Abstract
Cervical cancer is the most common cancer in Tanzania. After excluding human immunodeficiency virus, lower respiratory infections, malaria, diarrheal diseases, and tuberculosis, cervical cancer kills more women than any other form of illness in the country. Unfortunately, Tanzania has a low doctor-to-patient ratio (1:50,000) and nearly 7000 women die each year from this disease. The clinical problem is further magnified by the country's lack of resources and prevailing poverty, sporadic cervical cancer screening, prevalence of high-risk oncogenic human papillomavirus subtypes, and relatively high rates of human immunodeficiency virus co-infection. In recent years, addressing the cervical cancer problem has become a priority for the Tanzanian government. In this systematic review of 39 peer-reviewed publications that appeared in the PubMed/MEDLINE (NCBI) database from 2013 to 2018, we synthesize the growing body of literature to capture current trends in Tanzania's evolving cervical cancer landscape. Six domains were identified, including risk factors, primary prevention, barriers to screening, treatment, healthcare worker education, and sustainability. In addition to traditional risk factors associated with sexual behavior, acetowhite changes observed during visual inspection of the cervix with acetic acid, lower education, rural setting, and HIV positivity also have a noteworthy clinical impact.
Keywords: Visual inspection with acetic acid, VIA, Tanzania, Cervical cancer, HPV, HIV
Highlights
-
•
Poverty, fewer doctors, and HIV magnify the cervical cancer problem in Tanzania.
-
•
VIA can be effective but improved access, education, and follow-up is needed.
-
•
Tanzania is one of seven African countries that has rolled out the HPV vaccine.
1. Background
Cervical cancer is the fourth most common cancer in women globally, with over 500,000 new cases and an estimated 250,000 deaths each year. The burden of cervical cancer is disproportionately high in developing countries, which account for 85% of cases worldwide and nearly 90% of cervical cancer deaths. The estimated age-standardized rate of cervical cancer mortality in East Africa is higher than that of any other region in the world (Bray et al., 2018). In Tanzania, cervical cancer is the leading cause of female cancer with a striking 9772 new cases and 6695 deaths each year (Bruni et al., 2018).
In many high-income countries, cervical cancer rates declined substantially after the widespread introduction of the Papanicolaou smear in the mid 20th century (IARC Working Group, 2005). However, similar trends did not emerge in developing countries due to lack of resources for screening implementation (including cytopathology review) and low population coverage. The relatively high prevalence of human papillomavirus (HPV) and human immunodeficiency virus (HIV) co-infection in these countries also contributes to elevated cervical cancer rates (Walboomers et al., 1999; Bruni et al., 2010; Dartell et al., 2012). The World Health Organization advocates visual inspection of the cervix with acetic acid (VIA) as an alternative in settings where cytology-based screening methods are impractical. In 2012, the organization conducted a pilot in six African countries demonstrating that “screen and treat” with VIA and cryotherapy is effective and feasible (Mgomella et al., 2012).
There are three U.S. FDA-approved HPV vaccines, all providing coverage against infection by HPV types 16 and 18, which account for 70% of invasive cervical cancer cases worldwide. The vaccine has been licensed in over 60 countries and >100 million doses have been administered (Castle and Maza, 2016). Challenges to widespread deployment of the vaccine in developing countries include cost, healthcare infrastructure, and sociocultural acceptability (Agosti and Goldie, 2007).
The Tanzanian Ministry of Health and Social Welfare launched VIA cervical cancer screening and cryotherapy in over 300 sites nationally in 2011 (Fig. 1, Fig. 2) (Ministry of Health, 2016). On April 10, 2018, Tanzania became the seventh African country (following Uganda, Rwanda, Botswana, Mauritius, Seychelles, and South Africa) to introduce HPV vaccination into its immunization program (Fig. 3), with over 600,000 girls ages 9 to 14 years vaccinated in 2018. Low-cost doses of the quadrivalent vaccine are supplied by the Vaccine Alliance, Gavi. Approximately 15 USD is spent vaccinating each girl; for each woman presenting with an advanced cervical cancer, an estimated 2000 USD is required for supportive care (Kileo, 2018).
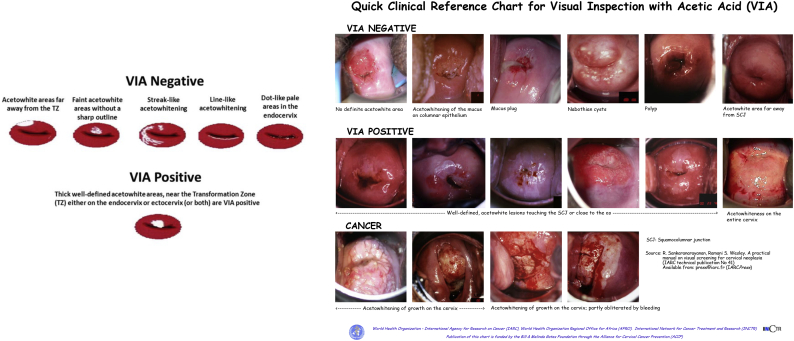
Fig. 1.
Visual inspection with acetic acid charts for healthcare provider training. Prepared by the World Health Organization's International Agency for Research on Cancer (IARC) and made available by the Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP).
Fig. 2.
Cervical cancer screen-and-treat in Northern Tanzania. A. Women in line for VIA screening at Buzuruga Health Centre in the Mwanza metropolitan area of the Ilemela District of northern Tanzania. Photograph taken with permission on July 4, 2018 during the University of California, Irvine-led cervical cancer screen-and-treat workshop. B. UCI medical students Elizabeth Crawford and Megan Bernstein with CDM high school student Sujata Tewari, together with Buzuruga Health Centre nurse Suzie and Brother Erick Oguta. C. University of California, Irvine Class of 2021 medical students on the ground in Mwanza, Tanzania for the July 2018 cervical cancer screen-and-treat workshop at Buzuruga Health Centre: From left to right: Justine Maher, Emma Cooper, Alexa Lucas, Danielle Zezoff, Elizabeth Crawford, Ariana Naaseh, Kevin Bera, Justine Chinn, Kayla White, Ava Runge, Andreea Dinicu.
