Abstract
Background
Ginseng (Panax ginseng Meyer) is an invaluable medicinal plant containing various bioactive metabolites (e.g., ginsenosides). Owing to its long cultivation period, ginseng is vulnerable to various biotic constraints. Biological control using endophytes is an important alternative to chemical control.
Methods
In this study, endophytic Trichoderma citrinoviride PG87, isolated from mountain-cultivated ginseng, was evaluated for biocontrol activity against six major ginseng pathogens. T. citrinoviride exhibited antagonistic activity with mycoparasitism against all ginseng pathogens, with high endo-1,4-β-D-glucanase activity.
Results
T. citrinoviride inoculation significantly reduced the disease symptoms caused by Botrytis cinerea and Cylindrocarpon destructans and induced ginsenoside biosynthesis in ginseng plants. T. citrinoviride was formulated as dustable powder and granules. The formulated agents also exhibited significant biocontrol activity and induced ginsenosides production in the controlled environment and mountain area.
Conclusion
Our results revealed that T. citrinoviride has great potential as a biological control agent and elicitor of ginsenoside production.
Keywords: Biocontrol agent, Formulation, Fungal endophyte, Ginsenoside, Mountain-cultivated ginseng
1. Introduction
Ginseng (Panax ginseng) is a highly valuable plant and has been used for medicinal purposes for thousands of years [1]. Ginseng produces bioactive metabolites such as ginsenosides, oleanic acids, and phenolic compounds, which have pharmacological properties, including anticancer, antiplatelet, and antimicrobial activities [2]. Mountain-cultivated ginseng (MCG) is propagated under natural conditions in the forest and requires a long period of cultivation [3]. Phytopathogens such as Botrytis cinerea, Cylindrocarpon destructans, Pythium spp., and Rhizoctonia solani cause serious problems in MCG cultivation [4], [5]. Therefore, suitable biocontrol agents (BCAs) are urgently required to control ginseng pathogens.
Fungal endophytes dwell within plants without causing apparent disease symptoms [6]. They propagate in the intercellular or intracellular spaces without harming the plants. Previous studies have found that some fungal endophytes improve the resistance of plants to insects and pathogens [7] and tolerance to abiotic stresses such as drought, extreme temperature, and salinity [8]. Endophytes can also produce various metabolites that have a range of biological activities, such as anticancer, antimicrobial, and antiviral activities [9]. Therefore, fungal endophytes might have potential functions as BCAs against microbial pathogens.
Trichoderma spp. are the most prevalent soil fungi [10] and are opportunistic avirulent plant symbionts because they have beneficial effects on plants [10], [11]. Trichoderma spp. are also used as BCAs against plant pathogenic fungi such as B. cinerea, Fusarium spp., Pythium spp., and Rhizoctonia spp. [12]. Trichoderma spp. use different modes of action as BCAs, including antibiosis, mycoparasitism, and induced resistance [11], [13]. Furthermore, Trichoderma-based BCAs can promote plant growth and have soil remediation activity; therefore, they have greater ability than viruses, bacteria, and protozoa [14]. Among the different species, T. citrinoviride is one of the most widespread soil fungi of the longibrachiatum clade and is a well-known producer of strong cellulases [15]. Therefore, T. citrinoviride is a good BCA against phytopathogens.
In this study, we examined the antagonism of T. citrinoviride, which was isolated from MCG, as a BCA against major ginseng pathogens. We also confirmed the biocontrol activity of T. citrinoviride formulations. Furthermore, we determined the ability of T. citrinoviride and its formulations to induce ginsenoside synthesis and ginseng defense responses.
2. Materials and methods
2.1. Fungal endophyte, ginseng pathogens, and ginseng plants
T. citrinoviride PG87 was isolated from the roots of MCG plants, which were collected from 24 sites in Korea [16]. Ginseng pathogens were procured from the Rural Development Administration Genebank Information Center (Suwon, Korea): Rhizoctonia solani KACC40123 (Korea Agricultural Culture Collection), Botrytis cinerea KACC43521, Alternaria panax KACC42461, Cylindrocarpon destructans KACC44656, Phytophthora cactorum KACC40166, and Pythium spp. KACC40581. B. cinerea and C. destructans were used as above- (stem and leaf) and below-ground (root) pathogens, respectively.
Two-yr-old ginseng roots were purchased from Geumsan Insamjohap (Geumsan, Korea), planted into sterile artificial ginseng soil mix (Shinsung Mineral Co., Ltd., Seongnam, Korea), and grown in a growth room (23°C, 150 ± 10 μmol/m2 sec, 16-h light) for 1–4 wk.
2.2. Mycoparasitism assay
T. citrinoviride was screened for mycoparasitic ability against ginseng pathogens using a microscope slide and dual-culture assays [5]. For the glass microscope slide assay, slides were covered with 50% potato dextrose agar (PDA) media and inoculated with PDA plugs (5-mm diameter) of T. citrinoviride and each of the six ginseng pathogens (3-cm apart). The pathogens alone were inoculated using PDA plugs as controls. Three replicate slides were used for each ginseng pathogen. After 3–7 d of incubation at 25°C, the contact area was observed under an Olympus BX51 TRF equipped with an Olympus digital Infinity1C camera at 1000 × magnification (Tokyo, Japan).
PDA plugs of both T. citrinoviride and each of the six ginseng pathogens were cocultured on a PDA plate at opposite edges of the petri dishes (6-cm apart) and incubated at 25°C. Antagonistic behavior was observed after 2–6 d of incubation. The pathogens alone were inoculated as controls. Mycelial growth inhibition (%) was calculated and compared with the control plates using the following equation: Mycelial growth inhibition (%) = [(growth of control – growth of treatment)/growth of control] × 100. Experiments were conducted with three sets of replication plates.
2.3. Dual culture of T. citrinoviride and B. cinerea
A B. cinerea PDA plug was inoculated on the edge of the PDA plate and incubated at 25°C for 4 d. Thereafter, a T. citrinoviride PDA plug was placed on the opposite edge of a PDA plate preinoculated with B. cinerea (6-cm apart) and incubated at 25°C for a further 3 d. T. citrinoviride and B. cinerea mycelia were harvested around the contact area using a sterile spatula. Fungal RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. cDNA was synthesized using 1 μg of total RNA and the GoScript Reverse Transcription System (Promega, Madison, WI, USA). The patterns of T. citrinoviride and B. cinerea gene expression were analyzed (primers sequences are shown in Table S1) as described previously [17]. The experiment was conducted twice with three biological replicates.
2.4. Activity assay of cell wall–degrading enzymes
T. citrinoviride was grown on PDA media at 25°C for 5 d. Then, five 5-mm plugs were inoculated into 50-mL basal media supplemented with one of the following carbon sources (1 g/L): cellulose microcrystalline, carboxymethy1 cellulose, B. cinerea cell wall, or C. destructans cell wall. Cell wall preparations of B. cinerea and C. destructans were prepared as previously described [18]. Each flask was incubated at 25°C with shaking at 120 rpm for 7 d to induce endo-1,4-β-D-glucanase, exo-1,4-β-D-glucanase, β-1,3-glucanase, and β-glucosidase activity. The supernatants were collected after filtration through Whatman no. 1 filter paper (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 7,000 g at 4°C for 20 min. Protein concentrations were measured using the Bradford assay (Sigma-Aldrich) [19]. Endo-1,4-β-D-glucanase, exo-1,4-β-D-glucanase, β-1,3-glucanase, and β-glucosidase activity was measured using 1% Avicel, carboxymethy1 cellulose, laminarin, and salicin as a substrate, respectively, and the reducing sugar was determined with dinitrosalicylic acid reagent (Sigma-Aldrich) [20]. One unit (U) of extracellular enzyme activity was defined as the amount of enzyme that produced 1 μmol reducing sugar/h under the above conditions. The experiment was conducted twice with three biological replicates.
2.5. Inoculation of ginseng plants with T. citrinoviride
To determine whether T. citrinoviride has any harmful effects on ginseng plants, we inoculated ginseng roots with T. citrinoviride potato dextrose broth (PDB) media and with the PDB media alone as a control. Briefly, 2-yr-old ginseng roots were surface-sterilized using 2% NaOCl for 10 min and rinsed three times with sterile distilled water. After sterilization, roots were inoculated with 1 mL 1 × 106 spores/mL of T. citrinoviride in PDB media by dipping for 3 h. After air-drying for 1 h on a clean bench, roots were planted in pots (5 × 5 × 11 cm) containing sterile artificial ginseng soil. Pots were placed in a growth room. After 3 wk of growth, ginseng roots were examined visually, and T. citrinoviride was reisolated. The ginseng plants (root, stem, and leaf) were surface-sterilized, cut into 1-cm pieces, and placed on a PDA plate. Plates were incubated for 10 d at 25°C, and fungal endophytes were identified using internal transcribed spacer sequences. The colonization frequency (CF%) was calculated as follows: CF (%) = (total number of T. citrinoviride-colonized segments/total number of segments) × 100.
After 1, 2, 3, and 4 wk of inoculation, the below- (root) and above-ground (stem and leaf) parts of ginseng plants were collected, and DNA, RNA, hormones, and ginsenosides were extracted. The experiment was conducted twice with three biological replicates.
2.6. Quantitative real-time PCR (QPCR)
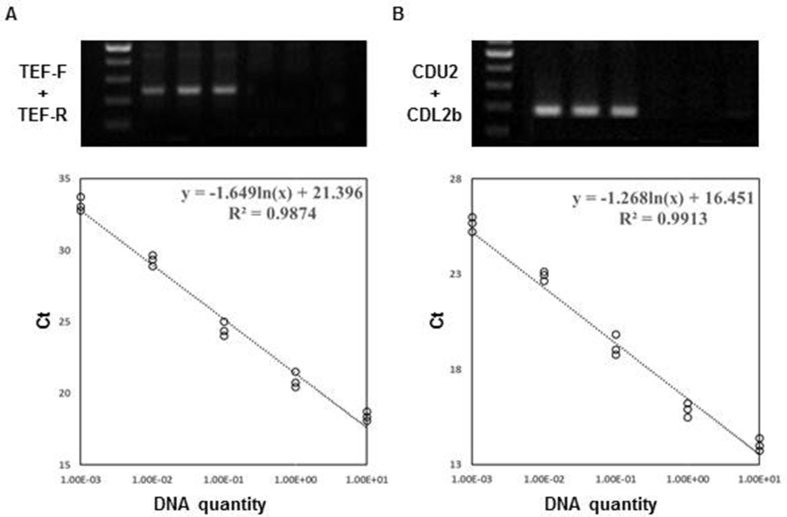
Quantitative real-time polymerase chain reaction (QPCR) was used to quantify the expression of target transcripts and DNA. Translation elongation factor alpha 1 (TEF1, KJ665454) was used to quantify T. citrinoviride using QPCR [21]. Primer 3 software from Biology Workbench (http://workbench.sdsc.edu/) was used to design primers (Table S1). Fungal genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), and 100 ng of template DNA was used to amplify the TEF1 fragment (approximately 250 bp) [22]. The amplified products were loaded onto 1.2% agarose gel and stained with ethidium bromide. The PCR products were purified from the excised gel using Wizard SV gel and a PCR Clean-up System (Promega) and processed for sequencing at Cosmo Genetech (Seoul, Korea). Generated sequences were analyzed using Clustal W (Biology Workbench). Specific primers (CDU1 and CDL1b) for C. destructans were designed from intergenic spacers as described previously [23]. The respective 152-bp product was sequenced and confirmed with the reported C. destructans sequence. Specificity of the primer sets for T. citrinoviride and C. destructans was confirmed by QPCR using a DNA template from ginseng root inoculated with the respective fungi. Ginseng root inoculated with PDB was used as a control.
