Abstract
Melanoma is an invasive and malignant type of tumor with unsatisfactory therapeutic outcomes. The present study aimed to detect the expression levels of microRNA (miR)-125b in formalin-fixed paraffin-embedded (FFPE) melanoma tissues and the association of its expression levels with the clinical features, diagnosis and prognosis of melanoma. Expression levels of miR-125b in 29 FFPE melanoma specimens (16 primary and 13 metastatic tumors), and 16 intradermal nevus (IDN) specimens as a control, were detected by reverse transcription-quantitative PCR. Associations among miR-125b expression and mortality, patient age and sex, tumor location and size, lymph node metastasis (LNM) and TNM stage were analyzed by t-test. The diagnostic value of miR-125b for melanoma was evaluated by receiver operating characteristic (ROC) curve analysis. Prognosis of patients in the microRNA-125b low- and high-expression groups was analyzed by Fisher's exact test. The association between miR-125b expression and the overall survival of patients with melanoma was assessed using Kaplan-Meier curve analysis and a Cox proportional hazards model. The results revealed that the expression levels of miR-125b in primary and metastatic melanomas were significantly lower than those in the IDN control group (P<0.05), and the expression levels of miR-125b in the metastatic group were significantly lower than those in the primary group (P<0.05). In addition, the expression levels of miR-125b were significantly associated with LNM (P=0.001) and TNM stage (P=0.004), but not with age, sex, tumor size or location (P>0.05). ROC curve analysis revealed that the area under the curve (AUC) was 0.880, with a 95% CI of 0.777–0.984 (P<0.05). The overall survival rate of the patients with a low expression level of miR-125b (20.0%) was lower than that of patients with a high expression level of miR-125b (64.3%) (P<0.05). miR-125b expression was an independent predictor of overall survival in patients with melanoma [hazard ratio (HR), 0.252; 95% CI, 0.087–0.729]. Overall, these findings indicated that a low expression level of miR-125b was associated with higher LNM and TNM stage in patients with melanoma, and that this has a certain diagnostic value. miR-125b may be used for the early screening of melanoma and determining the prognosis of patients with melanoma, and may be a potential target for the treatment of the disease.
Keywords: microRNA-125b, melanoma, formalin-fixed paraffin-embedded, prognosis
Introduction
Melanoma is an invasive and malignant type of tumor, which mainly occurs in the skin and oral mucosa (1). The number of cases of mortality associated with cutaneous melanoma in the United States in 2017 reached 9,730, and it accounted for 1.6% of all cases of cancer-associated mortality (2). Despite decades of research efforts and development of antimelanoma drugs, the therapeutic outcome of melanoma is generally unsatisfactory.
Early detection and monitoring of patients with melanoma are particularly important for melanoma therapy. MicroRNAs (miRNAs/miRs) are a class of small non-coding molecules, 18–25 nt in length, which have emerged as a powerful novel tool for the diagnosis and monitoring of patients with various types of cancer (3,4). Additionally, our previous studies demonstrated that expression levels of miR-125b are markedly downregulated in melanoma tissues and cell lines, and that miR-125b is able to inhibit the proliferation and invasion of melanoma cells (5,6). However, the functional role of miR-125b in melanoma tissues, and its association with the survival and prognosis of patients with melanoma, remain unclear.
The majority of miRNA samples in studies are derived from fresh tissues. However, since it is difficult to obtain fresh tissues due to limited sources, medical and research institutions have commonly used the formalin fixation plus paraffin embedding method to preserve pathological tissues. A number of previous studies identified that miRNAs in formalin-fixed paraffin-embedded (FFPE) tissues could still provide reliable expression profiles, as they exhibited a high correlation with those in fresh tissues (7); even tissues that have been preserved for >30 years can still provide reliable miRNA expression data (8). These characteristics of FFPE miRNAs are beneficial for improving the utilization of the largest bank of specimens to explore the development, progression and prognosis of human tumors.
The aim of the present study was to detect the expression of miR-125b in previously confirmed FFPE melanoma tissues, and to analyze the associations among miR-125b expression and the metastasis and prognosis of melanoma. This was performed in an attempt to provide novel predictors and interventional targets to improve the early screening level and efficacy of diagnosis and treatment of this rare but devastating human malignant disease.