Fig. 3.
Some of the first girls to receive the HPV vaccine following the campaign launch in Tanzania during April 2018. Here they pose with their vaccination booklets. Source: Jhpiego.
It is essential to understand Tanzania's evolving cervical cancer problem in order to facilitate appropriate future research, prevention, and treatment efforts. In this systematic review, we synthesize the growing body of literature to characterize the country's cervical cancer landscape from 2013 to 2018.
1.1. Introduction to Tanzania
Tanzania is known for majestic Mount Kilimanjaro and lush Serengeti plains, the stage upon which the second largest terrestial mammal migration in the world plays out each year. The people of Tanzania are descended from 120 indigenous ethnic groups and speak >100 different dialects, including Sukuma, Nyamwezi, Chagga, Haya, Hadza, and Maasai (Atlas, 2018). The country has the largest population in East Africa, with over one-third of its 55,451,343 residents living in the urban areas of Dar es Salaam, Mwanza, and Dodoma. Over 60% of the population is under 25, reflecting Tanzania's high birth rate (35.3 births /1000 population), high fertility rate (4.71 children per woman), and relatively low life expectancy (61.6 years for men, 64.6 years for women) (Central Intelligence Agency, 2019). Twenty-three percent live below the poverty line, with 80% of the poor living in rural areas. Agriculture is the largest sector, employing approximately 65% of the work force. The primary drivers of Tanzania's latest economic growth are the construction, communications, and financial sectors, due to the high annual rate of urbanization (5.22%) (World Bank Group, 2015). Much of the economic growth among individual households is attributable to increased education levels. Children complete an average of 8 years of school and overall literacy is approximately 80% (Central Intelligence Agency, 2019).
Tanzania gained independence from Britain on December 9, 1961. Ujamaa (Swahili for “family hood”) was an exercise in socialism developed by Julius Kambarage Nyerere who served as President of Tanzania from 1964 to 1985. The impetus of Ujamaa was on collective agriculture through villagization and increased national self-reliance. Although the mandate called for voluntary redistribution of individuals from urban districts to villages, what ensued were campaigns of persuasion, intimidation, and coercion. Regulations preventing physicians from treating patients privately in their homes after having worked in government-run clinics and hospitals led to the emigration (and expulsion) of many doctors (Kaufman, 1999). Ultimately, Ujamaa failed and by the time Nyerere left office, Tanzania had become one of Africa's poorest countries, with a low density of doctors, and highly dependent on international aid.
In modern day Tanzania, there continues to be a significant lack of access to allopathic medical services, with a physician density of 0.02 per 1000 and hospital bed density of 0.7 beds per 10,000 population. There are approximately 240 hospitals, 633 health centers, and 5469 dispensaries across the country (Boex et al., 2015). Insufficient surgical equipment, medications, and imaging capabilities are frequently reported (Nyberger et al., 2019; Penoyar et al., 2012; Galukande et al., 2010). The Ocean Road Cancer Institute (ORCI), Bugando Medical Center, the Kilimanjaro Christian Medical Center, and Arusha Lutheran Medical Center are among the few hospitals offering any form of cancer treatment services (Fig. 4) (The Foundation for Cancer Care in Tanzania, 2015).
Fig. 4.
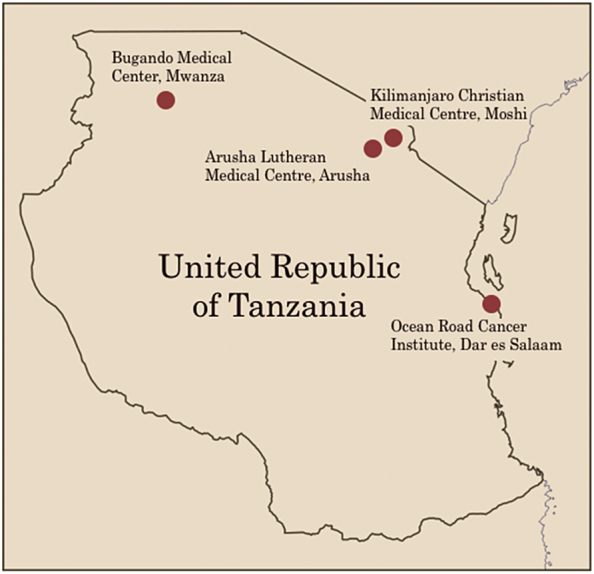
Map of the Republic of Tanzania indicating medical centers where cancer care is provided. Designed by Ava Runge.
Various methods of traditional medicine are commonly practiced in Tanzania. A study of 655 adults in Northern Tanzania found that a majority of respondents use traditional medicines due to lower cost, fewer side effects, ease of access, and perceived safety and efficacy (Stanifer et al., 2015). Recent violence against persons with albinism has been associated with “magical practices”, “witch doctors”, and traditional healers, spurring controversy surrounding the regulation and legitimacy of traditional medicine (Brocco, 2016).
2. Methods
Seven separate searches of PubMed/MEDLINE (NCBI) were conducted using the keyword “Tanzania” in combination with each of the following search terms: visual inspection with acetic acid, VIA, human papilloma, human papillomavirus, HPV, cervical cancer, cryotherapy, chemotherapy, and radiotherapy. Inclusion criteria included publication between 2013 and 2018, cervical cancer-specific content, and a focus on Tanzania. In order to fill in gaps related to invasive cervical cancer treatment, a secondary search of White Papers published by the Tanzanian government and non-governmental organizations (NGOs) was conducted.
3. Results
From 208 publications identified, 65 met the inclusion criteria. After eliminating 21 duplicative reports, a total of 39 peer-reviewed journal articles, one White Paper by the Foundation for Cancer Care in Tanzania, and one report from the Tanzanian government were included in this systematic review. Six domains were characterized: (1) cervical cancer awareness and primary prevention, (2) facilitators and barriers to screening, (3), cervical lesion prevalence and risk factors, (4) treatment of dysplasia and invasive cancer, (5) healthcare worker education, and (6) sustainability.
