In this work, we have investigated the role of bacterial motility with regard to antibiotic-tolerant bacterial aggregate formation. Previous work has convincingly demonstrated that P. aeruginosa flagellar motility promotes the formation of surface-adhered biofilms in many systems. In contrast, aggregate formation by P. aeruginosa was observed for nonmotile but not for motile cells in the presence of an exogenous scaffold. Here, we demonstrate that both wild-type P. aeruginosa and mutants that genetically lack motility spontaneously form antibiotic-tolerant aggregates in the absence of an exogenously added scaffold. Additionally, we also demonstrate that wild-type (WT) and nonmotile P. aeruginosa bacteria can coaggregate, shedding light on potential physiological interactions and heterogeneity of aggregates.
KEYWORDS: Pseudomonas aeruginosa, aggregates, antibiotic tolerance, biofilms
ABSTRACT
Pseudomonas aeruginosa is a bacterial pathogen that causes severe chronic infections in immunocompromised individuals. This bacterium is highly adaptable to its environments, which frequently select for traits that promote bacterial persistence. A clinically significant temporal adaptation is the formation of surface- or cell-adhered bacterial biofilms that are associated with increased resistance to immune and antibiotic clearance. Extensive research has shown that bacterial flagellar motility promotes formation of such biofilms, whereupon the bacteria subsequently become nonmotile. However, recent evidence shows that antibiotic-tolerant nonattached bacterial aggregates, distinct from surface-adhered biofilms, can form, and these have been reported in the context of lung infections, otitis media, nonhealing wounds, and soft tissue fillers. It is unclear whether the same bacterial traits are required for aggregate formation as for biofilm formation. In this report, using isogenic mutants, we demonstrate that P. aeruginosa aggregates in liquid cultures are spontaneously formed independent of bacterial flagellar motility and independent of an exogenous scaffold. This contrasts with the role of the flagellum to initiate surface-adhered biofilms. Similarly to surface-attached biofilms, these aggregates exhibit increased antibiotic tolerance compared to planktonic cultures. These findings provide key insights into the requirements for aggregate formation that contrast with those for biofilm formation and that may have relevance for the persistence and dissemination of nonmotile bacteria found within chronic clinical infections.
IMPORTANCE In this work, we have investigated the role of bacterial motility with regard to antibiotic-tolerant bacterial aggregate formation. Previous work has convincingly demonstrated that P. aeruginosa flagellar motility promotes the formation of surface-adhered biofilms in many systems. In contrast, aggregate formation by P. aeruginosa was observed for nonmotile but not for motile cells in the presence of an exogenous scaffold. Here, we demonstrate that both wild-type P. aeruginosa and mutants that genetically lack motility spontaneously form antibiotic-tolerant aggregates in the absence of an exogenously added scaffold. Additionally, we also demonstrate that wild-type (WT) and nonmotile P. aeruginosa bacteria can coaggregate, shedding light on potential physiological interactions and heterogeneity of aggregates.
INTRODUCTION
The Gram-negative bacterium Pseudomonas aeruginosa is one of the most common causes of nosocomial infections and infections of immunocompromised individuals (1). P. aeruginosa has been studied extensively for its ability to transition from a motile to a nonmotile state and its biofilm mode of growth in chronic infections (2–6). These shifts have been reported for the bacteria isolated from cystic fibrosis (CF) patients, chronic wounds, urinary catheters, and mechanical ventilator tubing (3, 7). Notably, these transitions within chronic infections are consistently linked to poor clinical outcomes, likely resulting from an inability to eradicate P. aeruginosa due to the high tolerance of bacterial biofilms to clinical antibiotic treatments (7, 8).
The genetics and traits that underlie bacterial biofilm formation have largely been identified through studies of the bacteria on exogenous surfaces and scaffolds, including agar, plastic dishes, and epithelial cell monolayers (2, 3, 7). However, emerging evidence shows that during infections and in natural environments, P. aeruginosa are able to clump as multicellular aggregates (9–14). Importantly, in the lungs of CF patients, P. aeruginosa persists in the form of aggregates in the mucus; ex vivo samples from chronic infections mostly recovered nonattached aggregates rather than planktonic bacteria (11, 13). Previous studies have also observed nonattached aggregates in otitis media, nonhealing wounds, and soft tissue fillers (9, 10). These aggregates share characteristics with surface-attached biofilms, such that they are also highly resilient to both antimicrobial treatment and host immune responses (15–20). The aggregate phenotype is not unique to P. aeruginosa and has been described for Achromobacter, Borrelia spp., Mycobacterium abscessus, Staphylococcus aureus, Staphylococcus epidermidis, and Vibrio cholerae in a multitude of in vitro and in vivo systems (21–26).
Over the course of chronic infections, P. aeruginosa undergoes adaptation and selection of certain traits in response to the host environment (27–29). Besides selection for biofilm mode of growth, loss of flagellar motility due to the absence or downregulation of flagellar gene expression is frequently observed in chronic isolates (4, 30). We have previously demonstrated that loss of flagellar swimming motility confers phagocytic resistance of bacteria to innate immune cells (31, 32). Relevant to P. aeruginosa biofilms, under many experimental conditions, flagellum-mediated motility promotes biofilm formation, and therefore surface-adhered biofilm formation is impaired in the absence of flagellar motility (2, 5, 33). In contrast, recent findings suggest that nonmotile P. aeruginosa cells can form aggregates within agar, while motile P. aeruginosa cells do not (16). However, the relative abilities of wild-type (WT) and nonmotile P. aeruginosa, as well as the contribution of the flagellum or flagellar motility, to the spontaneous formation of antibiotic-tolerant aggregates in the absence of an exogenously added scaffold remains unknown.
In order to assess the necessity of flagellar motility for aggregate formation, we used nonmotile mutants of two independent strains of P. aeruginosa and compared them to their respective isogenic wild-type strains. Here, we show that, in contrast to surface-attached biofilms, P. aeruginosa strains without flagella or flagellar motility can form nonattached aggregates that share characteristics with biofilms, including increased antibiotic tolerance. Importantly, aggregate formation did not require an exogenously added scaffold, and both wild-type and nonmotile mutant bacteria were able to form spontaneous, and even mixed, aggregates. This study expands our current understanding of the ecology and evolution of biofilms in the environment and in chronic infections and may provide insights into bacterial dispersal and persistence within an infected organ.
