Abstract
Extramedullary infiltration (EMI) is common in patients with acute myeloid leukemia (AML) and is closely associated with the prognosis of disease. We previously reported that patients carrying the AML1/ETO (A/E) fusion gene and expressing the amyloid precursor protein (APP) tended to develop EMI, and had a poor prognosis. In the present study, the relapse-free survival (RFS) time and overall survival (OS) time were significantly lower in patients with EMI. The results demonstrated that the EMI incidence was significantly higher (P<0.05), while the RFS and OS rates were significantly lower (P<0.05), in patients with high APP expression. Kasumi-1 cells, which are A/E+, and the APP gene were used as the in vitro cell model to detect the mechanism of action in detail. Following the knockdown of APP expression, cell migration was significantly reduced (P<0.05). Furthermore, western blotting demonstrated that the protein expression of phosphorylated extracellular-signal-regulated kinase (p-ERK), matrix metalloproteinase-2 (MMP-2) and c-Myc was markedly reduced following interference of APP, while the expression of CXCR4 and MMP-9 was not altered. Kasumi-1 cells were co-cultured with p-ERK or c-Myc inhibitors and demonstrated that the APP/p-ERK/c-Myc/MMP-2 pathway was involved in signal transduction and regulation of cell migration. MicroRNA-144 (miR-144) mimics and transfected Kasumi-1 cells were generated. Reverse transcription-quantitative polymerase chain reaction and western blotting demonstrated that miR-144 was a negative regulator of APP. Taken together, the findings of the present study suggest that miR-144 negatively targets the APP gene and regulates cell migration via the APP/p-ERK/c-Myc/MMP-2 pathway.
Keywords: microRNA-144, amyloid precursor protein, AML1/ETO+, migration, matrix metalloproteinase-2
Introduction
Due to the continued improvement of chemotherapeutic agents, the remission rate of leukemia has significantly improved (1–3). However, the recurrence rate remains as high as 30–40% (4). Extramedullary infiltration (EMI) of acute leukemia includes a wide variety of clinically significant phenomena that often pose therapeutic dilemmas. The acute myeloid leukemia 1 protein/protein ETO (AML1/ETO; A/E) fusion gene, caused by a t(8;21) translocation, accounts for 15% of AML cases and is identified in 15–26.7% of young patients with EMI (5), with a 5-year overall survival (OS) rate of 61% (6). It is hypothesized that being A/E+ is indicative of a good prognosis and it has been reported that ≤40% of A/E+ leukemia cases have EMI (7–9). Our previous study demonstrated that patients with high expression of amyloid precursor protein (APP) were more likely to develop EMI (10). The aim of the present study was to investigate the molecular mechanisms of EMI in detail in vitro.
C-X-C motif chemokine receptor 4 (CXCR4) serves an important role in cell migration, with certain studies hypothesizing that cell migration is predominantly dependent on the CXCR4/stromal-derived factor-1 (SDF-1) axis (11). CXCR4 and its ligand SDF-1 are primarily studied for their crucial roles in the homing of stem and progenitor cells in the bone marrow, chemotaxis, cell arrest, angiogenesis, metastasis and cell survival (12). Tavor et al (13) reported that AML cells constitutively secrete and express SDF-1-dependent cell surface elastase, which regulates their migration and proliferation. Our previous study revealed that A/E+ patients highly expressed CXCR4. Furthermore, it was found that APP regulated cell migration via matrix metalloproteinase (MMP)-2. MMP-2 and MMP-9 serve important roles in metastasis due to their capacity to degrade the extracellular matrix (14,15); they are hypothesized to be particularly important for cell migration, as these proteinases act on type IV collagen (11). Experimental evidence has demonstrated that MMP-2 and MMP-9 are not only involved in the invasion and metastasis of solid tumors, but are also overexpressed in a variety of acute and chronic leukemia (13,16), suggesting that they may serve an important role in breaking through the bone marrow barrier. In the present study, the different molecular expression levels of MMP-2 and MMP-9 were measured following interference with APP expression.
MicroRNA (miRNA/miR)-144 is an important transcriptional regulator in the process of hematopoiesis, and its abnormal expression is closely associated with the pathogenesis of hematological malignancies (17,18). At present, an increasing amount of research is focusing on miR-144 and its role in erythroid formation; however, there are relatively few studies pertaining to its role in leukemia (19,20). Liu et al (21) reported that miR-144 increased the sensitivity of leukemia cells to imatinib and was closely associated with the c-Myc gene. Liang et al (22) reported that miR-144 could inhibit human embryonic trophoblast cell invasion. Based on these results, we hypothesize that miR-144 may serve an important role in the migration of Kasumi-1 cells.
The present study investigated the association between miR-144 and the APP gene, and demonstrated that APP regulates cell migration through the APP/phosphorylated extracellular-signal-regulated kinase (p-ERK)/c-Myc/MMP-2 pathway. This finding may provide novel insights into the development of therapeutic strategies for the treatment of patients with AML, with particular focus on A/E+ patients with EMI.
Materials and methods
Patient characteristics
A/E+ AML patients (n=123), diagnosed according to the World Health Organization 2008 criteria (23), were enrolled in the present study between February 2002 and June 2013 at the Nanfang Hospital, Southern Medical University, (Guangzhou, China). All patients enrolled in the present study provided written informed consent, and the study was approved by the Ethics Committee of Nanfang Hospital (Guangzhou, China). Patients were examined using marrow cytology analysis, karyotype analysis and fluorescence in situ hybridization (FISH). All patients received follow-up until December 2013, with a median time of 46 months (6–141 months). As this was a retrospective study, certain data were not obtained for all individuals included in the present study.
