 0.82 |
| Response to SSB | ||||||||||||
| Linear | 0.17 | 0.81 | 0.22 | 0.25 | 0.65 | 0.25 | 0.58 | 0.32 | 0.25 | 0.034 | 0.95 | 0.44 |
| Quadratic | 0.35 | 0.81 | 0.39 | 0.70 | 0.26 | 0.49 | 0.005 | 0.53 | 0.98 | 0.27 | 0.60 | 0.61 |
Data represent least-square means (LSM) from 12 replicate cages per treatment. Means with different superscript capital letters within the same column in each phase differed significantly (P < 0.05). Doses (CFU/kg of feed) for B. subtilis DSM 29784: CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
Unless parameter in column 1 is P value or linear/quadratic response to SSB.
Total sugars = sum of lactose, glucose, xylose, and arabinose.
Total short-chain fatty acid (SCFA) = sum of acetic, propionic, isobutyric, and n-butyric acids.
Apparent retention of DM and the AMEn of diets were improved (P < 0.05) in birds consuming SSB compared to the control in the grower phase (Table 4). In the developer phase, the lower dose (LSSB) resulted in higher (P < 0.0001) apparent retention values for most components in birds consuming the probiotics. In the layer phase, a higher dose (MSSB and HSSB) evidently improved the apparent retention of most components (DM, N, GE, Ca, and total P) and the AMEn of diets compared to the control (Table 4).
TABLE 4.
Effect of B. subtilis DSM 29784 on apparent retention of components in Shaver White chickens at the end of the grower (week 10), developer (week 16), and layer (week 28) phasesa
| Component | Dose of B. subtilisb |
Pooled SE | P | |||
|---|---|---|---|---|---|---|
| CON | LSSB | MSSB | HSSB | |||
| Grower (wk 10) | ||||||
| DM (%) | 68.8B | 68.9B | 71.7A | 72.6A | 0.92 | 0.012 |
| N (%) | 41.8 | 37.5 | 41.9 | 47.5 | 2.54 | 0.071 |
| GE (%) | 77.5 | 77.9 | 79.0 | 78.9 | 0.74 | 0.434 |
| Ca (%) | 36.5A | 29.7B | 31.1AB | 26.2B | 1.52 | 0.0009 |
| Total P (%) | 18.8 | 22.7 | 26.4 | 25.3 | 2.01 | 0.084 |
| AMEn (kcal/kg diet) | 2,867B | 2,911AB | 2,991A | 2,942AB | 29.3 | 0.039 |
| Developer (wk 16) | ||||||
| DM (%) | 73.6B | 75.7A | 73.2B | 73.6B | 0.37 | <0.0001 |
| N (%) | 49.8B | 56.3A | 52.3AB | 54.8A | 1.27 | 0.004 |
| GE (%) | 81.0B | 82.9A | 80.7B | 81.4B | 0.27 | <0.0001 |
| Ca (%) | 39.5BC | 51.5A | 41.9B | 36.6C | 1.33 | <0.0001 |
| Total P (%) | 34.6B | 44.1A | 36.1B | 38.0B | 1.08 | <0.0001 |
| AMEn (kcal/kg diet) | 2,969BC | 3,044A | 2,929C | 2,984B | 11.6 | <0.0001 |
| Layer (wk 28) | ||||||
| DM (%) | 67.1B | 67.6B | 71.7A | 73.1A | 0.52 | <0.0001 |
| N (%) | 49.1C | 51.8BC | 56.7A | 53.6AB | 1.34 | 0.002 |
| GE (%) | 79.5C | 79.7C | 83.7A | 82.3B | 0.42 | <0.0001 |
| Ca (%) | 35.3B | 28.9B | 50.3A | 56.3A | 2.03 | <0.0001 |
| Total P (%) | 51.5B | 53.9B | 60.3A | 63.9A | 1.21 | <0.0001 |
| AMEn (kcal/kg diet) | 2,724C | 2,747C | 3,063A | 2,860B | 15.4 | <0.0001 |
Data represent least-square means (LSM) from 12 replicate cages per treatment. Means with different superscript capital letters within the same row differ significantly (P; < 0.05).
Doses (CFU/kg of feed) for B. subtilis DSM 29784: CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
Correlation of bacterial taxa with SCFA and apparent retention of components.
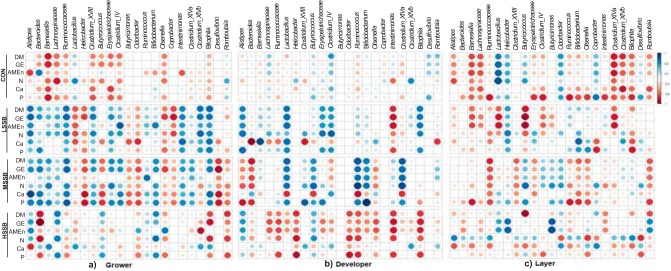
Significant correlations were observed between selected taxa with SCFA (Fig. 6) and AR (Fig. 7). In the grower phase, among the probiotic supplemented group, the LSSB-fed birds showed a positive correlation (P < 0.05) between acetate and Alistipes, Clostridium cluster XVIII, Ruminococcus, and Clostridium cluster XIVb. For the probiotic-fed group, propionate was positively correlated (P < 0.05) with bacterial species of Clostridium (clusters IV and XIVb) and Bifidobacterium for LSSB and with Lactobacillus for MSSB. Isobutyric acid was positively correlated (P < 0.05) with Butyricimonas and negatively correlated with Clostridium cluster XIVa for the LSSB-fed birds, negatively correlated with Bacteroids for MSSB, and positively correlated with Alistipes for HSSB (Fig. 6a). In the latter group, most SCFA were negatively correlated (P < 0.05) with Helicobacter (Fig. 6a). The CON-fed groups showed a positive correlation (P < 0.05; Fig. 6a) between most SCFA (except isobutyric acid) with members of Firmicutes (Lachnospiraceae, Ruminococcaceae, and Lactobacillus) and Bilophila (phylum Proteobacteria). However, Ruminococcus and species of Clostridium (XIVb and XIVa), were negatively correlated with most SCFA in the CON-fed birds.
FIG 6.
Spearman nonparametric rank correlations between concentration of SCFA (lactate, acetate, propionate, isobutyrate, and n-butyrate) and the relative abundances of bacterial taxa in ceca in the grower (a), developer (b), and layer (c) phases. All correlations presented were statistically significant (P < 0.05), with strong correlations indicated by large circles and weaker correlations indicated by small circles. The scale colors indicate whether the correlation is positive (closer to +1, blue circles) or negative (closer to −1, red circles) between the taxa and concentration of SCFA in each treatment. Doses (CFU/kg of feed) for B. subtilis DSM 29784: CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09).
FIG 7.