3.1. Domain I. Cervical cancer awareness and primary prevention
Seven studies focused on primary prevention, with the majority assessing cervical cancer knowledge and HPV vaccine feasibility. Overall awareness was high, with over 80% of women surveyed reporting they had heard about cervical cancer (Moshi et al., 2018; Mabelele et al., 2018). Key factors predicting awareness included having secondary education or higher, urban household, being affluent, having 1–4 children, and/or being 30–44 years old (Moshi et al., 2018). The majority perceived they were susceptible to cervical cancer, with urban women feeling more at risk than rural women (71% vs 62%). Most had received education on cervical cancer and the HPV vaccine from radio and television. Approximately one-third of women knew someone with cervical cancer (Mabelele et al., 2018; Cunningham et al., 2015).
Despite high general awareness, knowledge of specific risk factors and prevention methods was strikingly low. In a cross-sectional survey by Mabele et al., 82.7% scored <50% on their knowledge of cervical cancer, and the majority (82.4%) were unable to mention a single cervical cancer risk factor or symptom. Inaccurate responses suggested an abundance of misinformation in the surveyed community. Among risk factors, “long term oral contraceptive use” was most frequently mentioned, and “infection with HPV”, “uncircumcised partner”, and “weakened immunity” were least frequent. “Persistent vaginal malodorous discharge” was the most frequently recalled symptom; none mentioned vaginal bleeding during or after sexual intercourse, or unexplained weight loss (Mabelele et al., 2018; Cunningham et al., 2015).
Overall HPV vaccine-related awareness was low among women in both rural and urban Tanzania (<8%), although 93% of a 575-respondent survey said they would be highly accepting of a vaccine (Mabelele et al., 2018). Eighty percent believed their friends and family would support the vaccine, while a lower percentage (64%) believed their husbands would be accepting. The greatest concern was perceived vaccine costs, followed by unknown future side effects and short-term side effects. Additional concerns included social acceptability and possible encouragement of sexual promiscuity and/or earlier sexual debut (Cunningham et al., 2015).
An HPV vaccine training program for 17 African countries demonstrated the feasibility of adolescent immunization campaigns. The study found that school-based delivery was most cost-effective and manageable. Many countries packaged the vaccine with additional health interventions for adolescents, with vaccine rollout in Tanzania also offering deworming, vision screening, and dental services (Dochez et al., 2017). In addition, a stakeholder meeting of 14 Tanzanian school health interventions found education sessions on reproductive health to be suitable for integration with vaccine delivery. Suggestions to improve vaccination programs included decreasing the number of vaccine doses (from three to two doses) to minimize loss of follow-up, reducing vaccine cost, and reaching girls who have dropped out of school to earn money for their families (Dochez et al., 2017). Stakeholders also agreed that interventions for boys and age groups outside those receiving the vaccine were important (Watson-Jones et al., 2016).
While immunization cost is a commonly cited concern, a cost-effectiveness model by Kim et al. suggested that HPV vaccination is a high value intervention, with an estimated 41,200 DALYs (disability-adjusted life years) averted if 70% of pre-adolescent girls were vaccinated (Kim et al., 2013). HPV also appears to be contracted shortly after sexual debut, reinforcing the advantage of vaccinating the target population. Watson-Jones et al. reported 74% of sexually active women aged 10–25 years had HPV at the time of enrollment, with nearly 55% having high-risk subtypes (Watson-Jones et al., 2013). Houlihan et al. detected HPV in 24.1% of sexually-active girls aged 15–16 years. Interestingly, 8.4% of girls in this age group had HPV prior to self-reported sexual debut, suggesting underreporting of sexual activity (Houlihan et al., 2014; Houlihan et al., 2016b). This is consistent with the current dogma that it is critical to vaccinate girls before their first sexual encounter, and suggests that it may be pertinent to explore vaccination below Tanzania's target of 14 years.
Because less common high-risk HPV subtypes have been identified in women with HIV, it will also be important to evaluate vaccine efficacy in HIV positive individuals (Mujuni et al., 2016). In addition, while parasitic infections are common in Tanzania, one study reported that HPV immunogenicity 12 months following vaccination was not attenuated in the presence of malaria and helminth infections (Brown et al., 2014).
3.2. Domain II. Facilitators and barriers to cervical cancer screening
Seven studies investigated cervical cancer screening practices in Tanzania, with an emphasis on predictors of accessing such services. Screening methods deployed over the past six years were largely VIA-based, although Pap smears were occasionally used (Baldur-Felskov et al., 2018; Chambuso et al., 2017; Kafuruki et al., 2013; Liu et al., 2016). Multiple publications focused on improving screening programs through strengthening provider training (see Domain V).
A common trend was that a majority of women have heard of cervical cancer, but few (6–21%) have ever been screened (Cunningham et al., 2015; Koneru et al., 2017; Kileo et al., 2015). Women from urban areas were more likely to have been screened if they were older, had some form of health insurance, or had prior knowledge about cervical cancer. In rural areas, condom use was positively associated with screening (Cunningham et al., 2015). Perng et al. also found that women who attended screening services were older, had a poorer quality of life, listened regularly to the radio, had faced cost barriers to healthcare in the preceding year, and held a positive attitude toward screening (Perng et al., 2013). In a study of 512 primary school teachers in Dar es Salaam, women were more likely to use a cervical cancer screening service if they were multiparous, did not involve their spouse in health-related decision making, or reported over one lifetime sexual partner. Interestingly, Cunningham et al. found that older teachers (ages 40–49) were less likely to use cervical cancer screening services compared to younger teachers (ages 20–29) (Kileo et al., 2015). Knowledge of the screening process was varied and greater among those with previous screening history (Koneru et al., 2017). A large proportion of women reported that they would be willing to travel over two hours to visit a screening clinic. Significant barriers to screening included being unaware that preventative screening tests exist and concerns about being able to afford travel and testing costs (Cunningham et al., 2015). Fear, stigma, and misinformation surrounding cervical cancer also limited screening (Bateman et al., 2018).
