Abstract
Programmed death receptor ligand 1 (PD-L1), which belongs to the B7 family, is overexpressed in a variety of human cancer types and serves a crucial role in immune escape by malignant cells. Programmed death receptor 1 (PD-1) is a specific PD-L1 receptor. PD-1/PD-L1 signaling inhibits the antitumor effects of dendritic cell (DC) immunization for tumor treatment. The aim of the present study was to determine whether inhibiting PD-L1 may increase the immunologic anti-tumor effect of dendritic cells against pancreatic cancer. In the present study, PD-L1 levels in non-cancerous and malignant tissue samples were compared, and the impact of PD-L1 downregulation on human pancreatic cancer PaTu8988 cells was determined by lentivirus-based RNA interference and DC immunotherapy. PD-L1 expression in pancreatic specimens was assessed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and immunohistochemistry. PaTu8988 cells expressing reduced levels of PD-L1 were generated by lentivirus-based knockdown to assess the mechanism by which the inhibition of PD-L1 signaling in DC immunization affects therapeutic outcomes in pancreatic cancer-bearing SCID-hu mice. PD-L1 levels were markedly elevated in pancreatic adenocarcinoma samples compared with in non-cancerous tissue. PD-L1 silencing in pancreatic adenocarcinoma cells resulted in improved treatment outcomes of DC immunization in vitro and in vivo compared with traditional DC immunization. PD-L1 silencing enhances the antitumor response of cytotoxic T cells by increasing interferon γ production in vitro. In vivo, this method prevented tumor growth and lung metastasis, and prolonged survival in the SCID-hu model. In conclusion, the results of the present study suggested that suppressing PD-L1 in malignant cells during DC immunization may be a useful tool for immunotherapy in pancreatic adenocarcinoma.
Keywords: pancreatic cancer, RNA interference, programmed death receptor ligand 1, dendritic cell vaccine
Introduction
Pancreatic adenocarcinoma is a common and highly aggressive malignancy that affects the digestive system (1). Occult infiltration and early rapid dissemination make pancreatic adenocarcinoma the fourth deadliest cancer worldwide, with the mortality rate being ~7% in both sexes (2,3). Surgery is the exclusive method for radical treatment of pancreatic cancer, with adjuvant chemotherapeutics such as gemcitabine and S-1 often administered post-surgery (4). Despite recent therapeutic advances, the incidence of pancreatic adenocarcinoma is steadily increasing with the rate of new cases being ~3% in females, and the 5-year survival rate in definite cases is only ~8%, largely due to the low rate of early diagnosis and the limited resection rate of advanced tumors (3,5). It is estimated that by 2030, pancreatic cancer will overtake breast cancer to become the second deadliest malignant tumor in the USA (6). In addition, there are no effective therapeutic options currently available to eradicate metastasis (7).
Immunotherapy induces cancer-specific immune reactions in the host, with remarkable therapeutic effects, which are enhanced by cytokines and interleukins (8). Successful tumor immunotherapy should counteract immune evasion and tolerance in cancer cells. Immunotherapy aims to inhibit cancer cells by specifically activating antitumor immune reactions (9), which are controlled, in particular, by cytotoxic (CD8+) T cells. Their precursors, dendritic cells (DCs), are the most powerful antigen-presenting cells that stimulate naive T cells with high efficiency to produce CD8+ T cells (10). Immature DCs populate peripheral tissues to capture antigens, followed by maturation and migration through afferent lymphatic vessels into the draining lymph nodes (11). DCs are crucial in inducing tumor-specific immune responses, particularly through presentation of major histocompatibility complex-class I antigens and cytotoxic T cell production (12). DC-based tumor immunization represents a novel experimental immunotherapeutic approach in (13).
Programmed death receptor ligand 1 (PD-L1), which belongs to the B7 family of membrane proteins, was the first co-inhibitory protein the levels of which were reported to strongly correlate with cancer-specific survival (14). A number of studies have revealed the mechanisms by which tumors evade destruction by immune reactions associated with PD-L1 expression in cells (15–17). Therefore, the present study assessed whether lentivirus-mediated RNA interference (RNAi) targeting PD-L1 combined with DC vaccination may enhance T cell activity in pancreatic cancer. In addition, the effects of PD-L1 knockdown in cancer cells combined with DC vaccination on the inhibition of metastasis and survival were evaluated in a SCID-hu pancreatic tumor mouse model.
Materials and methods
Pancreatic cancer and clinical specimens
The present study was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University (Suzhou, China). A total of 46 patients with pancreatic adenocarcinoma surgically treated between January and December 2010 in the Department of General Surgery, The First Affiliated Hospital of Soochow University (Suzhou, China) were enrolled in the study. In the present study, the pancreatic cancer cases included 27 men and 19 women, with an average age of 61.5 years (range, 42–71 years). There were 26 patients with stage I–II tumors and 20 patients with stage III–IV tumors according to the 8th edition American Joint Committee on Cancer pancreatic cancer staging criteria (18). The criteria for including patients within the study included, patients who were diagnosed with pancreatic cancer prior to surgery and a diagnosis was confirmed via postoperative pathology. Exclusion criteria were: i) Treatment with chemotherapy or radiotherapy; ii) other pancreatic surgery prior to the surgical treatment of pancreatic adenocarcinoma. Pancreatic adenocarcinoma tissues were collected following signed written informed consent from the patients. Non-cancerous pancreatic tissue specimens were used as control samples. Hematoxylin and eosin (H&E) staining was performed to confirm diagnosis. During preparation and analysis, the tissue samples were divided in two by dissection under aseptic conditions; one part of the tissue specimens was submitted to fixation with 10% buffered methanol at room temperature for 24 h and used for PD-L1 detection by immunohistochemistry (IHC); the remaining part was frozen in liquid nitrogen for RNA extraction followed by reverse transcription-quantitative PCR (RT-qPCR) for PD-L1 mRNA level assessment. Peripheral blood samples were collected from healthy volunteers, which were prepared in order to extract peripheral blood mononuclear cells (PBMCs).
Immunohistochemical staining
Following overnight fixation with formalin at room temperature, the samples were embedded in paraffin and sectioned to 4-µm-thick slices using a microtome. The activity of endogenous peroxidase was inactivated by 3% H2O2 for 15 min, and 3% BSA was used to block non-specific binding at 37°C for 30 min. The sections were incubated overnight with rabbit anti-PD-L1 (dilution, 1:100; cat. no. ab205921; Abcam) primary antibody at 4°C, and further incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1000; cat. no. ab6721; Abcam) at 37°C for 30 min. The tissue sections were counterstained with hematoxylin, dehydrated in gradient concentrations of ethanol, and mounted in neutral resin. The presence of brown diaminobenzidine precipitates, which represent positive reactivity, was evaluated under a light microscope (magnification, ×200 and ×400). Brown cytoplasmic signals were considered to indicate PD-L1 expression.
