Abstract
Background
Endometrial stromal cell decidualization is critical for embryo implantation. Dysfunctional decidualization leads to implantation failure, miscarriage and even pregnancy associated disorders in subsequent pregnancy trimesters. Protein glycosylation is involved in many physiological and pathological processes. Protein O-fucosyltransferase 1 (poFUT1) is the key enzyme for the O-fucosylation of proteins. However, the role and mechanism of poFUT1 in human endometrial stromal cell decidualization remain elusive.
Methods
We employed immunohistochemistry to detect the level of poFUT1 in the uterine endometrium from those of the proliferative phase, secretory phase, early pregnancy women and miscarriage patients. Using human endometrial stromal cells (hESCs) and a mouse model, the underlying mechanisms of poFUT1 in decidualization was investigated.
Findings
The level of poFUT1 was increased in the stromal cells of the secretory phase relative to those in the proliferative phase of the menstrual cycle, and decreased in the stromal cells of miscarriage patients compared to women with healthy early pregnancies. Furthermore, we found that poFUT1 promoted hESCs decidualization. The results also demonstrated that poFUT1 increased O-fucosylation on Notch1 in hESCs, which activated Notch1 signaling pathway. Activated Notch1 (NICD), as a specific trans-factor of PRL and IGFBP1 promoters, enhanced PRL and IGFBP1 transcriptional activity, thus inducing hESCs decidualization.
Interpretation
Level of poFUT1 is lower in the uterine endometrium from miscarriage patients than early pregnancy women. poFUT1 is critical in endometrial decidualization by controlling the O-fucosylation on Notch1. Our findings provide a new mechanism perspective on poFUT1 in uterine decidualization that may be a useful diagnostic and therapeutic target for miscarriage.
Fund
National Natural Science Foundation of China (31770857, 31670810 and 31870794). Liaoning Provincial Program for Top Discipline of Basic Medical Sciences.
Keywords: poFUT1, O-fucosylation, Notch1, Decidualization, Miscarriage
Research in context.
Evidence before this study
Protein glycosylation is involved in many physiological and pathological processes. Protein O-fucosyltransferase 1 is the key enzyme for the O-fucosylation of proteins. The knockout of poFUT1 in mice results in severe growth retardation during early embryogenesis and causes embryonic lethality. We previous found that silence poFUT1 inhibit trophoblast invasion of the uterine epithelium. However, the role and mechanism of poFUT1 in human endometrial stromal cell decidualization remain elusive.
Added value of this study
We found that the level of poFUT1 increased in stromal cells in the secretory phase relative to those in the proliferative phase of the menstrual cycle and decreased in the stromal cells of miscarriage patients relative to women with healthy early pregnancies. Using hESC lines and a mouse model, we provided evidence that poFUT1 promoted hESC decidualization. We also demonstrated that poFUT1 increased O-fucosylation on Notch1 in hESC, which further activated the Notch1 signaling pathway. Activated Notch1 (NICD) is a specific trans-factor of the PRL and IGFBP1 promoters and may increase PRL and IGFBP1 transcriptional activity, further inducing hESC decidualization.
Implications of all the available evidence
Our findings provide a new mechanism perspective on poFUT1 in uterine decidualization. poFUT1 may serve as a new biomarker for clinical diagnosis and as a target for miscarriage treatment.
Alt-text: Unlabelled Box
1. Introduction
Successful embryo implantation in a receptive uterine endometrium requires the decidualization of the endometrial stromal cells (ESCs) [6,7]. When embryo implantation occurs in the uterus, ESCs undergo dramatic proliferation and differentiation into transient epithelioid decidual cells [35]. Decidual cells biosynthesize and secrete many important factors, such as signal messengers, growth factors, cytokines and chemokines, which are essential for the regulation of embryo implantation and placenta formation [3,8]. Among these molecules, prolactin (PRL) and insulin-like growth factor binding protein-1 (IGFBP1) are commonly used to assess the quality of the decidual response in ESCs [10,12]. Functional impairment of endometrial stromal decidualization leads to a variety of pregnancy disorders, including recurrent miscarriages, infertility, preeclampsia and intrauterine growth restriction [5,13,40]. According to an investigation by the World Health Organization, female infertility affects >48 million women [28]. Despite the developments in assisted reproductive technologies (ARTs), the implantation rate in ARTs is still limited. Even with the high-quality and mature embryos, an impaired decidualization process is a causal factor of implantation failure and infertility. Deepening the understanding of the intricate mechanism of decidualization not only helps to clarify the physiology of pregnancy but also aids in alleviating the intractable problems that are associated with infertility and miscarriages.
Protein glycosylation is an important form of co-translational and post-translational modifications, which are involved in a variety of physiological and pathological processes, such as tissue development, fertilization and tumor progression [32,37]. Glycosylation is an enzymatic reactionby which mono-or oligosaccharides residues are site-specifically added to the accecptor molecules, generally proteins and lipids. The different glycosylation modifications arecatalyzed by the specific glycosyltransferases [44]. Fucosylation is an important type of glycosylation, and it is classified into N-fucosylation and O-fucosylation. Fucosyltransferases (FUTs) are enzymes that catalyze the transfer of GDP-fucose residues to the acceptor molecules [4,33]. To date, thirteen FUT genes have been identified in the human genome [41]. FUT1–11 catalyzes N-linkage fucosylation. Protein O-fucosyltransferases 1 and 2 (poFUT1 and poFUT2) add O-linked fucose to the folded epidermal growth factor (EGF)-like repeats and thrombospondin type 1 repeats (TSR), respectively [9,17,45]. The glycobiology of implantation shows that fucosylation is a functional event present at the embryonic-maternal interface. The expression of FUT4 is eleveated in the uterine endometrium of the secretory phase compared to the proliferative phase in human, which contributes to the uterine receptivity [34]. Mice lacking FUT8 are severely growth restricted at birth and develop emphysema [47]. The knockout of poFUT1 in mice results in severe growth retardation during early embryogenesis and causes embryonic lethality [31,42]. Silencing poFUT1 inhibits trophoblast invasion of the uterine epithelium [26]. It is also reported that poFUT2 disruption in mice leads to teratomas. However, little information is available regarding the potential function of poFUT1 and O-fucosylation in the proliferation and differentiation of human endometrial stromal cells during decidualization.
Glycoproteins on the cell surface are closely associated with cellular functions, such as molecular recognization, cell-cell adhesion and signaling transduction. Notch1 is a transmembrane receptor glycoprotein that binds to the ligand Jagged-1. The binding induces the proteolytic cleavage and release of Notch intracellular domain (NICD), which enters nucleus of the cell and initiates the gene expression of target proteins. Notch1 is a type-1 transmembrane glycoprotein consisting of multiple EGF-like repeats, which are potential fucosylation sites. Studies reveal that poFUT1 is essential for the O-fucosylation on Notch1, further influencing Notch-ligand binding and Notch signaling [19,30,36,42,45]. The incapacity of poFUT1 causes the loss of Notch-ligand binding. The Notch 1 signaling pathway is a conserved cell signaling system, and plays an important role in variety developmental processes in cell fate determination, such as stem cell maintenance, cell lineage determination, and lymphoid development and differentiation [1]. Recent evidence has suggested that the developmental Notch signaling pathway is critically involved in human trophoblast function and placenta formation [16]. However, the underlying mechanism governing O-fucosylation on Notch during decidualization remains largely elusive.
In the present study, we revealed that poFUT1 expression was higher in the uterine endoemetrium of secretory phase than in the proliferative phase, and lower in the decidual cells of miscarriage patients compared with normal early pregnancy women. Employing hESCs and a mouse model, we further demonstrated that poFUT1 siRNA decreased O-fucosylation on Notch1, thus impairing hESC decidualization and embryo implantation. These findings suggest that poFUT1 and O-fucosylation are required for endometrial stromal cell decidualization.
2. Materials and methods
2.1. Tissue samples
The endometrial tissue samples of the proliferative phase, early secretory phase, late secretory phase, early pregnancy and miscarriage at the age of 25–38 were collected from The Second Affiliated Hospital of Dalian Medical University (Dalian, China), and signed informed consent forms were obtained from the subjects. The uterine endometrium of proliferative phase (n = 20), early secretory phase and late secretory phase (n = 20) were with regular menstrual cycle, and excluded from other gynecological abnormalities. The normal early pregnant group (n = 20) and miscarriage group (n = 20) were from women in 7–10 pregnancy weeks. The cases were confirmed by ultrasound detection and underwent induced abortion. The miscarriage group was consist of recurrent patients (n = 13) and first abortion (n = 7), and serum progesterone level was less 25 ng/ml. This study was approved by the Clinical Ethics Review Board of Dalian Medical University.
