Abstract
Vitiligo is a chronic autoimmune condition involving selective dysfunction and destruction of melanocytes in the skin, hair, or both. The typical presentation is well-demarcated depigmented skin patches. Given vitiligo is the most common cause of depigmentation worldwide and early disease responds best to treatment, prompt diagnosis and proactive management of vitiligo are critical. While a wide variety of treatments has demonstrated variable effectiveness in treating vitiligo, phototherapy remains standard of care because of its proven efficacy and favorable side effect profile. However, many patients with vitiligo are unable to access affordable, consistent, or convenient phototherapy. To address these issues, home-based phototherapy has emerged as a patient-centered alternative. The purpose of this review is to discuss management of vitiligo with a specific focus on access to home-based phototherapy (HBPT) for patients with this condition. Key challenges to HBPT include misperceptions around safety and efficacy, inadequate physician education and training, insurance and financial barriers, and appropriate patient selection. Solutions to these challenges are presented, such as approaches to improve physician education and increasing the evidence surrounding the effectiveness and safety of this treatment for vitiligo. In addition, various practical considerations are discussed to guide dermatologists on how to approach HBPT as a treatment option for patients with vitiligo.
Keywords: vitiligo, pigmentation disorders, phototherapy, photomedicine
Introduction
Vitiligo is a chronic condition involving an immune-mediated attack on melanocytes, resulting in selective dysfunction and destruction of melanocytes in skin, hair, or both.1 It is the most common cause of depigmentation worldwide with an estimated prevalence of 1–2% and no predilection for a particular age, race, or gender.1 The typical presentation is white skin patches or hair with distinct margins between normal pigmented and involved depigmented areas.1,2 The pathogenesis of vitiligo has not been clearly established but is likely multifactorial. Hypothesized causes include autoimmune processes, genetic influences, biochemical pathways, and environmental factors.1,3 The autoimmune theory is supported by strong evidence, including the clinical association of vitiligo with autoimmune disorders of various organ systems such as endocrine, gastrointestinal, and neurologic diseases.1,4,5 Vitiligo can also have a profound negative impact on quality of life (QoL) due to psychological trauma experienced by patients with vitiligo, resulting in low self-esteem, shame, depression, anxiety, and social isolation.6,7 Furthermore, vitiligo is associated with a significant economic burden involving high direct and indirect costs, ranging from work absenteeism to expenses related to accessing care.5 Given these consequences and that early disease responds best to treatment, prompt diagnosis and management of vitiligo are critical.1
Diagnosis and management of vitiligo
The first step in the diagnosis and management of vitiligo includes gathering a complete disease history, including onset, progression, response to prior treatments, other medical conditions, family history, and environmental exposures. Next, disease extent must be evaluated by examining the skin with both natural light and a Wood’s lamp. Depigmented skin of vitiligo will fluoresce brightly white under Wood’s lamp. Physical exam must include inspection of common sites of vitiligo, including the lips and perioral area, periocular areas, dorsal surface of the hands, fingers, flexor surface of the wrists, and inguinal and anogenital regions.8
The next step is to discuss treatment options, which depend on the location/subtype, percent body surface area (BSA) involved, and the impact on QoL.9 Since the pathogenesis of vitiligo is not fully understood, a variety of modalities have been attempted to stabilize progression and stimulate repigmentation.9–13 These include topical therapies9 (eg, topical corticosteroids,9,12,14,15 calcineurin inhibitors,9,16,17 vitamin D analogues9,12,18), systemic therapies19 (eg, corticosteroids,9,12 methotrexate9), surgical therapies20 (eg, melanocyte-keratinocyte transplantation,21–25 hair follicle transplant,26,27 punch, blister, split thickness grafting26–28), complementary and alternative therapies29 (eg, L-phenylalanine, khellin, biloba, folic acid, zinc, copper, vitamins B12, C, D, and E), and a variety of experimental therapies (eg, afamelanotide,19,30 topical prostaglandin E2,19,31 systemic and topical Janus kinase inhibitors,19,32–34 apremilast,19 topical Wnt agonists19).
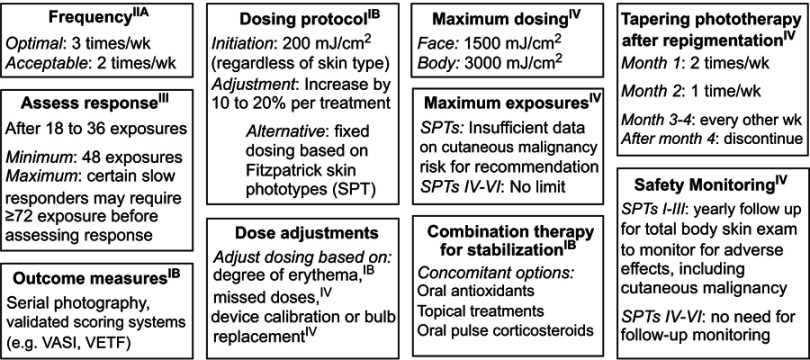
While these approaches have each demonstrated variable effectiveness, phototherapy (PT) remains standard of care for vitiligo because of its proven efficacy and favorable side effect profile.19,35–37 According to the most recent recommendations from the Vitiligo Working Group (VWG), indications for PT to treat vitiligo include 1) extensive disease, 2) rapidly spreading disease, and 3) patients with smaller areas of involvement who have not responded to other therapies.9 For these indications, the most effective modalities include narrowband UVB (NB-UVB), psoralen plus ultraviolet A (PUVA), and targeted UVB with the excimer laser. The VWG’s key recommendations for PT for vitiligo are summarized in Figure 1. Comparing PUVA to NB-UVB for extensive disease, several retrospective and prospective studies have shown superior repigmentation, color matching, safety in pediatric and pregnant patients, and fewer side effects with NB-UVB.11,38–45 Therefore, NB-UVB is considered a first-line therapy for vitiligo.
