Abstract
Top of line (TOL) corrosion is a common corrosion case found on pipe that flows wet gas in oil and gas industry. The present study aims to evaluate the impact of mono ethylene glycol (MEG) on the TOL corrosion rate of low carbon steel in acidic environment with increased temperature. The study was conducted at temperature 50 °C and 70 °C in a solution containing 2000 ppm of acetic acid and 50 % volume of MEG. There are four types of experimental conditions with variation in the temperature and MEG content were used. The result present that MEG effectively reduce the condensation rate and TOL corrosion rate of low carbon steel at elevated temperature environment. This revealed that increasing of TOL corrosion inhibition effect along the experimental period. Moreover, the increased level of severity was associated with increased temperature. The results concluded that this procedure resulted due to decrease in condensation rate that is likely to reduce the supply of electrolyte for the corrosion reaction.
Keywords: Materials science, TOL corrosion, Acetic acid, MEG, Condensation rate
1. Introduction
Top of line (TOL) corrosion problem has been reported on several gas fields around the world, such as Crossfield Gasfield (Canada) [1], Tunu Gasfield (Indonesia) [2], and Lacq Gasfield (France) [3]. This type of corrosion occurs by the condensation of corrosive vapor on the top of pipeline which is caused by the temperature difference between inside and outside the pipe [4]. In The temperature inside the pipe transporting wet gas is between 50 – 105 °C [5]; while, the outside temperature varies from the atmospheric until deep sea temperature that could reach 4 °C [6]. In higher temperature of gas inside the pipe will increase corrosion rate [7]. The pipeline used to transport the wet gas is usually made of low carbon steel. However, low carbon steel has relatively low corrosion resistance to acetic acid, which is one of the corrosive substances often found in wet gas flow with typical concentration of 300–2000 ppm [6]. On the other hand, the presence of CO2 on the top of the line corrosion was also mentioned by Singer et al [8], Ruiz [9] and Anuar [10]. One of the alternative mitigations to diminish corrosion rate is using inhibitors and the spent of industry attain $3.7 billion annually [11]. Mono Ethylene Glycol (MEG) is another substance commonly found on wet gas flow to inhibit corrosion. It has been injected to reduce the hydrate formation risk. Beside the hydrate-inhibition effect, high concentration of MEG (50–90%) exhibit corrosion inhibition effect on the pipe transporting wet gas [12].
In particular, 50% volume of MEG has shown to reduce the corrosion rate of low carbon steel immersed in acetic acid solution [13]. Several mechanisms of inhibiting effect of MEG have been discussed in the literature. For instance, MEG can be absorbed on the steel surface, which will prevent the steel to interact with the corrosive environment [12]. MEG is also able to decrease solubility of Fe ion leading to saturation of Fe ion and subsequently decreasing the corrosion rate [14]. In the TOL corrosion case, MEG has been reported to suppress the condensation rate by decreasing the vapor pressure of the liquid phase of wet gas [12, 14]. On the other hand, the modeling result of Svenningsen predicted that the concentration of MEG in the condensate will be lower than on the bulk solution [14].
It is important to investigate the effect of MEG on the TOL corrosion rate of low carbon steel in acetic acid environment at elevated temperature (i.e. 50 °C and 70 °C). Studies have been conducted to examine the effect of MEG on TOL corrosion; however, the present study aims to investigate the mechanism of MEG reducing TOL corrosion through the examination of factors that affects TOL corrosion like condensation rate and the composition of condensate by experimental chamber.
2. Methodology
The process of corrosion involves metal oxidation and concurrent reduction of corrosive species within the working solution. This shows significant correspondence between the rate of corrosion and rate of transfer between the aqueous oxidant and corroding metal surface. At top of the pipeline, the condensation occurs in a drop-wise condensation mode, rather than a film-wise condensation mode. This is likely to be observed through the morphology of corroded steel surface. The droplets contain a certain amount of MEG, when the water and MEG vapors are condensed at the top of the pipeline.
