Abstract
MicroRNA-145 (miR-145) and long non-coding RNA (lncRNA) growth arrest specific 5 (GAS5) function as tumor suppressors in prostate carcinoma. The aim of the present study was to investigate the role of miR-145 and lncRNA GAS5 in prostate carcinoma. In the present study, miR-145 and lncRNA GAS5 expression levels were demonstrated to be downregulated in tumor tissues compared with adjacent healthy tissues of patients with prostate carcinoma. miR-145 and lncRNA GAS5 expression levels were found to be positively and significantly correlated in tumor tissues, but not in adjacent healthy tissues. A follow-up study revealed that low miR-145 and lncRNA GAS5 expression levels were associated with poor survival. Overexpression of miR-145 resulted in upregulated lncRNA GAS5, whereas lncRNA GAS5 overexpression or silencing did not affect miR-145 expression. Overexpression of miR-145 and lncRNA GAS5 promoted apoptosis and inhibited cell proliferation in prostate carcinoma cell lines, whereas lncRNA GAS5 knockdown had an opposite effect. In addition, lncRNA GAS5 knockdown partially attenuated the effect of miR-145 overexpression of cancer cell proliferation and apoptosis. Therefore, miR-145 may inhibit cell proliferation and induce apoptosis in human prostate carcinoma by upregulating lncRNA GAS5.
Keywords: prostate carcinoma, microRNA-145, long non-coding RNA growth arrest specific 5, proliferation, apoptosis
Introduction
Prostate carcinoma is one of the most frequently diagnosed malignancies in men worldwide (1). As a heavy burden on public health, prostate carcinoma has an unacceptably high mortality rate (2). Patients with prostate carcinoma are mostly treated with androgen ablation therapies; although there is a high rate of initial response, emergence of castration-resistant prostate carcinoma in 10–20% of patients, results in failure of treatment (3). Prostate cancer affects 1 in 9 men during their lifetime and <30% of patients with prostate cancer with evidence of metastasis will live longer than 5 years in cases (2,3). In addition, early stages of prostate carcinoma lack do not exhibit any noticeable symptoms and the majority of present are diagnosed with tumor metastasis, which makes surgical intervention, a radical treatment for solid tumors, unsuitable (4,5).
Non-coding RNAs (ncRNAs) are involved in many physiological and pathological processes (6,7). ncRNAs are divided into subgroups based on their length and functions; long ncRNAs (lncRNAs) are ncRNAs >200 nucleotides (8). A growing body of literature has shown that lncRNAs participate in cancer biology through their interactions with multiple signaling molecules (8–10), including microRNAs (miRNAs), which is another subgroup of ncRNAs (11). miR-145 and lncRNA growth arrest specific 5 (GAS5) serve a role as tumor suppressor in prostate carcinoma (12,13). Mir-145 and GAS5 negatively regulate cancer progression and are downregulated in prostate carcinoma (12,13). The results of the present study provided further evidence that miR-145 and lncRNA GAS5 may function in prostate carcinoma and demonstrated that miR-145 may inhibit cell proliferation and induce apoptosis in human prostate carcinoma possibly by upregulating lncRNA GAS5.
Materials and methods
Tissue samples and cell culture
Tumor tissue and adjacent healthy tissue samples were collected from 62 patients with prostate carcinoma who were admitted to Ruijin Hospital, Shanghai Jiaotong University (Shanghai, China) between January 2010 and January 2013. Patients were aged between 38 and 70 years (mean, 51.2±5.3 years). Inclusion criteria: i) Patients diagnosed by pathological examinations in Ruijin Hospital, Shanghai Jiaotong University; ii) patients with complete medical records treated at Ruijin Hospital, Shanghai Jiaotong University; iii) patients who participated in the 5-year follow-up after discharge; iv) patients willing to participate. Exclusion criteria: i) Patients diagnosed with other diseases; ii) patients who were transferred to other hospitals during treatment; iii) patients who died from other causes during the follow-up period. There were 12 cases in stage I, 14 cases in stage II, 18 cases in stage III and 18 cases in stage IV. The study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University. All participants signed informed consent before admission.