Materials and methods
Sample collection and grouping
The present study included 29 FFPE melanoma tissue specimens and 16 FFPE intradermal nevus (IDN) tissue specimens, which were obtained from patients who underwent radical resection at The First Affiliated Hospital of Nanchang University (Nanchang, Jiangxi, China) between January 2014 and May 2017. All specimens were fixed with 20% neutral-buffered formalin for 24 h at room temperature (25–27°C) and stored following paraffin embedding. The thickness of paraffin sections was 5–10 µm. The inclusion criteria for patients with melanoma were: i) Patients who had not received radiotherapy or chemotherapy prior to surgery; ii) patients without other co-existing cancer, heart disease and/or diabetes; and iii) patients with complete clinicopathological data. FFPE tissue specimens of all patients were prepared by the same technician, and reviewed and confirmed by two pathologists. Clinicopathological data of each patient and survival data were collected during the follow-up period (6–32 months). The patients were divided into three groups according to the 8th Edition of the American Joint Committee on Cancer melanoma staging system (9): IDN (control; n=16), primary melanoma (stage I and II; n=16) and metastatic melanoma (stage III and IV; n=13). The 29 patients with melanoma were comprised of 13 males and 16 females, who ranged in age between 34 and 81 years, including 15 patients >60 years and 14 patients <60 years. The 16 patients with IDN were comprised of 7 males and 9 females, who ranged in age between 29 and 75 years, including 6 patients >60 years and 10 patients <60 years. The Ethics Committee of The First Affiliated Hospital of Nanchang University reviewed and approved the present study, and written informed consent was obtained from each patient at each examination phase. The present study complied with the principles of the Declaration of Helsinki.
miR-125b extraction
Expression levels of miR-125b in each group were detected by reverse transcription-quantitative PCR (RT-qPCR). Reagents, primers and instruments used in the present study included miR-125b extraction reagent, the miRNA prep Pure FFPE kit, miR-125b reverse transcription reagent, the miRcute Plus miRNA First-Strand cDNA Synthesis kit, the miR-125b PCR reagent kit and miRcute Plus miRNA qPCR Detection kit SYBR Green (all purchased from Beijing Wanter Biopharmaceutical Co., Ltd.). U6 was used as the internal control. FFPE tissues were sliced into 6–8 sections of 5–10 µm thickness (the first 2–3 sections were discarded) from which RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. All procedures were performed under ribozyme-free conditions. The total RNA was measured for optical density, and subsequently stored in RNA solution at −80°C.
RT-PCR
To each tube, 10 µl 2X miRNA RT Reaction Buffer, 4 µl total miRNA and 2 µl miRNA RT Enzyme mixed with 4 ml RNase-Free ddH2O were added, totaling 20 µl. PCR reaction solution of each sample was reverse transcribed under the conditions of 42°C for 60 min, followed by 95°C for 3 min, and then the obtained cDNA product was stored at −20°C.
qPCR
The reaction solution for PCR (20 µl) consisted of 10 µl 2X miRcute Plus miRNA Premix (with SYBR and ROX), 0.4 µl Reverse Primer (10 µm), 2 µl 50X ROX Reference Dye and 7.6 µl ddH2O. cDNA was added to the reaction solution and mixed gently. qPCR was conducted under the following conditions: Initial template denaturation at 95°C for 15 min for one cycle, 94°C for 20 sec for 5 cycles, 63–65°C for 30 sec for 5 cycles, 72°C for 34 sec for 5 cycles, 94°C for 20 sec for 45 cycles of cyclic denaturation and 60°C for 34 sec for 45 cycles of cyclic c annealing and extension. Relative miR-125b expression was measured using the 2−ΔΔCq method (10). The primers were as follows: miR-125b forward, 5′ddd-TGC CTC CCT GAG ACC CTA ACT TGT GA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; and U6 forward, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-AGGGGCCATGCTAATCTTCT-3′.