2.2. Cell culture
Human endometrial stromal cell (hESC) line was obtained from Professor Haibin Wang (Fujian Provincial Key Laboratory of Reproductive Health Research, Medical College of Xiamen University, , China) as a gift. hESCs were cultured in DMEM/F12 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C in a humidified chamber with 5% CO2. The culture medium was changed every 2-3 days. To induce decidualization in vitro, hESCs were treated with DMEM/F12 medium containing 2% FBS 10 nM estrogen (E2, Selleck, Chemicals, USA), 1 μM medroxyprogesterone 17-acetate (MPA, Selleck), and 0.5 mM dibutyryl cAMP (dbcAMP, Selleck).
2.3. Immunohistochemistry
Endometrial decidual tissue sections were incubated with xylol for deparaffinized and rehydrated in descending concentrations of ethanol following with antigen retrieval in citrate buffer (pH 6.0). 0.3% H2O2 was used to remove endogenous peroxidases by incubation for 10 min and blocking with 5% BSA (Beyotime, Shanghai, China) for 45 min at room temperature. Primary antibodies: poFUT1 (1:100, Proteintech, Wuhan, China), IGFBP1 (1:100, Santa Cruz, USA) and PCNA (1:100, Proteintech, Wuhan, China) were applied at 4 °C overnight in a wet chamber. After washing with PBS, slides were incubated with the second antibody for 45 min and visualized with diaminobenzidine. The slides were counterstained with hematoxylin, and the evaluation was performed. Images were captured under an inverted microscope.
2.4. Immunofluorescence
Immunofluorescent staining was performed after fixing the cells with 4% paraformaldehyde for 20 min. After blocking with 3% BSA (Beyotime, Shanghai, China) for 1 h, the cells were incubated with poFUT1 (1:100), IGFBP1 (1:100), PRL (1:100, Bioss, Beijing, China) and PCNA antibody (1:100) overnight at 4 °C. After washing with PBS, the cells were incubated with TRITC-conjugated goat anti-mouse IgG (1:100, ZSGB-BIO, Beijing, China) and FITC-conjugated goat anti-rabbit IgG (1:100, ZSGB-BIO) for 1 h at room temperature. Then slides were incubated with DAPI (1:1000, Beyotime) for 10 min at room temperature. Images were obtained under a fluorescence microscope (Olympus BX83, Japan).
2.5. Real-time PCR
Quantitative PCR (qPCR) was used to measure mRNA level. Total RNAs were extracted using Trizol (Takara, Japan) according to the manufacturer's protocol. RNA was reversely transcribed into cDNA using PrimeScriptTM RT reagent kit (TransGen Biotech, China), and then qPCR was performed using TransStart TipTop Green qPCR SuperMix (TransGen Biotech, China) according to the manufacturer's instructions. The primer sequences were as follows: poFUT1 Forward 5′- CAGCGAAGCCCAGATAAGAAG -3′, Reverse 5′- CTGTAGGAAGCTCTGAAGGAAAT -3′; IGFBP1 (Forward) 5′- TATGATGGCTCGAAGGCTCT -3′, (Reverse) 5′- CCATTCTTGTTGCAGTTTGG -3′; PRL (Forward) 5′- TCTCGCCTTTCTGCTTATTATAAC -3′, (Reverse) 5′- CGATTCGGCACTTCAGGAGCTT -3′; GAPDH (Forward) 5′- TCCTGTTCGACAGTCAGCCGCAT -3′, (Reverse) 5′- TGCAAATGAGCCCCAGCCTTCTCCA -3′. Quantified data were normalized to GAPDH, and the relative quantity was calculated using the 2-ΔΔCT method. Triplicate independent experiments were carried out.
2.6. Western blot
Cells were lysed. Protein concentration was determined with Coomassie protein assay reagent using bovine serum albumin as a standard. Total protein was run on a 10% SDS-PAGE gel followed by transferring to a nitrocellulose filter membrane (NC, Millipore, Billerica, MA, USA) and blocking with 5% non-fat dry milk in TBST for 2 h. The membrane was incubated with the primary antibody overnight followed by incubation with goat anti-rabbit IgG or goat anti-mouse IgM, and HRP-linked antibody (1:3000, Beyotime) for 1 h. An enhanced chemiluminescence (ECL) detection system (Bio-Rad) was used to visualize immunoreactive bands.
2.7. Enzyme-linked immunosorbent assay
Commercial enzyme-linked immunosorbent assay (ELISA) kits (Elabscience, Wuhan, China) were purchased and used to detect PRL and IGFBP1 level. Samples (100 μl each) were added into the micro plate wells and incubated at 37 °C for 90 min. The samples were removed and a biotinylated detection antibody (100 μl) was added and incubated for 1 h. After washing, 100 μl of HRP conjugate working solution was added to each well. Ninety microliters of substrate reagent were added after incubation for 30 min and washing. When an apparent gradient appeared, a stop solution (50 μl) was added, and the color turned to yellow immediately. The OD value of 450 nm (OD450) was determined and data were collected.
2.8. Cell proliferation assay
Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was used to assess the proliferation ability of hESCs. The cells in the logarithmic growth phase were plated in 96 well plates at 3000 cells per well, and transfected with 10 nM E2, 1 μM MPA, 0.5 mM dbcAMP, poFUT1 siRNA (50 nM) and poFUT1 cDNA (4 μg/ml). After culturing for 2 h in the incubator, the OD450 value was measured by a microplate reader for 5 days. Three parallel sample wells were applied for each set of experiments and averaged. The cell growth curve was plotted with time as the abscissa and OD450 as the ordinate.
2.9. Immunoprecipitation
Immunoprecipitation was conducted with protein G agarose beads (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Cells after transfection were lysed and incubated in immunoprecipitation lysis buffer (Beyotime) for 10 min at room temperature. The extracts were incubated with Notch1-antibody (2 μg/ml, Abcam, Cambridge, UK) at 4 °C overnight, and the immunoprecipitate was purified by protein G agarose beads with gentle rocking. The beads were washed for three times with extraction buffer and resuspended in 20 μl SDS loading buffer. The whole cell lysates and immunoprecipitates were incubated at 70 °C for 10 min followed with western blot analysis.
2.10. O-fucose assay
The Click-iT® Protein Reaction Buffer Kit (Invitrogen, Grand Island, NY, USA) was used to assess the level of O-fucose. In breaf, 6-alkynyl fucose (6AF) (50 μM) was added in the cell culture medium for 48 h. Lysate 200 μl (1% SDS, 50 mM Tris-HCl, pH 8.0) was added to the cell culture dish and lysed on ice for –30 min to collect cells. Click reactions were performed as on both the labeled cell lysate to tag glycoproteins containing 6AF with biotinylated azide at room temperature for 1 h. The tagged cell lysate was analyzed by immunoblot.
2.11. Chromatin immunoprecipitation (ChIP)
The hESCs were treated with poFUT1 siRNA and poFUT1 cDNA. Thereafter, hESCs (1 × 107) were cross-linked, collected, lysed, then sheared by sonication until the average length of DNA was 500 bp as evaluated by agarose gel electrophoresis. One percent of the chromatin fragments were stored at −20 °C to be used later for non-precipitated total chromatin (input) for normalization. The rest of the chromatin fragments were equally divided, and incubated overnight with 4 μg activited Notch1 (NICD) antibody, or 4 μg anti-rabbit IgG, as a negative control for nonspecific immunoprecipitation. The chromatin-antibody complex was incubated with protein A beads for 4 h, then the beads were washed repeatedly. The beads were suspended in elution buffer, and the precipitated protein/DNA complexes were eluted from the antibodies/beads. The cross-linking was reversed, and then proteins were digested using proteinase K. Purified DNA served as the template for PCR using various primer sets to amplify specific regions of the IGFBP1 and PRL promoter. IGFBP1 (Forward) 5′- GCCTCAGTGTTCTCCTCTATAAAGC -3′, (Reverse) 5′- GCCTGCCCCTTGGTCT -3′; PRL (Forward) 5′- ATATAGATTGAAATGTCTAGCAGGT -3′, (Reverse) 5′- TTTGGCTGTCTTATTCAATGTCA -3′.
2.12. Animal experiments
Mice (Kunming White Crossing species; 7–8 weeks) were obtained from the Experimental Animal Center of Dalian Medical University (Liaoning, China). The mouse was maintained in controlled conditions (22–25 °C, 60% humidity, 14 L:10D). Pregnant mice were obtained by housing one female and one male mouse. The day found the vaginal plug was defined as gestation day 1. On day 1 of pregnancy at 09:00, a mouse was anesthetized and 10 μl normal saline containing 1 nmol poFUT1 siRNA was injected into the right uterus horn while the left with scramble RNA in 10 μl normal saline. Samples of mouse endometrium (n = 6) from day 4 and day 8 were collected. The number of implanted embryos was counted and analyzed statistically. The procedures of animal experiments were carried out in conformity with the guidance for the care and use of laboratory animals in Dalian Medical University.
2.13. Statistical analysis
The analysis was performed using GraphPad Prism 6 statistical software, and each experiment was repeated 3 times. The results were expressed as mean ± SEM, and one-way analysis of variance was used between the comparison groups. The difference of *p < .05, **p < .01 was considered as a significant difference, and ***p < .001 was extremely significant.