The 22Rv1 human prostate carcinoma cell line was used in this study. Cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM containing 10% fetal bovine serum (cat. no. 30-2020; ATCC) at 37°C in a 5% CO2 incubator with 98% humidity.
Follow-up study
Following discharge, all patients were followed-up monthly for 5 years and overall survival was recorded. Three patients who died from other causes were not included; a total of 57 patients completed the follow-up.
Reverse transcription-quantitative PCR (RT-qPCR)
To detect the expression levels of miR-145, miRNAs were extracted from 0.1 g of tissue specimen from each sample and 1×105 cells using miRNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA), and PCR reaction systems were prepared using TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). To detect the expression levels of lncRNA GAS5, total RNAs were extracted from 0.1 g of tissue specimen from each sample and 1×105 cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), reverse transcription was performed using MMLV Reverse Transcriptase 1st-Strand cDNA Synthesis kit (Lucigen Corporation, Middleton, WI, USA), and SYBR® Green Real-Time PCR Master Mix (Thermo Fisher Scientific, Inc.) was used to prepare qPCR reaction systems. The thermocycling conditions were 95°C for 55 sec, followed by 40 cycles of 95°C for 25 sec and 55.5°C for 30 sec. Primers for miR-145, lncRNA GAS5 and endogenous controls U6 and β-actin were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Primer sequences were: miR-145 forward, 5′-CAGTGCGTGTCGTGGAGT-3′ and reverse, 5′-AGGTCCAGTTTTCCCAGG-3′; U6 forward, 5′-CGCTTCACGAATTTGCGTGTCA-3′ and reverse, 5′-GCTTCGGCAGCACATATACTAAAAT-3′; GAS5 forward, 5′-CACACAGGCATTAGACAGA-3′ and reverse, 5′-GCTCCACACAGTGTAGTCA-3′; β-actin forward, 5′-GACCTCTATGCCAACACAG-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′. Expression of miR-145 was normalized to U6 and expression of lncRNA GAS5 was normalized to β-actin using the 2−ΔΔCq method (14).
Cell transfection
lncRNA GAS5 pcDNA3.1 expression vector and siRNA (5′-GCAGAACCATAAAGATGGTCCA-3′) were designed and constructed by Sangon Biotech Co., Ltd. Empty vectors and negative control (NC) siRNA (5′-TTCTCCGAACGTGTCACGTTT-3′) were also provided by Sangon Biotech Co., Ltd. MISSION® microRNA mimic hsa-miR-145 and scrambled NC miRNAs were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Cell transfections were performed using Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.) at 37°C with vectors, siRNAs and miRNAs at doses of 10, 40 and 40 nM, respectively. Cells were incubated with the transfection mixtures for 6 h. Cells treated with Lipofectamine® 2000 reagent only were used as untreated control cells. Cells transfected with empty vectors, NC siRNAs, or scrambled NC miRNAs were used as transfection controls. The thresholds for successful transfections were 200% for lncRNA GAS5 and miR-145 overexpression and 50% for lncRNA GAS5 knockdown. Further experiments were performed 24 h post-transfection.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was detected using CCK-8 assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Cell suspensions (5×104 cells/ml) were cultivated in a 96-well plate (0.1 ml/well) under normal conditions (37°C; 5% CO2), and 15 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added into each well at 24, 48, 72 and 96 h. Cells were incubated with CCK-8 solution for 4 h. Optical density values at 450 nm were measured using a plate reader to determine cell proliferation rate.
Apoptosis assay
Apoptosis was detected using cell a apoptosis assay. Serum-free DMEM was used to prepare cell suspensions (5×104 cells/ml). Cells were transferred to a 6-well plate (10 ml/well), cultivated for 60 h, and cell digestion with 0.25% trypsin was performed. Annexin V-FITC (Dojindo, Kumamoto, Japan) and propidium iodide (PI) staining was performed at room temperature for 30 min in the dark, and then flow cytometry was performed using BD Biosciences 2 Laser 4 Color FacsCalibur Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) to detect apoptotic cells. Data were analyzed using BD Paint-A-Gate™ Pro Software (BD Biosciences).