Figure 1.
Key phototherapy recommendations from the Vitiligo Working Group.
Notes: Superscripts indicate the level of evidence for each set of recommendations. IIA, evidence from at least one controlled study without randomization; III: evidence from nonexperimental descriptive studies, such as comparative studies, correlation studies, and case–control studies; IV: evidence from expert committee reports or opinions or clinical experience of respected authorities, or both. IB indicates evidence from at least one randomized controlled trial.
Abbreviations: VASI, Vitiligo Area Scoring Index; VETF, Vitiligo European Task Force Assessment.
The mechanism of action for NB-UVB in vitiligo has not been fully elucidated. NB-UVB is thought to decrease expression of inflammatory ILsthat are increased in vitiligo (ie, IL-17 and IL-22) and increase FOXP3, which is decreased in vitiligo.37,46 Furthermore, NB-UVB stimulates the activation, proliferation, and migration of melanocytes from the outer root sheath of hair follicles to the epidermis; in addition, keratinocytes increase expression of basic fibroblast growth factor and endothelin-1, which further induces melanocyte proliferation and migration.43,47 Finally, NB-UVB increases tyrosinase and HMB-45 receptor expression on melanocytes, increasing melanin production.47,48
PT with NB-UVB is an excellent treatment option for vitiligo, but significant barriers prevent many patients from accessing this therapy. Most importantly, PT requires frequent visits (up to three times per week) to institution-based PT centers for several months to years. This can be logistically difficult and/or financially prohibitive for certain patients, especially those who do not live near PT centers, are unable to afford transportation, or cannot secure insurance coverage for PT.49 One alternative to institution-based PT is home-based PT (HBPT).
Home-based phototherapy for vitiligo
Given the obstacles in accessing office-based PT, HBPT has emerged as a patient-centered alternative. HBPT was introduced in 1979 for the treatment of psoriasis and was adapted for vitiligo in the 1990s.43,50 The benefits of HBPT for vitiligo are many. First, the ability to receive treatment at home reduces logistical burdens on patients as well as health care providers, such as PT nurses who benefit from a reduced daily patient load. Second, several studies have demonstrated that patients undergoing HBPT are more compliant.51,52 For example, a 2015 study among 44 vitiligo patients found that 92% in the HBPT group adhered to the protocol compared to only 70% in the institution-based group; the authors attributed this difference in compliance to HBPT patients’ ability to receive treatment at their convenience, in terms of time and location.53 Third, the safety of HBPT has been supported by various studies. In a 2010 study among 89 vitiligo patients receiving home- or institution-based PT, HBPT was found to be as safe as institution-based PT, illustrated by a similar occurrence of side effects between the two groups.43 Despite such advantages of HBPT, there are many challenges that have suppressed its widespread adoption.49 The following section will focus on challenges and solutions related to HBPT for patients with vitiligo.
Challenges and solutions for home-based phototherapy for vitiligo
Safety and efficacy
The safety and efficacy of HBPT for the treatment of vitiligo are a concern for both patients and clinicians.54 In fact, apprehension over safety is routinely identified as a barrier to prescribing HBPT.43 Adverse events (AEs) of HBPT include burning, erythema, xerosis, and pruritis in the short-term, and photoaging and photodamage with long-term use. Even though these AEs are the exact same as institution-based PT, many dermatologists are concerned that home devices might enable over-treatment, resulting in a higher occurrence of both short- and long-term AEs. In fact, a 2006 survey among 367 dermatologists revealed that 33% felt greater risks were associated with HBPT; moreover, 55% believed that institution-based PT is more efficacious than HBPT.55
Perceptions that HBPT has inferior safety and efficacy compared to office-based PT are not necessarily consistent with the evidence. Most of the safety data on HBPT is from studies on psoriasis, such as the PLUTO study that was the first multicenter, single-blinded, randomized controlled trial (RCT) comparing institution-based PT to HBPT.55 Patients in the HBPT arm experienced no difference in the number of side effects; furthermore, none of the patients reported feeling that HBPT was unsafe, and overall patient satisfaction was higher in the HBPT group.55 There was no statistical difference in skin improvement between the two groups,55 demonstrating that HBPT can be as effective as institution-based PT for the treatment of psoriasis.