Statistical analysis
All experiments were repeated three times. Data were analyzed using SPSS version 22.0 statistical software (IBM Corp.). Results are expressed as the means ± standard deviation. One-way ANOVA and a least significant difference post hoc test were used when making comparisons among multiple groups. An independent-sample t-test was used for comparisons between two groups. Categorical variables were assessed by Fisher's exact test. Receiver operating characteristic (ROC) curve analysis was used to evaluate the value of diagnosis and prognostic prediction. Kaplan-Meier survival curves were plotted to compare the survival of the miR-125b low-expression group and the high-expression group. A log-rank test was used to compare the survival curves of the two groups. Cox regression analysis was used to compare the overall survival in the miR-125b low- and high-expression groups. P<0.05 was considered to indicate a statistically significant.
Results
Comparison of miR-125b expression in different groups
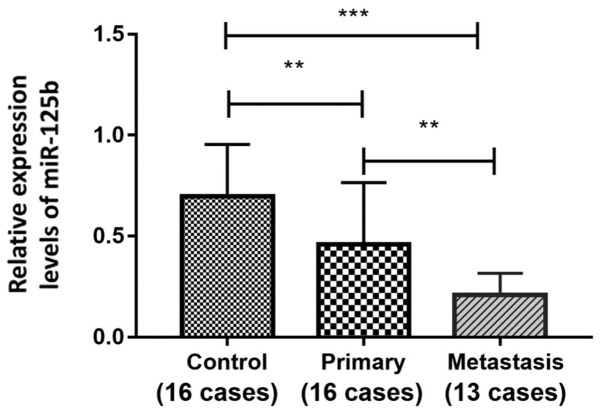
Expression levels of miR-125b in FFPE melanoma tissues were detected by RT-qPCR. As shown in Fig. 1, the expression levels of miR-125b in the primary melanoma group (n=16) and in the metastatic melanoma group (n=13) were significantly lower than that in the IDN group (n=16). Additionally, the expression level of miR-125b in the metastatic melanoma group was significantly lower than that in the primary melanoma group (0.21±0.11, 0.70±0.26 and 0.46±0.31, for metastasis, IDN and primary groups, respectively; P<0.05).
Figure 1.
Comparison of miR-125b expression in the control, primary and metastasis groups. Data were compared by one-way ANOVA and a least significant difference post hoc test. **P<0.01 and ***P<0.001. Control, intradermal nevus control group; metastasis, metastatic melanoma group; miR-125b, microRNA-125b; primary, primary melanoma group.
Associations among miR-125b expression and clinical features of patients with melanoma
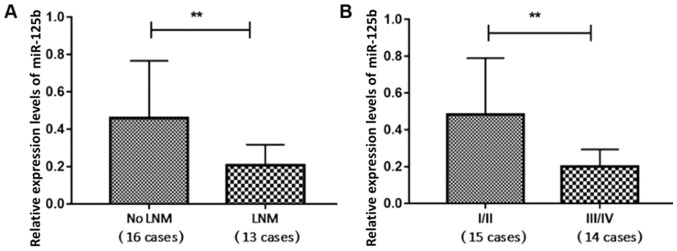
The clinicopathological features of the patients are listed in Table I. The miR-125b expression levels of the 16 patients with melanoma with no lymph node metastasis (LNM) and the 13 patients with melanoma with LNM were 0.46±0.31 and 0.21±0.11, respectively, revealing a significant difference between the two groups (P<0.05; Fig. 2A). The expression levels of miR-125b in the 15 cases of TNM stage I and II (I/II) melanoma and 14 cases of TNM stage III and IV (III/IV) were 0.47±0.31 and 0.21±0.09, respectively, revealing a significant difference between the two groups (P<0.05; Fig. 2B). Expression levels of miR-125b were associated with LNM and TNM stage, but not with patient age, sex, tumor size or the location of the lesion (acral vs. non-acral; Table I; Fig. 2).
Table I.
Associations between miR-125b expression and the clinical features of patients with melanoma.
| Clinical features | Number, n (%) | miR-125b expression (mean ± standard deviation) | P-value |
|---|---|---|---|
| Sex | 0.443 | ||
| Male | 13 (44.83) | 0.30±0.18 | |
| Female | 16 (55.17) | 0.38±0.32 | |
| Age, years | 0.874 | ||
| ≥60 | 15 (51.72) | 0.35±0.27 | |
| <60 | 14 (48.28) | 0.34±0.27 | |
| Tumor diameter, cm | 0.425 | ||
| ≥2 | 11 (37.93) | 0.29±0.29 | |
| <2 | 18 (62.07) | 0.38±0.26 | |
| Lymph node metastasis | 0.001a | ||
| No | 16 (55.17) | 0.46±0.31 | |
| Yes | 13 (44.83) | 0.21±0.11 | |
| TNM stage | 0.004a | ||
| I/II | 15 (51.72) | 0.47±0.31 | |
| III/IV | 14 (48.28) | 0.21±0.09 | |
| Lesion location | 0.991 | ||
| Acral | 17 (58.62) | 0.35±0.25 | |
| Non-acral | 12 (41.38) | 0.35±0.30 |
A t-test was used to compare the differences among groups.