3. Results
3.1. poFUT1 is differentially expressed in the uterine endometrium from menstrual cycle, normal early pregnancy and miscarriage
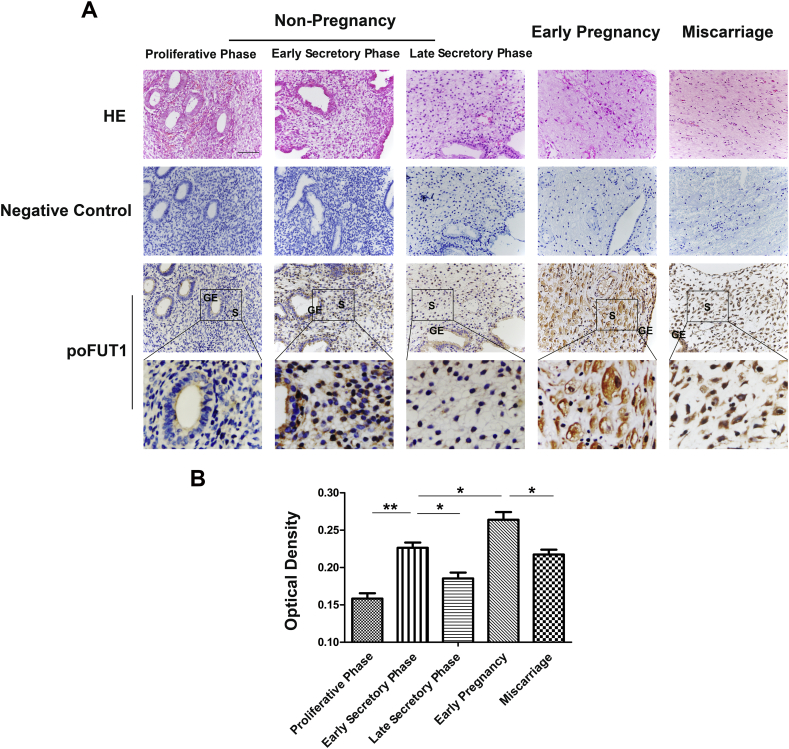
To address the dynamic alterations of poFUT1 in the uterine endometrium during different physiological and pathological states, we analyzed human endometrial biopsy samples by immunohistochemistry. The staining results revealed that the poFUT1 protein was highly expressed in endometrial stromal cells and glandular epithelium in the secretory phase of the menstrual cycle, but at lower level in the proliferative phase. Based on the fact that embryo implantation usually occurrs in the secretory phase, we also detected the expression of poFUT1 in stromal cells of early secretory phase and late secretory phase. The results showed that poFUT1 expression in early secretory phase was higher than that in late secretory phase (Fig. 1). Therefore, poFUT1 in stromal cells may play crucial for receiving an embryo. We further detected the poFUT1 expression in decidual cells of early pregnancy women and miscarriage patients. The immunohistochemistry staining showed that poFUT1 was widely expressed in the decidual cells. Compared with the cells from non-pregnant endometrium, early pregnancy decidual cells present with a rounded, epithelioid-like morphology and expressed more poFUT1. However, there was a lower level of poFUT1 in the decidual cells of miscarriage patients than in normal early pregnancies (Fig. 1).
Fig. 1.
poFUT1 is differentially expressed in the uterine endometrium from menstrual cycle, normal early pregnancies and miscarriage (A) Immunohistochemistry of poFUT1 in uterine endometrium. (B) Statistical analysis of poFUT1 expression. S: stroma; GE: gland epithelium. (bar: 50μm) *p<.05, **p<.01
3.2. poFUT1 is increased in human endometrial stromal cells after artificially induced decidualization
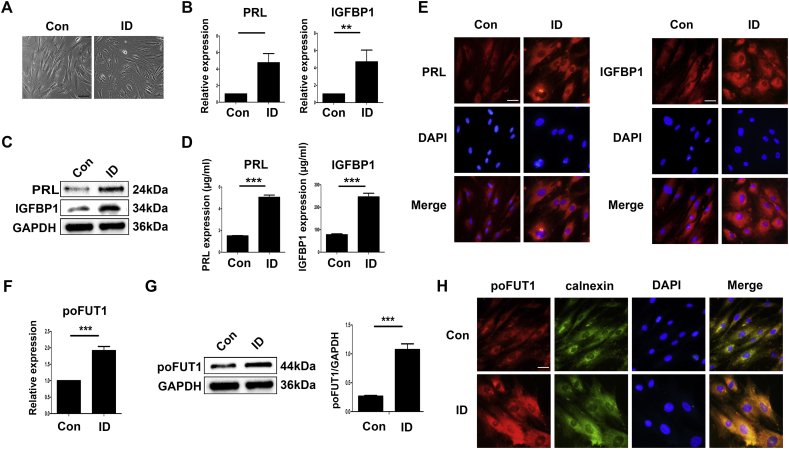
We continually detected the expression of poFUT1 in undecidualized hESCs and decidualized hESCs in vitro. The induction of decidualization (ID) was performedby EPCtreatment and achiceved by detection the decidual markers. As shown in Fig. 2, the dramatic morphological differentiation from elongate, fibroblast-like stromal mesenchymal cells into rounded, epithelioid-like hESCs was observed after decidualization (Fig. 2A). The induction of transcripts encoding prolactin (PRL) and insulin-like growth factor binding protein 1 (IGFBP1) (established decidual biomarkers), as evidenced by real-time PCR (Fig. 2B), western blot (Fig. 2C) and immunoflurescent staing (Fig. 2E), confirmed the decidualization of hESCs. We also detected PRL and IGFBP1 levels in the culture medium by ELISA. The data showed that the concentration of PRL and IGFBP1 was significantly increased after decidualization in vitro (Fig. 2D). The rapid induction of elevated transcription and expression levels of poFUT1 was observed by real-time PCR and western blot (Fig. 2F, G). Immunofluorescent staining also showed that poFUT1 increased during decidualization (Fig. 2H). Calnexin is an integral protein of the endoplasmic reticulum (ER), which as the ER marker. The process of protein O-fucosylation and poFUT1 has been showed to reside in the ER. The immunofluorescent staining showed that poFUT1 co-localized with calnexin in the ER apparatus (Fig. 2H).
Fig. 2.
poFUT1 is increased in human endometrial stromal cells (hESCs) after artificially induced decidualization. Decidualization induction of hESCs were performed with E2, MPA and dbcAMP (EPC) treatment. Morphological changes (A) and the relative transcript levels (B), proteins levels (C), and secreted levels (D) of PRL and IGFBP1 were determined by light microscopy, real-time PCR, western blot and ELISA, respectively. (E) Representative immunofluorescence staining of PRL and IGFBP1 in hESCs. The relative transcript levels (F) and proteins levels (G) of poFUT1 were determined by real-time PCR and western blot, respectively. (H) Representative immunofluorescence staining of poFUT1 in hESCs. (bar:50 μm).
3.3. poFUT1 promoteshESCs decidualization in vitro
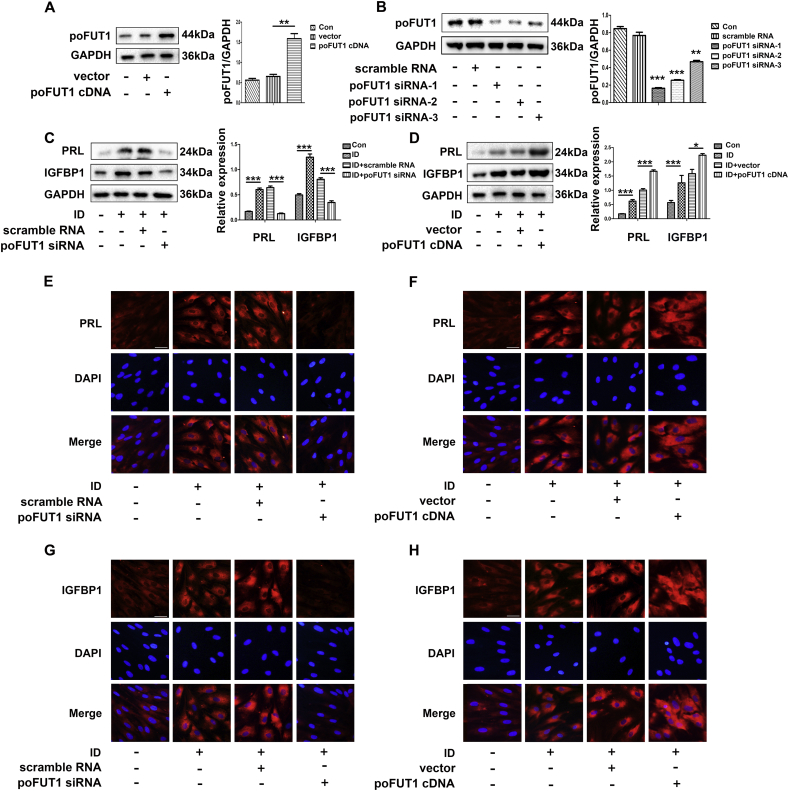
To determine whether poFUT1 is functionally required for hESC decidualizationin vitro, poFUT1 cDNA or poFUT1 siRNAs (-1 -2 -3) was transiently transfected into hESCs seprately prior to EPC induction. The western blot results showed that poFUT1 cDNA and poFUT1 siRNAs could significantly up- or down-regulate poFUT1 expression (Fig. 3A, B), respectively. Compared to those treated with scramble siRNA, hESCs treated with poFUT1 siRNA failed to undergo the typical cellular transformation in which mesenchymal cells took on epithelial morphology, the change that is an indicator of stromal decidualization following EPC treatment. Meanwhile, lower PRL and IGFBP1 levels were observed in hESCs in the poFUT1 siRNA transfection group (Fig. 3C, E, G). However, poFUT1 cDNA transfection enhanced the sensitivity of hESCs to EPC, and more PRL and IGFBP1 were detected (Fig. 3D, F, H). These results indicate that the inhibition of poFUT1 causes the disruption of hESCs decidualization; wherase increased poFUT1 can promote decidualization. The results suggest that poFUT1 is essential in the regulation of endometrial decidualization.
