The present study was conducted to evaluate changes in virulence properties in Escherichia coli O157:H7 adapted to sublethal essential oils (EOs). The results demonstrated reduced motility, biofilm-forming ability, and efflux pump activities in EO-adapted E. coli O157:H7, with no induction of antibiotic resistance or infection (adhesion and invasion) on Caco-2 cells. Reverse transcription-quantitative PCR results revealed changes in the expression of related virulence genes. Thus, the present study provides new insights into microbial virulence behavior following EO adaptation and suggests that Thy, Car, and TC sublethal exposure did not constitute a significant risk in inducing microbial virulence.
KEYWORDS: Caco-2 cell line, E. coli O157:H7, antibiotic resistance, biofilm, essential oil adaptation, motility
ABSTRACT
Essential oils (EOs) have demonstrated wide-spectrum antimicrobial activities and have been actively studied for their application in foods as alternative natural preservatives. However, information regarding microbial adaptive responses and changes in virulence properties following sublethal EO exposure is still scarce. The present study investigated the effect of sublethal thymol (Thy), carvacrol (Car), or trans-cinnamaldehyde (TC) adaptation on virulence gene expression and virulence properties of Escherichia coli O157:H7. The results demonstrated that E. coli O157:H7 grown to the early stationary phase in the presence of sublethal EO showed significantly (P < 0.05) reduced motility (reversible after stress removal), biofilm-forming ability, and efflux pump activity, with no induction of antibiotic resistance and no significant changes to its adhesion and invasion ability on a human colon adenocarcinoma (Caco-2) cell line. Reverse transcription-quantitative PCR revealed reduced expression of relevant virulence genes, including those encoding flagellar biosynthesis and function, biofilm formation regulators, multidrug efflux pumps, and type III secretion system components. This study demonstrated that Thy, Car, and TC at sublethal concentrations did not potentiate virulence in adapted E. coli O157:H7, which could benefit to their application in the food industry.
IMPORTANCE The present study was conducted to evaluate changes in virulence properties in Escherichia coli O157:H7 adapted to sublethal essential oils (EOs). The results demonstrated reduced motility, biofilm-forming ability, and efflux pump activities in EO-adapted E. coli O157:H7, with no induction of antibiotic resistance or infection (adhesion and invasion) on Caco-2 cells. Reverse transcription-quantitative PCR results revealed changes in the expression of related virulence genes. Thus, the present study provides new insights into microbial virulence behavior following EO adaptation and suggests that Thy, Car, and TC sublethal exposure did not constitute a significant risk in inducing microbial virulence.
INTRODUCTION
Escherichia coli O157:H7 is one of the leading causes of bacterial gastroenteritis in humans, resulting in an estimated 73,000 illness cases annually in the United States (1). Diseases caused by E. coli O157:H7 range from watery diarrhea to hemorrhagic colitis and life-threatening hemolytic uremic syndrome, with much higher hospitalization and fatality rates compared to other enteric pathogens such as Salmonella or Campylobacter spp. (1). According to the Centers for Disease Control and Prevention, foodborne transmission accounted for 52% of E. coli O157:H7 outbreaks, with contaminated beef, dairy products, ready-to-eat fresh produce, and fruits being the most commonly identified food sources (2). In addition, E. coli O157:H7 are able to form biofilm on various surfaces, which makes their elimination from food processing facilities impossible (3).
Given the importance of E. coli O157:H7 in human illness, it is critical that strategies controlling the risk from this pathogen are available. The increasing bacterial resistance to conventional preservatives and the consumer demand for natural food additives have encouraged research for finding novel antimicrobials. Essential oils (EOs) are gaining popularity in recent years, due to their natural origin, wide-spectrum antimicrobial activities, and generally recognized as safe (GRAS) status for food usage (4). In particular, thymol (Thy), carvacrol (Car), and trans-cinnamaldehyde (TC), the principal compounds of thyme, oregano, and cinnamon EOs, respectively, have been demonstrated to possess good antimicrobial activities against various foodborne pathogens, including E. coli O157:H7 (5), Staphylococcus aureus (6), Salmonella enterica serovar Typhimurium (7), Pseudomonas aeruginosa (3), and Campylobacter jejuni (8).
The main obstacle limiting the application of EOs in foods is that their antimicrobial dosages in food matrix are often higher than the organoleptically acceptable concentrations (9), which demands their use at sublethal concentrations in combination with other preservative methods (the hurdle approach) (10). In addition, their interaction with food components such as proteins and fats, as well as their potential loss during storage due to highly volatile nature (4), could further reduce the active concentration to below lethal level. It is well known that exposure of microorganisms to sublethal stresses such as biocides (11), weak acid, and mild heat (12) would induce microbial adaptive responses and result in increased stress tolerance and possibly higher virulence in stress-adapted bacteria (13). However, limited studies have been conducted to evaluate microbial adaptive response and virulence changes toward sublethal EO exposure.
Our previous study (14) using RNA sequencing revealed altered expression of various virulence genes in E. coli O157:H7 grown to early stationary phase in the presence of sublethal Thy, Car, or TC, including genes related to flagellar biosynthesis and function, chemotaxis, biofilm development, type III secretion systems (T3SS), and efflux pumps. However, the biological significance of these transcriptional changes has not yet been verified. Therefore, the present study aimed to understand the effect of sublethal EO (Thy, Car, TC) adaptation on E. coli O157:H7 virulence properties, including motility, biofilm formation, adherence to and invasion of the Caco-2 cell line, and antibiotic resistance. In addition, changes in the expression of related virulence genes were determined using reverse transcription-quantitative PCR (RT-qPCR).
RESULTS AND DISCUSSION
Changes in virulence gene expression in EO-adapted E. coli O157:H7.