Genomic DNA was extracted from inoculated ginseng roots using the DNeasy Plant Mini Kit (Qiagen). QPCR was then performed as described previously except for C. destructans, for which the following parameters were used: an initial denaturing period of 5 min at 94°C, followed by 30 cycles of 95°C for 30 s, 60°C for 40 s, and 72°C for 1 min, followed by 10 min at 72°C. Genomic DNA from T. citrinoviride or C. destructans (10, 1, 0.1, 0.01, and 0.001 ng) was also included to generate calibration curves. Dilutions with five different concentrations, each with three replicates, were used, and QPCR was performed twice. Threshold cycle (Ct) values were obtained from reactions containing fungal DNA and were correlated with the amount of DNA.
2.7. Hormone analysis
Ginseng hormones, zeatin (ZA), abscisic acid (ABA), and jasmonic acid (JA), were analyzed as previously described [24], [25]. Approximately 50 mg of frozen samples was extracted with 980 μL methanol:acetic acid (99:1, v/v) and 20 μL internal standard solution. The samples were centrifuged at 13,000 g for 5 min at 4°C. Supernatant (1 mL) was collected and filtered using a 0.45-μm nylon syringe filter (Sigma-Aldrich), and 10 μL of each sample was injected. The experiment was carried out using liquid chromatography–electrospray ionization–tandem mass spectrometry. The gradient profile was as follows: 0.01–2 min, 0–40%; 2–5 min, 40–60%; 5–13 min, 60–100%; 13–15 min, 100–20% eluent B. The flow rate was 0.5 mL/min, and the column was maintained at 40°C.
2.8. Ginsenoside analysis
Ginsenosides were analyzed as previously reported [26]. Methanol-dissolved samples were diluted 100 times to quantify major ginsenosides. Ginsenosides (Ro, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, F1, and F2; Ambo Institute, Seoul, Korea) were used as standards. High-performance liquid chromatography–grade acetonitrile, methanol, and water were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Formic acid, for ionization, was obtained from Duksan Pure Chemicals Co., Ltd. (Ansan, Korea). All ginsenoside standards were dissolved in methanol to obtain 50 μg/mL stock solutions and stored at −20°C until use. To achieve the coefficient of determination, calibration curves of each standard were prepared with 11 different concentrations (25–20 mg/mL). liquid chromatography–electrospray ionization–tandem mass spectrometry analysis was performed using an LCMS-8040 tandem quadruple mass spectrometer (Shimadzu, Kyoto, Japan) equipped with a degasser, binary pump, auto sampler, column oven, and UV detector. All ginsenosides were confirmed in negative electrospray mode. MS was operated in multiple reaction–monitoring mode using argon gas as the collision energy. Operating parameters were as follows: nebulizer gas flow 3 L/min, drying gas flow 15 L/min, desolvation line temperature 250°C, and heat block temperature 400°C. Data were analyzed by Labsolutions program (Shimadzu, Kyoto, Japan). The mobile phase consisted of formic acid in water (1:103, v/v; eluent A) and 100% acetonitrile (eluent B). Chromatographic separation was achieved on an ACE® UltraCore™ 2.5 Super C18 column (150 × 4.6 mm, 2.5 μm; Advanced Chromatography Technologies, Aberdeen, UK), and the gradient profile was as follows: 0.01–2 min, 25–35%; 2–6 min, 35%; 6–15 min, 35–90% B. The column was washed with 100% B for 5 min, followed by 25% B for 5 min after each injection. The flow rate and temperature were 0.5 L/min and 40°C, respectively, in all experiments, and 10 μL of each sample was injected. The ginsenosides contents are expressed in ng/g dry weight.
2.9. Biocontrol assay of B. cinerea and C. destructans in ginseng plants using T. citrinoviride
To study the induction of B. cinerea resistance, ginseng roots were inoculated with T. citrinoviride by root dipping as described and grown in a growth room for 2 wk. Then, a B. cinerea spore suspension (1 mL of 1 × 106 spores/mL) was sprayed on whole leaves of each plant. Ginseng plants were grown for 1 wk in a sealed plastic box (40 × 50 × 35 cm) with 100% humidity to aid disease progression. The relative infected area (RIA %) was measured, and leaves from ginseng plants inoculated with T. citrinoviride and control leaves (no T. citrinoviride root inoculation) were harvested, and the expression of ginseng and B. cinerea genes was examined.
To mimic field conditions, we inoculated ginseng shoots by spraying with T. citrinoviride spore suspension. After 12 h of spraying, plants were treated with B. cinerea spore suspension. For the control, PDB without T. citrinoviride was sprayed. After 1-week growth, the RIA (%) was measured, and leaf samples were harvested for subsequent expression analysis.
To investigate C. destructans suppression, ginseng roots were inoculated with T. citrinoviride spore suspension by pipetting into the soil within the pot. PDB was used as a control. After 24 h of inoculation, ginseng roots were pulled from the soil and wounded (1-mm depth, 0.25-mm diameter, 20 wounds) using sterilized needles [27]. A C. destructans spore suspension was poured into ginseng soil after wounding. After 3 wk of infection, ginseng roots were collected for fungal quantification and gene expression analysis.
2.10. Formulation of T. citrinoviride
T. citrinoviride was formulated in concurrence with Enbio (Gunpo, Korea). Two different formulations were selected for further tests: dustable powder (DP) and granule (GR) (Table S2). DP was formulated by mixing with white carbon, calcite, and pulverized T. citrinoviride. GR was formulated by mixing with white carbon, polyvinyl alcohol, corn starch, bentonite, calcite, and pulverized T. citrinoviride. The products were assembled through an extruder and dried with a floating dryer.
2.11. Application of DP and GR for the biocontrol of B. cinerea and C. destructans in ginseng
Biocontrol activity of the formulated DP and GR agents was tested against B. cinerea and C. destructans, respectively. After the growth of ginseng plants in soil for 2 wk, 0.5 g of DP was sprayed onto whole leaves in one set, and 0.5 g of GR was applied to the soil in another. After 1 wk, leaves from the set sprayed with DP were inoculated with B. cinerea spore suspension through foliar spraying. The second set of plants treated with GR was inoculated with C. destructans spore suspension by pipetting into the soil as described (20 wounds). Ginseng plants inoculated with B. cinerea and C. destructans were allowed to grow for a further 1 and 3 wk, respectively, after which tissue samples (above- and below-ground parts, respectively) were harvested to analyze RIA (%), quantification, and gene expression.
2.12. Application of DP and GR in a ginseng field
A field test was carried out at the MCG cultivation site (Jecheon, Korea) with the formulated T. citrinoviride agents. Three-yr-old MCG plants were grown under the same environmental conditions. During the growth period (May–June, 2016), DP and GR agents were applied to foliage five times weekly at 10 g/m2. Morbidity for each plant was recorded until harvest (August, 2016). The harvested MCG plants were prepared for experimentation as described. These experiments were conducted with six biological replicates.
2.13. Statistical analyses
Mean values were obtained from three to six replicates, and the standard errors are indicated by error bars. Means and significant differences were calculated using analysis of variance followed by Duncan's multiple range test at p = 0.05 with SAS v9.4 (Cary, NC, USA). Graphs were prepared using GraphPad Prism 6 project (San Diego, CA, USA).
3. Results
3.1. Mycoparasitism
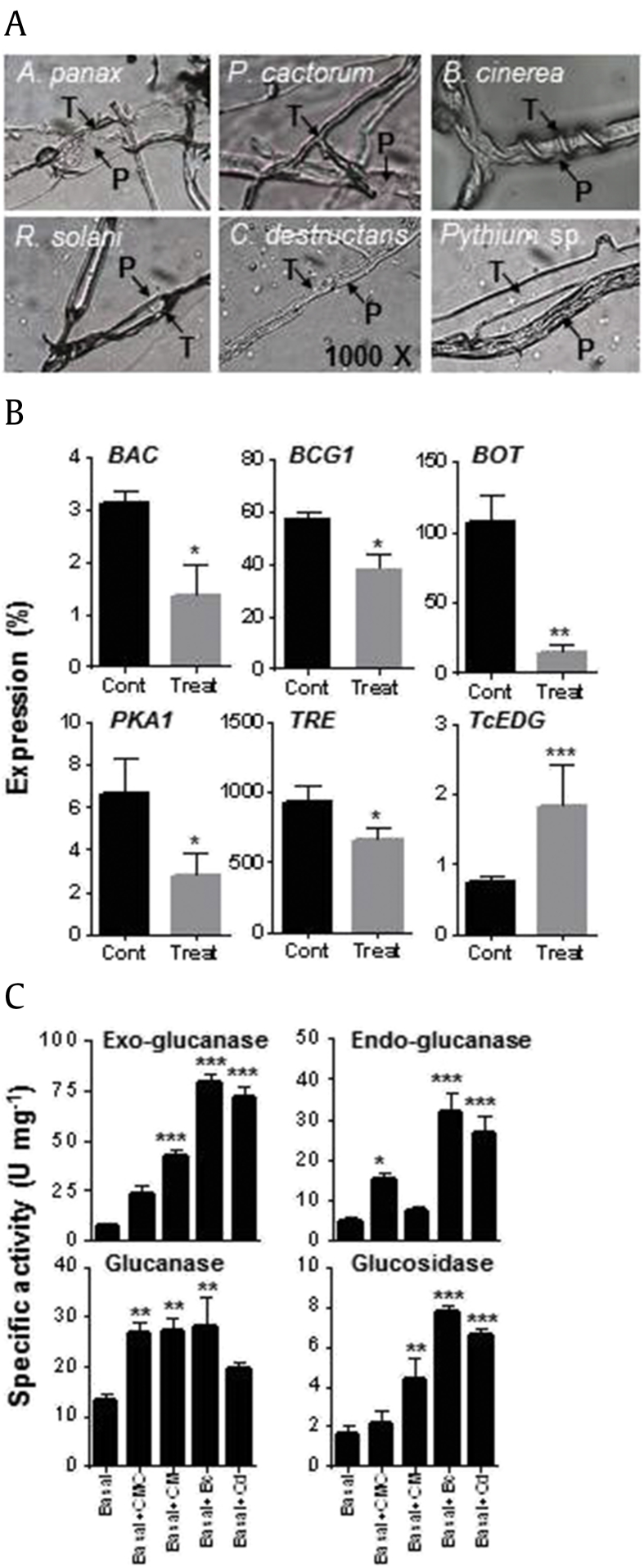
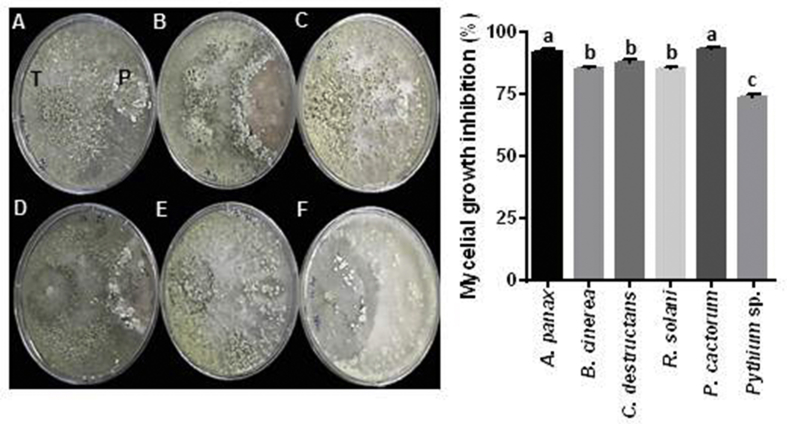
T. citrinoviride showed the highest inhibitory effect on the mycelial growth of six ginseng pathogens with rapid colonization and profuse sporulation (Fig. S1). Thus, the mycoparasitic ability of T. citrinoviride was evaluated against ginseng pathogens. The results revealed the unrestricted growth of T. citrinoviride along the hyphae of the pathogens in the contact area in agar slides (Fig. 1A). T. citrinoviride hyphae directly penetrated A. panax and P. cactorum hyphae without coiling. However, T. citrinoviride coiled around and did not penetrate B. cinerea and R. solani hyphae. A parallel association with appressorium formation was observed without coiling or penetration in C. destructans and Pythium spp.
Fig. 1.