2.1. Materials
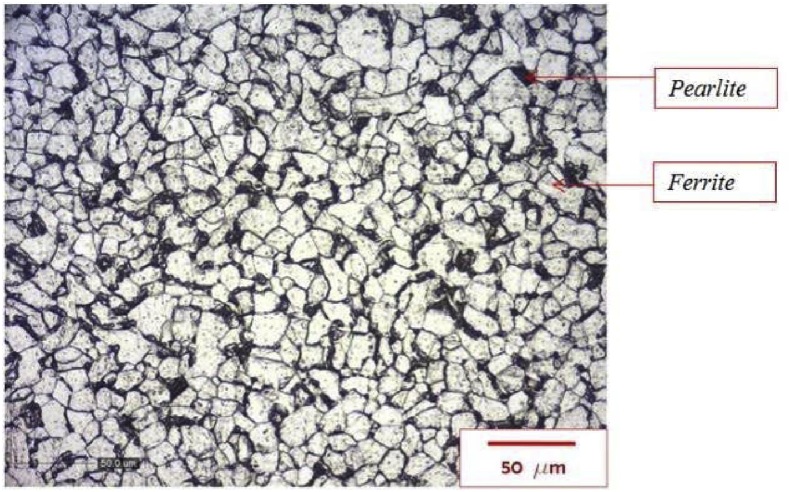
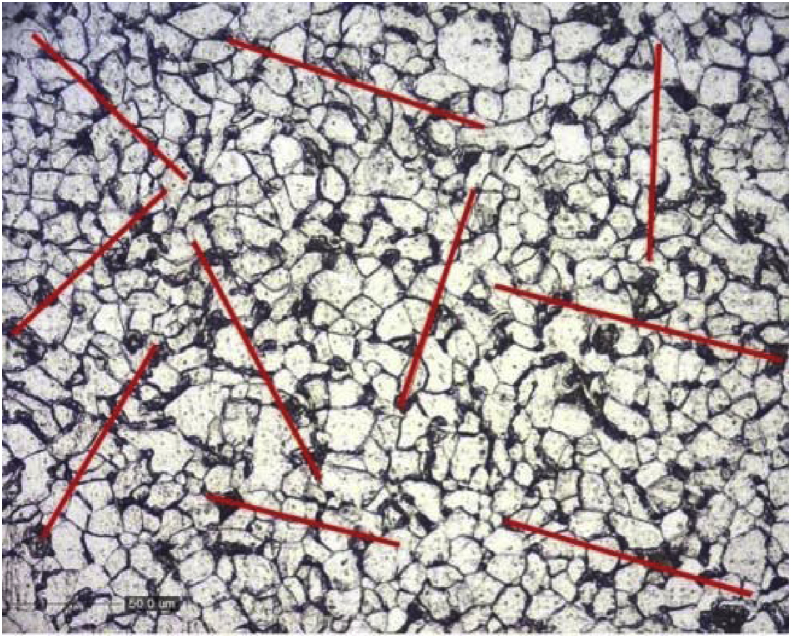
Carbon steel was employed as the corrosion specimen in this study. It was characterized by using the micro structure and hardness value. The micro structure of the corrosion specimen test is illustrated in Fig. 1. Line method that was proposed by Rosiwal [15] is used to determine carbon composition of the steel specimen. Fig. 2 has illustrated the lines obtained to measure carbon composition.
Fig. 1.
The microstructure of AISI 1018 steel of corrosion test specimen.
Fig. 2.
The lines that is used in line method.
The volume fraction of pearlite phase from the line method is 0.2238, which lead to 0.186% of carbon composition. Hence, the carbon content of the specimen is similar to AISI 1018 steel. Table 1 has shown the Vickers hardness value of specimen. The average hardness value of the specimen is lower than the hardness value of hot rolled AISI 1018 based on ASM handbook [16], due to the difference between the grain size of the specimen and AISI 1018.