Unfortunately, there has been no multicenter RCTs to compare institution-based versus HBPT in vitiligo.43 Smaller studies in vitiligo have yielded results consistent with PLUTO,49,56,57 but the majority of HBPT guidelines for vitiligo are based on specialist consensus36 and data from other skin diseases.11 Therefore, there remains a tremendous need for further studies.
Efforts in this direction have been led by the Home Intervention of Light therapy (Hi-Light) trials.56 A pilot study in 2014 examined the feasibility of conducting a multicenter RCT to investigate the use of hand-held HBPT devices to treat vitiligo at home. The study found a strong willingness of participants to adhere to treatments.56 A RCT including children and adults with early and limited vitiligo comparing the efficacy of potent topical corticosteroid, HBPT with a hand-held NB-UVB device, and a combination of the two is underway.58 Recruitment for this trial began in July 2015, and as of early 2019, results are not yet publicly available.
Physician education and training
Physicians are often reluctant to prescribe HBPT due to limited education on this topic during their training. A 2011 survey among 96 dermatology residents found that only 25% had dedicated didactics on HBPT, only 35% had received specific training on prescribing HBPT, and 73% had never prescribed a single home unit during their training.59 In fact, 20% stated that they were unaware that HBPT existed or did not know how to prescribe it to eligible patients.59 Such knowledge gaps translate into treatment gaps that prevent access to HBPT.60,61 Consistent with these findings, a 2017 survey among 42 dermatology residency program directors (PDs) found that 38% of programs reported providing less than 5 hours of phototherapy training over three years of residency, and formal instruction on HBPT was not provided in 31% of programs.61 The majority of PDs cited “lack of curriculum time” as the most common barrier providing such education.61
Consequently, surveys have shown that dermatologists are hesitant to prescribe HBPT for reasons ranging from the potential use of the equipment as a tanning bed to the risk of severe AEs occurring remotely from the hospital.62,63 Lack of familiarity with the robust safety profile of HBPT magnifies such fears and also raises legal concerns, given that the prescribing physician might be held liable.64 All of this contributes to an overall decline in prescribing HBPT that effectively limits vitiligo treatment options, disadvantaging providers and patients alike.49,56,57
The need to build physician knowledge around HBPT is evident. Specific educational aims for vitiligo should include a review of the efficacy and safety of HBPT, an overview of home devices available for vitiligo treatment, discussion of HBPT protocols for vitiligo, information on logistics of prescribing and obtaining insurance coverage for HBPT, and resources on active or upcoming clinical trials. By equipping dermatologists with such knowledge, concerns around medical or legal risks or logistical difficulties are likely to diminish as providers become more comfortable with prescribing and managing patients receiving HBPT.
Insurance and financial barriers
Insurance coverage for institution-based PT frequently presents challenges, and insurance approval for HBPT can be particularly difficult. A 2009 study reviewed three large insurance companies regarding the reimbursement of HBPT and found a wide variation in approval policies, more difficulty obtaining approval for HBPT compared to institution-based PT or biologic therapy, and more stringent requirements for HBPT.65 Such findings were unexpected considering HBPT is much less costly than biologic treatment and home devices can be utilized for years after purchase.65
According to a 2010 analysis, the out-of-pocket cost of HBPT devices without insurance coverage varies between $2,180 and $7,000 depending on the size and model.52 Updated estimates from a 2018 study that took into account device cost, shipping, set-up, technical support, and a warranty reported an upfront cost of $5,000 plus $1,000 every three to six years for light bulb replacements.66 Such costs are unaffordable for many patients.30,43,49,59,67 In a 2011 study, 43% of patients with a HBPT prescription did not purchase a device; 73% of these cases were due to high out-of-pocket expenses.59 Relatedly, the top reasons that providers did not prescribe HBPT were fear of high patient costs and frustration about the reimbursement policy of insurance companies.59
Given the potentially high out-of-pocket costs associated with HBPT, it is important to consider the overall savings of transitioning treatment from an institution to the home. From the patient’s perspective, the costs of receiving institution-based PT include but are not limited to lost wages from missing work, insurance co-payments, and travel expenses. A 2013 economic analysis of thrice weekly institution-based PT determined that purchasing a HBPT device was a more cost-effective option for patients living 20 miles or more from an institution-based PT center, even if the home device was not covered by insurance.68
From the provider’s perspective, HBPT reduces administrative costs and nursing time associated with the frequent office visits that institution-based PT requires. Relatedly, the option of HBPT may result in more patients pursuing this treatment before trying more expensive options, such as oral immunosuppressants or biologics.66 In fact, a 2018 study comparing biologics to HBPT in psoriasis found that a HBPT device costs $5,000 over three years whereas biologic therapy can cost over $180,000 for the same treatment duration.66
Considering the significant cost savings of HBPT, dermatologists should play an active role in advocating for their patients by working with insurance companies to increase coverage of home devices, especially for patients with vitiligo who are likely to benefit from long-term treatment with HBPT.