All patients completed 1–2 cycles of induction chemotherapy, the majority of whom received the ‘3+7’ regimen consisting of anthracyclines and cytarabine. Following remission, 109 patients received a median of 3 (range, 1–8) intrathecal injections of 10 mg methotrexate or 50 mg cytarabine plus 5 mg dexamethasone, which were used alternately. A total of 25 patients with central nervous system lymphoma received a lumbar intrathecal injection every other day until cerebrospinal fluid without blast cells was detected by microscopy (Table I).
Table I.
Characteristics of AML1/ETO+ patients with or without EML.
| Characteristics | EML+ | EML− | P-value |
|---|---|---|---|
| No. of patients | 36 | 87 | |
| Male/female, n | 26/10 | 46/41 | 0.048 |
| Median age (range), years | 30.5 (2–69) | 25.0 (3–74) | 0.295 |
| Median WBC (range), ×109 cells/l | 21.7 (1.8–79.3) | 13.6 (1.1–85.8) | 0.059 |
| C-KIT mutation, n (%)a | |||
| Positive | 9 (39.1) | 14 (23.7) | 0.163 |
| Negative | 14 (60.9) | 45 (76.3) | |
| FLT3-ITD, n (%)a | |||
| Positive | 1 (4.3) | 2 (3.4) | 0.836 |
| Negative | 22 (95.7) | 57 (96.6) | |
| Expression of APP gene, n (%) | |||
| High | 11 (91.7) | 20 (41.7) | 0.019 |
| Low | 1 (8.3) | 28 (58.3) | |
| Induction therapy, n (%) | |||
| DA | 10 (30.3) | 20 (23.3) | |
| IA | 15 (45.5) | 46 (53.5) | 0.648 |
| Other | 8 (24.2) | 20 (23.3) | |
| Consolidation therapy, n (%) | |||
| Regimen 1 | 17 (58.6) | 31 (38.8) | |
| Regimen 2 | 8 (27.6) | 34 (42.5) | 0.179 |
| Regimen 3 | 4 (13.8) | 15 (18.8) | |
| IT, n (%) | |||
| ≤2 | 11 (31.0) | 37 (42.5) | 0.415 |
| >2 | 25 (69.0) | 50 (57.5) | |
| Outcomes of therapy, % | |||
| Rate of RFS | 34.5 | 68.8 | <0.001 |
| Rate of OS | 48.3 | 73.8 | 0.003 |
c-kit mutation and FLT3-ITD mutation data were not collected for all patients. WBC, white blood cell; FLT3-ITD, mutation in the FMS-like tyrosine kinase 3 gene; DA, cytarabine plus daunorubicin; IA, cytarabine plus idarubicin; IT, intrathecal injection; OS, overall survival; RFS, relapse-free survival; APP, amyloid precursor protein; EML, extramedullary leukemia.
Patients without extramedullary leukemia (EML) were designated as the control group, and those with EML were designated as the study group. The complete remission (CR) rate, relapse-free survival (RFS) time and OS time were compared following two cycles of chemotherapy. RFS was calculated from time of CR until relapse and OS was defined as the time from diagnosis until mortality. APP expression was determined using bone marrow samples (n=65) of the AML/ETO+ patients prior to treatments via reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The prognosis was analyzed and compared between these two groups (Table II).
Table II.
Association between APP expression and survival.
| High expression of APP (n=30), % | Low expression of APP (n=30), % | P-value | |
|---|---|---|---|
| Cumulative CR rate after the second cycle of chemotherapy | 84.4 | 100.0 | 0.020 |
| Rate of RFS | 40.0 | 80.0 | 0.001 |
| Rate of OS | 60.0 | 83.3 | 0.029 |
CR, complete remission; RFS, relapse-free survival; OS, overall survival; APP, amyloid precursor protein.
Cell culture and detection
Kasumi-1 cells (American Type Culture Collection, Manassas, VA, USA), which are a human A/E+ cell line derived from AML, were plated at 3×105 cells/ml in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 4 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. FISH, morphological detection using Wright's dye, and karyotype analysis were performed as described previously (10).
Cell migration assay
The procedure was performed as described previously (10). To evaluate the migration ability the method was adjusted as follows: Non-invading cells were removed from the upper surface of the Transwell membrane with a cotton swab and invading cells on the lower membrane surface were fixed in methanol at the room temperature for 15 min. The membrane was then stained with 0.1% crystal violet for 15 min, washed with water three times and dried for 3 h at the room temperature. Images were captured using a light microscope (magnification, ×200) and cells in five randomly selected fields were counted. Each invasion experiment was performed in triplicate.
