Abstract
Sanfilippo syndrome type B, or mucopolysaccharidosis IIIB (MPS IIIB), is a rare autosomal recessive lysosomal storage disease caused by a deficiency of α-N-acetylglucosaminidase (NAGLU). Deficiency in NAGLU disrupts the lysosomal turnover of heparan sulfate (HS), which results in the abnormal accumulation of partially degraded HS in cells and tissues. BMN 250 (NAGLU-insulin-like growth factor 2 [IGF2]) is a recombinant fusion protein developed as an investigational enzyme replacement therapy for MPS IIIB. The IGF2 peptide on BMN 250 promotes enhanced targeting of the enzyme to lysosomes through its interaction with the mannose 6-phosphate receptor. The focus of these studies was to further characterize the ability of NAGLU-IGF2 to clear accumulated HS compared to unmodified NAGLU in primary cellular models of MPS IIIB. Here, we establish distinct primary cell models of MPS IIIB with HS accumulation. These cellular models revealed distinct NAGLU uptake characteristics that depend on the duration of exposure. We found that with sustained exposure, NAGLU uptake and HS clearance occurred independent of known lysosomal targeting signals. In contrast, under conditions of limited exposure duration, NAGLU-IGF2 was taken up more rapidly than the unmodified NAGLU into MPS IIIB primary fibroblasts, astrocytes, and cortical neurons, where it efficiently degraded accumulated HS. These studies illustrate the importance of using physiologically relevant conditions in the evaluation of enzyme replacement therapies in cellular models.
Keywords: Sanfilippo B, MPS IIIB, BMN 250, NAGLU-IGF2, NAGLU, heparan sulfate, non-reducing end
Introduction
Sanfilippo syndrome type B (Sanfilippo type B, mucopolysaccharidosis IIIB [MPS IIIB]) is a rare, lysosomal storage disease inherited in an autosomal recessive manner. The disease is caused by mutations in the gene encoding α-N-acetylglucosaminidase (NAGLU, EC 3.2.1.50). NAGLU is one of several key lysosomal enzymes required for the normal degradation of heparan sulfate (HS) throughout the body.1 NAGLU deficiency in Sanfilippo syndrome type B patients results in cellular accumulation of partially degraded HS, which can lead to severe progressive neurological decline, along with other pathological manifestations in peripheral tissues and organs, such as hepatomegaly in the liver. CNS pathology can cause behavioral disturbances, progressive dementia, loss of vision, deafness, and a shortened lifespan of 2 to 3 decades for affected individuals.2, 3 To date, there is no approved therapy for Sanfilippo type B.
BMN 250 (NAGLU-insulin-like growth factor 2 [IGF2]) is a recombinant fusion protein developed as an investigational therapeutic enzyme replacement therapy (ERT) for the treatment of Sanfilippo syndrome type B through intracerebroventricular (ICV) administration directly into the cerebrospinal fluid (CSF). Its use is currently being investigated in a clinical trial (ClinicalTrials.gov: NCT02754076), the purpose of which is to evaluate the safety, tolerability, and efficacy of BMN 250 administered to subjects with MPS IIIB via ICV delivery. ICV administration bypasses common challenges associated with delivery of therapeutics across the blood-brain barrier by introducing ERT directly into the CSF, providing local exposure to cells throughout the brain.
Endogenous NAGLU enzyme is thought to contain mannose 6-phosphate (M6P)-modified glycans, which act as a signal for receptor-mediated endocytosis and are targeted to the lysosome where HS is normally degraded. For reasons not completely understood, recombinant NAGLU, produced in Chinese hamster ovary (CHO) cells, has very little M6P-modified glycans to mediate the lysosomal delivery.4 To address this limitation, the IGF2-derived peptide was recombinantly fused to NAGLU to promote enhanced targeting of the enzyme to lysosomes compared to the unmodified enzyme, by taking advantage of the ability of the cation-independent M6P receptor to bind independently to IGF2.5, 6, 7, 8, 9 NAGLU-IGF2 had been shown in in vitro assays to effectively bind the M6P/IGF2 receptor with high affinity (50% inhibitory concentration [IC50] = 0.28 ± 0.05 nM) and to retain a similar KM value for a synthetic fluorogenic substrate to that of unmodified NAGLU (KM = 0.22 mM versus unmodified NAGLU KM = 0.2 mM).3 The NAGLU-IGF2 fusion protein combined with ICV administration has been shown to clear accumulated HS throughout the brain, in mouse models of Sanfilippo type B and reduces HS levels in many peripheral tissues and organs, including the liver.10, 11
Based on the current understanding of the native lysosomal delivery mechanism of NAGLU by the M6P receptor, we expected the unmodified NAGLU to be unable to clear lysosomal HS from the MPS IIIB cell models we tested because of its lack of an efficient lysosomal targeting feature. However, we found that, with sustained exposure, unmodified NAGLU that lacks M6P containing glycans reduced lysosomal HS in MPS IIIB-deficient cell cultures. However, in vivo, most recombinant lysosomal enzymes are rapidly cleared after administration, which could limit therapeutic exposure to distal regions, especially if the therapeutic enzyme lacks efficient mechanisms for cellular uptake. Thus, in these studies, we used relevant primary cellular models of MPS IIIB, to further understand the differences in lysosomal delivery of NAGLU-IGF2 and unmodified NAGLU.