Table 1.
Vickers hardness value of sample.
| No. | Hardness (VHN) |
|---|---|
| 1 | 110 |
| 2 | 109 |
| 3 | 109 |
| Average | 109.3 |
| AISI 1018 | 124 [16] |
Stainless steel, technical grade of acetic acid (98%v), demineralized water, and MEG (99.8%v) were used for the condensation test.
2.2. Experimental design
The present study has used four types of experimental conditions with variation in the temperature and MEG content. The volume of MEG that is used on the experiment is same as acetic acid solution volume (1:1 by volume). Table 2 has shown the detail of each condition. The corrosion rate of each treatment, that measured based on ASTM G-1, was calculated every two days for six days. This experiment also examines condensation rate of each treatment and MEG composition in the condensate. The study was executed in 2 different temperature, because the beginning of this work is dedicated to create the test equipment and doing test performance of this equipment.
Table 2.
Design of the experiment.
| Parameter | Day-2 | Day-4 | Day-6 |
|---|---|---|---|
| Temperature: 50 °C, Acetic Acid, 2000 ppm | 50-Day 2 | 50-Day 4 | 50-Day 6 |
| Temperature: 70 °C, Acetic Acid, 2000 ppm | 70-Day 2 | 70-Day 4 | 70-Day 6 |
| Temperature: 50 °C, Acetic Acid, 2000 ppm + MEG |
50-Day 2- MEG | 50-Day 4- MEG | 50-Day 6- MEG |
| Temperature:70oC, Acetic Acid, 2000 ppm + MEG | 70-Day 2- MEG | 70-Day 4- MEG | 70-Day 6- MEG |
2.3. Experimental equipment
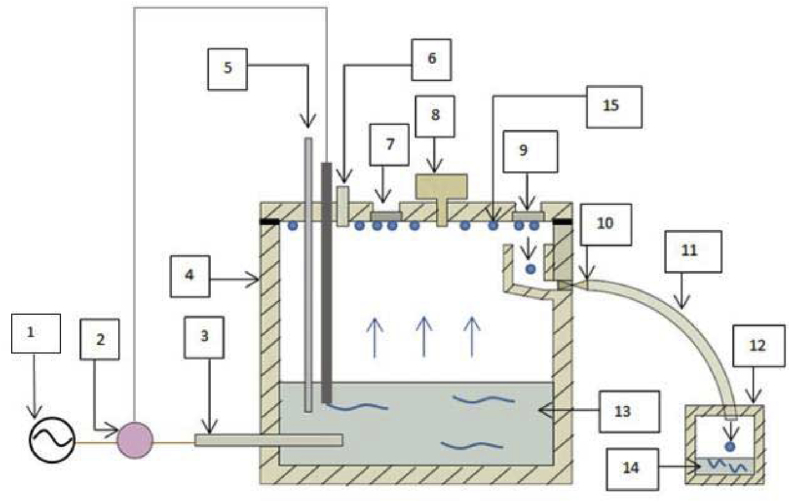
The experiment uses the chamber that can simulate the top of line corrosion by evaporation of heated solution. The scheme of chamber has been illustrated in Fig. 3.
Fig. 3.
Scheme of experimental chamber to simulate top of line corrosion condition.
(1) Electricity source, (2) Thermostat, (3) Element heater, (4) Chamber wall, (5) Thermometer, (6) Safety valve, (7) Corrosion test specimen, (8) Barometer, (9) Condensation test specimen, (10) Valve, (11) Hose, (12) Condensate collector, (13) Solution test, (14) Collected condensate, and (15) Formed condensate.
This chamber allowed to set the solution temperature by thermostat. During the experiment, condensate was formed on steel specimen surface and eventually it dropped to condensate collector. The collected condensate was examined for condensation rate and its composition. The corroded steel specimen was also checked for every specified time-based experimental design, to examine the corrosion rate of each treatment. The verification of materials using quantitative metallography is considered sufficient with this method because it is a commercial steel that classifies carbon content of steel.