Spearman nonparametric rank correlations between apparent retention of components and relative abundances of bacterial taxa in the ceca in the grower (a), developer (b), and layer (c) phases. All correlations presented were statistically significant (P < 0.05), with strong correlations indicated by large circles and weaker correlations indicated by small circles. The scale colors indicate whether the correlation is positive (closer to +1, blue circles) or negative (closer to −1, red circles) between the taxa and the apparent retention of components in each treatment. Doses (CFU/kg of feed) for B. subtilis DSM 29784: CON (control, no probiotic), LSSB (low SSB, 1.1E+08), MSSB (medium SSB, 2.2E+08), and HSSB (high SSB, 1.1E+09). AMEn, apparent metabolizable energy corrected for nitrogen.
In the developer phase, under probiotic treatment, LSSB showed a significant and positive correlation (P < 0.05) between lactate and the most abundant genus members of Bacteroidetes (Bacteroids and Barnesiella), as well as members of Firmicutes (Ruminococcaceae, Erysipelotrichaceae, Clostridium cluster XVIII and Clostridium cluster IV) (Fig. 6b). The concentrations of most SCFA were positively correlated (P < 0.05) with the genus Intestinimonas and Clostridium cluster XIVb for LSSB and MSSB and with the genera Odoribacter and Ruminococcus for HSSB. Negative correlations (P < 0.05) were observed between microbial taxon Erysipelotrichaceae, Clostridium cluster XIVa, and Romboutsia with most SCFA for all levels of probiotic (except lactic acid for LSSB). In the CON-fed birds, lactate was negatively correlated (P < 0.05) with most bacterial taxa, including the most abundant genus members of Bacteroidetes (Bacteroids and Barnesiella) and Clostridium cluster XVIII and Bifidobacterium. However, in the CON-fed group, the isobutyric acid level was positively correlated (P < 0.05) with Ruminococcaceae and the Clostridium cluster IV (Fig. 6b).
In the layer phase, most bacterial species were negatively correlated (P < 0.05; Fig. 6c) with most assayed SCFA for all treatments, particularly for the CON-fed group. Across all levels of the probiotic-fed group, Helicobacter (phylum Proteobacteria) was positively correlated (P < 0.05) with either lactate, propionate, or isobutyrate. Birds fed higher levels of the probiotic, particularly MSSB, had a strong and negative correlation (P < 0.05) between most SCFA and the major abundant members of Bacteroidetes (Alistipes, Bacteroides, and Barnesiella) but were positively correlated with Butyricimonas, Odoribacter, Olsenella, Desulfovibrio, and Romboutsia (Fig. 6c).
Apparent retention levels of most components for birds fed the control diet in the grower and developer phases were either negatively correlated or lacked correlation with most bacterial taxa (Fig. 7). On the other hand, during the grower phase (Fig. 7a), the probiotic-fed birds, particularly the LSSB and MSSB groups, resulted in a positive correlation (P < 0.05) between the AR of most components with the major (abundant) genus members of Bacteroidetes (Alistipes, Bacteroides, and Barnesiella) and Firmicutes (Lachnospiraceae and Ruminococcaceae). In addition, Clostridium species IV, XVIII, XIVa, and XIVb were positively correlated (P < 0.05) with AR for most components for the LSSB- and MSSB-fed birds (Fig. 7a). Similarly, in the developer phase, the probiotic-fed birds, particularly the LSSB and MSSB groups, showed a positive correlation (P < 0.05) between the AR of most components with selected taxa (Fig. 7b). The genus Lactobacillus (phylum Firmicutes) was positively correlated with the AR of most components in the developer for the probiotic-supplemented group, particularly the LSSB and MSSB levels. In addition, Bifidobacterium and Ruminococcus were positively correlated (P < 0.05) with the AR of most components for the MSSB (Fig. 7b). In the layer phase, across treatment, the HSSB-fed birds in particular showed a positive correlation (P < 0.05) of Helicobacter with AR data. For CON-fed birds, Lactobacillus was positively correlated (P < 0.05) with the AR of most components (Fig. 7c).
DISCUSSION
The use of probiotics in poultry diets has a direct role in modulating the composition of gut microbiota to promote the growth of beneficial species (11, 41–43). This study was part of a large project (40, 44), that evaluated the efficacy of feeding Shaver White chickens with various levels of B. subtilis DSM 29784 (no probiotic [CON], 1.1E+08 [LSSB], 2.2E+08 [MSSB], or 1.1E+09 [HSSB] CFU/kg of diet) in modulating the growth/production performance and AR of nutrients across different phases of the growth/production cycle. The present study aimed to further elucidate the impact of the probiotic on cecal microbial composition and its association with the concentration of SCFA and AR of components (data extracted from Neijat et al. [40]), during the grower, developer, and layer phases.
In agreement with results from previous studies in broilers (45), turkeys (46), geese (47), and laying hens (48), the present study revealed that the core phyla within the cecal microbiota, regardless of treatments and phases (grower, developer, and laying phase), were primarily Firmicutes and Bacteroidetes. Despite the lack of difference in the relative abundance of bacterial communities at the phylum level between treatments, distinct differences were evident at lower taxonomic levels, mainly at the genus level, despite their low relative abundance. Thus, the relative abundances of phylotypes at the genus level were considered for correlation assessment against the concentration of SCFA and AR of nutrient, as measured at different phases.
In the grower phase, a tendency for increased Shannon and Invsimpson indices, observed in probiotic-fed hens, particularly the LSSB and MSSB groups, might suggest higher species richness and evenness. In our previous report (40), supplementation of an increasing level of B. subtilis DSM 29784 linearly improved body weight and feed conversion ratio (FCR) of birds in the pullet phase, particularly in the grower phase. Similar observations in performance outcomes were noted in poults (49) and broilers (50) using the same product (B. subtilis DSM 29784). In the present study, taxonomies, mainly in Firmicutes but also from Bacteroidetes and Proteobacteria, which potentially improve hens’ performance (11, 13, 51) were specifically enriched in the probiotic-fed hens at the grower phase. As observed in the LEfSe analysis, differentially enriched (with greater effect size) members of the Firmicutes in birds fed the HSSB containing diet included the Lachnospiraceae, Faecalibacterium, and Clostridium clusters XVIII and XIVa. These microbes are associated with gut health (52) by modulating the immune system (43, 53, 54), improving metabolic efficiency (13, 55), and reducing colonization by undesirable microbes (56). Studies in pigs (57, 58) and humans (59, 60) have identified different genera of Firmicutes and Bacteroidetes as key indicators of growth performance based on the differences in their ability to harvest energy. Most members of Bacteroidetes help to promote intestinal digestion, nutrient utilization (51) and hind gut fermentation of substrates to produce SCFA (61), as well as promoting the conversion of the absorbed SCFA to more complex compounds in the liver (55). In the present study, the influential capabilities of Firmicutes and Bacteroidetes were evident from the positive correlations observed with AR of most components (DM, GE, AMEn, N, Ca, and total P). These variables were positively correlated with the major (abundant) members of Bacteroidetes (genera Alistipes, Bacteroides, and Barnesiella) and Firmicutes (families Lachnospiraceae and Ruminococcaceae, as well as the genus Clostridium clusters XVIII, XIVb, and XIVa) due to probiotic supplementation. These results may be suggestive of a direct effect of the strain in modulating gut microbiome, possibly linking to efficient intestinal absorption of components as has been described by Jacquier et al. (41) in broilers fed with B. subtilis DSM 29784, which supports a correlation between increased intestinal microvillus length and improved feed efficiency. This is in line with previous studies (10, 11) that show increased digestion and absorption of nutrients as among the major mechanisms responsible for enhanced growth performance in poultry involving probiotics supplementation.