RESULTS
P. aeruginosa (PAO1) flagella are dispensable for formation of nonattached aggregates.
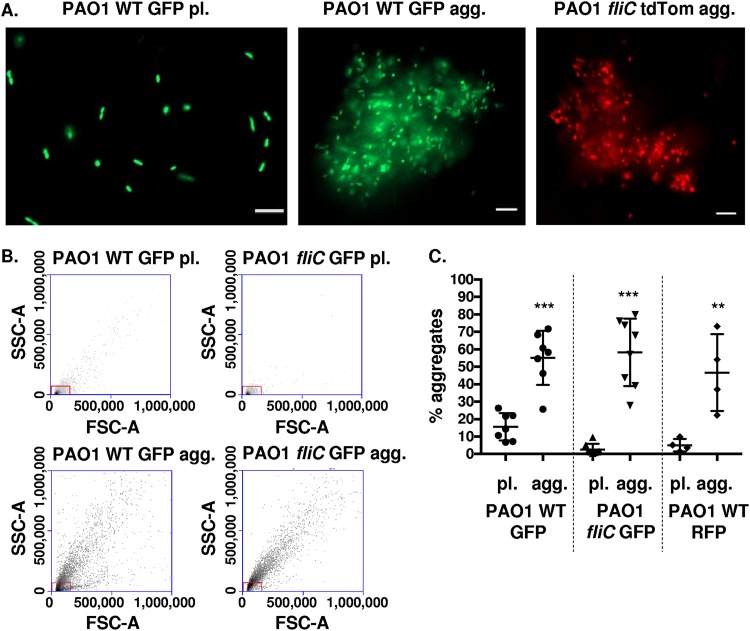
Previous studies demonstrated that the bacterial flagellum and flagellar motility promote, and in some cases are required for, establishment of surface-attached biofilms (2, 33). However, in the absence of an added scaffold, it remains unknown whether the flagellum has a functional role in the formation of nonattached aggregates. To address this, we monitored bacterial aggregate formation in the presence or absence of a flagellum, both qualitatively with the use of fluorescence microscopy and quantitatively by fluorescence-activated cell sorting (FACS) analyses. For both assays, we employed motile wild-type P. aeruginosa PAO1 and the isogenic flagellum-deficient and nonmotile fliC mutant (fliC encodes the flagellin structural gene [34]). The bacteria were grown in parallel in liquid cultures for 48 h (aggregate cultures) or subcultured from overnight cultures (planktonic or single-cell cultures) as a control. Representative images demonstrate that the nonmotile fliC mutant spontaneously formed nonattached aggregates that were visually comparable to those formed by the wild-type strain (Fig. 1A); these aggregates were distinct, based on size, from planktonic bacterial cultures of single bacterial cells. To validate and quantify the results obtained with microscopy, we employed FACS analyses. FACS analyses quantitatively measured the percentage of aggregated cells in the bacterial cultures, with nonaggregated planktonic bacteria excluded by gating on forward and side scatters (FSC and SSC, respectively). Using this methodology, we observed that both wild-type and nonmotile P. aeruginosa cells formed aggregates that constituted ∼20 to 70% of the total bacterial culture (Fig. 1B and C). Similar results were obtained following gating on green fluorescent protein (GFP)-positive bacteria before excluding planktonic bacteria based on the forward and side scatter plots (data not shown). Therefore, nonattached aggregates spontaneously form independent of the presence of bacterial flagella.
FIG 1.
Wild-type and nonmotile P. aeruginosa (PAO1) cells form aggregates. (A) P. aeruginosa bacteria were assayed for the formation of bacterial aggregates. Representative images of planktonic (pl.) PAO1 WT GFP bacteria (left), PAO1 WT GFP aggregate (agg.) bacterial culture (middle), and PAO1 fliC tdTomato agg. (right) culture. GFP-expressing PAO1 WT and tdTomato-expressing PAO1 fliC are shown in green and red colors, respectively (×20 magnification). Bar, 10 μm. (B) Flow cytometry to assess and quantify bacterial aggregate formation compared to planktonic cultures. Representative side-scatter area (SSC-A; y axis) and forward-scatter area (FSC-A; x axis) plots of PAO1 WT GFP (pl. and agg.) and PAO1 fliC GFP (pl. and agg.), as indicated. The red boxes indicate gates for planktonic bacteria. (C) Quantification of aggregate formation using the methodology shown in (B). The y axis, percentage aggregates, represents the percentage of bacteria outside the gate set in panel B. Data, analyzed using one-way analysis of variance (ANOVA) with Tukey’s post hoc analysis, are representative of at least four independent biological experiments (n ≥ 4). ***, P ≤ 0.0005; **, P ≤ 0.005, compared to planktonic bacterial cultures.
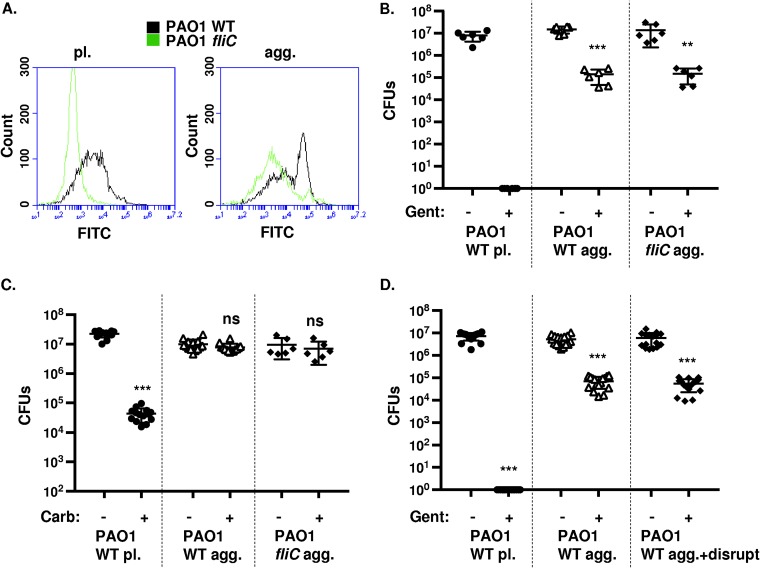
Based on data from surface-attached biofilms (5), motile bacteria likely become nonmotile or sessile when transitioning to an aggregate state. However, it is unknown whether flagella would still be present in the aggregate cultures. Therefore, we stained P. aeruginosa flagella in planktonic and aggregate cultures with anti-FliC antibodies and evaluated the relative shifts in fluorescence using FACS analyses. As expected, we observed distinct staining for flagella in planktonic PAO1 WT compared to the negative control, planktonic PAO1 fliC bacteria (Fig. 2A). Interestingly, a subset of the aggregated PAO1 WT bacteria still stained for the presence of flagella in contrast to the flagellum-deficient PAO1 fliC strain (Fig. 2A). These data indicate that wild-type P. aeruginosa may still have flagella present following aggregate formation.