Statistical analysis
All experiments were performed in triplicate and data are presented as the mean ± standard deviation. Comparisons of lncRNA GAS5 and miR-145 expression between tumors and adjacent healthy tissues were performed using Student's paired t-test. Comparisons among multiple groups were performed by one-way analysis of variance followed by Fisher's Least Significant Difference post hoc test. Correlation analyses between the expression levels of lncRNA GAS5 and miR-145 were performed using Pearson's correlation coefficient. Patients were divided into high (n=32) and low (n=30) lncRNA GAS5 expression groups, as well as high (n=28) and low (n=34) miR-145 expression groups according to Youden's index. Association analyses between GAS5 and miR-145 expression in tumor tissue and patients' clinicopathological data were performed by χ2 test. Kaplan-Meier method was used to plot survival curves, which were compared using logrank test. P<0.05 was considered to indicate a statistically significant difference.
Results
lncRNA GAS5 and miR-145 expression levels are downregulated in tumor tissues compared with adjacent healthy tissues in patients with prostate carcinoma
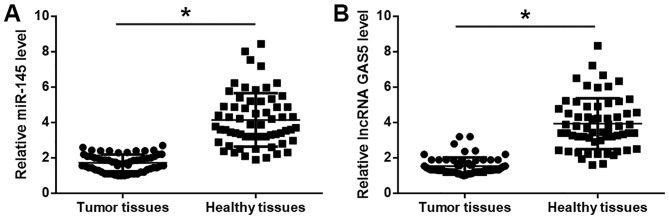
Expression levels of lncRNA GAS5 and miR-145 in 62 tumor tissues and adjacent healthy tissues were detected by RT-qPCR. Compared with adjacent healthy tissues, expression of lncRNA GAS5 (Fig. 1A) and miR-145 (Fig. 1B) were significantly downregulated in tumor tissues (P<0.05).
Figure 1.
Expression levels of lncRNA GAS5 and miR-145 are downregulated in tumor tissues compared with adjacent healthy tissues of patients with prostate carcinoma. (A and B) Compared with adjacent healthy tissues, expression of (A) lncRNA GAS5 and (B) miR-145 were significantly downregulated in tumor tissues. *P<0.05. lncRNA GAS5, long non-coding RNA growth arrest specific 5; miR-145, microRNA-145.
lncRNA GAS5 and miR-145 expression levels are positively correlated in tumor tissues
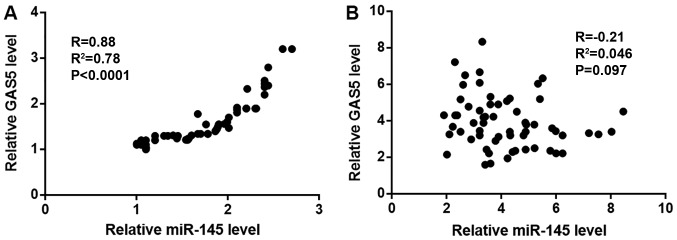
Correlation between the expression levels of lncRNA GAS5 and miR-145 was detected using Pearson's correlation coefficient. Expression levels of miR-145 and lncRNA GAS5 were positively and significantly correlated in tumor tissues (Fig. 2A). By contrast, no relationship was observed between lncRNA GAS5 and miR-145 expression levels in adjacent tissues (Fig. 2B).
Figure 2.
Expression levels of miR-145 and lncRNA GAS5 are correlated in tumor tissues. (A and B) Expression levels of miR-145 and lncRNA GAS5 were positively and significantly correlated in (A) tumor tissues, but not in (B) adjacent healthy tissues. lncRNA GAS5, long non-coding RNA growth arrest specific 5; miR-145, microRNA-145.