Target in vitro luciferase reporter assay
pMIR-REPORT plasmids for the miR-144 target APP 3′-untranslated region (UTR) were constructed as wild-type (WT) pmiR-APP containing two tandem repeats of miR-144 response element from the APP 3′-UTR and a mutant (MUT) pmiR-APP by replacing two nucleotides within the ‘seed sequence’ (psi-CHECK-APP-mut-UTR). The oligonucleotides were annealed and inserted into the pMIR-REPORT vector (Promega Corporation, Madison, WI, USA). The site-directed mutagenesis was performed using the Quick Change kit (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA). The empty vector (pMIR-REPORT) was used as a negative control. Cells were transfected using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol, with 0.2 µg Target reporter plasmids (catalog no. 64158; Addgene, Inc., Cambridge, MA, USA) and 0.01 µg pMIR-REPORT Control Plasmid (catalog no. 26280; Addgene, Inc.), and 200 nM miR-144-MIMIC (miR-34c precursor; Addgene, Inc.) and NC control (Shanghai GenePharma Co., Ltd.) per well on 96-well plates. Following 24 h of incubation, the cells were subjected to a luciferase reporter assay using a dual-luciferase reporter assay system (E1910; Promega Corporation). Renilla luciferase activity was normalized to Firefly luciferase activity for each sample. Each experiment was repeated at least three times in triplicate.
RT-qPCR of miR-144 and APP expression
Total RNA was extracted from kasumi-1 cells with TRIzol reagents according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and quantified by an ultraviolet spectrophotometer (UVP, LLC, Phoenix, AZ, USA) at 260 nm. The cDNA was synthesized from 1,000 ng total RNA using the PrimerScript RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). The PCR primer sequences were as follows: β-actin forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; and APP forward, 5′-TGGCCCTGGAGAACTACATC-3′ and reverse, 5′-TGGCCCTGGAGAACTACATC-3′. The procedure was performed as described previously (4). First, the stem-loop RT primer was hybridized to an miRNA molecule and then reverse transcribed with a MMLV reverse transcriptase. The RT products were amplified using conventional TaqMan PCR, according to the manufacturer's protocol (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A 20-µl RT reaction was incubated at 25°C for 30 min and at 94°C for 3 min. The qPCR was 45 cycles of denaturing at 94°C for 20 sec, annealing at 50°C for 25 sec and synthesis at 72°C for 20 sec. U6 (forward, 5′-CTCGCTTCGGCZGCZCZ-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′) was used as the internal control. The fold-change for miR-144 expression level was calculated using the 2−∆∆Cq method (24).
Knockdown of APP by lentiviral transduction
The complementary DNA sequence of APP was designed from the full-length APP sequence by Shanghai GeneChem Co., Ltd. (Shanghai, China). The siRNA target sequence of APP was 5′-CATCTTTGACCGAAACGAA-3′ and that of the negative control was 5′-TTCTCCGAACGTGTCACGT-3′ (NC). The procedure was performed as described previously (10).
p-ERK inhibitor and c-Myc inhibitor treated with kasumi-1 cells
In the present study, WT Kasumi-1 cells were treated with the p-ERK inhibitor PD98059 (Santa Cruz Biotechnology Inc., Dallas, TX, USA) and the c-Myc inhibitor 10058-F4 (Selleck Chemicals, Houston, TX, USA), respectively. Based on preliminary experiments, Kasumi-1 cells were treated with the p-ERK inhibitor at concentrations of 20 and 40 µM for 24 and 48 h. Also, Kasumi-1 cells were co-cultured with c-Myc inhibitor 10058-F4 at concentrations of 50 and 100 µM for 24 and 48 h. The cells were collected and western blotting was performed to detect the changes.
APP/p-ERK/c-Myc/MMP-2 pathway detected by western blotting
Protein concentrations of cell lysates were determined using the BCA protein assay reagent kit. Equal amounts (30 µg) of total protein were resolved by SDS-PAGE and transferred to a polyvinylidene fluoride membrane. Following blocking with 5% skimmed milk at room temperature for 2 h with shaking and washing with TBST (50 ml Tris HCl, 8 g NaCl, 0.2 g KCl, 1 ml 0.1% Tween-20 and 1,000 ml distilled water), the membrane was incubated overnight at 4°C with the following primary antibodies: APP (catalog no. ab76763; 1:2,000; Abcam, Cambridge, MA, USA), MMP-2 (catalog no. cs-13594; dilution of 1:1,000; Santa Cruz Biotechnology Inc.), MMP-9 (catalog no. cs-21733; dilution of 1:500; Santa Cruz Biotechnology Inc.), p-ERK (catalog no. ab201015; dilution of 1:500; Abcam), ERK (catalog no. ab54230; dilution of 1:500; Abcam), c-Myc (catalog no. sc-40; dilution of 1:1,000; Santa Cruz Biotechnology Inc.) and β-actin (catalog no. ab115777; 1:200; Abcam). Following washing with TBS-T, the membranes were incubated with secondary HRP-conjugated anti-rabbit IgG or anti-mouse IgG (1:100; OriGene Technologies, Inc. Beijing, China) for 2 h at room temperature. Proteins of interest were visualized by enhanced chemiluminescence HRP substrate (EMD Millipore, Billerica, MA, USA) and analyzed with an image analyzer (ChemiDoc MP Imaging system; Bio-Rad Laboratories, Inc.).