3.3. Domain III. Cervical lesion prevalence and risk factors
Five publications reported on VIA results involving 43,943 women. The cumulative VIA positivity was 9.2% (n = 4062) (Table 1). HIV infection was significantly associated with precancerous changes, with 16.8% of HIV+ women also VIA+ (523/3122) (Anderson et al., 2015; Bernstein et al., 2018; Dartell et al., 2014; Baldur-Felskov et al., 2018; Masalu et al., 2017; Chambuso et al., 2017; Kafuruki et al., 2013) (Table 2). High grade dysplastic lesions were often inferred by VIA screening results due to infrequency of pathologic confirmation. The average prevalence of VIA positivity or apparent high grade squamous intraepithelial lesions (HGSIL) ranged from 8.4 to 71.8% for HIV+ women and from 3.2 to 27.3% in HIV-negative women. Women with more advanced HIV (per WHO classification) demonstrated a relative higher risk of HGSIL. Risk for cervical intraepithelial neoplasia (CIN) was higher among those with baseline CD4 T lymphocyte count <200 cells/mm3 and with tobacco exposure (Kafuruki et al., 2013; Liu et al., 2016). HIV+ women with cervical cancer tended to be younger with lower parity, highlighting the postulated role of HIV in accelerating the disease course (Liu et al., 2016). Faber et al. reported HIV+ women were four times as likely to be infected by high risk HPV subtypes compared to HIV-negative women (Dartell et al., 2014; Dartell et al., 2013; Faber et al., 2017). HIV+ women may be more likely to suffer death unrelated to cervical cancer and/or receive preventive screening compared to HIV-negative patients. Lovgren et al. reported HIV+ women to be significantly more likely to attend screening than HIV-negative women (Lovgren et al., 2016). Gallagher et al. found no association between HPV infection and subsequent HIV acquisition (Gallagher et al., 2016).
Table 1.
VIA screen-and-treat for cervical cancer and precursor lesions in Tanzania (2013–2018).
| Author & year | Region | N | VIA + | Cryotherapy |
|---|---|---|---|---|
| Anderson et al. (2015) | Unspecified | 7449 | 532 (7.1%) | 450 (6.0%) |
| Bernstein et al. (2018) | Mwanza | 614 | 59 (9.6%) | 49 (7.9%) |
| Dartell et al. (2014) | Dar es Salaam | 3767 | 161 (4.3%) | Cryotherapy provided at follow-up visit; number treated not reported |
| Baldur-Felskov et al. (2018) | Dar es Salaam, Pwani, Mwanza, Mtwara | 3339 | 145 (4.3%) | Cryotherapy provided at time of screen; number treated not reported |
| Masalu et al. (2017) | Mwanza, Mara | 2342 | 302 (12.9%) | 34 (1.5%) |
Table 2.
HIV infection and VIA screening for cervical cancer and precursor lesions in Tanzania (2013–2018).
| Author & year | Region | N | HIV+ | HIV+ and VIA+ | HIV management | VIA+ management |
|---|---|---|---|---|---|---|
| Anderson et al. (2015) | Unspecified | 7449 | 1797 (24.1%) | 237 (3.2%) | Provider-initiated HIV counseling and treatment initiation | Cryotherapy |
| Referral for LEEP PRN | ||||||
| Dartell et al. (2014) | Dar es Salaam | 3767 | 334 (8.9%) | 43 (1.1%) | Provider-initiated HIV counseling and treatment initiation | Return to clinic for treatment within 3 months |
| Baldur-Felskov et al. (2018) | Dar es Salaam, Pwani, Mwanza, Mtwara | 3339 | 334 (8.9%) | 43 (1.1%) | Provider-initiated HIV counseling and treatment initiation | Cryotherapy |
| Follow-up not reported | ||||||
| Masalu et al. (2017) | Mwanza, Mara | 2342 | 192 (8.2%) | Not reported | Not reported | Cryotherapy |
| Referral for colposcopy, biopsy, or higher level of care | ||||||
| Chambuso et al. (2017) | Morogoro | 536 | 110 (20.5%) | 79 (14.7%) | Provider-initiated HIV counseling and treatment initiation | Cryotherapy, LEEP, referral for tertiary follow-up |
| Kafuruki et al. (2013) | Mwanza | 335a | 95 (28.4%) | 95 (28.4%) | On-site HIV treatment and management | Cryotherapy |
| Referral for higher level of care |
Study subjects were exclusively HIV-positive women.
Beyond HIV, Houlihan et al. found that spending the night away from home increased the risk for HPV-positivity among females aged 15–16 years, while exposure to pornographic movies decreased this risk (Houlihan et al., 2016a). Trichomonas vaginalis increased risk for HPV 16 infection 6.5-fold (Lazenby et al., 2014). Factors positively associated with a diagnosis of VIA+ or HGSIL included high-risk HPV+, increasing age, higher gravidity, and lower body mass index, as well as untreated sexually transmitted disease(s) (Baldur-Felskov et al., 2018; Faber et al., 2017). Risk factors for invasive cancer also included being housewives or manual laborers, uneducated, widowed or separated, older, of higher gravidity, and post-menopausal (Gard et al., 2014). Women were more likely to present with more advanced cancer if they regularly visited alternative health practitioners, lacked formal education, or lacked “personal initiative” to visit (allopathic) healthcare facilities (Mlange et al., 2016).