Factors in patient selection
As with all treatments, each patient must be thoroughly assessed to determine if he/she is an appropriate candidate for HBPT. Several medical conditions are relative or absolute contraindications to PT. Relative contraindications include a history of photodermatitis, immunosuppression, photodamaged skin, personal or family history of melanoma, and multiple non-melanoma skin cancers;43 a history of melanoma or multiple non-melanoma skin cancers are relative contraindications since the association between medical PT and skin cancer has not been conclusively demonstrated.52 Caution must be taken in patients taking photosensitizing medications such as thiazide diuretics, amiodarone, and antibiotics (eg, tetracyclines, sulfonamides, quinolones).52 Absolute contraindications include patients with a photosensitivity disorder (eg, xeroderma pigmentosum, lupus erythematosus, dermatomyositis, porphyria).
Certain patients may simply prefer to receive treatment at an institution. In fact, a 2010 retrospective analysis comparing HBPT to institution-based PT found that satisfaction with skin results was significantly lower in the HBPT group, despite similar repigmentation results and occurrence of AEs.43 Possible explanations include patients preferring institution-based PT because they feel that traveling to the doctor to receive treatment is the more traditional, appropriate, or effective approach. Alternatively, some patients prefer not to have a PT device in their home for reasons ranging from limited space to insecurities about properly using the device.
Lastly, social determinants of health must be evaluated, especially the patient’s level of education. This is relevant because patients being considered for HBPT must be able to follow instructions on operating home devices and adhere to prescribed protocols.52 In addition, HBPT patients must be able to return to the institution periodically for assessment and re-evaluation of treatment response.
It is essential to select appropriate patients for HBPT in order to optimize both safety and efficacy. First, all patients must be screened for the relative and absolute contraindications discussed. One simple solution is to develop a provider checklist for every patient considering HBPT. Second, the physician should engage the patient in a conversation about their openness to having a device in their home, given that some patients might not be comfortable with this arrangement. Third, the provider should assess the patient’s readiness to self-administer HBPT by evaluating their ability to follow directions or any psychological factors that might interfere with home-based treatment. Fourth, follow-up should be arranged at least once every three months to monitor therapeutic response and adjust dosing. Some guidelines suggest a contract to ensure follow-up compliance.52
Barring any of these issues, vitiligo patients with a history of good response to institution-based PT are ideal candidates for HBPT.43,49,52,69 It is recommended that any patients considering HBPT undergo a trial period of institution-based PT to ensure treatment response, ability to follow instructions, and reliability to follow-up.43 This will help assess whether or not the patient is likely to benefit from HBPT and also prepares them to recognize normal skin response versus AEs.52 Furthermore, the trial period can serve as an opportunity for patients to learn how to handle missed doses or other unexpected circumstances that can arise after transitioning to HBPT.
Practical considerations
Home device options
There are currently a variety of HBPT devices on the market, but many have not been rigorously tested and may not be approved by the US Food and Drug Administration (FDA). FDA-approved devices are proven to be safe and effective for use at home by patients and typically require a physician’s prescription. FDA-approved devices should be recommended over devices without FDA approval. Otherwise, the choice of device is based on the size and location of vitiligo lesions and the percentage of BSA affected.70 Other important considerations in device selection include the patient’s insurance policies and out-of-pocket expenses.
The three most common devices for NB-UVB HBPT are single- or multi-paneled booths, hand and foot units, and hand-held units.37,51,58 Such variety allows for personalized treatment, especially for localized or recalcitrant vitiligo.37 Multi-paneled units are expensive but tend to provide high-output power leading to shorter treatment times, whereas single-panel booths are less expensive but may require repositioning and longer treatment periods to ensure all affected areas are exposed to adequate doses of light.49 Devices for hands and feet can be very effective, but it is important to note that these areas are particularly difficult to treat. Relatedly, hand-held units are effective for recalcitrant lesions or intertriginous areas,49 but as with acral lesions, patients should be informed that certain sites may be resistant to PT, whether institution- or home-based.71 In these cases, proper expectations must be set with patients, including the development of treatment plans that combine PT with adjuvant options like oral medications and even surgery.72
Furthermore, it is important that clinicians are aware of financing options for HBPT devices to help patients offset out-of-pocket expenses. A letter of medical necessity is often needed from the physician for insurance authorization of HBPT devices. Templates for these letters are available on some manufacturer websites (National Biological and Daavlin), as well as organizations such as Vitiligo Support International. Several manufacturers offer financial assistance programs or affordable payment plans that are readily advertised on their websites, and non-profit organizations like the National Psoriasis Foundation also maintain a list of these and other programs.
Treatment pearls
A full review of the VWG’s recommendations on PT for the treatment of vitiligo36 is beyond the scope of this review, but dermatologists should follow the basic guidelines outlined in Figure 1 when prescribing HBPT for vitiligo. Furthermore, patients with home units can receive treatments on continuously alternating days, rather than forcing thrice-weekly treatments into weekdays as is the case for most institutional-based PT. As a result, patients on HBPT can receive higher weekly UV doses, potentially resulting in a faster response. Such a protocol is also safer than a thrice-weekly Monday to Friday schedule because the cumulative erythemogenic effect of more closely clustered UV exposure is avoided.49,52 HBPT can be combined with high potency topical steroids (five days a week for up to a month) or twice daily topical tacrolimus (0.1%) or pimecrolimus (1%).19 However, topical medications should not be applied immediately prior to receiving PT as this can alter the effectiveness of therapy. As with all regimens, close monitoring for AEs and routine follow-up to assess response is essential.
