Abstract
Expression of Ki-67 and P16 proteins in cervical cancer and precancerous lesions of young women and the diagnostic value for cervical cancer and precancerous lesions were investigated. A total of 64 paraffin-embedded specimens of uterus tissue from young female patients who were admitted to Jiading District Central Hospital Affiliated to Shanghai University of Medicine and Health Sciences from January 2015 to December 2017 were selected. According to pathological examination, the specimens were divided into chronic cervicitis group (control group, 10 cases), low-grade squamous intraepithelial lesion (LSIL) group (12 cases), high-grade squamous intraepithelial lesion (HSIL) group (20 cases) and squamous carcinoma of the cervix (SCC) group (22 cases). Expression of Ki-67 and P16 protein was detected by immunohistochemistry and the diagnostic values were analyzed. Positive rates of Ki-67 and P16 expression in HSIL and SCC groups were significantly higher than those in LSIL and control groups (P<0.05), but there was no significant difference between LSIL and control groups (P>0.05). Spearman's analysis showed that the expression levels of Ki-67 and P16 were positively correlated with the degree of cervical lesions (rs=0.725; rs=0.829), and their expression levels were also positively correlated (rs=0.772). Sensitivity and specificity analysis showed that the Ki-67 diagnosis has higher sensitivity (95.2%), but the specificity is poor (86.7%). Diagnosis using P16 has high specificity (94.6%), but the sensitivity is poor (85.4%). When the two were combined for diagnosis, sensitivity (94.8%) and specificity (93.2%) were both at a high level. The combined detection of Ki-67 and P16 protein has a high application prospect as an auxiliary diagnosis of SCC.
Keywords: Ki-67 protein, P16 protein, cervical cancer, precancerous lesions, auxiliary diagnosis
Introduction
Cervical cancer is one of the most common malignant tumors in gynecology worldwide. The latest clinical data show that the incidence of cervical cancer in young women is increasing year by year (1). Cervical squamous intraepithelial lesion (CSIL) is an important transitional stage of normal cervical tissue transforming to squamous carcinoma of the cervix (SCC) (2). According to the new classification criteria proposed by the LAST Project in 2012, CSIL is classified into low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) (3). LSIL is the same as cervical intraepithelial neoplasia (CIN) I in the traditional CIN classification standard, and represents a non-carcinogenic human papillomavirus (HPV) infection, which is generally resolved without treatment. HSIL (same as CIN II and III) is a precancerous lesion and often requires surgical intervention to inhibit further progression to SCC. Therefore, it is important to establish a detection method that can quickly and effectively separate LSIL, HSIL, and SCC, which is clinically important for the design of patient treatment plans.
Cell cycle-dependent protein kinase inhibitor P16 is a protein that can negatively regulate the cell cycle. HPV persistent infection causes overexpression of P16 (4), but P16 expression is also present in normal cells. P16 is of great significance for the screening of cervical cancer, but by itself may not be sufficient for diagnosis. Ki-67 is a nuclear antigen that can be detected in the non-G0 phase of the cell cycle, marking the process of cell proliferation (5). For normal tissues, the simultaneous expression of P16 and Ki-67 is less likely to occur (6).
The aim of the study was to explore the expression of Ki-67 and P16 protein in different cervical tissues, and provide reference for their applications in SCC screening. Results showed that the combined detection of Ki-67 and P16 protein has a high application prospect as an auxiliary diagnosis of SCC.
Patients and methods
General information
All paraffin specimens were selected from 64 female patients in the Department of Obstetrics and Gynecology who were admitted by Jiading District Central Hospital Affiliated to Shanghai University of Medicine and Health Sciences (Shanghai, China) from January 2015 to December 2017 due to abnormal TCT screening for colposcopic biopsy. According to the postoperative pathological examination (diagnostic criteria refer to the 2014 fourth edition of the female genital tumor WHO classification), the patients were divided into chronic cervicitis group (control group, 10 cases), LSIL group (12 cases), HSIL group (20 cases) and SCC group (22 cases). The selected patients had no serious internal or surgical diseases after examination, and were not treated with hormones, chemotherapy or radiotherapy within 3 months before admission. The general information of the four groups of patients is shown in Table I.