The relative abundance of genus Alistipes, a propionate producer (62), was positively correlated with propionate concentration for the B. subtilis DSM 29784-supplemented group compared to the control, indicating an enhanced ability to ferment undigested carbohydrate (19, 20). In a broiler study, the same strain of probiotic (B. subtilis DSM 29784) stimulated Ruminococcus, Anaerostipes, and Lachnospiraceae, which are known to produce butyrate (41). In the present study, enriched taxa with probiotic supplementation, particularly the HSSB dose, associated with butyrate production, including Faecalibacterium, Lachnospiraceae, and the Clostridium clusters XVIII and XIVa, were negatively correlated with the concentration butyrate. This, in part, may be due to the lack of accumulation of butyrate in the ceca, implying these beneficial microbes may have the ability to promote rapid utilization of the SCFA produced by the host (19, 55). However, for the CON-fed birds, although most of these enriched microbial taxa were positively correlated with the concentration of butyrate, this did not translate into improved growth performance compared to the probiotic-fed birds, as revealed in our previous report (40). In addition, the ceca of the CON-fed birds were enriched with bacterial communities of family Erysipelotrichaceae, a potentially undesirable bacterium associated with intestinal inflammation in humans (63). Furthermore, in the grower phase, both Bilophila and Akkermansia, recognized as mucin degraders (64), were enriched by the CON-fed birds; however, these genera were positively correlated with the AR of most dietary components, for the probiotic-supplemented groups, particularly for LSSB and MSSB, suggesting an improved nutrient absorptive capacity (36) in birds fed the probiotics. The excessive accumulation of mucin can act as a physical barrier for nutrient absorption (36); hence, B. subtilis DSM 29784 may be involved in the regulation of the mucin layer, supporting previous observations in broilers involving probiotic supplementation (65) that indicate the processes of mucin biosynthesis and degradation are mediated via changes in the gut bacterial populations.
Based on beta-diversity analysis between phases, irrespective of treatment, the bacterial community structure changed significantly with age (phase comparison), in agreement with previous work (2, 32), which could be attributable to the physiological need of the bird. Microbial ecology and/or abundance is sensitive to dietary manipulation (66, 67), which may also include diet change and the availability of nutrients. Coincidentally, during the developer phase, feed intake by birds was influenced by stocking density (40). Cage dimensions of 71.1 cm by 76.2 cm by 40.6 cm (depth by width by height) housing 14 birds during the developer stages (11 to 16 weeks) provided an adequate floor space of 387 cm2/bird based on breed recommendation for Shaver White chickens (6 to 18 weeks, 350 cm2/bird allocation). However, adequate feeder space to allow simultaneous feeding (68, 69) was compromised, availing only 5.4 cm/bird compared to the recommended feeder space of 7.5 cm/bird. Hence, decreased feed intake by birds may have impacted substrate availability for microbes (66, 67) which, in part, may explain the observed low diversity of bacterial community in the ceca for SSB- compared to CON-fed birds as the B. subtilis dose increased, which could have a selection pressure on some microbes while allowing the preferential enrichment of particular bacterial guilds in the probiotic-fed groups. This observation contrasted with previous reports by Guo et al. (48) and Li et al. (51) indicating higher species richness in hens fed B. subtilis-supplemented diets. Other researchers, using either multistrain (70) or single-strain (71) probiotics, reported no significant changes in the gut microbial profiles of chickens. In line with these findings, Ma et al. (72), by supplementing B. subtilis DSM 29784 (1.0E+09 spores/kg of diet) to broiler diets, also did not detect changes in the species diversity of cecal microbiota. Although the quantification of B. subtilis DSM 29784 in diets in the present study (based on plate counts) showed an increasing dose of the probiotic and is expected to be reflected in the gastrointestinal tracts of the birds, a metagenomic analysis based on partial 16S rRNA gene sequences, such as the V3-V4 hypervariable region as used in the this study, may limit the identification of bacterial communities to the genus or species level (73). At a higher taxonomic level (e.g., class), the relative abundance of Bacilli (phylum Firmicutes) was lower in probiotic-supplemented hens than in the controls, particularly in the developer and layer phases. This result may relate to a suggestion by Ballou et al. (74) that the supplementation of Firmicutes-based probiotics may not stimulate more rapid conversion to a Firmicutes-dominated microbiome in probiotic-fed animals. It is reported that B. subtilis vegetative cells are present in the gastrointestinal tracts of birds as normal residents (75). However, a decrease in the relative abundance of Bacilli in the probiotic-fed groups compared to the control, which was observed in the developer and layer phases, may suggest a feedback of lessened potential pathogens after its supplementation (72, 76), possibly creating a conducive environment in modifying the structure of the cecal microbiome. On the other hand, probiotics containing dead organisms have also been shown to express beneficial effects (77, 78), such as by inducing specific immune responses based on the ability of immune cells to recognize individual bacterial cell structural components (79). These theories may suggest possible indirect attributes of the probiotic strain used in the present study, which were evidenced through the improved AR of most components, in the developer phase for the probiotic supplemented group, particularly with the LSSB and MSSB, despite a decreased relative abundance of Bacilli in the former dose of supplementation, compared to the control group. Unlike in the grower phase, where abundant members of Firmicutes and Bacteroidetes were the major players in the improved performance (body weight and FCR) (40), the developer phase enriched members of the phylum Bacteroidetes but with less impact on these variables. In poultry nutrition, a complex interplay exists between the resident gut microbes and enterocyte function (61, 80). Because the mode of action of probiotics involves the inhibition of pathogens, favoring beneficial microbes and ultimately enhancing gut health (7, 31, 81), it is possible based on the results in the present study that B. subtilis DSM 29784 (depending on the dose) might provide a potential to modify the relative composition of gut microbial community based on the physiological status of the animal (30). Previous studies have indicated that supplementation of Lactobacillus under relatively ideal conditions may not have a beneficial effect (82, 83). However, considering the aforementioned constraint in the developer phase, competitive interactions may exist within a community as a result of substrate change (84) that could have favored the enrichment of Bifidobacterium with the LSSB supplementation and the highly positive correlation of Lactobacillus with AR of most components for LSSB- and MSSB-supplemented birds. Lactobacillus and Bifidobacterium have a strong ability to attach to epithelial tissue, suppressing colonization by undesirable bacteria (85, 86), and demonstrate antibacterial effects (bacteriocin related, bacteriostatic and bactericidal) (23, 83, 87). In addition, these genera are the primary producers of lactic acid (88, 89), which might explain the increased level of this acid in the LSSB- and MSSB-supplemented groups. The accumulation of lactic acid may be partly associated with a reduction of pH in the gut, creating a hostile environment to the harmful microbes and enhancing proteolytic digestion (90, 91), and formation of acidic anion that creates a complex with minerals, enhancing their digestion and absorption (92). Moreover, the improved AR data (40), coupled with their positive correlations with Lactobacillus for the LSSB- and MSSB-supplemented birds observed in the present study, may also relate to the ability of this beneficial bacterium to produce digestive enzymes, such as amylase, to enhance digestion (93); however, this requires further investigation.