Fig. 3.
poFUT1 promotes hESCs decidualization in vitro. (A, B) Western blot analysis of poFUT1 expression in hESCs after vector, poFUT1 cDNA (A) or scramble RNA, or poFUT1 siRNA (B) transfection. (C, D) Western blot analysis of PRL and IGFBP1 levels in hESCs transfected with vector, poFUT1 cDNA (C) or scramble RNA, or poFUT1 siRNA (D). (E, F, G, H) Representative immnofluorescence staining of poFUT1 in hESCs transfected with vector, poFUT1 cDNA (F, H) or scramble RNA, or poFUT1 siRNA (E, G). (bar:50 μm).
3.4. poFUT1 increases hESCs proliferation capability
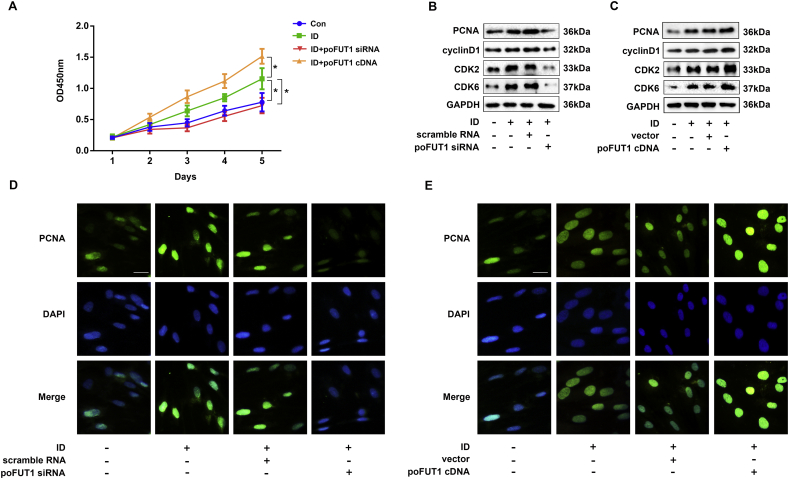
Since successful decidualization requires the coordinated progression of stromal cell proliferation and differentiation at the site of implantation, we then analyzed the effects on proliferation by poFUT1 in hESCs during decidualization (Fig 4). The CCK-8 results showed that hESCs proliferation ability increased in induced decidualization group compared with undecidualization group. The data also revealed poFUT1 cDNA transfection significantly enhanced the proliferation ability of hESCs; while poFUT1 siRNA transfection inhibited cell proliferation (Fig. 4A). Western blot analysis suggested that the expression of cell cyclin proteins (cyclin D1, CDK2, and CDK6) and PCNA increased after decidualization. Accordingly, poFUT1 cDNA transfection increased cell cyclin protein and PCNA expression; while poFUT1 siRNA transfection decreased the expression of these proteins (Fig. 4B, C). The results were further confirmed by immunofluorescent staining, PCNA located in the nucleus, and poFUT1 cDNA transfection increased the PCNA staining intensity while a significant reduction in PCNA staining intensity was observed in the poFUT1 siRNA transfection group (Fig. 4D, E).
Fig. 4.
poFUT1 increases hESCs proliferation ability. hESCs were transfected with vector, poFUT1 cDNA or scramble RNA, or poFUT1 siRNA. (A) CCK-8 assay analysis was used to evaluate the cell proliferation capabilities of hESCs. (B, C) Western blot analysis of the expression of PCNA, cyclin D1, CDK2, CDK6 and GAPDH in hESCs. (D, E) Representative immnofluorescent staining of PCNA in hESCs. (bar:50 μm).
3.5. poFUT1 increases the O-fucosylation of Notch, further promoting hESCs decidualization
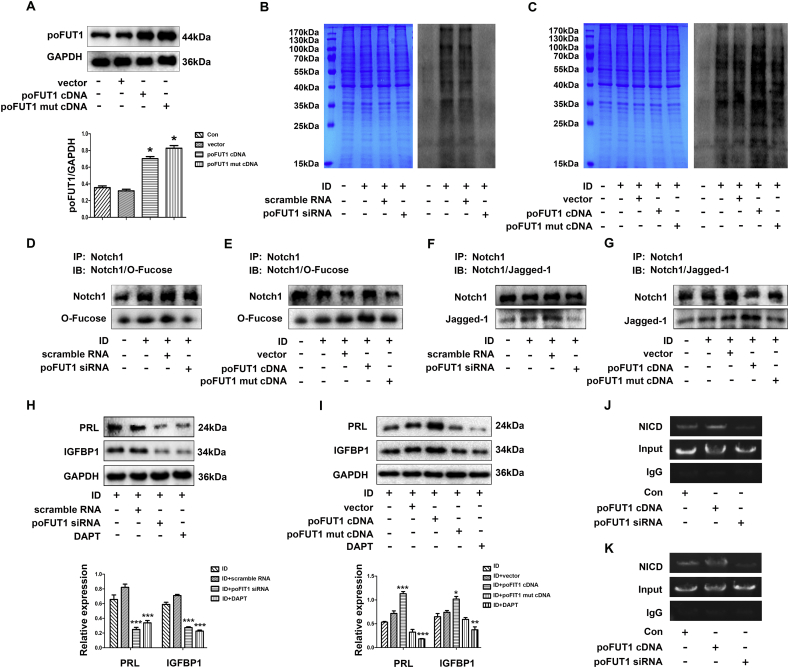
poFUT1 is mainly responsible for adding the O-fucose residue to specific glycoproteins through O-linkage. The Notch protein contains EGF-like repeats, which contain the specific target glycosylation sites of poFUT1. We hypothesised thatpoFUT1 could regulate the O-fucosylation on Notch1in hESCs. The recombinant poFUT1 mutant plasmid (poFUT1 MUT cDNA) were constructed, and then both poFUT1 mutation plasmids and the poFUT1 cDNA plasmids were transfected into hESCs, respectively. The western blot results showed that both transfection of poFUT1 cDNA and poFUT1 mutation plasmid increased poFUT1 protein expression (Fig. 5A). In addition, O-fucosylated glycans on glycoproteins were detected. The data showed that poFUT1 siRNA decreased O-fucose biosynthesis; whereas poFUT1 cDNA increased O-fucose biosynthesis, respectively (Fig. 5B, C). Although the poFUT1 mutation plasmid increased the poFUT1 protein level, O-fucose biosynthesis was not influenced (Fig. 5C). We further detected alterations in O-fucosylation on Notch1. Fig. 5D and E showed that the down-regulation of poFUT1 by specific siRNA transfection inhibited O-fucosylation on Notch1, whereas the upregulation of poFUT1 by poFUT1 cDNA enhanced O-fucosylation on Notch1, and the poFUT1 mutation decreased the O-fucosylation on Notch1 compared to the poFUT1 cDNA transfection.