Biocontrol activity of Trichoderma citrinoviride PG87 against ginseng pathogens. (A)T. citrinoviride was screened for mycoparasitic ability against ginseng pathogens on potato dextrose agar (PDA) slides. Glass slides covered with 50% PDA were inoculated with PDA plugs of T. citrinoviride and each of the six ginseng pathogens (3-cm apart). The contact area was observed under the microscope (1000 × magnification). T, T. citrinoviride; P, pathogen. (B) Quantitative real-time PCR (QPCR) analysis of Botrytis cinerea (BAC, BCG1, BOT, PKA1, and TRE) and T. citrinoviride (TcEDG) genes in dual culture. A B. cinerea PDA plug was inoculated on the edge of the PDA plate and incubated at 25°C for 4 d. Thereafter, a T. citrinoviride PDA plug was placed on the opposite edge of a PDA plate preinoculated with B. cinerea (6-cm apart) and incubated at 25°C for a further 3 d. T. citrinoviride and B. cinerea mycelia were harvested around the contact area, and fungal RNA was extracted for QPCR analysis. B. cinerea and T. citrinoviride ACTINs were used to normalize the relative expression levels. The experiment was conducted twice with three biological replicates. Cont, Control; Treat, Treatment. (C) Activity assay of cell wall–degrading enzymes (CWDEs) for T. citrinoviride. T. citrinoviride was grown for 7 d in basal media plus one of the following carbon sources: carboxymethy1 cellulose (CMC), cellulose microcrystalline (CM), B. cinerea cell wall (Bc), or C. destructans cell wall (Cd). The culture filtrates were assayed for endo-1,4-β-D-glucanase, exo-1,4-β-D-glucanase, β-1,3-glucanases, and β-glucosidases. The experiment was conducted twice with three biological replicates. Vertical bars indicate the means with standard error from six biological replications. *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
PCR, polymerase chain reaction.
3.2. Molecular interaction between T. citrinoviride and B. cinerea in dual culture
B. cinerea genes related to growth and virulence were analyzed in PDA dual culture with T. citrinoviride by QPCR (Fig. 1B). Five B. cinerea genes were analyzed using RNA extracted from the mycelia around the contact area. All five B. cinerea genes were significantly downregulated in the presence of T. citrinoviride. In contrast, TcEDG was strongly induced by B. cinerea.
3.3. Production of cell wall–degrading enzymes involved in mycoparasitism
The activities of cell wall–degrading enzymes (CWDEs) were determined in the PDB liquid culture filtrates of T. citrinoviride (Fig. 1C). Enzymatic activity was measured by the amount of reducing sugars released from different substrates. Enzymatic activity was substrate dependent, which could induce the activity of multiple enzymes. The highest activity was observed for endo-1,4-β-D-glucanase. Fungal cell wall from B. cinerea was the best substrate to induce enzymatic activity: 79, 32, 28, and 8 U/mL for exo-1,4-β-D-glucanase, endo-1,4-β-D-glucanase, β-1,3-glucanase, and β-glucosidases, respectively. As a substrate, C. destructans cell wall also induced high enzymatic activity, similar to B. cinerea cell wall, with the exception of β-1,3-glucanase.
3.4. Endophytism of T. citrinoviride in ginseng plants
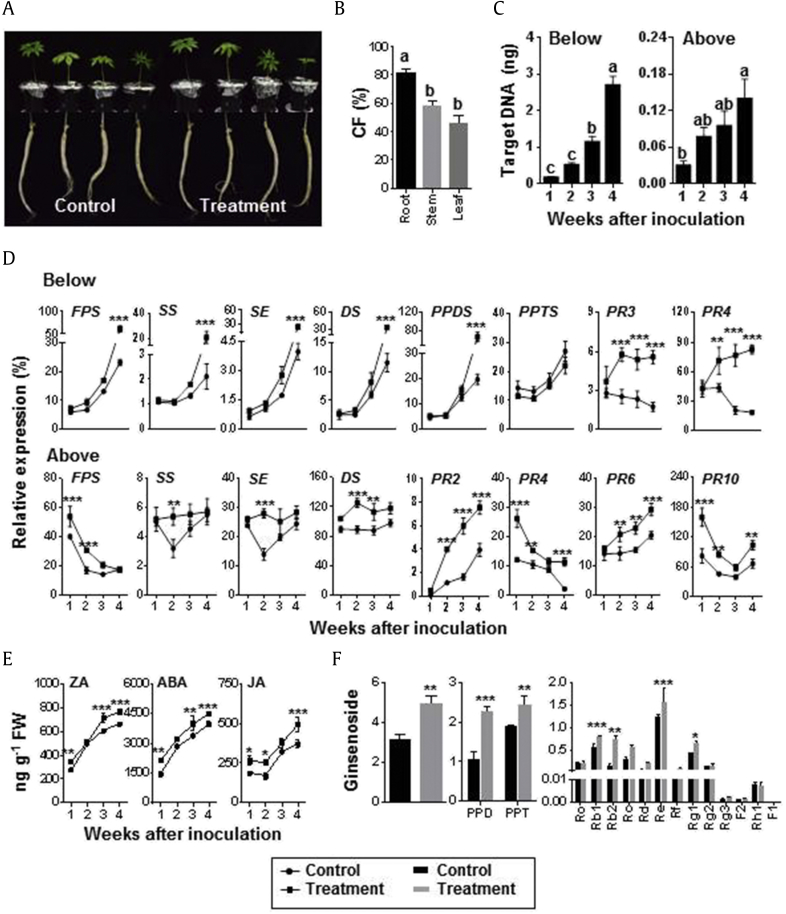
No disease symptoms or growth inhibition were observed in ginseng roots after 3-wk preinoculation with T. citrinoviride, as noted for both below- and above-ground parts (Fig. 2A). CF% varied significantly among different tissues. CF was higher in the roots than in the stem and leaf tissues (81.6 vs. 58 and 46.0%, respectively) (Fig. 2B). No negative effect was imparted by T. citrinoviride in ginseng plants, with the best colonization observed in root tissue. After the inoculation of ginseng plants with T. citrinoviride for 1, 2, 3, and 4 weeks, T. citrinoviride was quantified using QPCR using TEF1 primers. The quantities of standard DNA versus their respective Ct for T. citrinoviride were obtained, and calibration curves were constructed (Fig. S2). The amount of T. citrinoviride target DNA was presented as nanograms target DNA in 50 ng of total genomic DNA. Target DNA increased significantly with inoculation period (ranging from 0.17 ng to 2.69 ng) in the below-ground part (Fig. 2C). However, the increment in target DNA in the above-ground part was relatively lower (ranging from 0.03 ng to 0.14 ng) than in the root. Thus, T. citrinoviride colonized the below-ground part more than the above-ground part (5.30 and 0.28% per total DNA in 4 wk, respectively).
Fig. 2.
Endophytism of Trichoderma citrinoviride PG87 in ginseng plant. Ginseng roots were inoculated with T. citrinoviride spore suspension by dipping. Inoculated roots were planted into sterile artificial ginseng soil and grown for 1–4 wk. After 1, 2, 3, and 4 wk of inoculation, below- (root) and above-ground (stem and leaf) parts of ginseng plants were collected to extract DNA, RNA, hormones, and ginsenosides. (A) Photographs showing the absence of disease symptom in ginseng plants inoculated with T. citrinoviride for 3 wk. (B) Colonization frequency (%) of T. citrinoviride in three different tissues. After 3 wk, ginseng plants (root, stem, and leaf) were surface-sterilized, cut into 1-cm pieces, and placed on PDA plates for 10-d growth. Fungal endophytes were identified using internal transcribed spacer (ITS) sequence. (C) Quantification of T. citrinoviride colonization in below- and above-ground parts of ginseng plants using QPCR. Target DNA = ng of target DNA in 50 ng of total genomic DNA. (D) Expression of genes involved in ginsenoside biosynthesis (FPS, SS, SE, DS, PPDS, and PPTS) and defense (PR2, PR3, PR4, PR6, PR10) in the below- and above-ground parts of ginseng plants. Ginseng ACTIN was used to normalize the relative expression levels. (E) Alterations in hormone levels in ginseng roots. (F) Ginsenoside contents of ginseng roots after 4 wk of T. citrinoviride inoculation. The total ginsenoside contents and ginsenoside contents of PPD (Rb1, Rb2, Rc, Rd, Rg3, and F2) and PPT type (Re, Rf, Rg1, Rg2, Rh1, and F1). The experiment was conducted twice with three biological replications. Vertical bars indicate the means with standard error from six biological replications. Significance of the relative values is indicated by different letters on the means as per Duncan's multiple range test (p < 0.05). *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
ABA, abscisic acid; FW, fresh weight; JA, jasmonic acid; PPD, Protopanaxadiol; PPT, protopanaxatriol; QPCR, quantitative real-time polymerase chain reaction; ZA, zeatin.
3.5. In planta study of ginseng-induced disease resistance, hormones, and ginsenosides
Preinoculation of ginseng roots with T. citrinoviride by root dipping upregulated genes involved in ginsenoside biosynthesis (FPS, SS, SE, DS, PPDS, and PPTS) and defense (PR2, PR3, PR4, PR6, and PR10). The expression of five ginsenoside biosynthetic genes (FPS, SS, SE, DS, and PPDS) significantly increased 4 wk after inoculation compared with the controls in the below-ground parts, except for PPTS (Fig. 2D). Defense-related genes (PR3 and PR4) were significantly upregulated 2 wk after inoculation; induction was maintained up to 4 wk in below-ground parts, and a slight reduction was detected in the control. In contrast, FPS, SS, SE, and DS were significantly upregulated in above-ground parts after 1 and/or 2 wk of inoculation, with no significant changes observed after 4 wk (Fig. 2D). PR2 and PR6 expression was gradually upregulated in T. citrinoviride-inoculated plants compared with that in the control, except at 1 wk. PR4 and PR10 were significantly upregulated 1, 2, and 4 wk after inoculation compared with the control.
Phytohormones (ZA, ABA, and JA) were significantly upregulated in T. citrinoviride-inoculated ginseng roots (Fig. 2E). Notably, phytohormones were significantly increased at each time point, except at 2 or 3 wk after inoculation. The highest induction of phytohormones was observed 4 wk after inoculation.
Ginsenoside contents were evaluated in the T. citrinoviride-inoculated ginseng roots 4 wk after inoculation. The total ginsenosides in treated roots were significantly increased (1.57-fold) compared with those in the control (Fig. 2F). Protopanaxadiol (PPD)-type ginsenosides were induced more than protopanaxatriol (PPT)-type ginsenosides, which was mainly due to Rb2 induction. The highest induction was detected in ginsenoside Rb2. Ginsenosides Rc, Re, and Rg1 were also significantly induced.