Results
Unmodified NAGLU Can Clear HS Storage in Human MPS IIIB Patient Fibroblasts
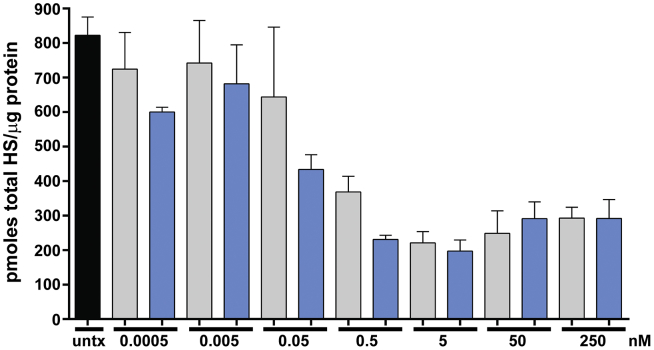
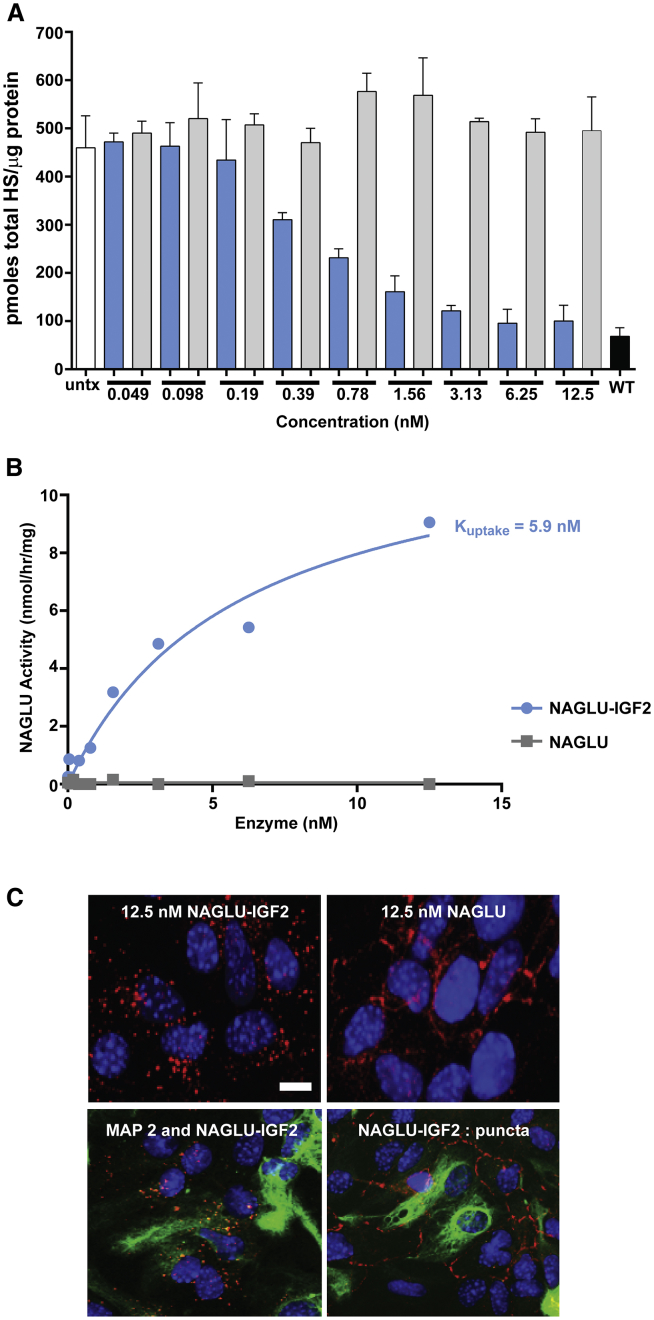
To investigate the cellular uptake and lysosomal clearance of HS by NAGLU, we established a cellular model of MPS IIIB based on patient-derived dermal fibroblasts (GM02931). The cultured primary fibroblasts were grown to confluence to increase lysosomal storage of HS, then treated for 9 days with NAGLU-IGF2 or unmodified NAGLU at concentrations starting near and extending beyond the Kuptake for NAGLU-IGF2 in fibroblasts, which has been reported as 5.4 ± 0.9 nM.3 Using this model, we measured a decrease in total HS storage, using both NAGLU-IGF2 and unmodified NAGLU in MPS IIIB patient fibroblasts (Figure 1). As expected, the IGF2-tagged NAGLU was able to reduce HS storage at doses as low as 0.05 nM. However, we were surprised to find that the unmodified version of NAGLU cleared HS storage at 10-fold higher concentrations (0.5 nM) under these conditions. This finding was unexpected in light of previous studies showing that recombinant unmodified NAGLU produced in CHO cells had little to no M6P and was not taken up by cultured fibroblasts.3 These findings suggest that unmodified NAGLU can be internalized by cells through a mechanism other than the M6P/IGF2 receptor after extended periods of exposure.
Figure 1.
Effect of Different Concentrations of NAGLU-IGF2 and Unmodified NAGLU on Total Heparan Sulfate Storage after Extended Exposure of MPS IIIB Fibroblasts
HS measurements were taken from confluent cultures of MPS IIIB-patient-derived fibroblasts that were either not treated (untx, black bar) or treated with increasing amounts of either NAGLU-IGF2 (blue bars) or unmodified NAGLU (gray bars) for 9 days in culture. Treatments were administered in triplicate, with the results shown as the mean ± SD.