Table I.
General information of patients.
| Groups | Age (years) | Median age (years) |
|---|---|---|
| Chronic cervicitis | 20–34 | 26 |
| LSIL | 19–34 | 25 |
| HSIL | 20–33 | 26 |
| SCC | 20–35 | 28 |
LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous carcinoma of the cervix.
The study was approved by the Ethics Committee of Jiading District Central Hospital Affiliated to Shanghai University of Medicine and Health Sciences. Patients who participated in this study had complete clinical data. Signed informed consents were obtained from the patients and/or the guardians.
Immunohistochemistry
The selected paraffin tissue was serially sliced to approximately 5 µm. After conventional baking and dewaxing, the endogenous peroxidase blocker was used to inactivate the peroxidase in tissue, and the antigen was repaired after high temperature treatment. After that, non-specific antigen (goat serum) was used for blocking at 37°C for 10 min. After incubating with primary antibodies (rabbit anti-human Ki-67 and P16 monoclonal antibodies; 1:100; cat. nos. ab16667 and ab51243, respectively; Abcam, Cambridge, MA, USA) for 1 h, secondary goat anti-rabbit polyclonal antibody (1:5,000; cat. no. ab207995; Abcam) was added and incubated for 30 min. After that, horseradish peroxidase-labeled streptomycin avidin (SP) was added and incubated for 30 min. DAB dye solution was quickly added, and staining time was controlled by observation under a microscope. Excess dye was rinsed off with PBS, the hematoxylin stain solution was added, followed by the addition of hydrochloric acid and observation under an optical microscope (Xuzhou Senpu Optoelectronics Technology Co., Ltd.).
Scoring criteria: P16 is mainly expressed in the nucleus and cytoplasm, and color is brown when expression is positive. When there is no brown granule in the cell, it is marked as (−); when it is mainly stained in the lesion or epithelial basal layer, it is marked as (+); when the coloration extends to 1/3-2/3 layer of the squamous epithelium of the cervix, it is marked as (++); when the coloration extends to 2/3 to whole layer of the squamous epithelium of the cervix, it is marked as (+++); Ki-67 is mainly expressed in nucleus, and color is also brown when the expression is positive. When 0 to 5% of cells are stained, it is marked as (−); when 6 to 25% of cells are stained, it is marked as (+); when 26 to 75% of cells are stained, it is marked as (++); when 76–100% of cells are stained, it is marked as (+++).
Statistical analysis
Experimental data were analyzed by SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Comparison of the countable data was performed by Fisher's exact test and χ2 test. Spearman was used for correlation rank analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of P16 protein in four groups of patients
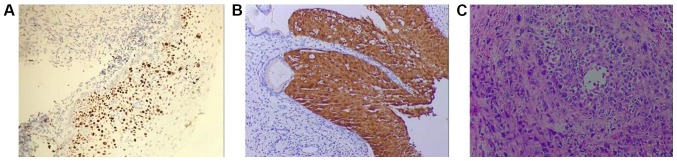
As shown in Fig. 1, in LSIL group, no brown particles appeared in cells (Fig. 1A). Staining of the HSIL group showed that stained part extended to 1/3 to 2/3 layer of squamous epithelium of the cervix (Fig. 1B). In SCC group, the stained granules were diffused throughout the whole cervical squamous epithelium (Fig. 1C). In the control, LISL, HISL and SCC groups, positive rate of P16 expression was 0, 9.09, 65.00 and 95.65%, respectively. Control group had significant difference compared with HSIL and SCC groups (P<0.05), and LISL group had significant difference compared with HSIL and SCC groups (P<0.05), there was also a significant difference between HSIL and SCC groups (P<0.05), but there was no significant difference between control and LISL groups (P>0.05) (Table II).
Figure 1.
Expression of P16 protein in LSIL, HSIL and SCC. (A) LISL; (B) HISL; (C) SCC (magnification, ×400). LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous carcinoma of the cervix.
Table II.
