Abstract
Ginsenosides, the major active ingredients of ginseng and other plants of the genus Panax, have been used as natural medicines in the East for a long time; in addition, their popularity in the West has increased owing to their various beneficial pharmacological effects. There is therefore a wealth of literature regarding the pharmacological effects of ginsenosides. In contrast, there are few comprehensive studies that investigate their pharmacokinetic behaviors. This is because ginseng contains the complicated mixture of herbal materials as well as thousands of constituents with complex chemical properties, and ginsenosides undergo multiple biotransformation processes after administration. This is a significant issue as pharmacokinetic studies provide crucial data regarding the efficacy and safety of compounds. Moreover, there have been many difficulties in the development of the optimal dosage regimens of ginsenosides and the evaluation of their interactions with other drugs. Therefore, this review details the pharmacokinetic properties and profiles of ginsenosides determined in various animal models administered through different routes of administration. Such information is valuable for designing specialized delivery systems and determining optimal dosing strategies for ginsenosides.
Keywords: Bioavailability, Ginsenosides, Pharmacokinetic, Protopanaxadiol, Protopanaxatriol
1. Introduction
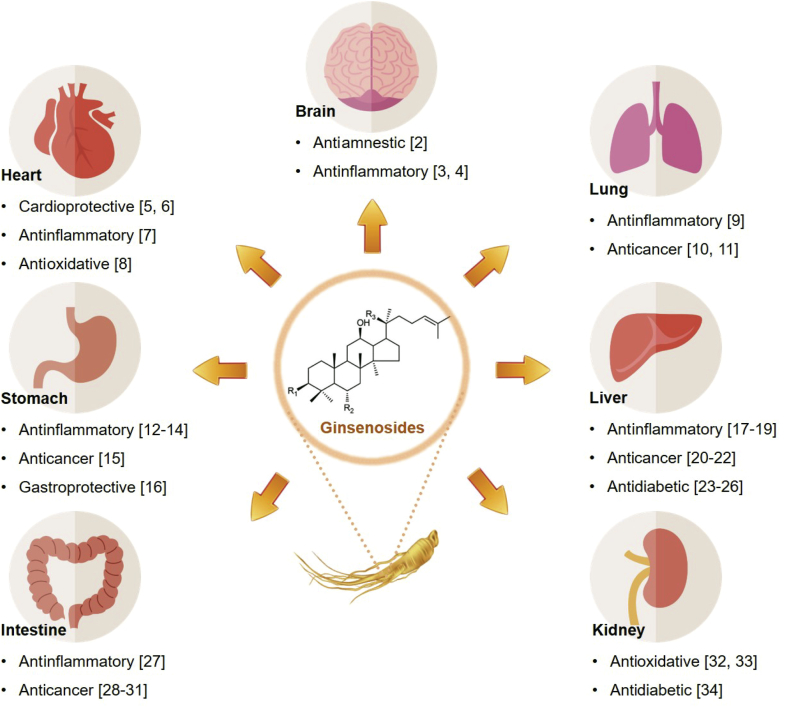
Panax ginseng Meyer (P. ginseng) and other ginsengs have long been used medicinally in East Asia and have gradually gained popularity in Western countries over the past several decades. P. ginseng contains various bioactive ingredients, including ginsenosides, polysaccharides, polyacetylenes, and polyphenols; of these, the ginsenosides are considered the main active components [1]. Hence, numerous studies have investigated the beneficial effects of ginsenosides and revealed various pharmacological activities, including antiinflammatory, anticancer, antidiabetic, and antioxidative effects, as illustrated in Fig. 1 [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34].
Fig. 1.
Therapeutic effects of ginsenosides and effective organs.
However, there have been very few studies reported to date which have investigated the pharmacokinetic characteristics of ginsenosides [35]. This is presumably attributable to the complex nature of biotransformation of ginsenosides during and after their absorption despite the fact that pharmacokinetic studies provide important data that can contribute to the evaluation of the efficacy and safety of ginsenosides and their related compounds [36]. In addition, as ginsenosides have complex chemical structures and as they are subdivided into several groups depending on their chemical forms, the study of their pharmacokinetic behavior is not simple [1]. For these reasons, there have been many complications in the design of the optimal dosage strategies for ginsenosides based on their pharmacokinetic behavior, and the assessment of their interactions with other drugs has been also limited [37]. Moreover, a proper evaluation of the pharmacokinetic properties of ginsenosides is considered to be extremely difficult because of their very low oral bioavailability, which is usually less than 10% (Table 1) [38], [39], [40], [41], [42], [43], [44], [45]. As the systemic exposure to ginsenosides impacts their efficacy and side effects, there is also a growing need to determine the optimal dosage regimens of ginsenosides [46].
Table 1.
Reported oral bioavailabilities of ginsenosides evaluated in animals
| Ginsenosides | Species | Oral dose (mg/kg) | Oral bioavailability (%) | References |
|---|---|---|---|---|
| Rb1 | Rat | 10 | 4.35 | [38] |
| Rb1 | Rat | 104 | 1.18 | [40] |
| Rb2 | Rat | 100 | 3.7 | [39] |
| Rd | Rat | 25 | 2.36 | [40] |
| Rh2 | Rat | 1 | 4.7 | [41] |
| Rh2 | Rat | 3 | 4.0 | [41] |
| Rh2 | Rat | 9 | 6.4 | [41] |
| Rh2 | Dog | 1 | 16.7 | [41] |
| Rh2 | Dog (male) | 1 | 17.6 | [43] |
| Rh2 | Dog (female) | 1 | 24.8 | [43] |
| Rg3 | Rat | 10 | 2.63 | [42] |
| Rg1 | Rat | 10 | 18.40 | [38] |
| Rg1 | Rat | 79 | 6.06 | [40] |
| Rh1 | Rat | 50 | 1.01 | [44] |
| Re | Rat | 13 | 7.06 | [40] |
| Re | Rat | 10 | 0.28 | [45] |
Therefore, this review comprehensively analyzed the pharmacokinetic behavior of each ginsenoside component, characterized in different animal models after administration through different routes, to provide the essential pharmacokinetic information and background required for the development of ginsenoside-based nutraceuticals and pharmaceuticals. In particular, we have specifically discussed the behavior of each ginsenoside component in the processes of absorption, distribution, metabolism, and excretion (ADME).