3. Results
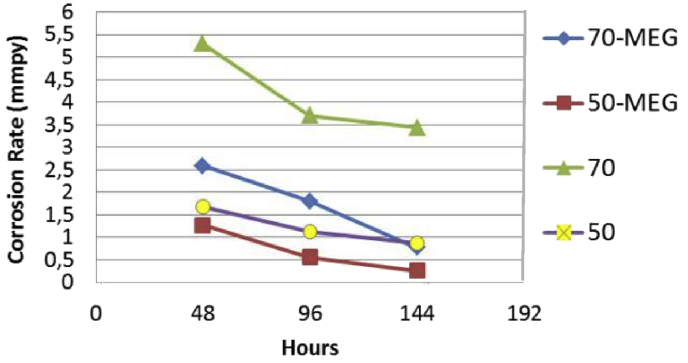
Addition of MEG into the solution of acetic acid decreased the TOL corrosion rate of specimen at 50 °C and 70 °C during the 144 hours experimental period, as shown by Fig. 4. Fig. 4 has also shown the increment of corrosion rate along the rise of the temperature.
Fig. 4.
The decreasing of TOL corrosion rate along with the addition of MEG.
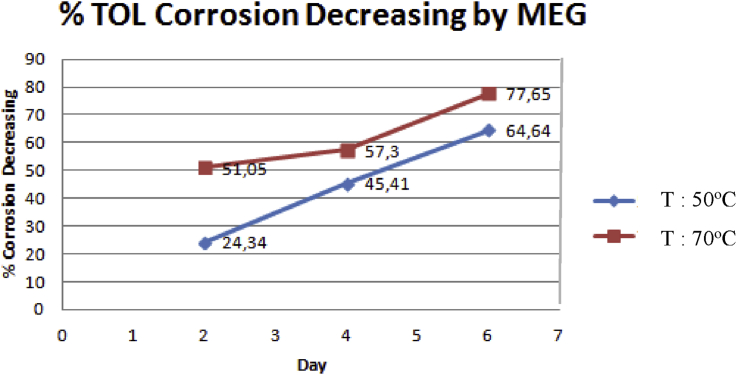
The experimental result on Fig. 4 could also be transformed to the percentage of TOL corrosion inhibition by addition of MEG as shown by Fig. 5 below. It shows that the addition of MEG could decrease the TOL corrosion on the second day by 51.05% at 70 °C and 24.34% at 50 °C. Fig. 5 has revealed the increasing of TOL corrosion inhibition effect along the experimental period.
Fig. 5.
The percentage of TOL corrosion rate decrease along with the addition of MEG.
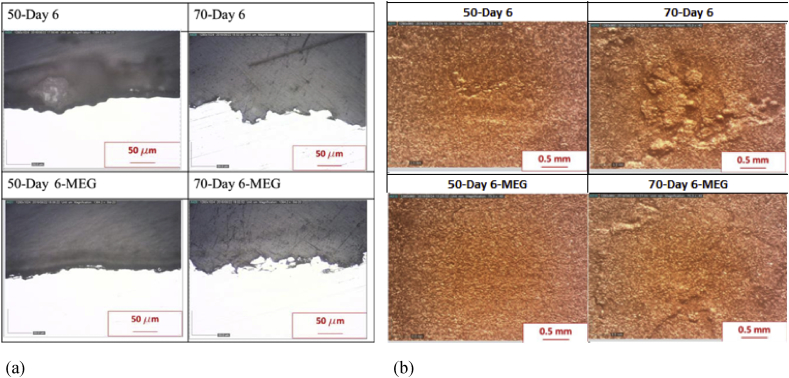
The inhibition effect of MEG on the TOL corrosion rate and the increment of TOL corrosion rate along with the rise of temperature is confirmed by surface observation of the specimen on Fig. 5. The decreasing of severity level on the specimen's surface along with the addition of MEG is shown in Fig. 6. Fig. 6 has shown the increasing of severity level along with the rise of the temperature.
