Colonization of FeOB on corrosion-resistant stainless steel types (304SS and 316SS) has been quantified from environmental deployments along salinity gradients in estuarine environments. Greater FeOB abundance at higher salinities and on the more-corrosion-resistant 316SS suggests that there may be a higher risk of biocorrosion at higher salinities and there may be a selective advantage from certain stainless steel alloy metals, such as molybdenum, for FeOB colonization. A novel species of FeOB described here was isolated from our stainless steel coupon deployments, and its genome sequence supports our environmental data, as genes involved in the potential selectiveness toward surface colonization of stainless steel might lead to higher rates of biocorrosion of manmade aquatic infrastructure. These combined results provide environmental constraints for FeOB colonization on anthropogenic iron sources and build on previous frameworks for biocorrosion prevention strategies.
KEYWORDS: iron-oxidizing bacteria, microbial surface colonization, stainless steel, Zetaproteobacteria, biocorrosion
ABSTRACT
Iron-oxidizing bacteria (FeOB) are some of the initial colonizing organisms during microbially influenced corrosion of steel infrastructure. To better understand the abiotic conditions under which FeOB colonize steel, an environmental study was conducted to determine the effects of salinity, temperature, dissolved oxygen levels, and steel type on FeOB colonization. Stainless steel (304 and 316 [i.e., 304SS and 316SS]) was used to determine the potential susceptibility of these specialized corrosion-resistant steels. Steel coupon deployments along salinity gradients in two river systems revealed attachment by FeOB at all sites, with greater abundance of FeOB at higher salinities and on 316SS, compared to 304SS. This may be due to the presence of molybdenum in 316SS, potentially providing a selective advantage for FeOB colonization. A novel Zetaproteobacteria species, Mariprofundus erugo, was isolated from these stainless steel samples. Genes for molybdenum utilization and uptake and reactive oxygen species protection were found within its genome, supporting the evidence from our FeOB abundance data; they may represent adaptations of FeOB for colonization of surfaces of anthropogenic iron sources such as stainless steel. These results reveal environmental conditions under which FeOB colonize steel surfaces most abundantly, and they provide the framework needed to develop biocorrosion prevention strategies for stainless steel infrastructure in coastal estuarine areas.
IMPORTANCE Colonization of FeOB on corrosion-resistant stainless steel types (304SS and 316SS) has been quantified from environmental deployments along salinity gradients in estuarine environments. Greater FeOB abundance at higher salinities and on the more-corrosion-resistant 316SS suggests that there may be a higher risk of biocorrosion at higher salinities and there may be a selective advantage from certain stainless steel alloy metals, such as molybdenum, for FeOB colonization. A novel species of FeOB described here was isolated from our stainless steel coupon deployments, and its genome sequence supports our environmental data, as genes involved in the potential selectiveness toward surface colonization of stainless steel might lead to higher rates of biocorrosion of manmade aquatic infrastructure. These combined results provide environmental constraints for FeOB colonization on anthropogenic iron sources and build on previous frameworks for biocorrosion prevention strategies.
INTRODUCTION
Neutrophilic iron-oxidizing bacteria (FeOB) play important roles in Earth’s natural iron cycle and in the corrosion of manmade iron structures (1). Corrosion by FeOB has been well documented for freshwater environments, which often are characterized by clogged and degrading pipelines in residential and industrial systems (1, 2), and more recently was documented on mild steel in estuarine and marine environments (3–5). Total damages due to corrosion in the United States have been estimated at $276 billion, with 50% reported to be microbiologically influenced (6). Sulfate-reducing bacteria are known to play a major role in biocorrosion, as studies have shown positive correlations between the abundance of sulfate-reducing bacteria and the severity of corrosion (7, 8). Recent evidence has shown that FeOB may be the initial colonizers in the succession of microbial biocorrosion communities (9) and thus may play equally important roles in biocorrosion. The steel industry has responded to corrosion with more consistent use of corrosion-resistant steels, such as the commonly used 304 stainless steel (304SS) or the more specialized 316SS, which is better rated for resistance to corrosion in salty marine environments (6).
During corrosion of ferrous metals, oxidation proceeds with oxygen as an electron acceptor and Fe(II) as an electron donor. Stainless steel is designed to combat abiotic oxidation of iron within the steel via the inclusion of alloying metals such as chromium, nickel, and molybdenum, which react with oxygen to form a surface oxide film (10). The formation of an oxide film by the alloying metals creates a barrier that limits accessibility to the electron-donating iron and thus greatly reduces the potential for electron transfer and subsequent oxidation and corrosion. This design, however, does not address the effects that microorganisms have in altering surface film characteristics via colonization, as some microorganisms are known to be resistant to metals such as chromium (11), which is present within the stainless steel alloy. Most FeOB are microaerophilic and thrive in low-oxygen environments, where they have a kinetic advantage over abiotic oxidation of iron (1). If FeOB are able to disrupt the oxide film on stainless steel, then they should be able to form their typical colonization biofilm, which is depleted in oxygen (5, 12), and subsequently utilize the underlying iron as a source of electrons, without competition from abiotic oxidation. The formation of this new microbial film by colonizing FeOB could allow other fouling materials and microorganisms, such as sulfate-reducing bacteria, to colonize and to contribute to this microbial corrosion community (12). This would likely result in a positive feedback loop in which the integrity of the steel surface is compromised by the formation of the biofilm, thus creating more biologically available iron to fuel the corrosion community. Such a scenario would further support the role of FeOB as primary colonizers on steel surfaces. This would be consistent with previous work by McBeth and Emerson, which showed FeOB as primary colonizers in microbial attachment succession on mild steel surfaces (9). Investigating the extent to which FeOB attach and colonize stainless steel will help determine the overall role and importance of FeOB in biocorrosion of steel alloys.
The two most common types of stainless steel, 304SS and 316SS, are rated differently based on their environmental applications. Stainless steel 304 is composed of 18% chromium, 8% nickel, 2% manganese, and <1% carbon, phosphorus, sulfur, silicon, and nitrogen, with the rest of the composition balanced by iron. The composition of 316SS differs, with 16.6% chromium, 10% nickel, 2% molybdenum, 2% manganese, and <1% carbon, phosphorus, sulfur, silicon, and nitrogen, balanced by iron (details obtained from Metal Samples Company, Alabama Specialty Products Inc., Munford, AL). Two significant differences to note between the stainless steel types are the addition of molybdenum in 316SS and the lower overall percentage of iron in 316SS. Molybdenum is considered more resistant to corrosive environments and therefore makes 316SS better rated for salty marine environments (6). It is commonly known that rust occurs more rapidly in saltwater, which is partly due to the role of sulfate-reducing bacteria, as described above, but is largely due to the higher conductivity of saltwater and the increased concentration of chloride ions. These ions provide a plentiful supply of electrons, which enable more redox reactions and hence more iron oxidation. Estuarine environments can potentially have high salinities and thus are more vulnerable to chemical iron oxidation. For this reason, estuarine infrastructure is often built using stainless steel, in hopes of resisting corrosion, but biological effects on stainless steel corrosion are often ignored due to a lack of research and understanding.