Under Limited Exposure Conditions, NAGLU-IGF2 Is More Efficient at Clearing Lysosomal HS Than Unmodified NAGLU in Human Patient MPS IIIB Fibroblasts
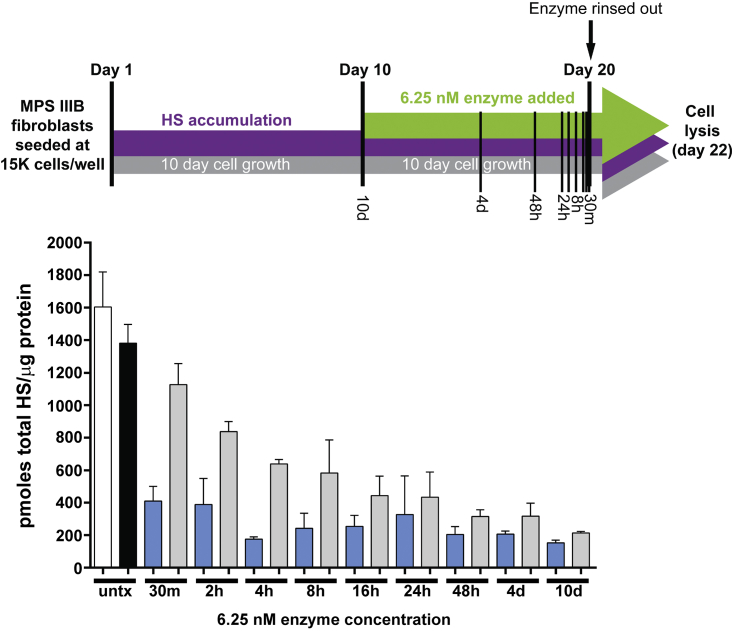
Limiting the time of exposure may be a more physiologically relevant model, because enzyme replacement therapy is rapidly cleared in vivo. In order to explore the nature of the cellular activity of unmodified NAGLU that we observed, we examined the effects of limited exposure time on the clearance of lysosomal HS in affected patient fibroblasts. Using information from earlier experiments conducted with extended exposure time,10, 12 we designed a time course study focusing on using a single concentration of 6.25 nM (equal to the Kuptake for NAGLU-IGF2 in fibroblasts), with exposures ranging from 30 min to 10 days (Figure 2). After the indicated time of NAGLU exposure, the culture medium was changed, and cells were cultured for an additional 48 h to allow for degradation of HS. The cells were then lysed and analyzed for total HS normalized to total protein. No cell loss was observed in any treatment group. We found that both NAGLU-IGF2 and unmodified NAGLU were able to eliminate lysosomal HS when the exposure time was 8 h or more. In fact, we observed no significant difference between the two enzymes in lowering HS levels (p > 0.05). However, with shorter exposure times, NAGLU-IGF2 was much more efficient at clearing HS than was unmodified NAGLU at the same dose (p < 0.03). These results are consistent with NAGLU-IGF2 having better cellular uptake characteristics than unmodified NAGLU, which can be seen under limited exposure conditions.
Figure 2.
Single Concentration Substrate Clearance Time Course in MPS IIIB Human Patient Fibroblasts
(Top) HS levels were measured in cultures of MPS patient dermal fibroblast that were either untreated (untx) or treated with 6.25 nM of either NAGLU-IGF2 (blue bars) or unmodified NAGLU (gray bars) for increasing amounts of time from 30 min up to 10 days, continuously (30 min; 2, 4, 8, 16, 24, and 48 h; and 4 and 10 days). (Bottom) In order to allow cells to accumulate lysosomal HS, fibroblasts were seeded at 15,000 cells/well and cultured for 10 days before the time course was initiated. Treatments were done in triplicate with the results (the mean ± SD) shown as picomoles total HS/microgram protein. For the untreated controls, we analyzed HS levels at both 10 days just before the start of treatment (white bar) and at the end of the treatment period (black bar), in order to detect significant increases in HS levels that might skew the treatment results. No significant increases were observed.
Primary Cellular Models of MPS IIIB and Accumulation of NAGLU Metabolic Substrates
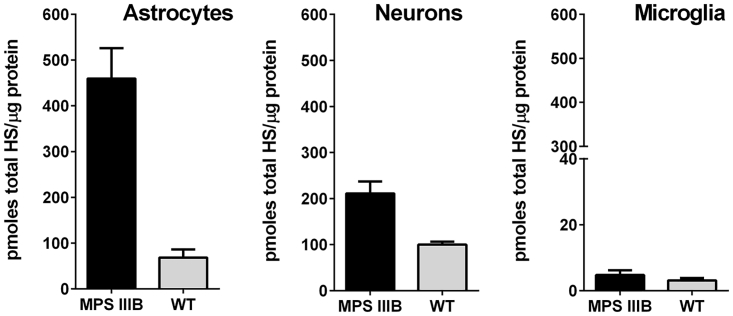
In order to better understand if the superior HS clearance observed with NAGLU-IGF2 in primary human MPS IIIB dermal fibroblasts is also observed in more disease-relevant cell types, we established cellular models of MPS IIIB based on primary neurons, astrocytes, and microglia isolated from the brains of MPS IIIB mouse embryos. Mouse cortical brain tissue was micro-dissected from embryonic day 18 (E18) homozygous Naglu−/− and wild-type embryos. The purity of these cultures was determined by observed cellular morphology and was deemed to be approximately 90% or higher. MPS IIIB primary mouse cortical neurons, astrocytes, and microglia were grown for 14 to 25 days in culture, along with age-matched wild-type cells to determine if HS accumulation would occur in culture (Figure 3). We found that MPS IIIB astrocytes cultured for 15–20 days stored the highest level of total HS in the three primary culture types, with a maximum of approximately 6.5 times more HS storage than in wild-type astrocytes. Overall, the levels of HS accumulation detected in MPS IIIB primary cortical neurons were lower than those observed in astrocytes, with a maximum accumulation of approximately 2-fold over wild type. These findings are in line with previous observations in an MPS IIIA primary neuron and astrocyte cell model where researchers observed much higher levels of HS storage in astrocytes than in neurons.13
Figure 3.
Heparan Sulfate Accumulation in Cultured Primary Astrocytes, Neurons, and Microglia Derived from NAGLU-Null Mouse E18 Embryos
Primary cultures of neurons, astrocytes, and microglia were established from brain tissues taken from wild-type and NAGLU-null mouse embryos (E18) and cultured to allow for accumulation of HS in affected cells. HS was prepared, and total HS was measured by quantitative HS analysis. Results are shown as mean picomoles total HS/microgram protein ± SD in triplicate wells.
Unlike both astrocytes and neurons, HS levels in microglia cultures showed no significant differences between affected and normal microglia, suggesting little or no storage in this cell type resulting from disease (Figure 3; Figure S1). The results for microglia were unexpected, since others have reported morphological signs of enlarged lysosomal features presumably containing disease-specific HS metabolites in affected microglia. However, we could not confirm this biochemically. Because of the apparent lack of disease-specific accumulation of HS in microglial cells under our culture conditions, we focused on MPS IIIB primary neurons and astrocytes for our remaining experiments.