RNA extraction and RT-qPCR
Total RNA extraction from 46 cases of resected pancreatic adenocarcinoma and non-cancerous pancreatic specimens was performed using the RNeasy® Mini kit (Qiagen GmbH) following the manufacturer's protocol. RNA amounts were assessed by UV spectrophotometry, and cDNA was obtained using the QuantiTect® Reverse Transcription kit (Qiagen GmbH) according to the manufacturer's protocol. qPCR was performed using SYBR Green real-time PCR Master Mix (Takara Bio, Inc.) on 7500 Fast Real-Time PCR system (Bio-Rad Laboratories, Inc.), and the experimental results were analyzed using the 2−ΔΔCq method to determine relative expression levels (19). GAPDH was used as a reference gene for normalization. The thermocycling conditions were as follows: Initial denaturation at 95°C for 15 sec, followed by 45 cycles of 95°C for 5 sec and 60°C for 30 sec. The primers used were: PD-L1 forward, 5′-ACTGGCATTTGCTGAACG-3′ and reverse, 5′-TCCTCCATTTCCCAATAGAC-3′ (148 bp); GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′ (121 bp).
Cells and culture
Pancreatic adenocarcinoma PaTu8988 cells were provided by the Institute of Biochemistry and Cell Biology (Chinese Academy of Science). The media contained 10% fetal bovine serum (Atlanta Biologicals, Inc.) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Inc.), and cells were cultured at 37°C in a humid atmosphere containing 5% CO2. Cells were harvested at 80–90% confluency using 0.25% trypsin. Small hairpin RNA (shRNA) of human PD-L1 (GenBank ID, NM_014143) harboring a lentivirus gene transfer vector that encodes green fluorescent protein (GFP) was obtained from Shanghai GeneChem Co., Ltd. The targeting sequence of PD-L1 was confirmed by sequencing to be 5′-GACCTATATGTGGTAGAGTAT-3′. The recombinant lentiviral vector expressing shRNA targeting PD-L1 (RNAi) and the non-targeted control mock lentivirus (NC) were adjusted to 5×109 transfection units/ml. PaTu8988 cells were seeded in 6-well plates (5×104 cells/well) overnight prior to transfection with lentiviral particles (multiplicity of infection, 100). The transfection was implemented at 37°C for 12 h, then culture medium was replaced by the normal culture medium, followed by incubation in a medium containing puromycin (5 µg/ml) for selecting stably-transfected cells. Fluorescent microscopy was used to evaluate GFP expression and assess infection efficiency 3 days after transfection. Non-transfected PaTu8988 cells were used as controls. PD-L1 mRNA knockdown in transfected cells was confirmed by RT-qPCR and immunoblotting.
Flow cytometric analysis
PaTu8988 cells that had not been subjected to any other treatments were digested with EDTA-pancreatin, collected and washed with PBS. Cells (1×106) were incubated with a rabbit phycoerythrin-conjugated anti-PD-L1 antibody (1:100; cat. no. ab228415; Abcam) for 30 min at 4°C, followed by incubation with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000; cat. no. ab150077; Abcam) for 30 min at 4°C in the dark. The collected cells were resuspended in 0.5 ml PBS and analyzed using Cytomics FC 500 flow cytometer (Beckman Coulter, Inc.). Data analysis was performed using FlowJo v10.0 software (FlowJo LLC).
Western blot analysis
A total of 1×107 cells were collected and washed twice prior to lysis on ice and then centrifuged for supernatant collection. The amounts of protein were assessed using Bio-Rad DC Protein assay (Bio-Rad Laboratories, Inc.). Proteins (50 µg per lane) were separated via SDS-PAGE (10% gel) and transferred onto polyvinylidene difluoride (PVDF) membranes. Following blocking with 5% milk dissolved in TBS containing 0.05% Tween-20 (TBST), at room temperature for 1 h, the membranes were incubated overnight with rabbit anti-PD-L1 (1:1,000; cat. no. ab228415; Abcam) and rabbit anti-GAPDH (1:1,000; cat. no. ab22555; Abcam) primary antibodies at 4°C. A horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (1:2,000; cat. no. ab6721; Abcam) was added for 1 h under shaker conditions at room temperature. Protein expression was determined using Enhanced Chemiluminescence Western Blot Detection Reagents (GE Healthcare).
CD8+ T cell antitumor response in vitro
PBMCs were obtained by Ficoll-Hypaque gradient centrifugation. By using a bead-labeled anti-CD8 monoclonal antibody CD8-PE (1:50; cat. no. 130113720; Miltenyi Biotec GmbH) and a MACS separation column (Miltenyi Biotec GmbH) following the manufacturer's protocol, CD8+ T lymphocytes were purified from PBMCs by immuno-magnetic reaction. The purity of CD8+ T cells was >95%, and they were used for T cell response experiments.
CD14+ monocytes (>95% purity) were obtained by magnetic cell separation (Miltenyi Biotec GmbH) and evaluated by flow cytometric analysis. The cells (3×105 cells/ml) were generated by following the classical technique for mature DC generation (20), and cultured in medium containing granulocyte macrophage colony-stimulating factor (GM-CSF; 25 ng/ml) and interleukin (IL)-4 (500 U/ml). Tumor necrosis factor (TNF)-α was supplemented at 5 days. Following two additional days of culture, the cells were prepared for T cell response experiments.
The control, NC and RNAi pancreatic cancer cell groups were washed with PBS three times following pre-treatment with mitomycin C (50 mg/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min to inhibit proliferation. Each group of pancreatic cancer cells (5×104 cells/group) was co-cultured with human CD8+ T lymphocytes (4×105 lymphocytes/well) and stimulated with CD14+ monocytes (8×104 monocytes/well). Cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS at 200 µl/well at 37°C in a humid environment containing 5% CO2 for 72 h. Co-cultures were performed three times in 96-well plates. At 72 h of co-culture, supernatants from the three groups were collected separately for ELISA of soluble interferon (IFN)-γ produced by CD8+ T lymphocytes using an ELISA kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Pancreatic cancer cells were also co-cultured with human CD8+ T lymphocytes without DCs as described above.