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA) was used for data analysis. Data were obtained from independent experiments and are expressed as the mean ± standard deviation. Statistical analysis was performed by one-way ANOVA followed by the Fisher's least significant difference test. Survival analyses were performed using Kaplan-Meier survival analysis. ROC curve analysis was used to calculate the cut-off value of APP, which served to divide the patients into a high expression group (with levels of APP ≥ cut-off value) and a low expression group (with levels of APP < cut-off value). Not normally distributed data were described by the median. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the A/E+ patients
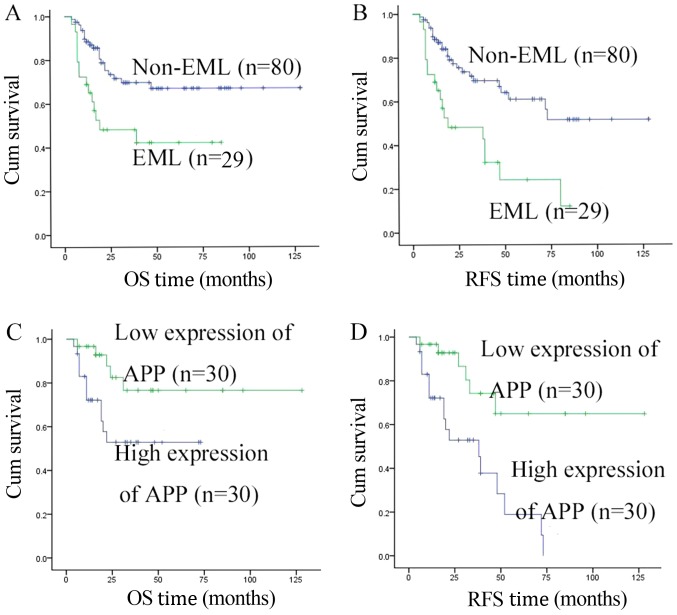
The 123 A/E+ patients were divided into two groups: 36 patients in the EML group (with EML), and 87 patients in the control group (without EML). In the EML group, 29 patients achieved a CR and finished the follow-up treatment after the CR. In the control group, 80 patients achieved a CR and finished the follow-up treatment after the CR. There was no significant difference (P>0.05) in the two-cycle induction chemotherapy regimens, the number of white blood cells or treatments following CR in these two groups (Table I). At the median follow-up time of 46 months (range, 6–141 months), the RFS and OS rates in the EML group were significantly lower than those in the control group (34.5 vs. 68.8%, P<0.001; 48.3 vs. 73.8%, P=0.003, respectively; Table I). These results were consistent with Fig. 1A and B.
Figure 1.
Characteristics of AML1/ETO+ patients at the median follow-up time of 46 months. (A) OS rate in the EML group was significantly lower than that in the control group (34.5 vs. 68.8%, P<0.001; 48.3 vs. 73.8%, P=0.003, respectively). (B) RFS rate in the EML group was significantly lower than that in the control group (34.5 vs. 68.8%, P<0.001). (C) OS rate in the high expression APP group was significantly lower than that in the low expression group (P=0.029). (D) RFS in the high expression APP group was significantly lower than that the low expression group (P=0.001). OS, overall survival; RFS, relapse-free survival; EML, extramedullary leukemia; AML, acute myeloid leukemia; APP, amyloid precursor protein.
Overall, 60 out of 65 patients with APP mRNA achieved a CR and finished the follow-up treatment after the CR. When we followed up for 35 months (6–96 months), the RFS rate in the high expression group was 40.0%, which was significantly lower compared with the low expression group with 80.0% (P=0.001). In addition, a similar result was observed for the OS rate (60.0 vs. 83.3%; P=0.029; Table II). These results were consistent with Fig. 1C and D.
Effect of APP on cell migration
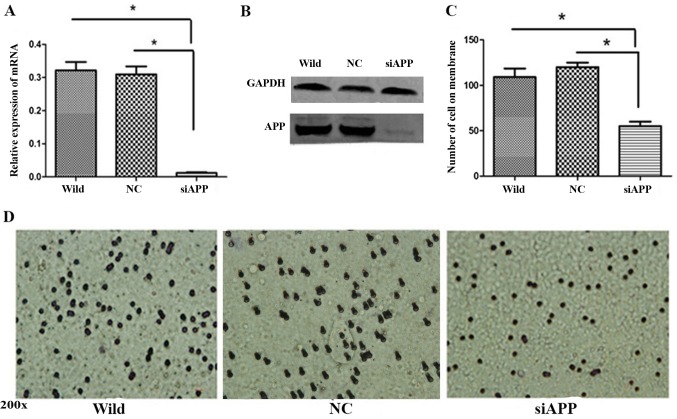
Kasumi-1 cells were transfected with siRNA against APP (siAPP) and scramble siRNA (NC) and WT Kasumi-1 cells were used as a control. RT-qPCR analysis demonstrated that the mRNA of APP was significantly reduced in the siAPP group (Fig. 2A, P<0.01), and western blotting revealed that the protein expression was markedly inhibited in the siAPP group (Fig. 2B).
Figure 2.
APP regulates Kasumi-1 cell migration. (A) RT-qPCR and (B) western blotting detected that the expression of APP was significantly reduced following siRNA interference. (C) Quantification and (D) representative images of transmigrated Kasumi-1 cells in response to siAPP treatment, all of the image were recorded with 200× magnification. *P<0.05. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; APP, amyloid precursor protein; si, small interfering; wild, wild-type; NC, negative control.
A Transwell assay was performed to evaluate cell migration following the interference in APP. A significant reduction in migrated cells in the siAPP group was observed when compared with the control groups. Following the assessment of five randomly-selected fields of view, the number of cells in the siAPP group was observed to be significantly lower than that in the control groups (P<0.05; Fig. 2C and D).