FIG 2.
Wild-type and nonmotile P. aeruginosa (PAO1) cells form antibiotic-tolerant aggregates. (A) Flow cytometry of PAO1 WT and PAO1 fliC planktonic (pl., left) and aggregate (agg., right) bacteria stained with anti-FliC antibodies. PAO1 WT is represented in black and PAO1 fliC in gray. (B and C) Survival of P. aeruginosa upon gentamicin (B) and carbenicillin (C) treatment, respectively. Cultures of planktonic and aggregate PAO1 WT and PAO1 fliC, as indicated, were exposed to 80 μg/ml gentamicin or 2,500 μg/ml carbenicillin for 20 min, and CFU were enumerated. (D) Survival of PAO1 WT agg. bacteria upon 80 μg/ml gentamicin treatment for 20 min, with or without prior physical disruption. Data, analyzed using one-way ANOVA with Tukey’s post hoc analysis, are representative of at least three independent biological experiments (n ≥6). ***, P ≤ 0.0005; **, P ≤ 0.005; ns, not significant compared to no antibiotic treatment.
Spontaneously formed P. aeruginosa aggregates are antibiotic tolerant.
To validate the increased antibiotic tolerance of the observed aggregates, we incubated bacterial cultures in the presence or absence of the bactericidal aminoglycoside antibiotic gentamicin and quantitatively assessed survival post treatment by counting viable CFU. As expected, and as a positive control for antibiotic efficacy, we were unable to recover live bacteria following the antibiotic treatment of planktonic PAO1 WT cultures, suggesting that single bacterial cells were readily killed by gentamicin (Fig. 2B). In contrast, both wild-type and fliC PAO1 aggregates displayed increased tolerance to killing by gentamicin compared to that of single cells (>1,000-fold) (Fig. 2B). We also tested the tolerance of bacterial cultures to carbenicillin, a bactericidal antibiotic that inhibits bacterial cell wall synthesis. The treatment of planktonic PAO1 WT cultures with carbenicillin decreased bacterial viability by nearly 1,000-fold (Fig. 2C). However, carbenicillin did not have a significant effect on the bacterial viability of either wild-type or fliC PAO1 aggregates (Fig. 2C).
Next, we investigated whether the tolerance of aggregated bacteria to gentamicin was due to protection conferred by the physiology of bacteria within the aggregates. With the use of established methodology (35), the aggregates were physically disrupted by forcing them through a 22-gauge needle several times prior to antibiotic treatment. Interestingly, disruption of aggregated PAO1 WT bacteria did not substantially increase susceptibility of bacteria to gentamicin (Fig. 2D), similar to previous results that measured colistin tolerance following mechanical disruption of aggregates (15). Thus, these findings collectively demonstrate that aggregates are formed by both wild-type and flagellum-deficient P. aeruginosa, are antibiotic tolerant, and suggest that aggregates share certain characteristics with surface-attached biofilms. Specifically, they demonstrate that the aggregates are gentamicin and carbenicillin tolerant and spontaneously form independent of flagellar surface expression and motility.
Wild-type and nonmotile P. aeruginosa (PAK) cells form nonattached aggregates.
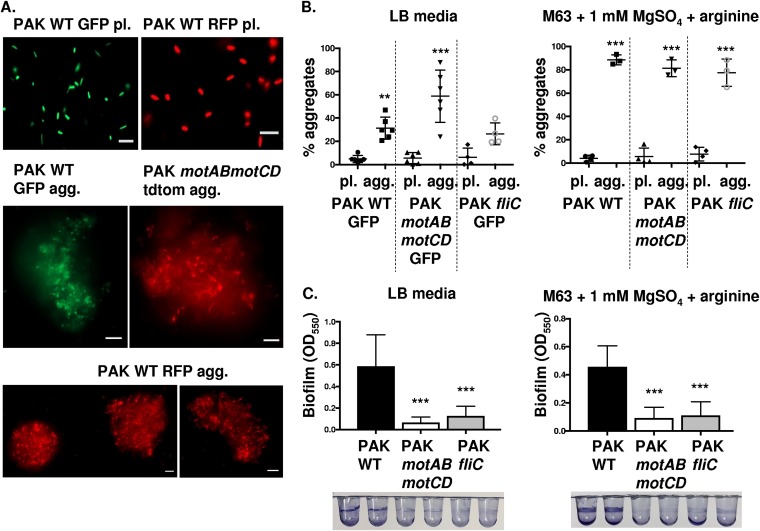
To determine whether the described findings are exclusive to P. aeruginosa strain PAO1 or represent a widespread phenomenon applicable to other strains, we evaluated aggregate formation by the swimming-competent wild-type PAK P. aeruginosa strain and nonmotile isogenic mutants. Similar to the results observed for PAO1, the WT and nonmotile mutants of the PAK strain, motAB motCD (have flagella but lack motility) and fliC (lack both flagella and swimming motility), all formed aggregates, as assessed by microscopy and FACS analyses (Fig. 3A and B). Moreover, the ability of wild-type and isogenic nonmotile PAK bacteria to form aggregates is observed within a variety of growth media (Fig. 3B). Interestingly, the ability to form aggregates was unaffected by the lack of swimming motility, either through phenotypic loss of flagellum (fliC) or loss of flagellar function (motAB motCD).
FIG 3.