Previous studies investigated the role of FeOB and sulfate-reducing bacteria in the corrosion of 316SS in industrial freshwater systems (14). Sulfate-reducing bacteria exhibited a higher corrosion rate than FeOB, but a combination of FeOB and sulfate-reducing bacteria yielded an even higher corrosion rate than sulfate-reducing bacteria alone. Stainless steel in the marine environment has been shown to be colonized by phylogenetically diverse groups of microorganisms (15), with evidence that other types of bacteria, such as manganese-oxidizing bacteria, also affect the occurrence of biocorrosion (16). These studies demonstrate that microbial community interactions on stainless steel are important, and further investigation of FeOB colonization as it varies in the environment will help determine the conditions under which stainless steel is most vulnerable to biocorrosion.
Salinity also plays an important role in the distribution of microbial species and community structures. Previous studies described the dispersal of FeOB lineages along an estuarine river in Maine, with salinity being the determining factor for FeOB community composition, according to 16S rRNA-tagged pyrosequencing results (17). Marine FeOB (Zetaproteobacteria) were limited to salinities greater than 5 ppt, whereas freshwater FeOB (Betaproteobacteria) were mostly limited to less than 5 ppt. This study builds on those results by presenting a long-term environmental study using generalized FeOB enrichment cultures (18) to describe variations in relative abundance and colonization of FeOB in the environment across steel types, salinity gradients, and fluctuations in temperature and dissolved oxygen (DO) levels.
The goal of this study was to assess the influence of abiotic factors on FeOB colonization and potential biocorrosion in estuarine environments. The differences in composition between two stainless steel types, 304SS (18% chromium and 8% nickel) and 316SS (16.6% chromium, 10% nickel, and 2% molybdenum), were hypothesized to cause differences in FeOB colonization due to differences in oxide film characteristics. The 304SS was hypothesized to have greater FeOB colonization due to its greater percentage of iron available for FeOB utilization and its lower rating for corrosion resistance in salty environments. The influence of salinity on FeOB colonization was unclear prior to this study, because FeOB lineages and their impacts on biocorrosion span both freshwater and marine environments (1–3). Due to a wider range of salinity tolerance (19), marine FeOB were expected to colonize at a greater number of sites. Both psychrophilic and thermophilic lineages of FeOB were reported previously (20, 21), indicating a wide range of potential colonization temperatures. Growth rates of other closely related neutrophilic FeOB are highest at 20°C to 30°C (19), and thus greater colonization was expected in this range of in situ temperatures. DO concentrations were expected to be lower at sites with greater FeOB abundance, as they are generally thought to be microaerophilic organisms. These results not only shed light on the environmental growth constraints of FeOB but also provide a framework that industrial and residential property owners can use to minimize biocorrosion of structural property.
RESULTS
Between February 2017 and July 2018, a total of 140 stainless steel coupon samples were collected from six sites on the Pamlico River and five sites on the Neuse River, in North Carolina, with a range of temperatures, salinities, and DO concentrations (Table 1). Coupon deployments lasted for a minimum of 6 weeks and a maximum of 8 weeks, depending on weather conditions and personnel availability. Samples were retrieved from water temperatures as low as 4.6°C and as high as 33.8°C, while salinities ranged from 0 ppt at the most inland site to 21.2 ppt at the site closest to the ocean. DO levels ranged from 2.84 to 13.5 mg/liter. FeOB colonization occurred at all sites and salinities that were sampled, across a wide range of temperatures and DO concentrations and on both stainless steel types, showing that their attachment to stainless steel in estuarine environments is widespread, although some salinities exhibited colonization more frequently and more abundantly than others (Fig. 1; also see Fig. S1 in the supplemental material). Some variation in salinity at individual sites was observed, as it is a tidally influenced estuarine environment, although site-specific salinity was consistent for the majority of the deployment time (Cedar Island in March 2018: range, 5 to 22 ppt; average, 14.8 ppt) (Fig. S2). Salinity was a significant environmental factor, as FeOB cell abundance increased with increasing salinity (linear regression test, df = 139, z = 4.29, P = 0.0001). DO concentrations did not significantly correlate with FeOB cell abundances (linear regression test, df = 139, z = −0.92, P = 0.36). Temperatures also did not significantly correlate with FeOB cell abundances (linear regression test, df = 139, z = 0.58, P = 0.58) (Fig. S3); however, site-specific seasonal trends in FeOB colonization revealed more FeOB colonization in spring and fall months (Fig. 2), suggesting that temperature may be a contributing factor. The average FeOB cell abundance at lower salinities (<10 ppt) (mean, 306 cells/coupon; standard deviation [SD], 701 cells/coupon) was significantly lower than the FeOB cell abundance at higher salinities (>10 ppt) (mean, 925 cells/coupon; SD, 1,534 cells/coupon) (t test, df = 74, t = −2.87, P = 0.0054). Chiu et al. reported salinity growth ranges for the estuarine FeOB Mariprofundus aestuarium and Mariprofundus ferrinatatus as 7 to 31.5 ppt, with an optimal range of 14 to 17.5 ppt (19); therefore, a threshold of above versus below 10 ppt was deemed appropriate for summarizing results in this study. The greatest FeOB abundance of 8,000 cells/coupon was recorded at a salinity of 19.7 ppt on 316SS. Abundance was significantly correlated with salinity whether deployment or retrieval salinity measurements were used in the model (data not shown). Continuous salinity data were collected for a select few deployments, in order to identify short-term fluctuations (one deployment is shown in Fig. S2, with similar trends for the remaining deployment data). Continuous salinity data were limited to a minimal number of deployments because of a lack of equipment availability.
TABLE 1.