Fig. 5.
poFUT1 increases the O-fucosylation of Notch1, further promoting hESCs decidualization. (A) Western blot analysis of poFUT1 levels in hESCs transfected with vector, poFUT1 cDNA or poFUT1 mut cDNA (mutant poFUT1 cDNA). (B, C) Western blot analysis of O-fucosylation in hESCs transfected with scramble, poFUT1 siRNA, or vector, poFUT1 cDNA or poFUT1 mut cDNA, alone or combined with ID. (ID: hESCs treated with E2, MPA and dbcAMP), CBB: Coomassie brilliant blue. (D, E) Immunoprecipitation and western blot analysis of O-fucosylation on Notch1 in hESCs transfected with scramble, poFUT1 siRNA, or vector, poFUT1 cDNA or poFUT1 mut cDNA, alone or combined with ID. Immunoprecipitation (IP): protein pulled down with an anti-Notch1 antibody. Immune blot (IB): detection of O-fucosylation by an O-fucose assay. (F, G) IP and western blot analysis of Notch1 and Jagged-1 in hESCs transfected with scramble, poFUT1 siRNA, or vector, poFUT1 cDNA or poFUT1 mut cDNA. Alone or combined with ID. IP: protein pulled down with an anti-Notch1 antibody. IB: detection of Jagged-1 by antibody. (H, I) Western blot analysis of PRL and IGFBP1 in hESCs transfected with scramble, poFUT1 siRNA, or vector, poFUT1 cDNA or poFUT1 mut cDNA combined with ID. (J, K) CHIP was performed in hESCs to confirm the interaction of NICD with the PRL (F) and (G) promoter. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Next, we asked whether poFUT1 knockdown could attenuate Notch1 signaling pathway activation and the downstream target protein expression. Herein, using the Notch1 antibody, we immunoprecipitated Notch1 and detected its binding with Jagged-1 ligand. The data demonstrated that Notch1 with low level of O-fucose combined less Jagged-1; whereas increased O-fucosylation on Notch1 promoted the receptor-ligand binding (Fig. 5F, G). Based on the above results, we propose that O-fucosylation on Notch1 determines the activation of the Notch1 signaling pathway.
Then, we want to know how O-fucosylation and Notch 1 influence hESCs decidualization. The western blot results showed that silencing poFUT1 by the specific siRNA or overexpressing poFUT1 by poFUT1 cDNA transfection could inhibit or increase PRL and IGFBP1 expression through Notch 1 signaling pathway. Meantime, DAPT (Notch1 signaling pathway inhibitor) significantly down-regulated PRL and IGFBP1 expression (Fig. 5H, I). To explore the mechanism by which hESCs decidualization is correlated with the O-fucosylation and activation of Notch1, we identified NICD, the active the Notch1, as the specific transcription factor that binds to both PRL and IGFBP1 promoter regions in hESCs by CHIP analysis. Chromosomal DNA from hESCs transfected with poFUT1 siRNA or poFUT1 cDNA was immunoprecipitated by with NICD antibody, and subjected to PCR using primers for the PRL and IGFBP1 promoter regions harboring the NICD binding sites (Fig. 5J, K). The results demonstrate that the PRL and IGFBP1 promoters have specific binding sites for NICD. The data manifest that poFUT1 controls the O-fucosylation on Notch1, thus regulating the activation of Notch1, as well as the downstream expression of decidual markers of human endometrial stromal cells.
3.6. poFUT1 controls decidualization in vivo
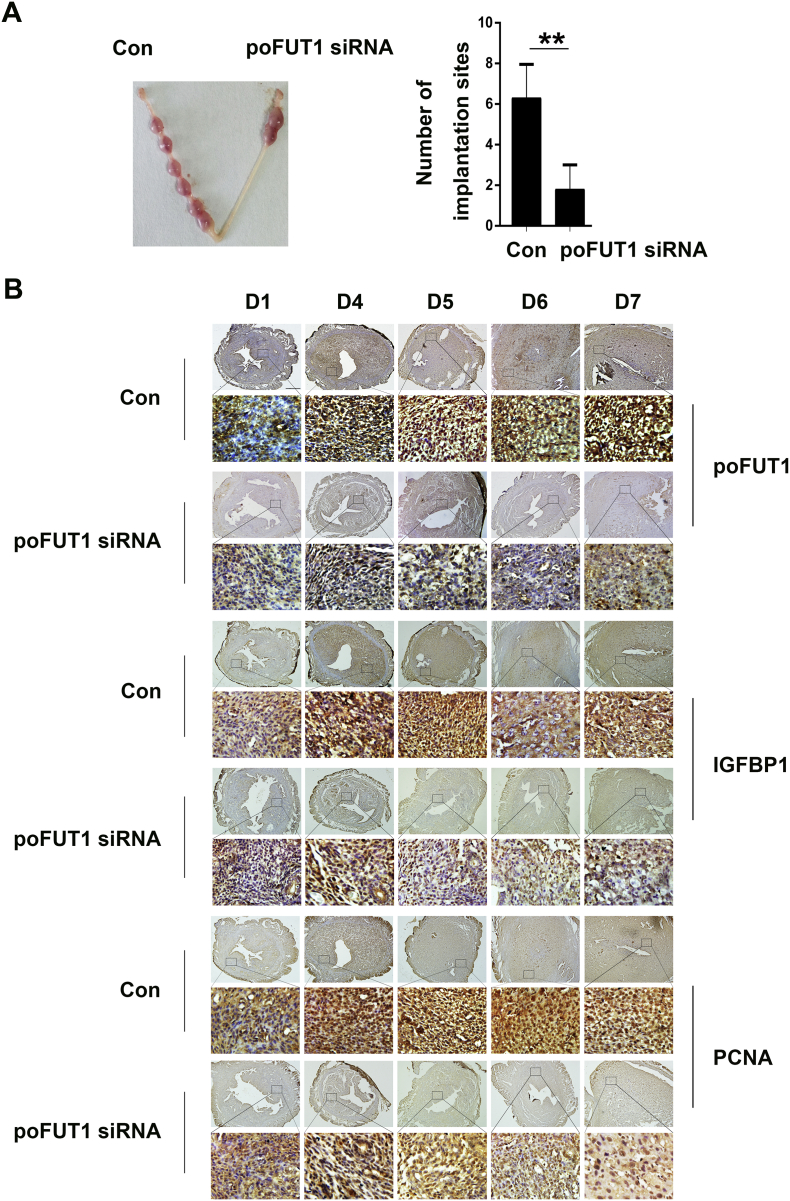
Having testified that poFUT1 siRNA could hamper hESCs decidualization by inhibiting the O-fucosylation and activation of Notch1 in vitro, we further investigated whether poFUT1 impeded decidualization and embryo implantation in vivo. A mouse model was used to observe the role of poFUT1 in endometrial decidualization. We administered poFUT1 siRNA or scramble RNA was administered to the mouse uterus on Day 1 of pregnancy as described in the Materials and methods. The decidualization was detected from Day 1 to Day 7, and the number of implantation sites were counted on Day 8. As shown in Fig. 6A, poFUT1 siRNA significantly inhibited the embryo implantation efficiency in the mice. In the poFUT1 siRNA administration group, lower levels of poFUT1 and IGFBP1 and PCNA were observed in the mouse uterine endometrium by immunohistochemistry (Fig. 6C). To confirm the results, the negative control was used (Suppl Fig 1). The results suggest that decidualization arrest was the cause of embryo implantation failure in the mice. Together, our cell and mouse studies support the proposal that poFUT1 siRNA inhibits O-fucosylation on Notch1, further decreasing NICD activity and hampering hESC decidualization.
Fig. 6.
poFUT1 siRNA inhibits decidualization in vivo. The murine uterus was injected with normal saline (NS) or poFUT1 siRNA on Day 3 of pregnancy. (A) The number of implanted embryos in the uterus on Day 8 of pregnancy. (B) Immunohistochemical analysis of poFUT1, IGFBP1 and PCNA in the uterine endometrium of pregnant mice. (bar: 200 μm).
4. Discussion
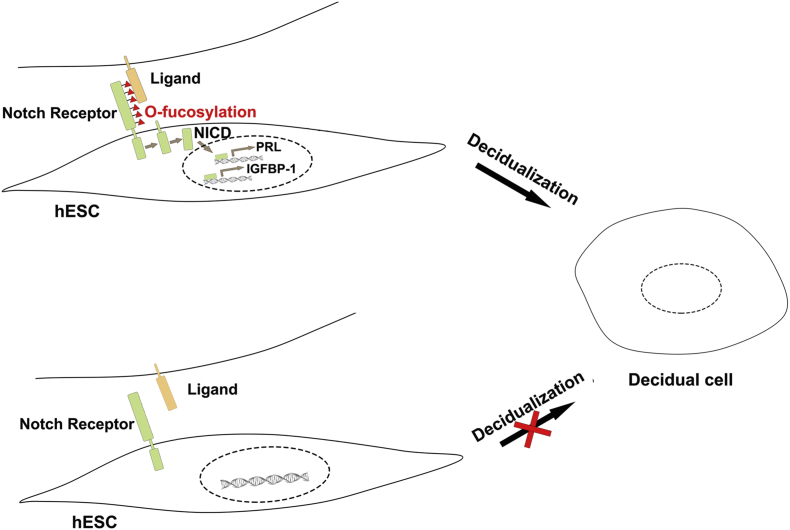
Successful pregnancy requires multiple competant events, including decidualization, implantation, and placentation, etc. The growth and differentiation of hESCs is the key step of decidualization. The decidualized cells faciliate embryo implantation anddevelopment prior to placenta formation [2,39]. With respect to the molecular basis governing this dynamic endometrial transformation, we demonstrated, herein, that the level of poFUT1 was significantly increased during the secretory phase of the endometrium compared with the proliferative phase of the endometrium, and the higher expression of poFUT1 in uterine endometrium of early secretory phase than late secretory phase in human. Meanwhile, a lower level of poFUT1 in the endometrium of miscarriage patients than in the endometrium of women with normal pregnancies was detected (Fig 1). Using hESCs and a mouse model, we also provided the evidence that poFUT1 controlled O-fucosylation on Notch1 is required for hESCs proliferation and decidualization, whereas silencing poFUT1 expression inhibits the O-fucosylation on Notch1 and inactivates Notch1 signaling pathway, thus hampering decidualizationand impairing embryo implantation (Fig. 7).