Fig. 6.
The surface condition of each specimen after pickling. (a) Cross section of specimen surface observed by light microscope, (b) top view of speciment surface observed by macro camera.
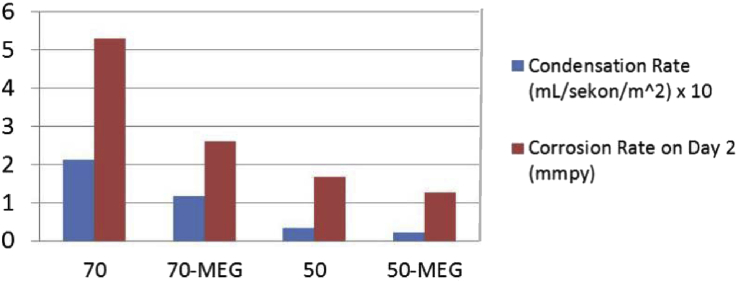
Condensation rate was also measured in this experiment and the results have been illustrated in Fig. 7. It shows that the addition of MEG decreased the condensation rate of the experiment conducted at the same temperatures. The increasing of temperature also increase the condensation rate of the experiment performed at the same solution.
Fig. 7.
The decreasing of condensation rate and TOL corrosion rate along with the addition of MEG.
The presence of MEG which has much lower vapor pressure than water and acetic acid at the same temperature tend to reduce the overall vapor pressure. The vapor pressure data of each pure component has been shown in Table 3. It was calculated based on Antoine equation. This experiment does not measure vapour pressure of each component as the limitation of experiment chamber capability. The vapour pressure data from Antoine equation could explain the mechanism of MEG in the solution and lower the condensation rate.
Table 3.
The vapor pressure of pure component at 50 °C and 70 °C.
| Component | Po at 50 °C mmHg) | Po at 70 °C (mmHg) |
|---|---|---|
| MEG | 0.7 | 2.8 |
| Water | 92.58 | 233.84 |
| Acetic Acid | 57.56 | 140.09 |
The vapor pressure data on Table 3 is obtained by Antoine formula below [17].
| Log Po = A – B/(T + C) | (1) |
Po = Vapor pressure of pure component
T = Temperature
A, B, and C = Constant for particular component
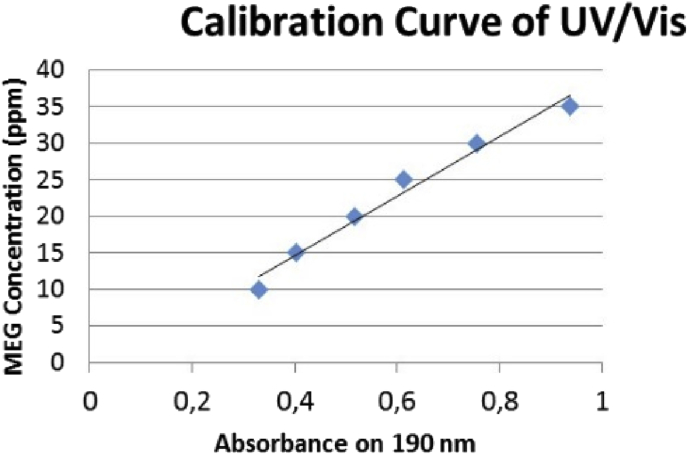
The concentration of MEG in condensate was measured by UV/VIS examination. The UV absorption on 190 nm is used to detect MEG content in water solution [18]. Fig. 8 shows calibration solution data used in this examination.
Fig. 8.
Calibration curve used to estimate MEG concentration in condensate.