The IGF2 Domain Is Essential for Rapid Uptake of NAGLU into MPS IIIB Primary Neurons
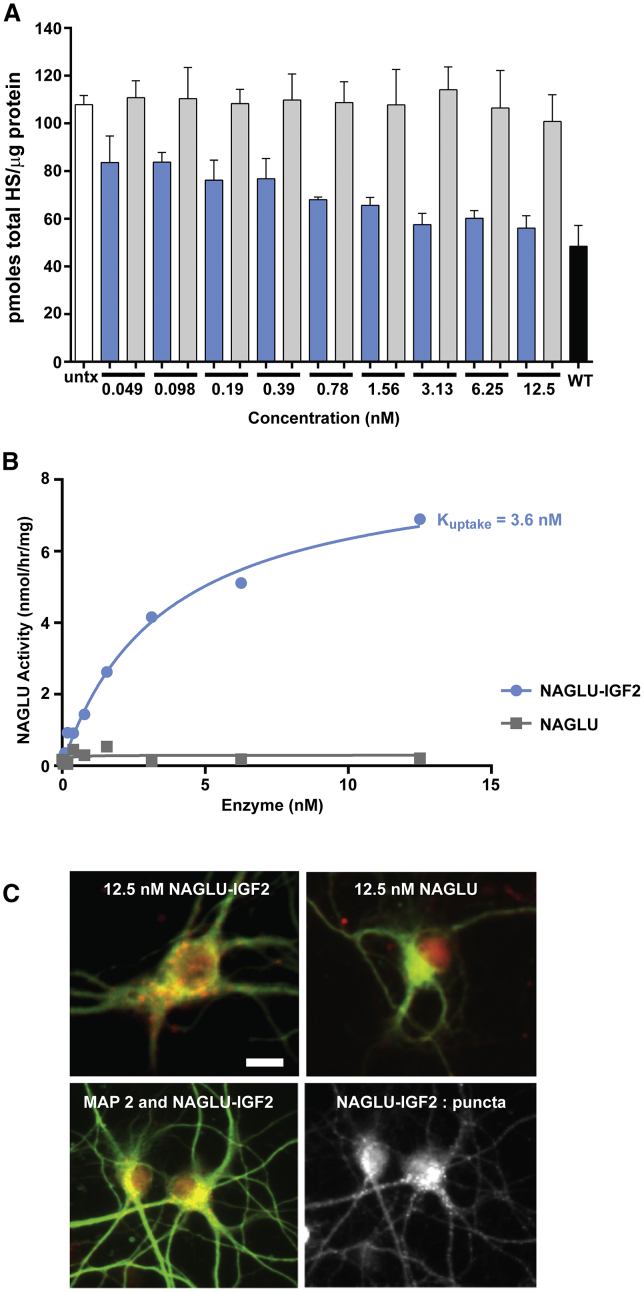
Using our primary mouse neuron cellular model of MPS IIIB, we examined HS clearance in response to different concentrations of NAGLU-IGF2 and unmodified NAGLU after just 30 min of exposure. We found that NAGLU-IGF2 gave a dose-dependent reduction of total HS, whereas unmodified NAGLU had no effect under these conditions (Figure 4A). The greatest decrease occurred at 3.13–12.5 nM with HS storage reduced to near wild-type levels. Measurement of cell associated NAGLU activity showed that NAGLU-IGF2 was readily taken up by MPS IIIB neurons after limited exposure, with Kuptake of 3.6 nM, whereas unmodified NAGLU exposure did not lead to an increase in cell-associated NAGLU activity (Figure 4B). Results for unmodified NAGLU activity in neurons were consistent with those observed in MPS IIIB fibroblasts (Figure 2). These finding were repeated in three independent primary cell isolates with similar findings (Figure S2).
Figure 4.
Effects of NAGLU-IGF2 and Unmodified NAGLU on MPS IIIB Primary Mouse Neurons
(A) HS levels in three replicate wells in a 96-well plate after transient 30 min exposure of MPS IIIB neurons to different concentrations of either NAGLU-IGF2 (blue bars) or unmodified NAGLU (gray bars) were measured 48 h after treatment and compared to levels in replicate vehicle-treated MPS IIIB neurons (white bar) or wild-type neurons (black bar). The results (the mean ± SD) are shown as picomoles total HS/microgram protein. (B) Cellular uptake of exogenous NAGLU-IGF2 (blue trace) or unmodified NAGLU (gray trace) after 30 min exposure by primary embryonic cortical neurons. (C) Immunofluorescence was measured after cortical neurons were exposed for 30 min to either NAGLU-IGF2 or unmodified NAGLU. Permeabilized cells were immunostained with an antibody to NAGLU (red; 1:800) and an antibody to the neuronal marker MAP2 (green; 1:500). Scale bar, 10 μm.
In order to verify that NAGLU-IGF2 is internalized by exposed neurons, NAGLU-specific immunofluorescence was measured. NAGLU staining in murine MPS IIIB primary cortical neurons exhibited a punctate appearance for NAGLU-IGF2, localized around the neuronal cell bodies and along the processes (Figure 4C). Punctate NAGLU staining was consistent with that reported previously in the literature from tissue biodistribution and colocalization studies with the lysosomal markers LAMP1 and LAMP2.10, 11 NAGLU staining for the unmodified enzyme in neurons showed a more diffuse staining pattern (Figure 4C). This also suggests that the untagged form of the enzyme is not taken up as efficiently into lysosomes when exposure conditions are limited, which is consistent with results from our quantitative HS storage assay and enzyme activity results.
The IGF2 Domain Is Essential for Rapid Uptake of NAGLU into MPS IIIB Primary Astrocytes
MPS IIIB mouse astrocytes exposed to NAGLU-IGF2 and unmodified NAGLU for 30 min showed substrate clearance effects similar to those seen in primary neurons. NAGLU-IGF2 decreased total HS in a dose-dependent manner starting at 0.39 nM up to the highest dose of 12.5 nM, whereas the unmodified NAGLU had no effect on HS storage under these same exposure conditions (Figure 5A). These results are consistent with the rapid and efficient uptake of NAGLU-IGF2 by astrocytes and its targeting to the lysosomes where HS is degraded. Corresponding enzyme uptake assay results were consistent with the HS reduction results demonstrating that NAGLU-IGF2 was taken up into MPS IIIB astrocytes in a dose-dependent manner (Figure 5B). The calculated Kuptake for NAGLU-IGF2 internalization by astrocytes (5.9 nM) was similar to that reported previously in MPS IIIB patient fibroblasts (5.4 and 6.5 nM).3, 10 However, both the levels of HS reduction and the enzyme uptake results for unmodified NAGLU showed no detectable NAGLU activity in MPS IIIB astrocytes, indicating that there was little to no uptake with limited exposure (Figure 5B). These experiments were repeated with three independent primary cell isolates with similar findings (Figure S3).