Fig. 7.
Model of poFUT1 and O-fucosylation effects in hESC decidualization of normal pregnancy and miscarriage.
Decidualization occurs in women during the secretory phase of the menstrual cycle, as well as during pregnancy. The successful decidualization of the endometrium is critical in protecting the embryo from maternal immunological rejection, and participates in the exchanges of nutrition, gas, and waste during gestation [46]. Many factors regulates human stromal cell decidualization. For example, Liao et al. found that NEDD8-mediated neddylation was required for human stromal proliferation and decidualization [25]. Kommagani et al. revealed that the promyelocytic leukemia zinc finger transcription factor is critical for hESCs decidualization [21]. In the current study, we found by immunohistochemisry that poFUT1 level washigher in human endometrial tissues in the secretory phase than in the proliferative phase, and the highest level was observed during pregnancy decidualization (Fig. 1). The defects in the progression of decidualization are a major cause of implantation failure. Studies have revealed that the deletion of BMP2 results in female infertility due to failed decidualization of the endometrial stroma [23]. BMPR1 and ALK2 deletions also result in female infertility due to impaired endometrial stromal cell decidualization [38]. Our results indicate that the staining of poFUT1 in decidual cells of miscarriage is weaker than those in normal pregnancy (Fig. 1). The data suggest that poFUT1 is associated with endometrial stromal cell decidualization, and can be used as a potential biomarker for miscarriage diagnosis.
The dynamic changes of specific fucosyltransferases are closely correlated with reproduction function. Accumulated evidence shows that variations in the fucosyltransferase gene expression levels play an important role in determining the implantation at the fetal–maternal interface [49,50]. Downregulation of FUT4 by miR-200c impairs uterine receptivity formation [52]. FUT8 knockout mice exhibit fetal growth restriction [24]. Progesterone and estrogen are the main hormones which control menstrual cycle and uterine endometrium receptivity,and both can influence FUTs expression. White and Kimber reported that progesterone strongly inhibited alpha-1,2-FUT activity [43,48].
The embryo implantation and endometrial decidualization may be precisely regulated by specific molecules or crosstalk between trophoblast cells and stromal cells. We previously revealed that poFUT1 was expressed in the invasive trophoblast cells, and decreased poFUT1 expression hampered trophoblast cells to invade into the endometrium [26]. Progesterone promotes embryo adhesion to the endometrium by upregulating poFUT1 expression. In the current study, we further found that poFUT1 was expressed differentially in the human endometrium of physiological and pathological states. We proposed that poFUT1 not only regulate trophoblast implantation, but also is involved in stromal cell decidualization at fetal-maternal interface. We showed that poFUT1 is required for endometrial stromal proliferation and decidualization, and alterations in its expression directly impair embryo implantation. In the current study, silencing poFUT1 inhibited hESC proliferation and decidualization, as evidenced by western blot, immunofluorescence and CCK-8 assays in vitro (Fig. 3). Additionally, the administration of poFUT1 siRNA to the mouse uterus inhibited the endometrial decidualization, further leading to embryo implantation failure (Fig. 6). The above data demonstrate that poFUT1 regulates the process of endometrial decidualization. Based on the roles of poFUT1 in endometrial decidualization and embryo implantation, poFUT1 play critical roles during embryo implantation at the fetal-maternal interface.
Alterations in the fucosylation of glycoproteins are associated with cellular functions, such as development, reproduction, and cancer metastasis. FUT9 is responsible for the generation of Lewis X (LeX),which contains α-1,3-fucose epitope in the mouse brain, and is involved in neural development [29]. The prevention of terminal α-1,3-fucosylation by knocking down FUT4 inhibits tumor growth [51]. In the current study, we found that poFUT1 increased O-fucosylation and facilitating decidualization in hESCs (Fig. 5). Fucosylation usually mediates glycoprotein folding and affects its functions, including molecule recognition, cell adhesion and signal transduction. Notch1 receptor contains multiple EGF-like repeats which are modified by O-fucosylation. Notch1 recognizes and binds the ligands (Jagged-1). Atfter binding the active Notch1 (NICD) is released into the cytoplasm [22]. Recent co-crystal structures have demonstrated that O-fucosylation on Notch1 (EGF8–12) participates in ligand Jagged-1 -binding [27]. Here, we further detected the role of poFUT1 in Notch1 signaling pathway. As shown in the results, poFUT1 siRNA inhibited O-fucosylation on Notch1, then weakened the binding of Jagged-1 to Notch1. In contrast, poFUT1 cDNA promoted O-fucosylation on Notch1 and strengthened Jagged-1 binding (Fig. 5). The results suggest that poUFT1 regulate O-fucosylation of Notch 1 in endometrial decidualization.
The Notch1 signaling pathway controls numerous biological processes, such as stem cell maintenance, cell lineage determination, and differentiation. Farnie et al. showed that breast cancer cells with a surface molecule of CD44+CD24− had stem cell characteristics and also highly expressed Notch [11]. Galectin-3 could maintain the stem cell characteristics of ovarian cancer stem cells by activating Notch1 [20]. According to reports in the literature, the activation of Notch signaling pathway is conducive to the maintenance of neural stem cell dryness, while inhibition of Notch signaling pathway promotes the neural differentiation of neural stem cells [14,18]. Here, we aimed to clarify how Notch1 mediated hESCs differentiation into the decidual cells. NICD usually translocates to the nucleus, and functions as a coactivator of the transcription factor of the target genes in placental development, such as HES1, HES5 and HEY1, etc. [15]. We explored whether NICD could regulate decidualization-associated gene expression. Using TFbind, INPUT and JASPAR softwares, we predicted that PRL and IGFBP1 promoter regions had NICD binding sites. By ChIP analysis, we identified the corresponding binding sites, and poFUT1 siRNA inhibited O-fucosylation on Notch1 and decreased the release of NICD, further lessening the transcriptional activity of PRL and IGFBP1 and hampering decidualization (Fig. 5).
In summary, we revealed that poFUT1 was increased in the secretory phase compared to the proliferative phase. Meanwhile, poFUT1 was expressed at lower levels in the uterine endometrium of miscarriage patients than that in normal early pregnancies. Moreover, we provides novel evidence showing that poFUT1 controls the O-fucosylation and activation ofNotch1. The inactive Notch 1 by downregulation of poFUT1 decreased PRL and IGFBP1 promoter activity, impaired decidualization and causes embryo implantation failure. poFUT1 can serves as a new biomarker for clinical diagnosis and as a target for the treatment of miscarriage patients.
The following are the supplementary data related to this article.
Supplementary Fig. 1.
Negative controls and HE staining for immunohistochemistry in mouse uterine.
Supplementary Table 1. Antibody information
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No: 31770857, 31670810 and 31870794) and Liaoning Provincial Program for Top Discipline of Basic Medical Sciences. We thank Professor Haibin Wang for providing human endometrial stromal cell.
Declaration of interests
The authors declare that they have no competing interests.
Author contributions
Shuai Liu and Qiu Yan designed the experiments. Yu Yang and Dandan Zhang performed the experiments. Yu Yang performed statistical analysis and wrote the manuscript. Huamin Qin collected the human endometrial tissues. All authors reviewed the manuscript.
Contributor Information
Shuai Liu, Email: liushuai_129@163.com.
Qiu Yan, Email: yanqiu63@126.com.