P<0.01. miR-125, microRNA-125b.
Figure 2.
Comparison of miR-125b expression. Comparison between (A) the no LNM group and the LNM group, and (B) the TNM stage I/II and III/IV groups. Data were compared by independent-sample t-test. **P<0.01. LNM, lymph node metastasis; miR-125b, microRNA-125b.
Diagnostic value of miR-125b expression in melanoma
In the ROC curve analysis, the AUC was 0.880, with a 95% CI of 0.777–0.984 (P<0.001), with a sensitivity and specificity of 79.3 and 93.7%, respectively, and a cutoff value of 0.73 (Fig. 3). These results suggested that miR-125b may be used as a predictor of diagnosis for patients with melanoma.
Figure 3.
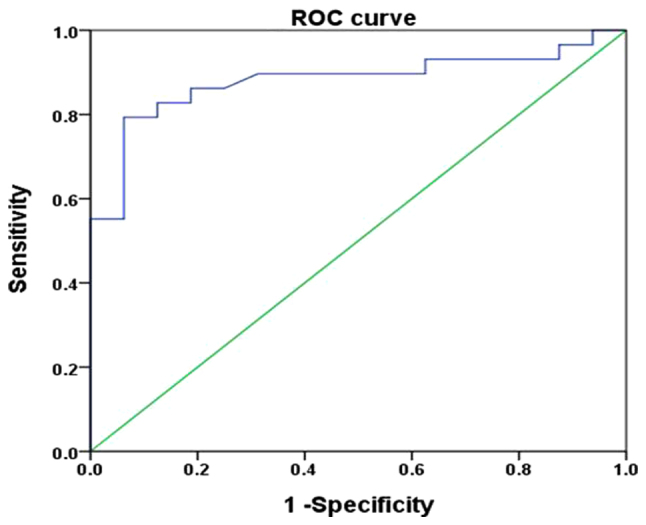
ROC curve analysis for predictive value of miR-125b in melanoma. The area under the curve was 0.880 (P<0.001), which revealed that miR-125b may be used as a predictor of melanoma diagnosis. miR-125b, microRNA-125b; ROC, receiver operating characteristic.
Association between miR-125b expression and the prognosis of patients with melanoma
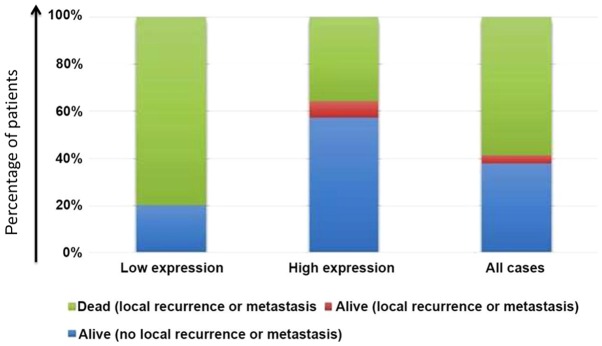
Using the median value (0.30) as the cut-off value, the expression levels of miR-125b were used to classify the patients into a high-expression group and a low-expression group. No patients were lost to follow-up or died of other causes. The mortality rate in the low-expression group was significantly higher than that in the high-expression group (P<0.05; Table II, Fig. 4).
Table II.
Comparison of prognosis of patients in the microRNA-125b low-expression and high-expression groups.
| Patient prognosis | Low expression | High expression | P-value |
|---|---|---|---|
| Alive (no local recurrence or metastasis) | 3 | 8 | 0.034a |
| Alive (local recurrence or metastasis) | 0 | 1 | |
| Dead (local recurrence or metastasis) | 12 | 5 | |
| Dead (other diseases or accidents) | 0 | 0 | |
| Dead (lost to follow-up) | 0 | 0 |
A Fisher's exact test was used for the analysis of categorical data.