To mitigate concerns regarding safety of HBPT, most manufacturers have built devices that require a code provided by a physician, and devices are equipped with safety features such as key-locked switches, timers limiting the length of treatment, and a system that allows only a set number of treatments before returning to the prescribing provider for follow-up.43,73 Despite these measures, patient education is critical to avoiding AEs and optimizing outcomes. In addition to a trial of institution-based phototherapy as discussed earlier, providers should also consider an after-hours, on-call service for any urgent patient concerns. Multiple studies have shown that when patients are adequately educated on HBPT, treatment compliance and incidence of AEs are no different than institution-based PT.51,53,62,74
Conclusion
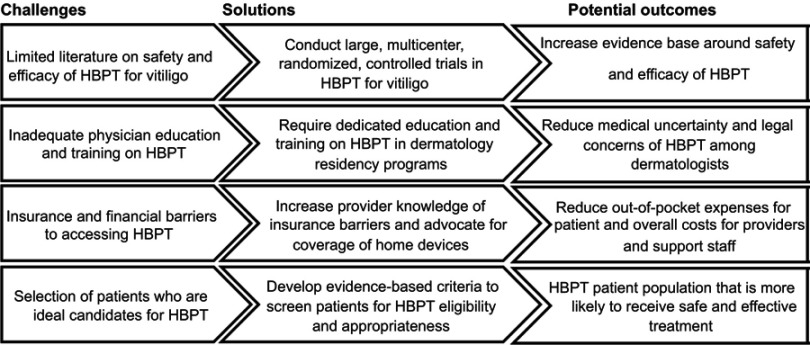
In conclusion, there are significant challenges to widespread adoption of HBPT for vitiligo. Key issues include misperceptions around safety and efficacy, inadequate physician training, insurance and financial barriers, and appropriate patient selection. As summarized in Figure 2, there are a variety of practical solutions to these challenges, ranging from improving physician education to increasing the evidence surrounding the effectiveness and safety of this treatment. We have discussed various practical considerations for dermatologists to increase access to HBPT for patients with vitiligo.
Figure 2.
Home-based phototherapy for vitiligo: challenges, solutions, and potential outcomes.
Abbreviation: HBPT, home-based phototherapy.
Disclosure
Tina Bhutani has received research funding from the National Psoriasis Foundation and has served as a research investigator and/or consultant for Eli Lilly, Janssen, Merck, Celgene and Regeneron. The authors report no other conflicts of interest in this work.
References
- 1.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(15)61137-0 [DOI] [PubMed] [Google Scholar]
- 2.Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35(2):257–265. doi: 10.1016/j.det.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillon AB, Sideris A, Hadi A, Elbuluk N. Advances in vitiligo: an update on medical and surgical treatments. J Clin Aesthet Dermatol. 2017;10(1):15–28. [PMC free article] [PubMed] [Google Scholar]
- 4.Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. doi: 10.1016/j.jaad.2015.08.063 [DOI] [PubMed] [Google Scholar]
- 5.Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73(5):883–885. [DOI] [PubMed] [Google Scholar]
- 6.Porter J, Beuf A, Nordlund JJ, Lerner AB. Personal responses of patients to vitiligo: the importance of the patient-physician interaction. Arch Dermatol. 1978;114(9):1384–1385. [PubMed] [Google Scholar]
- 7.Porter JR, Beuf AH, Lerner A, Nordlund J. Psychosocial effect of vitiligo: a comparison of vitiligo patients with “normal” control subjects, with psoriasis patients, and with patients with other pigmentary disorders. J Am Acad Dermatol. 1986;15(2 Pt 1):220–224. [DOI] [PubMed] [Google Scholar]
- 8.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE. Current and emerging treatments for vitiligo. J Am Acad Dermatol. 2017;77(1):17–29. [DOI] [PubMed] [Google Scholar]
- 10.Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38(5):419–431. doi: 10.1111/j.1346-8138.2010.01139.x [DOI] [PubMed] [Google Scholar]
- 11.Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2015;(2):Cd003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezzedine K, Whitton M, Pinart M. Interventions for vitiligo. JAMA. 2016;316(16):1708–1709. doi: 10.1001/jama.2016.12399 [DOI] [PubMed] [Google Scholar]
- 13.Bordere AC, Lambert J, van Geel N. Current and emerging therapy for the management of vitiligo. Clin Cosmet Investig Dermatol. 2009;2:15–25. [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelmaksoud A. Methotrexate for treatment of vitiligo. Dermatol Ther. 2017;30(6). doi: 10.1111/dth.12532 [DOI] [PubMed] [Google Scholar]
- 15.Garza-Mayers AC, Kroshinsky D. Low-dose methotrexate for vitiligo. J Drugs Dermatol. 2017;16(7):705–706. [PubMed] [Google Scholar]