Preparation of the dendritic cell vaccine
Pancreatic cancer cells were thawed and cultured in RPMI-1640 containing 10% fetal bovine serum (FBS) (Sijiqing Biological Manufacturer Co., Ltd.) overnight at 37°C with 5% CO2. Monocyte culture was performed in presence of GM-CSF + IL-4 + isotype immunoglobulin G (IgG) for five days, and cells were treated with tumor lysates (cancer cell/DC ratio, 1:3) for 16 h. The cells were washed with PBS, and DC maturation was stimulated by adding TNF-α + isotype IgG to the culture medium for two additional days.
Establishment and confirmation of the SCID-hu mouse model
All experiments involving animals were approved by the Animal Care and Use Committee of Soochow University (Suzhou, China) and complied with current guidelines for animal welfare of the China National Committee for Animal Experiments. A total of 36, 6-week-old female SCID mice were supplied by Shanghai SLAC Laboratory Animal Co., Ltd., and kept under specific pathogen-free conditions in an environment with controlled humidity at 25–28°C in the dark. The mice had constant access to food and water. The SCID-hu model of pancreatic cancer was established as previously described (21). In brief, hemocytopoiesis in SCID mice was inhibited by administration of cerebrotendinous xanthomatosis at 40 mg/kg/d. Following intraperitoneal injections for four successive days, PBMCs (107 cells/animal) were administered by injection to the abdominal cavity of each SCID mouse in order to generate the SCID-hu model. Human T and B cells and human IgG levels in peripheral blood were detected by fluorescence-assisted cell sorting and ELISA. Pancreatic adenocarcinoma model establishment was performed in SCID-hu mice by subcutaneous injection of 100 µl pancreatic cancer cell suspension from each group (control, NC and RNAi; 2×104 cells/mouse) for 4 weeks following engraftment. There were 12 mice per group. Tumor growth was monitored every two days. The animal studies were performed at the Laboratory Animal Center of Soochow University (Suzhou, China) according to a protocol approved by the Animal Care and Use Committee of Soochow University.
Treatment of tumor-bearing mice
Tumor diameter was ~1 cm four weeks post-cancer cell administration. Each group of tumor-bearing mice was randomly divided into two sub-groups (six mice per sub-group). In each group, one sub-group received subcutaneous injections of the DC vaccine (1×106 cells/mouse), while the other did not, which served as the control. Therapeutic DC vaccines were administered weekly for four consecutive weeks. Tumor sizes were measured by Vernier calipers twice weekly, and volumes were calculated as V=LxW2×0.5. Mice were sacrificed by cervical dislocation 5 weeks later, and tumors were harvested five weeks following the completion of the DC vaccine treatment. Additionally, the thoracic cavity of each mouse was carefully opened to expose the trachea; India ink was intratracheally administered for lung staining, and the lung was rinsed with Fekete's solution. White nodules in the lung indicated metastatic pancreatic cancer cells. The number of metastatic tumor nodules on the lung surface was determined for each mouse. PD-L1 expression levels in the tumors and lung surface metastatic tumor nodules were determined by RT-qPCR as described above.
Assessment of mouse survival following DC vaccine treatment
The CD8+ T cell-DC vaccine-SCID-hu mice were used to establish the pancreatic cancer model by subcutaneous injection of 100 µl pancreatic cancer cell suspension (control, NC and RNAi; 2×104 cells/mouse) four weeks following engraftment. There were 16 mice per group. Each group of tumor-bearing mice was randomly divided into two sub-groups (8 mice per sub-group) four weeks following tumor cell inoculation. In each group, one subgroup received the DC vaccine treatment as described above, whereas the other did not, which served as the control. The DC vaccines were administered weekly in all the treatment sub-groups for four consecutive weeks. Following the completion of treatment, the survival rates of mice were monitored for 10 weeks. The survival time for each mouse was recorded and the survival curve was drawn.
Statistical analysis
Data are expressed as the mean ± standard deviation. The in vitro experiments were repeated three times. SPSS software (version 10.0; SPSS, Inc.) was used for the statistical analysis. Differences in values and percentages among groups were compared using a paired t-test, χ2 test, Fisher's exact test or one-way analysis of variance followed by Dunnett's multiple comparison test, as appropriate. Survival curves and univariate analysis were estimated by the Kaplan-Meier method and the log-rank test using GraphPad Prism software (version 7.0; GraphPad Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
PD-L1 is found in most pancreatic adenocarcinoma tissues and absent in normal pancreatic tissues
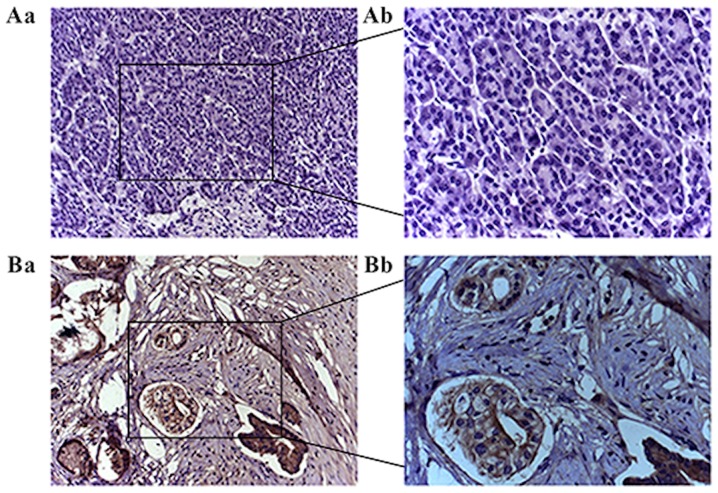
Relative expression levels of PD-L1 mRNA in pancreatic adenocarcinoma specimens were significantly increased compared with non-cancerous pancreatic tissue samples (P<0.001; Fig. 1). Immunohistochemical staining demonstrated significant overexpression of PD-L1 in pancreatic adenocarcinoma tissues compared with non-cancerous tissues (χ2=12.541; P<0.001; Fig. 2). PD-L1 was identified in >70% of cells in 34 out of 46 pancreatic cancer specimens, but not detected in normal pancreatic specimens (Fig. 2).
Figure 1.