2. Physicochemical characteristics of ginsenosides
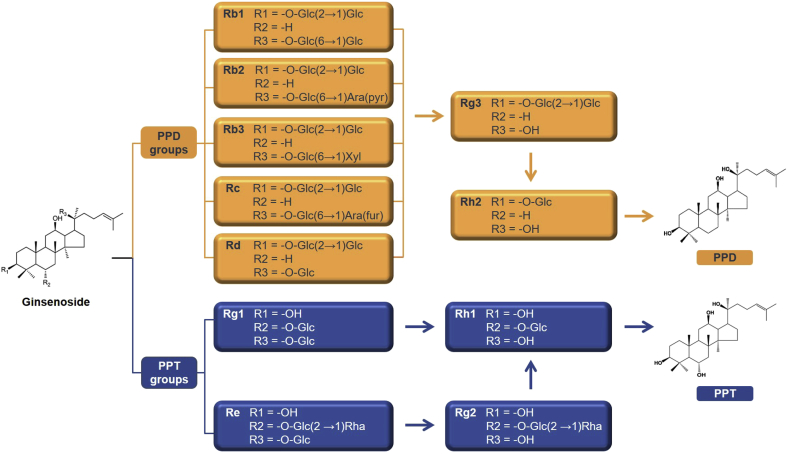
To achieve a better understanding of the pharmacokinetic behaviors of ginsenosides, it is helpful to first examine their physicochemical properties. Ginsenosides, classified as dammarane-type triterpene saponins, typically comprise 17 carbons in a 4-ring structure called the dammarane skeleton, with diverse sugar moieties, such as glucose, rhamnose, xylose, and arabinose, attached to the C-3 and C-20 positions [47]. As presented in Fig. 2, there are two major groups of ginsenosides: the protopanaxadiol (PPD) and protopanaxatriol (PPT) groups. Ginsenoside Rb1, Rb2, Rb3, Rc, and Rd represent the main PPD-type ginsenosides, whereas ginsenoside Rg1 and Re are the main PPT-type ginsenosides. In the PPD group, Rg3 is a metabolite of Rb1, Rb2, Rb3, Rc, and Rd, and Rg3 is subsequently metabolized to Rh2. In the PPT group, Rg2 is a metabolite of Re, and Rh1 is a metabolite of Rg1 and Re [48]. Some minor ginsenosides, such as F0, F11, and 25-OH-PPT, do not belong to either of the two groups mentioned previously and are considered “other” ginsenosides. PPD-type ginsenosides contain sugar moieties attached to the β-OH at the C-3 and/or C-20 position, whereas PPT-type ginsenosides include sugar moieties attached to the α-OH at C-6 and/or the β-OH at C-20 [49].
Fig. 2.
Chemical structures of protopanaxadiol (PPD)- and protopanaxatriol (PPT)-type ginsenosides and the main metabolic pathways of PPD and PPT groups. “Arrow (→)” denotes metabolic changes of ginsenosides in vivo.
In general, ginsenosides have low aqueous solubility, poor membrane permeability, and metabolic instability. The aqueous solubility of ginsenosides is dictated by the presence of a hydrophobic functional group comprising triterpenes or steroid aglycones and a hydrophilic functional group consisting of a sugar side chain; therefore, it is considerably affected by the number of attached sugar moieties within the structures. However, most ginsenosides generally exhibit low aqueous solubility as they comprise steroid aglycones and lack sugar moieties. In addition, ginsenosides commonly have low membrane permeability owing to their dammarane-type structure with a large skeleton. Moreover, ginsenosides are known to be chemically labile in the gastrointestinal (GI) tract because they can be degraded by hydrolytic enzymes [50], [51], [52]. Thus, the clinical applications of ginsenosides have been limited by low bioavailability caused predominantly by the unsuitable physicochemical properties of ginsenosides mentioned previously.
3. Pharmacokinetic behavior of ginsenosides
The optimization of drug dosage is significantly affected by its pharmacokinetic properties. After administration, the drug undergoes four major pharmacokinetic processes in the body: ADME. It is hard to evaluate the pharmacokinetic behavior of ginsenosides in these processes as P. ginseng contains numerous substances in addition to ginsenosides. Moreover, there are difficulties in the identification of the pharmacokinetic characteristics of ginsenosides due to the fact that ginsenosides have different properties depending on the attached sugar moieties. Despite these complications, we have tried to scrutinize the considerable quantity of pharmacokinetic data for ginsenosides and assessed these data with regard to ADME.