It was observed that adaptation of E. coli O157:H7 to sublethal concentrations of Thy, Car, or TC resulted in the upregulation of genes encoding multidrug efflux pumps (marA and acrB) and negative regulators of biofilm formation (tnaA and bssS). On the other hand, several virulence genes related to flagellar biosynthesis and functions (fliA and motA), chemotaxis (cheA and cheZ), and T3SS (sepD and escC) were downregulated in EO-adapted bacteria, compared to nonadapted controls (Fig. 1). Similar to these results, Johny et al. (15) reported that 30 min of incubation of Salmonella enterica serovar Enteritidis with sublethal TC (0.1 mg/ml) or eugenol (Eug; 0.4 mg/ml) repressed genes associated with bacterial virulence and colonization, including those conferring flagellum-associated motility, epithelial cell invasion, T3SS biosynthesis, and other surface virulence structures. It is known that exposure of E. coli O157:H7 to various plant-derived antimicrobials (Thy, Car, TC, and Eug) repressed genes that are critical for colonization in cattle gastrointestinal tract (16). Moreover, Amalaradjou et al. (17) revealed that sublethal TC downregulated genes that are critical for host tissue colonization in uropathogenic E. coli, thereby suppressing the attaching and invading ability of the bacteria.
FIG 1.
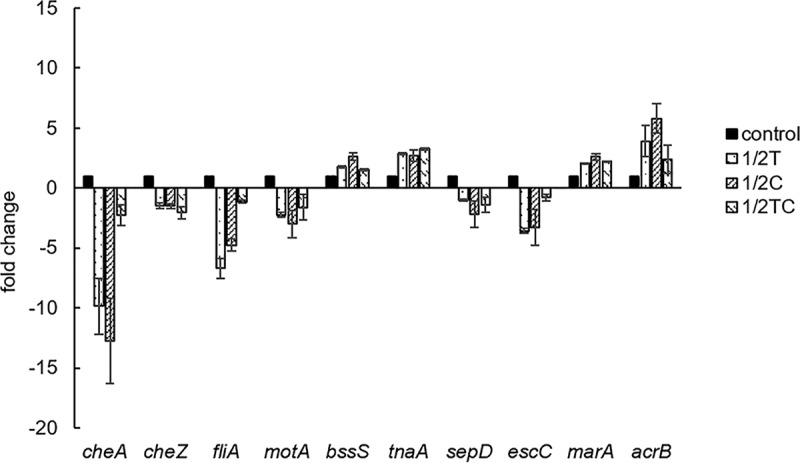
Relative expression levels of virulence genes related to chemotaxis (cheA and cheZ), flagella (fliA and motA), biofilm (bssS and tnaA), the T3SS (sepD and escC), and antibiotic resistance (marA and acrB) in E. coli O157:H7 adapted to sublethal concentrations of thymol (1/2T), carvacrol (1/2 C), or trans-cinnamaldehyde (1/2TC), compared to nonadapted cells (control).
To confirm whether the observed changes in virulence gene expression would lead to observable phenotype changes, we performed experiments on EO-adapted E. coli O157:H7 to assess its flagellum-mediated motility, biofilm-forming ability, T3SS-dependent intestinal cell infection (adhesion and invasion), and antibiotic resistance, which are critical virulence factors for full E. coli O157:H7 pathogenicity.
Changes in motility of EO-adapted E. coli O157:H7.
No significant difference (P > 0.05) in the diameters of motility halos was observed between nonadapted control and EO-adapted E. coli O157:H7 strains, when these bacteria were incubated in swim agar for 6 h at 37°C (Fig. 2). This observation contradicted our RT-qPCR results, where genes related to bacterial motility were found to be significantly downregulated. These include fliA (−1.1- to −6.7-fold), sigma factors that promote the biosynthesis of flagellar components (18); motA (−1.6- to −3.0-fold), components of flagellar stator complex that powers flagellar motor rotation (19); and cheA (−2.3- to −12.8-fold) and cheZ (−1.5- to −2.1-fold), components of a signaling complex that influences flagellar rotation and cell swimming behavior in response to external stimuli (20) (Fig. 1). Similar to our RT-qPCR results, Carraro et al. (21) found repression (−3.34- to −34.11-fold change) of various motility and chemotaxis genes in E. coli K-12 treated with polyphenols from olive mill waste. Burt et al. (5) reported that overnight incubation of E. coli O157:H7 with 1 mM Car inhibited flagellin synthesis, resulting in aflagellate and nonmotile cells, and cell motility was negatively correlated with Car concentration.
FIG 2.
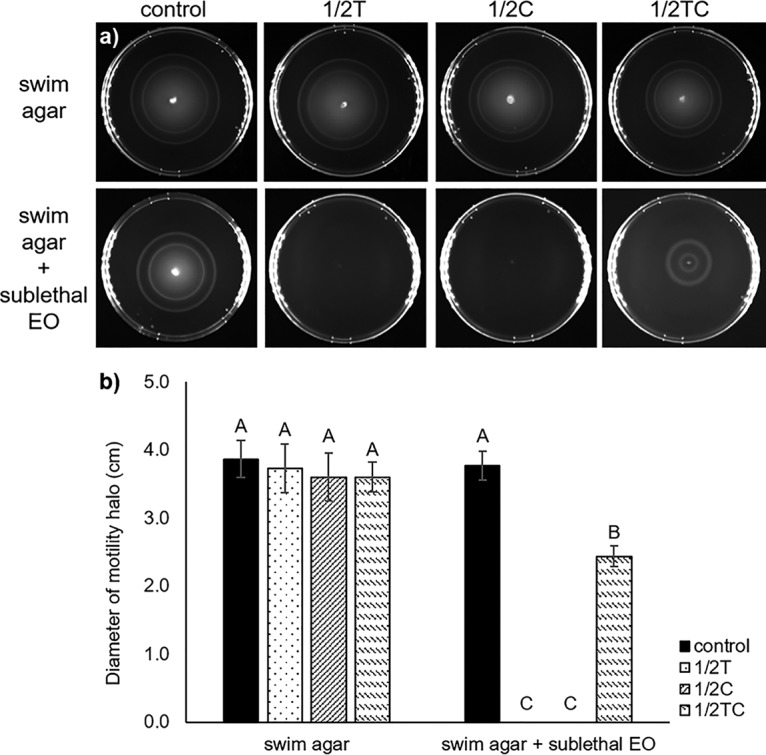
Representative image (a) and diameter (b) of motility halo of E. coli O157:H7, nonadapted (control) or adapted to sublethal thymol (1/2T), carvacrol (1/2 C), or trans-cinnamaldehyde (1/2TC), in swim agar without (recovery media) or with a sublethal level of EO (continuous stress media). Different capital letters (A to C) represent statistically significant (P < 0.05) differences between control and EO-adapted cells.