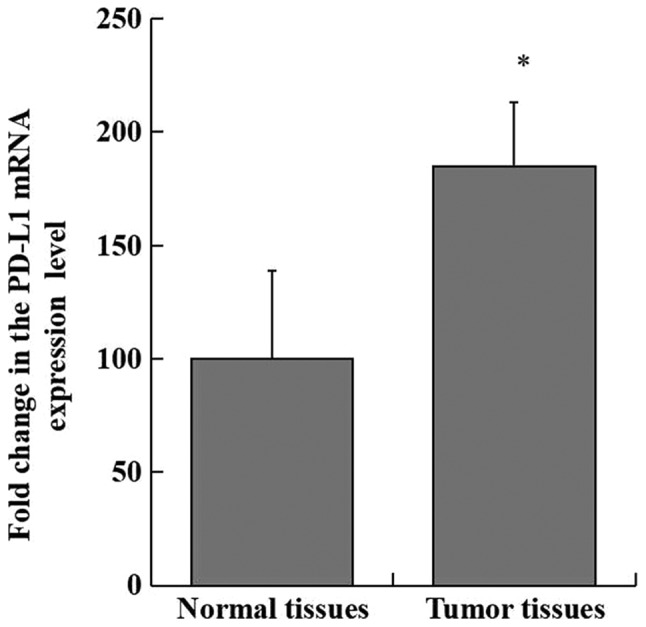
PD-L1 mRNA expression is increased in pancreatic adenocarcinoma compared with non-cancerous pancreatic tissues. Tumor tissues, pancreatic adenocarcinoma tissues obtained from patients with pancreatic adenocarcinoma. Normal tissues, paired precancerous tissue from patients with pancreatic adenocarcinoma. *P<0.01 vs. non-cancerous tissue.
Figure 2.
PD-L1 protein expression in clinical samples detected by immunohistochemistry. (A) No PD-L1 was observed in the non-cancerous pancreatic tissue. (B) PD-L1 was overexpressed in pancreatic adenocarcinoma. Magnification, A-a and B-a, ×100; A-b and B-b, ×200. PD-L1, programmed death receptor ligand.
Successful PD-L1 downregulation by RNA interference in PaTu8988 cells
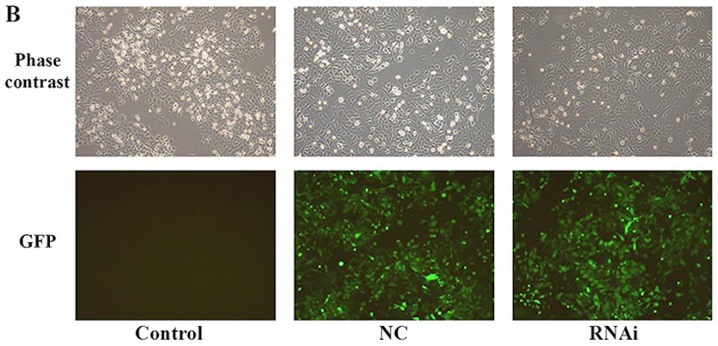
Flow cytometry was used to assess PD-L1 expression levels in PaTu8988 cell lines prior to lentiviral transfection (Fig. 3A). The results revealed that PD-L1 was highly expressed in PaTu8988 cell lines. Fluorescence microscopy was used to demonstrated that the lentiviral infection of PaTu8988 cells with RNAi and NC was successful and highly efficient (Fig. 3B). In addition, PD-L1 mRNA and protein expression levels were assessed; PD-L1 mRNA expression levels were significantly reduced in the RNAi group compared with the NC and control groups (25±7%; P<0.01; Fig. 4A and B); ~75% of gene expression was suppressed. The NC and control groups exhibited similar PD-L1 mRNA expression. PD-L1 protein expression levels detected by western blotting exhibited a similar pattern to mRNA expression (Fig. 4C). The present results indicated that RNAi downregulation of PD-L1 was specific and efficient.
Figure 3.
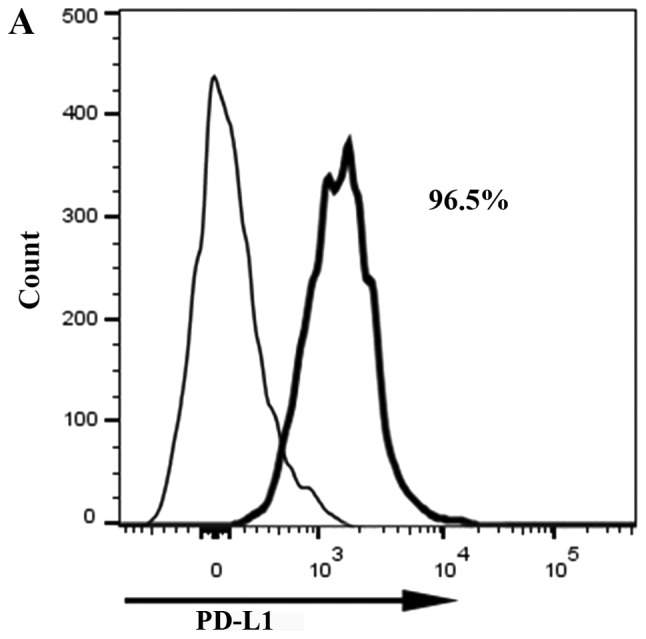
Efficiency of PD-L1 RNAi silencing. (A) PD-L1 expression levels in PaTu8988 cell lines prior to lentiviral infection evaluated by flow cytometry. (B) PaTu8988 cell infection with NC and RNAi lentiviruses. GFP expression was evaluated by fluorescence microscopy. Magnification, ×100. B7-H3, cluster of differentiation 276; GFP, green fluorescent protein; NC, negative control; PD-L1, programmed death receptor ligand 1; RNAi, cells transfected with short hairpin RNA targeting PD-L1.
Figure 4.