Furthermore, in the developer phase, at the genus level, Bacteroides and Barnesiella were the major enriched taxa with the supplementation of MSSB and LSSB, respectively. These genera are associated with immunomodulatory function (52, 80) involved in providing protection to the mucosal membrane by stimulating the helper T cells and the production of anti-inflammatory cytokines (94, 95). However, this beneficial protective function of probiotic has a nutrient and energy cost for the host because live microbes have nutrient requirements for their growth and proliferation (11). This phenomenon, in part, may explain the highly negative correlation of most bacterial taxa with the AR of components with birds supplemented with the HSSB dose.
In the layer phase, the enriched microbial communities were members primarily from low-abundance phyla, including Proteobacteria, Verrucomicrobia, Fusobacteria, Proteobacteria, Lentisphaerae, and Elusimicrobia, with a few members of the Firmicutes and Bacteroidetes present. This is in agreement with a previous work in laying hens by Guo et al. (48), who also noted a lack of significant differences in the relative proportions of the dominant phylum or members of the Bacteroidetes and Firmicutes but rather between the low-abundance communities of the phyla Tenericutes and Fusobacteria due to B. subtilis CGMCC 1.921 supplementation. In the layer phase, a reduction in species richness (Chao1) for the MSSB-fed birds compared to the other treatment groups may relate to stress during the egg laying period, which coincides with our previous observations (44), where the MSSB-fed birds showed an early initiation of egg laying compared to the rest. In the present study, although no significant differences were observed in the concentration of acetate or butyrate between treatments, positive correlations observed between these acids and the genera Odoribacter and Clostridium cluster IV for the MSSB-fed birds provide further evidence supporting the beneficial effect of the probiotic to improve nutrient utilization (18).
Although high association of Lactobacillus with AR of most components was evident for the CON-fed hens, this did not translate into improved retention values of components in these birds compared to the probiotic-fed hens. Borda-Molina et al. (96) reviewed the positive effects in the relationship between use of probiotic Bacillus sp. in chickens and its ability to increase Lactobacillus and Bifidobacterium counts in the ceca, resulting in better digestive efficiency. Moreover, the present results showed higher concentrations of lactic acid in the layer phase (for LSSB- and MSSB-fed birds compared to CON- or HSSB-fed birds) versus the concentration in either in the grower or the developer phases. This cecal fermentation end product might improve mineral availability (51, 97), possibly promoting egg production and maintaining eggshell quality. Furthermore, undesirable microbes such as Helicobacter were enriched with the supplementation of the probiotic in this phase; however, they were positively associated with the concentration of most SCFAs and the AR of most components, unlike for the control group, suggesting that the supplementation of B. subtilis DSM 29784 may enhance tolerance in birds to potentially undesirable microbes (e.g., Helicobacter).
In summary, this study demonstrates that supplementation of B. subtilis DSM 29784 modulates cecal microbiota by selectively enriching beneficial bacteria in different stages of growth, which in turn is critical in promoting the growth and performance of chickens. First, the grower phase revealed strong relationships between the bacterial taxa (particularly members of the Firmicutes and Bacteroidetes), SCFA, and nutrient retention variables associated with growth performance. The major bacterial taxa involved in this age group included Alistipes, Bacteroides, and phylum Bacteroidetes, as well as several members of the Firmicutes, particularly the Clostridium clusters XVIII, XIVb, and XIVa, which underscores the growth-promoting effect of this probiotic strain. Second, the preferential enrichment of specific genera or their interaction with the physiological variables, particularly for Bifidobacterium and Lactobacillus, was evident in the developer and layer phases. Consequently, stimulation of lactate production in these phases suggests the beneficial impact of this probiotic strain associated with a reduction of pH in the gut, which may control the growth of pathogenic bacteria while enhancing nutrient utilization.
MATERIALS AND METHODS
Animal ethics.
The experimental protocol describing the management and care of animals used in this study was in accordance with recommendations established by the Canadian Code of Practice for the Care and Use of Animals for Scientific Purposes (98) and reviewed and approved by the University of Guelph Animal Ethics Committee.
Birds, housing, and experimental diets.
A total of 720 day-old Shaver White pullets procured from a commercial farm (Archer’s Poultry Farm, Ltd., Brighton, CA) were placed on a standard commercial starter diet for 4 weeks. At the end of week 4, the birds were weighed and randomly allocated to four experimental treatments. Each treatment had 12 replicate cages. Each cage housed 15 pullets during the grower (5 to 10 weeks of age), 14 pullets during the developer (11 to 16 weeks of age), and 13 pullets during the layer (19 to 28 weeks of age) phases. The composition of the starter diet and the dimensions of housing cages used in this study have been previously described (44). At the beginning of week 5, the birds were randomly allocated to one of four experimental treatments. The experimental treatments included a corn-soybean basal diet containing varied levels of a single strain of B. subtilis (SSB) DSM 29784 (Alterion-NE50; Adisseo USA, Inc., Alpharetta, GA). The diets were formulated to contain no probiotic (control [CON]) or 1.1E+08 (low SSB [LSSB]), 2.2E+08 (medium SSB [MSSB]), and 1.1E+09 (high SSB [HSSB]) CFU/kg of diet. Water and feed were provided for ad libitum consumption throughout the experiment. The composition of the basal diet and the recovery test for different levels of the probiotic in the diets in all phases are provided as supplemental material (Tables S1 and S2, respectively).
Sample collection.
At the end of each phase, i.e., grower (week 10), developer (week 16), and layer (week 28), one bird per cage was randomly selected, weighed, and euthanized by cervical dislocation, and both ceca were removed to obtain the cecal contents. The cecal contents from each bird were collected aseptically in two separate clean and sterile vials on ice: one for analysis of the bacterial population and the other for analysis of their metabolites (SCFA and sugars). After collection, the samples were stored at –80°C until analyzed.
Analysis of short-chain fatty acids and simple sugars.