Wild-type and nonmotile P. aeruginosa (PAK) cells form aggregates, but nonmotile bacteria do not form surface-attached biofilms. (A) P. aeruginosa bacteria (PAK strain) were assayed for the formation of bacterial aggregates. Representative images of PAK WT GFP and RFP planktonic (pl.) bacteria, PAK WT GFP and RFP aggregate (agg.) bacteria, and PAK motAB motCD tdTomato agg. bacteria, as indicated. GFP-expressing bacteria are shown in green, while RFP- and tdTomato-expressing bacteria are shown in red (×20 magnification). Bar, 10 μm. (B) Flow cytometry to assay relative PAK bacterial aggregate formation compared to that of planktonic cultures in LB medium (left) or M63 medium (right). Quantification of aggregate formation is performed by the same methodology as in Fig. 1. (C) PAK WT, motAB motCD, and fliC bacteria were assessed for their ability to form surface-adhered biofilms in the same media as that in panel B. Images of representative wells are shown following staining with 0.1% crystal violet. Biofilm formation was quantified by the OD at 550 nm of the biofilm-associated crystal violet. Data, analyzed using one-way ANOVA with Tukey’s post hoc analysis, are representative of at least three independent biological experiments (n ≥3). ***, P ≤ 0.0005; **, P ≤ 0.005, compared to planktonic bacterial cultures in panel B and compared to PAK WT biofilm formation in panel C.
Previous research examining requirements of P. aeruginosa flagellum for biofilm formation revealed that the flagellum itself and flagellum-mediated motility are required to initiate surface-attached biofilm formation in several in vitro models (5). Therefore, as an important control for the differences between biofilms and aggregates, we tested the ability of swimming competent wild-type and nonswimming P. aeruginosa PAK strains to form biofilms on plastic 96-well round-bottom microtiter plates. The biofilm data corroborated previous findings that PAK motAB motCD and PAK fliC were impaired in biofilm formation compared to the wild-type strain in both LB and M63 media (Fig. 3C). These data demonstrate that mutants deficient for flagellar motility are capable of antibiotic-tolerant aggregates, but not formation of surface-attached biofilms. Moreover, formation of aggregates by nonmotile bacteria was recapitulated in multiple strains (PAO1 and PAK), suggesting that this may be broadly applicable in P. aeruginosa.
Wild-type and nonmotile P. aeruginosa can coaggregate.
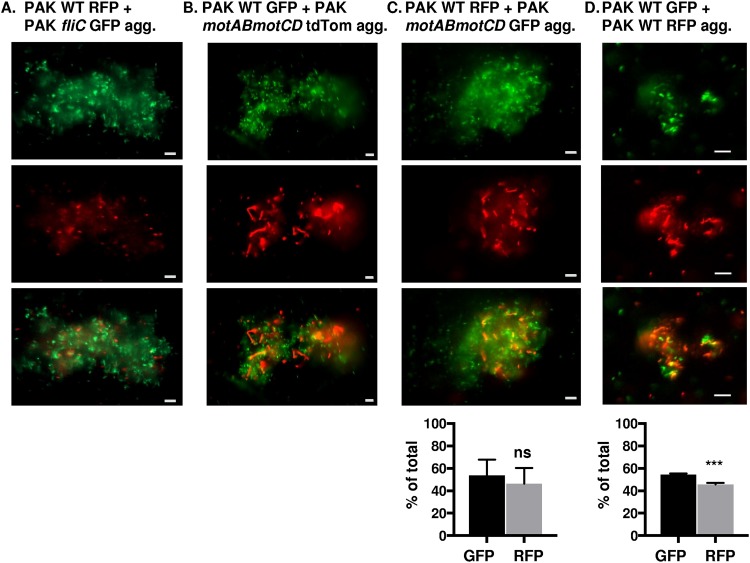
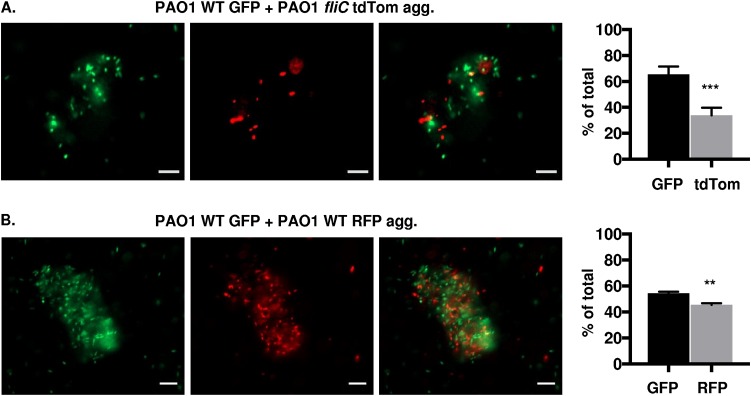
We extended our studies to elucidate whether wild-type and nonmotile P. aeruginosa can form mixed aggregates, as well as to evaluate the contributions from the different bacterial populations within the coaggregates. In order to address these questions, bacterial strains were mixed (1:1), followed by assessment of coaggregate formation 48 h later by fluorescence microscopy. PAK WT red fluorescent protein (RFP) and PAK fliC GFP, PAK WT GFP and PAK motAB motCD tandem dimer tomato (tdTomato), or PAK WT RFP and PAK motAB motCD GFP (Fig. 4A, B, and C, respectively) formed mixed aggregates, shown by GFP and RFP or tdTomato fluorescence within the same aggregates. This demonstrates that P. aeruginosa wild-type and nonmotile bacteria can coaggregate. These findings were validated in an independent strain, as mixed PAO1 WT GFP and PAO1 fliC tdTomato bacteria also formed coaggregates (Fig. 5A). As controls, and consistent with the aggregation of WT bacteria shown in Fig. 1 and 3, we showed that PAK WT GFP and PAK WT RFP (Fig. 4D) or PAO1 WT GFP and PAO1 WT RFP (Fig. 5B) wild-type bacteria also coaggregate. These results suggest that coaggregates can form regardless of bacterial motility.
FIG 4.