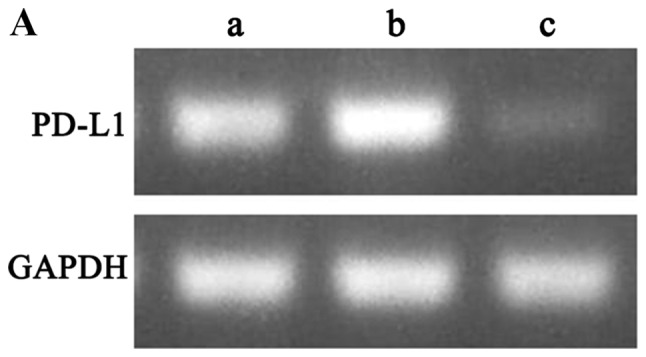
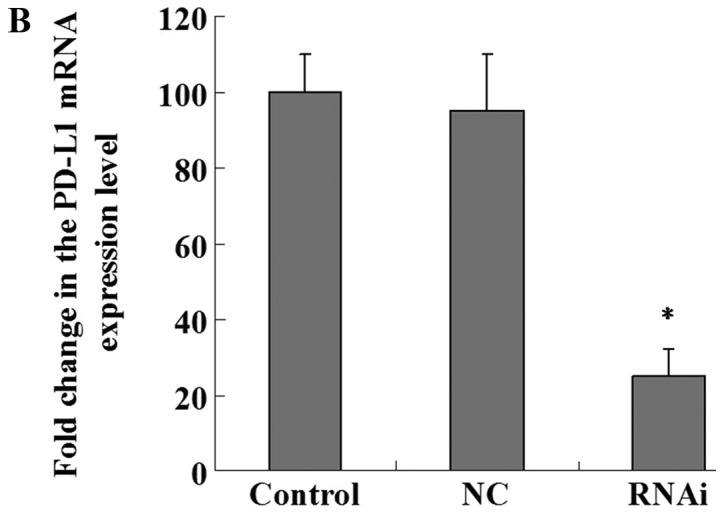
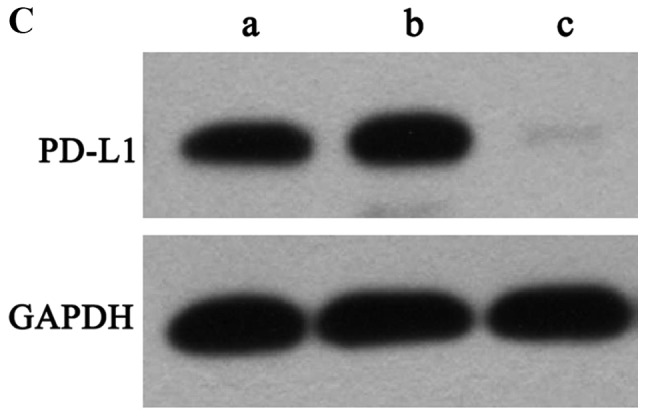
PD-L1 mRNA and protein expression levels are decreased following RNAi silencing. (A) Electrophoresis results of RT-qPCR products in the three groups of PaTu8988 cells (a, control; b, NC; c, RNAi). PB-L1 gene expression was reduced in the RNAi group compared with the control groups. (B) Quantification of PD-L1 mRNA levels by RT-qPCR following silencing with RNAi. PD-L1 mRNA levels were significantly decreased in the RNAi group. (C) PD-L1 protein expression levels detected by western blotting (a, control; b, NC; c, RNAi). PD-L1 protein expression in the RNAi group was reduced compared with that in the control and negative control groups. *P<0.01 vs. control. NC, negative control; PD-L1, programmed death receptor ligand 1; RNAi, cells transfected with short hairpin RNA targeting PD-L1; RT-qPCR, reverse transcription-quantitative PCR.
PD-L1 knockdown in PaTu8988 cells increases IFN-γ production by CD8+ T cells in co-culture with pancreatic adenocarcinoma cells and DCs
CD8+ T cells were simultaneously cultured with DCs and pancreatic adenocarcinoma cells to assess the role of PD-L1 expressed by pancreatic cancer cells in suppressing the effects of T-cells. Knockdown of PD-L1 significantly enhanced IFN-γ biosynthesis by CD8+ T lymphocytes (1,200±89 ng/ml) compared with the NC (345±54 ng/ml) and control (330±45 ng/ml) groups (P<0.01; Fig. 5). No statistically significant differences in IFN-γ levels were observed between the NC and control groups. Low levels of IFN-γ were detected in the three groups without co-culture with DCs.
Figure 5.
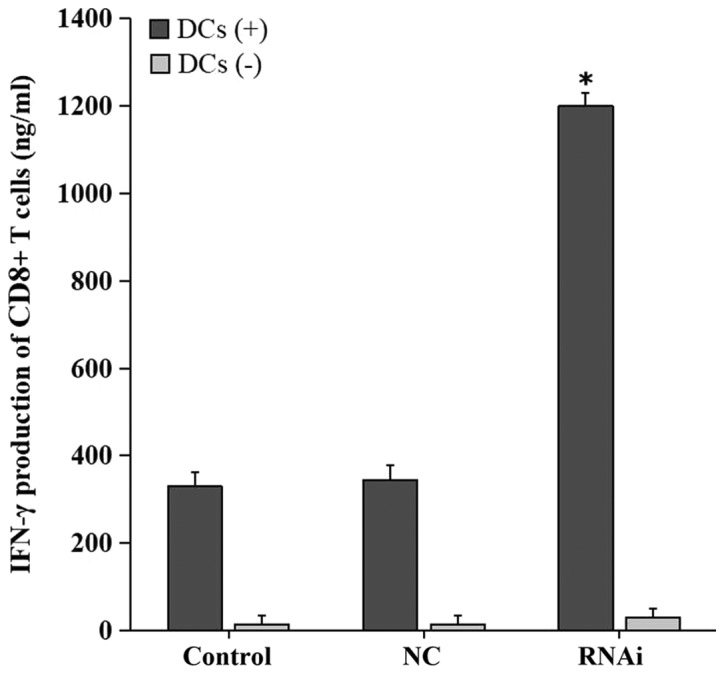
PD-L1 knockdown in cancer cells enhances T cell-associated IFN-γ secretion in vitro. CD8+ T lymphocytes were cultured in presence of tumor cells with or without mature DCs (DC+/−). IFN-γ levels in the supernatants were evaluated by ELISA. IFN-γ secretion by CD8+ T cells co-cultured with tumor cells of the RNAi group was significantly increased compared with that in the control groups. Data are from three independent experiments. *P<0.01 vs. control. DC, dendritic cells; IFN-γ, interferon γ; NC, negative control; RNAi, cells transfected with short hairpin RNA targeting programmed death receptor ligand 1.
