Abstract
High-intensity focused ultrasound (HIFU) treatment has recently been pursued to reduce radical treatment-related morbidity in low-to-intermediate-risk localized prostate cancer (PCa), especially in older men. The aim of this study was to develop a dedicated framework for HIFU therapy. All clinical data, such as risk categories, magnetic resonance with functional parametric imaging, and histopathology, are essential for driving proper HIFU treatment. All needed data can be added to the framework to localize areas that need to be treated. Once PCa areas have been featured, quantified, and located, planning can be adapted to drive accurate HIFU treatment. Our planning framework may be useful for all ablative therapies in order to standardize treatment for both clinical and scientific purposes.
Keywords: Ablation techniques, High-intensity focused ultrasound ablation, Planning techniques, Prostatic neoplasms
INTRODUCTION
Prostate cancer (PCa) is the second most common tumor after cutaneous malignancy in men, with an estimated 161,360 new cases in 2017, and is the third leading cause of cancer death in the United States [1]. The recent ProtecT trial showed that PCa-specific mortality was low at a median of 10 years irrespective of the treatment assigned (monitoring, surgery, or radiotherapy) in men with localized disease [2], but that treated men reported worse urinary, bowel, and sexual functions and significant effects on quality of life [3]. Therefore, it is clear that radical treatment of localized PCa in most men, particularly the elderly, may be an overtreatment with severe adverse events, because the survival benefit conferred by radical therapy is seen over 10 to 15 years compared with active monitoring [2]. Several forms of ablative therapy, mainly high-intensity focused ultrasound (HIFU), laser ablation, and brachytherapy, have been pursued to reduce treatment-related morbidity in patients with low-to-intermediate-risk PCa [4]. The medium-term efficacy of HIFU in men with localized intermediate-risk and high-risk PCa was recently demonstrated by Guillaumier et al. [5], who published a large, multicenter study of 5-year outcomes. They reported a failure-free survival rate of 88%, metastasis-free survival rate of 98%, and cancer-specific survival rate of 100% at 5 years [5]. The new Focal One® is a HIFU robot-assisted prostate tumorectomy device (EDAP TMS, Vaulx-en-Velin, France). It enables accurate and magnetic resonance (MR)-fused imaging. The MR target can be displayed on a live ultrasound image, treatment planning is adjustable in real time, HIFU shots can be followed live, and end-of-treatment imaging can be validated with contrast-enhanced ultrasound. To maximize such characteristics, however, treatment planning should be as accurate as possible. The objective of this study was thus to develop a dedicated HIFU framework. In fact, many frameworks are available in the literature and they have been developed and applied to reduce technology-induced errors [6].
MATERIALS AND METHODS
In line with these findings, our dedicated framework was necessary because we believed that 1) Treatments should be standardized, both for clinical and scientific purposes, and 2) Physicians should feature, quantify, and correctly locate PCa in the gland in order to correctly treat the lesions and likely improve oncological outcomes.