Low miR-145 and lncRNA GAS5 expression levels are associated with poor survival
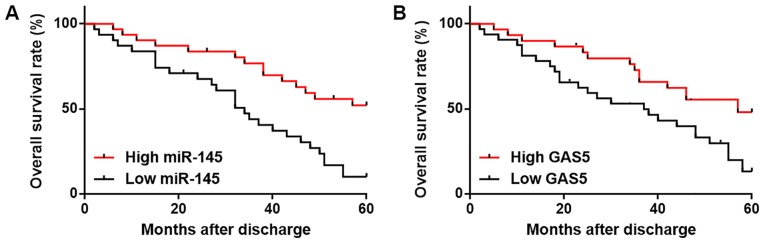
Patients were divided into high (n=32) and low (n=30) lncRNA GAS5 (cutoff value=1.58), as well as high (n=28) and low (n=34) miR-145 (cutoff value=1.64) expression groups according Youden's index. χ2 test revealed no significant association between expression levels of lncRNA GAS5 (Table I) and miR-145 (Table II) in tumor tissues and patient age or clinical stage. Kaplan-Meier method was used to plot survival curves, which were compared using logrank test. Patients in the low miR-145 (Fig. 3A) and low lncRNA GAS5 (Fig. 3B) groups exhibited significantly lower overall survival rates compared with patients in the high miR-145 and high lncRNA GAS5 groups (P=0.021).
Table I.
Association between lncRNA GAS5 expression levels in tumor tissue and patient clinicopathological characteristics.
| Expression | |||||
|---|---|---|---|---|---|
| Clinicopathological factor | Cases (n=62) | High | Low | χ2 | P-value |
| Age (years) | 0.59 | 0.44 | |||
| >50 | 30 | 17 | 13 | ||
| ≤50 | 32 | 15 | 17 | ||
| Stage | 0.78 | 0.85 | |||
| I | 12 | 7 | 5 | ||
| II | 14 | 8 | 6 | ||
| III | 18 | 8 | 10 | ||
| IV | 18 | 9 | 9 | ||
lncRNA GAS5, long non-coding RNA growth arrest specific 5.
Table II.
Association between miR-145 expression levels in tumor tissue and patient clinicopathological data.
| Expression | |||||
|---|---|---|---|---|---|
| Clinicopathological factor | Cases (n=62) | High | Low | χ2 | P-value |
| Age (years) | 1.56 | 0.21 | |||
| >50 | 30 | 16 | 14 | ||
| ≤50 | 32 | 12 | 20 | ||
| Stage | 1.16 | 0.76 | |||
| I | 12 | 5 | 7 | ||
| II | 14 | 6 | 8 | ||
| III | 18 | 10 | 8 | ||
| IV | 18 | 7 | 11 | ||
miR-145, microRNA-145.
Figure 3.
Low expression levels of miR-145 and lncRNA GAS5 are associated with poor survival. (A and B) Patients with low expression levels of (A) miR-145 and (B) lncRNA GAS5 exhibited lower survival rates compared with patients with high miR-145 and lncRNA GAS5 expression levels, respectively. lncRNA GAS5, long non-coding RNA growth arrest specific 5; miR-145, microRNA-145.
Overexpression of miR-145 results in upregulated lncRNA GAS5
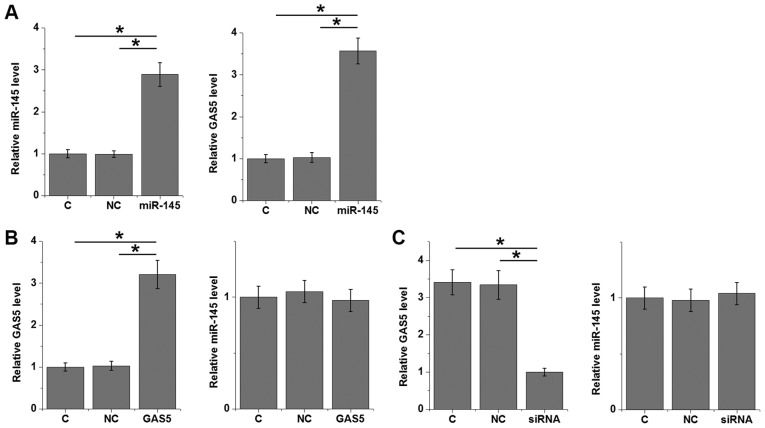
The significant and positive correlation between miR-145 and lncRNA GAS5 indicated a possible interaction between them. Overexpression experiments were performed in 22Rv1 prostate cancer cells to further investigate the interactions between miR-145 and lncRNA GAS5. Compared with control and NC groups, overexpression of miR-145 led to upregulated lncRNA GAS5 (P<0.05; Fig. 4A). By contrast, lncRNA GAS5 overexpression (P>0.05; Fig. 4B) and siRNA silencing (P>0.05; Fig. 4C) caused no significant changes in expression levels of miR-145.