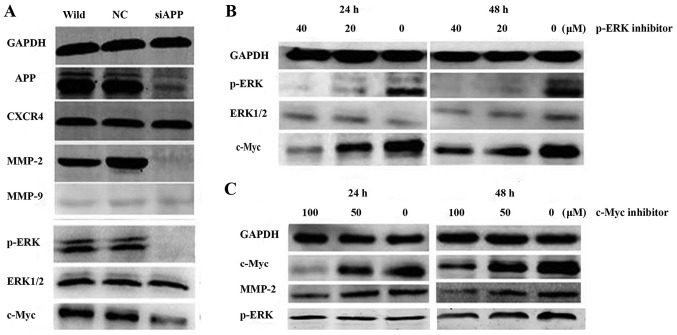
Mechanisms of APP regulate Kasumi-1 cell migration in vitro
The expression of MMP-2, p-ERK and c-Myc was markedly decreased following the interference of APP expression; however, there was no evident change in the expression of MMP-9 or CXCR4 (Fig. 3A). Furthermore, WT Kasumi-1 cells were treated with the p-ERK inhibitor PD98059 at concentrations of 20 and 40 µM for 24 and 48 h. The western blotting assay suggested that the expression levels of p-ERK and c-Myc were markedly reduced (Fig. 3B). Similarly, when the c-Myc inhibitor 10058-F4 was co-cultured with Kasumi-1 cells at concentrations of 50 and 100 µM for 24 and 48 h, the expression levels of MMP-2 and c-Myc were evidently decreased (Fig. 3C); however, there was no evident change in p-ERK expression. These results suggested that c-Myc was regulated by p-ERK.
Figure 3.
Western blotting detecting the pathway of APP/p-ERK/c-Myc/MMP-2. (A) MMP-2, p-ERK and c-Myc expression was significantly decreased after APP expression was attenuated; however, there was no change in the expression of MMP-9 or CXCR4. (B) Kasumi-1 cells treated with a p-ERK inhibitor at concentrations of 20 and 40 µM for 24 and 48 h. The western blotting assay suggested that the expression levels of p-ERK and c-Myc were significantly reduced. (C) The c-Myc inhibitor 10058-F4 was co-cultured with Kasumi-1 cells at a concentration of 50 and 100 µM for 24 and 48 h. The expression of MMP-2 and c-Myc was significantly decreased, but there was no change in p-ERK expression. APP, amyloid precursor protein; CXCR4, C-X-C motif chemokine receptor 4; MMP, matrix metalloproteinase; p-ERK, phosphorylated extracellular-signal-regulated kinase.
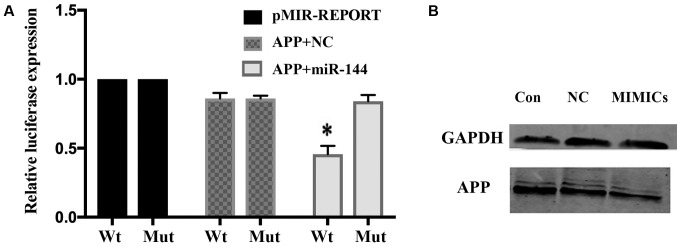
miR-144 negatively regulates APP expression
To examine whether miR-144 repression of APP is mediated by the direct interaction of miR-144 with the APP 3′-UTR, a WT or MUT miR-144/IL-6R response element was cloned into a pMIR-REPORT plasmid downstream of luciferase. miR-144-MIMIC was transfected into Kasumi-1 cells. The reporter activities of WT, but not MUT, were inversely associated with miR-144 expression. The relative luciferase expression in the cells that were co-transfected with psi-CHECK-APP-UTR and miR-144 oligonucleotide was significantly reduced compared with the NC (P<0.01; Fig. 4A). Western blotting demonstrated that the expression of the APP protein was decreased in the miR-144-MIMIC group (Fig. 4B).
Figure 4.
Connection between APP and miR-144. (A) Dual-luciferase reporter assay. The relative luciferase expression in cells that were co-transfected with psi-CHECK-APP-UTR and miR-144 oligonucleotide was significantly reduced. *P<0.01 vs. Mut APP+miR-144. (B) Western blotting showed that APP protein was decreased in the miR-144 MIMICs group. Wt, wild-type; Mut, mutant; NC, negative control; Con, control; APP, amyloid precursor protein; UTR, untranslated region; miR, microRNA.
Discussion
Previous studies have demonstrated that the 5-year OS rate of A/E+ AML patients is ~61% (25,26), and EMI rates among A/E+ AML patients have been reported to be as high as 15–26.7% (27). In our previous study, it was demonstrated that the APP gene was involved in the EMI of leukemia (10). The process of EMI includes the escape of the leukemia cell from the bone marrow, adhesion, degradation of the matrix and migration to other areas. High expression of MMPs in patients with acute leukemia is closely associated with invasion (16,28,29).
The present study examined the expression of other migration-associated proteins, which may assist in investigating the underlying mechanisms of APP in regulating leukemic EMI. The mechanisms of EMI are complicated, with previous studies reporting that leukemia cells that escaped from the bone marrow to the surrounding organs were associated with different signal molecules, including MMPs, CXCR4 and EZH2 (30–32). The SDF1-CXCR4 axis serves a crucial role in hematopoiesis and cell migration, and a number of studies have suggested that CXCR4 is closely associated with metastasis in leukemia and solid tumors (33–35). Möhle et al (36) reported that CXCR4 was highly expressed in Kasumi-1 cells. We consequently hypothesized that the central nervous system and other organs may attract leukemia cells from the bone marrow to their locations via CXCR4. However, in the present study, CXCR4 expression was not altered when the expression of APP was reduced by siRNA. Therefore, it may be concluded that the APP gene regulating Kasumi-1 cell migration is not directly associated with CXCR4. In this study, MMP-2 expressuin was significantly decreased after interference in APP, but there was no change in MMP-9 or CXCR4, which are known as tumor metastasis molecules. Therefore, the present study focused on the association between APP and MMP-2.