- 16.Dang YP, Li Q, Shi F, Yuan XY, Liu W. Effect of topical calcineurin inhibitors as monotherapy or combined with phototherapy for vitiligo treatment: a meta-analysis. Dermatol Ther. 2016;29(2):126–133. doi: 10.1111/dth.12295 [DOI] [PubMed] [Google Scholar]
- 17.Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009;60(4):604–608. doi: 10.1016/j.jaad.2008.10.059 [DOI] [PubMed] [Google Scholar]
- 18.Wat H, Dytoc M. Off-label uses of topical vitamin D in dermatology: a systematic review. J Cutan Med Surg. 2014;18(2):91–108. doi: 10.2310/7750.2014.14022 [DOI] [PubMed] [Google Scholar]
- 19.Passeron T. Medical and maintenance treatments for vitiligo. Dermatol Clin. 2017;35(2):163–170. doi: 10.1016/j.det.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 20.Matin R. Vitiligo in adults and children: surgical interventions. BMJ Clin Evid. 2015;2015:1717. [PMC free article] [PubMed] [Google Scholar]
- 21.Tawfik YM, Abd Elazim NE, Abdel-Motaleb AA, Mohammed RAA, Tohamy AMA. The effect of NB-UVB on noncultured melanocyte and keratinocyte transplantation in treatment of generalized vitiligo using two different donor-to-recipient ratios. J Cosmet Dermatol. 2018;18(2):638–646. . doi: 10.1111/jocd.12759 [DOI] [PubMed] [Google Scholar]
- 22.Lu N, Xu A, Wu X. Follow-up study of vitiligo patients treated with autologous epidermal sheet transplants. J Dermatolog Treat. 2014;25(3):200–204. doi: 10.3109/09546634.2012.671912 [DOI] [PubMed] [Google Scholar]
- 23.Khodadadi L, Shafieyan S, Sotoudeh M, et al. Intraepidermal injection of dissociated epidermal cell suspension improves vitiligo. Arch Dermatol Res. 2010;302(8):593–599. doi: 10.1007/s00403-010-1034-7 [DOI] [PubMed] [Google Scholar]
- 24.Orouji Z, Bajouri A, Ghasemi M, et al. A single-arm open-label clinical trial of autologous epidermal cell transplantation for stable vitiligo: A 30-month follow-up. J Dermatol Sci. 2018;89(1):52–59. doi: 10.1016/j.jdermsci.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Jin Y, Xu A, Wang P, Song X, Liu X. Long-term follow-up and correlated factors of vitiligo following autologous epidermal transplantation. Cutis. 2011;87(3):137–141. [PubMed] [Google Scholar]
- 26.Ezz-Eldawla R, Abu El-Hamd M, Saied SM, Hassanien SH. A comparative study between suction blistering graft, mini punch graft, and hair follicle transplant in treatment of patients with stable vitiligo. J Dermatolog Treat. 2018;1–6. doi: 10.1080/09546634.2018.1528329 [DOI] [PubMed] [Google Scholar]
- 27.Mohamed Mohamed EE, Younes AK, Osmand A, Mohamed R, Makki M, Younis M. Punch graft versus follicular hair transplantation in the treatment of stable vitiligo. J Cosmet Laser Ther. 2017;19(5):290–293. doi: 10.1080/14764172.2017.1303170 [DOI] [PubMed] [Google Scholar]
- 28.Linthorst Homan MW, Spuls PI, Nieuweboer-Krobotova L, et al. A randomized comparison of excimer laser versus narrow-band ultraviolet B phototherapy after punch grafting in stable vitiligo patients. J Eur Acad Dermatol Venereol. 2012;26(6):690–695. doi: 10.1111/j.1468-3083.2011.04147.x [DOI] [PubMed] [Google Scholar]
- 29.Cohen BE, Elbuluk N, Mu EW, Orlow SJ. Alternative systemic treatments for vitiligo: a review. Am J Clin Dermatol. 2015;16(6):463–474. doi: 10.1007/s40257-015-0153-5 [DOI] [PubMed] [Google Scholar]
- 30.Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol. 2015;151(1):42–50. doi: 10.1001/jamadermatol.2014.1875 [DOI] [PubMed] [Google Scholar]
- 31.Kapoor R, Phiske MM, Jerajani HR. Evaluation of safety and efficacy of topical prostaglandin E2 in treatment of vitiligo. Br J Dermatol. 2009;160(4):861–863. doi: 10.1111/j.1365-2133.2008.08923.x [DOI] [PubMed] [Google Scholar]
- 32.Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110–1112. doi: 10.1001/jamadermatol.2015.1520 [DOI] [PubMed] [Google Scholar]
- 33.Vu M, Heyes C, Robertson SJ, Varigos GA, Ross G. Oral tofacitinib: a promising treatment in atopic dermatitis, alopecia areata and vitiligo. Clin Exp Dermatol. 2017;42(8):942–944. doi: 10.1111/ced.2017.42.issue-8 [DOI] [PubMed] [Google Scholar]
- 34.Rothstein B, Joshipura D, Saraiya A, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol. 2017;76(6):1054–1060.e1051. doi: 10.1016/j.jaad.2017.02.049 [DOI] [PubMed] [Google Scholar]