Sampling site descriptions, including retrieval salinity, water temperature, and DO measurements
| Sitea | Latitude | Longitude | River system | Salinity (ppt) (range [average]) | Temp (°C) (range [average]) | DO level (mg/liter) (range [average]) |
|---|---|---|---|---|---|---|
| 1. Estuarium (Washington, NC) | 35.5399 | −77.0537 | Pamlico | 0–0.5 (0.2) | 6.6–28.8 (18.1) | 3.9–10.1 (6.5) |
| 2. Mallard Creek (Washington, NC) | 35.4754 | −76.9261 | Pamlico | 2.6–11 (5.9) | 4.6–31.2 (17.2) | 3.5–11 (6.7) |
| 3. Goose Creek (Washington, NC) | 35.4668 | −76.9249 | Pamlico | 1.2–8.0 (5.1) | 5.9–32.7 (22.1) | 4.2–12 (7.6) |
| 4. North Creek (Bath, NC) | 35.4286 | −76.7088 | Pamlico | 7.3–16.6 (10.8) | 8.4–32.6 (20.5) | 3.1–10.8 (6.9) |
| 5. Wright’s Creek (Belhaven, NC) | 35.4115 | −76.5891 | Pamlico | 9.1–17.9 (11.4) | 8–32 (20.6) | 3–11.6 (7.7) |
| 6. Swan Quarter (Swan Quarter, NC) | 35.3765 | −76.4845 | Pamlico | 10.5–19 (15.3) | 10–24.5 (19.1) | 5.4–8.6 (6.9) |
| 7. Fisher’s Landing (New Bern, NC) | 34.9988 | −76.9808 | Neuse | 1.8–5.4 (3.5) | 15.7–31.8 (24.9) | 6.5–13.5 (10.4) |
| 8. Cahooque Creek (Havelock, NC) | 34.9176 | −76.8527 | Neuse | 3.6–9.6 (5.9) | 8.6–33.8 (23.6) | 4.3–12 (8.4) |
| 9. Pin Oak Court (Merrimon, NC) | 34.9313 | −76.8023 | Neuse | 12.4–19.2 (15.9) | 23.7–32.4 (26.9) | 5.5–8.4 (7.2) |
| 10. Matthew’s Point (Havelock, NC) | 34.9096 | −76.7624 | Neuse | 7.9–15.4 (12.5) | 8.7–33.4 (21.6) | 4.4–13.2 (8.9) |
| 11. Cedar Island (Cedar Island, NC) | 35.0049 | −76.3054 | Neuse | 14–21.2 (17.3) | 10.9–30 (22.4) | 2.8–10.2 (6.4) |
See Fig. 5 for specific site locations.
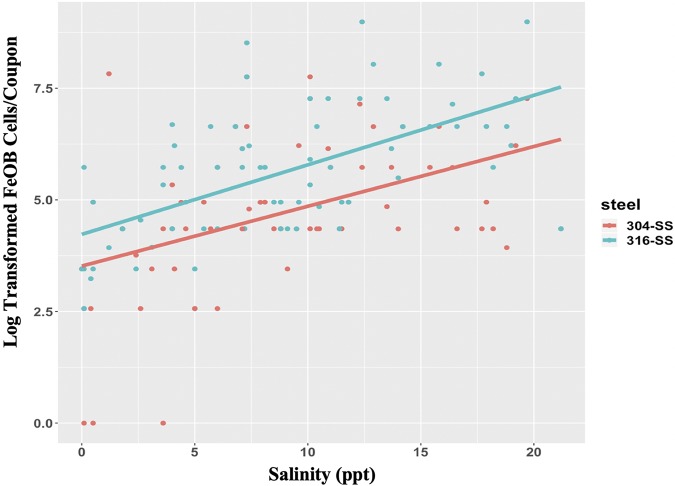
FIG 1.
Logarithmically transformed general linear model, showing greater FeOB abundance on 316SS, compared with 304SS (df = 139, z = 2.35, P = 0.018), and at higher salinities (df = 139, z = 4.29, P = 1.8e−5).
FIG 2.
FeOB abundance plots for several high-salinity sites according to retrieval month, to show seasonal trends. Cell abundance was equal or greater on 316SS for all retrievals except for February 2017 at Wright’s Creek. (A) Matthew’s Point exhibited the greatest cell abundance in May, July, August, and October. (B) Wright’s Creek exhibited the greatest cell abundance in February and April. (C) North Creek exhibited the greatest cell abundance in February and March. (D) Cedar Island exhibited the greatest cell abundance in May, July, and October.
FeOB cell abundance was significantly higher on 316SS and lower on 304SS (general linear model, df = 139, z = 2.35, P = 0.018; t test, df = 86, t = −2.46, P = 0.016) (Fig. 1). Dark orange rust coloration was visually evident on four different 316SS samples but never on 304SS samples (Fig. S4). This coloration is indicative of a compromised surface oxide film and exposure of the underlying iron. The 304SS sample (Fig. S4) exhibited high levels of algal and fish egg colonization but no evidence of corrosion. A total of 64.3% of sample retrievals exhibited a greater abundance of FeOB cells on 316SS, 8.6% of sample retrievals exhibited a greater abundance of FeOB cells on 304SS, and 27.1% of sample retrievals exhibited FeOB cell abundances on the two stainless steel types that were estimated to be equal (Fig. 2). This trend for steel type preference appears to be even stronger at higher salinities and during temperate months. Multiple iron oxide morphologies (twisted stalks and tubular sheaths) (Fig. S5) were observed in our enrichment cultures via epifluorescence microscopy, suggesting that multiple species of FeOB can colonize stainless steel. This finding supports the use of culture-based most probable number (MPN) methods, with cultivation of multiple types of FeOB, as quantitative PCR analysis of specific FeOB taxa may miss some of these organisms.
A novel Zetaproteobacteria operational taxonomic unit (OTU) (22) was successfully cultivated and identified from positive MPN enrichment cultures. Two different strains were isolated, from Mallard Creek (February 2017, 4 ppt, 316SS) and North Creek (February 2017, 7.3 ppt, 316SS). The two isolate strains represent a novel Zetaproteobacteria species, referenced here as Mariprofundus erugo strain P3 (isolated from Mallard Creek) and strain P7 (isolated from North Creek). The strains are members of the same OTU, as they are 99.6% identical in 16S rRNA gene sequences, and they exhibit 98.96% average nucleotide identity (ANI) and 98.3% average amino acid identity (AAI) across the whole genome. Although they are members of a new Zetaproteobacteria OTU, they are most similar to other isolates, including Mariprofundus ferrooxydans PV-1 (23) (16S rRNA gene sequence: 96.5% identity for PV-1 to P3 and 95.8% identity for PV-1 to P7; whole-genome sequence: ANI, 74.46% for PV-1 to P3 and 74.41% for PV-1 to P7; AAI, 73.68% for PV-1 to P3 and 73.4% for PV-1 to P7) (Fig. 3). The P3 and P7 genomes are both 99% complete and 0.84% redundant, with 0% strain heterogeneity (24). The ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) genes utilized for carbon fixation pathways in the M. erugo draft genomes are type II, indicating an affinity for low-oxygen and/or higher-CO2 conditions. The RubisCO amino acid sequence is phylogenetically most similar to the RubisCO sequence found in the freshwater FeOB Ferriphaselus sp. strain R-1 (25) (Fig. S6). It has been suggested that cyc2 is primarily responsible for energy acquisition via electron transport in marine FeOB (19, 26, 27). A homolog of the Cyc2 protein sequence from M. ferrooxydans PV-1 was found in the M. erugo draft genomes (75% AAI). Another FeOB isolate, Mariprofundus sp. strain DIS-1 (5), was isolated from mild steel coupons deployed in seawater off the coast of Maine. M. erugo has a higher G+C content (54.7%) than strain DIS-1 (48.6%), and strain DIS-1 has both type I and type II RubisCO genes, whereas the genome of M. erugo shows only type II. Thus, strain DIS-1 may be better adapted for both high and low oxygen affinities and also has genes to protect against reactive oxygen species such as catalase and catalase-peroxidase genes. Interestingly, M. erugo also contains coding sequences for protection against reactive oxygen species and oxidative stress, specifically cytochrome c551 peroxidase and superoxidase dismutase. These proteins may provide an advantage in colonizing steel in oxygenated waters, although the presence of only type II RubisCO genes suggests that the bacteria still prefer low-oxygen conditions while metabolically active in surface environments.