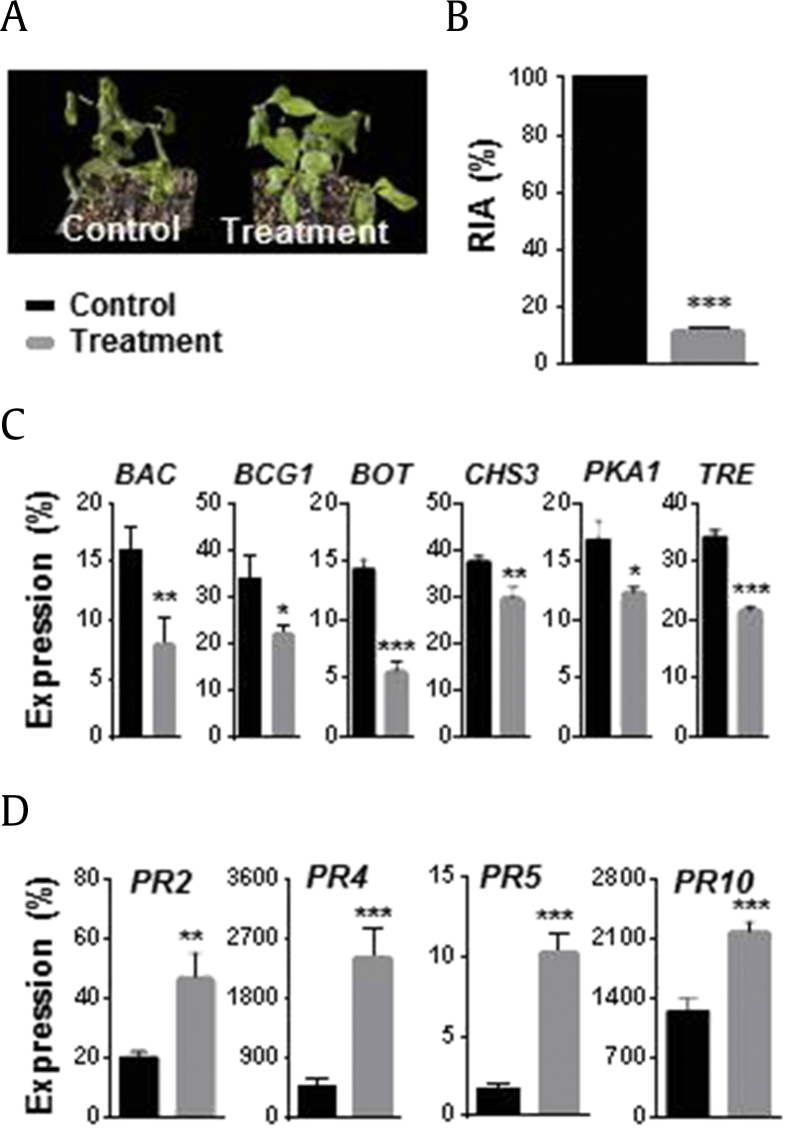
3.6. Induced disease resistance against B. cinerea by T. citrinoviride inoculation
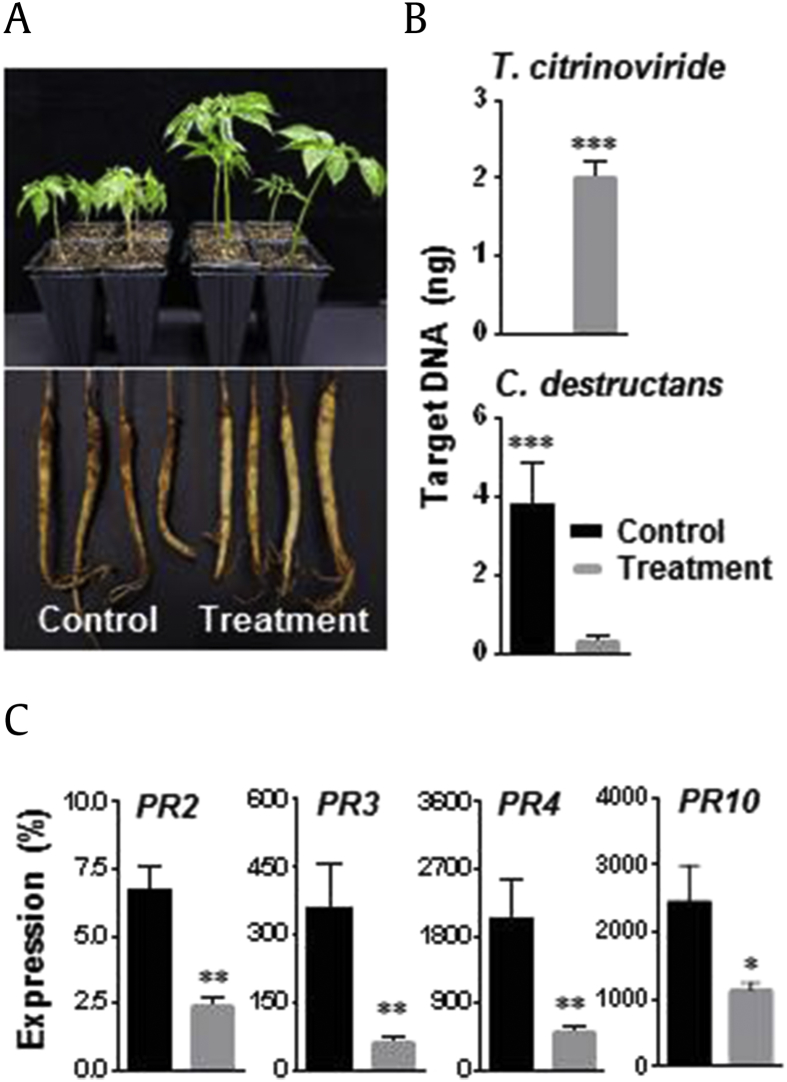
Ginseng roots were inoculated with T. citrinoviride by root dipping and grown for 2 wk before B. cinerea infection. T. citrinoviride-inoculated roots showed significantly reduced RIA 1 wk after B. cinerea foliar infection, with 100% and 11.5% RIA in the control and treatment groups, respectively (Fig. 3A and B). The expression of six genes involved in the growth and virulence of B. cinerea (BAC, BCG1, BOT, CHS3, PKA1, and TRE1) was significantly downregulated in the above-ground parts compared with that in the control (Fig. 3C). Conversely, the expression of ginseng defense-related genes (PR2, PR4, PR5, and PR10) was significantly upregulated in leaf samples from preinoculated plants (Fig. 3D).
Fig. 3.
Induced disease resistance in ginseng plants. Ginseng roots were inoculated with Trichoderma citrinoviride PG87 by root dipping and grown for 2 wk. Thereafter, Botrytis cinerea spore suspension was sprayed onto whole leaves. Ginseng plants were grown for 1 wk. Relative infected area (RIA %) was measured, and leaves were harvested to analyze the expression patterns of ginseng and B. cinerea genes. (A) Photographs showing the effect of induced disease resistance in ginseng leaves. (B) Relative infected area (RIA %). (C) Expression analysis of B. cinerea genes involved in growth and pathogenicity. (D) Expression analysis of ginseng pathogenesis-related (PR) genes. B. cinerea and ginseng ACTINs were used to normalize the relative expression levels. Vertical bars indicate the means with standard error from three biological replications. *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
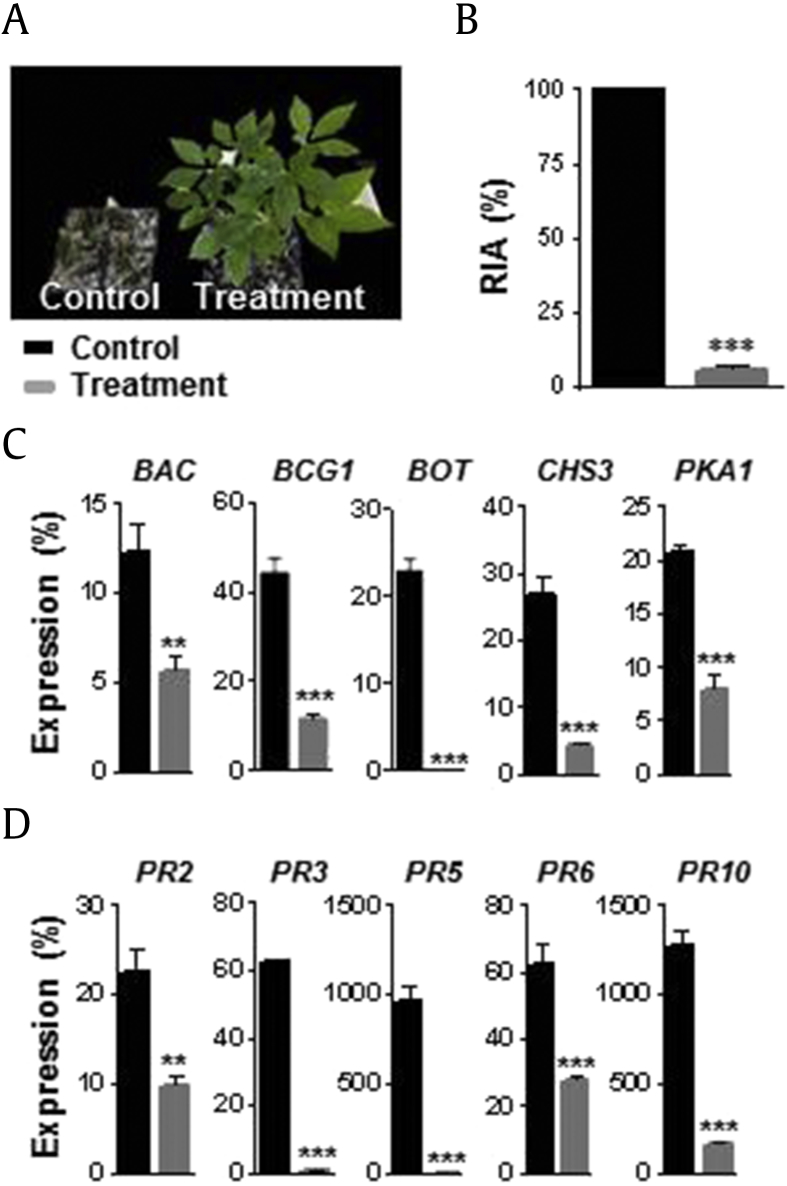
3.7. Suppression of B. cinerea by T. citrinoviride preinoculation
Ginseng leaves were inoculated with T. citrinoviride by leaf spraying and dried for 12 h before B. cinerea infection. T. citrinoviride-inoculated leaves showed significant resistance to B. cinerea (Fig. 4A). Minor infection was detected in the preinoculated leaf samples (5.7% RIA), whereas severe infection was observed in the control (Fig. 4B). Both B. cinerea (BAC, BCG1, BOT, CHS3, and PKA1) and ginseng (PR2, PR3, PR4, PR6, and PR10) genes were expressed at significantly lower levels in T. citrinoviride preinoculated ginseng plants than in the control (Fig. 4C and D).
Fig. 4.
Biocontrol activity of Trichoderma citrinoviride PG87 on leaf spot disease caused by Botrytis cinerea.T. citrinoviride spore suspension was sprayed onto ginseng leaves. After 12 h of spraying, B. cinerea spore suspension was sprayed onto ginseng leaves. After 1 wk of growth, relative infected area (RIA %) was measured, and leaf samples were harvested for expression analysis. (A) Photographs showing the biocontrol activity of T. citrinoviride against B. cinerea. (B) Relative infected area (RIA %). (C) Expression analysis of B. cinerea genes. (D) Expression analysis of ginseng PR genes. B. cinerea and ginseng ACTINs were used to normalize the relative expression levels. Vertical bars indicate the means with standard error from three biological replications. *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
3.8. Suppression of C. destructans by T. citrinoviride preinoculation
Ginseng roots were inoculated with T. citrinoviride by pipetting in soil. After 24 h, roots were pulled, wounded, replanted in soil, and infected with C. destructans by pouring the culture into soil. T. citrinoviride-inoculated roots showed a slight yellowish color without any rot symptoms 3 weeks after C. destructans infection (Fig. 5A). The above-ground parts of the control plants wilted, and the below-ground parts exhibited severe rot. T. citrinoviride colonization was confirmed by QPCR in treated roots (2 ng of target DNA per 50 ng of total DNA), whereas T. citrinoviride was not detected in control roots (Fig. 5B). A high amount of C. destructans DNA (3.7 ng) was detected in control roots, whereas a small amount (0.29 ng) was detected in treated roots. Ginseng defense-related genes (PR2, PR3, PR4, and PR10) were expressed at significantly lower levels in the treatment group than in the control (Fig. 5C).
Fig. 5.
Biocontrol activity of Trichoderma citrinoviride PG87 on root rot disease caused by Cylindrocarpon destructans. Ginseng roots were inoculated with T. citrinoviride spore suspension through pipetting into the soil within the pot. After 24 h of inoculation, ginseng roots were pulled out from soil, wounded, and infected with C. destructans spore suspension. After 3 wk of infection, ginseng roots were collected for fungal quantification and gene expression analysis. (A) Photographs showing the biocontrol activity of T. citrinoviride against C. destructans. (B) Quantification of T. citrinoviride and C. destructans in ginseng roots using QPCR. Target DNA = ng of target DNA in 50 ng of total genomic DNA. (C) Expression analysis of ginseng PR genes. The ginseng ACTIN was used to normalize the relative expression levels. Vertical bars indicate the means with standard error from three biological replications. *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
QPCR, quantitative real-time polymerase chain reaction.