- 35.Esmat S, Mostafa W, Hegazy RA, et al. Phototherapy: the vitiligo management pillar. Clin Dermatol. 2016;34(5):594–602. doi: 10.1016/j.clindermatol.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 36.Mohammad TF, Al-Jamal M, Hamzavi IH, et al. The Vitiligo Working Group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J Am Acad Dermatol. 2017;76(5):879–888. doi: 10.1016/j.jaad.2016.12.041 [DOI] [PubMed] [Google Scholar]
- 37.Mohammad TF, Silpa-Archa N, Griffith JL, Lim HW, Hamzavi IH. Home phototherapy in vitiligo. Photodermatol Photoimmunol Photomed. 2017;33(5):241–252. doi: 10.1111/phpp.2017.33.issue-5 [DOI] [PubMed] [Google Scholar]
- 38.Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A therapy vs Narrowband-UV-B therapy. Arch Dermatol. 2007;143(5):578–584. [DOI] [PubMed] [Google Scholar]
- 39.Sapam R, Agrawal S, Dhali TK. Systemic PUVA vs. narrowband UVB in the treatment of vitiligo: a randomized controlled study. Int J Dermatol. 2012;51(9):1107–1115. doi: 10.1111/j.1365-4632.2011.05454.x [DOI] [PubMed] [Google Scholar]
- 40.Parsad D, Kanwar AJ, Kumar B. Psoralen-ultraviolet A vs. narrow-band ultraviolet B phototherapy for the treatment of vitiligo. J Eur Acad Dermatol Venereol. 2006;20(2):175–177. doi: 10.1111/j.1468-3083.2006.01413.x [DOI] [PubMed] [Google Scholar]
- 41.Bhatnagar A, Kanwar AJ, Parsad D, De D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: an open prospective study. J Eur Acad Dermatol Venereol. 2007;21(5):638–642. doi: 10.1111/j.1468-3083.2006.02088.x [DOI] [PubMed] [Google Scholar]
- 42.Kwok YK, Anstey AV, Hawk JL. Psoralen photochemotherapy (PUVA) is only moderately effective in widespread vitiligo: a 10-year retrospective study. Clin Exp Dermatol. 2002;27(2):104–110. [DOI] [PubMed] [Google Scholar]
- 43.Wind BS, Kroon MW, Beek JF, et al. Home vs. outpatient narrowband ultraviolet B therapy for the treatment of nonsegmental vitiligo: a retrospective questionnaire study. Br J Dermatol. 2010;162:1142–1144. [DOI] [PubMed] [Google Scholar]
- 44.Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol. 2001;2(3):167–181. doi: 10.2165/00128071-200102030-00006 [DOI] [PubMed] [Google Scholar]
- 45.Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol. 1997;133(12):1525–1528. doi: 10.1001/archderm.1997.03890480045006 [DOI] [PubMed] [Google Scholar]
- 46.Hegazy RA, Fawzy MM, Gawdat HI, Samir N, Rashed LA. T helper 17 and Tregs: a novel proposed mechanism for NB-UVB in vitiligo. Exp Dermatol. 2014;23(4):283–286. doi: 10.1111/exd.2014.23.issue-4 [DOI] [PubMed] [Google Scholar]
- 47.Wu CS, Yu CL, Lan CC, Yu HS. Narrow-band ultraviolet-B stimulates proliferation and migration of cultured melanocytes. Exp Dermatol. 2004;13(12):755–763. doi: 10.1111/j.0906-6705.2004.00141.x [DOI] [PubMed] [Google Scholar]
- 48.De Francesco V, Stinco G, Laspina S, Parlangeli ME, Mariuzzi L, Patrone P. Immunohistochemical study before and after narrow band (311 nm) UVB treatment in vitiligo. Eur J Dermatol. 2008;18(3):292–296. [DOI] [PubMed] [Google Scholar]
- 49.Hum M, Kalia S, Gniadecki R. Prescribing home narrowband UVB phototherapy: a review of current approaches. J Cutan Med Surg. 2018;23(1):91–96. doi: 10.1177/1203475418800947. [DOI] [PubMed] [Google Scholar]
- 50.Larko O, Swanbeck G. Home solarium treatment of psoriasis. Br J Dermatol. 1979;101(1):13–16. doi: 10.1111/bjd.1979.101.issue-1 [DOI] [PubMed] [Google Scholar]
- 51.Eleftheriadou V, Ezzedine K. Portable home phototherapy for vitiligo. Clin Dermatol. 2016;34(5):603–606. doi: 10.1016/j.clindermatol.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 52.Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7(2):31–35. [PMC free article] [PubMed] [Google Scholar]