Based on RT-qPCR results and the previously reported studies, it was hypothesized that E. coli O157:H7 cells might have recovered and regained the lost motility when incubated in nutrient-rich swim agar (recovery media). To confirm this hypothesis, swim agar containing different sublethal EOs (continuous stress media) was prepared and used for incubation. As expected, swim agar containing sublethal Thy or Car was found to completely inhibit microbial motility, while agar containing sublethal TC significantly (P < 0.05) reduced motility compared to a control plate without EO (Fig. 2). This observation agrees with previously reported studies, wherein soft agar containing sublethal Car, TC, or Eug were found to significantly (P < 0.05) reduce the motility of S. Typhimurium and C. jejuni (8, 22).
Flagellar and chemotaxis systems are essential for full pathogenesis of E. coli, since flagellated pathogens are able to swim toward beneficial environment and away from toxic compounds, thereby having greater survival advantages (20). Moreover, flagella play essential role in adhesion, biofilm formation, secretion of effector molecules, and the host immune system defense, which enhances pathogen virulence during infection (23). However, bacterial motility and chemotaxis systems are physiologically expensive, requiring approximately 3% of total cellular proteins to carry out these functions (24). It is known that EOs, even at sublethal concentrations, could damage microbial membranes, dissipate membrane potential, and induce intracellular ATP leakage in treated bacteria (7). Therefore, when subjected to sublethal EO stress, it is expected that E. coli O157:H7 would repress its motility systems and conserve energy for vital cellular activities and damage repair until a suitable growth condition was provided. Moreover, the stronger motility inhibition observed in Thy- and Car-containing plates, compared to TC-containing plates, could be explained by the stronger bactericidal activities of Thy and Car compounds (4). Thus, the present results indicate that Thy, Car, and TC present at sublethal concentrations could reduce E. coli O157:H7 virulence by abolishing or reducing microbial motility.
Changes in biofilm forming ability of EO-adapted E. coli O157:H7.
It was observed that E. coli O157:H7 adapted to sublethal Thy, Car, or TC had significantly (P < 0.05) compromised biofilm-forming ability, since it could only produce 63 to 69% of the amount of biofilm compared to that of a nonadapted control (Fig. 3). It is well documented that E. coli O157:H7 is able to attach, colonize, and form biofilms on various food contact surfaces and equipment, which increases the risk of food contamination during processing (25). Past studies focused mainly on using EOs as antimicrobial treatment to prevent biofilm formation (26) or destroy existing biofilms (27), whereas the results obtained in this study demonstrated that preadaptation to sublethal level of Thy, Car, or TC had an extended impact on E. coli O157:H7 biofilm formation, even after EO removal and nutrient replenishment.
FIG 3.
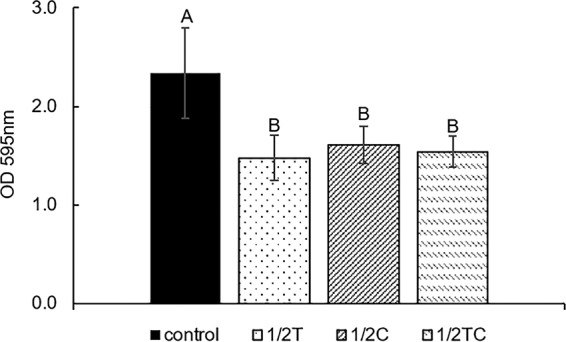
Biofilm-forming ability of E. coli O157:H7, nonadapted (control) or adapted in 0.16 mg/ml thymol (1/2T), carvacrol (1/2 C), or trans-cinnamaldehyde (1/2TC), in TSB. Different capital letters (A and B) represent statistically significant (P < 0.05) differences between control and EO-adapted cells.
In accordance with our biofilm assay results, RT-qPCR revealed enhanced expression of the genes tnaA (2.7- to 3.2-fold) and bssS (1.5- to 2.6-fold), which were negative regulators of biofilm formation. Gene tnaA encodes enzyme tryptophanase, which is involved in the production of indole (28), a signaling molecule that represses biofilm formation in E. coli (29). Similarly, BssS was reported to reduce bacterial biofilm formation by influencing cell signaling, and the deletion of the bssS gene was found to increase biofilm formation in E. coli (29). Consistent with these observations, our previous RNA sequencing study (14) revealed that multiple points of biofilm formation pathways were affected, including genes for the biosynthesis of flagella (flhCD, fliAZ, and flgM), poly-N-acetylglucosamine (csrB), colonic acid (wza), and curli (csgD).
Changes in the adhesion and invasion properties of EO-adapted E. coli O157:H7 for the Caco-2 cell line.