Expression of P16 protein in four groups of patients.
| Groups | n | – | + | ++ | +++ | Positive rate (%) |
|---|---|---|---|---|---|---|
| Control | 10 | 10 | 0 | 0 | 0 | 0 |
| LSIL | 11 | 10 | 1 | 0 | 0 | 9.09 |
| HSIL | 20 | 7 | 7 | 5 | 1 | 65.00a,b |
| SCC | 23 | 1 | 5 | 8 | 9 | 95.65a–c |
Compared with control group
P<0.05; compared with LSIL group
P<0.05; compared with HSIL group
P<0.05. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous carcinoma of the cervix.
Expression of Ki-67 protein in four groups of patients
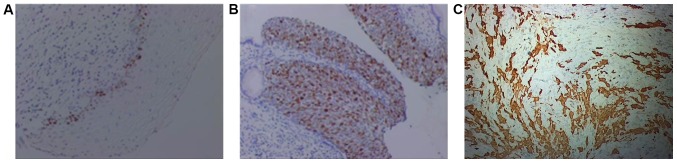
As shown in Fig. 2, only a small number of cells in LSIL group were stained brown (Fig. 2A), and in HSIL group more than 50% of cells were stained brown (Fig. 2B), in SCC group, stained particles diffused throughout the whole observation field (Fig. 2C). In control, LISL, HISL and SCC groups, positive rates of Ki-67 expression were 0, 18.18, 55.00 and 100%, respectively. Control group had significant difference compared with HSIL and SCC groups (P<0.05), and LISL group had significant difference compared with HSIL and SCC groups (P<0.05), there was also a significant difference between HSIL and SCC groups (P<0.05), but there was no significant difference between control and LISL groups (P>0.05) (Table III).
Figure 2.
Expression of Ki-67 protein in LSIL, HSIL and SCC. (A) LSIL; (B) HSIL; (C) SCC (magnification, ×400). LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous carcinoma of the cervix.
Table III.
Expression of Ki-67 protein in four groups of patients.
| Groups | n | – | + | ++ | +++ | Positive rate (%) |
|---|---|---|---|---|---|---|
| Control | 10 | 10 | 0 | 0 | 0 | 0 |
| LSIL | 11 | 9 | 2 | 0 | 0 | 18.18 |
| HSIL | 20 | 9 | 6 | 4 | 1 | 55.00a,b |
| SCC | 23 | 0 | 2 | 6 | 15 | 100.00a–c |
Compared with control group
P<0.05; compared with LSIL group
P<0.05; compared with HSIL group
P<0.05. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous carcinoma of the cervix.
Analysis of correlation between P16 and Ki-67
Correlation between expression levels of two tumor markers (P16/Ki-67) in patients with chronic cervicitis, LISL, HISL and SCC was analyzed by Spearman rank analysis. Results showed that the expression levels of P16 and Ki-67 were significantly and positively correlated with the degree of cervical lesions. Expression levels of P16 and Ki-67 were also positively correlated. Correlation indices were rs=0.725, P<0.001<0.05; rs=0.829, P<0.001; rs=0.772, P<0.001<0.05 (data not shown).
Diagnostic values of P16 and Ki-67 for cervical cancer and precancerous lesions
Based on pathological diagnosis, diagnostic values of P16 and Ki-67 for cervical cancer and precancerous lesions were evaluated. Sensitivity of P16 and Ki-67 alone was 85.4 and 95.2%, respectively, and area under the specific curves was 94.6 and 86.7%, respectively. Sensitivity of the combined detection was 94.8% and the specificity was 93.2% (Table IV).
Table IV.
Diagnostic values of P16 and Ki-67 alone and combined detection for cervical cancer and precancerous lesions.
| Indicators | Sensitivity (%) | Specificity (%) |
|---|---|---|
| P16 | 85.4 | 94.6 |
| Ki-67 | 95.2 | 86.7 |
| P16+Ki-67 | 94.8 | 93.2 |
Discussion
Cervical cancer is the only malignant tumor in the world with known causes and preventable measures. Numerous studies have shown that the occurrence of cervical cancer is associated with persistent infection of HPV (7,8), and the HPV screening is becoming increasingly popular. However, LSIL caused by general HPV infection usually resolves on its own and no additional treatment is required. Some of the LSIL will transform into HSIL, which will eventually lead to the occurrence of SCC (9). Therefore, it is extremely important to find an auxiliary diagnosis method that can indicate the extent of cervical lesions.