PD-L1 silencing enhances the efficacy of DC vaccination in a subcutaneous tumor model
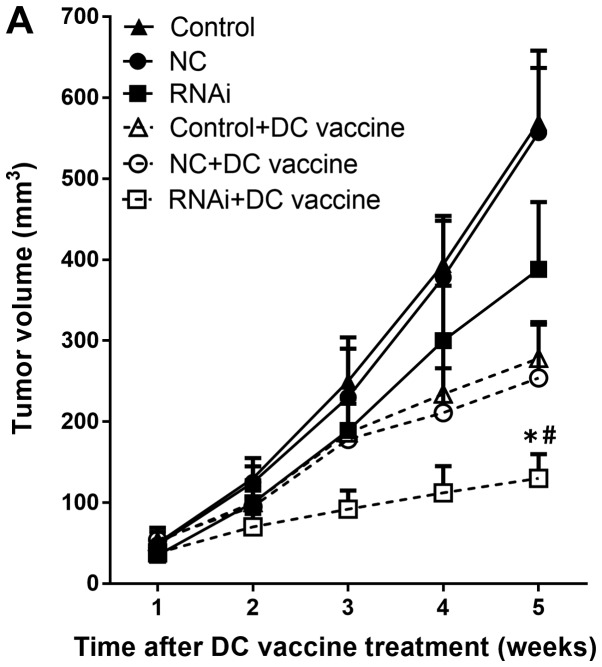
The effects of PD-L1 on the tumor immune environment have been demonstrated by in vitro experiments assessing pancreatic cancer cells (22). Thus, the present study assessed whether such effects are present in vivo. DC vaccines were administered in tumor-bearing mice weekly for four consecutive weeks. In each pancreatic cancer model mouse, DC vaccination treatment inhibited tumor growth compared with the untreated group (Fig. 6A). In addition, knockdown of PD-L1 combined with DC vaccination induced a more potent anti-tumor immunity compared with other groups: significant growth inhibition was observed in the RNAi + DC vaccine group five weeks following treatment (130±30 mm3) compared with the NC + DC vaccine (254±66 mm3) and control + DC vaccine (278±45 mm3) groups (P<0.01; Fig. 6A). The mRNA expression levels of PD-L1 were significantly downregulated in the RNAi + DC vaccine group compared with the control + DC group (P<0.01; Fig 6B).
Figure 6.
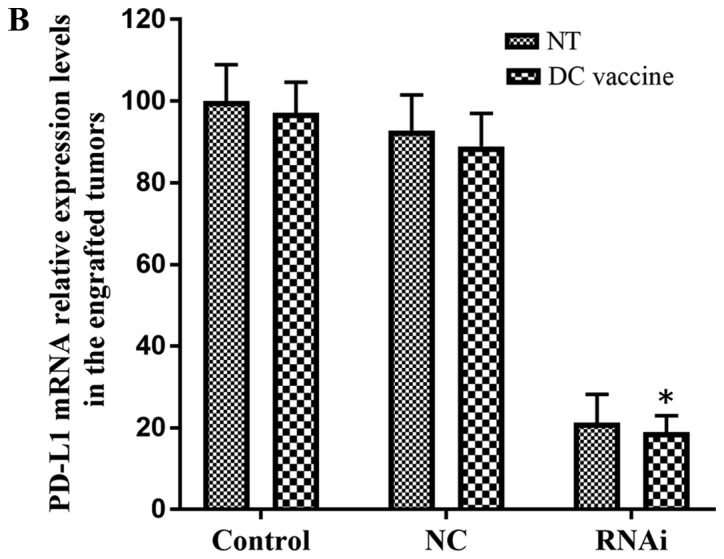
Subcutaneous tumor growth in the hu-SCID mouse model. (A) Growth curves of subcutaneously implanted pancreatic adenocarcinoma xenografts following various treatments (n=6 mice/group). The RNAi + DC vaccine group exhibited reduced tumor volumes compared with all other groups. (B) PD-L1 mRNA expression levels were downregulated in the RNAi + DC vaccine group compared with the control + DC group. #P<0.01 vs. RNAi + NT. *P<0.01 vs. control + DC vaccine. DC, dendritic cells; NC, negative control; NT, no treatment; PD-L1, programmed death receptor ligand 1; RNAi, cells transfected with short hairpin RNA targeting PD-L1.
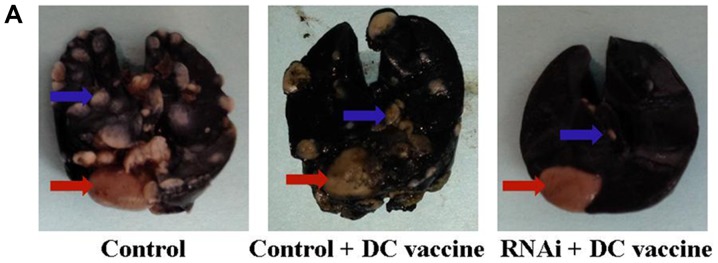
Compared with DC vaccination only treatment, PD-L1 knockdown combined with DC vaccination demonstrated improved therapeutic effects on lung metastasis. Lung metastasis was significantly reduced in the RNAi + DC vaccine group five weeks following treatment (4±3) compared with the NC + DC vaccine (23±9) and control + DC vaccine (25±7) groups (P<0.01; Fig. 7A and B). The non-treated (NT) RNAi group (33±6) also exhibited significantly reduced lung metastasis compared with the NC + NT (40±9) and control + NT (45±8) groups (P<0.05; Fig. 7B). In addition, PD-L1 mRNA expression levels were significantly downregulated in engrafted tumors and lung surface metastatic tumor nodules following treatment with RNAi + DC vaccine compared with the NC+ DC vaccine and control + DC vaccine groups (Figs. 6B and 7C). These data indicated that inhibition of PD-L1 expression combined with DC treatment may decrease pancreatic adenocarcinoma metastasis in vivo. This result supported the in vitro observations that PD-L1 may be critical in inhibiting CD8+ T cell antitumor immunological responses.
Figure 7.