Genes involved in T3SS biosynthesis and function, namely, sepD and escC, were downregulated 1.0- to 2.2-fold and 0.8- to 3.6-fold, respectively, in EO-adapted E. coli O157:H7 (Fig. 1). Genes sepD and escC encode essential components of T3SS, and the lack of these genes in E. coli mutants was found to impair the pathogen’s ability to adhere and induce attaching and effacing lesions in host cells (30, 31). The RT-qPCR results prompted us to study the adhesion and invasion abilities of EO-adapted bacteria for the Caco-2 cell line, since these T3SS-dependent virulence properties were essential for full E. coli O157:H7 pathogenicity (32). To our surprise, no significant reduction (P > 0.05) was found in the percentage of EO-adapted bacterial cells that attached to and/or invaded the Caco-2 cell line relative to this percentage for the nonadapted control (Fig. 4). In contrast to the present results, S. Typhimurium adapted to 0.08 and 0.12 mg/ml Car to log phase showed reduced invasion of, but no difference in adhesion to, human (Caco-2) and porcine (IPEC-J2) epithelial cell lines (22). Similarly, E. coli O157 (EDL933) and non-O157 enterohemorrhagic E. coli strains adapted to sublethal Car (0.016%) to stationary phase showed a 9.2 to 21.6% reduction in adherence to the human ileocecal carcinoma cell line HCT-8 (33). In contrast, van Alphen et al. (34) found that a 16-h preadaptation with sublethal Car (0.03 mg/ml) reduced C. jejuni invasion of INT-407 intestinal epithelial cells by 54.4% but had no effect on the invasiveness of Car-adapted E. coli. Therefore, it is possible that the EO-adaptive response is organism and strain dependent; more bacterial isolates should be evaluated in future studies to gain a better understanding of the microbial adaptive response to sublethal EO adaptation.
FIG 4.
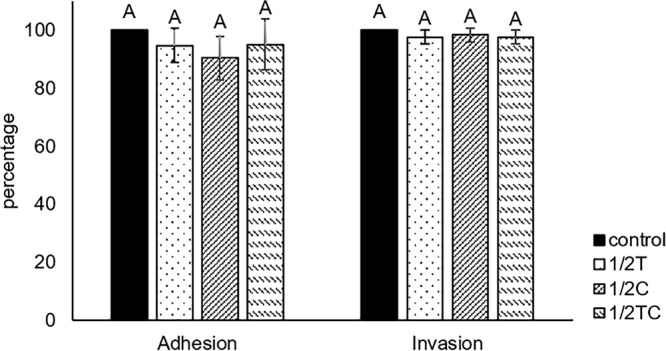
Adhesion and invasion ability of E. coli O157:H7, nonadapted (control) or adapted in 0.16 mg/ml thymol (1/2T), carvacrol (1/2C), or trans-cinnamaldehyde (1/2TC), to the Caco-2 cell line.
One possible reason for the discrepancies between RT-qPCR and Caco-2 assay results could be that the repression of the T3SS gene expression levels in EO-adapted bacteria was reversed during incubation with the Caco-2 cell line. Giron et al. (35) previously demonstrated that the presence of HeLa epithelial cells stimulated and restored the expression of surface adhesive structure in enteropathogenic E. coli strain due to signaling molecules produced by eukaryotic cells. Furthermore, since E. coli O157:H7 possesses a complex intestine adherence system consisting of more than 25 colonization factors (30), it is also possible that the production of other virulence factor masked the defects in T3SS and enabled normal microbial infection of the intestinal cell line.
Changes in antibiotic resistance and efflux pump activities of EO-adapted E. coli O157:H7.
The effects of sublethal EO exposure on the development of E. coli O157:H7 antibiotic resistance were minimal when evaluated by standard MIC assessment criteria (Table 1), where an MIC difference of ≤2-fold was not considered significant (36). In addition, both control and EO-adapted bacteria remained “susceptible” to all antibiotics evaluated according to the CLSI MIC breakpoint classification (36). On the other hand, the efflux pump genes marA and acrB were upregulated 2.1- to 2.6-fold and 2.4- to 5.8-fold, respectively, in EO-adapted bacteria compared to the nonadapted control (Fig. 1). In E. coli, the multiple antibiotic resistance (mar) gene marA is known to be a global regulator that controls the expression of more than 60 chromosomal genes, resulting in increased pathogen resistance to antibiotics and other environmental hazards (37). Among the MarA-regulated gene products, multidrug efflux pumps such as AcrAB can expel a wide variety of structurally unrelated antibiotics, including quinolones, β-lactams, tetracyclines, and aminoglycosides (38), and were found to play a major role in E. coli multidrug resistance (MDR) (39).
TABLE 1.
MICs of various antibiotics against E. coli O157:H7, either nonadapted (control) or adapted to sublethal thymol (1/2T), carvacrol (1/2C), or trans-cinnamaldehyde (1/2TC)
| Antibiotic | Avg MIC (μg/ml) |
|||
|---|---|---|---|---|
| Control | 1/2T | 1/2C | 1/2TC | |
| Tetracycline | 4 | 4 | 4 | 4 |
| Ampicillin | 5 | 4 | 5 | 4 |
| Nalidixic acid | 7 | 6 | 6 | 6 |
| Gentamicin | 6 | 6 | 4 | 5 |
| Chloramphenicol | 20 | 18 | 24 | 16 |
| Streptomycin | 22 | 19 | 19 | 21 |
| Novobiocin | 630 | 692 | 692 | 630 |
There has been a growing concern on the use of antibacterial agents in the food industry, as chronic exposure of bacteria to sublethal concentration of food preservatives (40) and disinfectants (41) has been found to increase microbial antibiotic resistance. Among the limited number of studies conducted on EO adaptation to date, inconsistent results were obtained for their induction on bacterial MDR development. Repeated exposure to EOs (tea tree EO, oregano EO, and cinnamon EO) or their major components (terpinen-4-ol, eugenol, and TC) has a minimal impact on antibiotic resistance development (MIC change ≤ 2-fold) in E. coli, P. aeruginosa, and S. aureus (42–44). In contrast, habituation of Salmonella spp., E. coli, S. aureus, and methicillin-resistant S. aureus to tea tree EO (45) or linalool (46) was found to increase the MIC by up to 32-fold in adapted bacteria versus standard antibiotics. Our results demonstrate that although sublethal Thy, Car, and TC induced the expression of efflux pump genes, they had little impact on the development of MDR in adapted E. coli O157:H7 and were thus not considered a potential risk to the health of the general public.