3.4. Domain IV. Treatment of cervical dysplasia and invasive cervical cancer
Unfortunately, treatment failure due to loss to follow-up was a common trend across the cervical pre-cancer and cancer care continuum. Masalu et al. found that 28.8% of women did not receive cryotherapy for significant VIA+ lesions (Masalu et al., 2017). A second study demonstrated that receiving immediate treatment for VIA+ lesions in a single visit resulted in significant reduction in loss to follow-up, as 82% of those who postponed cryotherapy did not return for the procedure (Anderson et al., 2015). Among women with locally advanced invasive cancer referred for adjuvant and/or primary radiation therapy, fewer patients were lost to follow-up compared to those with early stage disease (Gard et al., 2014). Those who did not complete treatment included women with a skilled occupation (compared to housewives), younger age, and those residing in Dar es Salaam.
A significant barrier to invasive cervical cancer treatment is the lack of access to modern cancer therapeutic modalities in Tanzania. The Ocean Road Cancer Institute (ORCI) in Dar es Salaam has historically been the only specialized cancer treatment center in the country offering both chemotherapy and radiotherapy, although in recent years a small number of hospitals have made progress in developing cancer care programs of their own. Bugando Medical Center (BMC) in Mwanza provides chemotherapy and surgery and has recently built a radiotherapy center, although it is unclear whether it currently has sufficient resources to operate it. In 2016, the Foundation for Cancer Care in Tanzania opened the Helmut & Rotraut Diefenthal Cancer Care Centre and the Mark & Linda Jacobson Infusion Centre at the Kilimanjaro Christian Medical Center (KCMC), expanding its services to include chemotherapy infusions. Both KCMC and the Arusha Lutheran Medical Center (ALMC) provide diagnostic biopsies and surgical tumor resection and also offer residency training programs in radiology and general surgery.
Despite the growth of Tanzania's cancer care programs, inadequate staffing is a significant constraint, with only six medical oncologists and apparently only one gynecologic oncologist working in the country as of 2014. Moreover, the geographic confinement of existing clinics to northern Tanzania and the high cost of travel make access to therapy problematic (if not impossible) for many women. Resources for chemotherapy are also a considerable challenge. At ORCI, over 70% of patients often cannot receive their prescribed medications due to lack of availability. Such medications used to treat cervical cancer in Tanzania are based on the World Health Organization's 2017 Essential Medicines list and include cisplatin, paclitaxel, bevacizumab, carboplatin, docetaxel, and gemcitabine (The Tanzanian Ministry of Health, 2017). Improving access to these medications and to other oncology services will require further governmental and NGO investment in cancer care. Improved transportation and education services to ensure women obtain treatment in a timely manner, as well as adequate monitoring and management of treatment-related toxicities, are also fundamental to the provision of meaningful and compassionate cancer therapy. Unfortunately, the existing infrastructure in Tanzania is not sufficient to meet these needs.
3.5. Domain V. Healthcare worker education
Another common theme is the impact of education on both patients and healthcare workers. In the past five years, researchers have conducted surveys to assess gaps in current knowledge of cervical cancer. Rick et al. implemented an immersive, one-day cancer training symposium in Arusha with 43 clinicians; 69% had never received any cervical cancer training (Rick et al., 2017). Pre- and post-test knowledge showed significant improvement in recognition of signs and symptoms of cervical cancer. Bernstein et al. reported similar results when training a group of 11 mid-level healthcare providers including nurses and clinical officers via a one-day classroom course, which was followed by supervised screen-and-treat practice during a mass screening campaign in Mwanza (Bernstein et al., 2018).
Before sweeping educational changes and new responsibilities are added on the existing medical workforce, common problems such as lack of adequately trained healthcare staff, task shifting, and concerns of “work overload” by personnel must be considered (Gallagher et al., 2018). Additionally, educational efforts must implement a standard that can be upheld uniformly across screening sites. Lavelle et al. found that high false positive rates of precancerous cervical lesions that were referred to Ocean Road Cancer Institute in Dar el Salaam were largely due to inconsistency in training among their referring clinics. The investigators stressed the importance of closely evaluating trained providers to identify gaps in knowledge, such as difficulty differentiating a screen-positive lesion from an active cervical infection (Lavelle et al., 2017). As technology and access to smart phone usage continue to increase in Africa, mobile technology will be integrated into healthcare education. Yeates et al. reported how five non-physician providers in Moshi were trained to perform VIA enhanced by smartphone cervicography; images were transferred digitally to regional experts and within one month the interpretation of VIA results by trainees and experts was found to be in agreement 96.8% of the time (Yeates et al., 2016).
3.6. Domain VI. Sustainability
Eight papers addressed the sustainability of interventions aimed at combatting cervical cancer, including screening, HPV testing, and HPV vaccination. Commonly identified barriers appear in Table 3. (Bateman et al., 2018; Tsu et al., 2018). Some investigators have encouraged small changes that can have a large impact. Bateman et al. studied patient (or peer) navigators, community members who take on the role of guiding patients through the healthcare system. They help educate women and their communities, advocate screening, encourage a dialogue regarding screening results, provide emotional support, assist with setting up appointments, arrange transport, and accompany women to the clinics to provide continuity and prevent loss to follow-up (Bateman et al., 2018). Koneru et al. also investigated peer navigators and found that while most women were unfamiliar with their services, the majority (88%) expressed a strong interest in being accompanied for cervical cancer evaluation and treatment. This may be explained in part by the high self-reported need for assistance with explanation of medical terms and the preference for a female in the room if a male healthcare provider is performing the exam. This same study found that approximately 90% of women agreed that receiving SMS texts and phone call reminders of appointments for cervical cancer screening and/or treatment would also be helpful (Koneru et al., 2017).
Table 3.
Potential barriers to sustainability of cervical cancer control in Tanzania.
| Type of Barrier | Examples |
|---|---|
| Structural | Lack of resources |
| Lack of facilities | |
| Unreliable power sources | |
| Systemic | Lack of education |
| Scarcity of trained healthcare providers | |
| Competing health needs | |
| Socioeconomic | Rural communities |
| Lack of transportation to screening clinics | |
| Financial limitations | |
| Sociocultural | Stigmata of diagnosis |
| Fear of screening | |
| Fear of vaccination |
Ngoma et al. emphasize that utilizing cost-effective programs is imperative to maintaining a financially sustainable cervical cancer program that can be subsidized by government funding. Accordingly, the investigators have encouraged programs such as screen-and-treat campaigns using VIA and cryotherapy (Ngoma et al., 2016). Additionally, Plotkin et al. have advocated for the integration of reciprocal point of care HIV testing with all HPV and cervical cancer screening to improve rates of diagnosis and treatment, and to reduce doctors' visits and costs to the patient (Plotkin et al., 2014).