3.9. Formulation of T. citrinoviride
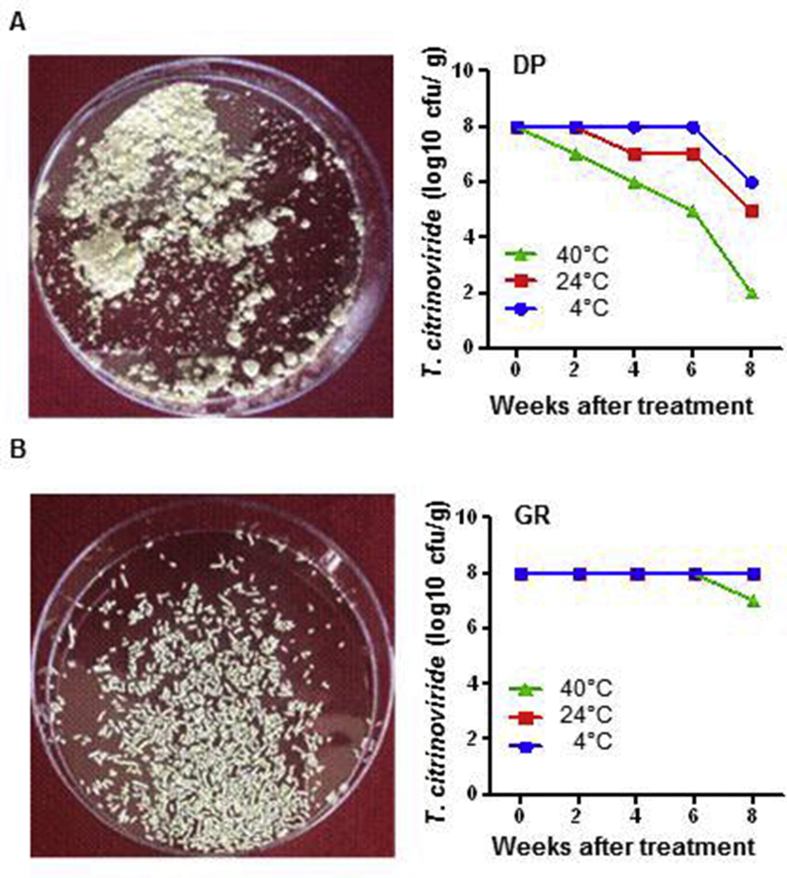
DP and GR agents were formulated and maintained at three different temperatures (4, 24, and 40°C) to confirm the survivability of T. citrinoviride (Fig. S3). After 8 wk at 40°C, the GR agent was highly preserved with a slight reduction in the T. citrinoviride concentration (108 to 107 cfu/g) compared with DP (108 to 102 cfu/g).
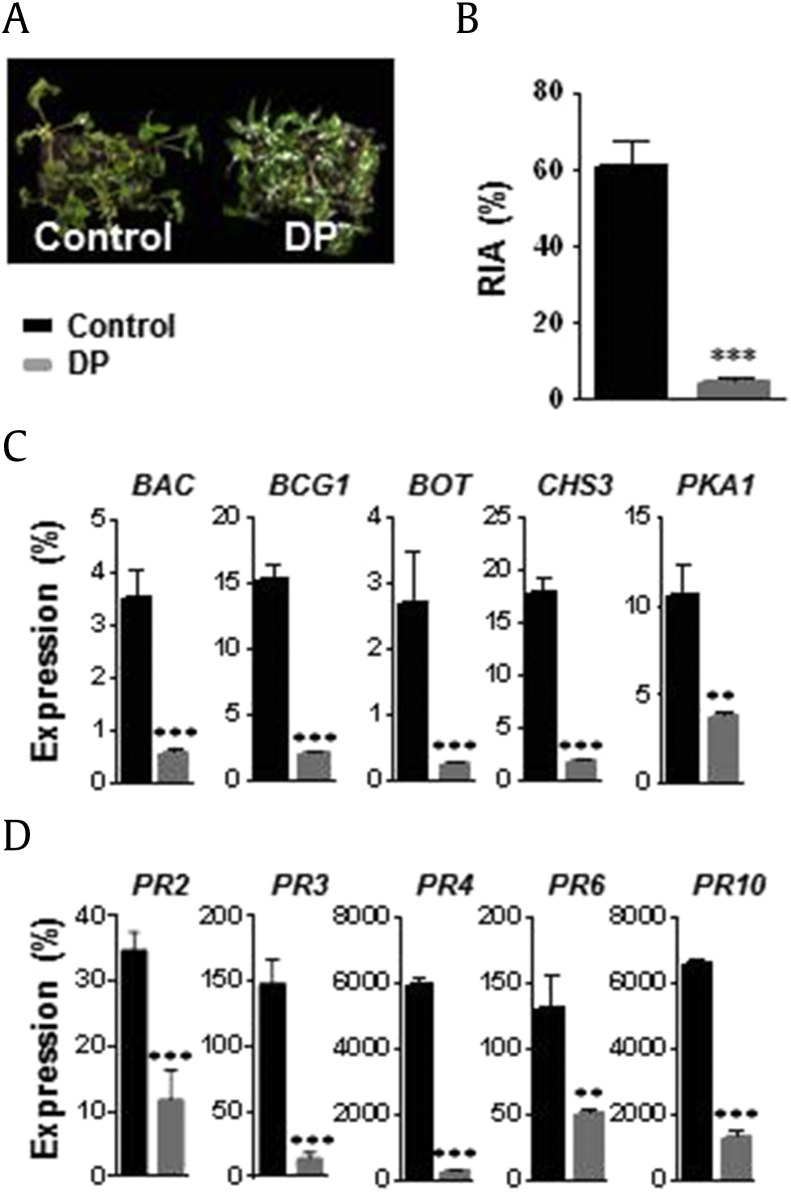
3.10. Suppression of B. cinerea by T. citrinoviride DP
Application of the DP agent significantly reduced disease symptoms (Fig. 6A). The RIA of control plants was 60.50%, compared with 4.15% in DP-treated ginseng leaves (Fig. 6B). The expression levels of B. cinerea (BAC, BCG1, BOT, CHS3, and PKA1) and ginseng (PR2, PR3, PR4, PR6, and PR10) genes were significantly reduced in DP-treated ginseng leaves (Fig. 6C and D).
Fig. 6.
Biocontrol activity of dustable powder (DP) agent of Trichoderma citrinoviride PG87 on leaf spot disease caused by Botrytis cinerea. After 1 wk of DP spraying, leaves were inoculated with B. cinerea spore suspension through foliar spraying. After 1 wk of inoculation, leaf samples were harvested for the analyses of relative infected area (RIA %), quantification, and gene expression. (A) Photographs showing the efficacy of DP agent in biocontrol of the leaf spot disease. (B) Relative infected area (RIA %). (C) Expression analysis of B. cinerea genes. (D) Expression analysis of ginseng PR genes. B. cinerea and ginseng ACTINs were used to normalize the relative expression levels. Vertical bars indicate the means with standard error from three biological replications. *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
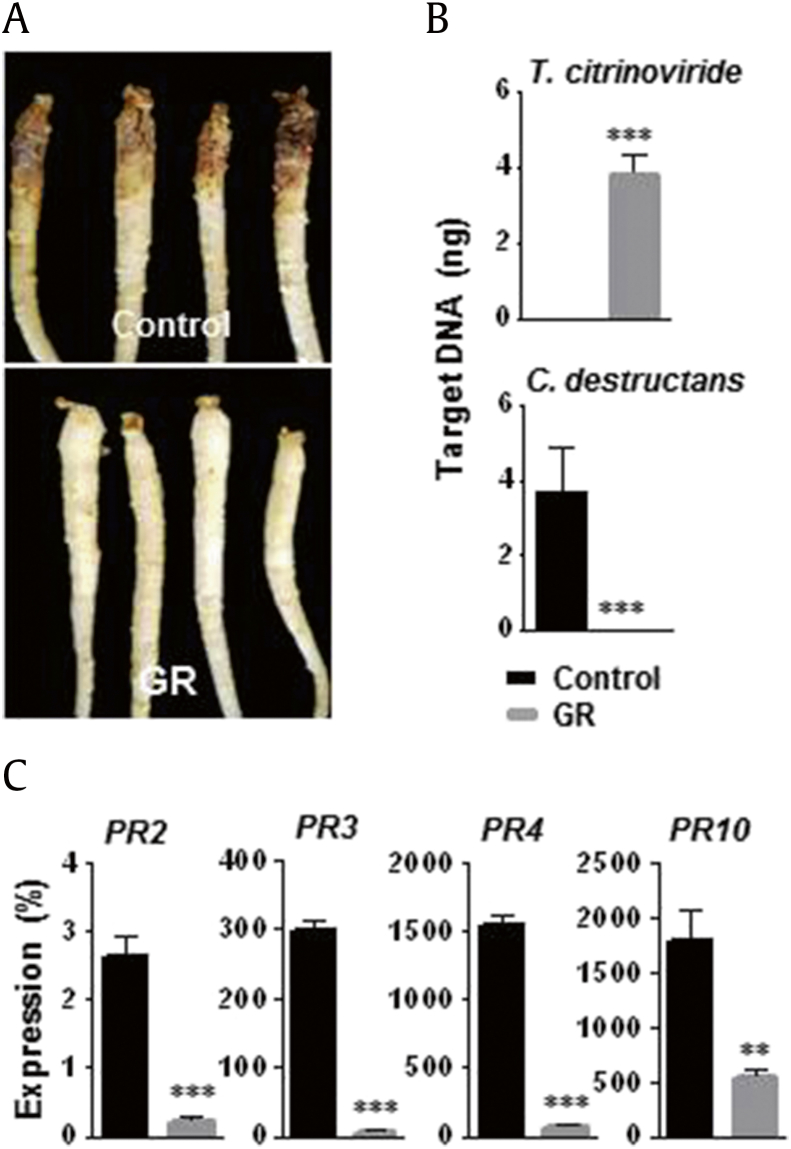
3.11. Suppression of C. destructans by T. citrinoviride GR
The GR agent showed greater antagonistic activity against C. destructans root rot disease than the control (Fig. 7A). No root rot symptoms were observed in roots treated with GR before C. destructans infection. T. citrinoviride target DNA was detected only in GR-treated ginseng roots (3.89 ng of target DNA per 50 ng of total DNA) (Fig. 7B). C. destructans colonization was confirmed in control roots (3.66 ng per 50 ng of total DNA) and was not detected in GR-treated ginseng roots. Ginseng genes involved in defense (PR2, PR3, PR4, and PR10) were expressed at significantly lower levels in GR-treated ginseng roots than in the control (Fig. 7C).
Fig. 7.
Biocontrol activity of granule (GR) agent of Trichoderma citrinoviride PG87 on root rot disease caused by Cylindrocarpon destructans. After 1 wk of GR application into soil, roots were inoculated with C. destructans spore suspension by pipetting into the soil. After 3 wk of inoculation, root samples were harvested for the analyses of quantification and gene expression. (A) Photographs representing the efficacy of GR agent in biocontrol of the root rot disease. (B) Quantification of T. citrinoviride and C. destructans in ginseng roots using QPCR. (C) Expression analysis of ginseng PR genes. The ginseng ACTIN was used to normalize the relative expression levels. Vertical bars indicate the means with standard error from three biological replications. *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
QPCR, quantitative real-time polymerase chain reaction.