P<0.05.
Figure 4.
Constituent ratios of patient prognoses. The mortality rate in the low-expression group was significantly higher than that in the high-expression group. Differences were examined by Fisher's exact test (P<0.05).
Kaplan-Meier analysis demonstrated that the 1- and 2-year cumulative survival rates in the high-expression group were 100 and 62.3% compared with 33.3 and 11.1% in the low-expression group, respectively. A log-rank test identified a P-value of 0.005, indicating that the survival time of patients in the low-expression group was significantly shorter than that of patients in the high-expression group (Fig. 5).
Figure 5.
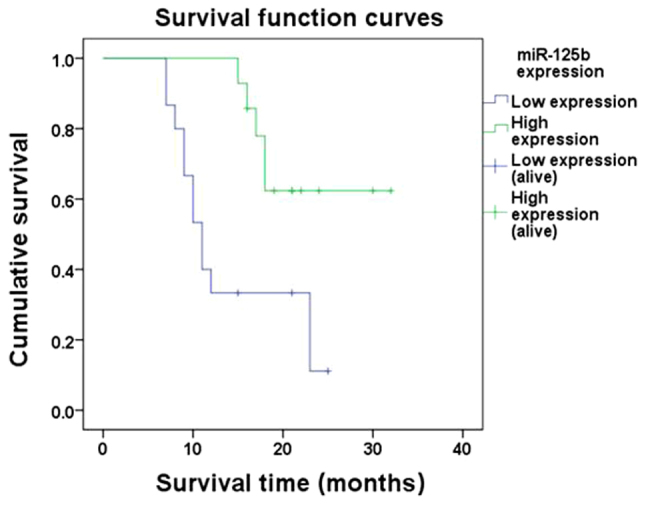
Kaplan-Meier survival curve analysis revealed that the survival time of patients with melanoma in the miR-125b low-expression group was shorter than that in the miR-125b high-expression group. The difference was statistically significant, as determined by a log-rank test (P<0.05).
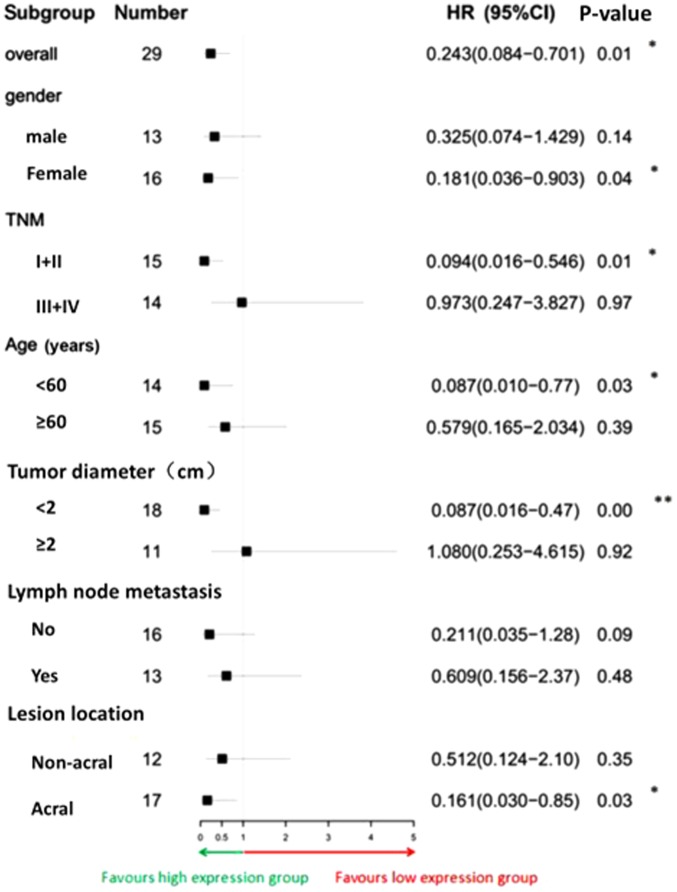
Survival of the patients was further analyzed by Cox proportional hazards model (Fig. 6). The overall risk of mortality of the miR-125b high expression group was significantly lower than that of the low expression group (HR, 0.243; 95% CI, 0.084–0.701). In the five subgroups of female sex, TNM stage I/II, age <60 years, tumor diameter <2 cm and tumor located at the extremities, the risk of mortality in the high expression group was significantly lower than that in the low expression group (P<0.05). The associations between clinical features and expression of miR-125b and overall survival of patients with melanoma were analyzed by univariate and multivariate Cox regression analysis. In univariate analysis, lesion location, lymph node metastasis and miR-125b expression were significantly associated with overall survival of patients. However, further multivariate analysis revealed that lesion location and lymph node metastasis could not enter the Cox regression equation. The results of this indicated that only miR-125b expression was an independent predictor of overall survival in patients with melanoma (HR, 0.252; 95% CI, 0.087–0.729; Table III).