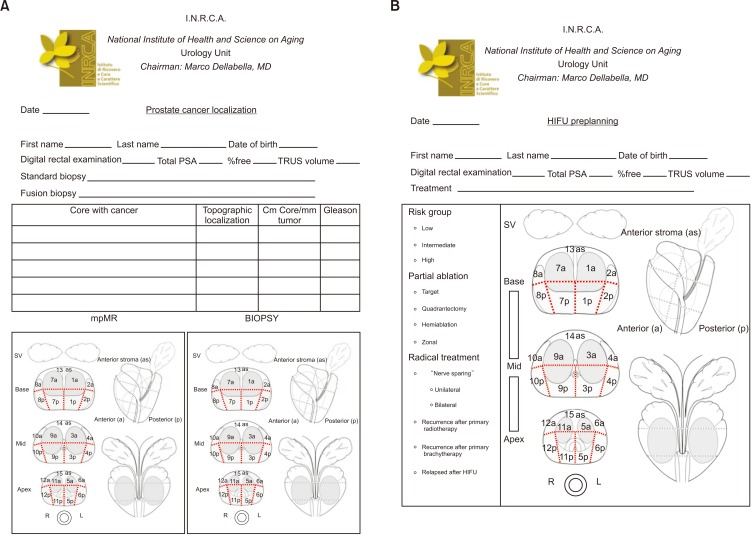
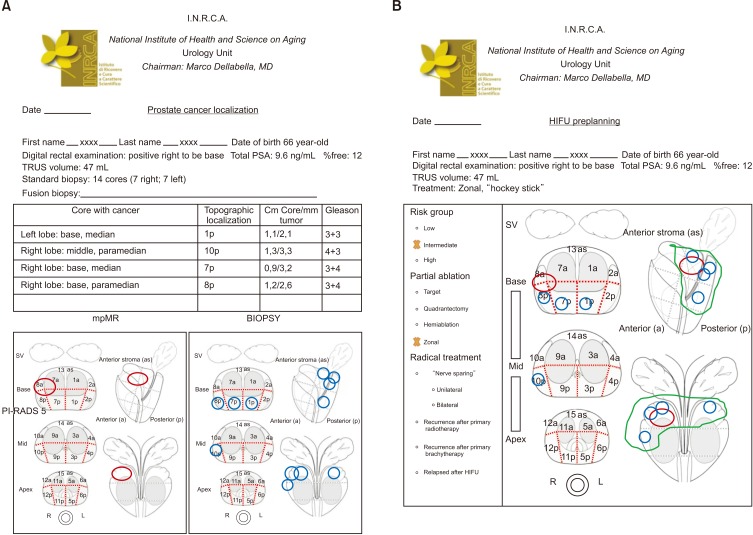
We started to use the Focal One® HIFU device in December 2014 and we adopted this dedicated framework (Fig. 1). The study was approved by INRCA Ethics Committee (approval number: DGEN 44/2018). In our framework, it is possible to insert all needed information regarding the patient's characteristics. Featuring in our framework means to report the patient's age, multiparametric MR (mpMR) imaging lesions according to Prostate Imaging–Reporting and Data System version 2 (PI-RADS v2) score 3 to 5 [7], prostate-specific antigen (PSA) value, number of positive biopsies, and corresponding Gleason score [8]. These parameters help to stratify each man with localized and locally advanced PCa into low, intermediate, or high-risk groups for biochemical recurrence [8]. Quantifying implies tumor volume at mpMR, number of cores with cancer, the length/proportion of carcinoma-positive cores, the extent of tumor involvement per biopsy core, and the percentage of Gleason score [8]. Lastly, locating means to identify the lesion with mpMR (unifocal/multifocal, right/left) and to correctly report the localization of each single core of the prostate biopsy (right/left, base, middle, apex, anterior/posterior) [8]. Despite guideline recommendations for 10 to 12 cores, not all patients receive sufficient systematic biopsies [8]. Therefore, even negative biopsy findings may be added to the framework to get an idea of the mapping performed.
Fig. 1. (A) Prostate cancer localization: clinical, biopsy, and multiparametric magnetic resonance (mpMR) imaging data have to be dotted in 27 sectors according to Dickinson et al. [9]. (B) High-intensity focused ultrasound (HIFU) preplanning: mpMR lesions and biopsy findings are both dotted in the plan. Their localization drives ablation in radical (whole gland) or partial (target, quadrantectomy, hemiablation, zonal) treatment. Picture modified with permission from Dickinson et al. [9]. PSA, prostate-specific antigen; TRUS, transrectal ultrasound; SV, seminal vesicles.
RESULTS
The framework was developed with the intent of translating all the above data in a simple way. It is made up of two sheets. In the first one (Fig. 1A), the physician has to report the patient's information: name and date of birth, PSA, findings on the digital rectal examination, prostate volume, and type of biopsy (systematic or MR-fusion) with the number of cores. In the panel, one has to write the localization of cores with cancer and their corresponding topographic layout in the 27 sectors according to Dickinson et al. [9], mm of tumor/length of the positive core, and Gleason score. All these data are dotted in the biopsy picture. Likewise, lesions detected during mpMR are dotted with their corresponding PI-RADS score in the MR figure. The second sheet (Fig. 1B) is the HIFU planning, where cancer cores and mpMR lesions are reported in Dickinson's sectors. Both data drive ablation treatment, which can be partial or radical. In radical ablation, the whole gland is ablated. In partial ablation, we have defined four treatments:
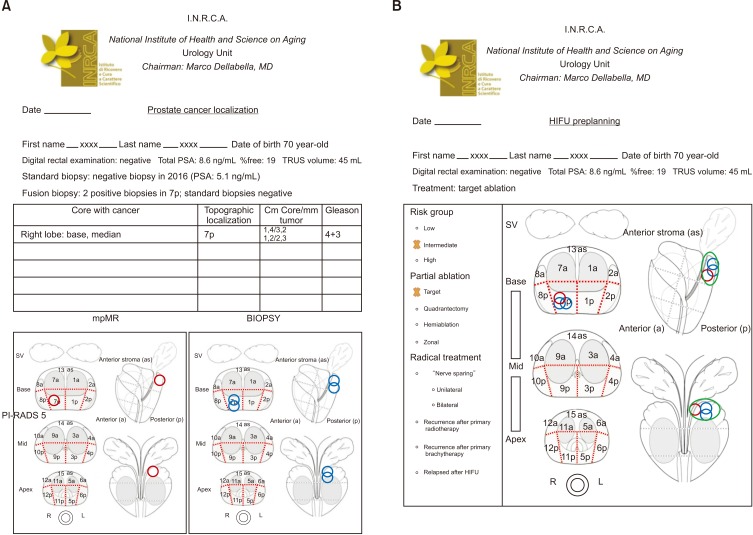
1) Target: this is the typical focal therapy, that is, treatment of the index lesion discovered at fusion biopsy (Fig. 2).