In the screening of early cervical cancer molecular markers, P16, a tumor suppressor gene, can participate in the regulation of normal cell cycle and is of concern to researchers (10,11). It has been reported that P16 has a predictive value for the extent of cervical lesions (12,13) and has been recommended by the American College of Pathology, the American Society of Colposcopy and Cervical Pathology as a biomarker for the diagnosis of CSIL. In the present study, P16 was not expressed or expressed in a small amount in common cervical tissues and LSIL tissues, whereas in HSIL tissues, P16 protein was expressed in the 1/3 to 2/3 layers of the cervical squamous epithelium, and in SCC tissues. P16 expression was positive in the whole squamous epithelium of the cervix, which indicated that the expression intensity of P16 was highly correlated with the degree of cervical tissue lesions. It was confirmed that there was a positive correlation between expression intensity of P16 and the degree of cervical tissue lesions. This study showed that there was no significant difference in the expression of P16 between normal cervical tissue and LSIL tissue, suggesting that P16 does not fully reflect the severity of cervical lesions.
As a gene capable of promoting cell proliferation, abnormal expression of Ki-67 protein usually indicates abnormal cell proliferation (14,15). Results of this study showed that Ki-67 expression was not detected in normal cervical tissues, but expression was detected in LSIL, HSIL and SCC, and the positive rate of Ki-67 expression was significantly increased with the increased degree of cervical lesions. From the results of immunohistochemistry, it can be seen that there are only a few brown cells in the LSIL tissue. As the disease progresses, the brown particles diffuse throughout the whole observation field. Ki-67 can also be used as a biomarker for cervical lesions, which has important diagnostic significance for tumor growth, grading, proliferation and prognosis. However, some studies have also shown that Ki-67 is expressed in non-cancerous proliferative cervical tissues (16), and in this study, LISL patients also have a positive expression rate of 18.18%. Therefore, in the identification of cervical cancer lesions, Ki-67 and P16 can be combined for diagnosis.
In summary, the expression intensity of P16 and Ki-67 was positively correlated with the degree of cervical lesions, and the sensitivity and specificity of the combination of P16 and Ki-67 were satisfactory. Therefore, the combination of P16 and Ki-67 can identify patients with high risk of SCC and reduce the rate of misdiagnosis, which is of high value for the differential diagnosis of young women with SCC and CSIL. However, studies with larger sample size are needed to further confirm the conclusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QS wrote the manuscript. QS and LX performed immunohistochemistry. RY and YM performed the research, collected and analyzed the data of this study. QS and LQ were responsible for data analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Jiading District Central Hospital Affiliated to Shanghai University of Medicine and Health Sciences (Shanghai, China). Patients who participated in this study had complete clinical data. Signed informed consents were obtained from the patients and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sharkas G, Arqoub K, Khader Y, Nimri O, Shroukh W, Jadallah H, Saheb T. Trends in the incidence of cervical cancer in Jordan, 2000–2013. J Oncol. 2017;2017:6827384. doi: 10.1155/2017/6827384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aho I, Kivelä P, Haukka J, Sutinen J, Heikinheimo O. Declining prevalence of cytological squamous intraepithelial lesions of the cervix among women living with well-controlled HIV-most women living with HIV do not need annual PAP smear screening. Acta Obstet Gynecol Scand. 2017;96:1330–1337. doi: 10.1111/aogs.13207. [DOI] [PubMed] [Google Scholar]