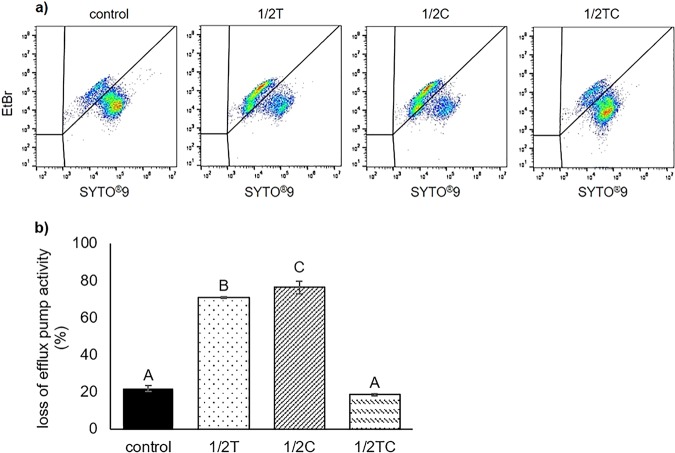
To better understand the seemingly contradictory results from the MIC assay and RT-qPCR, we measured the loss of efflux pump activity in nonadapted and EO-adapted bacteria with SYTO9/ethidium bromide (EtBr) stain using flow cytometry. SYTO9 enters both live and dead bacteria, binds to nucleic acids, and emits green fluorescence. On the other hand, EtBr enters only cells with inactive efflux pumps, displaces SYTO9 from nucleic acid, and emits red fluorescence (47). It was observed that significantly (P < 0.05) greater amounts of E. coli O157:H7 lost their efflux pump activities after adaptation to sublethal Thy (70.9% ± 0.4%) or Car (76.2% ± 3.5%) compared to nonadapted controls (21.9% ± 0.1%), whereas no significant difference (P > 0.05) was found between nonadapted controls and TC-adapted cells (18.4% ± 0.5%) (Fig. 5). Previous studies suggested that some natural products were also substrates for multidrug efflux pumps and could “block” the activities of these efflux pumps from expelling antibiotics by competitive binding and saturation of the pumps, thereby restoring the susceptibility of MDR bacteria against standard antibiotics (48, 49). Results from the present study provided a different perspective, i.e., that EOs (particularly Thy and Car) could directly disrupt efflux pump activities, which could be the consequence of EO-protein interaction or due to the depletion of cellular energy after membrane disruption (47). Therefore, the enhanced expression of efflux pump genes could be the bacteria’s attempt to balance stresses imposed by EOs, so they could continue to expel harmful intracellular compounds (e.g., metabolic by-products) and maintain a normal cellular environment condusive for bacterial growth.
FIG 5.
Representative flow cytometry plots (a) and calculated percentage loss of efflux pump activity (b), assessed using SYTO9/EtBr stain, in nonadapted E. coli O157:H7 (control) and cells adapted to sublethal thymol (1/2T), carvacrol (1/2C), or trans-cinnamaldehyde (1/2TC). Different capital letters (A to C) represent statistically significant (P < 0.05) differences between control and EO-adapted cells.
Conclusion.
The present results show that E. coli O157:H7 grown to the early stationary phase in the presence of sublethal Thy, Car, or TC shows altered virulence gene expression, temporary suppression of motility (reversible after stress removal and nutrient replenishment), and reduced biofilm-forming ability. No significant effect was found on the abilities of EO-adapted E. coli O157:H7 to adhere to and invade the Caco-2 cell line. Furthermore, EOs (especially Thy and Car) at sublethal concentrations reduced efflux pump activities but did not induce antibiotic resistance in adapted bacteria. Overall, adaptation to sublethal Thy, Car, or TC did not enhance the virulence of E. coli O157:H7, and thus these EOs could be explored further as natural antimicrobials or disinfectants for application in the food industry.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
A frozen stock of E. coli O157:H7 (ATCC 35150) was purchased from the American Type Culture Collection (ATCC; Manassas, VA) and stored at –80°C in Cryoinstant vials with porous beads (DeltaLab, Barcelona, Spain). Frozen culture was activated in sterile tryptic soy broth (TSB; Oxoid, Hampshire, England) by two consecutive transfers (37°C, 24 h), and daily transfer was performed to maintain cell viability. Cells were washed twice in 1× phosphate-buffered saline (PBS; Vivantis, Inc., Oceanside, CA) with centrifugation (5,000 × g, 4°C, 5 min) and diluted in TSB to an appropriate concentration before use.
Preparation of essential oil-adapted cells.
Thy, Car, and TC were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). Stock solutions of EOs were prepared in 95% ethanol at 100 mg/ml and stored at 4°C in the dark until use. To prepare EO-adapted bacteria, an overnight E. coli O157:H7 culture was inoculated (107 CFU/ml) into 10 ml of fresh TSB containing 1.6% (vol/vol) ethanol (nonadapted control) or 0.16 mg/ml Thy, Car, or TC, a sublethal concentration that was determined to have no inhibitory effect on bacterial growth (data not shown), followed by incubation at 37°C until the early stationary phase. In the present study, “EO-adapted bacteria” is defined as a “bacterial population that is able to grow in the presence of EO to stationary phase, with a final population of 9.0 ± 0.5 log CFU/ml, within 24 h of incubation.”
Effect of EO adaptation on virulence gene expression.
Early-stationary-phase nonadapted and EO-adapted E. coli O157:H7 cultures were stabilized with RNAprotect bacterial reagent (Qiagen, Hilden, Germany), and RNA extraction was performed using an RNeasy minikit (Qiagen) with on-column DNase digestion according to the manufacturer’s protocols. The quality and concentration of extracted RNA were verified using NanoDrop 1000 (Thermo Fisher Scientific, Inc., Wilmington, DE), and the integrity was checked by agarose gel electrophoresis. Template cDNA was synthesized from 1 μg of extracted total RNA by using the GoScript reverse transcription system (A5000; Promega) and used as the template for qPCR with a SYBR Select Master mix kit (catalog no. 4472953; Applied Biosystems). Each qPCR mixture (total volume, 25 μl) consisted of 1 μl of cDNA, 1 μl of each forward and reverse primer, 10 μl of SYBR green supermix, and 7 μl of nuclease-free water. The 16S rRNA gene was used as an endogenous control for normalization within samples. Forward and reverse PCR primers for the virulence genes were designed using Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/) (Table 2).