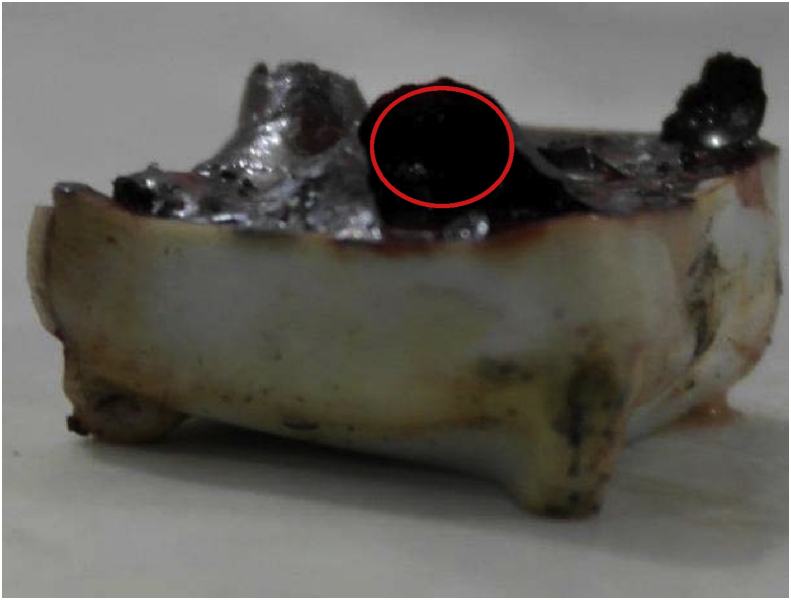
The concentration of MEG in condensate at day 2 has been shown in Table 4, revealing that the concentration of MEG in condensate was much lower as compared to the solution. Table 4 has also shown that the concentration of MEG in condensate is higher at temperature of 70 °C as compared to its counterpart at 50 °C. This idea is supported by the fact that MEG have much lower ability to re-evaporate than water. The cavity made by corrosion product give space for the condensate to be trapped for some period and suggest the possibility for the accumulation mechanism to happen (Fig. 9).
Table 4.
MEG concentration on condensate at day 2 base on UV-Vis examination.
| Absorbance on 190 nm | MEG Concentration (ppm) | MEG Concentration (%vol) | |
|---|---|---|---|
| 70 °C - MEG | 0.97 | 38.304 | 0.0034 |
| 50 °C - MEG | 0.42 | 15.593 | 0.0014 |
Fig. 9.
The area marked by red circle shows the cavity formed by corrosion product which leads the condensate to be trapped for some period.
4. Discussion
The results of present study have shown that the measured condensation rate was having the same trend with the TOL corrosion rate. This phenomenon was confirmed by Nyborg experimental result by the formula below [13].
| CR = f. R condensation. [Fe2+] | (2) |
CR = TOL corrosion rate
f = correction factor
R condensation = condensation rate
[Fe2+] = Fe ion concentration in condensate
The decreasing of condensation rate will reduce the electrolyte supply for the corrosion reaction. The decrement of the condensation rate along with the addition of MEG can be explained by the decreasing of vapor pressure in the solution. This phenomenon can be explained by the Raoult formula below (with the assumption of ideal solution) [17].
| Psolution = ∑ (Xcomponent . Pocomponent) | (3) |
P = Vapor pressure of solution
X = Fraction of component in the solution
Po = Vapor pressure of pure component
This theory is in agreement with the simulation study conducted by Svenningsen, who reported the reduction of vapor pressure along with the rise of MEG concentration on water-MEG solution [14]. Sufficient amount of MEG in the condensate will also effectively reduce the corrosion rate.
The corrosion inhibition of MEG on the low carbon steel immersed in acetic acid solution from Sarah Fauziana's report is used to understand the effect of MEG on the condensate [13]. Fauziana suggested that the addition of 50%v MEG reduces the condensation rate by 28% at 50 °C. In the present study, the effect of MEG concentration on the reduction of TOL corrosion rate was on the same level (24.34%) as Fauziana's report as the concentration of MEG in condensate was much lower than 50%v. This phenomena indicated the different inhibition mechanism of MEG happened for TOL corrosion case considered in the present study.