Cecal digesta samples collected in each phase were analyzed for short-chain fatty acids (SCFA), including both nonvolatile (succinic, lactic, fumaric, and formic acids) and volatile (acetic, propionic, isobutyric, and butyric acids) SCFA, as well as simple sugars (lactose, citric, glucose, xylose, and arabinose), as described previously in our lab (99). Briefly, cecal digesta samples were thawed, and ∼0.1 g of each sample was resuspended with 1 ml of 0.005 N H2SO4 (1:10 [wt/vol]) in microcentrifuge vials, vigorously vortexed, and then stored overnight at 4°C. The samples were further vortexed until completely dissolved and then centrifuged at 20,200 × g (Thermo Scientific Sorvall, Germany) for 15 min. The supernatants (400 μl) from each sample were transferred to amber high-pressure liquid chromatography (HPLC) vials and then diluted with an equal volume of 0.005 N H2SO4 buffer. The resulting cecal digesta fluid was assayed for SCFA and simple sugars using an HP1100 series HPLC (Woldbronn, Germany) equipped with an ROA LC column (300 by 7.8 mm; Phenomenex, Torrance, CA) and an Agilent 1260 Infinity RID detector (Woldbronn, Germany). Samples (20 μl) were injected and run at a temperature of 60°C using 0.005 N H2SO4 buffer as the mobile phase flowing at the rate of 0.5 ml/min ml/min (isocratic) for a total run time of 35 min.
Analysis of apparent retention of components.
The apparent retention (AR) of components such as dry matter (DM), gross energy (GE), nitrogen (N), calcium (Ca), and total phosphorus (total P), as well as the apparent metabolizable energy of diets corrected for nitrogen (AMEn) was evaluated using titanium dioxide as an indigestible marker (5 g/kg of diet) as described by Olukosi et al. (100). Briefly, fresh fecal samples obtained over a period of 3 consecutive days were weighed and oven dried at 60°C for 48 h and then weighed after drying to determine fecal DM (40). The fecal samples were subsequently pooled, subsampled, and finely ground and, along with feed samples, analyzed for titanium, DM, GE, N, and minerals (Ca and total P), as mentioned previously (40). The titanium concentration was measured as described previously (101). Apparent retention of components and the AMEn were calculated according to equations provided by Kiarie et al. (102).
DNA extraction.
A total of 96 DNA samples (n = 8 per treatment per phase) were extracted using QIAamp DNA stool minikit (Qiagen, Inc., Valencia, CA) in accordance with the manufacturer’s instructions except that we included a bead-beating step for the mechanical lysis of the microbial cells to increase the DNA yield. The quality of each DNA sample was verified by using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE), and concentrations were measured using a Qubit fluorometer. All DNA samples were then stored at –80°C until analyzed.
Sequencing and data analysis.
The profiles of microbial communities in the ceca were assessed by high-throughput sequencing of the V3-V4 hypervariable region of the 16S rRNA gene by using a MiSeq platform (Illumina, San Diego, CA). Amplicon library preparation and MiSeq sequencing were performed according to the standard Illumina protocol using a dual-indexing strategy for multiplexed sequencing (103); this was conducted at the University of Guelph’s Genomics Facility, Advanced Analysis Centre (Guelph, Ontario, Canada). For raw sequencing data, an ∼250-bp length of each pair of the reads was received in FASTQ format, and the sequences were curated using Mothur v.1.39.5 (104), as described in the MiSeq SOP (105). Briefly, contigs were generated from the forward and reverse reads using the make.contig command, followed by screening to remove sequences with ambiguous bases and those with a length inconsistent with the target region using the screen.seqs command. Only unique sequences were used for computational purposes. Sequences were then aligned using the align.seqs command to a customized 16S rRNA gene reference sequence from the SILVA v132 bacterial database (106). After poorly aligned sequences were removed, reads were taxonomically assigned at an 80% threshold, and nontarget reads (e.g., archaea, mitochondria, chloroplast) were also removed. Chimeric sequences were removed using VSEARCH v2.8.2, the final quality reads were clustered into OTUs at a 97% similarity cutoff, and rarefaction curves were calculated (Fig. S1). Prior to downstream OTU-based analyses, reads were normalized by subsampling, based on the lowest number of sequences per sample (n = 29,734). The numbers of reads in two samples from the developer phase and one sample from the layer phase were too low and were thus excluded from further analyses.
Alpha-diversity (Chao1 estimates, observed species, Shannon index, and Invsimpson index) and beta-diversity (nonmetric multidimensional scaling [NMDS]) indices were analyzed in mothur to illustrate the relationships between treatments (probiotic level) within each phase, as well as between phases accessing all samples. STAMP (v2.1.3; Parks et al. [107]) was used to identify and visualize taxa with a discriminating feature between treatments, mainly comparing CON versus SSB supplementation groups. Furthermore, the LEfSe algorithm (108) within the mothur program was used to compare treatment effects in each phase. Venn diagrams were also generated to compare the numbers of common and unique OTUs between treatment groups (Fig. S2).
Correlation of bacterial taxa versus SCFA concentration and apparent retention of components.
Spearman’s nonparametric correlation analysis was used to investigate the associations between bacterial taxa, SCFA concentration, and apparent retention of components (data extracted from Neijat et al. [40]) of birds fed diets supplemented with different levels of the probiotic during grower (week 10), developer (week 16), and layer (week 28) phases. The correlation heatmaps were created using R as described by Zhang et al. (109).
Statistical analyses.
Statistical analyses of the SCFA concentrations, AR values, and alpha-diversity indices were tested using by one-way analysis of variance (ANOVA) using the PROC MIXED procedure of SAS version 9.4 (SAS Institute, Inc., Cary, NC), with the probiotic levels (none, 1.1E+08, 2.2E+08, and 1.1E+09 CFU/kg of diet) as fixed factors and the birds per cage as a random factor. The clustering patterns in NMDS were analyzed in mothur, and the significance of the clusters was tested using analyses of molecular variance (AMOVA). The NMDS ordinations were then plotted using R program. Statistical analysis of metagenomic profiles was used to compare the relative abundances of microbial taxa between two pairwise treatment groups, i.e., CON versus SSB supplementation groups. The significance of variations was assessed using White’s nonparametric t test and P values adjusted using Benjamin (P values of <0.05 [confidence intervals of 95%] were considered significant), and finally extended error bars were plotted. LDA values, using the LEfSe algorithm, were generated using the nonparametric factorial Kruskal-Wallis sum-rank test. An LDA score of >2 and a P value of <0.05 were applied. Correlations between bacterial taxa and physiological variables (SCFA and AR) were calculated by nonparametric Spearman’s rank correlation analysis using R. The differences between treatments were considered significant at a P value of <0.05.
Accession number(s).
The raw sequence read of bacterial 16S rRNA genes of the 96 samples obtained in this study has been submitted to the Sequence Read Archive database of the National Center for Biotechnology Information as FASTQ files under study accession number PRJNA506873.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Ontario Agri-Food Innovation Alliance and Adisseo USA, Inc. (Alpharetta, GA). In addition, technical support provided during the study by A. Yitbarek (Department of Pathobiology, University of Guelph, Guelph, Ontario, Canada) and members of the Monogastric Nutrition Lab (Department of Animal Biosciences, University of Guelph) are greatly appreciated.
M.N., R.B.S., and E.K. conceived the study and, together with A.W., J.B., and P.T., designed the experiment. M.N. conducted the animal study, collected and processed samples, and performed laboratory analyses. Bioinformatics and microbiota statistical analyses were performed by J.H. Statistical integration of physiological and microbiota data and interpretation was conducted by M.N., J.H., and E.K. M.N. wrote the manuscript and received feedback from all authors. All authors read and approved the final version of the manuscript. E.K. was the grant holder, directed the study, and had overall editorial responsibility.