Figure 5.
Effects of NAGLU-IGF2 and Unmodified NAGLU on MPS IIIB Primary Mouse Astrocytes
(A) HS levels in three replicate wells in a 96-well plate after a transient 30 min exposure of MPS IIIB astrocytes to different concentrations of either NAGLU-IGF2 (blue bars) or unmodified NAGLU (gray bars) were measured 48 h after treatment and compared to levels in replicate vehicle-treated MPS IIIB astrocytes (white bar) or wild-type astrocytes (black bar). The results (the mean ± SD) are shown as picomoles total HS/microgram protein. (B) Cellular uptake of exogenous NAGLU-IGF2 (blue trace) or unmodified NAGLU (gray trace) by primary embryonic astrocytes after a 30 min exposure. (C) Immunofluorescence was measured in permeabilized astrocyte cultures previously exposed for 30 min with either NAGLU-IGF2 or unmodified NAGLU. NAGLU antibody (red; 1:800), astrocyte marker GFAP (green; 1:500), and Hoechst dye for nuclei (blue; 1 μg/mL). Scale bar, 10 μm.
Immunofluorescence for cell-associated NAGLU showed results similar to those observed in primary neurons. NAGLU immunostaining in MPS IIIB astrocytes gave a punctate appearance for NAGLU-IGF2, consistent with rapid uptake and localization after 30 min of enzyme exposure (Figure 5C). Similar punctate NAGLU-IGF2 staining and colocalization with the lysosomal markers LAMP1 and LAMP2 have been reported previously from brain tissue in Naglu−/− mice after ICV administration in in vivo studies and in wild-type cultured neurons.3, 10 After limited exposure, unmodified NAGLU showed a distinct ring-like pattern of staining that was different from that of NAGLU-IGF2. This finding is suggestive of unmodified NAGLU remaining along the outer plasma membrane and not being taken up into cells under limited exposure conditions.
Similar short-exposure experiments were carried out on age-matched wild-type neurons and astrocytes. As expected, unaffected normal neurons and astrocytes had significantly lower levels of total HS than were found in their corresponding MPS IIIB cell types and, as expected, no effect was observed with either NAGLU-IGF2 or unmodified NAGLU (Figure S4).
Discussion
Results from this study add to our overall understanding of the biochemical effects of NAGLU-IGF2 in MPS IIIB brain-specific primary cells. In addition, we have described three cell-based models of MPS IIIB involving primary human fibroblasts, mouse primary neurons, and mouse primary astrocytes. Each of these systems may be useful in modeling cell-type-specific aspects of this complex disease and in developing therapeutic approaches designed to treat it.
Our characterization of cultured primary cells from the MPS IIIB mouse highlight the fact that not all cells store the same amount of HS in the absence of intrinsic NAGLU activity. When isolated in culture, we found that astrocytes accumulated more HS in culture, whereas neurons stored less, and microglial storage was not significant. Since it is not completely understood what cell types drive the disease phenotype in MPS IIIB, it is important that therapies such as ERT with recombinant NAGLU reach all cell types that accumulate lysosomal HS.
In comparing the differential uptake and cellular activity of NAGLU-IGF2 and unmodified NAGLU, we discovered that both exposure time and dose significantly affect cellular uptake of recombinant NAGLU and the subsequent clearance of lysosomal HS. We first observed that after 9 days of exposure, unmodified NAGLU cleared lysosomal HS in affected human fibroblasts at a 10-fold higher concentration than that necessary for NAGLU-IGF2. Thus, with sustained exposure, we were able to eliminate the dependence on the M6P/IGF2 mechanism of lysosomal targeting. We next evaluated the rate at which equimolar amounts of NAGLU-IGF2 and unmodified NAGLU are taken up by MPS IIIB fibroblasts and found that NAGLU-IGF2 is taken up more rapidly. Just 30 min of exposure to NAGLU-IGF2 was sufficient to clear HS storage, whereas unmodified NAGLU required 8 h of exposure to reach similar reductions. In cell types that may play important roles in the neurological deficits seen in this disease—i.e., neurons and astrocytes—we observed, even at concentrations exceeding the Kuptake for NAGLU-IGF2, that unmodified NAGLU had no significant effect on HS storage after 30 min exposures. On the contrary, NAGLU-IGF2 showed significant HS reduction, even at concentrations below the Kuptake in both neurons and astrocytes. These results demonstrate the superior uptake characteristics of NAGLU-IGF2 over unmodified NAGLU.
Since most recombinant lysosomal enzymes in vivo are rapidly cleared after administration, it is important that recombinant enzymes are efficiently taken up by the cells and delivered to the appropriate subcellular compartment containing the therapeutic target. Our cellular models incorporating short exposure times were potentially more physiologically relevant than models using sustained exposure. Since in these models NAGLU-IGF2 had a lower effective concentration and was more rapidly taken up compared to unmodified NAGLU, it would be interesting to compare the two in vivo to see if these distinctions hold and which may have the highest clinical benefit. It would also be interesting to see how such differences apply to other lysosomal enzymes and other modes of lysosomal delivery.
Materials and Methods
NAGLU-IGF2 and Unmodified Enzyme
Recombinant human NAGLU-IGF2 fusion protein and unmodified NAGLU were expressed in CHO cells and purified as described elsewhere (U.S. Patent 9,376,480 B2).3 Purified protein stocks (20.6 and 21.3 mg/mL) were stored frozen at −80°C in artificial CSF solution (vehicle): 1 mM Na2HPO4/NaH2PO4, 148 mM NaCl, 3 mM KCl, 0.8 mM MgCl2, and 1.4 mM CaCl2 (pH 7.2) and kept on ice before each experiment was initiated.
Human Fibroblast Cell Culture
A human MPS IIIB dermal fibroblast cell line (GM02931) derived from a patient punch biopsy was purchased from Coriell Institute (Camden, NJ, USA) and was cultured per the supplier’s instructions in Eagle’s minimum essential medium supplemented with 15% (v/v) fetal bovine serum (FBS).