3.1. Absorption
Many pharmacokinetic studies have shown that ginsenosides are very poorly absorbed and exhibit low bioavailability. Xie et al [42] investigated the pharmacokinetic behaviors of ginsenoside Rg3 and its metabolites in rats. Ginsenoside Rg3 is deglycosylated to ginsenoside Rh2 and then to PPD in vivo [53]. The authors administered an oral dose of 10 mg/kg of Rg3 or an intravenous dose of 1 mg/kg of Rg3 to rats. In the plasma concentration–time curve, Rg3 appeared first, followed by Rh2 and PPD after 1 h and 4 h, respectively. In addition, Rh2 and PPD were present at considerably lower concentrations than Rg3. This observation indicated that Rg3 was metabolized to Rh2 and then to PPD in rats; these metabolic changes are summarized in Fig. 2. The Cmax and Tmax values of Rg3 were 104.07 ± 59.95 ng/mL and 4.40 ± 1.67 h, respectively. In this study, the oral bioavailabilities of Rg3 and Rh2 were separately evaluated. The oral bioavailability of Rg3 was 2.63%, and it was not possible to measure the oral bioavailability of Rh2 because of the very low exposure levels to the systemic circulation [42]. Similarly, Ren et al [54] investigated the pharmacokinetic properties of PPD, the metabolite of ginsenoside Rg3 and Rh2. PPD was administered orally to rats at a dose of 2 mg/kg, and the plasma levels of PPD were measured by HPLC equipped with atmospheric pressure chemical ionization–mass spectrometry. In their study, the Cmax and Tmax of PPD were 130.2 ± 41.5 ng/mL and 150.0 ± 73.5 min, respectively. The oral bioavailability of PPD was evaluated as 36.8 ± 12.4%, which was unusually higher than that of ginsenosides Rg3 and Rh2. These results showed that Rg3 and Rh2 were rarely absorbed in the GI tract, whereas PPD was well absorbed. Such results suggest that PPD may be largely responsible for the major pharmacological effects of Rg3 and Rh2.
Several studies have investigated the possible differences in pharmacokinetic profiles and properties among ginsenoside epimers. Peng et al [53] assigned the rats into four groups and investigated the administration of 20(S)-Rg3 and 20(R)-Rg3 through the oral and intravenous routes. For intravenous administration, a 5 mg/kg dose of 20(S)-Rg3 and 20(R)-Rg3 was injected to the rats. After the intravenous administration, plasma concentrations of 20(S)-Rg3 and 20(R)-Rg3 rapidly decreased from 2 to 30 min; after 1 h, neither epimer was detectable. Cmax values were reported to be 70,096.2 ± 7,204.6 ng/mL for 20(S)-Rg3 and 46,439.2 ± 14,365.5 ng/mL for 20(R)-Rg3, with Tmax of 0.033 h for both epimers. As for the oral administration of Rg3 epimers, there were different results from those obtained after intravenous administration. After the oral administration of 20(S)-Rg3 to rats at a dose of 50 mg/kg, the Cmax and Tmax values were 98.1 ± 40.5 ng/mL and 5.7 ± 2.0 h, respectively, and the oral bioavailability of 20(S)-Rg3 was only 0.17%. In contrast, after the oral administration of 50 mg/kg of 20(R)-Rg3 to rats, traces of 20(R)-Rg3 could not be identified in plasma, which prevented the evaluation of the oral bioavailability of 20(R)-Rg3. However, instead of 20(R)-Rg3, very low levels of 20(S)-Rg3 and 20(R)-PPD could be detected. These results possibly indicated that the majority of 20(R)-Rg3 was converted to 20(S)-Rg3 by chiral inversion and 20(R)-PPD by deglycosylation in vivo. Although the mechanism of chiral inversion of Rg3 is still unknown, it was confirmed to be a single direction inversion from 20(R)-Rg3 to 20(S)-Rg3. The chiral inversion rate was calculated as 7.9% and 9.7% after intravenous and oral administration, respectively, in this study. The study reported that the chiral inversion of ginsenosides could occur in systemic circulation and during the absorption processes in the GI tract.
There were differences in the absorption of ginsenosides depending on their particle size or the animal species. Gu et al [41] examined the pharmacokinetic properties of ginsenoside Rh2 in rats and dogs, which are the rodent and nonrodent species generally used in nonclinical studies. As the oral bioavailability of ginsenosides was known to be very low owing to their poor aqueous solubility and low membrane permeability, the researchers therefore used micronized Rh2 to improve the absorption rate. Micronization reduced the particle size of Rh2 and increased the dissolution rates. Consequently, the absorption rate of Rh2 was greatly enhanced [54]. After the intravenous and oral administration of Rh2 at doses of 0.1 mg/kg and 1 mg/kg, respectively, to dogs, the calculated oral bioavailability of native Rh2 was approximately 16%, whereas that of micronized Rh2 was approximately 32%. These results indicate that the oral bioavailability of Rh2 was doubled by micronization. In addition, the Cmax of micronized Rh2 was approximately twice that of native Rh2. These results showed that the micronization effectively enhanced the oral bioavailability of ginsenosides in dogs. However, as the absorption processes of Rh2 in rats were more complicated than in dogs, the oral bioavailability evaluated in rats was only 4.7% after an oral dose of 1 mg/kg micronized Rh2. Moreover, the measured Tmax values provided an explanation of the difference in the oral bioavailability in dogs and rats. The Tmax values in rats were much longer than those in dogs, which were each 6 h and 1.8 h. In other words, the faster absorption of micronized Rh2 was more prominent in dogs than in rats. Such results were thought to be attributable to the anatomical and physiological differences between rats and dogs [41].