R.B.S., A.W., J.B., and P.T. are employees of Adisseo.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00402-19.
REFERENCES
- 1.Berg RD. 1996. The indigenous gastrointestinal microflora. Trends Microbiol 4:430–435. doi: 10.1016/0966-842X(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 2.Rehman HU, Vahjen W, Awad WA, Zentek J. 2007. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr 61:319–335. doi: 10.1080/17450390701556817. [DOI] [PubMed] [Google Scholar]
- 3.Savage DC. 1977. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 4.Pluske JR, Pethick DW, Hopwood DE, Hampson DJ. 2002. Nutritional influences on some major enteric bacterial diseases of pig. Nutr Res Rev 15:333–371. doi: 10.1079/NRR200242. [DOI] [PubMed] [Google Scholar]
- 5.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrigan A, de Leeuw M, Penaud-Frézet S, Dimova D, Murphy RA. 2015. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl Environ Microbiol 81:3460–3470. doi: 10.1128/AEM.04194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson JA, Burkholder KM. 2003. Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 8.Gibson GR, Fuller R. 2000. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr 130:391S–395S. doi: 10.1093/jn/130.2.391S. [DOI] [PubMed] [Google Scholar]
- 9.Araya M, Morelli L, Reid G, Sanders ME, Stanton C. 2002. Joint FAO/WHO Working Group report on guidelines for the evaluation of probiotics in food, London, Ontario, Canada. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 10.Li L-L, Hou Z-P, Li T-J, Wu G-Y, Huang R-L, Tang Z-R, Yang C-B, Gong J, Yu H, Kong X-F, Pan E, Ruan Z, Xhu W-Y, Deng Z-Y, Xie M, Deng J, Yin F-G, Yin Y-L. 2008. Effects of dietary probiotic supplementation on ileal digestibility of nutrients and growth performance in 1- to 42-day-old broilers. J Sci Food Agric 88:35–42. doi: 10.1002/jsfa.2910. [DOI] [Google Scholar]
- 11.Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K. 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci 89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- 12.Fuller R. 2001. The chicken gut microflora and probiotic supplements. J Poult Sci 38:189–196. doi: 10.2141/jpsa.38.189. [DOI] [Google Scholar]
- 13.Gheisar MM, Hosseindoust A, Kim I. 2016. Effects of dietary Enterococcus faecium on growth performance, carcass characteristics, faecal microbiota, and blood profile in broilers. Vet Med (Praha) 61:28–34. doi: 10.17221/8680-VETMED. [DOI] [Google Scholar]
- 14.Sanderson IR. 2004. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr 134:2450S–2454S. doi: 10.1093/jn/134.9.2450S. [DOI] [PubMed] [Google Scholar]
- 15.Waititu SM, Yitbarek A, Matini E, Echeverry H, Kiarie E, Rodriguez-Lecompte JC, Nyachoti CM. 2014. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult Sci 93:625–635. doi: 10.3382/ps.2013-03575. [DOI] [PubMed] [Google Scholar]
- 16.Chen HM, Lifschitz CH. 1989. Preparation of fecal samples for assay of volatile fatty acids by gas-liquid chromatography and high-performance liquid chromatography. Clin Chem 35:74–76. [PubMed] [Google Scholar]
- 17.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 18.Thanh N, Loh T, Foo H, Hair-Bejo M, Azhar B. 2009. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br Poult Sci 50:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. 2017. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndou SP, Tun HM, Kiarie E, Walsh MC, Khafipour E, Nyachoti CM. 2018. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta- and mucosa-associated microbiota in pigs. Sci Rep 8:5880. doi: 10.1038/s41598-018-24043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrier DE, Hinton A Jr, Ziprin RL, Beier RC, DeLoach JR. 1990. Effect of dietary lactose on cecal pH, bacteriostatic volatile fatty acids, and Salmonella typhimurium colonization of broiler chicks. Avian Dis 34:617–625. doi: 10.2307/1591254. [DOI] [PubMed] [Google Scholar]
- 22.Rhee SH, Pothoulakis C, Mayer EA. 2009. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaway T, Edrington T, Anderson R, Harvey R, Genovese K, Kennedy C, Venn D, Nisbet D. 2008. Probiotics, prebiotics, and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev 9:217–225. doi: 10.1017/S1466252308001540. [DOI] [PubMed] [Google Scholar]
- 24.O’Hara AM, Shanahan F. 2007. Gut microbiota: mining for therapeutic potential. Clin Gastroenterol Hepatol 5:274–284. doi: 10.1016/j.cgh.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol 69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikulski D, Jankowski J, Naczmanski J, Mikulska M, Demey V. 2012. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult Sci 91:2691–2700. doi: 10.3382/ps.2012-02370. [DOI] [PubMed] [Google Scholar]
- 27.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, Percy NJ, Ophel-Keller K. 2011. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol 77:5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torok VA, Hughes RJ, Ophel-Keller K, Ali M, MacAlpine R. 2009. Influence of different litter materials on cecal microbiota colonization in broiler chickens. Poult Sci 88:2474–2481. doi: 10.3382/ps.2008-00381. [DOI] [PubMed] [Google Scholar]
- 30.Kers JG, Velkers FC, Fischer EAJ, Hermes GDA, Stegeman JA, Smidt H. 2018. Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol 9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller R. 1989. Probiotics in man and animals. J Appl Bacteriol 66:365–378. [PubMed] [Google Scholar]
- 32.Pedroso A, Menten J, Lambais M. 2005. The structure of bacterial community in the intestines of newly hatched chicks. J Appl Poult Res 14:232–237. doi: 10.1093/japr/14.2.232. [DOI] [Google Scholar]
- 33.Lumpkins BS, Batal AB, Lee MD. 2010. Evaluation of the bacterial community and intestinal development of different genetic lines of chickens. Poult Sci 89:1614–1621. doi: 10.3382/ps.2010-00747. [DOI] [PubMed] [Google Scholar]
- 34.Kaminiska BZ. 1979. Food intake in the young chick, p 199–206. In Boorman KN, Freeman BM (ed), Food intake regulation in poultry. British Poultry Science, Ltd, Edinburg, Scotland. [Google Scholar]
- 35.Pan D, Yu Z. 2014. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montagne L, Piel C, Lalles J. 2004. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr Rev 62:105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 37.Lan Y, Verstegen MWA, Tamminga S, Williams BA. 2005. The role of the commensal gut microbial community in broiler chickens. Worlds Poult Sci J 61:95–104. doi: 10.1079/WPS200445. [DOI] [Google Scholar]
- 38.Yang Y, Iji PA, Choct M. 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World Poult Sci 65:97–114. doi: 10.1017/S0043933909000008. [DOI] [Google Scholar]