Wild-type and nonmotile P. aeruginosa (PAK) form coaggregates. Fluorescence microscopy of mixed fluorescently tagged PAK bacteria inoculated together and assessed for coaggregate formation 48 h post mixed liquid inoculation. Representative images of mixed-strain aggregates composed of (A) PAK WT RFP and fliC GFP, (B) PAK WT GFP and motAB motCD tdTomato, (C) PAK WT RFP and motAB motCD GFP, or (D) PAK WT GFP and RFP bacteria, as indicated. GFP-expressing bacteria are shown in green, while RFP- and tdTomato-expressing bacteria are shown in red (×20 magnification). GFP and RFP or tdTomato channels are represented individually in the top and middle rows, respectively, while the combined channels are shown in the bottom row. Bar, 10 μm. (C and D, bottom) Quantification of fluorescent bacteria (GFP or RFP) within aggregates, represented as the percentage of total bacteria. Data were analyzed using an unpaired t test with Welch’s correction (n ≥ 4). ***, P ≤ 0.0005; ns, not significant compared to GFP bacteria.
FIG 5.
P. aeruginosa (PAO1) cells form coaggregates independent of flagella. Fluorescence microscopy of the indicated strains of PAO1 bacteria, inoculated together and assessed for coaggregate formation 48 h post mixed liquid inoculation. Representative images of mixed-strain aggregates composed of (A) PAO1 WT GFP and fliC tdTomato and (B) PAO1 WT GFP and WT RFP bacteria, as indicated. GFP-expressing bacteria are shown in green, RFP- and tdTomato-expressing bacteria are shown in red (×20 magnification). GFP and RFP or tdTomato channels are represented individually in the first and second columns, respectively, while the combined channels are shown in the third column. Graphs in panels A and B show quantification of fluorescent bacteria (GFP, RFP, or tdTomato) within aggregates, represented as the percentage of total bacteria. Bar, 10 μm. Data were analyzed using unpaired t test with Welch’s correction (n ≥3). ***, P ≤ 0.0005; **, P ≤ 0.005, compared to GFP bacteria.
A biofilm-deficient, nonmotile P. aeruginosa clinical isolate forms aggregates.
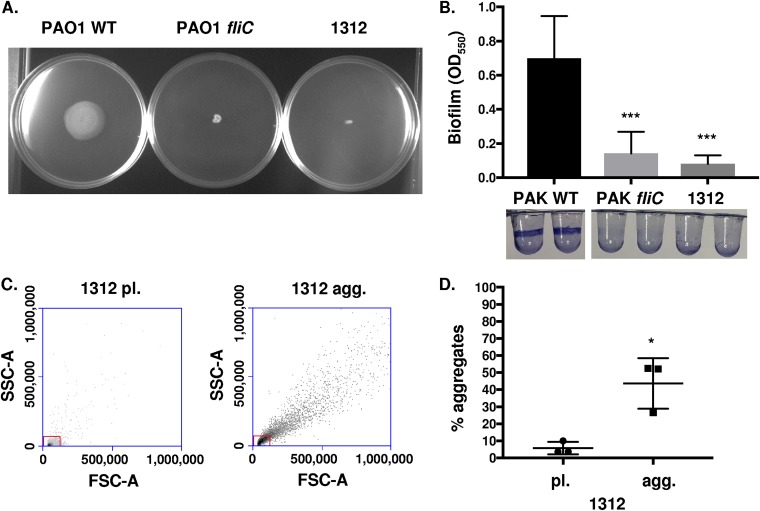
Our previous experiments have demonstrated that nonmotile mutants of defined laboratory reference strains form aggregates but not surface-adhered biofilms. We hypothesized that nonmotile clinical isolates of P. aeruginosa would also form aggregates. Motility assays identified that clinical isolate 1312, obtained from sputum, lacked swimming motility, with swimming-competent PAO1 WT employed as a positive control and PAO1 fliC as a negative control (Fig. 6A). Isolate 1312 was then evaluated for the capability to form surface biofilms. In accordance with previous results, the motile PAK WT strain formed biofilms while 1312, PAK fliC, and PAK motAB motCD were deficient in biofilm formation (Fig. 6B). Finally, the aggregate-forming capacity of 1312 was quantitatively assessed with the use of flow cytometry. As seen with the reference strains, 1312 readily formed aggregates (Fig. 6C and D). Therefore, these data derived from a clinical isolate corroborate that nonswimming P. aeruginosa strains can form aggregates even when they are unable to form surface-attached biofilms.
FIG 6.
Formation of aggregates by a nonmotile P. aeruginosa clinical isolate. (A) PAO1 WT, PAO1 fliC, and clinical isolate 1312 of P. aeruginosa, as indicated, were assayed for swimming motility in LB media containing 0.3% agar. (B) P. aeruginosa clinical isolate 1312 was assessed for its ability to form surface-adhered biofilms, with PAK WT and PAK fliC bacteria used as positive and negative controls, respectively. Staining with 0.1% crystal violet was used to view and quantitate biofilm formation. (C) Flow cytometry was used to quantify isolate 1312 bacterial aggregate formation compared to that of planktonic cultures, based on scatter. The boxes delineate the gate for planktonic bacteria. (D) Quantification of aggregate formation by isolate 1312, performed by the same methodology as that in Fig. 1. Data for panel B were analyzed using one-way ANOVA with Tukey’s post hoc analysis, while data for panel D were analyzed using an unpaired t test with Welch’s correction. All data are representative of at least three independent biological experiments (n ≥3). ***, P ≤ 0.0005; *, P ≤ 0.05, compared to PAK WT in panel B and pl. 1312 in panel D.
DISCUSSION
Several notable bacterial phenotypic changes are consistently observed during chronic P. aeruginosa infections, including progressive loss of flagellar motility, mucoid phenotype, and conversion from free swimming and planktonic to sessile and biofilm modes of growth (27–29). Infectivity studies have demonstrated that P. aeruginosa require functional flagella to initially establish infection and colonize a host (36, 37). Moreover, in vitro studies have also shown that loss of flagellar motility impedes surface attachment and therefore biofilm formation (2, 33). The observed conversion to a nonmotile biofilm during chronic infection likely contributes to bacterial persistence through multiple mechanisms, such as production of protective exopolysaccharide and enhanced resistance to antibiotics, phagocytic/immune clearance, and redox components (3, 36). Most of the elucidated mechanisms have been well documented using models of surface-attached bacterial biofilms; however, supported by recent literature, the prevalence of surface-independent and nonattached aggregates in P. aeruginosa infections is now being appreciated (7, 9–13).