Deriving Mouse Primary Cell Cultures from Embryonic Brain Tissue
Mouse cortical brain tissue was micro-dissected and pooled from a time-pregnant litter of E18 homozygous Naglu−/− embryos (Naglu−/−, B6.129S6-Naglutm1Efn/J, 00827; The Jackson Laboratory).14 A separately micro-dissected and pooled set of cortices from an age-matched wild-type (Naglu+/+, C57BL/6J, 00664; The Jackson Laboratory) litter of the same genetic background was included in parallel experimental conditions as a control for each experiment performed. Protocols were approved by the Institutional Animal Care and Use Committee and in accordance with State Office of Laboratory Animal Welfare regulations. Briefly, pooled cortical tissue from each genotype was separately digested then dissociated into a single-cell suspension, according to previously described primary neuronal culture methods.15, 16 To initiate neuronal cultures, cells were seeded directly in serum-free medium (referred to as neuronal growth medium [NGM]; see below). For astrocytes and microglia, cells were seeded and grown in serum-containing medium (referred to as astrocyte growth medium [AGM]; see below). Both types of prepared media were 0.22 μm sterile filtered before use, and the cells were handled and maintained according to standard aseptic technique in an incubator with conditions set at 37°C with 5% CO2 for the duration of all experiments.
Primary Cortical Neurons
Dissociated cells from separately pooled MPS IIIB and wild-type cortical preparations were seeded into sterile poly-D lysine/laminin-coated, 96-well tissue culture (TC) plates (Corning) at cell densities ranging from 20,000 to 100,000 cells/well, depending on the assay endpoint, in NGM, which consisted of serum-free NeuroBasal, B27 supplement+1% GlutaMAX, and 1% penicillin/streptomycin (Thermo Scientific). Cortical neurons were fed every 3–4 days in 150 μL/well medium and grown for a total of 14 days to ensure maximum neurite outgrowth and synaptic maturity before enzyme exposure experiments were initiated. On culture day 14, MPS IIIB and wild-type primary neurons were incubated with a range of serially diluted NAGLU-IGF2 and unmodified NAGLU for 30 min in adjacent wells in NGM. After enzyme exposure, the cells were gently rinsed twice in 200 μL/well 1× Hanks’ balanced salt solution (HBSS; Thermo Scientific), then fed with fresh NGM. Cell culture plates used for quantitative HS assay and enzyme activity were incubated for an additional 48 h at 37°C with 5% CO2 to allow for complete enzyme uptake and subsequent HS degradation before cell lysis. Culture plates used for immunofluorescence were fixed 1 h after enzyme incubation was completed in 4% paraformaldehyde (PFA; Electron Microscopy Science) in 1× Dulbecco’s PBS (DPBS; Thermo Scientific), to investigate enzyme uptake and localization shortly after 30 min of limited exposure.
Primary Cortical Astrocytes
Dissociated cells from pooled cortices were seeded directly into sterile poly-D lysine/laminin-coated, 96-well TC plates in DMEM+15% FBS+1% GlutaMAX and 1% penicillin/streptomycin (Thermo Scientific) AGM at an initial seeding density of 50,000–80,000 cells/well. Astrocytes were grown to post confluence (15–20 days) in order to promote maximum cellular HS accumulation and storage before enzyme exposure experiments were initiated. Astrocytes were incubated with serially diluted NAGLU-IGF2 and unmodified NAGLU in AGM for 30 min in adjacent wells. After enzyme exposure, cells were rinsed twice in 200 μL/well 1× HBSS and fed with fresh AGM. Culture plates used for HS quantification and enzyme activity were incubated for an additional 48 h after enzyme incubation was complete, to allow for complete enzyme uptake and subsequent HS degradation before cell lysis. Culture plates used for immunofluorescence were fixed 1 h after enzyme exposure was completed in 4% PFA in 1× DPBS, to investigate enzyme uptake and localization shortly after a limited (30 min) exposure.
Primary Cortical Microglia
Dissociated cells were seeded directly in serum-containing AGM and cultured for 21 days in laminin-coated T-75 flasks (Corning) in the presence of 25 ng/mL recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems) to promote microglia survival and proliferation in culture. Mixed microglia-astrocyte cultures were fed once every 5–7 days in 15 mL glia growth medium including GM-CSF. When the number of microglia were sufficient to subculture (microglia covering approximately two-thirds flask area), they were separated from the primary astrocyte monolayer by mechanical shaking via a platform shaker (VWR) set at 300 rpm for 2 h at 37°C, as described previously.17 AGM containing dislodged microglia was collected from multiple T-75 flasks and centrifuged at 4,500 rpm to collect cells. Microglia were then rinsed in 1× HBSS, counted, and re-suspended in fresh AGM and then seeded into poly-D lysine/laminin-coated tissue culture plates at a density of 50,000–80,000 cells/well. One to 3 days later, microglia were exposed to NAGLU-IGF2 and unmodified enzyme for 30 min in adjacent wells. Culture plates used for HS quantification and enzyme activity were incubated for an additional 48 h after enzyme exposure was complete to allow for complete enzyme uptake and subsequent glycosaminoglycan degradation. Culture plates used for immunofluorescence were fixed 1 h after enzyme exposure was completed, using 4% PFA in 1× DPBS to stain for the microglia marker Iba1 (1:1,000; Wako).
Enzyme Exposure and Sample Collection
Diluted treatment stocks of each enzyme were made up fresh before each experiment in room temperature NGM for neurons and AGM for astrocytes at a starting concentration of 12.5 nM from concentrated frozen enzyme aliquots, then serially diluted for a total of nine treatment concentrations for each enzyme plus an untreated group. A sufficient volume of medium containing each test concentration was pre-loaded into sterile, 12-channel reagent trays (VWR) to enable rapid and simultaneous treatment in triplicate wells at all concentrations using 100 μL/well. Each cell culture type was exposed to NAGLU-IGF2 and unmodified NAGLU in adjacent wells for 30 min at 37°C under 5% CO2 incubator conditions on separate 96-well plates for each endpoint analysis: HS quantification, enzyme activity, and immunocytochemistry.