Figure 4.
Overexpression of miR-145 results in upregulated lncRNA GAS5. (A) Overexpression of miR-145 led to upregulated lncRNA GAS5 in 22Rv1 prostate cancer cells. (B) lncRNA GAS5 overexpression and (C) siRNA silencing induced no significant changes in expression levels of miR-145. *P<0.05. C, untransfected control; lncRNA GAS5, long non-coding RNA growth arrest specific 5; miR-145, microRNA-145; NC, negative control.
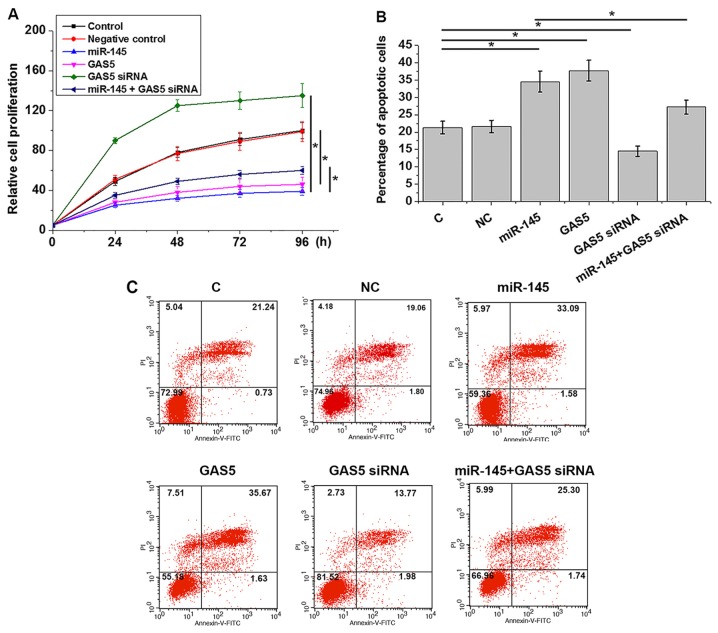
Overexpression of miR-145 inhibits cell proliferation and promotes apoptosis through lncRNA GAS5
Compared with control and NC groups, overexpression of miR-145 and lncRNA GAS5 led to significantly inhibited proliferation (Fig. 5A) and increased apoptosis (Fig. 5B) of 22Rv1 prostate cancer cells (P<0.05). lncRNA GAS5 knockdown attenuated the effects of miR-145 overexpression of cell proliferation (Fig. 5A) and apoptosis (Fig. 5B and C) (P<0.05).
Figure 5.
Overexpression of miR-145 inhibits cell proliferation and promotes cell apoptosis through lncRNA GAS5. (A and B) Overexpression of miR-145 and lncRNA GAS5 led to (A) significantly inhibited proliferation and (B) increased apoptosis of 22Rv1 prostate cancer cells. lncRNA GAS5 knockdown attenuated the effects of miR-145 overexpression on (A) cell proliferation and (B) apoptosis. (C) Representative images of apoptosis assay results. *P<0.05. C, untransfected control; lncRNA GAS5, long non-coding RNA growth arrest specific 5; miR-145, microRNA-145; NC, negative control.
Discussion
miR-145 and lncRNA GAS5 have exhibit similar functions in regulating the behaviours of cancerous cells during the development of prostate carcinoma (12,13); however, no studies examining a potential interaction have been published, to the best of our knowledge. The present study provided further evidence to support the role of miR-145 and lncRNA GAS5 as tumor suppressors in prostate carcinoma. The results also revealed that miR-145 inhibited cell proliferation and induced apoptosis in human prostate carcinoma possibly by upregulating lncRNA GAS5.