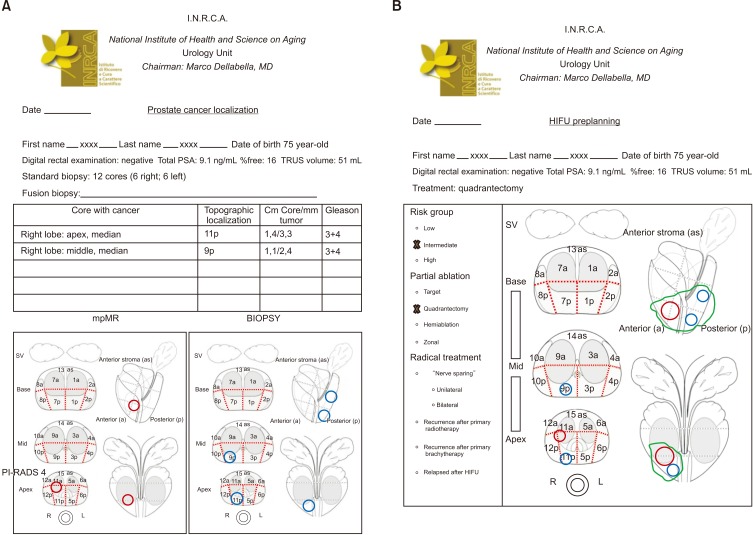
2) Quadrantectomy: half a lobe is ablated. This treatment includes complete ablation of lesions/positive core localized in middle-base or middle-apex areas (Fig. 3).
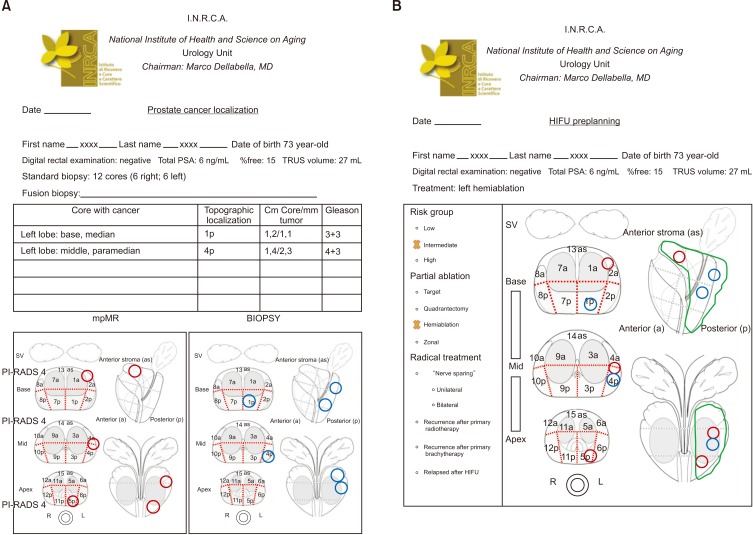
3) Hemiablation: one lobe is entirely treated (Fig. 4).
4) Zonal: this treatment covers several lesions and/or positive cores localized in multiple areas (Fig. 5), such as a right quadrantectomy and left base (e.g., “hockey stick” ablation).
Fig. 2. Target ablation. (A) Red circles: localization of one PI-RADS v2 score 4 lesion found at multiparametric magnetic resonance (mpMR). Blue circles: cancer core localization. (B) Green area encloses ablation target. PI-RADS, Prostate Imaging–Reporting and Data System; PSA, prostate-specific antigen; TRUS, transrectal ultrasound; HIFU, high-intensity focused ultrasound; SV, seminal vesicles.
Fig. 3. Right quadrantectomy, middle-apex. (A) Red circles: localization of one PI-RADS v2 score 4 lesion in the transitional zone found at multiparametric magnetic resonance (mpMR). Blue circles: cancer core localization. (B) Green area encloses ablation target. PI-RADS, Prostate Imaging–Reporting and Data System; PSA, prostate-specific antigen; TRUS, transrectal ultrasound; HIFU, high-intensity focused ultrasound; SV, seminal vesicles.
Fig. 4. Left hemiablation. (A) Red circles: localization of three PI-RADS v2 score 4 lesions found at multiparametric magnetic resonance (mpMR). Blue circles: cancer core localization. (B) Green area encloses ablation target. PI-RADS, Prostate Imaging–Reporting and Data System; PSA, prostate-specific antigen; TRUS, transrectal ultrasound; HIFU, high-intensity focused ultrasound; SV, seminal vesicles.