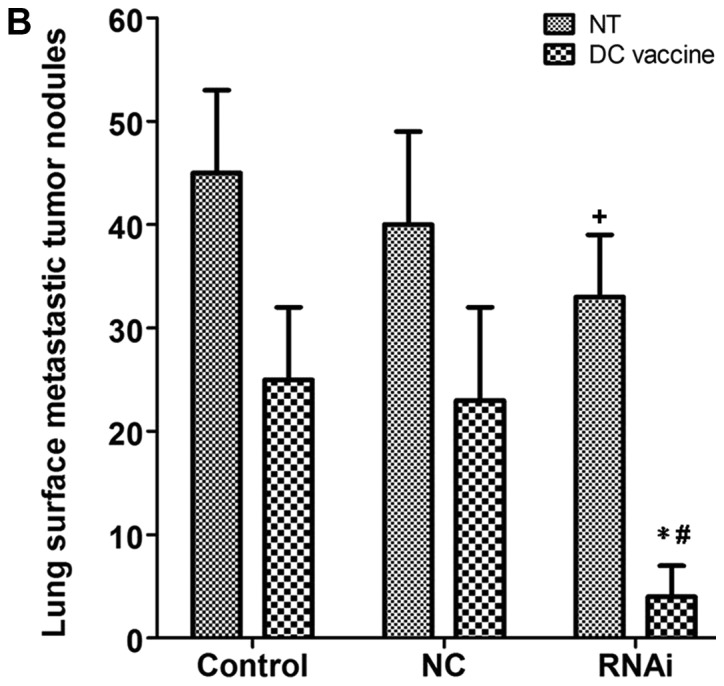
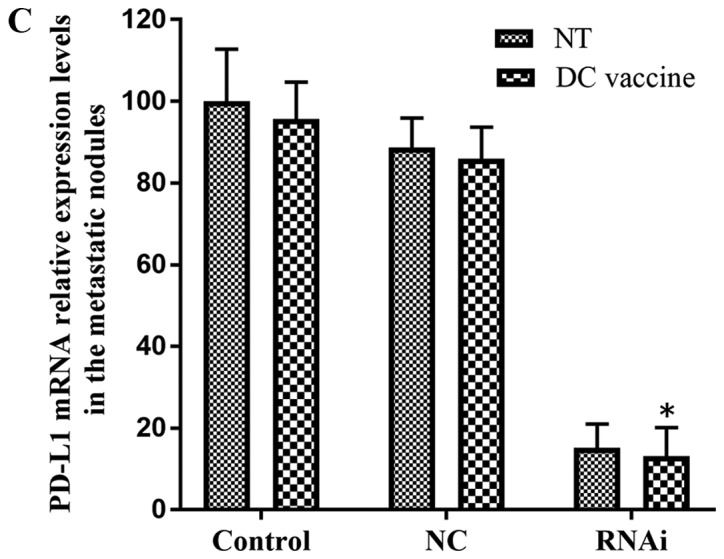
Lung surface metastatic nodules in the subcutaneous tumor hu-SCID mouse model. (A) Representative images of lung surface metastatic nodules. (B) The number of lung surface metastatic nodules in the RNAi + DC vaccine group was significantly decreased compared with that in the control + DC vaccine and the RNAi + NT groups. (C) PD-L1 mRNA expression in the RNAi + DC vaccine group was significantly reduced compared with that in the control + DC vaccine group. *P<0.01 vs. control + DC vaccine; #P<0.01 vs. RNAi + NT; +P<0.05 vs. control + NT. DC, dendritic cells; NC, negative control; NT, no treatment; PD-L1, programmed death receptor ligand 1; RNAi, cells transfected with short hairpin RNA targeting PD-L1.
In mouse survival experiments, PD-L1 knockdown combined with DC vaccination prolonged the survival period of tumor-bearing mice (Fig. 8). Although DC vaccination exhibited certain antitumor effects compared with untreated groups, PD-L1 knockdown had a synergistic effect in prolonging the survival period of the animals (P<0.01; Fig. 8).
Figure 8.
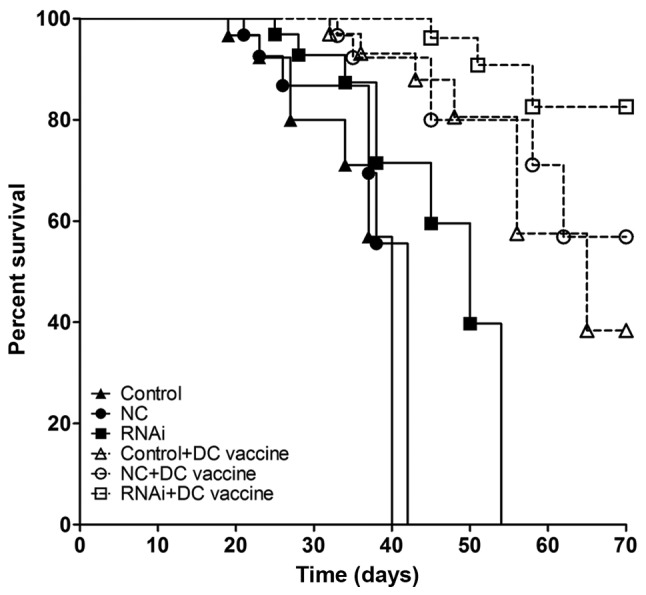
Knockdown of PD-L1 combined with DC vaccine increases hu-SCID mouse survival time. In the pancreatic adenocarcinoma mouse model, DC vaccine increased survival time compared with that in non-treated tumor-bearing animals. Compared with the RNAi alone and control + DC vaccination groups, the RNAi +DC vaccination group exhibited longer survival times.
Discussion
Antitumor immunity is mostly regulated by immune cells, particularly cancer-specific T cells (23). Although cancer-specific T lymphocytes exist in cancer patients and are activated by immunization, cancer cells are able to suppress T cell cytotoxicity by selecting certain immune checkpoints as the main immune resistance pathway (24). Therefore, blocking immune checkpoints is one of the most promising methods of inducing antitumor immune reactions (25). Among numerous immune checkpoints, PD-1 and its associated ligand PD-L1 have emerged as a promising target pair (26). PD-1/PD-L1 inhibition with monoclonal antibodies resulted in a breakthrough in cancer treatment, as it was revealed that antibody-mediated blockade of PD-L1 induced durable tumor regression and prolonged stabilization of disease in those patients with advanced disease stages (27,28).
As an immune-modulatory cell surface protein, PD-L1 binds PD-1 to negatively regulate cell-dependent immunity (29,30). Overexpression of PD-L1 has been described in various human malignancies, including melanoma and glioblastoma, lung, breast, colon, ovary and renal cell cancers, impairing the antitumor effects of T cells (14,16,31–34). Aberrant PD-L1 expression is related to cancer aggressiveness and poor prognosis, which suggests that PD-L1 may serve an important role in tumor progression. The results of the immunohistochemical staining in the present study revealed that PD-L1 was overexpressed in pancreatic adenocarcinoma compared with normal pancreatic tissues. RT-qPCR was also used to verify the results of the immunohistochemical staining; the results coincided with those in a study by Loos et al (35), which revealed that PD-L1 may regulate Tregs and IFN-γ to inhibit antitumor responses in pancreatic cancer, the mRNA expression levels of PD-L1 were upregulated in pancreatic adenocarcinoma.