3.12. Application of DP and GR agents to MCG fields
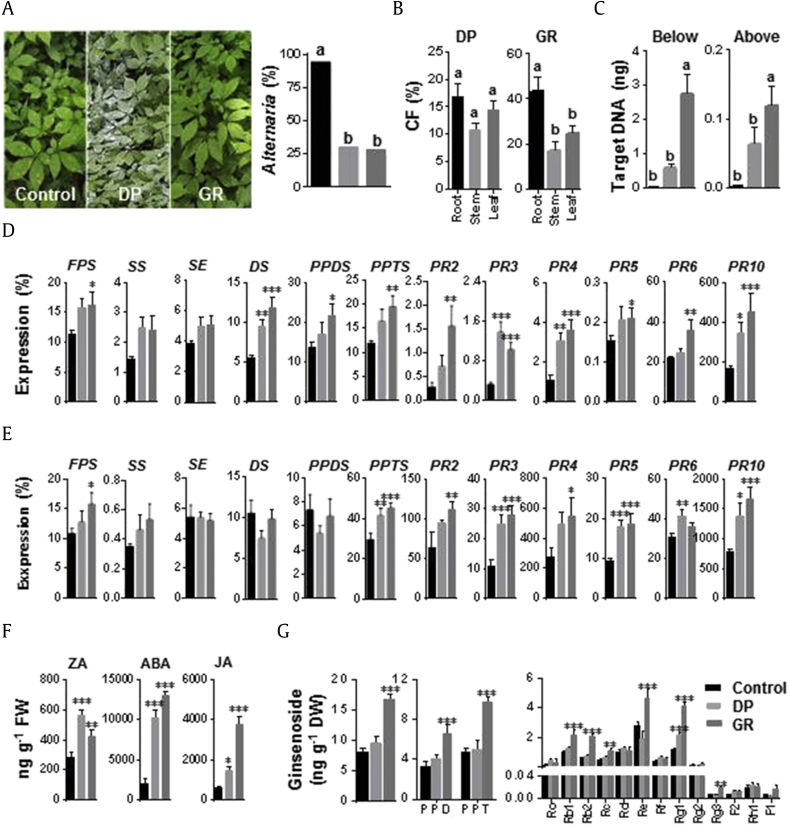
DP and GR agents were sprayed onto foliage in the field. The GR agent was dropped instantaneously into soil, while most of the DP agent remained in aerial plant parts. During treatment, Alternaria blight disease, caused by Alternaria panax, occurred naturally in aerial parts of control plants (94% Alternaria incidence), whereas blight disease was significantly inhibited in DP- and GR-treated plants (30 and 28%, respectively) (Fig. 8A). Two months from the last application, DP- and GR-treated MCG roots showed no disease symptoms or growth inhibition.
Fig. 8.
Biocontrol activity and endophytism of Trichoderma citrinoviridePG87–formulated agents (dustable powder, DP and granule, GR) in mountain-cultivated ginseng (MCG) field. (A) Photographs showing the efficacy of DP and GR agents in biocontrol of Alternaria panax disease and Alternaria incidence (%) in MCG leaves. (B) Colonization frequency (%) of T. citrinoviride in three different tissues of DP- and GR-treated MCG plants. (C) Quantification of T. citrinoviride colonization in below- and above-ground parts of MCG plants using QPCR. (D) Expression of ginseng genes involved in ginsenoside biosynthesis (FPS, SS, SE, DS, PPDS, and PPTS) and defense (PR2, PR3, PR4, PR5, PR6, and PR10) in below-ground parts of ginseng. (E) Expression of ginseng genes involved in ginsenoside biosynthesis (FPS, SS, SE, DS, PPDS, and PPTS) and defense (PR2, PR3, PR4, PR5, PR6, and PR10) in above-ground parts of ginseng. Ginseng ACTIN was used to normalize the relative expression levels. (F) Changes of hormone levels in ginseng roots. (G) The total and each ginsenoside content of PPD (Rb1, Rb2, Rc, Rd, Rg3, and F2) and PPT type (Re, Rf, Rg1, Rg2, Rh1, and F1). These experiments were conducted with six biological replications. Vertical bars indicate the means with standard error. Significance of the relative values is indicated by different letters on the means as per the Duncan's multiple range test (p < 0.05). *p < 0.1, **p < 0.05, and ***p < 0.01 represent significant difference with respect to control.
ABA, abscisic acid; JA, jasmonic acid; PPD, Protopanaxadiol; PPT, protopanaxatriol; QPCR, quantitative real-time polymerase chain reaction; ZA, zeatin.
CF% in DP-treated plants was invariable among the different tissues and was much lower than that in GR-treated plants (Fig. 8B). In GR-treated plants, CF in roots was higher (43%) than in other tissues (leaf, 24%; stem, 16%). T. citrinoviride target DNA was observed at higher levels in GR- versus DP-treated plants, with 2.70 and 0.11 ng in below- and above-ground parts, respectively (Fig. 8C).
In below-ground parts, genes involved in ginsenoside biosynthesis (FPS, DS, PPDS, and PPTS) were expressed at significantly higher levels in GR-treated plants (Fig. 8D). Notably, DS was induced twofold compared with the control. The expression of all PR genes was significantly higher in GR-treated plants, except for PR3, which was most highly induced in DP-treated plants. In above-ground parts, only FPS and PPTS were significantly induced by DP or GR (Fig. 8E). Most PR genes were significantly upregulated by GR application in aerial parts, except for PR6.
Application of DP and GR significantly induced ZA, ABA, and JA in below-ground parts (Fig. 8F). ZA was highly induced by DP, whereas JA was significantly induced by GR.
The GP agent significantly induced ginsenosides in below-ground parts (Fig. 8G). Both PPD and PPT types were notably increased in GR-treated roots compared with those in DP-treated roots and the control. Ginsenosides Rb1, Rb2, Rc, Re, Rg1, and Rg3 were significantly induced after GR treatment, resulting in high amounts of total ginsenosides. The DP agent showed no effect, except for the induction of Rg1.
4. Discussion
Fungal endophytes living in host plants are valuable natural resources that can be exploited as BCAs because of their beneficial effects on host plant development, growth, and fitness [6], [28]. In agriculture, BCAs are recognized as environmental-friendly alternatives to chemical pesticides to control plant pathogens [29]. Although ginseng is an important medicinal plant, research exploring its endophytic community remains in its infancy. Endophytes can induce phytochemical production and defense resistance against pathogens. Herein, we investigated the biocontrol activity of T. citrinoviride isolated from MCG. We found that treatment of ginseng plants with T. citrinoviride induced disease resistance and ginsenoside accumulation. We conducted in vitro and in vivo experiments and confirmed the beneficial effects of T. citrinoviride in ginseng plants. Several Trichoderma spp. are being widely used in agriculture as BCAs against phytopathogens [11]. We observed significant antagonistic behavior of T. citrinoviride against ginseng pathogens on pathogen growth through a dual-culture assay. Coiling and/or penetration of T. citrinoviride mycelia were observed around or into the hyphae of different ginseng pathogens in the contact area of the dual culture. Coiling is a common response of mycoparasitic Trichoderma [30].
BAC stimulates the formation of cyclic adenosine monophosphate (cAMP), and regulates pathogenicity, morphogenesis, and differentiation [31]. Induced cAMP is bound to the regulatory subunits of PKA1, leading to the phosphorylation of target molecules, and this catalytic subunit controlled the growth and virulence of B. cinerea [32]. BCG1 is important for signal transduction and is involved in the regulation of pathogenicity and vegetative growth of B. cinerea [33]. Consistent with this, the BCG1 mutant was shown to lose its pathogenicity and could not produce the phytotoxin botrydial [34]. BOT1 is involved in botrydial biosynthesis and in the generation of B. cinerea disease symptoms and is controlled by signal transduction [35]. During spore germination, TRE1 regulates carbohydrate metabolism through the decomposition of intercellular trehalose [36]. QPCR confirmed a significant reduction in the expression of B. cinerea genes involved in growth and virulence in ginseng plants pretreated with T. citrinoviride compared with that in the control. The findings of previous studies strongly support the downregulation of B. cinerea genes by fungal endophyte metabolites [37].
Mycoparasitism requires increased levels of CWDEs, and consistent with this, T. citrinoviride EDG was highly induced in the dual-culture assay. These results indicated that endoglucanase contributes to the antagonistic activity of T. citrinoviride against B. cinerea. The results of the enzyme assay revealed that T. citrinoviride produces large amounts of enzymes with high β-1,3-glucan-, β-1,4-glucan-, and β-glucoside-degrading activity. These enzymes may attack the cell wall of pathogenic fungi [38]. T. citrinoviride produced enzymes with strong activity in the presence of B. cinerea and C. destructans cell wall components. The lowest level of β-glucosidase activity was observed with all carbon sources compared with other enzymes. The activity of endo-1,4-β-D-glucanase was higher than that of other enzymes when the cell wall components of pathogens were used as a carbon source. This suggests that endo-1,4-β-D-glucanase is important in the degradation of B. cinerea and C. destructans cell walls. In addition, β-1,3-glucanase might contribute to inhibit P. cactorum and Pythium spp., because 80–90% of the oomycetes cell wall is β-1,3-glucan [39]. Strong enzyme activity of T. citrinoviride is an advantageous property of BCA.
No harmful effect of T. citrinoviride inoculation was observed in ginseng plants. This suggests that T. citrinoviride functions as a beneficial endophyte in the host. We confirmed the endophytic nature of T. citrinoviride by reisolation after inoculation. T. citrinoviride was also detected in the above-ground part of the ginseng plant (stem and leaf) after inoculation through root dipping. This is also an advantageous property of a BCA. QPCR confirmed the existence of T. citrinoviride as an endophyte in all ginseng tissues.
We analyzed the various plant responses to T. citrinoviride inoculation, including the levels of ginsenoside and hormones and the expression patterns of genes involved in ginsenoside biosynthesis and defense. The major pharmacologically active compounds in ginseng are triterpene ginsenosides. Ginsenosides have many pharmacological activities, such as anticancer, neuroprotective, antioxidant, and antidiabetic properties [40]. Based on aglycones, ginsenosides are classified into two groups, namely dammarane and oleanane types. Dammarane-type ginsenosides are further classified into PPD type (Rb1, Rb2, Rc, Rd, Rg3, and F2) and PPT type (Re, Rf, Rg1, Rg2, Rh1, and F1) [36]. Elicitors (methyl jasmonate, salicylic acid, and biotic factors) increase the expression or activities of these enzymes [41], [42]. In the present study, T. citrinoviride inoculation by root dipping significantly induced the expression of genes involved in ginsenoside biosynthesis (FPS, SS, SE, DS, and PPDS) in the below-ground parts of ginseng plants, with a slight induction observed in the above-ground parts. This indicates that T. citrinoviride functions as an elicitor to stimulate ginsenoside production, resulting in a 1.57-fold induction compared with the control. The PPD type was more highly induced than the PPT type. T. citrinoviride inoculation also significantly induced phytohormones (ZA, ABA, and JA) in the below-ground parts 4 wk after root inoculation. ZA and ABA contribute to root development through cell differentiation or elongation and plant immune signaling [43]. ABA acts synergistically on the JA response pathway on wounding or herbivore attack [44]. JA plays a major role in the response to wounding and in systemic acquired resistance [44]. ZA and JA also induce ginsenoside biosynthesis [45], [46]. Furthermore, we observed the induction of genes responsible for disease resistance, including PR2, PR3, PR4, PR5, PR6, and PR10. Pathogenesis-related proteins (PRs) exhibit antimicrobial activity and contribute toward plant resistance against pathogen infection. PR proteins constitute β-1,3-glucanases, chitinases, thaumatin-like proteins peroxidases, and ribosome-inactivating proteins [47]. Among these, β-1,3-glucanases and chitinases are the most important in many plant species as they degrade the major component of pathogen cell walls, β-1,3-glucan and chitin [48]. PR2 is composed of β-1,3-glucanases and is rapidly activated and accumulated in response to pathogen infection [49]. As chitinases, PR3 and PR4 are specialized in the degradation of fungal cell walls and exhibit synergism with β-1,3-glucanases [50]. PR5 consists of thaumtin-like proteins, which have roles in the plant defense against various stress conditions and pathogen infection [51]. PR6 and PR10 act as a protease inhibitor and a ribonuclease, respectively, with essential roles in plant defense, development, and disease resistance [52]. We found that PR2, PR5, PR6, and PR10 were significantly upregulated in the above-ground parts of plants, whereas only PR3 and PR4 were induced and retained constantly in the below-ground parts after T. citrinoviride inoculation by root dipping. These results support the notion that T. citrinoviride inoculation can induce ginseng plant immunity against pathogens through PR gene induction. Therefore, T. citrinoviride inoculation has a “priming effect”. Priming occurs when pretreatment with a mild stress or chemical improves the efficiency of plant defense mechanisms. The priming effect of fungal endophytes is well known in diverse plant species [53]. The priming effect and induction of disease resistance were confirmed using an in planta assay against B. cinerea. Leaves of ginseng plants preinoculated with T. citrinoviride by root dipping had significantly reduced symptoms on subsequent infection with B. cinerea (100 vs. 11.5% RIA in the control and treatment groups, respectively). Furthermore, genes involved in defense (PR2, PR4, PR5, and PR10) were significantly induced in ginseng leaves preinoculated with T. citrinoviride by root dipping compared with the control. In addition, the expression of B. cinerea genes (BAC, BCG1, BOT, CHS3, PKA1, and TRE1) was significantly reduced in ginseng leaves preinoculated with T. citrinoviride by root dipping. CHS3, which encodes chitin synthase 3, is important for hyphal growth [54]. The expression of CHS3 was inhibited in preinoculated plant leaves, indicating the suppression of B. cinerea growth. Previous studies have shown induced systemic resistance to B. cinerea through enhanced levels of PR proteins or phytohormones in plants preexposed to Trichoderma harzianum [55], [56]. These results confirmed that the enhanced levels of phytohormones and PR genes after T. citrinoviride preinoculation may explain the improved disease resistance against B. cinerea via priming.