Fig. 5. Zonal ablation. (A) Red circles: localization of one PI-RADS v2 score 5 lesion found at multiparametric magnetic resonance (mpMR). Blue circles: cancer core localization. (B) Green area encloses ablation target (hockey stick). PI-RADS, Prostate Imaging–Reporting and Data System; PSA, prostate-specific antigen; TRUS, transrectal ultrasound; HIFU, high-intensity focused ultrasound; SV, seminal vesicles.
DISCUSSION
In the present study we did not discuss the appropriate treatment ranges of localized PCa. The appropriate treatment ranges are obviously those available in guidelines and consensus conferences. Our framework is useful as a tool for identifying the correct and tailored treatment plan for an individual patient, using simplified topographic planning, because it is easy, reproducible, and standardized. It quickly gives a graphical view of cancer localization in the gland and may be useful in each man affected by localized PCa for counseling and therapeutic chance. The main aim of this framework is not to replace the Focal One® MR and fusion importing program; rather, its usefulness is in pre-therapy planning that can homogenize the treatment of patients referred from other centers. In fact, those patients may have had nonfusion biopsies or biopsy data that cannot be imported into Focal One®. All patients with a standard biopsy have a subsequent prostate mpMR before HIFU treatment. The results of this mpMR can be
1) Negative for suspected cancer.
2) Positive for suspected cancer (PI-RADS v2 3, 4, and 5) and concordant with the results of biopsy.
3) Positive for suspected cancer (PI-RADS v2 3, 4, and 5) and discordant compared with the results of biopsy.
In the last case, patients are submitted to fusion biopsy. All these situations and the index lesion are well and clearly displayed in our framework. Moreover, treatment margins in the target ablation area are not reported in the framework because the picture of the prostate is not a scale image. Those margins are considered during real-time treatment. To the best of our knowledge, this is the first HIFU dedicated framework. However, this framework may be useful for all available ablative therapies, regardless of the energy used (e.g., HIFU, brachytherapy, cryotherapy). In fact, it may be the right way to choose the correct source of energy according to the proposed à la carte model reported by Sivaraman and Barret [10]. Indeed, they proposed to improve outcomes by using different sources of energy, according to cancer location: brachytherapy for apical, cryotherapy for anterior, and HIFU for posterior tumors [10]. Several conceptual frameworks similar to ours are available, most of which have been developed in medical oncology [11,12,13,14,15]. For example, one of the most important was developed in 2013 by the American Society of Clinical Oncology, with the aim of assisting medical oncologists in comparing the clinical benefit, toxicity, and cost of medical treatments [11]. Moreover, Slomiany et al. [16] and Chandra et al. [17] reported the importance and utility of those oncological frameworks not only in clinical trials but also in clinical practice.
CONCLUSIONS
In conclusion, we believe that our framework may be a powerful and useful tool to be adopted for providing a standardized approach to assist urologists in ablative treatment planning, considering that focal therapy for PCa is gaining popularity worldwide.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 3.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valerio M, Cerantola Y, Eggener SE, Lepor H, Polascik TJ, Villers A, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017;71:17–34. doi: 10.1016/j.eururo.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74:422–429. doi: 10.1016/j.eururo.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borycki EM, Kushniruk AW, Bellwood P, Brender J. Technology-induced errors. The current use of frameworks and models from the biomedical and life sciences literatures. Methods Inf Med. 2012;51:95–103. doi: 10.3414/ME11-02-0009. [DOI] [PubMed] [Google Scholar]
- 7.Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Prostate cancer: state of the art imaging and focal treatment. Clin Radiol. 2017;72:665–679. doi: 10.1016/j.crad.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Sivaraman A, Barret E. Focal therapy for prostate cancer: an “À la Carte” approach. Eur Urol. 2016;69:973–975. doi: 10.1016/j.eururo.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology Statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) with NCCN Evidence Blocks [Internet] [cited 2019 Mar 30]. Available from: https://www.nccn.org/evidenceblocks/default.aspx.
- 13.Memorial Sloan Kettering Cancer Center Drug Pricing Lab. The Drug Abacus tool [Internet] [cited 2019 Mar 30]. Available from: https://drugpricinglab.org/tools/drug-abacus/
- 14.Institute for Clinical and Economic Review. Overview of the ICER value assessment framework and update for 2017-2019 [Internet] [cited 2019 Mar 30]. Available from: https://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-Updated-050818.pdf.
- 15.Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26:1547–1573. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
- 16.Slomiany M, Madhavan P, Kuehn M, Richardson S. Value frameworks in oncology: comparative analysis and implications to the pharmaceutical industry. Am Health Drug Benefits. 2017;10:253–260. [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra A, Shafrin J, Dhawan R. Utility of cancer value frameworks for patients, payers, and physicians. JAMA. 2016;315:2069–2070. doi: 10.1001/jama.2016.4915. [DOI] [PubMed] [Google Scholar]