TABLE 2.
Primers used in this study
| Function | Gene | Sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| Housekeeping | 16S rRNA | F: AGAGGATGACCAGCCACAC | 194 |
| R: CGGGTAACGTCAATGAGCAAAG | |||
| Chemotaxis | cheA | F: AGGCTATTGCGTACCAGGTG | 109 |
| R: AACTCGGCAAGCAGGTAGAA | |||
| cheZ | F: ATCGTCCACCTGATCCTGAC | 137 | |
| R: AGTTGCTGATGGTGCTGTTG | |||
| Flagella | fliA | F: CTATTGCCTGTGCCACTTCA | 141 |
| R: GCTATGACGCCCTACAAGGA | |||
| motA | F: GGTGCGTAACCGTTCAGATT | 135 | |
| R: TTTCCTCGGCATTTTATTGG | |||
| Biofilm | bssS | F: TCCTGCTCGGACTTATTTGG | 104 |
| R: ATTCAGACTCATCCGCTCGT | |||
| tnaA | F: CAAAGAAGCCTTCGATACGG | 135 | |
| R: GCGGAGTTACTGGTGATGGT | |||
| T3SS | sepD | F: TGCTTTCTTGCACGATTTTG | 109 |
| R: GGCCAGGATATTGCAGAGTC | |||
| escC | F: GAGAAAAGGTGGCGTCTCTG | 93 | |
| R: CGGTAAGGCTATTCGAGCTG | |||
| Efflux pumps | marA | F: AATACATCCGCAGCCGTAAG | 146 |
| R: CGGCGGAACATCAAAGTAAT | |||
| acrB | F: ATCAATGGGCGAGATTTCTG | 137 | |
| R: GCATTGCGGAGATAATGGTT |
Real-time PCR was performed using a StepOnePlus real-time PCR system (Applied Biosystems), with the following thermal cycling conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. Specificity of PCR was determined with melting-curve analysis (60 to 95°C with a heating rate of 0.3°C/s) and agarose gel electrophoresis. The relative changes in gene expression in EO-adapted cells compared to nonadapted control were calculated using the 2–ΔΔCT method (50).
Effect of EO adaptation on motility.
The swimming motility of E. coli O157:H7 was evaluated as previously described (26). Swim agar plates were prepared by adding 25 ml of motility agar (Luria-Bertani broth containing 0.3% agar; Sigma-Aldrich) into a petri dish (94 by 16 mm; Sigma-Aldrich) and allowed to solidify overnight at room temperature. Then, 25 μl of an early-stationary-phase E. coli O157:H7 culture (ca. 109 CFU/ml), nonadapted or EO adapted, was inoculated into the center of swim agar plate. The diameter of each motility halo (bacterial migration distance from the site of inoculation) was measured after 6 h of incubation at 37°C.
Effect of EO adaptation on biofilm formation.
Early-stationary-phase nonadapted and EO-adapted E. coli O157:H7 cells were diluted to a final concentration of 105 CFU/ml in fresh TSB, and 200 μl of the diluted culture was placed in a sterile 96-well plate (Thermo Fisher Scientific). After 24 h of incubation at 37°C, the amount of biofilm formed was quantified using a crystal violet staining method according to Lee et al. (51). Briefly, each well was washed three time with sterile deionized (DI) water to remove loosely attached cells and then stained with 200 μl of 0.1% (wt/vol) crystal violet solution for 20 min at room temperature. Subsequently, the staining solution was discarded, rinsed three times with DI water, and incubated with 200 μl of 95% ethanol at 4°C for 15 min to extract dye from biofilm. Absorption was then measured at 595 nm using a Multiskan FC microplate photometer (Thermo Fisher Scientific).
Effect of EO adaptation on the ability to adhere to and invade the Caco-2 cell line.
The Caco-2 human colon adenocarcinoma cells (ATCC HTB-37), purchased from the ATCC, were maintained in Dulbecco modified Eagle medium (Invitrogen/Life Technologies, Carlsbad, CA) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Invitrogen) at 37°C in 5% CO2. The growth medium was changed every 3 days, and cells were passaged once a week. For infection experiments, Caco-2 cells were seeded into 12-well cell culture plate (Sigma-Aldrich) at 105 cells per well and grown to semiconfluence before use.
Bacterial infection assay of Caco-2 cell line was performed according to Inamuco et al. (22). For adhesion assay, semiconfluence Caco-2 monolayers were washed three times with 1× PBS, and each well was infected with 1 ml 106 CFU/ml nonadapted or EO-adapted E. coli O157:H7 (multiplicity of infection = 10). After 2 h of incubation at 37°C in 5% CO2, the infected monolayers were washed three times with 1× PBS and lysed in 0.1% (vol/vol) Triton X-100 (Sigma-Aldrich) for 15 min at room temperature. The resulting suspensions were plated onto tryptic soy agar (TSA; Oxoid) with appropriate dilutions, and the number of adhesion bacteria was counted after 24 h of incubation at 37°C. For invasion assay, the infected Caco-2 monolayers were washed three times with 1× PBS and then incubated with 1 ml of 250 μg/ml gentamicin (Sigma-Aldrich) at 37°C in 5% CO2 for 2 h to kill extracellular bacteria. The treated Caco-2 monolayers were then washed and lysed, and the number of invasion bacteria was counted by TSA plating, as described above. The percentages of adhesion and invasion bacteria were calculated by dividing the number of adhered or invasion cell over inoculum, and final results are expressed as percentages relative to that of control (control = 100%).
Effect of EO adaptation on antibiotic resistance.
Antibiotic resistance of nonadapted and EO-adapted E. coli O157:H7 was evaluated using the Clinical and Laboratory Standards Institute (CLSI) microdilution method (36). Briefly, a 2-fold serial dilution of each antibiotic was prepared in a sterile 96-well plate, and equal volumes (100 μl) of antibiotic and bacterial solution (final inoculum level, 105 CFU/ml) were mixed. After 24 h of incubation at 37°C, 10 μl of 0.01% (wt/vol) resazurin (Sigma-Aldrich) solution was added to each well, followed by incubation for 2 h at 37°C before visual examination of the color change. The MIC in this study was defined as “the minimum EO concentration that inhibited microbial growth, as determined by a lack of color change in resazurin indicator.”