Figure 6.
Forest plot of HRs for survival of patients with melanoma. The microRNA-125b low expression group was compared with the high expression group. Data were analyzed by Cox proportional hazards model. *P<0.05 and **P<0.01. CI, confidence interval; HR, hazard ratio.
Table III.
Univariate and multivariate Cox regression analyses of miR-125b expression, clinicopathological features and overall survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Clinicopathological feature | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex | 0.979 | |||
| Male | Reference | |||
| Female | 1.013 (0.390–2.628) | |||
| Age, years | 0.080 | |||
| <60 | Reference | |||
| ≥60 | 2.471 (0.898–6.797) | |||
| Tumor diameter, cm | 0.124 | |||
| <2 | Reference | |||
| ≥2 | 2.116 (0.814–5.501) | |||
| Lymph node metastasis | 0.012 | 0.266 | ||
| No | Reference | |||
| Yes | 3.822 (1.339–10.912) | |||
| TNM stage | 0.053 | |||
| I/II | Reference | |||
| III/IV | 2.703 (0.985–7.413) | |||
| Lesion location | 0.041 | 0.185 | ||
| Non-acral | Reference | |||
| Acral | 0.364 (0.138–0.960) | |||
| miR-125b expression | 0.010 | 0.011 | ||
| Low expression | Reference | Reference | ||
| High expression | 0.243 (0.084–0.707) | 0.252 (0.087–0.729) | ||
HR, hazard ratio; CI, confidence interval; miR-125b, microRNA-125b.
Discussion
The lifetime risk of melanoma has been estimated to be 1 in 75 and rising each year with >60,000 Americans developing melanoma in 2010 (11). The case-fatality rate is extremely high in patients with metastatic melanoma (12). Risk factors of melanoma include excessive ultraviolet light exposure, photosensitive skin, improper management of pigmented nevi that may induce malignant alterations, the existence of large numbers of abnormally developed nevi and a family history of skin cancer. It is necessary to identify effective approaches for the early detection and treatment of high-risk populations of melanoma.
With the advantages of efficiency and accuracy, various biomarkers have been the most valued tool in the diagnosis and management of melanoma. miR-125b is an miRNA that serves an essential role in tumor progression. Previous studies have demonstrated that miR-125b may be a cancer suppressor gene of multiple tumors, and suggested that miR-125b may be associated with the development and progression of various types of cancer, including prostate, oral and ovarian cancer (13–15). Kappelmann et al (16) first reported that the expression level of miR-125b in melanoma cells obtained from primary and metastatic sources was lower than that in normal human melanocytes. Further experiments demonstrated that the downregulatory mechanism of miR-125b in melanoma cells inhibited cell proliferation, which suggested that miR-125b may be a potential tumor suppressor in melanoma. Our previous studies (5,6) demonstrated that the expression of miR-125b was able to inhibit the proliferation and invasion of melanoma cells. Based on the aforementioned research regarding RNA-protein-cell interactions (5,6), the present study aimed to identify the functional role of miR-125b in melanoma tissue and its association with the survival and prognosis of patients with melanoma.
In the present study, expression levels of miR-125b were detected in FFPE tissues from 29 patients with melanoma and 16 patients with IDN as the control. The results of the present study revealed that the expression levels of miR-125b in the former were significantly lower than those in in the latter. This result, together with previous findings (5,6), demonstrated that miR-125b served an anti-oncogenic role in the occurrence and development of melanoma.