Improving sustainability also requires addressing education, funding, and government engagement in women's health. Dochez et al. and McCree et al. propose that better education is needed to not only combat stigma and overcome sociocultural and religious beliefs, but also to improve the number of healthcare providers and the training levels and proficiency benchmarks of those professionals (Dochez et al., 2017; McCree et al., 2015). Tsu et al. stress the importance of research support and have called upon the government to spend capital on HPV and cervical cancer on the basis of evidence-driven protocols (Tsu et al., 2018).
4. Discussion
Collectively, investigators interested in the cervical cancer problem in Tanzania have made great strides in recent years as evidenced by the sheer volume of published data covered in our systematic review. The prevalence of cervical cancer and its risk factors in Tanzania harmonize with current epidemiological observations in similar resource-poor settings. Lower education, living in rural settings, older age, higher gravidity, a high number of sexual partners, HIV positivity, and other sexually transmitted co-infections were all identified as significant risk factors in the development of high-risk HPV infection, VIA positivity, cervical dysplasia, or invasive cancer.
It would appear that Tanzania's HPV vaccine rollout will succeed from both social acceptability and cost-effectiveness perspectives. The high awareness of the cervical cancer burden indicates that primary prevention will be met with enthusiasm, although raising awareness of the HPV vaccine will be paramount to its success. However, women unable to access vaccination and/or VIA programs and those who slip through the cracks of a suboptimal infrastructure will face significant challenges in receiving therapy as a consequence of structural, systemic, socioeconomic, and cultural barriers.
Despite the obstacles faced by Tanzanian women, the current literature generates a sense of optimism about the efforts being made to advance knowledge of both patients and healthcare workers and to implement new models that improve access, screening, and follow-up. A significant barrier to screening is predicated on lack of awareness and misinformation, suggesting that more robust education campaigns may increase screening adoption. Education on specific cervical cancer risk factors and symptoms is an unmet need and its improvement may lead women to seek screening services sooner. The increased utilization of screen-and-treat clinics as well as the budding use of patient navigators and smart phone technology are examples of progress being made in the face of a problem that sometimes seems impossibly large. Ultimately, the sustainability of projects aimed at combatting cervical cancer will depend on overcoming barriers common to resource-poor settings with cost-effective, community-driven programs that incorporate education for both patients and healthcare providers.
Author contributions
-
1.
Original concept: Runge, Bernstein, Lucas, Tewari.
-
2.
PUBMED database search: Runge, Bernstein, Lucas.
-
3.
Preparation of first and second manuscript drafts: Runge, Bernstein, Lucas.
-
4.
Critical review of second draft and preparation of third draft: Runge, Bernstein, Lucas, Tewari.
-
5.
Critical review of third draft and preparation of final (4th draft): Runge, Bernstein, Lucas, Tewari.
-
6.
Preparation of the revised manuscript: Runge, Bernstein, Lucas, Tewari.
-
7.
Preparation of Table 1, Table 2, Table 3: Bernstein, Tewari.
- 8.
-
9.
Preparation of Fig. 4: Runge.
-
10.
Final approval of manuscript: Runge, Bernstein, Lucas, Tewari.
References
- Agosti J.M., Goldie S.J. 2007. Introducing HPV Vaccine in Developing Countries — Key Challenges and Issues; pp. 1908–1910. [DOI] [PubMed] [Google Scholar]
- Anderson J., Wysong M., Estep D. Evaluation of cervical cancer screening programs in Côte d'Ivoire, Guyana, and Tanzania: effect of HIV status. PLoS ONE. 2015;10(9):1–13. doi: 10.1371/journal.pone.0139242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldur-Felskov B., Mwaiselage J., Faber M.T. Factors associated with a cervical high-grade lesion on cytology or a positive visual inspection with acetic acid among more than 3300 Tanzanian women. Tropical Med. Int. Health. 2018;00(00):1–9. doi: 10.1111/tmi.13184. [DOI] [PubMed] [Google Scholar]
- Bateman L.B., Blakemore S., Koneru A. Barriers and facilitators to cervical cancer screening, Diagnosis, Follow-Up Care and treatment: perspectives of human immunodeficiency virus-positive women and health care practitioners in Tanzania. Oncologist. 2018 doi: 10.1634/theoncologist.2017-0444. theoncologist.2017–0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M., Hari A., Aggarwal S. Implementation of a human papillomavirus screen-and-treat model in Mwanza, Tanzania: training local healthcare workers for sustainable impact. Int. Health. 2018;10(3):197–201. doi: 10.1093/inthealth/ihy014. [DOI] [PubMed] [Google Scholar]
- Boex J., Fuller L., Malik A. Decentralized local health services in Tanzania. Urban Inst. 2015:1–28. (published in April 2015 on www.urban.org) [Google Scholar]
- Bray F., Ferlay Jacques, Soerjomataram Isabelle, Siegel R.L., Torre L.A., Jemal A., Global Cancer Statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brocco G. Albinism, stigma, subjectivity and global-local discourses in Tanzania. Anthropol. Med. 2016;23(3):229–243. doi: 10.1080/13648470.2016.1184009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Baisley K., Kavishe B. Impact of malaria and helminth infections on immunogenicity of the human papillomavirus-16/18 AS04-adjuvanted vaccine in Tanzania. Vaccine. 2014;32(5):611–617. doi: 10.1016/j.vaccine.2013.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni L., Diaz M., Castellsagué X., Ferrer E., Bosch F.X., de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010 doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, de Bosch FX SS. Human Papillomavirus and related diseases report. 2018;(December).