- 39.Walugembe M, Hsieh JCF, Koszewski NJ, Lamont SJ, Persia ME, Rothschild MF. 2015. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult Sci 94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- 40.Neijat M, Shirley RB, Welsher A, Barton J, Thiery P, Kiarie E. 2019. Growth performance, apparent retention of components, and excreta dry matter content in Shaver White pullets (5 to 16 week of age) in response to dietary supplementation of graded levels of a single strain Bacillus subtilis probiotic. Poult Sci 2019:pez080. doi: 10.3382/ps/pez080. [DOI] [PubMed] [Google Scholar]
- 41.Jacquier V, Nelson A, Jlali M, Rhayat L, Brinch KS, Devillard E. 2019. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult Sci 98:2548–2554. doi: 10.3382/ps/pey602. [DOI] [PubMed] [Google Scholar]
- 42.Jayaraman S, Das PP, Saini PC, Roy B, Chatterjee PN. 2017. Use of Bacillus subtilis PB6 as a potential antibiotic growth promoter replacement in improving performance of broiler birds. Poult Sci 96:2614–2622. doi: 10.3382/ps/pex079. [DOI] [PubMed] [Google Scholar]
- 43.Teo AY, Tan HM. 2007. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT). J Appl Poult Res 16:296–303. doi: 10.1093/japr/16.3.296. [DOI] [Google Scholar]
- 44.Neijat M, Shirley R, Kiarie E. 2018. Performance and apparent retention of nutrients in Shaver White pullets and laying hens in response to dietary supplementation of graded levels of a single strain Bacillus probiotic. Poult Sci 97(E-Suppl 1):85. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Y, Xiang Y, Zhou W, Chen J, Li K, Yang H. 2016. Microbial community mapping in intestinal tract of broiler chicken. Poult Sci 96:1387–1393. [DOI] [PubMed] [Google Scholar]
- 46.D’Andreano S, Sànchez Bonastre A, Francino O, Cuscó Martí A, Lecchi C, Grilli G, Giovanardi D, Ceciliani F. 2017. Gastrointestinal microbial population of turkey (Meleagris gallopavo) affected by hemorrhagic enteritis virus. Poult Sci 96:3550–3558. doi: 10.3382/ps/pex139. [DOI] [PubMed] [Google Scholar]
- 47.Yang H, Xiao Y, Gui G, Li J, Wang J, Li D. 2018. Microbial community and short-chain fatty acid profile in gastrointestinal tract of goose. Poult Sci 97:1420–1428. doi: 10.3382/ps/pex438. [DOI] [PubMed] [Google Scholar]
- 48.Guo J, Dong X, Liu S, Tong J. 2018. High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult Sci 97:2543–2556. doi: 10.3382/ps/pey112. [DOI] [PubMed] [Google Scholar]
- 49.Mohammadigheisar M, Shirley RB, Barton J, Welsher A, Thiery P, Kiarie E. 2019. Growth performance and gastrointestinal responses in heavy Tom turkeys fed antibiotic free corn-soybean meal diets supplemented with multiple doses of a single strain Bacillus subtilis probiotic (DSM29784). Poult Sci 2019:pez305. doi: 10.3382/ps/pez305. [DOI] [PubMed] [Google Scholar]
- 50.Rhayat L, Jacquier V, Brinch KS, Nielsen P, Nelson A, Geraert PA, Devillard E. 2017. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult Sci 96:2274–2280. doi: 10.3382/ps/pex018. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Zhang H, Chen Y, Yang M, Zhang L, Lu Z, Zhou Y, Wang T. 2015. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim Feed Sci Technol 208:119–131. doi: 10.1016/j.anifeedsci.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Park SH, Lee SI, Ricke SC. 2016. Microbial populations in naked neck chicken ceca raised on pasture flock fed with commercial yeast cell wall prebiotics via an Illumina MiSeq platform. PLoS One 11:e0151944. doi: 10.1371/journal.pone.0151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farnell M, Donoghue A, De Los Santos FS, Blore P, Hargis B, Tellez G, Donoghue D. 2006. Upregulation of oxidative burst and degranulation in chicken heterophils stimulated with probiotic bacteria. Poult Sci 85:1900–1906. doi: 10.1093/ps/85.11.1900. [DOI] [PubMed] [Google Scholar]
- 54.Kabir S, Rahman MM, Rahman M, Rahman M, Ahmed S. 2004. The dynamics of probiotics on growth performance and immune response in broilers. Int J Poult Sci 3:361–364. doi: 10.3923/ijps.2004.361.364. [DOI] [Google Scholar]
- 55.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Songer JG, Anderson MA. 2006. Clostridium difficile: an important pathogen of food animals. Anaerobe 12:1–4. doi: 10.1016/j.anaerobe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Han GG, Lee J-Y, Jin G-D, Park J, Choi YH, Chae BJ, Kim EB, Choi Y-J. 2017. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl Microbiol Biotechnol 101:5903–5911. doi: 10.1007/s00253-017-8304-7. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen R, Andersen AD, Mølbak L, Stagsted J, Boye M. 2013. Changes in the gut microbiota of cloned and non-cloned control pigs during development of obesity: gut microbiota during development of obesity in cloned pigs. BMC Microbiol 13:30. doi: 10.1186/1471-2180-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. 2009. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meehan CJ, Beiko RG. 2014. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol 6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polansky O, Sekelova Z, Faldynova M, Sebkova A, Sisak F, Rychlik I. 2016. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl Environ Microbiol 82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaakoush NO. 2015. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol 5:84. doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smirnov A, Perez R, Amit-Romach E, Sklan D, Uni Z. 2005. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J Nutr 135:187–192. doi: 10.1093/jn/135.2.187. [DOI] [PubMed] [Google Scholar]
- 66.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. 2007. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thogerson CM, Hester PY, Mench JA, Newberry RC, Okura CM, Pajor EA, Talaty PN, Garner JP. 2009. The effect of feeder space allocation on productivity and physiology of Hy-Line W-36 hens housed in conventional cages. Poult Sci 88:1793–1799. doi: 10.3382/ps.2009-00011. [DOI] [PubMed] [Google Scholar]
- 69.Widowski TM, Caston LJ, Casey-Trott TM, Hunniford ME. 2017. The effect of space allowance and cage size on laying hens housed in furnished cages. II. Behavior at the feeder. Poult Sci 96:3816–3823. doi: 10.3382/ps/pex198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Priyankarage N, Silva S, Gunaratne S, Kothalawala H, Palliyaguru M, Gunawardana G. 2003. Efficacy of probiotics and their effects on performance, carcase characteristics, intestinal microflora and Salmonella incidence in broilers. Br Poult Sci 44:26–27. doi: 10.1080/713655283. [DOI] [Google Scholar]