Effect of EO adaptation on efflux pump activity.
To validate the effect of sublethal EO adaptation on E. coli O157:H7 efflux pump activity, flow cytometry analysis was performed using SYTO9 (Molecular Probes; Thermo Fisher Scientific) and EtBr (Sigma-Aldrich) (52). Briefly, nonadapted and EO-adapted E. coli O157:H7 cell cultures were diluted in 0.2-μm-pore-size-filtered PBS to 106 CFU/ml, and a 200-μl aliquot was withdrawn and stained with SYTO9/EtBr mixed probes at working concentrations of 5 and 30 μM, respectively. After incubation for 15 min at room temperature, measurements were made using a BD Accuri C6 flow cytometry device (Accuri Cytometers, Inc., Ann Arbor, MI). Signals for SYTO9 and EtBr were collected at the FL1 and FL3 detectors, respectively.
Statistical analysis.
Experiments were performed in duplicate and repeated for at least three times (n = 6). Results are presented as averages ± the standard deviations, and statistically significance differences (P < 0.05) were determined by one-way analysis of variance with Tukey post hoc test, using the IBM SPSS statistical software (v20; SPSS, Inc./IBM Corporation, Armonk, NY).
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Lim JY, Yoon JW, Hovde CJ. 2010. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J Microbiol Biotechnol 20:5–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis 11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YG, Lee JH, Kim SI, Baek KH, Lee J. 2015. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int J Food Microbiol 195:30–39. doi: 10.1016/j.ijfoodmicro.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Burt SA, van der Zee R, Koets AP, de Graaff AM, van Knapen F, Gaastra W, Haagsman HP, Veldhuizen EJA. 2007. Carvacrol induces heat shock protein 69 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl Environ Microbiol 73:4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles JR, Roller S, Murray DB, Naidu AS. 2005. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 71:797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, Wright A. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 8.Upadhyay A, Arsi K, Wagle BR, Upadhyaya I, Shrestha S, Donoghue AM, Donoghue DJ. 2017. trans-Cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front Microbiol 8:713. doi: 10.3389/fmicb.2017.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yemiş GP, Candoğan K. 2017. Antibacterial activity of soy edible coatings incorporated with thyme and oregano essential oils on beef against pathogenic bacteria. Food Sci Biotechnol 26:1113–1121. doi: 10.1007/s10068-017-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza EL. 2016. The effects of sublethal doses of essential oils and their constituents on antimicrobial susceptibility and antibiotic resistance among food-related bacteria: a review. Trends Food Sci Technol 56:1–12. doi: 10.1016/j.tifs.2016.07.012. [DOI] [Google Scholar]
- 11.Joynson JA, Forbes B, Lambert R. 2002. Adaptive resistance to benzalkonium chloride, amikacin, and tobramycin: the effect on susceptibility to other antimicrobials. J Appl Microbiol 93:96–107. doi: 10.1046/j.1365-2672.2002.01667.x. [DOI] [PubMed] [Google Scholar]
- 12.Álvarez-Ordóñez A, Fernández A, López M, Arenas R, Bernardo A. 2008. Modifications in membrane fatty acid composition of Salmonella typhimurium in response to growth conditions and their effect on heat resistance. Int J Food Microbiol 123:212–219. doi: 10.1016/j.ijfoodmicro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Doyle MP, Busta F, Cords BR, Davidson PM, Hawke J, Hurd HS, Isaacson RE, Matthews K, Maurer J, Meng J. 2006. Antimicrobial resistance: implications for the food system: an expert report, funded by the IFT Foundation. Compr Rev Food Sci Food Saf 5:71–137. [Google Scholar]
- 14.Yuan W, Seng ZJ, Kohli GS, Yang L, Yuk HG. 2018. Stress resistance development and genome-wide transcriptional response of Escherichia coli O157:H7 adapted to sublethal thymol, carvacrol, and trans-cinnamaldehyde. Appl Environ Microbiol 84:e01616-18. doi: 10.1128/AEM.01616-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johny AK, Frye JG, Donoghue A, Donoghue DJ, Porwollik S, McClelland M, Venkitanarayanan K. 2017. Gene expression response of Salmonella enterica serotype Enteritidis phage type 8 to subinhibitory concentrations of the plant-derived compounds trans-cinnamaldehyde and eugenol. Front Microbiol 8:1828. doi: 10.3389/fmicb.2017.01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskaran SA, Venkitanarayanan K. 2014. Plant-derived antimicrobials reduce Escherichia coli O157:H7 virulence factors critical for colonization in cattle gastrointestinal tract in vitro. Biomed Res Int 2014:212395. doi: 10.1155/2014/212395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amalaradjou MA, Narayanan A, Venkitanarayanan K. 2011. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J Urol 185:1526–1531. doi: 10.1016/j.juro.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 18.Campos A, Matsumura P. 2001. Extensive alanine scanning reveals protein-protein and protein-DNA interaction surfaces in the global regulator FlhD from Escherichia coli. Mol Microbiol 39:581–594. doi: 10.1046/j.1365-2958.2001.02248.x. [DOI] [PubMed] [Google Scholar]
- 19.Braun TF, Blair DF. 2001. Targeted disulfide cross-linking of the MotB protein of Escherichia coli: evidence for two H+ channels in the stator complex. Biochemistry 40:13051–13059. doi: 10.1021/bi011264g. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson JS, Hazelbauer GL, Falke JJ. 2015. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol 23:257–266. doi: 10.1016/j.tim.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carraro L, Fasolato L, Montemurro F, Martino ME, Balzan S, Servili M, Novelli E, Cardazzo B. 2014. Polyphenols from olive mill waste affect biofilm formation and motility in Escherichia coli K-12. Microb Biotechnol 7:265–275. doi: 10.1111/1751-7915.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inamuco J, Veenendaal AKJ, Burt SA, Post JA, Tjeerdsma-van Bokhoven JLM, Haagsman HP, Veldhuizen E. 2012. Sublethal levels of carvacrol reduce Salmonella Typhimurium motility and invasion of porcine epithelial cells. Vet Microbiol 157:200–207. doi: 10.1016/j.vetmic.