FIG 3.
Maximum likelihood phylogenetic tree showing the relatedness of the 16S rRNA genes of Mariprofundus erugo strains P3 and P7 (bold) to those of other known FeOB. Numbers on branches indicate bootstrap values.
Mariprofundus erugo strain P3 and P7 iron oxide morphologies were imaged with scanning electron microscopy (Fig. 4). Both strains exhibited twisted stalk morphologies, characterized as helical spirals. Additional species of FeOB also colonized the stainless steel coupons in this study, as evident in the multiple iron oxide morphologies observed through enrichment culture microscopy (Fig. S5). FeOB from marine environments that exhibit sheath morphologies, like those found in the enrichment cultures, have been observed previously (28, 29) but have not yet been taxonomically identified; therefore, isolation and identification represent an ongoing project.
FIG 4.
Representative images of Mariprofundus erugo. (A) Pure culture of strain P7 forming orange flocculant clumps suspended in liquid estuary medium with zero-valent iron under microaerophilic conditions. (B) Strain P7 cells under fluorescence after staining with SYTO13. (C) Scanning electron microscopy image of strain P3. (D) Scanning electron microscopy image of strain P7, representing diversity in the iron oxide morphologies produced by both strains of M. erugo.
DISCUSSION
The extensive long-term study presented here describes increases in FeOB colonization and abundance on stainless steel at higher salinities, more so on 316SS than on 304SS. This study reports on FeOB colonizing stainless steel in the natural environment, showing that two commonly used stainless steel types exhibited FeOB colonization at all salinities (0 to 21.2 ppt), all temperatures (4.6°C to 33.8°C), and all DO concentrations (2.84 to 13.5 mg/liter) that were sampled. Moreover, these results show that FeOB not only are capable of colonizing across a wide range of conditions but also are largely resilient to short-term fluctuations in environmental conditions. The variations in abundance of FeOB across salinity gradients not only reveal the differences in biocorrosion risks but also suggest optimal environmental conditions for FeOB growth, which could lead to greater proportions of FeOB within the overall microbial community. McBeth et al. reported the presence or absence of FeOB lineages along a salinity gradient (17), and Xu et al. revealed the importance of FeOB during the corrosion of stainless steel in freshwater industrial systems (14). This study builds on those to quantify variations in FeOB colonization and potential biocorrosion at different salinities. The stainless steel microbial communities in this study were also analyzed via 16S rRNA gene sequencing, but FeOB lineages were rarely found within the sequence data, always in small proportions (<0.1%). This finding supports their role as primary colonizers during early community succession and likely rare community members after initial colonization. Sulfate-reducing bacteria were found within the 16S rRNA gene sequence data but also in small community proportions (1 to 2%) and with no significant difference in proportions between 304SS and 316SS (t test, df = 24, t = −0.177, P = 0.86). Previous reports of FeOB cell succession during biocorrosion revealed complex community relationships. McBeth and Emerson found that zetaproteobacteria represented 12% of the microbial community on mild steel coupons after 9 days of deployment in coastal seawater and only 0.9% after 22 days (9), whereas Barco et al. detected no zetaproteobacteria on mild steel after 2 weeks in coastal seawater and 0 to 8% after 1 month (29). FeOB cell abundance data from this study present a reliable data set that reinforces the fact that FeOB are recurring members of biocorrosion communities, and the data can be used further to describe FeOB preferences for different conditions.
Our results showing greater abundances of FeOB at higher salinities for 140 total samples indicate that stainless steel may be more vulnerable to biocorrosion at higher salinities. This trend toward higher salinities further suggests that the marine class of FeOB, Zetaproteobacteria, may be more likely to colonize stainless steel than the freshwater class of FeOB, Betaproteobacteria. This is further supported by the 16S rRNA gene sequence data, within which no iron-oxidizing Betaproteobacteria taxa were found. This trend could also be influenced to some extent by the fact that marine systems are generally more iron limited than terrestrial freshwater systems. Zetaproteobacteria are known to tolerate salinities down to 3.5 ppt (19), but they may have a higher optimal salinity for growth. Results from this study showed multiple iron oxide morphologies that were confirmed via microscopy, which suggests that multiple species of FeOB beyond the Zetaproteobacteria class are capable of colonizing stainless steel. Occasional large fluctuations in salinity, such as at Cedar Island (the site with highest salinity), were likely due to heavy rainfall events in combination with tidal cycles. The attached microbial communities would be affected by these fluctuations, and their presence at the time of retrieval suggests that FeOB colonizing stainless steel seem to withstand salinity variations within tidally influenced estuarine environments. The environmental variability likely contributes to the variability in the FeOB abundance data at higher salinities.
Temperature and DO levels were not statistically significant variables affecting trends in FeOB cell abundance, but these variables did seem to have qualitative seasonal effects and possible correlations with salinity while being less important factors overall. Samples taken at temperatures less than 10°C exhibited extremely low abundances, while samples taken at moderate to high temperatures (22°C to 33°C) had greater abundance (>3,000 FeOB cells/coupon) and typically aligned with high salinity measurements. This finding suggests that FeOB may have optimal growth and colonization conditions in the spring through fall months in regions similar to North Carolina but only when salinity is also higher and thus more favorable. Temperature did not seem to be a determining factor for FeOB growth at sites with lower salinity (<10 ppt). During winter months, the general metabolism of FeOB may be slower overall, as for many other types of organisms, resulting in lower abundance and lower risk for subsequent biocorrosion. It is possible that FeOB remain dormant in the sediment as “seeds” until environmental conditions are more favorable, which may be a common strategy, particularly during winter months. DO exhibited lower concentrations at sites with higher FeOB cell abundance, which supports the microaerophilic lifestyle of FeOB, but the bulk DO measurements likely do not accurately reflect the microenvironment formed by colonizing microorganisms on the surface of the stainless steel.