The MAPK signaling pathway serves an important role in tumor development, invasion, metastasis and chemo-resistance (16,37). When cells are activated by external stimuli, cell membrane receptors are triggered and the signals transduce into the cytoplasm. In the nucleus, p-ERK binds to c-Myc and other transcription factors to regulate cell proliferation, differentiation and tumor development (38–41). Numerous studies have demonstrated that MMPs are one of the regulatory proteins downstream of the MAPK signaling pathway (39,40). Therefore, we hypothesized that the APP gene may regulate MMP-2 through the MAPK signaling pathway in leukemia EMI. The present study also demonstrated that p-ERK and c-Myc expression decreased at the same time, following the interference in APP, suggesting that the APP gene activated the MAPK/ERK signal transduction pathway. In order to understand the details behind the function of APP in A/E+ leukemia cell migration, wild-type Kasumi-1 cells were treated with the MEK inhibitor PD98059 at concentrations of 20 and 40 µM for 24 and 48 h. The western blot assay suggested that the expression levels of p-ERK and c-Myc were significantly reduced. Similarly, when the c-Myc inhibitor 10058-F4 was co-cultured with Kasumi-1 cells at concentrations of 50 and 100 µM for 24 and 48 h, the expression levels of MMP-2 and c-Myc were significantly decreased; however, there was no change in p-ERK expression. These results suggested that c-Myc was regulated by p-ERK. Based on this, it was concluded that APP regulated Kasumi-1 cell migration via the p-ERK/c-Myc/MMP-2 pathway.
An increasing number of studies have reported that miRNAs overexpressed in tumors may be considered as oncogenes, but only a few oncogenic miRNAs have been well characterized. These oncogenic miRNAs are called ‘oncomirs’. Several studies and clinical analyses suggest that miRNAs may function as a novel class of oncogenes or tumor suppressor genes (42–44). TargetScan (Massachusetts Institute of Technology, Cambridge, MA, USA), an online software used to predict binding sites on mRNAs, predicted that the 3′-UTR of the APP gene incorporated a target site of miR-144 with six complement oligo-nucleotide bases. In a study by Garcia et al (45) on human embryonic trophoblast cell invasion, it was found that miR-144 could inhibit cell migration. Furthermore, the present study identified that the expression of MMP-2 and MMP-9 was significantly decreased by the inhibition of p-ERK expression. This is consistent with the present results showing that miR-144 negatively regulates the APP gene.
In summary, miR-144 negatively regulates the APP gene and then regulates EMI in patients with A/E+ AML. The pathological molecular mechanism may be via the APP/p-ERK/c-Myc/MMP-2 pathway. These results provide a novel insight into the mechanism of EMI and could render a potential therapeutic method to delay or prevent EMI, and may be useful in preventing and treating A/E+ AML EMI.
Acknowledgements
The authors would like to acknowledge Dr Jie Yu Ye (Department of Hematology, Nanfang Hospital, Southern Medical University) for their assistance with revising the language of this manuscript.
Funding
This work was supported by the grants from National High Technology Research and Development Program of China (863) (grant no. 2012AA02A505), National Natural Science Foundation of China (grant no. 81400103), Natural Science Foundation of Guangzhou province (grant no. 2014A030310061).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LJ wrote the article and performed the majority of the experiments. WM performed the luciferase reporter assay and knockdown of APP by lentiviral transfection. GY analyzed the clinical data. CY and LL performed the polymerase chain reaction experiments. ZW performed western blot analysis. FM designed the all experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All patients provided written informed consent. The study was approved by the Ethics Committee of Nanfang Hospital (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang L, Xu J, Tian X, Lv T, Yuan G. Analysis of efficacy and prognostic factors of CLAG treatment in chinese patients with refractory or relapsed acute myeloid leukemia. Acta Haematol. 2019;141:43–53. doi: 10.1159/000493250. [DOI] [PubMed] [Google Scholar]
- 2.Low M, Lee D, Coutsouvelis J, Patil S, Opat S, Walker P, Schwarer A, Salem H, Avery S, Spencer A, Wei A. High-dose cytarabine (24 g/m2) in combination with idarubicin (HiDAC-3) results in high first-cycle response with limited gastro- intestinal toxicity in adult acute myeloid leukaemia. Intern Med J. 2013;43:294–297. doi: 10.1111/j.1445-5994.2012.02868.x. [DOI] [PubMed] [Google Scholar]
- 3.Sasine JP, Schiller GJ. Emerging strategies for high-risk and relapsed/refractory acute myeloid leukemia: Novel agents and approaches currently in clinical trials. Blood Rev. 2015;29:1–9. doi: 10.1016/j.blre.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH. Central nervous system disease in acute lymphoblastic leukemia: Prophylaxis and treatment. Hematology Am Soc Hematol Educ Program. 2006;2006:142–146. doi: 10.1182/asheducation-2006.1.142. [DOI] [PubMed] [Google Scholar]