On the other hand, the increasing percentage of TOL corrosion inhibition effect by MEG addition indicated the contribution of MEG in the condensates on specimen surface. There is a possibility that MEG after condensation could be absorbed on the specimen surface included at the internal upper pipe walls [12, 19], especially if content of MEG is high (90%) that decrease of condensation rate and increase MEG content in condensing phase. While, water re-evaporate during the experiment, results in the accumulation of MEG by the time. It was mentioned by Boris et al. [20] and Ajayi [21] that the use of MEG as inhibitor will be effective in the presence of organic acid in a saturated vapor pressure that correlates with this study. The study of Ivonye revealed that the content of MEG must be higher than 50% to have an effective effect on TOL case [22].
The accumulation of MEG during the experimental period may result in other corrosion inhibition mechanisms, which are the absorption on the specimen surface [12] and the reduction of Fe ion solubility [14]. A precaution must be taken in case a very low pH of solution, where the presence of MEG looks like ineffective [23]. However, the inhibition mechanisms will contribute to the enhanced inhibition effect of the TOL corrosion rate. The decrease of organic acid also contribute to the decrease of corrosion rate [24], it has a correlation with the needs of MEG content that also decreases. A similar study investigated the phenomena of corrosion by acetic acid and carbon dioxide under dew condition at the top of horizontal pipeline [8]. The results showed the top of the line corrosion was significantly affected by the rate of condensation, when the influence is not that strong at the bottom [8]. It has been shown that MEG is commonly used in the oil and gas industry because of being injected in the subsea flowlines for preventing hydrate formation [9]. Another study showed that increase in the condensation rate also increases the corrosion rate [10].
5. Conclusion
The present study has evaluated the effect of Mono Ethylene Glycol (MEG) on the TOL corrosion rate of low carbon steel in acidic environment with increased temperature. The results showed the reduction of TOL corrosion rate of the specimen after addition of MEG into the solution of acetic acid at 50 °C and 70 °C. The mechanism is more likely caused by the reduction of condensation rate which will reduce the supply of electrolyte for the corrosion reaction. The increasing inhibition effect phenomena of MEG during experimental period, indicated the accumulation of MEG that could lead to another inhibition mechanism to happen. The results also showed that the addition of MEG could decrease the TOL corrosion on the second day by 51.05% at 70 °C and 24.34% at 50 °C. Moreover, at same temperature, the addition of MEG reduces the condensation rate of the experiment. These results have clearly concluded that all the concentrations of MEG have significant inhibiting impact in the rate of corrosion. However, future studies need to evaluate the impact of MEG on the formation of protective corrosion film along with examining the inhibition effect of MEG in the presence of corrosion scales.