For fibroblast time course experiments, cells were grown to post confluence for 10 days, to maximize accumulated HS storage before enzyme exposure experiments were initiated. Subsets of triplicate wells were then exposed to both enzymes diluted to the same concentration (6.25 nM) for various lengths of time from 30 min up to 10 days (30 min; 2, 4, 8, 16, 24, and 48 hours, and 4 and 10 days) of continuous exposure in culture. An exception was made for the 10 day exposure group, where enzyme-containing medium was removed on culture day five and refreshed with new growth medium and enzyme to avoid buildup of cytotoxic metabolic products in treatment wells.
After each exposure interval was complete, the enzyme-containing medium was aspirated, and the cells were gently rinsed twice in 200 μL/well HBSS. Cells were then fed fresh medium and incubated under normal culture conditions for an additional 48 h, to allow for complete enzyme uptake and subsequent degradation of HS before cell lysis for quantitative HS analysis or enzyme activity assays were performed. Each cell plate was lysed and collected specifically, depending on which type of assay was performed. For quantification of total HS, cells were rinsed twice in 1× HBSS then lysed in 40 μL/well sterile TC grade water. From each 40 μL lysate, 5 μL was transferred to another 96-well plate for protein quantitation, using a bicinchoninic acid assay kit (Thermo Scientific), per the manufacturer’s instructions. Plates containing the remaining lysates were immediately sealed in adhesive microplate foil film (VWR) and stored at −80°C until the start of the assay. For enzyme activity, cells were rinsed twice in 1× HBSS then lysed in 57.5 μL/well M-PER Extraction Reagent (Thermo Scientific), and the plates were stored immediately using the same method. The results of quantitative analysis of total HS and enzyme activity were calculated and normalized to total protein/well. Cell plates used for immunofluorescent imaging were rinsed twice after enzyme exposure, fed with fresh growth medium, and incubated for an additional hour under normal culture conditions before being fixed in 100 μL/well in 4% PFA in 1× DPBS, to allow for investigation of cellular uptake and localization. Immunocytochemistry was performed to confirm general cell type, morphology, and enzyme localization after a limited (30 min) exposure. Cells were fixed for 15 min at room temperature in a fume hood, then rinsed twice in 200 μL/well 1× DPBS, sealed in plastic film, and stored at 4°C until immunostaining was initiated.
Quantitative Analysis of HS
Total HS was quantified using the glycan reductive isotope labeling-liquid chromatography-mass spectrometry (GRIL-LC/MS) assay previously described.18, 19 Briefly, glycosaminoglycans were isolated from cellular lysates by anion exchange chromatography and enzymatically depolymerized with a mixture of heparin lyases I, II, and III (IBEX Technologies). The digestion products were then tagged by reductive amination with isotopically labeled aniline (Sigma-Aldrich) and quantified by LC/MS, by comparing ion intensities to those of differentially isotope-labeled standards. The quantity of the most abundant disaccharides found in HS (D0A0, D0S0, D0A6, D0S6, D2S0, and D2S6)20 that were enzymatically liberated was used to determine total HS. Total HS data was expressed as the average picomoles total HS per microgram total protein from triplicate sample wells per condition ±SD.
Enzyme Activity Assay and Kuptake Determination
Enzyme activity was determined using a 4MU (4-methylumbelliferyl-N-acetyl-α-glucosaminidase) synthetic fluorogenic substrate (EMD Millipore), according to a published protocol.3, 21, 22 To determine the Kuptake, the concentration of enzyme mediating half-maximal uptake was measured. Cells were first exposed to various concentrations of either NAGLU-IGF2 or native NAGLU for 30 min (dose range, 0.049–12.5 nM) and then the enzyme activity present in cells was plotted into a Michaelis-Menten curve, by GraphPad Prism software (La Jolla, CA).
Immunocytochemistry
Previously fixed cells were permeabilized in 0.1% Triton X-100 (Sigma) in 1× DPBS for 10 min at room temperature, rinsed with 200 μL/well 1× DPBS, and blocked in 10% normal goat serum (Thermo Scientific) for 1 h at room temperature. Primary antibodies were incubated for 2 h at room temperature or overnight at 4°C in 1% bovine serum albumin (BSA; Sigma) in 1× DPBS and incubated at the following concentrations in 1% BSA in 1× DPBS: for neurons, mouse anti-MAP2, at 1:500 (EMD Millipore); for BMN 250/unmodified NAGLU, rabbit anti-NAGLU, at 1:800 (Abcam); for astrocytes, rabbit anti-glial fibrillary acidic protein (GFAP), at 1:1,000 (Dako); and for microglia, rabbit anti-Iba1, at 1:1,000 (Wako Pure Chemical Industries). When two primary antibodies of the same species were multiplexed in the same wells (NAGLU and GFAP), APEX antibody conjugation kit for Alexa Fluor 488 fluorescent probe was used according to the manufacturer’s specifications (Thermo Scientific), conjugated to GFAP. The cells were rinsed twice in 200 μL/well 1× DPBS for 5 min and then incubated with rabbit Alexa Fluor 594 or mouse Alexa Fluor 488 secondary antibodies (Thermo Scientific) at 1:1,000 for 1 h at room temperature, with or without 1 μg/mL Hoechst dye (Thermo Scientific) included as a stain for nuclei.
Data Analysis
The statistical significance between corresponding treatments with NAGLU-IGF2 and unmodified NAGLU was determined using p values calculated with the paired t test.
Author Contributions
Conceptualization: R.L., H.P., and J.H.L.; methodology: R.L., H.P., and G.Y.; investigation: H.P. and A.L.; resources: T.M.C., B.Y., J.H., and M.J.L.; writing–original draft: H.P.; writing–review and editing: B.E.C and R.L.; supervision, R.L., B.E.C., G.Y., M.A.-S. and J.H.L.; and project administration: R.L.
Conflicts of Interest
All authors are either current or former employees of and have interest in BioMarin Pharmaceutical, Inc., which is developing BMN 250 as a potential commercial therapeutic agent. M.A.-S., T.M.C., and J.H.L., together with others, hold a patent for compositions and methods for treating Sanfilippo type B, comprising therapeutic NAGLU-IGF2 fusion proteins (U.S. Patent 9,376,480 B2).