Xu et al [38] performed a study to compare the pharmacokinetic properties of ginsenosides Rb1 and Rg1. Ginsenoside Rb1 is categorized to the PPD group, and ginsenoside Rg1 is classified as the PPT group, as illustrated in Fig. 2. After the oral administration of ginsenosides at a dose of 50 mg/kg in rats, Rb1 was absorbed more slowly than Rg1. The comparison of the calculated pharmacokinetic parameters of Rb1 and Rg1 revealed that the Tmax and oral bioavailability of Rb1 were 1.5 h and 4.35%, respectively, and the Tmax and bioavailability of Rg1 were 1 h and 18.40%, respectively [38]. These results showed that the PPT group of ginsenosides exhibited better oral bioavailability than the PPD group. Such results are thought to be related to the slower metabolic changes of PPT-type ginsenosides (i.e., a slower disappearance from systemic circulation) than those of PPD-type ginsenosides [37].
3.2. Tissue distribution
Li et al [55] investigated the pharmacokinetic characteristics of ginsenosides Rg1, Rb1, and Rd in rat tissues after both oral and intravenous administration. The tissue distribution profiles of ginsenosides were evaluated at different time points after the administration of Rg1, Rb1, and Rd. After oral administration, the highest levels of these three ginsenosides were observed in the liver, followed by the lung, kidney, and spleen. On the other hand, when the ginsenosides were administered intravenously, their tissue concentrations varied depending on administration time in all organs other than the liver, which showed the highest distribution of the ginsenosides tested. Ginsenosides were distributed more rapidly after intravenous administration than after oral administration. Similarly, Feng et al [56] examined the tissue distribution profiles of Rg1 after intravenous administration to rats; their study also indicated that the highest levels of ginsenosides were observed in the liver, followed by the lung, spleen, and kidney.
In contrast, very low levels of ginsenosides were detected in the brain. Hao et al [57] reported the pharmacokinetic data of ginsenoside Rb1 evaluated in the plasma and brain after the intravenous administration at 20 mg/kg in rats. The plasma profiles of Rb1 revealed rapid distribution phases and prolonged terminal phases. The brain concentrations of Rb1 increased up to 1 h, and then there was a prolonged decrease in plasma levels of Rb1. The area under the curve (AUCplasma/AUCbrain) of ginsenoside Rb1 was measured as 47.1, which indicated that minimal transport of the ginsenoside occurred from the plasma to the brain. This can be attributed to the role of the well-known blood–brain barrier function.
High plasma protein binding is another important factor that can influence the tissue distribution of ginsenosides and their interactions with other drugs. Plasma protein binding is therefore a crucial factor that should be considered in pharmacokinetic evaluation [58]. Gu et al [41] examined the pharmacokinetic properties of ginsenoside Rh2, including plasma protein binding in rats. In this study, the binding of Rh2 to rat plasma proteins was reported to be 68.4 ± 4.9%, 72.6 ± 3.2%, 72.8 ± 1.2%, and 71.7 ± 4.0% at doses of 50, 100, 200, and 400 ng/mL, respectively. The plasma protein binding rates of Rh2 in rats were similar (approximately 70%) at all doses tested, which presumably indicated saturation of the binding ability of plasma proteins [41]. In another study assessing the plasma protein binding of Rh2, there were significant species-specific differences between plasma protein binding rates in rats and humans. This study found that Rh2 showed considerable species-dependent plasma protein binding rates of approximately 70% in rats and 27% in humans [59].
In general, the tissue distribution of ginsenosides reported tended to be low in most tissues, excluding the liver, and extremely low in the brain. To facilitate the distribution of ginsenosides to the brain, the administration of ginsenosides through routes that can avoid the blood–brain barrier, such as intranasal or intratympanic administration, has been studied [60]. More specifically, the greater distribution of ginsenosides to the liver may be attributable to the existence of the active uptake mechanisms of ginsenosides [49]. Collectively, the distribution of ginsenosides could be altered by their plasma protein binding properties; in addition, there were species-specific differences between plasma protein binding properties in animals and humans.
3.3. Metabolism
Ginsenosides have been known to exert various pharmacological effects in the body. However, the metabolic pathways of ginsenosides have only recently been reported. The typical metabolic processes of ginsenosides were reported to be deglycosylation and oxidation, which mainly occur in the liver. In addition, numerous studies confirmed that the predominant metabolism of ginsenosides occurred in the GI tract. This was thought to occur because intestinal microorganisms and enzymes are responsible for the metabolic degradation of ginsenosides in the GI tract [61].
Feng et al [56] studied the metabolic pathways of ginsenoside Rg1 in the rat intestine. The researchers demonstrated that ginsenoside Rg1 was easily biotransformed in vivo. They also revealed that Rg1 underwent multiple deglycosylations, which resulted in the formation of PPT via Rh1 or F1. In another study, Yang et al [62] studied the metabolic pathways of ginsenoside Re, one of the PPT-type ginsenosides such as Rg1. Rg1 and Re have similar chemical structures differing only in the C-20–attached rhamnose sugar [62]. In addition, both ginsenosides have common characteristics in that they were converted to Rh1 or F1 in the GI tract and that oxidation and deglycosylation were the major metabolic changes of the ginsenosides in rats. Ginsenosides Rb1, Rb2, Rc, and Rd, which are PPD-type ginsenosides, are all transformed to compound K in the GI tract. The other PPD-type ginsenosides Rg3 and Rg5 were converted to Rh2 and Rh3, respectively [37].
Furthermore, the metabolic pathways of ginsenosides exhibit different patterns depending on their administration routes. Yang et al [63] investigated the influence of administration route on the metabolic profile of ginsenoside Rd in animal models and humans by liquid chromatography–electrospray ionization–tandem mass spectrometry. After oral administration of Rd, deglycosylation was the major metabolic pathway, and ginsenoside Rg3 was the dominant metabolite. In contrast, after intravenous administration of Rd, oxidation and deglycosylation were the major metabolic pathways, and ginsenoside Rb1 was the dominant metabolite.