Analysis of the associations between miR-125b expression and the clinical features of patients with melanoma identified that the expression levels of miR-125b in patients with LNM were significantly lower than those in patients with primary melanoma without LNM and distant metastases (P<0.05). In addition, the expression levels of miR-125b in patients with high TNM stages were lower than those in patients with low TNM stages. There were no significant associations among the expression levels of miR-125b and sex, age, tumor size or lesion location (P>0.05). This suggested that miR-125b expression was closely associated with LNM and the TNM stage of melanoma. Glud et al (17) detected the expression levels of miR-125b in 28 paired patients with T2N0M0 and T2N1M0 by RT-qPCR, and revealed that the expression levels of miR-125b in the T2N1M0 patients were significantly downregulated compared with the expression levels in the T2N0M0 patients (P<0.05), which is consistent with the findings of the present study, further indicating that miR-125b is involved in LNM and the TNM stage of melanoma.
Expression levels of miR-125b in melanoma cell lines are lower than those in melanocytes (16). Therefore, it may be possible to use miR-125b expression as a diagnostic marker of melanoma. In the present study, ROC curves were generated according to the expression levels of miR-125b in patients with melanoma, by using sensitivity as the ordinate and 1-specificity as the horizontal ordinate, to explore the association between sensitivity and specificity, knowing that the larger the AUC value, the more accurate the diagnosis would be (18). The AUC value reported in the present study was 0.880, with a 95% CI of 0.777–0.984 (P<0.001), a sensitivity of 79.3% and a specificity of 93.7%, which indicated that miR-125b may be a valuable marker for the diagnosis of melanoma. However, larger sample size studies are required to confirm if miR-125b could be used as a diagnostic indicator for melanoma. Pathological evaluation remains the gold standard for the diagnosis of melanoma.
It is important to predict the prognosis of patients with melanoma. Since miR-125b has an impact on the proliferation and invasion of melanoma cells, the association between the level of miR-125b expression and the prognosis of patients with melanoma may be explored. The 1- and 2-year survival rates of patients with melanoma with high expression levels of miR-125b were 100 and 62.3%, respectively, compared with 33.3 and 11.1% in those patients with low expression, indicating that the survival time of patients in the low-expression group was significantly shorter than that of patients in the high-expression group. The clinical outcomes of patients with low or high expression levels of miR-125b were statistically analyzed, and the results revealed that the proportion of deaths in the high-expression group was significantly lower than that in the low-expression group. These findings suggested that low expression levels of miR-125b increased the risk of mortality and reduced the survival time of patients with melanoma. Univariate and multivariate Cox regression analysis revealed that the expression of miR-125b was an independent predictor for the overall survival of patients with melanoma. There was a direct link between the level of miR-125b expression and the survival of patients with melanoma.
The present study demonstrated that the expression levels of miRNA-125b in melanoma tissues were decreased, particularly in metastatic melanoma tissue. The abnormal miRNA-125b expression was associated with lymph node metastasis and TNM stage and its low expression was closely associated with the prognosis of patients with melanoma. As miR-125b expression is relatively stable in melanoma, it is relatively easy to obtain a specimen and the methods for miRNA extraction/use are easy to follow. Therefore, miR-125b may be used as an independent factor to predict the prognosis of patients with melanoma, and it is a potential diagnostic biomarker and therapeutic target of melanoma.
Acknowledgements
Not applicable.