The interaction between lncRNAs and miRNAs are frequently reported in the development of human cancers. For example, during the development of hepatocellular cancer, miR-29a inhibits lncRNA maternally expressed 3 expression through promoter hypermethylation (15); miRNA-218-5p is negatively regulated by lncRNA colon cancer-associated transcript 1 to promote gallbladder cancer development (16). The present study focused on miR-145 and lncRNA GAS5 since they have similar functions in inhibiting prostate carcinoma (12,13). The results indicated that miR-145 may indirectly upregulate lncRNA GAS5 in prostate carcinoma cells. In a recent study, Xue et al demonstrated that lncRNA GAS5 targets miR-103 through the AKT serine/threonine kinase/mammalian target of rapamycin pathway to inhibit the progression of prostate carcinoma (17). Therefore, miRNAs may be upstream and downstream of lncRNA GAS5 to participate in the development of prostate carcinoma.
The present study failed to elucidate the molecular mechanism of the regulation of lncRNA GAS5 by miR-145 in prostate carcinoma cells. It has been reported that Notch-1 negatively regulates lncRNA GAS5 to promote breast cancer cell proliferation (18). In addition, miRNA-145 targets Notch signaling to induce apoptosis in glioma cells (19). However, no significant changes in expression levels of Notch-1 are observed after miRNA-145 overexpression (data not shown), which suggests that Notch-1 is unlikely the mediator between miRNA-145 and lncRNA GAS5 in prostate carcinoma. Notably, the present study demonstrated that expression levels of miR-145 and lncRNA GAS5 were positively and significantly correlated in tumor tissues, but not in adjacent healthy tissues. It is hypothesized that one of the existing prostate carcinoma-related factors may mediate the interactions between lncRNA GAS5 and miR-145; however, future studies are required to identify these factors.
The present study failed to perform the knockdown of miR-145 due to a technical problem. This experiment needs to be performed in future studies to further validate the conclusions of the present study. More cell lines also need to be used to analyze the effects of different expression levels of lncRNA GAS5 on cancer cell behaviors.
In conclusion, miR-145 and lncRNA GAS5 may function as tumor suppressors in prostate carcinoma. miR-145 may inhibit cell proliferation and induce apoptosis in human prostate carcinoma by upregulating lncRNA GAS5.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Shanghai Municipal Commission of Health and Family Planning (grant no. 201540081).
Availability of data and materials
The analyzed datasets generated during the present study are available from the corresponding author on reasonable request.
Author's contributions
XX and WH designed experiments. XX, JD and XH performed experiments. XX, CF and WH analyzed data. WH wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University (Shanghai, China). All participants signed informed consent before admission.
Patient consent for publication
All patients signed informed consent for the possible publication of this study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS, Kallio HML, Högnäs G, Annala M, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: Regulators of disease. J Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 7.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 8.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 9.Gutschner T, Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Nangia-Makker P, Farhana L, Majumdar APN. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol Cancer. 2017;16:155. doi: 10.1186/s12943-017-0725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaman MS, Chen Y, Deng G, Shahryari V, Suh SO, Saini S, Majid S, Liu J, Khatri G, Tanaka Y, Dahiya R. The functional significance of microRNA-145 in prostate cancer. Br J Cancer. 2010;103:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832:1613–1623. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM, Patel T. MicroRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30:4750–4756. doi: 10.1038/onc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi: 10.1038/cddis.2014.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue D, Zhou C, Lu H, Xu R, Xu X, He X. lncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol. 2016 doi: 10.1007/s13277-016-5429-8. [DOI] [PubMed] [Google Scholar]
- 18.Pei J, Wang B. Notch-1 promotes breast cancer cells proliferation by regulating lncRNA GAS5. Int J Clin Exp Med. 2015;8:14464–14471. [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y, Li J, Xu T, Zhou DD, Zhang L, Wang X. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. Oncotarget. 2017;8:61510–61527. doi: 10.18632/oncotarget.18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the present study are available from the corresponding author on reasonable request.