In addition to a priming effect, T. citrinoviride could directly inhibit B. cinerea growth and pathogenicity on ginseng leaves. Preinoculation of ginseng leaves with T. citrinoviride induced significant resistance against B. cinerea. Significant downregulation of B. cinerea BOT1 also confirmed the direct antimicrobial activity of T. citrinoviride against B. cinerea. BOT1 deletion significantly reduced B. cinerea virulence in plants [35]. Interestingly, ginseng defense genes were significantly downregulated in T. citrinoviride-treated leaves. This is probably because the growth and pathogenicity of B. cinerea were directly suppressed by T. citrinoviride at an early time point. Therefore, priming is not necessary. This result also demonstrates the potential of T. citrinoviride as a BCA. Similar direct inhibitory activity of T. citrinoviride was found in ginseng root against C. destructans. Preinoculation of ginseng roots with T. citrinoviride significantly downregulated ginseng defense-related genes.
Several species of Trichoderma (T. harzianum, T. hamatum, and T. viride) have been exploited as BCAs with various mechanisms, including mycoparasitism, antibiosis, induced resistance, and competition for nutrients. However, this is the first report to show the potential of endophytic T. citrinoviride as a BCA. Generally, BCAs are grown in their natural habitat and combat both aerial and below-ground diseases [57]. BCA formulation is a key process for functional success [58]. Effective formulation requires the consideration of various components, such as the biocontrol organism, pathogen, environment, application practices, and equipment. Typically, dry formulations (powder or granule) provide a long shelf life and convenience for storage and treatment [59]. Therefore, we developed DP and GR agents for application onto aerial and below-ground tissues of MCG, respectively. The GR agent showed consistent stability for microbe survival and physical properties in the field. Application of formulated T. citrinoviride exhibited a greater antagonistic effect on B. cinerea and C. destructans compared with the application of T. citrinoviride spore suspension. The DP agent was superior for foliar disease caused by B. cinerea, whereas the GR agent was better for root disease caused by C. destructans.
The formulated BCAs were also tested in the MCG field under natural conditions. The GR agent applied to the ginseng plant eventually reached the soil and more heavily colonized the plant compared with the DP agent. The incidence of foliar disease caused by A. panax, which occurred naturally during the application period of formulated T. citrinoviride agents, was significantly reduced by DP and GR agents. The GR agent exhibited better colonization in all ginseng tissues. These results indicate that the GR agent has greater potential as a BCA for the control of ginseng pathogens both in the aerial and below-ground parts of MCG. In addition, GR treatment induced a significant accumulation of ginsenosides, which were induced more than twofold compared with the control root.
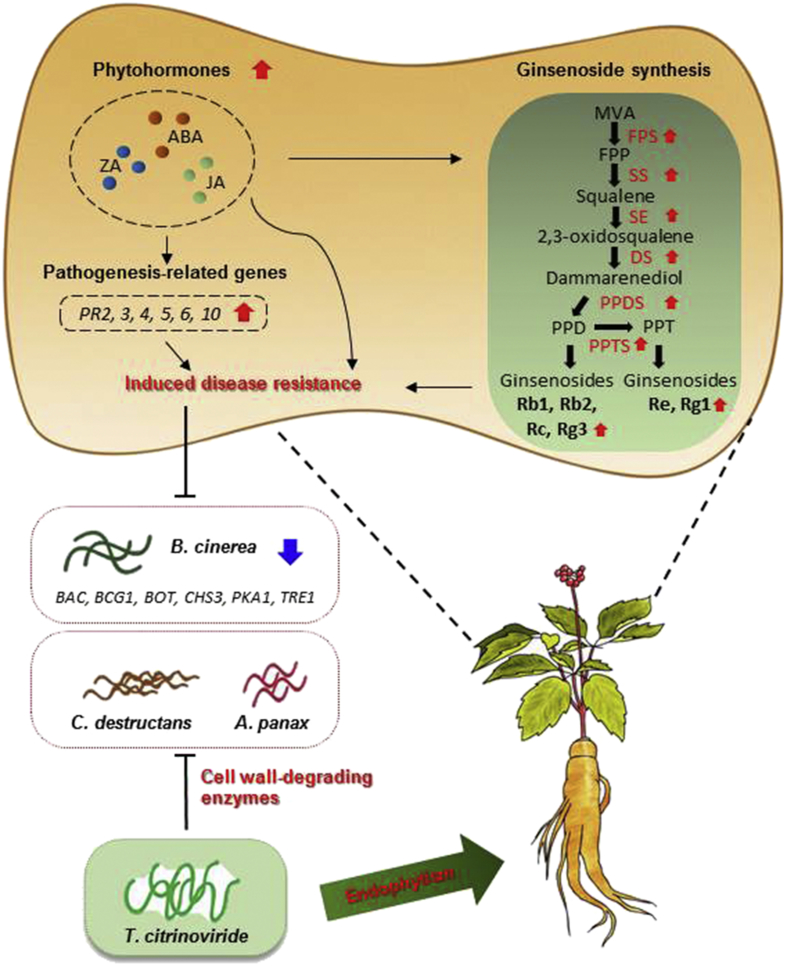
In summary, the fungal endophyte isolated from MCG, T. citrinoviride, has great potential as a BCA against ginseng pathogens (Fig. 9). T. citrinoviride can grow heavily inside ginseng plants, where it lives as a beneficial endophyte. T. citrinoviride can directly inhibit the growth of ginseng pathogens and induce disease resistance and ginsenoside accumulation in ginseng plants. Various mechanisms might be involved in biocontrol activity against ginseng pathogens, such as inhibition of B. cinerea genes involved in growth and virulence, production of CWDEs and phytohormones, and priming. Taken together, our results revealed that T. citrinoviride-colonizing MCG has great potential as a BCA and an elicitor of ginsenoside production. In accordance with green agriculture, this would represent a sustainable model of MCG cultivation.
Fig. 9.
Summary of the efficacy of Trichoderma citrinoviride PG87 in biocontrol of ginseng pathogens.T. citrinoviride, fungal endophyte of mountain-cultivated ginseng, has great potential as biocontrol agent against ginseng pathogens. T. citrinoviride can heavily colonize ginseng plants as a beneficial symbiont. T. citrinoviride can directly inhibit the growth of ginseng pathogens through mycoparasitism (e.g., cell wall–degrading enzymes) and induce disease resistance and ginsenoside accumulation in ginseng plants. Various mechanisms might be involved in biocontrol activity against ginseng pathogens: downregulation of B. cinerea genes involved in growth and virulence, upregulation of ginseng genes involved in pathogenesis-related proteins (PRs), production of cell wall-degrading enzymes and phytohormones, priming effect, and so forth.
ABA, abscisic acid; FPP, farnesyl pyrophosphate; JA, jasmonic acid; MVA, mevalonate; PPD, Protopanaxadiol; PPT, protopanaxatriol; ZA, zeatin.
Conflicts of interest
The authors declare that there are not conflicts of interest.
Acknowledgments
This study was carried out with the support of the “Forest Science and Technology” project (Project No. S121414L030110) provided by Korea Forest Service.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2018.03.002.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Mycelial growth inhibition (%) of 6 ginseng pathogens in dual culture assay with endophytic Trichoderma citrinoviride isolated from mountain-cultivated ginseng plants. (A)Alternaria panax, (B)Botrytis cienrea, (C)Cylindrocarpon destructans, (D)Rizoctonia solani, (E)Phytophthora cactorum, (F)Pythium sp. T, T. citrinoviride; P, pathogen. Vertical bars indicate the means with standard error from 3 biological replications. Different letters on the means indicated significantly different from each other according to Duncan’s multiple range test (p < 0.05).
Fig. S2.
Specificity assays for primers and calibration curves. (A) PCR amplification of DNA extracted from ginseng roots 3 wk after T. citrinoviride inoculation to assess the specificity of TEF-F/TEF-R primer set, showing a 250-bp amplified product. Calibration curve was calculated from threshold cycle (Ct) values from T. citrinoviride genomic DNA. Each point is from three technical replicates. (B) PCR amplification of DNA extracted from ginseng roots 3 wk after C. destructans inoculation to assess the specificity of IGS primer set, showing a 152-bp amplified product. Calibration curve was calculated from threshold cycle (Ct) values from C. destructans genomic DNA. Each point is from 3 technical replicates.
Fig. S3.
Formulation of dustable powder (DP) and granule (GR) from T. citrinoviride and their survival test in different temperatures. (A) Formulated T. citrinoviride as dustable powder and survival graph in different temperature. (B) Formulated T. citrinoviride as granule and survival graph in different temperature. Each point is from 3 biological replicates.