Stainless steel has proved to be more vulnerable to colonization by FeOB and potentially to subsequent biocorrosion than previously considered, and vulnerability is greatest at salinities greater than 10 ppt. While 316SS may be more resistant to chemical corrosion, the results in this study suggest that 316SS may be a more favorable substrate for FeOB colonization; therefore, more research should be done to determine whether 316SS exhibits a higher rate of biocorrosion in marine environments. The direct effect of FeOB colonization on biocorrosion also was not specifically addressed in this study but could be an important topic of future research. The preference of FeOB for 316SS was surprising not only because 316SS is rated as being more corrosion resistant but also because 316SS has less iron (by weight) overall available for FeOB to utilize. Stainless steel 316 is known in the steel industry as being more corrosion resistant in marine environments, in part due to the added component of molybdenum, which hardens and strengthens the steel, thus decreasing the potential damage from the chloride ions in saltwater (6). Hayes et al. proposed that molybdenum in stainless steel may provide more of a stabilization effect for the oxide film, whereas chromium provides more of the corrosion resistance effect (30). From a biological perspective, it is possible that molybdenum may have a metabolic benefit for FeOB, leading to greater colonization and abundance of FeOB cells, which create a low-oxygen microenvironment that promotes colonization by other corrosive microorganisms, such as sulfate-reducing bacteria. From a mechanistic perspective, it is possible that FeOB may be removing molybdenum from the stainless steel as a micronutrient for growth (31), thus disturbing the stabilization effect of molybdenum on the oxide film and exposing an inherently smaller amount of chromium available for corrosion resistance. Ongoing studies will help to characterize the responses of M. erugo to individual stainless steel alloy metals, such as molybdenum, at various concentrations.
Molybdenum is often incorporated into enzymes for proper metabolic functioning in bacteria (31) and could have undiscovered metabolic benefits for FeOB as well. While typically not considered a limited micronutrient in marine systems, molybdenum is extremely limited in freshwater systems (32). Thus, molybdenum should theoretically be limited in estuarine environments, where these two opposing systems meet. Some marine FeOB are known to have genes for molybdenum cofactor biosynthesis and for transport of molybdenum (23, 33), suggesting that molybdenum may be a necessary enzyme cofactor, similar to the situation in other organisms. Interestingly, the draft genome of strain P3 has three different protein-coding sequences for molybdenum transport (ModA, ModB, and ModC) and one protein-coding sequence for molybdenum binding (ModE) (see Fig. S7 in the supplemental material), none of which is present in M. ferrooxydans PV-1. These sequences are found in Mariprofundus sp. strain EKF-M39, however (33). These sequences were not found in the P7 strain genome, but that may be due to genome incompleteness. These proteins could be utilized during colonization of stainless steels that contain molybdenum alloys. These four molybdenum-based proteins are all located on the same operon in the genome sequence, indicating that they are more likely to be conserved sequences than if they were distant from each other within the genome. This sequence cluster is also neighboring to sequences for the cobalt-zinc-cadmium resistance protein CzcA, an RND efflux periplasmic transporter protein, the transcriptional regulator protein AcrR, and two hypothetical proteins that are found in multiple marine and freshwater FeOB. These molybdenum-related proteins may allow the molybdenum alloy in 316SS to have a metabolic benefit for the FeOB cells, thus being favored over 304SS. Because FeOB are thought to “prime” the surface of steel, creating more favorable colonization conditions for other corrosive microorganisms, such as sulfate-reducing bacteria (9), further investigation of colonization by sulfate-reducing bacteria on different types of stainless steel would be useful. If FeOB are beneficial to corrosion communities and their members, as suspected, then quantitative MPN experiments with sulfate-reducing bacteria on stainless steel could be hypothesized to demonstrate correlation trends similar to those found in this study.
Of 13 relatively abundant Zetaproteobacteria OTUs that are globally distributed, only 2 have previously been linked to corrosion (12). With the discovery of a third corrosion-associated OTU, M. erugo increases support for the theory that there may be selective pressure on these ancient organisms as a result of colonizing new anthropogenic iron sources. As of now, M. erugo is the only reported FeOB species isolated from stainless steel. Genomic evidence, such as molybdenum-related genes, and protection against reactive oxygen species and oxidative stress suggest that it may be adapted specifically for oxygenated surface environments made of advanced steel types, although its lifestyle may not be strictly limited to such environments. The high sequence similarity of the Cyc2 protein sequences of strains P3 and P7 to that of M. ferrooxydans PV-1 (75% AAI) suggests that the general iron oxidation mechanisms of M. erugo may be conserved among Zetaproteobacteria strains. The high sequence similarity of the RubisCO amino acid sequences of strains P3 and P7 to that of freshwater FeOB suggest a common evolutionary history and potential adaptation for estuarine environments. The given genomic evidence suggests either horizontal gene transfer from (or to) freshwater FeOB due to the proximity of estuarine environments to freshwater species or a transition of marine descendants, followed by adaptation and niche expansion. The MPN results show greater FeOB cell abundance at higher salinities but also seemingly strong resilience to wide salinity fluctuations, which means that FeOB are important organisms to consider when planning biocorrosion prevention strategies for nearly all types of estuarine environments. All of these results combined can be further utilized to improve our understanding of the Zetaproteobacteria class of microorganisms, which is more diverse than previously identified.
MATERIALS AND METHODS
Field deployments.
Sampling sites were chosen at regular intervals along two estuarine rivers, in order to maximize the diversity of environmental treatment factors (Table 1 and Fig. 5). Stainless steel coupons (1/2 inch by 3 inches by 1/16 inch) (12) were secured via cable ties inside modified 50-ml conical centrifuge tubes. The bottom conical part of the tube was cut off and the cap was left off, leaving an open-ended cylinder, with one coupon inside, for water to flow through freely, while the coupon was protected from attachment of larger organisms and debris. One coupon tube of each stainless steel type (304SS and 316SS) was secured vertically to a small plastic crate and staked into the ground at five sites along the Neuse River and six sites along the Pamlico River. Coupons were kept above the sediment layer and within the water column. There were continual coupon deployments and retrievals every 6 to 8 weeks for 1.5 years, in order to ensure adequate time for colonization of FeOB and full seasonal cycle variability but not long enough for other microorganisms to take over the community (9). Salinity, water temperature, and bulk water DO concentrations at each site were recorded upon retrieval of the coupon, using an electronic YSI 30 instrument (Xylem Inc.) for temperature and salinity measurements and a YSI ProODO instrument (Xylem Inc.) for DO concentration measurements. Salinity fluctuated throughout the deployment period, but the larger fluctuations stabilized quickly and the majority of the measurements for the deployment time remained near the salinity average (see Fig. S2 in the supplemental material). While still submerged, the plastic crate was gently turned on its side and the cable ties securing the centrifuge tubes with coupons were cut in preparation for sample collection, in an effort not to disturb the biological material attached to the coupons. Any coupons that were buried in sediment due to heavy storms or were exposed due to low water levels were not used for analysis. Coupons were collected with sterilized forceps, placed in a new centrifuge tube containing 50 ml of water from the site of collection, and placed on ice for transport back to the laboratory for processing. Field sampling was approved by the North Carolina Division of Marine Fisheries (permit 706671).
FIG 5.