- 3.Luttmer R, Berkhof J, Dijkstra MG, van Kemenade FJ, Snijders PJ, Heideman DA, Meijer CJ. Comparing triage algorithms using HPV DNA genotyping, HPV E7 mRNA detection and cytology in high-risk HPV DNA-positive women. J Clin Virol. 2015;67:59–66. doi: 10.1016/j.jcv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kim NR, Lin Z, Kim KR, Cho HY, Kim I. Epstein-Barr virus and p16INK4A methylation in squamous cell carcinoma and precancerous lesions of the cervix uteri. J Korean Med Sci. 2005;20:636–642. doi: 10.3346/jkms.2005.20.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carreras R, Alameda F, Mancebo G, García-Moreno P, Mariñoso ML, Costa C, Fusté P, Baró T, Serrano S. A study of Ki-67, c-erbB2 and cyclin D-1 expression in CIN-I, CIN-III and squamous cell carcinoma of the cervix. Histol Histopathol. 2007;22:587–592. doi: 10.14670/HH-22.587. [DOI] [PubMed] [Google Scholar]
- 6.Iaconis L, Hyjek E, Ellenson LH, Pirog EC. p16 and Ki-67 immunostaining in atypical immature squamous metaplasia of the uterine cervix: correlation with human papillomavirus detection. Arch Pathol Lab Med. 2007;131:1343–1349. doi: 10.5858/2007-131-1343-PAKIIA. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Wang G, Zhang L, Cong J, Hou J, Liu C. LncRNA PVT1 promotes the growth of HPV positive and negative cervical squamous cell carcinoma by inhibiting TGF-β1. Cancer Cell Int. 2018;18:70. doi: 10.1186/s12935-018-0567-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulk S, Berkhof J, Bulkmans NW, Zielinski GD, Rozendaal L, van Kemenade FJ, Snijders PJ, Meijer CJ. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br J Cancer. 2006;94:171–175. doi: 10.1038/sj.bjc.6602915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano T, Masuda N, Oyama T, Nakajima T. Overexpression of p16 and p14ARF is associated with human papillomavirus infection in cervical squamous cell carcinoma and dysplasia. Pathol Int. 2002;52:375–383. doi: 10.1046/j.1440-1827.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi-Lavina M, Chapman-Fredricks J, Gomez-Fernandez C, Ganjei-Azar P, Manoharan M, Jorda M. P16 expression in squamous cell carcinomas of cervix and bladder. Appl Immunohistochem Mol Morphol. 2010;18:344–347. doi: 10.1097/PAI.0b013e3181d2bbd7. [DOI] [PubMed] [Google Scholar]
- 11.Tozawa-Ono A, Yoshida A, Yokomachi N, Handa R, Koizumi H, Kiguchi K, Ishizuka B, Suzuki N. Heat shock protein 27 and p16 immunohistochemistry in cervical intraepithelial neoplasia and squamous cell carcinoma. Hum Cell. 2012;25:24–28. doi: 10.1007/s13577-011-0040-1. [DOI] [PubMed] [Google Scholar]
- 12.Cheah PL, Looi LM, Mun KS, Abdoul Rahman N, Teoh KH. Implications of continued upregulation of p16(INK4a) through the evolution from high-grade squamous intraepithelial lesion to invasive squamous carcinoma of the cervix. Malays J Pathol. 2011;33:83–87. [PubMed] [Google Scholar]
- 13.Wang Z, Dong J, Eyzaguirre EJ, Tang WW, Eltorky MA, Qiu S. Detection of human papilloma virus subtypes 16 and P16(ink4a) in invasive squamous cell carcinoma of the fallopian tube and concomitant squamous cell carcinoma in situ of the cervix. J Obstet Gynaecol Res. 2009;35:385–389. doi: 10.1111/j.1447-0756.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu JQ, Zhou Q, Zheng YF, Bao Y. Expression of vimentin and Ki-67 proteins in cervical squamous cell carcinoma and their relationships with clinicopathological features. Asian Pac J Cancer Prev. 2015;16:4271–4275. doi: 10.7314/APJCP.2015.16.10.4271. [DOI] [PubMed] [Google Scholar]
- 15.Gertych A, Joseph AO, Walts AE, Bose S. Automated detection of dual p16/Ki67 nuclear immunoreactivity in liquid-based Pap tests for improved cervical cancer risk stratification. Ann Biomed Eng. 2012;40:1192–1204. doi: 10.1007/s10439-011-0498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson B, Goldberg I, Lerner-Geva L, Gotlieb WH, Ben-Baruch G, Novikov I, Kopolovic J. Expression of topoisomerase II and Ki-67 in cervical carcinoma - clinicopathological study using immunohistochemistry. APMIS. 2000;108:209–215. doi: 10.1034/j.1600-0463.2000.d01-46.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.