Although previous studies have focused on the role of the GI tract in the metabolism of ginsenosides, the liver plays an equally important role in the biotransformation of ginsenosides in the GI tract. Ginsenosides were metabolized in the liver, mostly by oxygenation caused by hepatic cytochrome P450 3A4, the most abundant form of cytochrome P450 [64]. These studies commonly indicated that the GI tract and liver are the major metabolic organs in which deglycosylation and/or oxidation occur. In addition, the intestinal flora that excrete β-glucosaccharase are also involved in the hydrolysis of the glycosidic bond of ginsenosides [60].
3.4. Excretion
Ginsenosides are excreted mainly via bile, urine, and feces. He et al [65] administered an oral dose of 25 mg/kg of ginsenoside Rg1 to rats, and the researchers monitored the metabolites Rh1 and PPT, along with Rg1 in bile, urine, and feces. Rg1 was excreted in urine for up to 12 h and in bile and feces for up to 48 h. Ginsenoside Rg1 is known to be metabolized to PPT via Rh1. However, in this study, Rh1 and PPT were not detectable in urine or bile. The mean recovery of Rg1 was the highest in feces, at 40.11 ± 12.74%, followed by bile and urine, at 6.88 ± 2.56% and 0.038 ± 0.021%, respectively. In contrast, Feng et al [56] reported that the mean recovery of Rg1 was the highest in bile, at 60.77%, followed by urine and feces, at 27.95% and 7.64%, respectively, after the intravenous administration of Rg1. Qian et al [66] were not able to detect ginsenoside Rg3 in rat plasma and urine at 1.5 h after oral administration in rats owing largely to the rapid clearance of Rg3 from the body. Only 0.97–1.15% of the administered Rg3 was recovered in feces after oral administration at a dose of 100 mg/kg.
There were some differences in the elimination process of ginsenosides depending on the animal species. Gu et al [41] analyzed the excretion processes of ginsenoside Rh2 in dogs and rats. After the intravenous administration at a dose of 0.1 mg/kg, the systemic clearances of Rh2 in dogs and rats were measured as 2.1 and 20.9 mL/min/kg, respectively. In addition, the body extraction ratio, which refers to the body clearance divided by cardiac output, was calculated as 0.02 and 0.09 in dogs and rats, respectively. Differences in species showed that the excretion processes of ginsenosides in rats were more extensive than those in dogs [67]. In this study, the cumulative excretion rates of the intact Rh2 excreted in bile and feces were calculated after intravenous and oral administrations at doses of 0.1 and 1 mg/kg to rats, respectively. As a result, approximately 30% of the administered Rh2 was excreted in bile in the intact form after intravenous administration, whereas only approximately 1% of Rh2 was recovered in feces in the unchanged form after oral administration. These results indicated that biliary excretion was the crucial excretion pathway of Rh2 [41].
The biliary tract and kidney play an important role in the excretion of ginsenosides. Many studies have reported that most ginsenosides and their metabolites undergo rapid and extensive biliary excretion through active transport. In contrast with excretion via the biliary tract, the renal excretion of most ginsenosides is generally slow. However, ginsenosides Ra3, Rb1, Rc, and Rd showed slow but extensive renal excretion, whereas ginsenoside Rg1 exhibited rapid renal excretion, including active tubular secretion. It is thought that such differences in the rate of excretion resulted from the attachment of sugar moieties in the ginsenoside. For example, ginsenoside Rb1, a PPD-type ginsenoside with five sugar moieties, showed slow biliary excretion owing to its large molecular size, which led to a reduced chance of uptake by the biliary transporters. The slow excretion rate of ginsenosides enables their circulation in the plasma for a long time [49].
4. Conclusions
At present, there is a lack of comprehensive studies on the pharmacokinetic characteristics of ginsenosides as they have complicated structures and exist in many different chemical forms which are subdivided into several groups. Furthermore, these characteristics of ginsenosides also result in complex biotransformation pathways in the body. In general, most ginsenosides are poorly absorbed owing to their unsuitable physicochemical properties. Hence, ginsenosides commonly exhibit low oral bioavailability. Although there were slight differences in the pharmacokinetic properties and profiles of ginsenosides depending on the experimental conditions, such as administration routes and animal species, the low bioavailability, which is a common characteristic of ginsenosides, makes the clinical application of ginsenosides difficult. Several studies on the advanced delivery systems of ginsenosides have recently been conducted to facilitate the clinical usefulness of ginsenosides. However, we realized that the scientific information on pharmacokinetic characteristics of ginsenosides is still lacking. Therefore, in this review, we have tried to provide comprehensive information on the pharmacokinetic properties and profiles of ginsenosides, which can be helpful for the development of efficient dosage forms and diverse delivery systems of ginsenosides to improve their bioavailability. The optimized dosage forms and the delivery systems of ginsenosides will certainly maximize the therapeutic potentials of ginsenosides.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (No. 2015R1A5A1008958). This research was also supported by the Chung-Ang University Research Scholarship Grants in 2017.