Map of coupon deployment sites. The red stars represent coupon deployment sites (numbered 1 to 11; see Table 1) on the Neuse River and the Pamlico River in North Carolina. The coupon attachment setup is shown in the upper right corner. Map obtained from Google Earth 9.0 and modified with site locations.
Sample processing.
Using enrichment medium that is reported to grow nearly all types of neutrophilic microaerophilic FeOB allowed for a cost-effective and minimally biased method of processing hundreds of samples from each stainless steel type and from different salinities. After retrieval, samples from 7 of the 11 sites were randomly selected for FeOB enrichment culturing and abundance estimates, in order to account for sample loss (physical loss or burial/exposure), incubator space, and equipment limitations in the laboratory. Standardized FeOB enrichment medium (18) was prepared and autoclaved. Vitamins and trace minerals (ATCC MD-VS and MD-TMS) were added after autoclaving, according to the medium recipes. For sites with salinity of less than 5 ppt at the time of collection, freshwater medium (∼0.4 ppt) was used for enrichment cultures; for sites with salinities of more than 5 ppt, estuary medium (∼18 ppt) was used (34). This threshold of 5 ppt was deemed appropriate due to reports from McBeth et al. and Chiu et al. showing freshwater FeOB tolerance up to 5 ppt and marine FeOB tolerance down to 5 ppt (17, 19).
An MPN method utilizing a series of replicates and dilutions was used to estimate relative abundances of FeOB colonization on stainless steel. The most vulnerable salinities and stainless steel types for FeOB attachment and subsequent biocorrosion were determined by recording differences in the relative abundance of FeOB for each sample, without the bias of measuring only certain species or classes of FeOB (i.e., Zetaproteobacteria). Stainless steel microbial communities are less dense and thus less ideal for 16S rRNA gene sequencing to identify FeOB, which are already considered to be low-abundance rare species (1). Similarly, quantifying colonization using other molecular methods such as quantitative PCR, which requires selecting for a specific lineage of bacteria prior to analysis, is troublesome when many different possible lineages of FeOB may be present on any given sample, at potentially very low abundances. The MPN methods used in this study thus allowed us to quantify FeOB colonization with the least potential bias. Each coupon was aseptically scraped and vortex-mixed, and 1.5 ml of the 50 ml of sample was inoculated into petri dishes containing 13.5 ml of medium, for a total plate volume of 15 ml. Each sample was plated in 3 replicates, with 4 dilutions of each replicate (12 plates total for each steel type sample and 24 plates total for each site retrieval), for MPN estimates. For each dilution transfer, the previous plate was mixed via a combination of circular and zig-zag motions to ensure random mixing, followed by pipetting in a random fluid motion so as to not pipette entirely from the same spot on the plate. Five milligrams of zero-valent iron powder (−200 mesh, <74 μm; Alfa Aesar, Thermo Fisher Scientific) was then added to each plate as a source of iron for any FeOB that might be present in the sample.
After inoculation, MPN enrichment cultures were stored in sealed containers containing BD GasPak EZ Campy container system sachets, to obtain microaerobic conditions (∼1% O2 in the headspace). Containers were incubated at 20°C for 21 days to ensure growth by any FeOB that might be present (18). An incubation temperature of 20°C was chosen for all sample cultures, in order to avoid slow growth rates that may affect the results after 21 days and to align with previously reported optimal growth temperatures (19). Positive growth for each plate was first determined visually and then, if necessary, confirmed with both light microscopy (for distinctive biological iron oxide morphologies) and fluorescence microscopy (for distinctive bean-shaped cells attached to the iron oxides), with additional confirmation via subsequent enrichment plate transfer. Positive growth by FeOB appears in very distinctive, suspended, flocculant, orange clumps (Fig. 4), which indicate iron oxides of biological origin and can be described as intertwined stalks, tubular sheaths, short dreads, or even amorphic forms (35–38).
Data analysis.
An MPN calculator (39) was used to estimate abundance based on which of the 12 plates for each sample exhibited growth after the incubation period. Raw MPN abundance data were visualized in R (40) using ggplot (41) and then glm2 (42) for significant trends in salinity, temperature, DO levels, and steel type. The raw count data were skewed across orders of magnitude, with several data points having high leverage in the linear trend; therefore, the data were logarithmically transformed for interpretation of statistical differences in salinity and steel type. After logarithmic transformation, the data exhibited a more random distribution, without skewness or high leverage points, and a better fit for general linear model analysis.
Species isolation and sequencing.
Serial dilution isolation methods were used to isolate FeOB species from Mallard Creek (February 2017, 4 ppt, 316SS) and North Creek (February 2017, 7.3 ppt, 316SS). The 16S rRNA gene was sequenced from each isolate to identify its lineage, followed by whole-genome sequencing of the novel FeOB isolate M. erugo to investigate potential stainless steel alloy-related genes. Several high-FeOB-abundance samples for each steel type, from high- and low-salinity sites, and from each river were chosen to attempt isolation of pure cultures of FeOB species present in each sample. Two rounds of dilution to extinction led to the isolates described above. A total of 5 dilutions were used, from 10−1 to 10−5, in 10-fold dilution increments. Individual orange clumps of biological iron oxides were selectively pipetted and transferred to 1.5-ml centrifuge tubes containing 1 ml of the same enrichment medium used in the MPN plates. All centrifuge dilution tubes were then vortex-mixed, and 100 μl was pipetted from each dilution into separate petri plates containing 15 ml of enrichment medium and 5 mg of zero-valent iron mesh. Once growth was visible in the plates (typically after 3 to 5 days of incubation in microaerophilic containers, as described above), material from the most diluted plate exhibiting growth was selectively transferred, and the serial dilution was repeated. Pure cultures were confirmed using epifluorescence microscopy to identify the presence of a single cell morphology. Cell samples were dyed with 50 nM SYTO 13 green fluorescent nucleic acid stain (Invitrogen) and viewed with an Eclipse Ni-E upright microscope (Nikon Instruments Inc.), under ×1,000 magnification, using fluorescein isothiocyanate (FITC) excitation and emission spectra (peak wavelength, ∼500 nm). Microscopic images were visualized using NIS-Elements BR 4.60.00 imaging software and a Y-T TV microscope camera (Nikon Instruments Inc.). Pure cultures were additionally confirmed by inoculation onto nutrient agar (9 g/liter nutrient broth [Remel] and 15 g/liter bacteriological agar [VWR International, LLC]) to confirm the absence of heterotrophic contaminant microbes. Samples for scanning electron microscopy were mounted on a 0.2-μm-pore-size polycarbonate filter, air dried, and coated with platinum. Samples were imaged at ×4,000 magnification with a Thermo Scientific Apreo scanning electron microscope, with an accelerating voltage of 2 kV.