- 53.Tien Guan ST, Theng C, Chang A. Randomized, parallel group trial comparing home-based phototherapy with institution-based 308 excimer lamp for the treatment of focal vitiligo vulgaris. J Am Acad Dermatol. 2015;72(4):733–735. doi: 10.1016/j.jaad.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 54.Eleftheriadou V, Whitton ME, Gawkrodger DJ, et al. Future research into the treatment of vitiligo: where should our priorities lie? Results of the vitiligo priority setting partnership. Br J Dermatol. 2011;164(3):530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koek MB, Buskens E, Steegmans PH, van Weelden H, Bruijnzeel-Koomen CA, Sigurdsson V. UVB phototherapy in an outpatient setting or at home: a pragmatic randomised single-blind trial designed to settle the discussion. The PLUTO study. BMC Med Res Methodol. 2006;6:39. doi: 10.1186/1471-2288-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials. 2014;15:51. doi: 10.1186/1745-6215-15-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cameron H, Yule S, Moseley H, Dawe RS, Ferguson J. Taking treatment to the patient: development of a home TL-01 ultraviolet B phototherapy service. Br J Dermatol. 2002;147(5):957–965. [DOI] [PubMed] [Google Scholar]
- 58.Haines RH, Thomas KS, Montgomery AA, et al. Home interventions and light therapy for the treatment of vitiligo (HI-Light Vitiligo Trial): study protocol for a randomised controlled trial. BMJ Open. 2018;8(4):e018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yentzer BA, Feldman SR. Trends in home phototherapy adoption in the US: monetary disincentives are only the tip of the iceberg. J Dermatolog Treat. 2011;22(1):27–30. [DOI] [PubMed] [Google Scholar]
- 60.Anderson KL, Huang KE, Huang WW, Feldman SR. Training for prescribing in-office and home phototherapy. Photodermatol Photoimmunol Photomed. 2015;31(6):325–332. [DOI] [PubMed] [Google Scholar]
- 61.Goyal K, Nguyen MO, Reynolds RV, et al. Perceptions of U.S. dermatology residency program directors regarding the adequacy of phototherapy training during residency. Photodermatol Photoimmunol Photomed. 2017;33(6):321–325. [DOI] [PubMed] [Google Scholar]
- 62.Koek MB, Buskens E, Bruijnzeel-Koomen CA, Sigurdsson V. Home ultraviolet B phototherapy for psoriasis: discrepancy between literature, guidelines, general opinions and actual use. Results of a literature review, a web search, and a questionnaire among dermatologists. Br J Dermatol. 2006;154(4):701–711. [DOI] [PubMed] [Google Scholar]
- 63.Ungureanu S, Arzpayma P, Edwards C, Anstey AV. Home phototherapy in the U.K.’s National Health Service: time to reach out. Br J Dermatol. 2017;176(5):1339–1340. [DOI] [PubMed] [Google Scholar]
- 64.Franken SM, Vierstra CL, Rustemeyer T. Improving access to home phototherapy for patients with psoriasis: current challenges and future prospects. Psoriasis (Auckl). 2016;6:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yentzer BA, Yelverton CB, Simpson GL, et al. Paradoxical effects of cost reduction measures in managed care systems for treatment of severe psoriasis. Dermatol Online J. 2009;15(4):1. [PubMed] [Google Scholar]
- 66.Hyde K, Cardwell LA, Stotts R, Feldman S. Psoriasis treatment cost comparison: biologics versus home phototherapy. Am J Pharm Benefits. 2018;10(1):18–21. [Google Scholar]
- 67.Koek MB, Sigurdsson V, van Weelden H, Steegmans PH, Bruijnzeel-Koomen CA, Buskens E. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yentzer BA, Gustafson CJ, Feldman SR. Explicit and implicit copayments for phototherapy: examining the cost of commuting. Dermatol Online J. 2013;19(6):18563. [PubMed] [Google Scholar]
- 69.Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72(5):868–878.e861. [DOI] [PubMed] [Google Scholar]
- 70.Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop Report. Br J Dermatol. 2004;151(2):283–297. [DOI] [PubMed] [Google Scholar]
- 71.El-Zawahry BM, Esmat S, Bassiouny D, et al. Effect of procedural-related variables on melanocyte-keratinocyte suspension transplantation in nonsegmental stable vitiligo: a clinical and immunocytochemical study. Dermatol Surg. 2017;43(2):226–235. [DOI] [PubMed] [Google Scholar]
- 72.Esmat SM, El-Tawdy AM, Hafez GA, et al. Acral lesions of vitiligo: why are they resistant to photochemotherapy? J Eur Acad Dermatol Venereol. 2012;26(9):1097–1104. [DOI] [PubMed] [Google Scholar]
- 73.Rajpara AN, O’Neill JL, Nolan BV, Yentzer BA, Feldman SR. Review of home phototherapy. Dermatol Online J. 2010;16(12):2. [PubMed] [Google Scholar]
- 74.Shan X, Wang C, Tian H, Yang B, Zhang F. Narrow-band ultraviolet B home phototherapy in vitiligo. Indian J Dermatol Venereol Leprol. 2014;80:336–338. [DOI] [PubMed] [Google Scholar]