References
- 1.Park S.E., Na C.S., Yoo S.A., Seo S.H., Son H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J Ginseng Res. 2017;41:36–42. doi: 10.1016/j.jgr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y., Shen L.H., Zhang J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J., Jiang Y., Wu L., Lu T., Xu G., Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 4.Park J.S., Shin J.A., Jung J.S., Hyun J.W., Le T.K.V., Kim D.H., Park E.M., Kim H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012;341:59–67. doi: 10.1124/jpet.111.189035. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y.J., Zhang X.L., Li M.H., Iqbal J., Bourantas C.V., Li J.J., Su X.Y., Muramatsu T., Tian N.L., Chen S.L. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharm. 2013;62:50–57. doi: 10.1097/FJC.0b013e31828f8d45. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y.H., Han B., Yu X.F., Qu S.C., Sui D.Y. Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury in rats. Pharm Biol. 2011;49:900–906. doi: 10.3109/13880209.2011.554845. [DOI] [PubMed] [Google Scholar]
- 7.Zhu D., Wu L., Li C.R., Wang X.W., Ma Y.J., Zhong Z.Y., Zhao H.B., Cui J., Xun S.F., Huang X.L. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem. 2009;108:117–124. doi: 10.1002/jcb.22233. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Shao Z.H., Xie J.T., Wang C.Z., Ramachandran S., Yin J.J., Aung H., Li C.Q., Qin G., Vanden Hoek T. The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch Pharm Res. 2012;35:1259–1267. doi: 10.1007/s12272-012-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Qiao L.F., Li S.S., Yang G.T. Protective effect of ginsenoside Rb1 against lung injury induced by intestinal ischemia-reperfusion in rats. Molecules. 2013;18:1214–1226. doi: 10.3390/molecules18011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong V.K.W., Cheung S.S.F., Li T., Jiang Z.H., Wang J.R., Dong H., Yi X.Q., Zhou H., Liu L.A. Asian ginseng extract inhibits in vitro and in vivo growth of mouse Lewis lung carcinoma via modulation of ERK-p53 and NF-κB signaling. J Cell Biochem. 2010;111:899–910. doi: 10.1002/jcb.22778. [DOI] [PubMed] [Google Scholar]
- 11.Geng L., Fan J., Gao Q.L., Yu J., Hua B.J. Preliminary study for the roles and mechanisms of 20(R)-ginsenoside Rg3 and PEG-PLGA-Rg3 nanoparticles in the Lewis lung cancer mice. Beijing Da Xue Xue Bao Yi Xue Ban. 2016;48:496–501. [PubMed] [Google Scholar]
- 12.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Hong S., Aravinthan A., Kim J.H., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J.J., Ding L.L., Wang B.C., Ren G.Y., Sun A.N., Deng C., Wei X.H., Mani S., Wang Z.T., Dou W. Notoginsenoside R1 attenuates experimental inflammatory bowel disease via pregnane X receptor activation. J Pharmacol Exp Ther. 2015;352:315–324. doi: 10.1124/jpet.114.218750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan S.J., Yu W.K., Lin Z.L., Chen Q.Y., Shi J.L., Dong Y., Duan K.P., Bai X.W., Xu L., Li J.S. Anti-inflammatory effect of ginsenoside Rb1 contributes to the recovery of gastrointestinal motility in the rat model of postoperative ileus. Biol Pharm Bull. 2014;37:1788–1794. doi: 10.1248/bpb.b14-00441. [DOI] [PubMed] [Google Scholar]
- 15.Hwang J.W., Baek Y.M., Jang I.S., Yang K.E., Lee D.G., Yoon S.J., Rho J., Cho C.K., Lee Y.W., Kwon K.R. An enzymatically fortified ginseng extract inhibits proliferation and induces apoptosis of KATO3 human gastric cancer cells via modulation of Bax, mTOR, PKB and IκBα. Mol Med Rep. 2015;11:670–676. doi: 10.3892/mmr.2014.2704. [DOI] [PubMed] [Google Scholar]
- 16.Lee S., Kim M.G., Ko S.K., Kim H.K., Leem K.H., Kim Y.J. Protective effect of ginsenoside Re on acute gastric mucosal lesion induced by compound 48/80. J Ginseng Res. 2014;38:89–96. doi: 10.1016/j.jgr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou Y.L., Tsai Y.H., Lin Y.H., Chao J.C.J. Ginseng extract and ginsenoside Rb1 attenuate carbon tetrachloride-induced liver fibrosis in rats. BMC Complem Altern Med. 2014:14. doi: 10.1186/1472-6882-14-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao T.Z., Chen F., Bo L.L., Xie Q., Yi W.J., Zou Y., Hu B.J., Li J.B., Deng X.M. Ginsenoside Rg1 protects mouse liver against ischemia-reperfusion injury through anti-inflammatory and anti-apoptosis properties. J Surg Res. 2014;191:231–238. doi: 10.1016/j.jss.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y., Chu S.F., Li J.W., Li J.P., Zhang Z., Xia C.Y., Heng Y., Zhang M.J., Hu J.F., Wei G.N. Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J Ethnopharmacol. 2015;173:231–240. doi: 10.1016/j.jep.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Park H.M., Kim S.J., Kim J.S., Kang H.S. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem Toxicol. 2012;50:2736–2741. doi: 10.1016/j.fct.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Ko H., Kim Y.J., Park J.S., Park J.H., Yang H.O. Autophagy inhibition enhances apoptosis induced by ginsenoside Rk1 in hepatocellular carcinoma cells. Biosci Biotech Biochem. 2009;73:2183–2189. doi: 10.1271/bbb.90250. [DOI] [PubMed] [Google Scholar]