Here, we report that antibiotic-tolerant aggregates form, in contrast to biofilms, in both the presence and absence of flagellar motility. During in vivo bacterial adaptation, conditions in chronic infections (e.g., CF airways) select against bacterial functions considered essential for biofilm formation, including motility (36, 38–41). Related to this, neutrophil elastase, which is secreted during inflammation, was shown to reduce bacterial motility and repress expression of P. aeruginosa flagellin (16, 36). The selection of bacteria resistant to phagocytosis may also explain the progressive recovery of nonmotile bacterial isolates (32, 36). Importantly, P. aeruginosa isolates recovered from chronically infected patients are frequently impaired for biofilm formation but were able to form aggregates in agar gels (16). We also demonstrated a potential strategy by which motile and nonmotile bacteria may contribute to the persistence of P. aeruginosa in chronic infections.
Moreover, these studies open new avenues to understanding bacterial dispersion as aggregates. When bacteria disperse to seed new biofilms, either single motile cells or aggregates of cells can be sloughed off from the established biofilms (3). Based on the flagellar motility requirement to form surface-adhered biofilms, we would have hypothesized that bacteria would have to become motile before seeding a new antibiotic-tolerant biofilm. However, our data suggest that nonmotile bacteria in aggregates may be dispersed without regaining motility and can even persist as nonattached aggregates in infected organs, which can be tested in subsequent studies. Moreover, in comparison to studies using agar scaffolds, both wild-type and nonmotile bacteria can form and contribute to aggregates (16). These findings may integrate with a previous proposal that, within an infection and if competition with planktonic bacteria is high, preformed biofilm aggregates may have a fitness advantage for accessing nutrients and would be less vulnerable to antibiotics and immune responses than detached single cells (42). The aggregates would have a higher net fitness in that scenario, since cells at the exterior of the aggregates would have better access to growth resources by extending vertically above the surface (42).
Formation of surface-independent aggregates likely provides bacteria with a protected mode of colonization of new niches in a host environment (42). Previous studies demonstrated that overexpression of biofilm matrix components (alginate, Pel, and Psl), frequently expressed by clinical isolates, protect P. aeruginosa bacteria growing as aggregates against antibiotics (17). P. aeruginosa exopolysaccharide Psl has been proposed as a central mediator for the recruitment of planktonic cells around preformed aggregates, since PAO1 Psl-deficient, but not Pel-deficient, bacteria exhibited diminished aggregate formation in liquid cultures (18). In contrast, in viscous environments (with polymers, agar gels, or sputum media) that restrain bacterial motility, bacteria are still able to form aggregates independent of the production of exopolysaccharides or surface adhesins (pili) (16, 43, 44). Interestingly, extracellular DNA (eDNA) has been shown to maintain the cohesiveness of the aggregates, since treatment of bacterial cultures with DNase markedly reduced aggregation in multiple experimental models (15, 18, 43). Additionally, Staudinger et al. (16) demonstrated that host factors associated with the CF lung environment promote bacterial aggregation, which in turn contributes to reduced killing by neutrophils and the host defense oxidants, H2O2 and HOCl (15, 16). Aggregation may also be associated with recurrent inflammation in chronic P. aeruginosa infections, as well as with protection from antimicrobial products (17, 18, 35, 42).
Therefore, aggregates display many of the same phenotypes as surface-attached biofilms, such as increased antibiotic tolerance and resilience toward immune responses. We also demonstrated that wild-type and nonmotile P. aeruginosa bacteria can coaggregate, shedding light on potential physiological interactions and heterogeneity of aggregates. These findings reveal the impact of bacterial phenotypic changes in chronic infections and could potentially extend to other bacteria. For instance, Escherichia coli, Staphylococcus aureus, and multispecies aggregates (E. coli and P. aeruginosa) have been shown to produce aggregates in liquid batch cultures (18, 45).
In this report, we provided novel insights into the requirements for aggregate formation by elucidating that aggregates can form independent of flagellar motility, unlike surface-attached biofilms. These three-dimensional aggregates spontaneously formed in liquid cultures, were composed of variable sizes, and were tolerant to the antibiotics gentamicin and carbenicillin. This study may provide insights into the dispersal and persistence of nonmotile P. aeruginosa within chronic clinical infections.
MATERIALS AND METHODS
Bacteria.
P. aeruginosa strains on the PAO1 background and the P. aeruginosa clinical isolate from sputum (1312) were kindly provided by G. O’Toole and D. Hogan (Dartmouth Medical School, Hanover, NH), while PAK strains were provided by B. Kazmierczak (Yale University, New Haven, CT). The clinical isolate was obtained with informed consent. Bacterial strains expressing green fluorescent protein (GFP), red fluorescent protein (RFP), and tandem dimer tomato (tdTomato) were generated by transformation as previously described (31). For planktonic liquid bacterial cultures, bacteria were cultured overnight in a shaking incubator at 37°C and subsequently subcultured to log phase for 2 h in fresh LB. For aggregate liquid bacterial cultures, bacteria were cultured for 48 h in a shaking incubator at 37°C in LB (unless indicated otherwise) before further analyses. Alternatively, where indicated, bacteria were cultured in M63 medium, which in all cases was supplemented with 1 mM MgSO4 and arginine. For microscopy experiments with mixed aggregates, bacteria were cultured overnight in a shaking incubator at 37°C, and liquid cultures were subsequently mixed in a 1:1 ratio in fresh LB after normalizing optical density (OD) read at 600 nm (OD600). Coaggregate formation was assessed 48 h post mixed liquid inoculation.
Generation of antiserum against P. aeruginosa flagella.
Polyclonal antiserum against flagella from the pilA PA14 strain of P. aeruginosa was elicited in a rabbit using flagella isolated from an SDS-PAGE gel. Flagella were sheared from bacteria by vortexing in phosphate-buffered saline (PBS) buffer and precipitated using 0.1 volume each of 5 M NaCl and 30% (wt/vol) polyethylene glycol (PEG); molecular weight [MW], ∼8,000) on ice for at least 90 min. Flagella were collected by centrifugation, run on a 12% SDS-PAGE gel, and stained with Gel Code Blue (Thermo Scientific), and the FliC flagellin protein at 51 kDa was excised. Covance (NJ, USA) performed the rabbit immunizations and sera acquisition.