- 71.Lee K-W, Lillehoj HS, Jang SI, Lee S-H. 2014. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res Vet Sci 97:304–308. doi: 10.1016/j.rvsc.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Ma Y, Wang W, Zhang H, Wang J, Zhang W, Gao J, Wu S, Qi G. 2018. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci Rep 8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vinje H, Almøy T, Liland KH, Snipen L. 2014. A systematic search for discriminating sites in the 16S ribosomal RNA gene. Microb Inform Exp 4:2. doi: 10.1186/2042-5783-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ballou AL, Ali RA, Mendoza MA, Ellis JC, Hassan HM, Croom WJ, Koci MD. 2016. Development of the chick microbiome: how early exposure influences future microbial diversity. Front Vet Sci doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong HA, Duc LH, Cutting SM. 2005. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Gadde UD, Oh S, Lee Y, Davis E, Zimmerman N, Rehberger T, Lillehoj HS. 2017. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res Vet Sci 114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Coconnier MH, Bernet MF, Chauviere G, Servin AL. 1993. Adhering heat-killed human Lactobacillus acidophilus, strain LB, inhibits the process of pathogenicity of diarrhoeagenic bacteria in cultured human intestinal-cells. J Diarrhoeal Dis Res 11:235–242. [PubMed] [Google Scholar]
- 78.Zhang JL, Xie QM, Ji J, Yang WH, Wu YB, Li C, Ma JY, Bi YZ. 2012. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult Sci 91:2755–2760. doi: 10.3382/ps.2012-02339. [DOI] [PubMed] [Google Scholar]
- 79.Taverniti V, Guglielmetti S. 2011. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mountzouris KC, Tsirtsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K. 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci 86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- 82.Leeson S, Major D. 1990. Canadian researchers study need for feed criteria. Feedstuffs 62:1–29. [Google Scholar]
- 83.Nahashon SN, Nakaue HS, Mirosh LW. 1996. Nutrient retention and production parameters of Single Comb White Leghorn layers fed diets with varying crude protein levels and supplemented with direct-fed microbials. Anim Feed Sci Technol 61:17–26. doi: 10.1016/0377-8401(96)00956-X. [DOI] [Google Scholar]
- 84.Umu ÖC, Frank JA, Fangel JU, Oostindjer M, Da Silva CS, Bolhuis EJ, Bosch G, Willats WG, Pope PB, Diep DB. 2015. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 3:16. doi: 10.1186/s40168-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin L, Ho Y, Abdullah N, Jalaludin S. 1998. Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult Sci 77:1259–1265. doi: 10.1093/ps/77.9.1259. [DOI] [PubMed] [Google Scholar]
- 86.Yang J-j, Kun Q, Dong W, Zhang W, Wu Y-j, Xu Y-y. 2017. Effects of different proportions of two Bacillus sp. on the growth performance, small intestinal morphology, caecal microbiota, and plasma biochemical profile of Chinese Huainan Partridge Shank chickens. J Integr Agric 16:1383–1392. doi: 10.1016/S2095-3119(16)61510-1. [DOI] [Google Scholar]
- 87.Kawai Y, Ishii Y, Arakawa K, Uemura K, Saitoh B, Nishimura J, Kitazawa H, Yamazaki Y, Tateno Y, Itoh T, Saito T. 2004. Structural and functional differences in two cyclic bacteriocins with the same sequences produced by lactobacilli. Appl Environ Microbiol 70:2906–2911. doi: 10.1128/AEM.70.5.2906-2911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibson G, Wang X. 1994. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhu X, Joerger R. 2003. Composition of microbiota in content and mucus from cecae of broiler chickens as measured by fluorescent in situ hybridization with group-specific, 16S rRNA-targeted oligonucleotide probes. Poult Sci 82:1242–1249. doi: 10.1093/ps/82.8.1242. [DOI] [PubMed] [Google Scholar]
- 90.Kiarie E, Walsh M, Nyachoti C. 2016. Performance, digestive function, and mucosal responses to selected feed additives for pigs. J Anim Sci 94:169–180. doi: 10.2527/jas.2015-9835. [DOI] [Google Scholar]
- 91.Roy CC, Kien CL, Bouthillier L, Levy E. 2006. Short-chain fatty acids: ready for prime time? Nutr Clin Pract 21:351–366. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- 92.Edwards H, Baker D. 1999. Effect of dietary citric acid on zinc bioavailability from soy products using an egg white diets with zinc sulfate hepatahydrate as the stander. Poult Sci 78(Suppl 1):576. [Google Scholar]
- 93.Jin L, Ho Y, Ali M, Abdullah N, Ong K, Jalaludin S. 1996. Adhesion of Lactobacillus isolates to intestinal epithelial cells of chicken. Lett Appl Microbiol 22:229–232. doi: 10.1111/j.1472-765X.1996.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 94.Hegazy AN, West NR, Stubbington MJT, Wendt E, Suijker KIM, Datsi A, This S, Danne C, Campion S, Duncan SH, Owens BMJ, Uhlig HH, McMichael A, Bergthaler A, Teichmann SA, Keshav S, Powrie F. 2017. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 153:1320–1337. doi: 10.1053/j.gastro.2017.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, Lee MD, Collett SR, Johnson TJ, Cox NA. 2014. The chicken gastrointestinal microbiome. FEMS Microbiol Lett 360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- 96.Borda-Molina D, Seifert J, Camarinha-Silva A. 2018. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput Struct Biotechnol J 16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee KW, Lee SH, Lillehoj HS, Li GX, Jang SI, Babu US, Park MS, Kim DK, Lillehoj EP, Neumann AP, Rehberger TG, Siragusa GR. 2010. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult Sci 89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- 98.CCAC. 2009. The care and use of farm animals in research, teaching, and testing. Canadian Council on Animal Care, Ottawa, Ontario, Canada. [Google Scholar]
- 99.Leung H, Arrazola A, Torrey S, Kiarie E. 2018. Utilization of soy hulls, oat hulls, and flax meal fiber in adult broiler breeder hens. Poult Sci 97:1368–1372. doi: 10.3382/ps/pex434. [DOI] [PubMed] [Google Scholar]
- 100.Olukosi O, Bolarinwa O, Cowieson A, Adeola O. 2012. Marker type but not concentration influenced apparent ileal amino acid digestibility in phytase-supplemented diets for broiler chickens and pigs. J Anim Sci 90:4414–4420. doi: 10.2527/jas.2011-4801. [DOI] [PubMed] [Google Scholar]
- 101.Myers W, Ludden P, Nayigihugu V, Hess B. 2004. A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J Anim Sci 82:179–183. doi: 10.2527/2004.821179x. [DOI] [PubMed] [Google Scholar]
- 102.Kiarie E, Romero L, Ravindran V. 2014. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase. Poult Sci 93:1186–1196. doi: 10.3382/ps.2013-03715. [DOI] [PubMed] [Google Scholar]
- 103.Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J. 2014. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2012. The SILVA ribosomal RNA gene database project: improved data processing and Web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. 2014. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Z, Tun HM, Li R, Gonzalez BJM, Keenes HC, Nyachoti CM, Kiarie E, Khafipour E. 2018. Impact of xylanases on gut microbiota of growing pigs fed corn- or wheat-based diets. Anim Nutr 4:339–350. doi: 10.1016/j.aninu.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.