- 5.Boissel N, Leroy H, Brethon B, Philippe N, de Botton S, Auvrignon A, Raffoux E, Leblanc T, Thomas X, Hermine O, et al. Incidence and prognostic impact of c-Kit, FLT3 and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML) Leukemia. 2006;20:965–970. doi: 10.1038/sj.leu.2404188. [DOI] [PubMed] [Google Scholar]
- 6.Prébet T, Boissel N, Reutenauer S, Thomas X, Delaunay J, Cahn JY, Pigneux A, Quesnel B, Witz F, Thépot S, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: A collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27:4747–4753. doi: 10.1200/JCO.2008.21.0674. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse and overall survival in adult patients with de novo acute myeloid leukemia: Results from cancer and leukemia group B. Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 8.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, et al. Karyotypic analysis predicts outcome of pre- remission and postremission therapy in adult acute myeloid leuke- mia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 9.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 10.Jiang L, Yu G, Meng W, Wang Z, Meng F, Ma W. Overexpression of amyloid precursor protein in acute myeloid leukemia enhances extramedullary infiltration by MMP-2. Tumor Biol. 2013;34:629–636. doi: 10.1007/s13277-012-0589-7. [DOI] [PubMed] [Google Scholar]
- 11.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: Differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu SH, Gu Y, Pascual B, Yan Z, Hallin M, Zhang C, Fan C, Wang W, Lam J, Spilker ME, et al. A novel CXCR4 antagonist IgG1 antibody (PF-06747143) for the treatment of hematologic malignancies. Blood Adv. 2017;1:1088–1100. doi: 10.1182/bloodadvances.2016003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavor S, Petit I, Porozov S, Goichberg P, Avigdor A, Sagiv S, Nagler A, Naparstek E, Lapidot T. Motility, proliferation, and egress to the circulation of human AML cells are elastase dependent in NOD/SCID chimeric mice. Blood. 2005;106:2120–2127. doi: 10.1182/blood-2004-12-4969. [DOI] [PubMed] [Google Scholar]
- 14.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Suminoe A, Matsuzaki A, Hattori H, Koga Y, Ishii E, Hara T. Expression of matrix metalloproteinase (MMP) and tissue inhibitor of MMP (TIMP) genes in blasts of infant acute lymphoblastic leukemia with organ involvement. Leuk Res. 2007;31:1437–1440. doi: 10.1016/j.leukres.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 17.Su Z, Si W, Li L, Zhou B, Li X, Xu Y, Xu C, Jia H, Wang QK. MiR-144 regulates hematopoiesis and vascular development by targeting meis1 during zebrafish development. Int J Biochem Cell Biol. 2014;49:53–63. doi: 10.1016/j.biocel.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Papapetrou EP, Korkola JE, Sadelain M. A genetic strategy for single and combinatorial analysis of miRNA function in mammalian hematopoietic stem cells. Stem cells. 2010;28:287–296. doi: 10.1002/stem.257. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Zhu X, Ward CM, Starlard-Davenport A, Takezaki M, Berry A, Ward A, Wilder C, Neunert C, Kutlar A, Pace BS. MiR-144-mediated NRF2 gene silencing inhibits fetal hemoglobin expression in sickle cell disease. Exp Hematol. 2019;70:85–96.e5. doi: 10.1016/j.exphem.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trecul A, Morceau F, Gaigneaux A, Schnekenburger M, Dicato M, Diederich M. Valproic acid regulates erythro-megakaryocytic differentiation through the modulation of transcription factors and microRNA regulatory micro-networks. Biochem Pharmacol. 2014;92:299–311. doi: 10.1016/j.bcp.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Wang S, Chen R, Wu Y, Zhang B, Huang S, Zhang J, Xiao F, Wang M, Liang Y. Myc induced miR-144/451 contributes to the acquired imatinib resistance in chronic myelogenous leukemia cell K562. Biochem biophys Res Commun. 2012;425:368–373. doi: 10.1016/j.bbrc.2012.07.098. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Lin Q, Luo F, Wu W, Yang T, Wan S. Requirement of miR-144 in CsA induced proliferation and invasion of human trophoblast cells by targeting titin. J cell Biochem. 2014;115:690–696. doi: 10.1002/jcb.24710. [DOI] [PubMed] [Google Scholar]
- 23.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK, National Cancer Research Institute Adult Leukaemia Working Group Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 26.Zhen T, Wu CF, Liu P, Wu HY, Zhou GB, Lu Y, Liu JX, Liang Y, Li KK, Wang YY. Targeting of AML1-ETO in t(8;21) leukemia by oridonin generates a tumor suppressor-like protein. Sci Transl Med. 2012;4:127ra38. doi: 10.1126/scitranslmed.3003562. [DOI] [PubMed] [Google Scholar]