Acknowledgments
We gratefully acknowledge Sherry Bullens, Timothy Graham, and Henry Lopez for helping to make these experiments possible and Valoree Brovelli for contract assistance. We also wish to acknowledge Pascale M.N. Tiger, Diana S. Cheung, and Eric Chen for enzyme production.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.05.008.
Supplemental Information
References
- 1.Neufeld E.F., Neunzer J. McGraw Hill; 2001. The mucopolysaccharidoses. In The Metabolic and Molecular Basis of Inherited Disease, Eighth Edition; pp. 3421–3452. [Google Scholar]
- 2.Valstar M.J., Ruijter G.J., van Diggelen O.P., Poorthuis B.J., Wijburg F.A. Sanfilippo syndrome: a mini-review. J. Inherit. Metab. Dis. 2008;31:240–252. doi: 10.1007/s10545-008-0838-5. [DOI] [PubMed] [Google Scholar]
- 3.Kan S.H., Troitskaya L.A., Sinow C.S., Haitz K., Todd A.K., Di Stefano A., Le S.Q., Dickson P.I., Tippin B.L. Insulin-like growth factor II peptide fusion enables uptake and lysosomal delivery of α-N-acetylglucosaminidase to mucopolysaccharidosis type IIIB fibroblasts. Biochem. J. 2014;458:281–289. doi: 10.1042/BJ20130845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao K.W., Neufeld E.F. Purification and characterization of recombinant human alpha-N-acetylglucosaminidase secreted by Chinese hamster ovary cells. Protein Expr. Purif. 2000;19:202–211. doi: 10.1006/prep.2000.1230. [DOI] [PubMed] [Google Scholar]
- 5.Kiess W., Blickenstaff G.D., Sklar M.M., Thomas C.L., Nissley S.P., Sahagian G.G. Biochemical evidence that the type II insulin-like growth factor receptor is identical to the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 1988;263:9339–9344. [PubMed] [Google Scholar]
- 6.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 7.Morgan D.O., Edman J.C., Standring D.N., Fried V.A., Smith M.C., Roth R.A., Rutter W.J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987;329:301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- 8.Oshima A., Nolan C.M., Kyle J.W., Grubb J.H., Sly W.S. The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J. Biol. Chem. 1988;263:2553–2562. [PubMed] [Google Scholar]
- 9.Tong P.Y., Tollefsen S.E., Kornfeld S. The cation-independent mannose 6-phosphate receptor binds insulin-like growth factor II. J. Biol. Chem. 1988;263:2585–2588. [PubMed] [Google Scholar]
- 10.Aoyagi-Scharber M., Crippen-Harmon D., Lawrence R., Vincelette J., Yogalingam G., Prill H., Yip B.K., Baridon B., Vitelli C., Lee A. Clearance of heparan sulfate and attenuation of cns pathology by intracerebroventricular bmn 250 in sanfilippo type b mice. Mol. Ther. Methods Clin. Dev. 2017;6:43–53. doi: 10.1016/j.omtm.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kan S.H., Aoyagi-Scharber M., Le S.Q., Vincelette J., Ohmi K., Bullens S., Wendt D.J., Christianson T.M., Tiger P.M., Brown J.R. Delivery of an enzyme-IGFII fusion protein to the mouse brain is therapeutic for mucopolysaccharidosis type IIIB. Proc. Natl. Acad. Sci. USA. 2014;111:14870–14875. doi: 10.1073/pnas.1416660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoyagi-Scharber M., Christianson T., Wendt D.J., Tiger P.M.N., Yip B.K., Holtzinger J., Chen Z., Woloszynek J., Cheung D.S., Lo M.J. Engineering of a recombinant NAGLU fusion protein with insulin-like growth factor 2 leads to improved cellular uptake via a glycosylation-independent lysosomal targeting pathway. Mol. Genet. Metab. 2014;111:S20. [Google Scholar]
- 13.Dwyer C.A., Scudder S.L., Lin Y., Dozier L.E., Phan D., Allen N.J., Patrick G.N., Esko J.D. Neurodevelopmental changes in excitatory synaptic structure and function in the cerebral cortex of sanfilippo syndrome iiia mice. Sci. Rep. 2017;7:46576. doi: 10.1038/srep46576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H.H., Yu W.H., Rozengurt N., Zhao H.Z., Lyons K.M., Anagnostaras S., Fanselow M.S., Suzuki K., Vanier M.T., Neufeld E.F. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding alpha-N-acetylglucosaminidase. Proc. Natl. Acad. Sci. USA. 1999;96:14505–14510. doi: 10.1073/pnas.96.25.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer G.J. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci. Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 16.Brewer G.J., Torricelli J.R., Evege E.K., Price P.J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 17.Barger S.W., Basile A.S. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J. Neurochem. 2001;76:846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence R., Olson S.K., Steele R.E., Wang L., Warrior R., Cummings R.D., Esko J.D. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J. Biol. Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence R., Brown J.R., Al-Mafraji K., Lamanna W.C., Beitel J.R., Boons G.J., Esko J.D., Crawford B.E. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat. Chem. Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence R., Lu H., Rosenberg R.D., Esko J.D., Zhang L. Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat. Methods. 2008;5:291–292. doi: 10.1038/nmeth0408-291. [DOI] [PubMed] [Google Scholar]
- 21.Marsh J., Fensom A.H. 4-Methylumbelliferyl alpha-N-acetylglucosaminidase activity for diagnosis of Sanfilippo B disease. Clin. Genet. 1985;27:258–262. doi: 10.1111/j.1399-0004.1985.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 22.Yogalingam G., Luu A.R., Prill H., Lo M.J., Yip B., Holtzinger J., Christianson T., Aoyagi-Scharber M., Lawrence R., Crawford B.E., LeBowitz J.H. BMN 250, a fusion of lysosomal alpha-N-acetylglucosaminidase with IGF2, exhibits different patterns of cellular uptake into critical cell types of Sanfilippo syndrome B disease pathogenesis. PLoS ONE. 2019;14:e0207836. doi: 10.1371/journal.pone.0207836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.