The PD-L1/PD-1 interaction controlling cancer cell immune escape may be increased in pancreatic adenocarcinoma considering the high and ubiquitous expression of PD-L1 on cancer cells. The negative modulatory function of PD-L1 in cancer cells for tumor-specific T lymphocytes has been previously reported with transfected human cancer cells and animal experiments, which demonstrated altered tumor growth and metastasis control by immune cells (15–17,36). PD-L1 negatively regulates T-cell functions by binding PD-1; additionally, PD-1 is upregulated on activated T and B cells (37–39). Co-ligation of PD-1 reduces cytokine amounts and T cell proliferation (20,40–42). To determine the role of PD-L1 in antitumor immune evasion by pancreatic adenocarcinoma, a stable PD-L1-silenced pancreatic adenocarcinoma cell line was established in the present study by lentivirus-mediated RNA interference. In an in vitro experiment, CD8+ T lymphocytes were cultured in presence of mature DCs and pancreatic cancer cells, and the levels of secreted IFN-γ were detected. The results demonstrated that PD-L1 knockdown in pancreatic adenocarcinoma cells induced significant upregulation of IFN-γ, which is one of the most effective antitumor cytokines (43).
The in vitro results of the present study were further confirmed in vivo. In a SCID-hu subcutaneous tumor mouse model, PD-L1 RNAi knockdown combined with DC treatment decreased tumor growth and lung metastasis, and PD-L1 RNAi knockdown without DC treatment was also demonstrated to inhibit lung metastasis. Compared with the control and control + DC vaccine groups, the RNAi + DC vaccine group exhibited significantly reduced tumor volume and lung metastasis. Furthermore, knockdown of PD-L1 expression combined with DC treatment significantly prolonged the survival period of hu-SCID xenograft mice compared with the control and control + DC groups.
Previous findings indicate that PD-L1 pathway inhibition improves DC-mediated antitumor immunity. Curiel et al (31) demonstrated that PD-L1 pathway blockade enhances myeloid dendritic cell (MDC)-associated T cell activation, downregulates IL-10 as well as IL-2 and upregulates IFN-γ. The authors concluded that PD-L1 pathway inhibition in MDCs in the tumor microenvironment constitutes a potential tool for tumor immunotherapy. Ge et al (44) revealed that blocking PD-L1 signaling enhances DC maturation and proliferation as well as secreted IL-12 levels, induces DC-primed T cell responses and reverses cancer cell-associated T cell impairment. In the present study, PD-L1 silencing during DC immunization resulted in improved treatment outcomes compared with routine DC immunization, reducing cancer cell proliferation and increasing survival time in a pancreatic cancer-bearing SCID-hu model. These findings suggested that PD-L1 knockdown combined with DC immunization may reverse the immune suppression effects exerted by pancreatic cancer cells and enhance T cell function. Following PD-L1 knockdown combined with DC immunization, IFN-γ secretion was increased along with the proliferation of CD8+ T cells, which may subsequently result in antitumor immune effects in the tumor microenvironment. However, PD-L1 knockdown was only performed in pancreatic cancer cells using lentivirus-mediated RNAi, whereas the PD-L1 pathway in DCs was not blocked, e.g. with PD-L1-specific antibodies. Nevertheless, a significant promotion of antitumor immunity was achieved by PD-L1 silencing combined with DC vaccine treatment, resulting in decreased cancer cell proliferation and metastasis, and enhanced survival in model animals. The present study provided novel insights into DC vaccine treatment, which may aid in the development of new immunotherapies for cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science Foundation of China (grant nos. 31770985 and 81302146), the Postdoctoral Science Foundation Grant of China (grant no. 2016M591913), the Provincial Natural Science Foundation of Jiangsu Province (grant no. BK20161225), the Scientific Research Program of Jiangsu Provincial Commission of Health and Family Planning (grant no. H201620), the Science and Technology Program of Suzhou City (grant no. SYS201539), Jiangsu Provincial Medical Youth Talent (grant no. QNRC2016732), the Jiangsu Provincial “Six Peaks Talent” Program (grant no. 2016-WSW-043) and the Suzhou Municipal Project of Gusu Health Talent, Young Top Talent (grant no. 2018-057).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XZhao designed the study, proposed the research project and is the guarantor. JW performed the experiments and drafted the manuscript. MS performed the experiments and analyzed the data. XZhu, HZ, DM and ZZ assisted in preparing and taking part in the experiments. All authors were involved in study design and data interpretation, and have reviewed the final version of the manuscript.
Ethics approval and consent to participate
All patients were informed about the study and provided written informed consent. The present study was approved by the Ethics Committee of First Affiliated Hospital of Soochow University (Suzhou, China). All experiments were performed in accordance with relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J. 2017;23:333–342. doi: 10.1097/PPO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 5.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 6.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 7.Wells A, Grahovac J, Wheeler S, Ma B, Lauffenburger D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol Sci. 2013;34:283–289. doi: 10.1016/j.tips.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patard JJ. Dendritic cells for the treatment of metastatic renal cell carcinoma: At a low ebb? Eur Urol. 2006;50:11–13. doi: 10.1016/j.eururo.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alloatti A, Kotsias F, Magalhaes JG, Amigorena S. Dendritic cell maturation and cross-presentation: Timing matters. Immunol Rev. 2016;272:97–108. doi: 10.1111/imr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allgeier T, Garhmmer S, Nossner E, Wahl U, Kronenberger K, Dreyling M, Hallek M, Mocikat R. Dendritic cell-based immunogens for B-cell chronic lymphocytic leukemia. Cancer Lett. 2007;245:275–283. doi: 10.1016/j.canlet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicatorof tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 17.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7-H1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD81 T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.CAN-03-3259. [DOI] [PubMed] [Google Scholar]
- 18.Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide V, He J, et al. Multi-institutional validation study of the American joint commission on cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185–191. doi: 10.1097/SLA.0000000000001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KG, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 21.Ge Y, Xi H, Zhang XG. Vaccination with immature dendritic cells combined with CD40 mAb induces protective immunity against B lymphoma in SCID-hu mice. Biomed Pharmacother. 2010;64:487–492. doi: 10.1016/j.biopha.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med(Berl) 2016;94:509–522. doi: 10.1007/s00109-015-1376-x. [DOI] [PubMed] [Google Scholar]
- 25.Ito A, Kondo S, Tada K, Kitano S. Clinical development of immune checkpoint inhibitors. Biomed Res Int. 2015;2015:605478. doi: 10.1155/2015/605478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1:1223–1225. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 31.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 32.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med (Berl) 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 33.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 34.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 35.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, W Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 37.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 38.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD42/CD82) thymocytes. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 40.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1: PD-L1 inhibitory pathway affects both CD4 (+) and CD8 (+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Selenko-Gebauer N, Majdic O, Szekeres A, Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, et al. B7-h1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 43.Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-γ mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013;182:2345–2354. doi: 10.1016/j.ajpath.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge Y, Xi H, Ju S, Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in SCID-hu mice. Cancer Lett. 2013;336:253–259. doi: 10.1016/j.canlet.2013.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.