- Castle P.E., Maza M. Prophylactic HPV vaccination: past, present, and future. Camb. Univ. Press Epidemiol. Infect. 2016;2016:449–468. doi: 10.1017/S0950268815002198. [DOI] [PubMed] [Google Scholar]
- Central Intelligence Agency . 2019. The World Factbook: Tanzania. [Google Scholar]
- Chambuso R.S., Shadrack S., Lidenge S.J., Mwakibete N., Medeiros R.M. Influence of HIV/AIDS on cervical Cancer: a retrospective study from Tanzania. J. Glob. Oncol. 2017;3(1):72–78. doi: 10.1200/JGO.2015.002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.S., Skrastins E., Fitzpatrick R. Cervical cancer screening and HPV vaccine acceptability among rural and urban women in Kilimanjaro region. Tanzania. BMJ Open. 2015;5(3):1–9. doi: 10.1136/bmjopen-2014-005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartell M., Rasch V., Kahesa C. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the protect study. Sex. Transm. Dis. 2012 doi: 10.1097/OLQ.0b013e31823b50ad. [DOI] [PubMed] [Google Scholar]
- Dartell M., Rasch V., Munk C. Risk factors for high-risk human papillomavirus detection among HIV-negative and HIV-positive women from Tanzania. Sex. Transm. Dis. 2013;40(9):737–743. doi: 10.1097/OLQ.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Dartell M.A., Rasch V., Iftner T. Performance of visual inspection with acetic acid and human papillomavirus testing for detection of high-grade cervical lesions in HIV positive and HIV negative Tanzanian women. Int. J. Cancer. 2014;135(4):896–904. doi: 10.1002/ijc.28712. [DOI] [PubMed] [Google Scholar]
- Dochez C., Burnett R.J., Mbassi S.M. Improving skills and institutional capacity to strengthen adolescent immunisation programmes and health systems in African countries through HPV vaccine introduction. Papillomavirus Res. 2017;4(June):66–71. doi: 10.1016/j.pvr.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M.T., Munk C., Mwaiselage J. Risk factors for HIV positivity among more than 3,400 Tanzanian women. Women Health. 2017;57(6):650–664. doi: 10.1080/03630242.2016.1202882. [DOI] [PubMed] [Google Scholar]
- Gallagher K.E., Baisley K., Grosskurth H. The association between cervical human papillomavirus infection and subsequent HIV Acquisition in Tanzanian and Ugandan Women: a nested case-control study. J. Infect. Dis. 2016;214(1):87–95. doi: 10.1093/infdis/jiw094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher K.E., Erio T., Baisley K., Lees S., Watson-Jones D. The impact of a human papillomavirus (HPV) vaccination campaign on routine primary health service provision and health workers in Tanzania: a controlled before and after study. BMC Health Serv. Res. 2018;18(1):1–10. doi: 10.1186/s12913-018-2976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galukande M., von Schreeb J., Wladis A. Essential surgery at the district hospital: a retrospective descriptive analysis in three African countries. PLoS Med. 2010;7(3) doi: 10.1371/journal.pmed.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.C., Soliman A.S., Ngoma T. Most women diagnosed with cervical cancer by a visual screening program in Tanzania completed treatment: evidence from a retrospective cohort study. TT - BMC Public Health. 2014;14:910. doi: 10.1186/1471-2458-14-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan C.F., De Sanjosé S., Baisley K. Prevalence of human papillomavirus in adolescent girls before reported sexual debut. J. Infect. Dis. 2014;210(6):837–845. doi: 10.1093/infdis/jiu202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan C.F., Baisley K., Bravo I.G. The incidence of human papillomavirus in Tanzanian adolescent girls before reported sexual debut. J. Adolesc. Health. 2016;58(3):295–301. doi: 10.1016/j.jadohealth.2015.10.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan C.F., Baisley K., Bravo I.G. Rapid acquisition of HPV around the time of sexual debut in adolescent girls in Tanzania. Int. J. Epidemiol. 2016;45(3):762–773. doi: 10.1093/ije/dyv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group . Int Agency Res Cancer World Heal Organ; 2005. IARC Handbooks of Cancer Prevention Volume 10: Cervix Cancer Screening. [Google Scholar]
- Kafuruki L., Rambau P., Massinde A., Masalu N. Prevalence and predictors of cervical intraepithelial Neoplasia among HIV infected women at Bugando medical Centre Mwanza-Tanzania. Infect. Agent Cancer. 2013;8(1):45. doi: 10.1186/1750-9378-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M.T. Julius Nyerere of Tanzania dies; preached African socialism to the world. New York Times. 1999:1–132. Oct 15. [Google Scholar]
- Kileo N. Tanzania rolls out vaccination against cervical cancer. World Health Organ. 2018:1–9. [Google Scholar]
- Kileo N.M., Michael D., Neke N.M., Moshiro C. Utilization of cervical cancer screening services and its associated factors among primary school teachers in Ilala municipality, Dar es Salaam Tanzania. BMC Health Serv. Res. 2015;15(1):1–9. doi: 10.1186/s12913-015-1206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Campos N.G., O'Shea M., Diaz M., Mutyaba I. Model-based impact and cost-effectiveness of cervical cancer prevention in sub-saharan Africa. Vaccine. 2013;31(S5):F60–F72. doi: 10.1016/j.vaccine.2012.07.093. [DOI] [PubMed] [Google Scholar]