Funding
This study was supported by the Jiangxi Province Key Research and Development Program (grant no. 20161BBG70162), the Jiangxi Province Science Foundation for Distinguished Young Scholars (grant no. 20171BCB23090) and the Jiangxi Province Natural Science Foundation (grant no. 20171ACB21053).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JZ, JY and QJ designed the study and performed the experiments. HL, SN and SL collected the data. YX and CZ analyzed the data. JY and QJ prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee of the First Affiliated Hospital of Nanchang University reviewed and approved the present study, and written informed consent was obtained from each patient at each examination phase. The present study complied with the principles of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Myles ZM, Buchanan N, King JB, Singh S, White A, Wu M, Ajani U. Anatomic distribution of malignant melanoma on the non-Hispanic black patient, 1998–2007. Arch Dermatol. 2012;148:797–801. doi: 10.1001/archdermatol.2011.3227. [DOI] [PubMed] [Google Scholar]
- 2.NIH National Cancer Institute. Surveillance, Epidemiology, and End Results Program, Cancer Stat Facts: Melanoma of the Skin (EB/OL) https://seer.cancer.gov/statfacts/html/melanhtml. 2017
- 3.Golabchi K, Soleimani-Jelodar R, Aghadoost N, Momeni F, Moridikia A, Nahand JS, Masoudifar A, Razmjoo H, Mirzaei H. MicroRNAs in retinoblastoma: Potential diagnostic and therapeutic biomarkers. J Cell Physiol. 2018;233:3016–3023. doi: 10.1002/jcp.26070. [DOI] [PubMed] [Google Scholar]
- 4.Gholamin S, Mirzaei H, Razavi SM, Hassanian SM, Saadatpour L, Masoudifar A, ShahidSales S, Avan A. GD2-targeted immunotherapy and potential value of circulating microRNAs in neuroblastoma. J Cell Physiol. 2018;233:866–879. doi: 10.1002/jcp.25793. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Lu L, Xiong Y, Qin W, Zhang Y, Qian Y, Jiang H, Liu W. MLK3 promotes melanoma proliferation and invasion and is a target of microRNA-125b. Clin Exp Dermatol. 2014;39:376–384. doi: 10.1111/ced.12286. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Na S, Liu C, Pan S, Cai J, Qiu J. MicroRNA-125b suppresses the epithelial-mesenchymal transition and cell invasion by targeting ITGA9 in melanoma. Tumour Biol. 2016;37:5941–5949. doi: 10.1007/s13277-015-4409-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Wang L, Briggs C, Sicinska E, Gaston SM, Mamon H, Kulke MH, Zamponi R, Loda M, Maher E, et al. DNA degradation test predicts success in whole-genome amplification from diverse clinical samples. J Mol Diagn. 2007;9:441–451. doi: 10.2353/jmoldx.2007.070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Ahlfen S, Missel A, Bendrat K, Schlumpberger M. Determinants of RNA quality from FFPE samples. PLoS One. 2007;2:e1261. doi: 10.1371/journal.pone.0001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, HessK R, Sullivan DC, et al., editors. 8th. Springer; New York, NY: 2017. AJCC cancer staging manual. [DOI] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Wu S, Singh RK. Resistance to chemotherapy and molecularly targeted therapies: Rationale for combination therapy in malignant melanoma. Curr Mol Med. 2011;11:553–563. doi: 10.2174/156652411800615153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L, Grob JJ, Malvehy J, Newton-Bishop J, Stratigos AJ, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline-update 2016. Eur J Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Fredsøe J, Rasmussen AKI, Thomsen AR, Mouritzen P, Høyer S, Borre M, Ørntoft TF, Sørensen KD. Diagnostic and prognostic MicroRNA biomarkers for prostate cancer in cell-free urine. Eur Urol Focus. 2018;4:825–833. doi: 10.1016/j.euf.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Chang SM, Hu WW. Long non-coding RNA MALAT1 promotes oral squamous cell carcinoma development via microRNA-125b/STAT3 axis. J Cell Physiol. 2018;233:3384–3396. doi: 10.1002/jcp.26185. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Zhang X, Ma Y, Zhao X, Li B, Wang H. Ascites promotes cell migration through the repression of miR-125b in ovarian cancer. Oncotarget. 2017;8:51008–51015. doi: 10.18632/oncotarget.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappelmann M, Kuphal S, Meister G, Vardimon L, Bosserhoff AK. MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene. 2013;32:2984–2991. doi: 10.1038/onc.2012.307. [DOI] [PubMed] [Google Scholar]
- 17.Glud M, Rossing M, Hother C, Holst L, Hastrup N, Nielsen FC, Gniadecki R, Drzewiecki KT. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res. 2010;20:479–484. doi: 10.1097/CMR.0b013e32833e32a1. [DOI] [PubMed] [Google Scholar]
- 18.Ma H, Bandos AI, Gur D. On the use of partial area under the ROC curve for comparison of two diagnostic tests. Biom J. 2015;57:304–320. doi: 10.1002/bimj.201400023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.