References
- 1.Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]; Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake, Notch signaling: cell fate control and signal integration in development. Science, 1999. 284(5415): p. 770-6. [DOI] [PubMed]
- 2.Brosens J.J. Uterine selection of human embryos at implantation. Sci Rep. 2014;4:3894. doi: 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brosens, J.J., et al., Uterine selection of human embryos at implantation. Sci Rep, 2014. 4: p. 3894. [DOI] [PMC free article] [PubMed]
- 3.Clark D.A. Cytokines, decidua, and early pregnancy. Oxf Rev Reprod Biol. 1993;15:83–111. [PubMed] [Google Scholar]; Clark, D.A., Cytokines, decidua, and early pregnancy. Oxf Rev Reprod Biol, 1993. 15: p. 83-111. [PubMed]
- 4.Clerc F. Human plasma protein N-glycosylation. Glycoconj J. 2016;33(3):309–343. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clerc, F., et al., Human plasma protein N-glycosylation. Glycoconj J, 2016. 33(3): p. 309-43. [DOI] [PMC free article] [PubMed]
- 5.Corbeil L.B. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell. 1985;17(1):53–68. doi: 10.1016/0040-8166(85)90015-1. [DOI] [PubMed] [Google Scholar]; Corbeil, L.B., et al., Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell, 1985. 17(1): p. 53-68. [DOI] [PubMed]
- 6.Damjanov I. Decidua and implantation of the embryo from a historical perspective. Int J Dev Biol. 2014;58(2–4):75–78. doi: 10.1387/ijdb.140075id. [DOI] [PubMed] [Google Scholar]; Damjanov, I., Decidua and implantation of the embryo from a historical perspective. Int J Dev Biol, 2014. 58(2-4): p. 75-8. [DOI] [PubMed]
- 7.De Clercq K., Hennes A., Vriens J. Isolation of mouse endometrial epithelial and stromal cells for in vitro decidualization. J Vis Exp. 2017;(121) doi: 10.3791/55168. [DOI] [PMC free article] [PubMed] [Google Scholar]; De Clercq, K., A. Hennes, and J. Vriens, Isolation of Mouse Endometrial Epithelial and Stromal Cells for In Vitro Decidualization. J Vis Exp, 2017(121). [DOI] [PMC free article] [PubMed]
- 8.Ding N.Z. De novo synthesis of sphingolipids is essential for decidualization in mice. Theriogenology. 2018;106:227–236. doi: 10.1016/j.theriogenology.2017.09.036. [DOI] [PubMed] [Google Scholar]; Ding, N.Z., et al., De novo synthesis of sphingolipids is essential for decidualization in mice. Theriogenology, 2018. 106: p. 227-236. [DOI] [PubMed]
- 9.Du J. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev Biol. 2010;346(1):25–38. doi: 10.1016/j.ydbio.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Du, J., et al., O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev Biol, 2010. 346(1): p. 25-38. [DOI] [PMC free article] [PubMed]
- 10.Eyal O. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod. 2007;76(5):777–783. doi: 10.1095/biolreprod.106.053058. [DOI] [PubMed] [Google Scholar]; Eyal, O., et al., Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod, 2007. 76(5): p. 777-83. [DOI] [PubMed]
- 11.Farnie G., Clarke R.B. Mammary stem cells and breast cancer--role of notch signalling. Stem Cell Rev. 2007;3(2):169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]; Farnie, G. and R.B. Clarke, Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev, 2007. 3(2): p. 169-75. [DOI] [PubMed]
- 12.Fazleabas A.T., Kim J.J., Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment--a review. Placenta. 2004;25(Suppl A):S26–S31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]; Fazleabas, A.T., J.J. Kim, and Z. Strakova, Implantation: embryonic signals and the modulation of the uterine environment--a review. Placenta, 2004. 25 Suppl A: p. S26-31. [DOI] [PubMed]
- 13.Gellersen B., Brosens J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]; Gellersen, B. and J.J. Brosens, Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev, 2014. 35(6): p. 851-905. [DOI] [PubMed]
- 14.Geng X. Electroacupuncture in the repair of spinal cord injury: inhibiting the notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res. 2015;10(3):394–403. doi: 10.4103/1673-5374.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]; Geng, X., et al., Electroacupuncture in the repair of spinal cord injury: inhibiting the Notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res, 2015. 10(3): p. 394-403. [DOI] [PMC free article] [PubMed]
- 15.Haider S., Pollheimer J., Knofler M. Notch signalling in placental development and gestational diseases. Placenta. 2017;56:65–72. doi: 10.1016/j.placenta.2017.01.117. [DOI] [PubMed] [Google Scholar]; Haider, S., J. Pollheimer, and M. Knofler, Notch signalling in placental development and gestational diseases. Placenta, 2017. 56: p. 65-72. [DOI] [PubMed]
- 16.Haider S. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc Natl Acad Sci U S A. 2016;113(48):E7710–e7719. doi: 10.1073/pnas.1612335113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Haider, S., et al., Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc Natl Acad Sci U S A, 2016. 113(48): p. E7710-e7719. [DOI] [PMC free article] [PubMed]
- 17.Holdener B.C., Haltiwanger R.S. Protein O-fucosylation: structure and function. Curr Opin Struct Biol. 2019;56:78–86. doi: 10.1016/j.sbi.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Holdener, B.C. and R.S. Haltiwanger, Protein O-fucosylation: structure and function. Curr Opin Struct Biol, 2019. 56: p. 78-86. [DOI] [PMC free article] [PubMed]
- 18.Kageyama R. Dynamic notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11(11):1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]; Kageyama, R., et al., Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci, 2008. 11(11): p. 1247-51. [DOI] [PubMed]
- 19.Kakuda S., Haltiwanger R.S. Deciphering the fringe-mediated notch code: identification of activating and inhibiting sites allowing discrimination between ligands. Dev Cell. 2017;40(2):193–201. doi: 10.1016/j.devcel.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kakuda, S. and R.S. Haltiwanger, Deciphering the Fringe-Mediated Notch Code: Identification of Activating and Inhibiting Sites Allowing Discrimination between Ligands. Dev Cell, 2017. 40(2): p. 193-201. [DOI] [PMC free article] [PubMed]
- 20.Kang H.G. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget. 2016;7(42):68229–68241. doi: 10.18632/oncotarget.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kang, H.G., et al., Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget, 2016. 7(42): p. 68229-68241. [DOI] [PMC free article] [PubMed]
- 21.Kommagani R. The promyelocytic leukemia zinc finger transcription factor is critical for human endometrial stromal cell Decidualization. PLoS Genet. 2016;12(4) doi: 10.1371/journal.pgen.1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kommagani, R., et al., The Promyelocytic Leukemia Zinc Finger Transcription Factor Is Critical for Human Endometrial Stromal Cell Decidualization. PLoS Genet, 2016. 12(4): p. e1005937. [DOI] [PMC free article] [PubMed]
- 22.Kopan R., Ilagan M.X. The canonical notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kopan, R. and M.X. Ilagan, The canonical Notch signaling pathway: unfolding the activation mechanism. Cell, 2009. 137(2): p. 216-33. [DOI] [PMC free article] [PubMed]
- 23.Lee K.Y. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27(15):5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, K.Y., et al., Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol, 2007. 27(15): p. 5468-78. [DOI] [PMC free article] [PubMed]
- 24.Li W. Reduced alpha4beta1 integrin/VCAM-1 interactions lead to impaired pre-B cell repopulation in alpha 1,6-fucosyltransferase deficient mice. Glycobiology. 2008;18(1):114–124. doi: 10.1093/glycob/cwm107. [DOI] [PubMed] [Google Scholar]; Li, W., et al., Reduced alpha4beta1 integrin/VCAM-1 interactions lead to impaired pre-B cell repopulation in alpha 1,6-fucosyltransferase deficient mice. Glycobiology, 2008. 18(1): p. 114-24. [DOI] [PubMed]
- 25.Liao Y. NEDD8-mediated neddylation is required for human endometrial stromal proliferation and decidualization. Hum Reprod. 2015;30(7):1665–1676. doi: 10.1093/humrep/dev117. [DOI] [PubMed] [Google Scholar]; Liao, Y., et al., NEDD8-mediated neddylation is required for human endometrial stromal proliferation and decidualization. Hum Reprod, 2015. 30(7): p. 1665-76. [DOI] [PubMed]
- 26.Liu S. LIF upregulates poFUT1 expression and promotes trophoblast cell migration and invasion at the fetal-maternal interface. Cell Death Dis. 2014;5:e1396. doi: 10.1038/cddis.2014.335. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, S., et al., LIF upregulates poFUT1 expression and promotes trophoblast cell migration and invasion at the fetal-maternal interface. Cell Death Dis, 2014. 5: p. e1396. [DOI] [PMC free article] [PubMed]
- 27.Luca V.C., Kim B.C., Ge C. Notch-jagged complex structure implicates a catch bond in tuning ligand sensitivity. 2017;vol. 355(6331):1320–1324. doi: 10.1126/science.aaf9739. [DOI] [PMC free article] [PubMed] [Google Scholar]; Luca, V.C., B.C. Kim, and C. Ge, Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. 2017. 355(6331): p. 1320-1324. [DOI] [PMC free article] [PubMed]