References
- 1.Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res. 2010;34:259–263. [Google Scholar]
- 2.Park J.D., Rhee D.K., Lee Y.H. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochem Rev. 2005;4:159–175. [Google Scholar]
- 3.Kwon K.R., Park W.P., Kang W.M., Jeon E.Y., Jang J.H. Identification and analysis of differentially expressed genes in mountain cultivated ginseng and mountain wild ginseng. J Acupunct Meridian Stud. 2011;4:123–128. doi: 10.1016/S2005-2901(11)60018-6. [DOI] [PubMed] [Google Scholar]
- 4.Cho K.M., Hong S.Y., Lee S.M., Kim Y.H., Kahng G.G., Lim Y.P., Kim H., Yun H.D. Endophytic bacterial communities in ginseng and their antifungal activity against pathogens. Microb Ecol. 2007;54:341–351. doi: 10.1007/s00248-007-9208-3. [DOI] [PubMed] [Google Scholar]
- 5.Sathiyaraj G., Srinivasan S., Subramanium S., Kim Y.J., Kim Y.J., Kwon W.S., Yang D.C. Polygalacturonase inhibiting protein: isolation, developmental regulation and pathogen related expression in Panax ginseng C.A. Meyer. Mol Biol Rep. 2010;37:3445–3454. doi: 10.1007/s11033-009-9936-1. [DOI] [PubMed] [Google Scholar]
- 6.Li H.Y., Wei D.Q., Shen M., Zhou Z.P. Endophytes and their role in phytoremediation. Fungal Divers. 2012;54:11–18. [Google Scholar]
- 7.Ownley B.H., Gwinn K.D., Vega F.E. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl. 2010;55:113–128. [Google Scholar]
- 8.Ek-Ramos M.J., Zhou W., Valencia C.U., Antwi J.B., Kalns L.L., Morgan G.D., Kerns D.L., Sword G.A. Spatial and temporal variation in fungal endophyte communities isolated from cultivated cotton (Gossypium hirsutum) PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0066049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunatilaka A.A.L. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae H., Roberts D.P., Lim H.S., Strem M.D., Park S.C., Ryu C.M., Melnick R.L., Bailey B.A. Endophytic Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol Plant Microbe Interact. 2011;24:336–351. doi: 10.1094/MPMI-09-10-0221. [DOI] [PubMed] [Google Scholar]
- 11.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma species–opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 12.Zeilinger S., Omann M. Trichoderma biocontrol: signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul Syst Biol. 2007;1:227–234. doi: 10.4137/grsb.s397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinale F., Marra R., Scala F., Ghisalberti E.L., Lorito M., Sivasithamparam K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett Appl Microbiol. 2006;43:143–148. doi: 10.1111/j.1472-765X.2006.01939.x. [DOI] [PubMed] [Google Scholar]
- 14.Cavalcante R.S., Lima H.L.S., Pinto G.A.S., Gava C.A.T., Rodrigues S. Effect of moisture on Trichoderma conidia production on corn and wheat bran by solid state fermentation. Food Bioprocess Technol. 2008;1:100–104. [Google Scholar]
- 15.Lin H., Travisano M., Kazlauskas R.J. Experimental evolution of Trichoderma citrinoviride for faster deconstruction of cellulose. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0147024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury E.K., Jeon J., Ok Rim S., Park Y.H., Lee S.K., Bae H. Composition, diversity and bioactivity of culturable bacterial endophytes in mountain-cultivated ginseng in Korea. Sci Rep. 2017;7:10098. doi: 10.1038/s41598-017-10280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae H., Kim S.H., Kim M.S., Sicher R.C., Lary D., Strem M.D., Natarajan S., Bailey B.A. The drought response of Theobroma cacao (cacao) and the regulation of genes involved in polyamine biosynthesis by drought and other stresses. Plant Physiol Biochem. 2008;46:174–188. doi: 10.1016/j.plaphy.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Cao R., Liu X., Gao K., Mendgen K., Kang Z., Gao J., Dai Y., Wang X. Mycoparasitism of endophytic fungi isolated from reed on soilborne phytopathogenic fungi and production of cell wall-degrading enzymes in vitro. Curr Microbiol. 2009;59:584–592. doi: 10.1007/s00284-009-9477-9. [DOI] [PubMed] [Google Scholar]
- 19.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 21.Saroj D.B., Dengeti S.N., Aher S., Gupta A.K. A rapid, one step molecular identification of Trichoderma citrinoviride and Trichoderma reesei. World J Microbiol Biotechnol. 2015;31:995–999. doi: 10.1007/s11274-015-1839-9. [DOI] [PubMed] [Google Scholar]
- 22.Park S.U., Lim H.S., Park K.C., Park Y.H., Bae H. Fungal endophytes from three cultivars of Panax ginseng Meyer cultivated in Korea. J Ginseng Res. 2012;36:107–113. doi: 10.5142/jgr.2012.36.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z., Hao Z., Zeng Y., Guo L., Huang L.-Q., Wang Y., Chen B.-D. Molecular quantification of Cylindrocarpon destructans in the rhizosphere of Panax notoginseng for predicting plant growth response. Microbiol China. 2015;42:598–607. [Google Scholar]
- 24.Ghosh R., Mishra R.C., Choi B., Kwon Y.S., Bae D.W., Park S.-C., Jeong M.-J., Bae H. Exposure to sound vibrations lead to transcriptomic, proteomic and hormonal changes in Arabidopsis. Sci Rep. 2016;6:33370. doi: 10.1038/srep33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasote D.M., Ghosh R., Chung J.Y., Kim J., Bae I., Bae H., Kasote D.M., Ghosh R., Chung J.Y., Kim J. Multiple reaction monitoring mode based liquid chromatography-mass spectrometry method for simultaneous quantification of brassinolide and other plant hormones involved in abiotic stresses. Int J Anal Chem. 2016;2016:1–8. doi: 10.1155/2016/7214087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh J.Y., Kim Y.J., Jang M.G., Joo S.C., Kwon W.S., Kim S.Y., Jung S.K., Yang D.C. Investigation of ginsenosides in different tissues after elicitor treatment in Panax ginseng. J Ginseng Res. 2014;38:270–277. doi: 10.1016/j.jgr.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang Y., Kim S.G., Kim Y.H. Biocontrol efficacies of Bacillus species against Cylindrocarpon destructans causing ginseng root rot. Plant Pathol J. 2011;27:333–341. [Google Scholar]
- 28.Zhang Q., Zhang J., Yang L., Zhang L., Jiang D., Chen W., Li G. Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control. 2014;72:98–108. [Google Scholar]
- 29.Alabouvette C., Olivain C., Steinberg C. Biological control of plant diseases: the European situation. Eur J Plant Pathol. 2006;114:329–341. [Google Scholar]
- 30.Troian R.F., Steindorff A.S., Ramada M.H.S., Arruda W., Ulhoa C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol Lett. 2014;36:2095–2101. doi: 10.1007/s10529-014-1583-5. [DOI] [PubMed] [Google Scholar]
- 31.Lengeler K.B., Davidson R.C., D'souza C., Harashima T., Shen W.C., Wang P., Pan X., Waugh M., Heitman J. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64:746–785. doi: 10.1128/mmbr.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimpel A., Gronover C.S., Williamson B., Stewart J.A., Tudzynski B. The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol Plant Pathol. 2002;3:439–450. doi: 10.1046/j.1364-3703.2002.00137.x. [DOI] [PubMed] [Google Scholar]
- 33.Schulze Gronover C., Schorn C., Tudzynski B. Identification of Botrytis cinerea genes up-regulated during infection and controlled by the Galpha subunit BCG1 using suppression subtractive hybridization (SSH) Mol Plant Microbe Interact. 2004;17:537–546. doi: 10.1094/MPMI.2004.17.5.537. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher J., Kokkelink L., Huesmann C., Jimenez-Teja D., Collado I.G., Barakat R., Tudzynski P., Tudzynski B. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol Plant Microbe Interact. 2008;21:1443–1459. doi: 10.1094/MPMI-21-11-1443. [DOI] [PubMed] [Google Scholar]
- 35.Siewers V., Viaud M., Jimenez-Teja D., Collado I.G., Gronover C.S., Pradier J.-M., Tudzynski B., Tudzynski P. Functional analysis of the cytochrome P450 monooxygenase gene bcbot1 of Botrytis cinerea indicates that botrydial is a strain-specific virulence factor. Mol Plant Microbe Interact. 2005;602:602–612. doi: 10.1094/MPMI-18-0602. [DOI] [PubMed] [Google Scholar]
- 36.Argüelles J.C. Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch Microbiol. 2000;174:217–224. doi: 10.1007/s002030000192. [DOI] [PubMed] [Google Scholar]
- 37.Park Y.H., Chung J.Y., Ahn D.J., Kwon T.R., Lee S.K., Bae I., Yun H.K., Bae H. Screening and characterization of endophytic fungi of Panax ginseng Meyer for biocontrol activity against ginseng pathogens. Biol Control. 2015;91:71–81. [Google Scholar]
- 38.McQuilken M.P., Gemmell J. Enzyme production by the mycoparasite Verticillium biguttatum against Rhizoctonia solani. Mycopathologia. 2004;157:201–205. doi: 10.1023/b:myco.0000020590.20040.4a. [DOI] [PubMed] [Google Scholar]
- 39.Shi J., Liu A., Li X., Chen W. Control of Phytophthora incotianae disease, induction of defense responses and genes expression of papaya frutis treated with Pseudomonas putida MGP1. J Sci Food Agric. 2013;93:568–574. doi: 10.1002/jsfa.5831. [DOI] [PubMed] [Google Scholar]
- 40.Leung K.W., Wong A.S.-T. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali M.B., Yu K.W., Hahn E.J., Paek K.Y. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006;25:613–620. doi: 10.1007/s00299-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y., Liu Q., Zang P., Li X., Ji Q., He Z., Zhao Y., Yang H., Zhao X., Zhang L. An endophytic bacterium isolated from Panax ginseng C.A. Meyer enhances growth, reduces morbidity, and stimulates ginsenoside biosynthesis. Phytochem Lett. 2015;11:132–138. [Google Scholar]
- 43.Garay-Arroyo A., De La Paz Sanchez M., Garcia-Ponce B., Azpeitia E., Alvarez-Buylla E.R. Hormone symphony during root growth and development. Dev Dyn. 2012;241:1867–1885. doi: 10.1002/dvdy.23878. [DOI] [PubMed] [Google Scholar]
- 44.Pieterse C.M.J., Van der Does D., Zamioudis C., Leon-Reyes A., Van Wees S.C.M. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 45.Lian M., Chakrabarty D., Paek K. Effect of plant growth regulators and medium composition on cell growth and saponin production during cell suspension culture of mountaint ginseng (Panax ginseng C.A. Mayer) J Plant Biol. 2002;45:201–206. [Google Scholar]
- 46.Hu X., Neill S., Cai W., Tang Z. Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension cultured cells of Panax ginseng. Physiol Plant. 2003:414–421. [Google Scholar]
- 47.Pulla R.K., Lee O.R., In J.G., Kim Y.J., Senthil K., Yang D.C. Expression and functional characterization of pathogenesis-related protein family 10 gene, PgPR10-2, from Panax ginseng C.A. Meyer. Physiol Mol Plant Pathol. 2010;74:323–329. [Google Scholar]
- 48.Saboki E., Usha K., Singh B. Pathogenesis related (PR) proteins in plant defense mechanism age-related pathogen resistance. Curr Res Technol Adv. 2011:1043–1054. [Google Scholar]
- 49.Elsharkawy M.M., Shimizu M., Takahashi H., Hyakumachi M. The plant growth-promoting fungus Fusarium equiseti and the arbuscular mycorrhizal fungus Glomus mosseae induce systemic resistance against cucumber mosaic virus in cucumber plants. Plant Soil. 2012;361:397–409. [Google Scholar]
- 50.Mauch F., Mauch-Mani B., Boller T. Antifungal hydrolases in pea tissue 1. Plant Physiol. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y.J., Lee J.H., Jung D.Y., Sathiyaraj G., Shim J.S., In J.G., Yang D.C. Isolation and characterization of pathogenesis-related protein 5 (PgPR5) gene from Panax ginseng. Plant Pathol J. 2009;25:400–407. [Google Scholar]
- 52.Lee O.-R., Sathiyaraj G., Kim Y.-J., In J.-G., Kwon W.-S., Kim J.-H., Yang D.C. Defense genes induced by pathogens and abiotic stresses in Panax ginseng C.A. Meyer. J Ginseng Res. 2011;35:1–11. [Google Scholar]
- 53.Saikkonen K., Saari S., Helander M. Defensive mutualism between plants and endophytic fungi? Fungal Divers. 2010;41:101–113. [Google Scholar]
- 54.Soulié M.C., Perino C., Piffeteau A., Choquer M., Malfatti P., Cimerman A., Kunz C., Boccara M., Vidal-Cros A. Botrytis cinerea virulence is drastically reduced after disruption of chitin synthase class III gene (Bcchs3a) Cell Microbiol. 2006;8:1310–1321. doi: 10.1111/j.1462-5822.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 55.Martínez-Medina A., Fernández I., Sánchez-Guzmán M.J., Jung S.C., Pascual J.A., Pozo M.J. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perazzolli M., Dagostin S., Ferrari A., Elad Y., Pertot I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol Control. 2008;47:228–234. [Google Scholar]
- 57.Shoresh M., Harman G.E., Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 58.Leggett M., Leland J., Kellar K., Epp B. Formulation of microbial biocontrol agents – an industrial perspective. Can J Plant Pathol. 2011;33:101–107. [Google Scholar]
- 59.Sabaratnam S., Traquair J.A. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplants. Biol Control. 2002;23:245–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.