- 22.Toh D.F., Patel D.N., Chan E.C.Y., Teo A., Neo S.Y., Koh H.L. Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells. Chin Med UK. 2011;6:4. doi: 10.1186/1749-8546-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokozawa T., Kobayashi T., Oura H., Kawashima Y. Studies on the mechanism of the hypoglycemic activity of ginsenoside-Rb2 in streptozotocin-diabetic rats. Chem Pharm Bull (Tokyo) 1985;33:869–872. doi: 10.1248/cpb.33.869. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.J., Yuan H.D., Chung S.H. Ginsenoside Rg1 suppresses hepatic glucose production via AMP-activated protein kinase in HepG2 cells. Biol Pharm Bull. 2010;33:325–328. doi: 10.1248/bpb.33.325. [DOI] [PubMed] [Google Scholar]
- 25.Seo Y.S., Shon M.Y., Kong R., Kang O.H., Zhou T., Kim D.Y., Kwon D.Y. Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J Ethnopharmacol. 2016;190:231–240. doi: 10.1016/j.jep.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Wei S., Li W., Yu Y., Yao F., A L., Lan X., Guan F., Zhang M., Chen L. Ginsenoside compound K suppresses the hepatic gluconeogenesis via activating adenosine-5′ monophosphate kinase: a study in vitro and in vivo. Life Sci. 2015;139:8–15. doi: 10.1016/j.lfs.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Yang X.L., Guo T.K., Wang Y.H., Gao M.T., Qin H., Wu Y.J. Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch Pharm Res. 2012;35:1231–1239. doi: 10.1007/s12272-012-0714-6. [DOI] [PubMed] [Google Scholar]
- 28.Li B., Zhao J., Wang C.Z., Searle J., He T.C., Yuan C.S., Du W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301:185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C.Z., Yuan C.S. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S.Y., Kim G.T., Roh S.H., Song J.S., Kim H.J., Hong S.S., Kwon S.W., Park J.H. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci Biotechnol Biochem. 2009;73:811–816. doi: 10.1271/bbb.80637. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.M., Lee S.Y., Yuk D.Y., Moon D.C., Choi S.S., Kim Y., Han S.B., Oh K.W., Hong J.T. Inhibition of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res. 2009;32:755–765. doi: 10.1007/s12272-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 32.Yokozawa T., Liu Z.W., Dong E. A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron. 1998;78:201–206. doi: 10.1159/000044911. [DOI] [PubMed] [Google Scholar]
- 33.Baek S.H., Shin B.K., Kim N.J., Chang S.Y., Park J.H. Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo. J Ginseng Res. 2017;41:233–239. doi: 10.1016/j.jgr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang K.S., Ham J., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui J.F., Garle M., Bjorkhem I., Eneroth P. Determination of aglycones of ginsenosides in ginseng preparations sold in Sweden and in urine samples from Swedish athletes consuming ginseng. Scand J Clin Lab Invest. 1996;56:151–160. doi: 10.3109/00365519609088602. [DOI] [PubMed] [Google Scholar]
- 36.He W., Wang J., Zhang L., Liu Z. Biotransformation of ginsenosides and their aglycones. Int J Biomed Pharmaceut Sci. 2012;6:45–55. [Google Scholar]
- 37.Qi L.W., Wang C.Z., Du G.J., Zhang Z.Y., Calway T., Yuan C.S. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818–822. doi: 10.2174/138920011797470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 39.Takino Y. Studies on the pharmacodynamics of ginsenoside-Rg1, -Rb1 and -Rb2 in rats. Yakugaku Zasshi. 1994;114:550–564. [PubMed] [Google Scholar]
- 40.Li X., Wang G., Sun J., Hao H., Xiong Y., Yan B., Zheng Y., Sheng L. Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional Chinese medicine (TCM) in rats. Biol Pharm Bull. 2007;30:847–851. doi: 10.1248/bpb.30.847. [DOI] [PubMed] [Google Scholar]
- 41.Gu Y., Wang G.J., Sun J.G., Jia Y.W., Wang W., Xu M.J., Lv T., Zheng Y.T., Sai Y. Pharmacokinetic characterization of ginsenoside Rh2, an anticancer nutrient from ginseng, in rats and dogs. Food Chem Toxicol. 2009;47:2257–2268. doi: 10.1016/j.fct.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Xie H.T., Wang G.J., Sun J.G., Tucker I., Zhao X.C., Xie Y.Y., Li H., Jiang X.L., Wang R., Xu M.J. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr B. 2005;818:167–173. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 43.Xie H.T., Wang G.J., Lv H., Sun R.W.J.G., Jiang X.L., Li H., Wang W., Huang C.R., Xu M.J. Development of a HPLC-MS assay for ginsenoside Rh2, a new anti-tumor substance from natural product and its pharmacokinetic study in dogs. Eur J Drug Metab Pharmacokinet. 2005;30:63–67. doi: 10.1007/BF03226409. [DOI] [PubMed] [Google Scholar]
- 44.Lai L., Hao H., Liu Y., Zheng C., Wang Q., Wang G., Chen X. Characterization of pharmacokinetic profiles and metabolic pathways of 20(S)-ginsenoside Rh1 in vivo and in vitro. Planta Med. 2009;75:797–802. doi: 10.1055/s-0029-1185400. [DOI] [PubMed] [Google Scholar]