DNA was extracted from individual isolated FeOB strains using a MoBio DNeasy PowerSoil kit (Qiagen, Inc.). The 16S rRNA gene was PCR amplified using universal 16S primers 8F and 1492R (43, 44). The PCR products were purified using a QIAquick PCR purification kit (Qiagen, Inc.). The 16S rRNA gene was sequenced by GeneWiz (South Plainfield, NJ) for identification of the isolates. The sequence data were then aligned and cleaned using Sequencher (Gene Codes Corp., Ann Arbor, MI). The aligned 16S rRNA gene sequences were then imported into BLAST (45) to identify the closest neighboring species. The aligned 16S rRNA gene sequences were also imported into the ZetaHunter program (22) to identify the Zetaproteobacteria OTU to which the isolates were most similar.
The genomes of both isolates were sequenced by CGEB Integrated Microbiome Resource (Dalhousie University, Halifax, Canada) using an Illumina MiSeq system. The paired-end reads were trimmed using Trimmomatic (46) and quality filtered using FastQC (47). The reads were then assembled using SPAdes (48) and annotated using RASTtk (49–51). The draft genome sequence of P3 consists of 59 scaffolds; it has a genome size of 2,898,355 bp, a G+C content of 54.7%, and 2,831 protein coding sequences. The draft genome sequence of P7 consists of 115 scaffolds, with 2,986,037 bp, a G+C content of 54.7%, and 3,019 protein coding sequences. ANIs were calculated using OrthoANI (52), and AAIs were calculated using a web-based calculator (http://lycofs01.lycoming.edu/~newman/AAI). Maximum likelihood phylogenetic trees for 16S rRNA and RubisCO gene comparisons with similar bacterial lineages were constructed using MEGAX (53), using the Tamura-Nei nucleotide substitution model for 16S rRNA and the Jones-Taylor-Thornton amino acid substitution model for RubisCO, each with 1,000 bootstrap iterations.
DNA was extracted from corrosion coupon material from February 2017 and May 2017 retrievals by using a MoBio DNeasy PowerSoil kit. DNA samples were sent to CGEB Integrated Microbiome Resource for 16S rRNA gene sequencing using improved internal transcribed spacer marker gene primers (54), on an Illumina MiSeq system. Microbial community data were processed and analyzed using mothur 1.41.3 (55) and the associated curation pipeline (56).
Data availability.
The GenBank accession numbers for the 16S rRNA gene sequences of Mariprofundus erugo strain P3 and Mariprofundus erugo strain P7 are MK554583 and MK554584, respectively. The NCBI accession numbers for M. erugo P3 and M. erugo P7 whole-genome sequences are SAMN11634928 and SAMN11634956, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Field and Blakeslee laboratories (East Carolina University) for help with sample collection, as well as Debbie Powell (University of Delaware) for help with scanning electron microscopy. We also thank Matthew’s Point Marina and the North Creek Landing Homeowners Association for allowing us to use their facilities.
We thank the Oak Ridge Associated Universities and East Carolina University Coastal Maritime Council for financial support.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00483-19.
REFERENCES
- 1.Emerson D, Fleming EJ, McBeth JM. 2010. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol 64:561–583. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- 2.Rao TS, Sairam TN, Viswanathan B, Nair K. 2000. Carbon steel corrosion by iron oxidising and sulphate reducing bacteria in a freshwater cooling system. Corros Sci 42:1417–1431. doi: 10.1016/S0010-938X(99)00141-9. [DOI] [Google Scholar]
- 3.McBeth JM, Little BJ, Ray RI, Farrar KM, Emerson D. 2011. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl Environ Microbiol 77:1405–1412. doi: 10.1128/AEM.02095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dang H, Chen R, Wang L, Shao S, Dai L, Ye Y, Guo L, Huang G, Klotz MG. 2011. Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environ Microbiol 13:3059–3074. doi: 10.1111/j.1462-2920.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 5.Mumford AC, Adaktylou IJ, Emerson D. 2016. Peeking under the Iron Curtain: development of a microcosm for imaging the colonization of steel surfaces by Mariprofundus sp. strain DIS-1, an oxygen-tolerant Fe-oxidizing bacterium. Appl Environ Microbiol 82:6799–6807. doi: 10.1128/AEM.01990-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little BJ, Lee JS. 2007. Microbiologically influenced corrosion, vol 3 John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 7.Marty F, Gueuné H, Malard E, Sánchez-Amaya JM, Sjögren L, Abbas B, Quillet L, van Loosdrecht MC, Muyzer G. 2014. Identification of key factors in accelerated low water corrosion through experimental simulation of tidal conditions: influence of stimulated indigenous microbiota. Biofouling 30:281–297. doi: 10.1080/08927014.2013.864758. [DOI] [PubMed] [Google Scholar]
- 8.Enning D, Venzlaff H, Garrelfs J, Dinh HT, Meyer V, Mayrhofer K, Hassel AW, Stratmann M, Widdel F. 2012. Marine sulfate‐reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14:1772–1787. doi: 10.1111/j.1462-2920.2012.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBeth JM, Emerson D. 2016. In situ microbial community succession on mild steel in estuarine and marine environments: exploring the role of iron-oxidizing bacteria. Front Microbiol 7:767. doi: 10.3389/fmicb.2016.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton WA. 2003. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19:65–76. doi: 10.1080/0892701021000041078. [DOI] [PubMed] [Google Scholar]
- 11.Cervantes C, Campos-García J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R. 2001. Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347. doi: 10.1111/j.1574-6976.2001.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 12.Emerson D. 2019. The role of iron-oxidizing bacteria in biocorrosion: a review. Biofouling 34:989–1000. doi: 10.1080/08927014.2018.1526281. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Xu C, Zhang Y, Cheng G, Zhu W. 2007. Localized corrosion behavior of 316L stainless steel in the presence of sulfate-reducing and iron-oxidizing bacteria. Mater Sci Eng A 443:235–241. doi: 10.1016/j.msea.2006.08.110. [DOI] [Google Scholar]
- 15.Vandecandelaere I, Nercessian O, Faimali M, Segaert E, Mollica A, Achouak W, De Vos P, Vandamme P. 2010. Bacterial diversity of the cultivable fraction of a marine electroactive biofilm. Bioelectrochemistry 78:62–66. doi: 10.1016/j.bioelechem.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Baker PW, Ito K, Watanabe K. 2003. Marine prosthecate bacteria involved in the ennoblement of stainless steel. Environ Microbiol 5:925–932. doi: 10.1046/j.1462-2920.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 17.McBeth JM, Fleming EJ, Emerson D. 2013. The transition from freshwater to marine iron‐oxidizing bacterial lineages along a salinity gradient on the Sheepscot River, Maine, USA. Environ Microbiol Rep 5:453–463. doi: 10.1111/1758-2229.12033. [DOI] [PubMed] [Google Scholar]