- 27.Abdel Rahman H, Farrag SA, EI-Attar IA. AML1/ETO fusion gene in de novo pediatric acute myeloid leukemia: Clinical significance and prognostic implications. J Egypt Natl Canc Inst. 2007;19:39–47. [PubMed] [Google Scholar]
- 28.Pan YX, Yang L, Wen SP, Liu XJ, Luo JM. Expression and clinical significance of MMP-2 and MMP-9 in B acute lymphoblastic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:640–643. doi: 10.7534/j.issn.1009-2137.2014.03.012. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 29.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20:4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takayama K, Tsutsumi S, Suzuki T, Horie-Inoue K, Ikeda K, Kaneshiro K, Fujimura T, Kumagai J, Urano T, Sakaki Y, et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009;69:137–142. doi: 10.1158/0008-5472.CAN-08-3633. [DOI] [PubMed] [Google Scholar]
- 31.Krause K, Karger S, Sheu SY, Aigner T, Kursawe R, Gimm O, Schmid KW, Dralle H, Fuhrer D. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J Endocrinol. 2008;198:291–299. doi: 10.1677/JOE-08-0005. [DOI] [PubMed] [Google Scholar]
- 32.Iannetti A, Ledoux AC, Tudhope SJ, Sellier H, Zhao B, Mowla S, Moore A, Hummerich H, Gewurz BE, Cockell SJ, et al. Regulation of p53 and Rb links the alternative NF-kB pathway to EZH2 expression and cell senescence. PLoS Genet. 2014;10:e1004642. doi: 10.1371/journal.pgen.1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahara F, Kitaura J, Uchida T, Nishida C, Togami K, Inoue D, Matsukawa T, Kagiyama Y, Enomoto Y, Kawabata KC, et al. Hes1 promotes blast crisis in chronic myelogenous leukemia through MMP-9 upregulation in leukemic cells. Blood. 2014;123:3932–3942. doi: 10.1182/blood-2013-01-476747. [DOI] [PubMed] [Google Scholar]
- 34.Ugarte-Berzal E, Bailón E, Amigo-Jiménez I, Albar JP, García-Marco JA, García-Pardo A. A novel CD44-binding peptide from the pro-matrix metalloproteinase-9 hemopexin domain impairs adhesion and migration of chronic lymphocytic leukemia cells. J Biol Chem. 2014;289:15340–15349. doi: 10.1074/jbc.M114.559187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douglass S, Meeson AP, Overbeck-Zubrzycka D, Brain JG, Bennett MR, Lamb CA, Lennard TW, Browell D, Ali S, Kirby JA. Breast cancer metastasis: Demonstration that FOXP3 regulates CXCR4 expression and the response to CXCL12. J Pathol. 2014;234:74–85. doi: 10.1002/path.4381. [DOI] [PubMed] [Google Scholar]
- 36.Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- 37.Vlad A, Deglesne PA, Letestu R, Saint-Georges S, Chevallier N, Baran-Marszak F, Varin-Blank N, Ajchenbaum-Cymbalista F, Ledoux D. Down-regulation of CXCR4 and CD62 L in chronic lymphocytic leukemia cells is triggered by B-cell receptor ligation and associated with progressive disease. Cancer Res. 2009;69:6387–6395. doi: 10.1158/0008-5472.CAN-08-4750. [DOI] [PubMed] [Google Scholar]
- 38.Sarkissyan S, Sarkissyan M, Wu Y, Cardenas J, Koeffler HP, Vadgama JV. IGF-1 regulates cyr61 induced breast cancer cell proliferation and invasion. PLoS One. 2014;9:e103534. doi: 10.1371/journal.pone.0103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin W, Lu Y, Li Q, Wang J, Zhang H, Chang G, Lin Y, Pang T. Down-regulation of the P-glycoprotein relevant for multidrug resistance by intracellular acidification through the crosstalk of MAPK signaling pathways. Int J Biochem Cell Biol. 2014;54:111–121. doi: 10.1016/j.biocel.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Liu D, Zhang J, Fong C, Yang M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater Sci Eng C Mater Biol Appl. 2014;42:70–77. doi: 10.1016/j.msea.2013.12.066. [DOI] [PubMed] [Google Scholar]
- 41.Verykokakis M, Papadaki C, Vorgia E, Le Gallic L, Mavrothalassitis G. The RAS-dependent ERF control of cell proliferation and differentiation is mediated by c-Myc repression. J Biol Chem. 2007;282:30285–942. doi: 10.1074/jbc.M704428200. [DOI] [PubMed] [Google Scholar]
- 42.Gao Z, Zhang P, Xie M, Gao H, Yin L, Liu R. miR-144/451 cluster plays an oncogenic role in esophageal cancer by inhibiting cell invasion. Cancer Cell Int. 2018;18:184. doi: 10.1186/s12935-018-0679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witten LW, Cheng CJ, Slack FJ. miR-155 drives oncogenesis by promoting and cooperating with mutations in the c-kit oncogene. Oncogene. 2019;38:2151–2161. doi: 10.1038/s41388-018-0571-y. [DOI] [PubMed] [Google Scholar]
- 44.Shirafkan N, Shomali N, Kazemi T, Shanehbandi D, Ghasabi M, Baghbani E, Ganji M, Khaze V, Mansoori B, Baradaran B. microRNA-193a-5p inhibits migration of human HT-29 colon cancer cells via suppression of metastasis pathway. J Cell Biochem. 2018 Dec 2; doi: 10.1002/jcb.28164. (Epub ahead of print). doi: 10.1002/jcb.28164. [DOI] [PubMed] [Google Scholar]
- 45.Garcia TY, Gutierrez M, Reynolds J, Lamba DA. Modeling the dynamic AMD-associated chronic oxidative stress changes in human ESC and iPSC-derived RPE cells. Invest Ophthalmol Vis Sci. 2015;56:7480–7482. doi: 10.1167/iovs.15-17251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.