- 45.Joo K.M., Lee J.H., Jeon H.Y., Park C.W., Hong D.K., Jeong H.J., Lee S.J., Lee S.Y., Lim K.M. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J Pharm Biomed Anal. 2010;51:278–283. doi: 10.1016/j.jpba.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Kim H.K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res. 2013;37:451–456. doi: 10.5142/jgr.2013.37.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20–27. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Liu H., Yang J., Du F., Gao X., Ma X., Huang Y., Xu F., Niu W., Wang F., Mao Y. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 50.Dai L., Liu K.F., Si C.L., Wang L.Y., Liu J., He J., Lei J.D. Ginsenoside nanoparticle: a new green drug delivery system. J Mater Chem B. 2016;4:529–538. doi: 10.1039/c5tb02305j. [DOI] [PubMed] [Google Scholar]
- 51.Li L.A., Chen X.Y., Li D., Zhong D.F. Identification of 20(S)-protopanaxadiol metabolites in human liver microsomes and human hepatocytes. Drug Metab Dispos. 2011;39:472–483. doi: 10.1124/dmd.110.036723. [DOI] [PubMed] [Google Scholar]
- 52.Xiong J., Sun M.J., Guo J.X., Huang L.S., Wang S.J., Meng B.Y., Ping Q.N. Active absorption of ginsenoside Rg1 in vitro and in vivo: the role of sodium-dependent glucose co-transporter 1. J Pharm Pharmacol. 2009;61:381–386. doi: 10.1211/jpp/61.03.0014. [DOI] [PubMed] [Google Scholar]
- 53.Peng M., Li X.N., Zhang T., Ding Y., Yi Y.X., Le J., Yang Y.J., Chen X.J. Stereoselective pharmacokinetic and metabolism studies of 20(S)- and 20(R)-ginsenoside Rg3 epimers in rat plasma by liquid chromatography-electrospray ionization mass spectrometry. J Pharmaceut Biomed. 2016;121:215–224. doi: 10.1016/j.jpba.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Ren H.C., Sun J.G., Wang G.J., A J.Y., Xie H.T., Zha W.B., Yan B., Sun F.Z., Hao H.P., Gu S.H. Sensitive determination of 20(S)-protopanaxadiol in rat plasma using HPLC-APCI-MS: application of pharmacokinetic study in rats. J Pharm Biomed Anal. 2008;48:1476–1480. doi: 10.1016/j.jpba.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 55.Li L., Sheng Y.X., Zhang J.L., Wang S.S., Guo D.A. High-performance liquid chromatographic assay for the active saponins from Panax notoginseng in rat tissues. Biomed Chromatogr. 2006;20:327–335. doi: 10.1002/bmc.567. [DOI] [PubMed] [Google Scholar]
- 56.Feng L., Wang L., Hu C., Jiang X. Pharmacokinetics, tissue distribution, metabolism, and excretion of ginsenoside Rg1 in rats. Arch Pharm Res. 2010;33:1975–1984. doi: 10.1007/s12272-010-1213-2. [DOI] [PubMed] [Google Scholar]
- 57.Hao K., Gong P., Sun S.Q., Hao H.P., Wang G.J., Dai Y., Chen Y.C., Liang Y., Xie L., Li F.Y. Mechanism-based pharmacokinetic-pharmacodynamic modeling of the estrogen-like effect of ginsenoside Rb1 on neural 5-HT in ovariectomized mice. Eur J Pharm Sci. 2011;44:117–126. doi: 10.1016/j.ejps.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Toutain P.L., Bousquet-Melou A. Volumes of distribution. J Vet Pharmacol Ther. 2004;27:441–453. doi: 10.1111/j.1365-2885.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 59.Gu Y., Wang G., Sun J., Jia Y., Xu M., Wang W. In vitro assessment of plasma protein binding of 20(R)-ginsenoside Rh2 by equilibrium dialysis and LC-MS analysis: a case of species differences. Biol Pharm Bull. 2006;29:951–956. doi: 10.1248/bpb.29.951. [DOI] [PubMed] [Google Scholar]
- 60.Zeng M.F., Pan L.M., Qi S.M., Cao Y.T., Zhu H.X., Guo L.W., Zhou J. Systematic review of recent advances in pharmacokinetics of four classical Chinese medicines used for the treatment of cerebrovascular disease. Fitoterapia. 2013;88:50–75. doi: 10.1016/j.fitote.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 62.Yang L., Xu S.J., Liu C.J., Su Z.J. In vivo metabolism study of ginsenoside Re in rat using high-performance liquid chromatography coupled with tandem mass spectrometry. Anal Bioanal Chem. 2009;395:1441–1451. doi: 10.1007/s00216-009-3121-1. [DOI] [PubMed] [Google Scholar]
- 63.Yang L., Deng Y., Xu S., Zeng X. In vivo pharmacokinetic and metabolism studies of ginsenoside Rd. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;854:77–84. doi: 10.1016/j.jchromb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Hao H., Lai L., Zheng C., Wang Q., Yu G., Zhou X., Wu L., Gong P., Wang G. Microsomal cytochrome p450-mediated metabolism of protopanaxatriol ginsenosides: metabolite profile, reaction phenotyping, and structure-metabolism relationship. Drug Metab Dispos. 2010;38:1731–1739. doi: 10.1124/dmd.110.033845. [DOI] [PubMed] [Google Scholar]
- 65.He C., Feng R., Sun Y., Chu S., Chen J., Ma C., Fu J., Zhao Z., Huang M., Shou J. Simultaneous quantification of ginsenoside Rg1 and its metabolites by HPLC-MS/MS: Rg1 excretion in rat bile, urine and feces. Acta Pharm Sin B. 2016;6:593–599. doi: 10.1016/j.apsb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qian T., Cai Z., Wong R.N., Mak N.K., Jiang Z.H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 67.Toutain P.L., Bousquet-Melou A. Plasma clearance. J Vet Pharmacol Ther. 2004;27:415–425. doi: 10.1111/j.1365-2885.2004.00605.x. [DOI] [PubMed] [Google Scholar]