- 18.Emerson D, Floyd MM. 2005. Enrichment and isolation of iron‐oxidizing bacteria at neutral pH. Methods Enzymol 397:112–123. doi: 10.1016/S0076-6879(05)97006-7. [DOI] [PubMed] [Google Scholar]
- 19.Chiu BK, Kato S, McAllister SM, Field EK, Chan CS. 2017. Novel pelagic iron-oxidizing zetaproteobacteria from the Chesapeake Bay oxic-anoxic transition zone. Front Microbiol 8:1280. doi: 10.3389/fmicb.2017.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards KJ, Rogers DR, Wirsen CO, McCollom TM. 2003. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic α-and γ-Proteobacteria from the deep sea. Appl Environ Microbiol 69:2906–2913. doi: 10.1128/AEM.69.5.2906-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brierley JA. 1978. Thermophilic iron-oxidizing bacteria found in copper leaching dumps. Appl Environ Microbiol 36:523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllister SM, Moore RM, Chan CS. 2018. ZetaHunter, a reproducible taxonomic classification tool for tracking the ecology of the Zetaproteobacteria and other poorly resolved taxa. Microbiol Res Announc 7:e00932-18. doi: 10.1128/MRA.00932-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer E, Emerson D, Webb EA, Barco RA, Kuenen JG, Nelson WC, Chan CS, Comolli LR, Ferriera S, Johnson J, Heidelberg JF, Edwards KJ. 2011. Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium. PLoS One 6:e25386. doi: 10.1371/journal.pone.0025386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krepski ST, Hanson TE, Chan CS. 2012. Isolation and characterization of a novel biomineral stalk‐forming iron‐oxidizing bacterium from a circumneutral groundwater seep. Environ Microbiol 14:1671–1680. doi: 10.1111/j.1462-2920.2011.02652.x. [DOI] [PubMed] [Google Scholar]
- 26.Barco RA, Emerson D, Sylvan JB, Orcutt BN, Meyers MEJ, Ramírez GA, Zhong JD, Edwards KJ. 2015. New insight into microbial iron oxidation as revealed by the proteomic profile of an obligate iron-oxidizing chemolithoautotroph. Appl Environ Microbiol 81:5927–5937. doi: 10.1128/AEM.01374-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAllister SM, Moore RM, Gartman A, Luther GW, Emerson D, Chan CS. 2018. Marine Fe-oxidizing Zetaproteobacteria: historical. ecological, and genomic perspectives bioRxiv 416842. doi: 10.1101/416842. [DOI] [PMC free article] [PubMed]
- 28.Fleming EJ, Davis RE, McAllister SM, Chan CS, Moyer CL, Tebo BM, Emerson D. 2013. Hidden in plain sight: discovery of sheath-forming, iron-oxidizing Zetaproteobacteria at Loihi Seamount, Hawaii, USA. FEMS Microbiol Ecol 85:116–127. doi: 10.1111/1574-6941.12104. [DOI] [PubMed] [Google Scholar]
- 29.Barco RA, Hoffman CL, Ramírez GA, Toner BM, Edwards KJ, Sylvan JB. 2017. In‐situ incubation of iron‐sulfur mineral reveals a diverse chemolithoautotrophic community and a new biogeochemical role for Thiomicrospira. Environ Microbiol 19:1322–1337. doi: 10.1111/1462-2920.13666. [DOI] [PubMed] [Google Scholar]
- 30.Hayes JR, Gray JJ, Szmodis AW, Orme CA. 2006. Influence of chromium and molybdenum on the corrosion of nickel-based alloys. Corrosion 62:491–500. doi: 10.5006/1.3279907. [DOI] [Google Scholar]
- 31.Friedrich CG, Meyer O, Chandra TS. 1986. Molybdenum-dependent sulfur oxidation in facultatively lithotrophic thiobacteria. FEMS Microbiol Lett 37:105–108. doi: 10.1111/j.1574-6968.1986.tb01775.x. [DOI] [Google Scholar]
- 32.Glass JB, Axler RP, Chandra S, Goldman CR. 2012. Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front Microbiol 3:331. doi: 10.3389/fmicb.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field EK, Sczyrba A, Lyman AE, Harris CC, Woyke T, Stepanauskas R, Emerson D. 2015. Genomic insights into the uncultivated marine Zetaproteobacteria at Loihi Seamount. ISME J 9:857. doi: 10.1038/ismej.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field EK, Kato S, Findlay AJ, MacDonald DJ, Chiu BK, Luther IIG, Chan CS. 2016. Planktonic marine iron oxidizers drive iron mineralization under low‐oxygen conditions. Geobiology 14:499–508. doi: 10.1111/gbi.12189. [DOI] [PubMed] [Google Scholar]
- 35.Chan CS, Fakra SC, Edwards DC, Emerson D, Banfield JF. 2009. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim Cosmochim Acta 73:3807–3818. doi: 10.1016/j.gca.2009.02.036. [DOI] [Google Scholar]
- 36.Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ. 2011. Lithotrophic iron-oxidizing bacteria produce stalks to control mineral growth: implications for biosignature formation. ISME J 5:717–727. doi: 10.1038/ismej.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato S, Ohkuma M, Powell DH, Krepski ST, Oshima K, Hattori M, Shapiro N, Woyke T, Chan CS. 2015. Comparative genomic insights into ecophysiology of neutrophilic, microaerophilic iron oxidizing bacteria. Front Microbiol 6:1265. doi: 10.3389/fmicb.2015.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori JF, Scott JJ, Hager KW, Moyer CL, Kusel K, Emerson D. 2017. Physiological and ecological implications of an iron- or hydrogen-oxidizing member of the Zetaproteobacteria, Ghiorsea bivora, gen. nov., sp. nov. ISME J 11:2624–2636. doi: 10.1038/ismej.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curiale M. 2012. MPN calculator. http://mpn-calculator.freedownloadscenter.com/windows.
- 40.R Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- 41.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. [Google Scholar]
- 42.Marschner IC. 2011. glm2: fitting generalized linear models with convergence problems. R J 3:12–15. [Google Scholar]
- 43.Lane DJ. 1991. 16S/23S rRNA sequencing In Stackebrandt E, Goodfellow M (eds), Nucleic acid techniques in bacterial systematics, p 115–175. John Wiley & Sons, New York, NY. [Google Scholar]
- 44.Turner S, Pryer KM, Miao VP, Palmer JD. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 45.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 46.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 48.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomason JA, Stevens R, Vonstein V, Wattam AR, Xia F. 2015. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, Gilbert JA, Jansson JK, Caporaso JG, Fuhrman JA, Apprill A, Knight R. 2015. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1:e00009-15. doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GenBank accession numbers for the 16S rRNA gene sequences of Mariprofundus erugo strain P3 and Mariprofundus erugo strain P7 are MK554583 and MK554584, respectively. The NCBI accession numbers for M. erugo P3 and M. erugo P7 whole-genome sequences are SAMN11634928 and SAMN11634956, respectively.