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Chaban B, Hughes HV, Beeby M. 2015. The flagellum in bacterial pathogens: for motility and a whole lot more. Semin Cell Dev Biol 46:91–103. doi: 10.1016/j.semcdb.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Colin R, Sourjik V. 2017. Emergent properties of bacterial chemotaxis pathway. Curr Opin Microbiol 39:24–33. doi: 10.1016/j.mib.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Dourou D, Beauchamp CS, Yoon Y, Geornaras I, Belk KE, Smith GC, Nychas GJE, Sofos JN. 2011. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int J Food Microbiol 149:262–268. doi: 10.1016/j.ijfoodmicro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Niu C, Gilbert ES. 2004. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol 70:6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, Bailey RH. 2013. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Prot 76:205–212. doi: 10.4315/0362-028X.JFP-12-196. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs H, Chao D, Yanofsky C, Saier MH. 1994. Mechanism of catabolite repression of tryptophanase synthesis in Escherichia coli. Microbiology 140:2125–2134. doi: 10.1099/13500872-140-8-2125. [DOI] [PubMed] [Google Scholar]
- 29.Domka J, Lee J, Wood TK. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl Environ Microbiol 72:2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol 30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 31.O’Connell CB, Creasey EA, Knutton S, Elliott S, Crowther LJ, Luo W, Albert MJ, Kaper JB, Frankel G, Donnenberg MS. 2004. SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Mol Microbiol 52:1613–1625. doi: 10.1111/j.1365-2958.2004.04101.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaper JB, Nataro JP, Mobley H. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 33.Stratakos AC, Sima F, Ward P, Linton M, Kelly C, Pinkerton L, Stef L, Pet I, Corcionivoschi N. 2018. The in vitro effect of carvacrol, a food additive, on the pathogenicity of O157 and non-O157 Shiga toxin-producing Escherichia coli. Food Control 84:290–296. doi: 10.1016/j.foodcont.2017.08.014. [DOI] [Google Scholar]
- 34.van Alphen LB, Burt SA, Veenendaal AKJ, Bleumink-Pluym NMC, van Putten JPM. 2012. The natural antimicrobial carvacrol inhibits Campylobacter jejuni motility and infection of epithelial cells. PLoS One 7:e45343. doi: 10.1371/journal.pone.0045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giron JA, Torres AG, Freer E, Kaper JB. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol 44:361–379. doi: 10.1046/j.1365-2958.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 36.Clinical Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing, 16th informational supplement. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37.Barbosa TM, Levy SB. 2000. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J Bacteriol 182:3467–3474. doi: 10.1128/JB.182.12.3467-3474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li XZ, Nikaido H. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okusu H, Ma D, Nikaido H. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol 178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Critzer FJ, Dsouza DH, Golden DA. 2008. Transcription analysis of stx1, marA, and eaeA genes in Escherichia coli O157:H7 treated with sodium benzoate. J Food Prot 71:1469–1474. doi: 10.4315/0362-028X-71.7.1469. [DOI] [PubMed] [Google Scholar]
- 41.Bore E, Hébraud M, Chafsey I, Chambon C, Skjaeret C, Moen B, Møretrø T, Langsrud Ø, Rudi K, Langsrud S. 2007. Adapted tolerance to benzalkonium chloride in Escherichia coli K-12 studied by transcriptome and proteome analyses. Microbiology 153:935–946. doi: 10.1099/mic.0.29288-0. [DOI] [PubMed] [Google Scholar]
- 42.Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N. 2005. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob 4:20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becerril R, Nerin C, Gomez-Lus R. 2012. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathog Dis 9:699–705. doi: 10.1089/fpd.2011.1097. [DOI] [PubMed] [Google Scholar]
- 44.Hammer KA, Carson CF, Riley TV. 2012. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single-and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother 56:909–915. doi: 10.1128/AAC.05741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon MAS, Blair IS, Moore JE, McDowell DA. 2006. Habituation to sub-lethal concentrations of tea tree oil (Melaleuca alternifolia) is associated with reduced susceptibility to antibiotics in human pathogens. J Antimicrob Chemother 59:125–127. doi: 10.1093/jac/dkl443. [DOI] [PubMed] [Google Scholar]
- 46.Kalily E, Hollander A, Korin B, Cymerman I, Yaron S. 2017. Adaptation of Salmonella enterica serovar Senftenberg to linalool and its association with antibiotic resistance and environmental persistence. Appl Environ Microbiol 83:e03398-16. doi: 10.1128/AEM.03398-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viveiros M, Martins A, Paixao L, Rodrigues L, Martins M, Couto I, Fahnrich E, Kern WV, Amaral L. 2008. Demonstration of intrinsic efflux activity of Escherichia coli K-12 AG100 by an automated ethidium bromide method. Int J Antimicrob Agents 31:458–462. doi: 10.1016/j.ijantimicag.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Fadli M, Chevalier J, Saad A, Mezrioui NE, Hassani L, Pages JM. 2011. Essential oils from Moroccan plants as potential chemosensitizers restoring antibiotic activity in resistant Gram-negative bacteria. Int J Antimicrob Agents 38:325–330. doi: 10.1016/j.ijantimicag.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Lorenzi V, Muselli A, Bernardini AF, Berti L, Pagès JM, Amaral L, Bolla JM. 2009. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob Agents Chemother 53:2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JH, Cho MH, Lee J. 2011. 3-Indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol 13:62–73. doi: 10.1111/j.1462-2920.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim MJ, Bang WS, Yuk HG. 2017. 405±5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol 62:124–132. doi: 10.1016/j.fm.2016.10.002. [DOI] [PubMed] [Google Scholar]