Declarations
Author contribution statement
Bambang Widyanto & I Gede Bagus Eka Suputra Wiguna: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Bich N.N., Szklarz K.E. 1988. Crossfield Corrosion Experience. USA: NACE/Corrosion 88. Paper No. 196. [Google Scholar]
- 2.Supriyatman D., Achmad J., Gunaltun Y. 1999. Top of Line Corrosion in Multiphase Gas Line, a Case History. USA: NACE/Corrosion 99. Paper No 036. [Google Scholar]
- 3.Estavoyer M. NACE; USA: 1981. Corrosion Problems at Lacq Sour Gas Field; p. 905. [Google Scholar]
- 4.Keeper Gate. 10 October 2012. Top of Line Corrosion. GAT2004-GKP-2012. [Google Scholar]
- 5.Frenier W.W. Society of Petroleum Engineers; 2014. Multifaceted Aproaches for Controlling Top of the Line Corrosion in Pipelines. [Google Scholar]
- 6.Nybrorg R., Dugstad A. NACE/Corrosion 2007; USA: 2007. Top of Line Corrosion and Water Condensation Rates in Wet Gas Pipelines. Paper No. 07555. [Google Scholar]
- 7.Singer M., Nesic S., Gunaltun Y. CORROSION/2004; 2004. Top of the Line Corrosion in Presence of Acetic Acid and Carbon Dioxide. paper, (04377) [Google Scholar]
- 8.Singer M. NACE Corrosion; 2009. CO2 Top of Line Corrosion in Presence of Acetic Acid A Parameter Study. paper no 09292. [Google Scholar]
- 9.Ruiz R.A. The Department of Chemical and Biomolecular Engineering and the Russ College of Engineering and Technology; 2017. The Effect of Monoethylene Glycol (MEG) on CO2 Corrosion Mechanism, Master of Science Thesis. [Google Scholar]
- 10.Anuar S.S.A. Condensation rate effect on top of line corrosion in CO2 environment. ARPN J. Eng. Appl. Sci. Malaysia. 2016;11(20) October 2016. [Google Scholar]
- 11.TWI, Ltd . oct. 10, 2012. Corrosion and Corrosion Mitigation.http://www.twi.co.uk/industries/oil-and-gas/pipeline-integritymanagement/corrosion-and-corrosionmitigation [Google Scholar]
- 12.Ehsani H. Curtin University; Australia: 2013. Influence of Monoethylene Glycol (MEG) on the Corrosion Inhibition of Wet-Gas Flow Lines. [Google Scholar]
- 13.Fauziana Sarah. Institute of Technology Bandung; 2016. The Influence of Water Melon Extract to Corrosion Rate of API 5LX 65 Steel in NaCl Solution Saturated with CO2 and Containing Acetic Acid and Mono Ethylene Glycol (MEG), Bandung, Master Thesis on Materials Engineering Study Program. [Google Scholar]
- 14.Svenningsen G. 2014. Modelling of Top of Line Corrosion with Organic Acid and Glycol. Corrosion 2014/Paper No. 057. [Google Scholar]
- 15.Vandervoort G. McGraw-Hill; New York: 1999. Metallography Principles and Practice. [Google Scholar]
- 16.ASM . ASM International; 1990. ASM Vol 1 Iron and Steel Properties. [Google Scholar]
- 17.Atkins P. OUP Oxford; Oxford: 2010. Physical Chemistry. [Google Scholar]
- 18.Zhang Jianbin. China; Beijing: 2008. Hydrogen Bonding Interactions between Ethylene Glycol and Water: Density, Excess Molar Volume, and Spectral Study. [Google Scholar]
- 19.Guo S., Farelas F., Singer M. 2016. Effect of Monoethylene Glycol on Sweet Top of the Line Corrosion. NACE Corrosion 2016, paper no 7891. [Google Scholar]
- 20.Miksic Boris A. Chemical Treatment; August 2013. “Material Performance”. Vapor Corrosion Inhibitors for Top of the Line Corrosion. [Google Scholar]
- 21.Ajayi F.O. Faculty of Engineering and Physical Sciences, the University of Manchester; 2015. Mitigating Corrosion Risks in Oil and Gas Equipment by Electrochemical protection: Top of the Line Corrosion. PhD thesis. [Google Scholar]
- 22.Ivonye Ikechukwu Cyril. Institute of Engineering, Thermofluids, Surfaces and Interfaces, Scool of Mechanical Engineering, The University of Leeds; July 2014. Corrosion Proceses and Mechanisms in the Presence of Monoethylene Glycol (MEG) PhD Thesis. [Google Scholar]
- 23.Gonzales J.J., Afonso M.E. 2000. Corrosion Ofcarbon Steel in Monoethylene Glycol. NACE corrosion 2000, paper no 00498. [Google Scholar]
- 24.Jevremović I., Singer M., Achour M., Nešić S., Mišković-Stanković V. Development of an effective method for internal pipeline corrosion control in the presence of CO2. Zaštita materijala. 2016;57(2):195–204. [Google Scholar]