- Koneru A., Jolly P.E., Blakemore S. Acceptance of peer navigators to reduce barriers to cervical cancer screening and treatment among women with HIV infection in Tanzania. Int. J. Gynecol. Obstet. 2017;138(1):53–61. doi: 10.1002/ijgo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle A.E., Su D., Kahesa C., Soliman A.S. Needs for professional education to optimize cervical Cancer screenings in low-income countries: a case study from Tanzania. J. Cancer Educ. 2017:1–6. doi: 10.1007/s13187-017-1276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby G.B., Taylor P.T., Badman B.S. An association between trichomonas vaginalis and high-risk human papillomavirus in rural tanzanian women undergoing cervical cancer screening. Clin. Ther. 2014;36(1):38–45. doi: 10.1016/j.clinthera.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Liu E., McCree R., Mtisi E. Prevalence and risk factors of cervical squamous intraepithelial lesions among HIV-infected women in Dar es Salaam Tanzania. Int. J. STD AIDS. 2016;27(3):219–225. doi: 10.1177/0956462415584466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren K., Soliman A.S., Ngoma T., Kahesa C., Meza J. Characteristics and geographic distribution of HIV-positive women diagnosed with cervical cancer in Dar es Salaam Tanzania. Int. J. STD AIDS. 2016;27(12):1049–1056. doi: 10.1177/0956462415606252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabelele M.M., Materu J., Mahande M.J. Knowledge towards cervical cancer prevention and screening practices among women who attended reproductive and child health clinic at Magu district hospital. Lake zone Tanzania. 2018;62 doi: 10.1186/s12885-018-4490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masalu N., Serra P., Amadori D. Setting up a community-based cervical screening service in a low-income country: a pilot study from North-Western Tanzania. Int. J. Public Health. 2017;62(7):755–762. doi: 10.1007/s00038-017-0971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCree R., Giattas M.R., Sahasrabuddhe V.V. Expanding cervical Cancer screening and treatment in Tanzania: Stakeholders' perceptions of structural influences on scale-up. Oncologist. 2015;20(6):621–626. doi: 10.1634/theoncologist.2013-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mgomella G., Chikamata D., Lucas E. Prevention of cervical cancer through screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy. World Health Organ. 2012:1–33. [Google Scholar]
- Ministry of Health . United Republic of Tanzania the National Road Map Strategic Plan to Improve Health in Tanzania (2016–2020) 2016. Community development, gender, elderly and children. (June 2016) [Google Scholar]
- Mlange R., Matovelo D., Rambau P., Kidenya B. Patient and disease characteristics associated with late tumour stage at presentation of cervical cancer in northwestern Tanzania. BMC Womens Health. 2016;16(1):1–6. doi: 10.1186/s12905-016-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshi F.V., Vandervort E.B., Kibusi S.M. Cervical Cancer awareness among women in Tanzania: an analysis of data from the 2011-12 Tanzania HIV and malaria indicators survey. Int. J. Chronic. Dis. 2018;2018:1–7. doi: 10.1155/2018/2458232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujuni F., Mirambo M.M., Rambau P. Variability of high risk HPV genotypes among HIV infected women in Mwanza, Tanzania- the need for evaluation of current vaccine effectiveness in developing countries. Infect Agent Cancer. 2016;11(1):1–7. doi: 10.1186/s13027-016-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoma T., Kahesa C., Mwaiselage J. 2016. Cost-effectiveness of Screening and Treatment for Cervical Cancer in Tanzania: Implications for Other sub-Saharan African Countries; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberger K., Jumbam D.T., Dahm J. The situation of safe surgery and anaesthesia in Tanzania: a systematic review. World J. Surg. 2019;43(1):24–35. doi: 10.1007/s00268-018-4767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penoyar T., Cohen H., Kibatala P. Emergency and surgery services of primary hospitals in the United Republic of Tanzania. BMJ Open. 2012;2(1) doi: 10.1136/bmjopen-2011-000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng P., Perng W., Ngoma T. Promoters of and barriers to cervical cancer screening in a rural setting in Tanzania. Int. J. Gynecol. Obstet. 2013;123(3):221–225. doi: 10.1016/j.ijgo.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin M., Besana G.V., Yuma S. Integrating HIV testing into cervical cancer screening in Tanzania: an analysis of routine service delivery statistics. BMC Womens Health. 2014;14(1):120. doi: 10.1186/1472-6874-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick T.J., Deming C.M., Helland J.R., Hartwig K.A. Cancer training for frontline healthcare providers in Tanzania. J. Cancer Educ. 2017:1–5. doi: 10.1007/s13187-017-1274-8. [DOI] [PubMed] [Google Scholar]
- Stanifer J.W., Patel U.D., Karia F. The determinants of traditional medicine use in northern Tanzania: a mixed-methods study. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0122638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Foundation for Cancer Care in Tanzania Meeting the challenge of cancer care in northern Tanzania. Tanzania Cancer Care.Org. 2015:1–64. https://www.iccp-portal.org/sites/default/files/resources/FCCT-White-Paper.pdf [Google Scholar]
- The Tanzanian Ministry of Health . Standard Treatment Guidelines and National Essential Medicines List: Tanzania Mainland. 2017. Development, gender, elderly, and children. [Google Scholar]
- Tsu V.D., Njama-Meya D., Lim J., Murray M., de Sanjose S. Opportunities and challenges for introducing HPV testing for cervical cancer screening in sub-Saharan Africa. Prev. Med. (Baltim) 2018;114(March):205–208. doi: 10.1016/j.ypmed.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J.M.M., Jacobs M.V., Manos M.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999 doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Watson-Jones D., Baisley K., Brown J. High prevalence and incidence of human papillomavirus in a cohort of healthy young African female subjects. Sex. Transm. Infect. 2013;89(5):358–365. doi: 10.1136/sextrans-2012-050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson-Jones D., Lees S., Mwanga J. Feasibility and acceptability of delivering adolescent health interventions alongside HPV vaccination in Tanzania. Health Policy Plan. 2016;31(6):691–699. doi: 10.1093/heapol/czv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Atlas . 2018. Ethnic Groups of Tanzania. [Google Scholar]
- World Bank Group . 2015. Tanzania Mainland Poverty Assessment. [Google Scholar]
- Yeates K.E., Sleeth J., Hopman W. Evaluation of a smartphone-based training strategy among health care workers screening for cervical Cancer in northern Tanzania: the Kilimanjaro method. J. Glob. Oncol. 2016;2(6):356–364. doi: 10.1200/JGO.2015.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]