FACS analyses for bacterial flagella.
Equal numbers of planktonic or aggregate PAO1 WT and fliC P. aeruginosa bacteria (as determined by OD600) were filtered through 80-μm filters. Bacteria were blocked with 4% goat normal serum in PBS + 2% fetal bovine serum (FBS) for 15 min on ice, followed by incubation with the anti-FliC polyclonal antisera described above (1:250 to 1:500) for 20 min on ice. After washing, the bacteria were incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG secondary antibody (Pharmingen) for 20 min on ice (1:100). The stained bacteria were analyzed by flow cytometry (FACS analyses) for the acquisition of fluorescence as an indication of presence of flagella on bacteria.
Antibiotic susceptibility assays.
Planktonic and aggregate bacterial cultures (as indicated in Fig. 2) were exposed to 80 μg/ml gentamicin or 2,500 μg/ml carbenicillin for 20 min in a 37°C water bath, followed by mechanical disruption by vortexing (15) before plating on LB agar plates. After overnight incubation at 37°C, CFU were counted to quantitate bacterial survival following antibiotic treatment compared to untreated bacteria. Where indicated, the aggregates were physically disrupted by forcing them through a 22-gauge needle several times prior to antibiotic treatment (35).
Microscopy.
GFP-expressing (green) and RFP- or tdTomato-expressing (red) PAO1 and PAK bacterial strains were visualized using fluorescence microscopy. Microscopy was performed on an inverted Zeiss Axiovert 200 microscope with a 20× objective. White scale bars representing 10 μm were added on all images. For microscopy experiments with mixed aggregates, quantification of fluorescent bacteria was performed using ImageJ/Fiji software. The bacteria in each fluorescence channel are graphed as percentage of total bacteria.
FACS-based bacterial aggregate quantification.
Planktonic and aggregate PAO1 and PAK P. aeruginosa cultures (as indicated) were filtered through 80-μm filters before performing FACS analyses. Gating was based on the planktonic PAO1 or PAK WT GFP bacterial cultures, which mostly contained single or nonaggregated bacteria (confirmed by fluorescence microscopy).
Bacterial swimming motility.
The phenotypic assay for swimming motility was performed as previously described in 0.3% LB agar plates (31). Images were collected at 48-h postinoculation to monitor the formation of bacterial halos to assess the relative swimming motilities of the bacterial strains.
Biofilm formation assays.
Biofilm formation assays were performed using 96-well round-bottom microtiter plates (CoStar 2797) as previously described, utilizing M63 medium supplemented with 1 mM MgSO4 and arginine (46, 47) or LB media, as indicated. Following incubation inside a humidified chamber at 37°C for 18 h, the supernatants were removed, and the wells were stained with 0.1% (wt/vol) crystal violet solution and rinsed with distilled water. This assay was conducted three times for each strain, and the representative images are shown. For quantification, biofilms were dissolved in 30% acetic acid in water and a spectrophotometer was used to read absorbance at 550 nm.
Statistical analyses.
Means plus or minus standard deviations (SD) derived from multiple independent experiments, with technical replicates, are shown for each graph. Sample sizes for each experiment are noted in the figure legends. As indicated, unpaired Student's t tests with Welch’s correction or one-way analysis of variance (ANOVA) with Tukey’s post hoc analyses were performed using Prism 7.02 to determine statistical significance of the data. Statistical significance is represented in figures by asterisks.
ACKNOWLEDGMENTS
We thank Sherry Kuchma, George O’Toole, Deborah Hogan, and David Leib (Geisel School of Medicine at Dartmouth) and Brandon Brown (Morehouse College and ASURE Program) for providing input and reagents. This work was facilitated by the Dartmouth Lung Biology Translational Research Core and the DartLab Immune Monitoring Lab.
This work was supported by grants from the National Institutes of Health (NIH; grants T32AI007363, R21 AI121820, R03 AI135358, and R21 AI137656) and from the Cystic Fibrosis Foundation Research Development Program (grants STANTO19R0 and STANTO11R0). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PØ, Høiby N. 2013. The in vivo biofilm. Trends Microbiol 21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Luzar MA, Thomassen MJ, Montie TC. 1985. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun 50:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttenplan SB, Kearns DB. 2013. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev 37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belas R. 2014. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol 22:517–527. doi: 10.1016/j.tim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Mulcahy LR, Isabella VM, Lewis K. 2014. Pseudomonas aeruginosa biofilms in disease. Microb Ecol 68:1–12. doi: 10.1007/s00248-013-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoiby N. 2011. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med 9:32. doi: 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazli M, Bjarnsholt T, Kirketerp-Moller K, Jorgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker-Nielsen T. 2009. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol 47:4084–4089. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homoe P, Bjarnsholt T, Wessman M, Sorensen HC, Johansen HK. 2009. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur Arch Otorhinolaryngol 266:1533–1538. doi: 10.1007/s00405-009-0940-9. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 12.Kragh KN, Alhede M, Jensen PØ, Moser C, Scheike T, Jacobsen CS, Seier Poulsen S, Eickhardt-Sørensen SR, Trøstrup H, Christoffersen L, Hougen H-P, Rickelt LF, Kühl M, Høiby N, Bjarnsholt T. 2014. Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun 82:4477–4486. doi: 10.1128/IAI.01969-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam J, Chan R, Lam K, Costerton JW. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun 28:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schleheck D, Barraud N, Klebensberger J, Webb JS, McDougald D, Rice SA, Kjelleberg S. 2009. Pseudomonas aeruginosa PAO1 preferentially grows as aggregates in liquid batch cultures and disperses upon starvation. PLoS One 4:e5513. doi: 10.1371/journal.pone.0005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhede M, Kragh KN, Qvortrup K, Allesen-Holm M, van Gennip M, Christensen LD, Jensen PØ, Nielsen AK, Parsek M, Wozniak D, Molin S, Tolker-Nielsen T, Høiby N, Givskov M, Bjarnsholt T. 2011. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One 6:e27943. doi: 10.1371/journal.pone.0027943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staudinger BJ, Muller JF, Halldórsson S, Boles B, Angermeyer A, Nguyen D, Rosen H, Baldursson O, Gottfreðsson M, Guðmundsson GH, Singh PK. 2014. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 189:812–824. doi: 10.1164/rccm.201312-2142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goltermann L, Tolker-Nielsen T. 2017. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob Agents Chemother 61:e02696-16. doi: 10.1128/AAC.02696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kragh KN, Alhede M, Rybtke M, Stavnsberg C, Jensen PO, Tolker-Nielsen T, Whiteley M, Bjarnsholt T. 2017. Inoculation method could impact the outcome of microbiological experiments. Appl Environ Microbiol doi: 10.1128/AEM.02264-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhede M, Bjarnsholt T, Jensen PO, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Hoiby N, Rasmussen TB, Givskov M. 2009. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155:3500–3508. doi: 10.1099/mic.0.031443-0. [DOI] [PubMed] [Google Scholar]
- 20.Nichols WW, Evans MJ, Slack MP, Walmsley HL. 1989. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol 135:1291–1303. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen SM, Norskov-Lauritsen N, Bjarnsholt T, Meyer RL. 2016. Achromobacter species isolated from cystic fibrosis patients reveal distinctly different biofilm morphotypes. Microorganisms 4:E33. doi: 10.3390/microorganisms4030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Domenico EG, Cavallo I, Bordignon V, D’Agosto G, Pontone M, Trento E, Gallo MT, Prignano G, Pimpinelli F, Toma L, Ensoli F. 2018. The emerging role of microbial biofilm in Lyme neuroborreliosis. Front Neurol 9:1048. doi: 10.3389/fneur.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clary G, Sasindran SJ, Nesbitt N, Mason L, Cole S, Azad A, McCoy K, Schlesinger LS, Hall-Stoodley L. 2018. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob Agents Chemother 62:e01782-17. doi: 10.1128/AAC.01782-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid G, Schwarz-Faulkner S, McGroarty JA, Bruce AW. 1990. Bacterial aggregation in relation to peritoneal dialysis. Perit Dial Int 10:21–24. [PubMed] [Google Scholar]
- 25.Bjarnsholt T, Tolker-Nielsen T, Givskov M, Janssen M, Christensen LH. 2009. Detection of bacteria by fluorescence in situ hybridization in culture-negative soft tissue filler lesions. Dermatol Surg 35(Suppl 2):1620–1624. doi: 10.1111/j.1524-4725.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 26.Faruque SM, Biswas K, Udden SM, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ. 2006. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A 103:6350–6355. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winstanley C, O’Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Hoiby N, Molin S. 2012. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol 10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 29.Hauser AR, Jain M, Bar-Meir M, McColley SA. 2011. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev 24:29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahenthiralingam E, Campbell ME, Speert DP. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 62:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amiel E, Lovewell RR, O’Toole GA, Hogan DA, Berwin B. 2010. Pseudomonas aeruginosa evasion of phagocytosis is mediated by loss of swimming motility and is independent of flagellum expression. Infect Immun 78:2937–2945. doi: 10.1128/IAI.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovewell RR, Collins RM, Acker JL, O'Toole GA, Wargo MJ, Berwin B. 2011. Step-wise loss of bacterial flagellar torsion confers progressive phagocytic evasion. PLoS Pathog 7:e1002253. doi: 10.1371/journal.ppat.1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 34.Farinha MA, Ronald SL, Kropinski AM, Paranchych W. 1993. Localization of the virulence-associated genes pilA, pilR, rpoN, fliA, fliC, ent, and fbp on the physical map of Pseudomonas aeruginosa PAO1 by pulsed-field electrophoresis. Infect Immun 61:1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pestrak MJ, Chaney SB, Eggleston HC, Dellos-Nolan S, Dixit S, Mathew-Steiner SS, Roy S, Parsek MR, Sen CK, Wozniak DJ. 2018. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog 14:e1006842. doi: 10.1371/journal.ppat.1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovewell RR, Patankar YR, Berwin B. 2014. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M. 2014. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet 10:e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deligianni E, Pattison S, Berrar D, Ternan NG, Haylock RW, Moore JE, Elborn SJ, Dooley JS. 2010. Pseudomonas aeruginosa cystic fibrosis isolates of similar RAPD genotype exhibit diversity in biofilm forming ability in vitro. BMC Microbiol 10:38. doi: 10.1186/1471-2180-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee B, Haagensen JA, Ciofu O, Andersen JB, Hoiby N, Molin S. 2005. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Clin Microbiol 43:5247–5255. doi: 10.1128/JCM.43.10.5247-5255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaber JA, Carty NL, McDonald NA, Graham ED, Cheluvappa R, Griswold JA, Hamood AN. 2004. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J Med Microbiol 53:841–853. doi: 10.1099/jmm.0.45617-0. [DOI] [PubMed] [Google Scholar]
- 41.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kragh KN, Hutchison JB, Melaugh G, Rodesney C, Roberts AE, Irie Y, Jensen PO, Diggle SP, Allen RJ, Gordon V, Bjarnsholt T. 2016. Role of multicellular aggregates in biofilm formation. mBio 7:e00237-16. doi: 10.1128/mBio.00237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Secor PR, Michaels LA, Ratjen A, Jennings LK, Singh PK. 2018. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 115:10780–10785. doi: 10.1073/pnas.1806005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darch SE, Kragh KN, Abbott EA, Bjarnsholt T, Bull JJ, Whiteley M. 2017. Phage inhibit pathogen dissemination by targeting bacterial migrants in a chronic infection model. mBio 8:e00240-17. doi: 10.1128/mBio.00240-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haaber J, Cohn MT, Frees D, Andersen TJ, Ingmer H. 2012. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PLoS One 7:e41075. doi: 10.1371/journal.pone.0041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernier SP, Ha D-G, Khan W, Merritt JH, O’Toole GA. 2011. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbiol 162:680–688. doi: 10.1016/j.resmic.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merritt JH, Kadouri DE, O’Toole GA. 2005. Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1:Unit 1B.1. [DOI] [PMC free article] [PubMed] [Google Scholar]