- 28.Mascarenhas M.N. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12) doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mascarenhas, M.N., et al., National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med, 2012. 9(12): p. e1001356. [DOI] [PMC free article] [PubMed]
- 29.Ohata S. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 2009;136(10):1653–1663. doi: 10.1242/dev.033290. [DOI] [PubMed] [Google Scholar]; Ohata, S., et al., Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development, 2009. 136(10): p. 1653-63. [DOI] [PubMed]
- 30.Okajima T. Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science. 2005;307(5715):1599–1603. doi: 10.1126/science.1108995. [DOI] [PubMed] [Google Scholar]; Okajima, T., et al., Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science, 2005. 307(5715): p. 1599-603. [DOI] [PubMed]
- 31.Okamura Y., Saga Y. Pofut1 is required for the proper localization of the notch receptor during mouse development. Mech Dev. 2008;125(8):663–673. doi: 10.1016/j.mod.2008.04.007. [DOI] [PubMed] [Google Scholar]; Okamura, Y. and Y. Saga, Pofut1 is required for the proper localization of the Notch receptor during mouse development. Mech Dev, 2008. 125(8): p. 663-73. [DOI] [PubMed]
- 32.Oliveira-Ferrer L., Legler K., Milde-Langosch K. Role of protein glycosylation in cancer metastasis. Semin Cancer Biol. 2017;44:141–152. doi: 10.1016/j.semcancer.2017.03.002. [DOI] [PubMed] [Google Scholar]; Oliveira-Ferrer, L., K. Legler, and K. Milde-Langosch, Role of protein glycosylation in cancer metastasis. Semin Cancer Biol, 2017. 44: p. 141-152. [DOI] [PubMed]
- 33.Pennarubia F. In vitro acellular method to reveal O-fucosylation on EGF-like domains. Glycobiology. 2018 Nov 29 doi: 10.1093/glycob/cwy106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]; Pennarubia, F., et al., In vitro acellular method to reveal O-fucosylation on EGF-like domains. Glycobiology, 2018. [DOI] [PubMed]
- 34.Ponnampalam A.P., Rogers P.A. Expression and regulation of fucosyltransferase 4 in human endometrium. Reproduction. 2008;136(1):117–123. doi: 10.1530/REP-07-0548. [DOI] [PubMed] [Google Scholar]; Ponnampalam, A.P. and P.A. Rogers, Expression and regulation of fucosyltransferase 4 in human endometrium. Reproduction, 2008. 136(1): p. 117-23. [DOI] [PubMed]
- 35.Ramathal C.Y. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ramathal, C.Y., et al., Endometrial decidualization: of mice and men. Semin Reprod Med, 2010. 28(1): p. 17-26. [DOI] [PMC free article] [PubMed]
- 36.Rampal R. Highly conserved O-fucose sites have distinct effects on Notch1 function. J Biol Chem. 2005;280(37):32133–32140. doi: 10.1074/jbc.M506104200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rampal, R., et al., Highly conserved O-fucose sites have distinct effects on Notch1 function. J Biol Chem, 2005. 280(37): p. 32133-40. [DOI] [PMC free article] [PubMed]
- 37.Reily C. Glycosylation in health and disease. Nat Rev Nephrol. 2019 Mar 11 doi: 10.1038/s41581-019-0129-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; Reily, C., et al., Glycosylation in health and disease. Nature Reviews Nephrology, 2019. [DOI] [PMC free article] [PubMed]
- 38.Rigueur D. The type I BMP receptor ACVR1/ALK2 is required for chondrogenesis during development. J Bone Miner Res. 2015;30(4):733–741. doi: 10.1002/jbmr.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rigueur, D., et al., The type I BMP receptor ACVR1/ALK2 is required for chondrogenesis during development. J Bone Miner Res, 2015. 30(4): p. 733-41. [DOI] [PMC free article] [PubMed]
- 39.Sahu M.B. Decidual cells from women with preeclampsia exhibit inadequate decidualization and reduced sFlt1 suppression. Pregnancy Hypertens. 2019;15:64–71. doi: 10.1016/j.preghy.2018.11.003. [DOI] [PubMed] [Google Scholar]; Sahu, M.B., et al., Decidual cells from women with preeclampsia exhibit inadequate decidualization and reduced sFlt1 suppression. Pregnancy Hypertens, 2019. 15: p. 64-71. [DOI] [PubMed]
- 40.Salker M. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]; Salker, M., et al., Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One, 2010. 5(4): p. e10287. [DOI] [PMC free article] [PubMed]
- 41.Schneider M., Al-Shareffi E., Haltiwanger R.S. Biological functions of fucose in mammals. Glycobiology. 2017;27(7):601–618. doi: 10.1093/glycob/cwx034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schneider, M., E. Al-Shareffi, and R.S. Haltiwanger, Biological functions of fucose in mammals. Glycobiology, 2017. 27(7): p. 601-618. [DOI] [PMC free article] [PubMed]
- 42.Shi S., Stanley P. Protein O-fucosyltransferase 1 is an essential component of notch signaling pathways. Proc Natl Acad Sci U S A. 2003;100(9):5234–5239. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shi, S. and P. Stanley, Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A, 2003. 100(9): p. 5234-9. [DOI] [PMC free article] [PubMed]
- 43.Sidhu S.S., Kimber S.J. Hormonal control of H-type alpha(1-2)fucosyltransferase messenger ribonucleic acid in the mouse uterus. Biol Reprod. 1999;60(1):147–157. doi: 10.1095/biolreprod60.1.147. [DOI] [PubMed] [Google Scholar]; Sidhu, S.S. and S.J. Kimber, Hormonal control of H-type alpha(1-2)fucosyltransferase messenger ribonucleic acid in the mouse uterus. Biol Reprod, 1999. 60(1): p. 147-57. [DOI] [PubMed]
- 44.Spiro R.G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43r–56r. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]; Spiro, R.G., Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology, 2002. 12(4): p. 43r-56r. [DOI] [PubMed]
- 45.Stahl M. Roles of Pofut1 and O-fucose in mammalian notch signaling. J Biol Chem. 2008;283(20):13638–13651. doi: 10.1074/jbc.M802027200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stahl, M., et al., Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem, 2008. 283(20): p. 13638-51. [DOI] [PMC free article] [PubMed]
- 46.Tong J. Transcriptomic profiling in human decidua of severe preeclampsia detected by RNA sequencing. 2018;119(1):607–615. doi: 10.1002/jcb.26221. [DOI] [PubMed] [Google Scholar]; Tong, J., et al., Transcriptomic Profiling in Human Decidua of Severe Preeclampsia Detected by RNA Sequencing. 2018. 119(1): p. 607-615. [DOI] [PubMed]
- 47.Wang X. Phenotype changes of Fut8 knockout mouse: core fucosylation is crucial for the function of growth factor receptor(s) Methods Enzymol. 2006;417:11–22. doi: 10.1016/S0076-6879(06)17002-0. [DOI] [PubMed] [Google Scholar]; Wang, X., et al., Phenotype changes of Fut8 knockout mouse: core fucosylation is crucial for the function of growth factor receptor(s). Methods Enzymol, 2006. 417: p. 11-22. [DOI] [PubMed]
- 48.White S., Kimber S.J. Changes in alpha (1-2)-fucosyltransferase activity in the murine endometrial epithelium during the estrous cycle, early pregnancy, and after ovariectomy and hormone replacement. Biol Reprod. 1994;50(1):73–81. doi: 10.1095/biolreprod50.1.73. [DOI] [PubMed] [Google Scholar]; White, S. and S.J. Kimber, Changes in alpha (1-2)-fucosyltransferase activity in the murine endometrial epithelium during the estrous cycle, early pregnancy, and after ovariectomy and hormone replacement. Biol Reprod, 1994. 50(1): p. 73-81. [DOI] [PubMed]
- 49.Yu M. Novel function of pregnancy-associated plasma protein a: promotes endometrium receptivity by up-regulating N-fucosylation. Sci Rep. 2017;7(1):5315. doi: 10.1038/s41598-017-04735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yu, M., et al., Novel function of pregnancy-associated plasma protein A: promotes endometrium receptivity by up-regulating N-fucosylation. Sci Rep, 2017. 7(1): p. 5315. [DOI] [PMC free article] [PubMed]
- 50.Zhang Y.M. Baicalin promotes embryo adhesion and implantation by upregulating fucosyltransferase IV (FUT4) via Wnt/beta-catenin signaling pathway. FEBS Lett. 2015;589(11):1225–1233. doi: 10.1016/j.febslet.2015.04.011. [DOI] [PubMed] [Google Scholar]; Zhang, Y.M., et al., Baicalin promotes embryo adhesion and implantation by upregulating fucosyltransferase IV (FUT4) via Wnt/beta-catenin signaling pathway. FEBS Lett, 2015. 589(11): p. 1225-33. [DOI] [PubMed]
- 51.Zhang Z. Suppression of FUT1/FUT4 expression by siRNA inhibits tumor growth. Biochim Biophys Acta. 2008;1783(2):287–296. doi: 10.1016/j.bbamcr.2007.10.007. [DOI] [PubMed] [Google Scholar]; Zhang, Z., et al., Suppression of FUT1/FUT4 expression by siRNA inhibits tumor growth. Biochim Biophys Acta, 2008. 1783(2): p. 287-96. [DOI] [PubMed]
- 52.Zheng Q. MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and alpha1,3-fucosylation. Cell Death Differ. 2017;24(12):2161–2172. doi: 10.1038/cdd.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zheng, Q., et al., MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and alpha1,3-fucosylation. Cell Death Differ, 2017. 24(12): p. 2161-2172. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Antibody information